Abstract
Objective:
This study investigates the change of endothelial cell morphology and function at the rabbit basilar bifurcations in response to sustained high blood flow after bilateral common carotid artery ligation.
Methods:
Fifteen adult female New Zealand white rabbits were divided into experimental and sham control groups. The experimental group was subjected to bilateral common carotid artery ligation to increase the compensatory basilar artery flow. Basilar artery flow was monitored by transcranial Doppler after surgery. The endothelial cells at the arterial bifurcations were studied morphologically by electron microscopy and immunohistochemistry using β-catenin antibodies. Basilar artery flow increased significantly following common carotid artery ligation.
Results:
Early-stage basilar artery bifurcation aneurysms were present in all rabbits at three months after ligation. The endothelial cells changed from a fusiform to column shape at the basilar artery bifurcation. Gaps between endothelial cells of the experimental group appeared wider in the electron microscopic photographs compared with those of the control group. The expression of endothelial β-catenin at the arterial bifurcations also decreased.
Conclusion:
This study is the first to present endothelial cell changes of basilar artery bifurcation in response to sustained high blood flow in rabbits. Endothelial cell impairment possibly initiates aneurysm formation.
Keywords: Electron, endothelium, immunohistochemistry, intracranial aneurysm, rabbit experiment
Abstract
Objetivo:
Este estudio investiga el cambio de morfología de la célula endotelial y la función en las bifurcaciones basilares del conejo en respuesta al flujo sanguíneo alto sostenido después de la ligadura de la arteria carótida común bilateral.
Métodos:
Quince conejos blancos adultos hembras de Nueva Zelanda fueron divididos en un grupo experimental y un grupo de control con procedimiento simulado. El grupo experimental fue sometido a la ligadura de la arteria carótida común bilateral para aumentar el flujo de la arteria basilar compensatoria. El flujo de la arteria basilar fue monitoreado mediante Doppler transcraneal después de la cirugía. Las células endoteliales en las bifurcaciones arteriales fueron estudiadas morfológicamente mediante microscopia electrónica e inmunohistoquímica usando anticuerpos β-catenina. El flujo de la arteria basilar aumentó considerablemente tras la ligadura de la arteria carótida común.
Resultados:
Aneurismas de la bifurcación de la arteria basilar producidos en etapa temprana, se hallaban presentes en todos los conejos tres meses después de la ligadura. Las células endoteliales cambiaron de forma fusiforme a forma de columna en la bifurcación de la arteria basilar. Los espacios entre las células endoteliales del grupo experimental aparecían con mayor amplitud en las fotografías microscópicas electrónicas comparados con los del grupo de control. También disminuyó la expresión de β-catenina endotelial en las bifurcaciones arteriales.
Conclusión:
Este estudio es el primero en presentar cambios de la célula endotelial de la bifurcación de la arteria basilar en respuesta al flujo sanguíneo alto sostenido en conejos. El deterioro de la célula endotelial posiblemente inicia la formación de aneurismas.
INTRODUCTION
Intracranial aneurysms are a major cause of subarachnoid haemorrhage, which is characterized by high morbidity and mortality rates. Despite the catastrophic consequences of their rupture, the mechanisms underlying the formation, growth and rupture of cerebral aneurysms remain unclear. Given the poor prognosis of ruptured intracranial aneurysms, clarification of the pathological changes that lead to their formation is of paramount importance. Although hypertension and haemodynamic shear stress appear to be major aneurysmogenic factors (1, 2), considerable controversy still exists regarding the arterial layer critical for intracranial aneurysm formation, factors that damage this layer, and aneurysmal wall formation.
Endothelial cells (ECs), which form the inner lining of blood vessels, are known sensors of wall shear stress (3, 4) and are critical for vascular homeostasis. Most flow-endothelium research to date has focussed on EC exposure to shear stresses of low or normal values. These values are relevant to studies of atherogenesis, which preferentially occurs in low-flow regions in sinuses along the arterial tree. Atherogenesis is characterized by disturbed flow or flow recirculation and reattachment, high oscillatory shear index, and high wall shear stress gradients (5–7). Atherogenic ECs exhibit increased motility, proliferation, permeability, turnover, surface adhesion and altered protein expression responses that would make atherogenesis-prone arteries in vivo (8–14). However, limited information can be found on EC behaviour in the vicinity of the arterial bifurcation apex, a location prone to saccular aneurysm formation in the cerebral vasculature (15–18). The haemodynamics at the bifurcation apex is quite different. With the blood flow impinging at the apex and accelerating downstream, the apex environment constitutes an area exposed to flow stagnation and low wall shear stress, but high wall shear stress gradients, as well as an adjacent area experiencing high wall shear stress and high wall shear stress gradients (19, 20). In contrast to the well-studied EC function under low wall shear stress and disturbed flow at arterial sinuses, few studies exist on the effect of impinging flow on EC function. In the current study, in vivo experiments on the effects of impinging flow on EC morphology, alignment and movement were performed.
SUBJECTS AND METHODS
Animal model
Basilar bifurcation aneurysm in rabbits was experimentally induced according to the method described by Gao et al (15). Adult female New Zealand white rabbits (body weight 3 kg to 4 kg) were subjected to bilateral carotid artery ligation to obtain different degrees of flow rate increase in basilar artery. Animal survival rates with bilateral common carotid artery ligation were improved by first subjecting the left common carotid arteries to ligation, followed by right common carotid artery occlusion two weeks later in our experiment. Ten rabbits were randomly selected for bilateral common carotid artery ligation, whereas the five remaining rabbits comprised the sham control group; their bilateral common carotid arteries were exposed but not ligated. This study was carried out in strict compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Nanchang University.
Basilar artery flow measurements
Basilar artery blood flow was measured using transcranial Doppler on 0, 1, 4, 7, 14, 28, 35, 42, 49, 56, 70 and 84 days after surgery.
Tissue preparation
The basilar arteries were isolated from the rabbits under general anaesthesia at 1.5 and three months after bilateral common carotid artery ligation. At each time point, three of the five basilar arteries were fixed in 4% formaldehyde for 24 hours and routinely processed for paraffin embedding for immunohistochemistry, whereas the remaining two basilar arteries were fixed in 2.5% glutaraldehyde for electron microscopy.
Scanning electron microscopy
Dissected tissues were fixed in 2.5% glutaraldehyde for two hours in 0.1 M phosphate buffer (pH 7.4) and refixed for two hours at 4 °C with 1% osmium tetroxide in cacodylate buffer. The samples were rinsed in water, dehydrated in a graded series of ethanol to propylene oxide, and then infiltrated and embedded in epoxy resin. Ultrathin sections were contrasted with uranyl acetate and lead citrate, and examined using a Hitachi H-600 electron microscope.
Immunohistochemical investigation of β-catenin expression
The sections were preincubated with normal goat serum, and then incubated for 60 minutes with monoclonal β-catenin antibodies after eliminating endogenous peroxidase activity by 0.3% H2O2 for 10 minutes. The sections were washed in trisphosphate buffered saline (TPBS, pH 7.6, three times, three minutes/time) and subsequently incubated for 10 minutes with anti-rabbit secondary antibody. The sections were then washed in TPBS for five minutes (three times), and a brown stain was produced by treating the sections with 3, 39-diaminobenzidine (DAB). All incubations were performed at room temperature. Entire sections of the arterial segments were examined using an Olympus microscope at 20× magnification to quantify the EC.
Statistical analysis
All data are represented as mean ± SD. Statistical analysis was performed via t-test.
RESULTS
Early-stage basilar artery bifurcation aneurysms were present in all rabbits at three months after bilateral common carotid artery ligation.
Basilar artery flow analysis
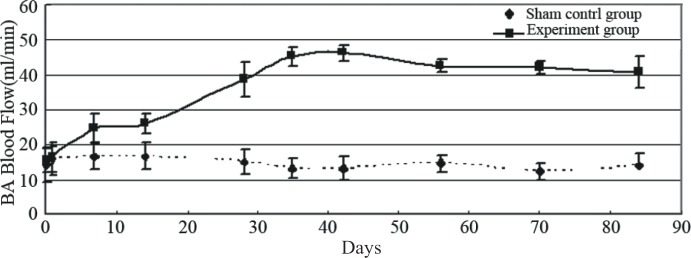
Basilar artery flow significantly increased within two weeks after bilateral common carotid artery ligation from about 15 ml/minute to more than 40 ml/minute. In addition, high blood flow was sustained at least three months after surgery (Fig. 1).
Fig. 1. Line graph showing the basilar artery (BA) blood flow after the common carotid artery ligation.
Ultrastructural changes of endothelial cells at basilar artery bifurcation
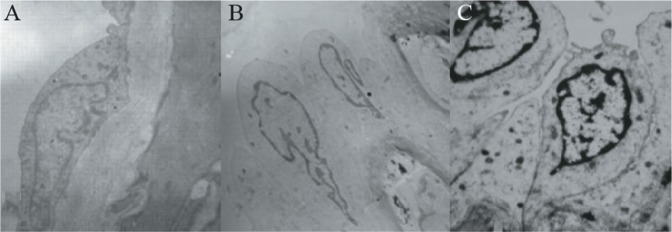
Scanning electron micrographs showed that ECs of the basilar artery bifurcations in sham-operated rabbits were fusiform-shaped. The integrity of the endothelium was evidenced by the absence of cracks between ECs (Fig. 2A). Compared with the basilar artery bifurcations in the sham-operated group, the ECs changed from a fusiform to a column shape at 1.5 months after bilateral common carotid artery ligation in both rabbits (Fig. 2B). The gaps between ECs appeared wider in both rabbit groups at three months after surgery. The EC nuclei began to show margination, indicating EC apoptosis (Fig. 2C).
Fig. 2. Scanning electron micrographs. (A) The endothelial cells of the basilar artery bifurcation were fusiform in the sham operation group; (B) 1.5 months after bilateral common carotid artery ligation, the endothelial cells of basilar artery bifurcation were morphologically changed from fusiform to column shape; (C) three months after bilateral common carotid artery ligation, the endothelium presents wide gaps between the endothelial cells (ECs), and ECs and endothelial cell nucleus showed margination.
β-catenin expression of endothelial cells at the basilar arterial bifurcation
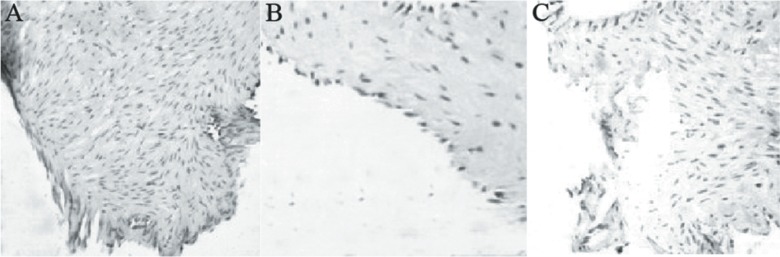
The β-catenin in the sham control group was mainly expressed in the plasmalemma (Fig. 3A). However, the β-catenin was only expressed in the nucleus at 1.5 months after bilateral common carotid artery ligation (Fig. 3B). Early-stage rabbit basilar artery bifurcation aneurysm was present and the EC and β-catenin expression at the rabbit basilar arterial bifurcations could hardly be found at three months after bilateral common carotid artery ligation (Fig. 3C).
Fig. 3. The expression of beta-catenin at the basilar artery bifurcation. (A) In the sham control, the beta-catenin was mainly expressed on the plasmalemma of endothelial cells; (B) 1.5 months after the rabbit bilateral common carotid artery ligation, beta-catenin was mainly expressed in the nucleus of endothelial cells. (C) three months after the rabbit left common carotid artery, the whole intima was corroded and endothelial cells and the expression of beta-catenin almost disappeared.
DISCUSSION
The mechanism of intracranial aneurysm formation is still unclear. The complex haemodynamic environment at the arterial bifurcations is responsible for intracranial aneurysm formation, which is characterized by high wall shear stress (a frictional force on the endothelium from blood flow) and high wall shear stress gradient. Endothelial injury and inflammatory response induced by haemodynamic changes preceded intracranial aneurysm formation. Endothelial injury is the basic step in the pathogenesis of aneurysm, which was revealed in an experimental study on a rat renal hypertension induced intracranial aneurysm model (21). Recently, a study demonstrated that high haemodynamic environment at arterial bifurcations induced nascent aneurysms by bilateral common carotid artery ligation in rabbits (15). A research in vitro cell model found that the EC density of an artery bifurcation was reduced because of impinging flow mimicking. The EC behaviour in an impinging flow environment can lead to aneurysm-initiating vascular remodelling (22). However, what happened to the ECs in vivo still needs to be clarified.
Endothelial cells are capable of responding to fluid shear stress by morphological alterations and cytoskeletal component distribution (23). Physiological fluid-induced shear stress transforms polygonal, cobblestone-shaped ECs of random orientation into fusiform ECs aligned to the blood flow direction, decreases EC proliferation, and increases vasodilator production. The exposure of ECs to high supra-physiological shear stress levels results in EC dysfunction and reduced vasodilator production, with a possible progression to EC degeneration (24–26)
In this study, EC changes in the morphology, intercellular junction and intensity of β-catenin expressed in response to high flow after bilateral common carotid artery ligation were observed at basilar artery bifurcations. The EC morphology at basilar bifurcations in the scanning electron micrographs showed changes from fusiform to column shape at 1.5 months after bilateral common carotid artery ligation. The increasing shear stress from sustained high blood flow is important in EC morphological alteration. At this time, β-catenin expression in the ECs was displaced from plasmalemma to the nucleus. Early aneurysmal changes at basilar bifurcations were observed at three months after bilateral common carotid artery ligation. The EC nucleus at the basilar artery bifurcations began to show margination that indicated EC apoptosis, whereas the ECs as well as the β-catenin expression at the rabbit basilar artery bifurcations almost disappeared. In ECs, β-catenin is mainly a structural component of adherent junctions, which binds to cadherin and composes the β-catenin-cadherin complex to sustain EC homeostasis (27). The ECs maintain contact with one another through a complex network of transmembrane adhesion proteins anchored to the actin cytoskeleton. The defect of β-catenin at the cell membrane indicates EC instability. Growing evidence indicates that endothelial cell-to-cell adhesion is accompanied by intracellular signalling. Sparse and confluent cells present a different functional phenotype (27). Confluent cells have an epithelioid morphology and are resistant to apoptotic stimuli. Furthermore, their motility and paracellular permeability to plasma solutes and/or inflammatory cells are reduced. Gene profiles of sparse and confluent cells also show that several genes are regulated by cellcell contacts, of which several are implicated in cell growth, apoptosis, matrix and cytoskeletal remodelling. In addition, the intracellular signalling axis serves in activating gene transcription by translocation of β-catenin to the nucleus, and is a signal for the T-cell factor (TCF)-/lymphoid-enhancer factor (LEF) transcription factor activation (28). β-catenin localization at the plasma membrane is controlled by a cytosolic multiprotein complex consisting of proteins axin, adenomatosis polyposis coli (APC), and glycogen synthase kinase-3 (28–30). This complex regulates β-catenin phosphorylation when released into the cytosol, which is a signal for ubiquitination and lysosomal degradation of β-catenin. This mechanism is crucial for mitogenic quiescence maintenance, whereby if targeted to the nucleus, β-catenin promotes the transcription of TCF-/LEF-dependent genes, including cell cycle regulatory proteins (eg cyclin D1), growth factors (eg vascular endothelial growth factor [VEGF]), matrix proteins (eg fibronectin, versican), proteases (eg metalloproteinase (MMP)-2, -7, -9), and proinflammatory enzymes and mediators [eg cyclooxygenase (COX)-2 and interleukin (IL)-8] (28, 29). These endothelial changes are similar to the initial stage of aneurysm formation. Endothelial cell apoptosis possibly causes morphological alterations, EC dysfunction and intercellular adherent junctions at the basilar artery bifurcations.
Another structural change of ECs was the appearance of wide gaps between ECs at three months after bilateral common carotid artery ligation. The wider gaps can increase vascular permeability, resulting in inflammatory cells coming into the vessel walls. The inflammatory cells can release mass of proteases, which hydrolyse the structural protein of the arterial wall, thus promoting intracranial aneurysm formation (31, 32).
In conclusion, EC structure injury and apoptosis by sustained high blood flow ultimately caused early-stage intracranial aneurysm in basilar bifurcations after bilateral common carotid artery ligation. Morphologic and functional changes of ECs after sustained high blood flow possibly precede aneurysm formation at arterial bifurcations. Moreover, the EC possibly plays a key role in the pathogenesis of intracranial aneurysm formation.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 30960397).
REFERENCES
- 1.Burleson AC, Turitto VT. Identification of quantifiable hemodynamic factors in the assessment of cerebral aneurysm behavior. On behalf of the Subcommittee on Biorheology of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1996;76:118–123. [PubMed] [Google Scholar]
- 2.Inagawa T. Trends in surgical and management outcomes in patients with aneurysmal subarachnoid hemorrhage in Izumo city, Japan, between 1980-1989 and 1990-1998. Cerebrovasc Dis. 2005;19:39–48. doi: 10.1159/000081910. [DOI] [PubMed] [Google Scholar]
- 3.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 4.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971;177:109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- 6.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 7.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 8.Chiu JJ, Wang DL, Chien S, Skalak R, Usami S. Effects of disturbed flow on endothelial cells. J Biomech Eng. 1998;120:2–8. doi: 10.1115/1.2834303. [DOI] [PubMed] [Google Scholar]
- 9.Chiu JJ, Chen CN, Lee PL, Yang CT, Chuang HS, Chien S, et al. Analysis of the effect of disturbed flow on monocytic adhesion to endothelial cells. J Biomech. 2003;36:1883–1895. doi: 10.1016/s0021-9290(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 10.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 11.DePaola N, Davies PF, Pritchard WF, Jr, Florez L, Harbeck N, Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA. 1999;96:3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol. 2004;286:H1916–H1922. doi: 10.1152/ajpheart.00897.2003. [DOI] [PubMed] [Google Scholar]
- 13.LaMack JA, Himburg HA, Li XM, Friedman MH. Interaction of wall shear stress magnitude and gradient in the prediction of arterial macromolecular permeability. Ann Biomed Eng. 2005;33:457–464. doi: 10.1007/s10439-005-2500-9. [DOI] [PubMed] [Google Scholar]
- 14.Tardy Y, Resnick N, Nagel T, Gimbrone MA, Jr, Dewey CF., Jr Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler Thromb Vasc Biol. 1997;17:3102–3106. doi: 10.1161/01.atv.17.11.3102. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Hoi Y, Swartz DD, Kolega J, Siddiqui A, Meng H. Nascent aneurysm formation at basilar terminus induced by hemodynamics. Stroke. 2008;39:2085–2090. doi: 10.1161/STROKEAHA.107.509422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto N, Handa H, Nagata I, Hazama F. Experimentally induced cerebral aneurysms in rats: Part V. Relation of hemodynamics in the circle of Willis to formation of aneurysms. Surg Neurol. 1980;13:41–45. [PubMed] [Google Scholar]
- 17.Krex D, Schackert HK, Schackert G. Genesis of cerebral aneurysmsan update. Acta Neurochir (Wien) 2001;143:429–448. doi: 10.1007/s007010170072. [DOI] [PubMed] [Google Scholar]
- 18.Stehbens WE. Histopathology of cerebral aneurysms. Arch Neurol. 1963;8:272–285. doi: 10.1001/archneur.1963.00460030056005. [DOI] [PubMed] [Google Scholar]
- 19.Meng H, Swartz DD, Wang Z, Hoi Y, Kolega J, Metaxa EM, et al. A model system for mapping vascular responses to complex hemodynamics at arterial bifurcations in vivo. Neurosurgery. 2006;59:1094–1100. doi: 10.1227/01.NEU.0000245599.92322.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007;38:1924–1931. doi: 10.1161/STROKEAHA.106.481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamous MA, Naqahiro S, Kitazato KT, Satoh K, Satomi J. Vascular corrosion casts mirroring early morphological changes that lead to the formation of saccular cerebral aneurysm: an experimental study in rats. J Neurosurg. 2005;102:532–535. doi: 10.3171/jns.2005.102.3.0532. [DOI] [PubMed] [Google Scholar]
- 22.Jamous MA, Naqahiro S, Kitazato KT, Tamura T, Aziz HA, Shono M, et al. Endothelial injury and inflammatory response induced by hemodynamic changes preceding intracranial aneurysm formation: experimental study in rats. J Neurosurg. 2007;107:405–411. doi: 10.3171/JNS-07/08/0405. [DOI] [PubMed] [Google Scholar]
- 23.Szymanski MP, Metaxa E, Meng H, Kolega J. Endothelial cell layer subjected to impinging flow mimicking the apex of an arterial bifurcation. Ann Biomed Eng. 2008;36:1681–1689. doi: 10.1007/s10439-008-9540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cucina A, Sterpetti AV, Pupelis G, Fragale A, Lepidi S, Cavallaro A, et al. Shear stress induces changes in the morphology and cytoskeleton organization of arterial endothelial cells. Eur J Vasc Endovasc Surg. 1995;9:86–92. doi: 10.1016/s1078-5884(05)80230-8. [DOI] [PubMed] [Google Scholar]
- 25.Kazuo K, Yumiko K, Shigeo O. Role of stress fibers and focal adhesions as a mediator for mechano-signal transduction in endothelial cell in situ. Vasc Health Risk Manage. 2008;4:1273–1282. doi: 10.2147/vhrm.s3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levesque MJ, Cornhill JF, Nerem RM. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials. 1990;11:702–707. doi: 10.1016/0142-9612(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 27.Nerem RM, Harrison DG, Taylor WR, Alexander RW. Hemodynamics and vascular endothelial biology. J Cardiovasc Pharmacol. 1993;21:S6–S10. doi: 10.1097/00005344-199321001-00002. [DOI] [PubMed] [Google Scholar]
- 28.Liebner S, Cavallaro U, Dejana E. The multiple languages of endothelial cell-to-cell communication. Arterioscler Thromb Vasc Biol. 2006;26:1431–1438. doi: 10.1161/01.ATV.0000218510.04541.5e. [DOI] [PubMed] [Google Scholar]
- 29.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javaraman T, Paget A, Shin YS, Li X, Mayer J, Chaudhry H, et al. TNFalpha-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc Health Risk Manag. 2008;4:805–817. doi: 10.2147/vhrm.s2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, et al. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H1329–H1335. doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]