Abstract
Thiazolidinediones, a class of medications indicated for the treatment of type 2 diabetes mellitus, reduce inflammation and have been shown to provide a therapeutic benefit in animal models of Parkinson disease. We examined the association between treatment with thiazolidinediones and the onset of Parkinson disease in older individuals. We performed a cohort study of 29,397 Medicare patients enrolled in state pharmaceutical benefits programs who initiated treatment with thiazolidinediones or sulfonylureas during the years 1997 through 2005 and had no prior diagnosis of Parkinson disease. New users of thiazolidinediones were propensity score matched to new users of sulfonylureas and followed to determine whether they were diagnosed with Parkinson disease. We used Cox proportional hazards models to compare time to diagnosis of Parkinson disease in the propensity score–matched populations. To assess the association with duration of use, we performed several analyses that required longer continuous use of medications. In the primary analysis, thiazolidinedione users had a hazard ratio for a diagnosis of Parkinson disease of 1.09 (95% confidence interval: 0.71, 1.66) when compared with sulfonylurea users. Increasing the duration-of-use requirements to 10 months did not substantially change the association; the hazard ratios ranged from 1.00 (95% confidence interval: 0.49, 2.05) to 1.17 (95% confidence interval: 0.60, 2.25). Thiazolidinedione use was not associated with a longer time to diagnosis of Parkinson disease than was sulfonylurea use, regardless of duration of exposure.
Keywords: cohort study, Parkinson disease, thiazolidinediones
Parkinson disease is a common age-related neurodegenerative movement disorder that affects more than 1% of individuals older than 70 years of age (1). Parkinson disease results from progressive degeneration of dopaminergic neurons, primarily in the substantia nigra. Risk factors for Parkinson disease include genetic predisposition and exposure to certain environmental toxins that are believed to cause neuroinflammation (2, 3). Animal and epidemiologic studies have suggested that nervous system inflammation plays a pivotal role in the development of Parkinson disease (4, 5).
Degeneration of dopamine neurons can cause microglial cells to overreact at toxic levels, which can exacerbate inflammation and lead to further neuronal degeneration in a positive feedback loop (6). The thiazolidinediones (TZDs) rosiglitazone, pioglitazone, and troglitazone, which are used to treat type 2 diabetes mellitus because of their insulin-sensitizing properties, might reduce inflammation by acting as agonists on peroxisome proliferator–activated receptors γ, which are found in neurons and glial cells throughout the central nervous system, including the substantia nigra (7). TZDs have been found to suppress microglial activity in animals by interfering with the inflammatory feedback loop and preventing neurodegeneration (6).
The widespread use of TZDs to treat diabetes makes it feasible to study whether these drugs are associated with a lower incidence of Parkinson disease in humans. In the present study, our objective was to assess whether treatment with TZDs was associated with a delayed diagnosis of Parkinson disease in humans by examining a large cohort of older individuals. To address potential confounding by diabetes status (8–11), patients who were treated with sulfonylureas were used as an active comparison group.
METHODS
Database
The study cohort was drawn from a database of Medicare beneficiaries who were enrolled in state pharmaceutical assistance programs in New Jersey and Pennsylvania between January 1, 1997, and December 31, 2005. These programs provide medications to low-income elderly individuals who do not qualify for Medicaid. We linked the prescription claims data from these programs to claims data from Medicare Parts A and B. These linked longitudinal databases comprise claims for medical procedures, diagnoses, dispensed drugs, and other health care services for approximately 1.1 million individuals. The strengths and limitations of longitudinal insurance claims databases in relation to epidemiologic research have been outlined in detail previously (12).
Cohort
We identified a cohort of new users of either TZDs or sulfonylureas between January 29, 1997 (the date on which the TZDs were first approved for use by the FDA), and December 31, 2005 (the end of the study period). The TZD category contained troglitazone, rosiglitazone, and pioglitazone, and the sulfonylurea category contained acetohexamide, chlorpropamide, glimepiride, glipizide, glyburide, tolazamide, and tolbutamide. New use was defined as a first prescription dispensation of either a TZD or sulfonylurea with no dispensation of a drug from either class in a 180-day baseline period immediately preceding that date. The index date was defined as the first pharmacy dispensation of either a TZD or a sulfonylurea.
We excluded patients who were prescribed drugs from both exposure groups of interest simultaneously on the index date and those who had a diagnosis of Parkinson disease, who had a diagnosis of extrapyramidal symptoms, or who received an antiparkinsonian medication that was recorded in the data at any time before the index date. To exclude cases of early-onset Parkinson disease, we restricted our sample to patients who were 65 years of age or older on the index date. We restricted the primary study cohort to patients with no recorded dispensation of diabetes medication during the 180-day baseline period to more closely align diabetes progressions between the exposure groups by focusing on patients who initiated monotherapy. We also created a secondary cohort by removing the restriction on diabetes drug use in the baseline period; these patients also had no prior use of TZDs or sulfonylureas in the baseline period.
Outcome and follow-up
Parkinson disease was defined as having an inpatient or outpatient diagnosis code of 332.0 from the International Classification of Diseases, Ninth Revision (paralysis agitans, excluding secondary parkinsonism), and having 2 or more prescriptions for medications to treat Parkinson disease filled (see Appendix Table 1) (13). The date of Parkinson disease diagnosis was defined as the date the diagnosis code was given or the date on which the drug was first dispensed, whichever occurred first.
We conducted multiple analyses with different classifications of follow-up time. First, we conducted an intention-to-treat (ITT) analysis in which follow-up began the day after the index date and continued until the first of diagnosis of Parkinson disease, death, loss of eligibility, or the end of the study period (December 31, 2005). This was the “any-exposure” ITT analysis. We then conducted additional analyses among subsets of patients with increasing durations of exposure to the index medication to examine potential duration-response associations. For example, we identified patients with at least 3 months of continuous use of the index drug and began follow-up at 3 months. We repeated this analysis with successively longer minimum-use requirements by adding 1 month to each analysis, up to a maximum of 10 months (Figure 1). We added a 60-day grace period to the end of the supply (in days) of each prescription to bridge serial refills in order to define continuous use.
Figure 1.
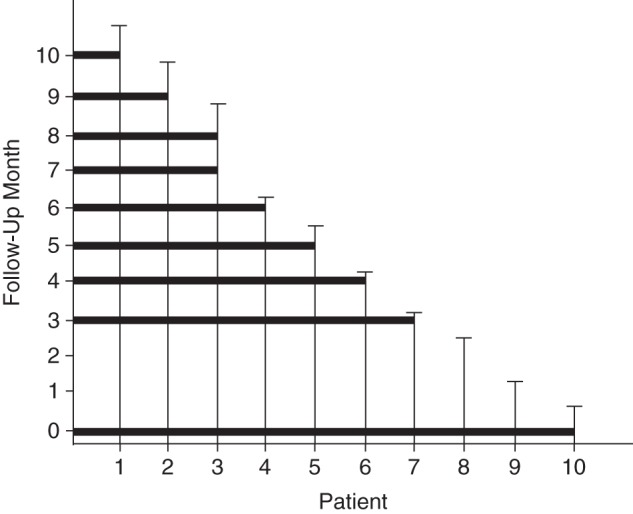
Graphical illustration of continuous-use analyses for 10 hypothetical patients. Vertical lines represent the follow-up time in months for each hypothetical patient. Hypothetical patients included in a given analysis requiring a certain duration of use are those whose follow-up time line is encompassed in the black box drawn across from that follow-up month. For example, all 10 patients are included in the any-exposure analysis because they are all under follow-up at time point 0. Patients 1–7 are included in the analysis restricted to those with at least 3 months of drug exposure and patients 1–3 are included in the analysis restricted to those with at least 4 months of drug exposure.
We also conducted as-treated analyses, in which we censored patients upon discontinuation of the index medication in addition to the censoring reasons in the ITT analyses. We used a 60-day grace period to bridge serial prescriptions and added 60 days to the end of the supply period of the last prescription refill to define days at risk. We conducted analogous duration-response as-treated analyses as described above for the ITT analyses by focusing on subsets of patients with increasing durations of exposure before the start of follow-up. All ITT and as-treated analyses were performed for both the primary and secondary cohorts, which were defined by whether diabetes drug use was allowed during the baseline period.
Covariates
A total of 81 covariates were assessed during the 180-day baseline period. These covariates included demographic characteristics (e.g., age, sex, and race); health care utilization variables, such as number of hospitalizations and days hospitalized; comorbid conditions, such as cardiovascular or renal disease; a combined comorbidity score (14); and use of prescription drugs, including medications for cardiovascular disease and drugs that can induce parkinsonism. Because this was a cohort of patients who were beginning treatment for diabetes and because diabetes might be a risk factor for Parkinson disease, we included diabetes-specific covariates, such as the number of doctor and hospital visits due to diabetes, prior insulin and oral antidiabetic treatment history, and indicators of common diabetic complications (e.g., retinopathy, neuropathy, nephropathy, and cardiovascular complications).
Statistical analysis
We used propensity score (PS) matching to account for measured differences between users of TZD and sulfonylurea in each analysis. PS predict the probability of receiving the treatment of interest using measured patient characteristics. To estimate the propensity scores, we used logistic regression models, which included all of the baseline covariates described in Table 1. To adjust for changes in prescribing patterns over time, we also included the year of study entry in the PS. Patients who used TZDs were matched to sulfonylurea patients by PS using an optimal nearest neighbor–matching algorithm. We matched each patient who used a TZD to up to 10 patients who used a sulfonylurea and had PS values within a caliper defined as 0.2 times the standard deviation of the logit of the PS, as proposed originally by Rosenbaum and Rubin (15) and later by Austin (16). Within each matched cohort, we fit a Cox regression model stratified by the matching ratio to estimate hazard ratios and 95% confidence intervals. We fit separate PS models and separately performed matching in the any-exposure cohort and in each subset of patients defined by the minimum-use requirements to ensure covariate balance in each analysis cohort.
Table 1.
Baseline Covariates for Medicare Enrollees Treated With Thiazolidinediones or Sulfonylureas in New Jersey and Pennsylvania, 1997–2005
| Characteristic | Unmatched |
Matcheda |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfonylurea Users (n = 24,167) |
TZD Users (n = 5,230) |
Absolute Difference, % | Sulfonylurea Users (n = 5,225) |
TZD Users (n = 5,225) |
Absolute Difference, % | |||||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |||
| Demographic Characteristics | ||||||||||||||
| Age, years | 78.69 (7.00) | 77.63 (6.88) | 1.06 | 77.51 (6.89) | 77.63 (6.88) | 0.12 | ||||||||
| Female sex | 17,623 | 72.92 | 3,815 | 72.94 | 0.02 | 3,792 | 72.57 | 3,811 | 72.94 | 0.37 | ||||
| White race | 21,281 | 88.06 | 4,433 | 84.76 | 3.30 | 4,416 | 84.52 | 4,431 | 84.80 | 0.28 | ||||
| Health Service Utilization | ||||||||||||||
| No. of days hospitalized | 3.04 (6.41) | 2.16 (5.37) | 0.88 | 2.20 (5.29) | 2.17 (5.37) | 0.03 | ||||||||
| No. of hospitalizations | 0.36 (0.56) | 0.27 (0.50) | 0.09 | 0.28 (0.50) | 0.27 (0.50) | 0.01 | ||||||||
| No. of days in a nursing home | 1.31 (5.81) | 0.98 (4.89) | 0.33 | 0.90 (4.51) | 0.98 (4.90) | 0.08 | ||||||||
| No. of nursing home admissions | 0.09 (0.30) | 0.06 (0.25) | 0.03 | 0.06 (0.24) | 0.06 (0.25) | 0.00 | ||||||||
| No. of outpatient physician visits | 4.81 (4.27) | 4.98 (4.08) | 0.17 | 4.94 (4.22) | 4.98 (4.09) | 0.04 | ||||||||
| No. of different medications prescribed | 6.90 (4.49) | 7.07 (4.39) | 0.17 | 7.02 (4.53) | 7.06 (4.39) | 0.04 | ||||||||
| No. of office visits for diabetes | 2.21 (2.88) | 2.21 (2.64) | 0.00 | 2.21 (2.81) | 2.21 (2.64) | 0.00 | ||||||||
| No. of hospitalizations for diabetes | 0.26 (0.59) | 0.20 (0.54) | 0.06 | 0.20 (0.53) | 0.20 (0.54) | 0.00 | ||||||||
| Prior Medication Use | ||||||||||||||
| ACE inhibitors | 7,625 | 31.55 | 1,631 | 31.19 | 0.36 | 1,658 | 31.73 | 1,630 | 31.20 | 0.53 | ||||
| Angiotensin receptor blockers | 2,480 | 10.26 | 948 | 18.13 | 7.87 | 895 | 17.13 | 944 | 18.07 | 0.94 | ||||
| α-blockers | 1,683 | 6.96 | 410 | 7.84 | 0.88 | 411 | 7.87 | 408 | 7.81 | 0.06 | ||||
| Antiarrhythmics | 748 | 3.10 | 126 | 2.41 | 0.69 | 127 | 2.43 | 126 | 2.41 | 0.02 | ||||
| Antiplatelet drugs | 1,474 | 6.10 | 469 | 8.97 | 2.87 | 467 | 8.94 | 469 | 8.98 | 0.04 | ||||
| β-blockers | 8,497 | 35.16 | 2,036 | 38.93 | 3.77 | 1,997 | 38.22 | 2,033 | 38.91 | 0.69 | ||||
| Bisphosphonates | 1,067 | 4.42 | 379 | 7.25 | 2.83 | 363 | 6.95 | 376 | 7.20 | 0.25 | ||||
| Calcium channel blockers | 8,134 | 33.66 | 1,661 | 31.76 | 1.90 | 1,682 | 32.19 | 1,661 | 31.79 | 0.40 | ||||
| COX-2 inhibitors | 2,180 | 9.02 | 801 | 15.32 | 6.30 | 773 | 14.79 | 799 | 15.29 | 0.50 | ||||
| Digoxin | 4,228 | 17.49 | 675 | 12.91 | 4.58 | 690 | 13.21 | 675 | 12.92 | 0.29 | ||||
| Estrogen | 744 | 3.08 | 168 | 3.21 | 0.13 | 179 | 3.43 | 168 | 3.22 | 0.03 | ||||
| H2 antagonists | 2,745 | 11.36 | 372 | 7.11 | 4.25 | 348 | 6.66 | 372 | 7.12 | 0.46 | ||||
| Drugs that cause hepatotoxicity | 14,215 | 58.82 | 2,817 | 53.86 | 4.96 | 2,779 | 53.19 | 2,814 | 53.86 | 0.67 | ||||
| Loop diuretics | 6,547 | 27.09 | 1,220 | 23.33 | 3.76 | 1,267 | 24.25 | 1,220 | 23.35 | 0.90 | ||||
| Nonselective NSAIDs | 3,231 | 13.37 | 594 | 11.36 | 2.01 | 585 | 11.20 | 590 | 11.29 | 0.09 | ||||
| Nitrates | 4,963 | 20.54 | 836 | 15.98 | 4.56 | 825 | 15.79 | 836 | 16.00 | 0.21 | ||||
| Other lipid-lowering drugs | 1,018 | 4.21 | 388 | 7.42 | 3.21 | 342 | 6.55 | 386 | 7.39 | 0.84 | ||||
| Other cardiovascular drugs | 921 | 3.81 | 184 | 3.52 | 0.29 | 191 | 3.66 | 184 | 3.52 | 0.14 | ||||
| Parkinsonism-inducing medications | 1,783 | 7.38 | 302 | 5.77 | 1.61 | 294 | 5.63 | 302 | 5.78 | 0.15 | ||||
| Potassium-sparing agents | 2,048 | 8.47 | 432 | 8.26 | 0.21 | 440 | 8.42 | 432 | 8.27 | 0.15 | ||||
| Proton pump inhibitors | 2,527 | 10.46 | 819 | 15.66 | 5.20 | 839 | 16.06 | 816 | 15.62 | 0.44 | ||||
| Statins | 5,984 | 24.76 | 2,020 | 38.62 | 13.86 | 1,955 | 37.42 | 2,015 | 38.56 | 1.14 | ||||
| Thiazides | 5,463 | 22.61 | 1,433 | 27.40 | 4.79 | 1,375 | 26.32 | 1,430 | 27.37 | 1.05 | ||||
| Thyroid hormone replacement | 3,108 | 12.86 | 743 | 14.21 | 1.35 | 765 | 14.64 | 743 | 14.22 | 0.42 | ||||
| Warfarin | 3,066 | 12.69 | 539 | 10.31 | 2.38 | 533 | 10.20 | 539 | 10.32 | 0.12 | ||||
| Clinical Conditions | ||||||||||||||
| Combined comorbidity score | 1.70 (2.52) | 1.38 (2.39) | 0.32 | 1.41 (2.40) | 1.38 (2.39) | 0.03 | ||||||||
| Alzheimer disease | 1,711 | 7.08 | 343 | 6.56 | 0.52 | 348 | 6.66 | 343 | 6.56 | 0.10 | ||||
| Atrial fibrillation | 942 | 3.90 | 150 | 2.87 | 1.03 | 163 | 3.12 | 150 | 2.87 | 0.25 | ||||
| Alcohol abuse | 89 | 0.37 | 18 | 0.34 | 0.03 | 17 | 0.33 | 18 | 0.34 | 0.01 | ||||
| Angina | 2,665 | 11.03 | 469 | 8.97 | 2.06 | 470 | 9.00 | 468 | 8.96 | 0.04 | ||||
| History of CABG before baseline period | 1,362 | 5.64 | 274 | 5.24 | 0.40 | 265 | 5.07 | 274 | 5.24 | 0.17 | ||||
| CABG during baseline period | 221 | 0.91 | 35 | 0.67 | 0.24 | 36 | 0.69 | 35 | 0.67 | 0.02 | ||||
| Congestive heart failure | 6,359 | 26.31 | 1,068 | 20.42 | 5.89 | 1,098 | 21.01 | 1,067 | 20.42 | 0.59 | ||||
| Chronic obstructive pulmonary disease | 2,409 | 9.97 | 381 | 7.28 | 2.69 | 413 | 7.90 | 381 | 7.29 | 0.61 | ||||
| Cancer | 4,166 | 17.24 | 850 | 16.25 | 0.99 | 836 | 16.00 | 850 | 16.27 | 0.27 | ||||
| Chronic kidney disease | 904 | 3.74 | 259 | 4.95 | 1.21 | 277 | 5.30 | 258 | 4.94 | 0.36 | ||||
| Coronary artery disease | 9,417 | 38.97 | 1,835 | 35.09 | 3.88 | 1,810 | 34.64 | 1,835 | 35.12 | 0.48 | ||||
| Depression | 693 | 2.87 | 142 | 2.72 | 0.15 | 139 | 2.66 | 142 | 2.72 | 0.06 | ||||
| End-stage renal disease | 7 | 0.03 | 4 | 0.08 | 0.05 | 5 | 0.10 | 3 | 0.06 | 0.04 | ||||
| Gastroparesis | 44 | 0.18 | 14 | 0.27 | 0.09 | 15 | 0.29 | 14 | 0.27 | 0.02 | ||||
| Gout | 771 | 3.19 | 143 | 2.73 | 0.46 | 142 | 2.72 | 143 | 2.74 | 0.02 | ||||
| HIV/AIDS | 11 | 0.05 | 2 | 0.04 | 0.01 | 3 | 0.06 | 2 | 0.04 | 0.02 | ||||
| Hyperlipidemia | 10,131 | 41.92 | 3,075 | 58.80 | 16.88 | 3,072 | 58.79 | 3,070 | 58.76 | 0.03 | ||||
| Hypothyroidism | 4,419 | 18.29 | 1,126 | 21.53 | 3.24 | 1,143 | 21.88 | 1,122 | 21.47 | 0.41 | ||||
| Illicit drug use | 28 | 0.12 | 4 | 0.08 | 0.04 | 1 | 0.02 | 4 | 0.08 | 0.06 | ||||
| Inflammatory arthritis | 5,835 | 24.14 | 1,238 | 23.67 | 0.47 | 1,272 | 24.34 | 1,237 | 23.67 | 0.67 | ||||
| Nephropathy | 10 | 0.04 | 1 | 0.02 | 0.02 | 2 | 0.04 | 1 | 0.02 | 0.02 | ||||
| Neuropathy | 1,000 | 4.14 | 257 | 4.91 | 0.77 | 250 | 4.78 | 256 | 4.90 | 0.12 | ||||
| History of MI before baseline period | 1,323 | 5.47 | 216 | 4.13 | 1.34 | 218 | 4.17 | 216 | 4.13 | 0.04 | ||||
| MI during baseline period | 1,057 | 4.37 | 157 | 3.00 | 1.37 | 151 | 2.89 | 157 | 3.00 | 0.11 | ||||
| Obesity | 804 | 3.33 | 210 | 4.02 | 0.69 | 206 | 3.94 | 209 | 4.00 | 0.06 | ||||
| Peripheral vascular disease | 3,387 | 14.01 | 628 | 12.01 | 2.00 | 654 | 12.52 | 628 | 12.02 | 0.50 | ||||
| Palpitations | 708 | 2.93 | 159 | 3.04 | 0.11 | 167 | 3.20 | 158 | 3.02 | 0.18 | ||||
| Peptic ulcer | 1,460 | 6.04 | 328 | 6.27 | 0.23 | 346 | 6.62 | 326 | 6.24 | 0.38 | ||||
| Retinopathy | 892 | 3.69 | 233 | 4.46 | 0.77 | 256 | 4.90 | 232 | 4.44 | 0.46 | ||||
| Stroke | 2,260 | 9.35 | 422 | 8.07 | 1.28 | 435 | 8.33 | 422 | 8.08 | 0.25 | ||||
| Transient ischemic attack | 1,116 | 4.62 | 214 | 4.09 | 0.53 | 243 | 4.65 | 214 | 4.10 | 0.55 | ||||
| Angiography | 811 | 3.36 | 150 | 2.87 | 0.49 | 158 | 3.02 | 150 | 2.87 | 0.15 | ||||
| Dialysis | 113 | 0.47 | 35 | 0.67 | 0.20 | 39 | 0.75 | 35 | 0.67 | 0.08 | ||||
| Stent | 177 | 0.73 | 42 | 0.80 | 0.07 | 41 | 0.78 | 42 | 0.80 | 0.02 | ||||
| Hypertension | 17,396 | 71.98 | 3,974 | 75.98 | 4.00 | 3,956 | 75.71 | 3,969 | 75.96 | 0.25 | ||||
| Liver disease | 859 | 3.55 | 187 | 3.58 | 0.03 | 208 | 3.98 | 186 | 3.56 | 0.42 | ||||
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; AIDS, acquired immunodeficiency syndrome; CABG, coronary artery bypass grafting; COX-2, cyclooxygenase-2; HIV, human immunodeficiency virus; MI, myocardial infarction; NSAID, nonsteroidal antiinflammatory drug; SD, standard deviation; TZD, thiazolidinedione.
a The matched covariate comparison is based on patients who were included in the any-exposure analysis. Covariate information for sulfonylurea patients is based on a random sample of 1 sulfonylurea user from each matched set, as described in the Statistical Analysis section.
To assess the PS estimation and matching processes, we examined PS distributions for overlap between treatment groups and covariate balance using the absolute differences in means or proportions between groups. Because variable ratio matching ensures covariate balance only within a matched set, covariate balance between exposure groups after variable ratio PS matching cannot be assessed using the marginal matched population. Therefore, we randomly sampled 1 sulfonylurea initiator from each matched set in the any-exposure analysis along with the corresponding TZD initiator and compared the covariates among this random sample (17).
RESULTS
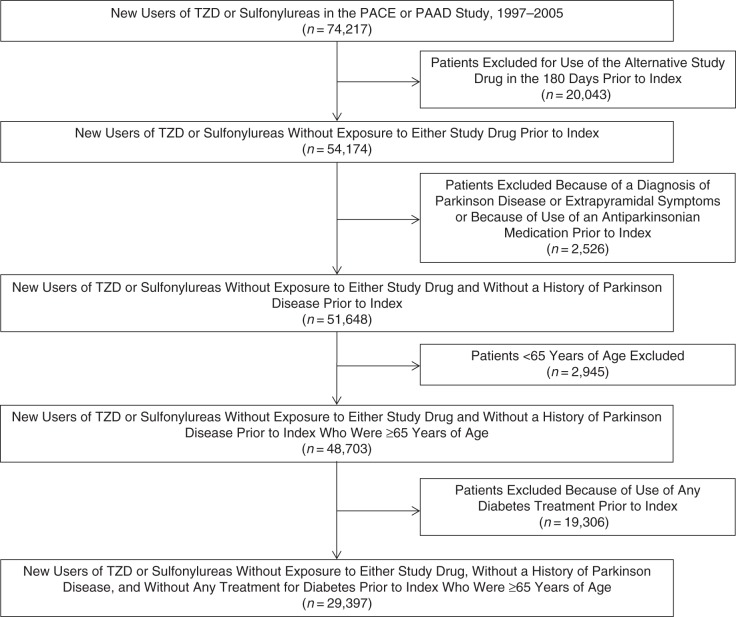
We identified 29,397 total patients for the primary study cohort, of whom 5,230 were new users of TZDs and 24,167 were new users of sulfonylureas (Figure 2). The mean age of the patients in the primary cohort was 78.5 years, 73% of the patients were female, and 88% were white. Before PS matching, new users of TZDs were more likely than were new users of sulfonylureas to have used cardiovascular medications such as statins (39% vs. 25%) and angiotensin receptor blockers (18% vs. 10%), and they were more likely to have been diagnosed with hyperlipidemia (59% vs. 42%) (Table 1). We identified 46,890 eligible patients for the secondary cohort, in which 40% of TZD users had used insulin during the baseline period compared with only 9% of sulfonylurea users (Web Table 1, available at http://aje.oxfordjournals.org/). In both cohorts, differences in observed covariates were substantially reduced after PS matching. PS distributions for the primary and secondary cohorts are shown in Web Figures 1 and 2, respectively.
Figure 2.
Patient selection flow chart for Medicare enrollees treated with thiazolidinediones or sulfonylureas in New Jersey and Pennsylvania, 1997–2005. PAAD, Pharmaceutical Assistance to the Aged and Disabled; PACE, Program of All-Inclusive Care for the Elderly; TZD, thiazolidinedione.
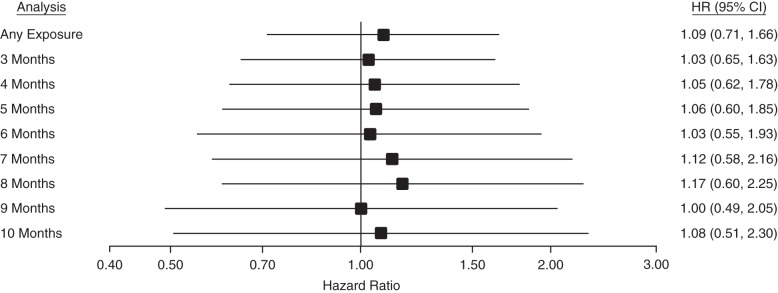
We observed 204 diagnoses of Parkinson disease during follow-up (mean, 2.97 years) in the primary cohort. In the any-exposure PS-matched ITT analysis, in which we began follow-up for Parkinson disease the day after the index date, TZD users in the primary cohort had a hazard ratio of 1.09 (95% confidence interval: 0.71, 1.66) for Parkinson disease diagnosis when compared with sulfonylurea users. Adding minimum durations of use to the models resulted in similar point estimates, all of which were slightly greater than 1 but had confidence intervals that included 1 (Figure 3). Hazard ratios ranged from 1.00 (95% confidence interval: 0.49, 2.05) to 1.17 (95% confidence interval: 0.60, 2.25) among analyses with different minimum-use requirements. The total numbers of new users and Parkinson disease events included in each ITT analysis of the primary cohort are displayed in Table 2. Results from ITT analyses in the secondary cohort were similar, with hazard ratios ranging from 1.02 (95% confidence interval: 0.67, 1.56) to 1.30 (95% confidence interval: 0.84, 2.02) (Web Table 2).
Figure 3.
Hazard ratios (HRs) for Parkinson disease diagnosis from intention-to-treat analyses of the primary variable ratio–matched cohort of Medicare enrollees treated with thiazolidinediones or sulfonylureas in New Jersey and Pennsylvania, 1997–2005. Bars, 95% confidence intervals (CIs).
Table 2.
Number of Patients and Parkinson Disease Outcomes Included in Intention-to-Treat Analyses, by Exposure Group, Medicare Enrollees Treated With Thiazolidinediones or Sulfonylureas in New Jersey and Pennsylvania, 1997–2005
| Analysis | No. of TZD Users | No. of TZD Events | No. of Sulfonylurea Users | No. of Sulfonylurea Events |
|---|---|---|---|---|
| Any exposure | 5,225 | 27 | 20,283 | 129 |
| 3 Months | 4,746 | 22 | 18,412 | 121 |
| 4 Months | 3,590 | 17 | 13,914 | 92 |
| 5 Months | 3,267 | 15 | 12,089 | 84 |
| 6 Months | 2,956 | 12 | 11,595 | 66 |
| 7 Months | 2,664 | 11 | 10,653 | 61 |
| 8 Months | 2,477 | 11 | 9,973 | 58 |
| 9 Months | 2,289 | 9 | 9,252 | 58 |
| 10 Months | 2,128 | 8 | 8,657 | 52 |
Abbreviation: TZD, thiazolidinedione.
When analyses were performed on the primary cohort using the as-treated approach, hazard ratios were consistently less than 1 regardless of the continuous-use requirement, but the confidence intervals were wide and always included 1. Hazard ratios from the as-treated in the primary cohort ranged from 0.54 (95% confidence interval: 0.16, 1.77) to 0.84 (95% confidence interval: 0.25, 2.83) (Web Table 3). In the secondary cohort, hazard ratios from the as-treated analysis ranged from 0.94 (95% confidence interval: 0.46, 1.92) to 1.32 (95% confidence interval: 0.65, 2.65) (Web Table 4).
DISCUSSION
In the present large, longitudinal, population-based cohort study, we did not observe an inverse association between the use of TZDs and the time to diagnosis of Parkinson disease relative to what was seen with use of sulfonylureas, regardless of restrictions on prior diabetes drug use or duration of continuous medication use. Results from the present study, which to our knowledge is the first in which the association between use of TZDs and the diagnosis of Parkinson disease in humans has been examined, suggest that despite data from animal studies that indicated a possible protective effect of TZDs, these drugs might not delay time to Parkinson disease diagnosis in humans.
Although both pioglitazone and rosiglitazone have been shown to completely prevent the death of dopaminergic brain cells in rats (18, 19), differences between animal models and Parkinson disease in humans might explain the discordance between findings in animal and human studies. Animal studies often utilize a chemically induced model of Parkinson disease in which neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and rotenone, which destroy dopaminergic neurons and produce symptoms similar to those in humans with Parkinson disease, are used. Although the chemical models of Parkinson disease can closely mimic the primary symptoms of Parkinson disease in humans, they differ in ways that might affect the utility of TZDs as an effective treatment. For example, the chemical inducers are often administered as acute doses, which contrasts with the slow, progressive neurodegenerative nature of Parkinson disease in humans (20).
Observational studies can be an important tool for identifying benefits of drugs used in large populations. For example, observational studies of aspirin in the 1970s were instrumental in the discovery of the drug's cardioprotective benefits (21). Our observational study has several strengths, including the large population-based cohort and the multiple years of follow-up time. We were able to use an active comparison group by focusing on patients who were also beginning treatment for diabetes with sulfonylureas. By comparing patients with similar indications for treatment at the same time in the course of treatment, an active comparison design reduces both measured and unmeasured confounding due to underlying indication (22). The large size of the database further allowed for the restriction of the primary cohort to those patients who received no treatment for diabetes during the baseline period. Although this restriction likely led to better control of confounding by diabetes severity, it resulted in the inclusion of fewer patients and therefore reduced statistical power. However, results in the secondary cohort in which we did not apply this restriction were similar to those in the primary cohort. Finally, the use of PS allowed us to adjust for a large number of potential confounders despite the small number of outcomes.
The results of our study should be interpreted in the context of limitations of observational studies and administrative claims data. Because our study was observational, we cannot rule out the possibility that the results might be affected by unmeasured confounding. The exact cause of Parkinson disease is unknown, but possible risk factors such as genetic predisposition, environmental toxin exposure, body size, and smoking status are not well measured in claims data and therefore we could not explicitly control for them in the analyses. However, we do not expect there to be large differences in these factors between new users of TZDs and new users of sulfonylureas.
One possible explanation for the slightly higher point estimates could be uncontrolled confounding by diabetes severity, because diabetes might be a risk factor for Parkinson disease (8–11). In the secondary cohort, it was evident from the differences in insulin use during the baseline period that the patients who began using TZDs were more advanced in their diabetes disease course. Restricting the primary cohort to patients who did not use diabetes drugs in the baseline period reduced differences in the number diabetes complications between exposure groups, but measures of diabetes disease severity, such as hemoglobin A1c and blood glucose values, are not available in the database. We further mitigated observed differences between exposure groups by using PS matching. Similar effect estimates in both the primary and secondary cohort suggest that our results were not substantially affected by differences in diabetes severity between exposure groups. Additionally, upward bias due to confounding by diabetes severity might have been counteracted by a downward bias due to the fact that patients with severe diabetes would be more likely to die from complications of diabetes itself or from the adverse effects of TZDs (23) before being diagnosed with Parkinson disease.
Another limitation of our study is possible exposure misclassification. Because patients with diabetes often modify their oral hypoglycemic drug regimens, it is likely that some patients either changed exposure groups or used both treatments simultaneously during the course of follow-up. We attempted to mitigate exposure misclassification by performing as-treated analyses, and the results were similar to those of the ITT analyses.
Because Parkinson disease is an insidious condition with no well-defined date of onset, we can only approximate the onset date using our claims-based definition. However, we have no reason to believe that there are differences in surveillance for Parkinson disease between patients taking sulfonylureas and those taking TZDs. Our study focused on cases of incident Parkinson disease, and although the use of TZDs was not associated with any benefit in delaying a diagnosis of Parkinson disease in our study, it might still have a therapeutic benefit in slowing the progression of the disease in patients who have already been diagnosed, as is being examined in the ongoing randomized Pioglitazone in Early Parkinson's Disease clinical trial (clinicaltrials.gov identifier: NCT01280123). Last, the results of our study, in which we examined the claims records of Medicare patients using TZDs to treat diabetes, might not be generalizable to younger patients or those without diabetes.
Although randomized controlled trials are considered the gold standard for making causal inferences about treatment efficacy, they are also expensive and time-consuming. In light of the null findings from the present study, additional observational studies of the association of TZDs with Parkinson disease in humans, which can be completed faster and at a lower cost than randomized trials, should be conducted to determine whether future randomized trials are warranted.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (John G. Connolly, Katsiaryna Bykov, Joshua J. Gagne).
This work was conducted with the support of a KL2/Catalyst Medical Research Investigator Training award (an appointed KL2 award) from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR001100).
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Conflict of interest: none declared.
Appendix Table 1.
Antiparkinsonian Medications Used in Outcome Definition
| Drug Class | Medication |
|---|---|
| Catechol-O-methyl transferase inhibitors | Amantadine |
| Entacapone | |
| Tolcapone | |
| Dopamine precursors | Levodopa |
| Levodopa and carbidopa combined | |
| Carbidopa | |
| Carbidopa, levodopa, and entacapone combined | |
| Dopamine receptor agonists | |
| Ergot derivatives | Bromocriptine |
| Cabergoline | |
| Pergolide | |
| Nonergot derivatives | Apomorphine |
| Pramipexole | |
| Ropinirole | |
| Monoamine oxidase B inhibitors | Rasagiline |
| Selegiline |
REFERENCES
- 1.Pringsheim T, Jette N, Frolkis A et al. . The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;2913:1583–1590. [DOI] [PubMed] [Google Scholar]
- 2.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;3639423:1783–1793. [DOI] [PubMed] [Google Scholar]
- 3.Noyce AJ, Bestwick JP, Silveira-Moriyama L et al. . Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;726:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology. 2010;7412:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;84:382–397. [DOI] [PubMed] [Google Scholar]
- 6.Carta AR. PPAR-γ: therapeutic prospects in Parkinson's disease. Curr Drug Targets. 2013;147:743–751. [DOI] [PubMed] [Google Scholar]
- 7.Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;1231:131–145. [DOI] [PubMed] [Google Scholar]
- 8.Cereda E, Barichella M, Pedrolli C et al. . Diabetes and risk of Parkinson's disease: a systematic review and meta-analysis. Diabetes Care. 2011;3412:2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cereda E, Barichella M, Pedrolli C et al. . Re: “Diabetes and risk of Parkinson's disease” [letter] Mov Disord. 2013;282:257. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, Fu DL, Li HQ et al. . Diabetes and risk of Parkinson's disease: an updated meta-analysis of case-control studies. PLoS One. 2014;91:e85781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palacios N, Ascherio A. Re: “Diabetes and risk of Parkinson's disease” [letter] Mov Disord. 2013;282:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;584:323–337. [DOI] [PubMed] [Google Scholar]
- 13.Noyes K, Liu H, Holloway R et al. . Accuracy of Medicare claims data in identifying Parkinsonism cases: comparison with the Medicare current beneficiary survey. Mov Disord. 2007;224:509–514. [DOI] [PubMed] [Google Scholar]
- 14.Gagne JJ, Glynn RJ, Avorn J et al. . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;647:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;391:33–38. [Google Scholar]
- 16.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;102:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagne JJ, Choudhry NK, Kesselheim AS et al. . Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med. 2014;1616:400–407. [DOI] [PubMed] [Google Scholar]
- 18.Breidert T, Callebert J, Heneka MT et al. . Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J Neurochem. 2002;823:615–624. [DOI] [PubMed] [Google Scholar]
- 19.Schintu N, Frau L, Ibba M et al. . PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009;295:954–963. [DOI] [PubMed] [Google Scholar]
- 20.Hattori N, Sato S. Animal models of Parkinson's disease: similarities and differences between the disease and models. Neuropathology. 2007;275:479–483. [DOI] [PubMed] [Google Scholar]
- 21.Regular aspirin intake and acute myocardial infarction. Br Med J. 1974;15905:440–443. [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss S, Patrick AR, Stürmer T et al. . Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 suppl 2):S131–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consoli A, Formoso G. Do thiazolidinediones still have a role in treatment of type 2 diabetes mellitus? Diabetes Obes Metab. 2013;1511:967–977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.