Abstract
Hematology and serum chemistry values were obtained from 28 male and 22 female stray dogs in Chittagong Metropolitan area, Bangladesh. The goal of the study was to establish reference value for hematology and serum chemistry for these semi wild animals in relation to age, sex, reproductive stage and body condition. No significant differences were found for mean values of hemoglobin, packed cell volume, mean corpuscular hemoglobin concentration, white blood cell, differential leukocyte count, total protein, albumin, glucose, cholesterol, phosphorus and potassium among or between sexes, ages, reproductive states or body conditions. Significant differences were noted for erythrocyte sedimentation rate (p<0.02) between sexes. Among different age groups significant differences were found for total red blood cell count (p<0.001). Different body conditions have significant differences in red blood cell count, mean corpuscular volume and mean corpuscular hemoglobin (p<0.001). Pregnant and non-pregnant females differed significantly in their red blood cell count, mean corpuscular volume and mean corpuscular hemoglobin (p<0.001).
Keywords: Hematology, Serum chemistry, Reference value, Stray dog, Bangladesh
Introduction
Hematology and serum clinical chemistry analyses are used for health assessment of domestic animals and a wide range of captive wildlife (Smith, 2000; Fowler and Miller, 2003; Kaneko et al., 2008).
However, for semi-wild animals, such as stray dogs, no published information is available in Bangladesh or other regions of south-east Asia. Dog population is estimated to be 37.5 million in the south-east Asia region with an increase of about 10% annually (Ali et al., 1977). There are no available data for stray dog populations in Bangladesh (WHO, 1998). Numerous wildlife species are natural reservoirs of rabies virus and on rare occasions act as a source of transmission to humans.
Blood is an important medium in assessing the health status of animals. Both the physiological and pathological conditions of animals can be assessed by the evaluation of hematological and biochemical analyses of the blood. Though ample work has been done on establishing the baseline values of biochemical and hematological parameters of dogs, there has been little work done on stray dogs and no studied groups in Bangladesh.
The aim of this first study was to establish reference values for selected hematology and clinical chemistry analyses that may contribute to the assessment of feral animal health in Bangladesh in the future.
Materials and Methods
Study area
Dogs were captured in Chittagong metropolitan area in Bangladesh. The study was conducted in an area containing 200 small homes where humans live in close contact with dogs. Dogs in this area forage primarily on household wastes and have no vaccination history.
Animals
Once identified, the most individuals were captured using baiting methods, then restrained and muzzled. Upon capture, blood samples were collected, animals were classified by age and sex and geographic location was recorded. Body condition score was assessed for each individual.
Sample processing
Blood samples were collected from the jugular or cephalic vein of each animal and immediately dispensed in two tubes, one with EDTA and the other was plain to obtain serum. Hematological parameters including: red blood cells (RBCs) and white blood cells (WBCs) count and hemoglobin concentration (Hb) were manually estimated according to Campbell (1995). Packed cell volume (PCV) was determined according to Howlett et al. (2002). Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated as described by Campbell (1995).
Blood smears were immediately prepared from EDTA blood samples and were stained with Diff Quick stain (EMD Chemicals, Inc., Gibbstown, New Jersey 08027, USA) (Fig. 1) and 200 leukocytes were differentiated in smears prepared from each animal.
Fig. 1.
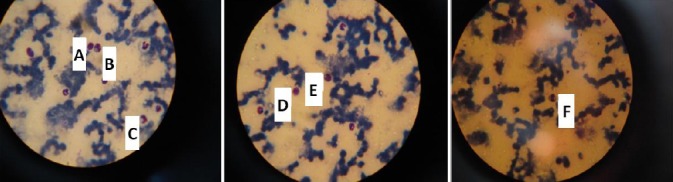
Normal blood cells stained with Diff Quik, 100x. A = Lymphocyte, B = Mature neutrophil, C = Band neutrophil, D = Basophil, E = Monocyte and F = Eosinophil.
Biochemical evaluation
Serum total protein, albumin, phosphorus, potassium, cholesterol and glucose were analyzed in a biochemical analyzer (Model: PLD-951/951A/951B).
Statistical analysis
All statistical analyses were performed using PASW Statistics 18.0 software (SPSS Inc., Chicago, IL, USA). Mean and standard deviation for hematology and serum chemistry values were generated initially by using the combined dataset (n=50). Independent t-tests for equality of means were used to test for a significant effect of sex or pregnancy status on blood variables. T-statistics, mean differences between groups, standard errors of those differences, and p-values were reported. One-way ANOVAs were used to test for significant interactions of age and BCS against blood cell counts and chemistry values. F-statistics, degrees of freedom and p-values were reported. Significance was determined when p<0.05.
Results
All stray dogs seemed healthy. The mean hematology and serum chemistry values were within the reference range for dogs with the exception of the total protein which was higher (92.6±21.6, 52-78 mg/dl) (Table 1).
Table 1.
Mean values of hematology and serum chemistry of 50 stray dogs
| Test | Mean±SD | Observation range | Reference range |
|---|---|---|---|
| RBC (×106 cells/µl) | 6.1±2.8 | 3.4-14.8 | 5.5-8.5 |
| Hb (g/dl) | 12.4±2.0 | 7.0-15.6 | 14.2-19.2 |
| PCV (%) | 40.1±10.2 | 24-62 | 29-55 |
| ESR (% in first hour) | 3.4±0.3 | 3-4 | 0-6 |
| MCV(fl) | 78.2±35.6 | 20.2-104.2 | 65-80 |
| MCH (pg) | 23.4±8.0 | 8.5-34.2 | 12.2-25.4 |
| MCHC (%) | 32.8±9.4 | 18.9-52.1 | 32-36 |
| WBC (x103) | 10.2±3.7 | 4.2-18.1 | 5.9-16.6 |
| Band neutrophil (%) | 13.0±7.4 | 1-30 | 0-4 |
| Mature neutrophil (%) | 39.0±15.8 | 7-69 | 51-84 |
| Monocyte (%) | 3.0±2.9 | 0-11 | 1-9 |
| Lymphocyte (%) | 47.0±13.9 | 16-72 | 8-38 |
| Eosinophil (%) | 5.1±2.8 | 0-10 | 0-9 |
| Basophil (%) | 0.9±0.8 | 0-2 | 0-1 |
| Total protein (mg/dl) | 92.6±21.6 | 56.8-182.3 | 52-78 |
| Albumin (mg/dl) | 26.0±3.9 | 19.5-38.0 | 23-31 |
| Glucose (mg/dl) | 55.9±27.8 | 17.9-139.4 | 80-120 |
| Cholesterol (mmol/l) | 76.1±13.6 | 53.7-112.0 | 110-330 |
| Phosphorus (mg/dl) | 3.3±2.2 | 1.9-12.9 | 2.5-7.7 |
| Potassium (mmol/l) | 4.1±0.9 | 2.5-7.0 | 4.4-6.1 |
Hematology and serum chemistry values of male and female dogs showed no significant differences except the ESR values, which were significantly higher in male dogs (3.6±0.3) as compared to their female counterparts (3.3±0.2) (p<0.02) (Table 2).
Table 2.
Hematology and serum chemistry values of male and female stray dogs (sample sizes in parentheses)
| Hematology/serum biochemistry | Female (n=22) | Male (n=28) | F | DF | p |
|---|---|---|---|---|---|
| RBC (×106 cells/µl) | 6.7±3.3 | 5.3±1.6 | 3.28 | 48 | 0.07 |
| Hb (g/dl) | 12.4±2.1 | 12.5±2.0 | 0.01 | 48 | 0.89 |
| PCV (%) | 40.0±10.0 | 40.4±10.6 | 0.01 | 48 | 0.89 |
| ESR(% in first hour) | 3.3±0.2 | 3.6±0.3 | 5.81 | 48 | 0.02* |
| MCV(fl) | 77.4±42.8 | 79.3±24.5 | 0.03 | 48 | 0.85 |
| MCH (pg) | 22.4±9.6 | 24.7±5.3 | 1.04 | 48 | 0.31 |
| MCHC (%) | 32.9±9.6 | 32.8±9.4 | 0.003 | 48 | 0.95 |
| WBC (x103) | 11.0±4.0 | 9.3±3.0 | 2.75 | 48 | 0.07 |
| Band neutrophil (%) | 13.6±7.9 | 12.3±6.8 | 0.40 | 48 | 0.52 |
| Mature neutrophil (%) | 40.3±17.2 | 37.3±14.1 | 0.45 | 48 | 0.50 |
| Monocyte (%) | 3.3±2.8 | 2.6±2.9 | 0.59 | 48 | 0.44 |
| Lymphocyte (%) | 47.5±17.7 | 46.3±7.0 | 0.09 | 48 | 0.76 |
| Eosinophil (%) | 4.6±2.5 | 5.9±3.1 | 2.58 | 48 | 0.11 |
| Basophil (%) | 1.0±0.8 | 0.6±0.7 | 2.93 | 48 | 0.09 |
| Total protein (mg/dl) | 93.9±27.0 | 90.8±11.9 | 0.25 | 48 | 0.61 |
| Albumin (mg/dl) | 26.6±4.3 | 25.2±3.4 | 1.44 | 48 | 0.23 |
| Glucose (mg/dl) | 57.9±31.6 | 53.3±22.6 | 0.34 | 48 | 0.56 |
| Cholesterol (mmol/l) | 74.5±13.6 | 78.3±13.6 | 0.95 | 48 | 0.33 |
| Phosphorus (mg/dl) | 3.3±2.0 | 3.4±2.4 | 0.009 | 48 | 0.92 |
| Potassium (mmol/l) | 4.0±1.0 | 4.2±0.9 | 0.74 | 48 | 0.39 |
Pregnant and non-pregnant dogs showed significant differences in RBC (10.5±4.1 4.1±0.4 x106 cells/µl), MCV (38.4±20.7 112.4±34.6 fl), MCH (13.8±5.5 29.8±5.3 pg) (p<0.001), total protein (116.8±45.9 87.4±10.6 mg/dl) (p<0.004) and glucose (47.2±19.7 86.5±48.2 mg/dl) (p<0.02) values. Other hematology and serum chemistry values did not show significant differences (Table 3).
Table 3.
Hematology and serum chemistry values of pregnant and non-pregnant stray bitches (sample sizes in parentheses)
| Hematology/serum biochemistry | Non-pregnant (n=5) | Pregnant (n=7) | F | DF | p |
|---|---|---|---|---|---|
| RBC (×106 cells/µl) | 4.1±0.4 | 10.5±4.1 | 18.26 | 47 | <0.001* |
| Hb (g/dl) | 12.2±1.2 | 12.7±0.5 | 0.09 | 47 | 0.91 |
| PCV (%) | 45.6±9.6 | 34.0±7.5 | 2.11 | 47 | 0.13 |
| ESR(% in first hour) | 3.2±0.1 | 3.6±0.6 | 2.80 | 47 | 0.07 |
| MCV(fl) | 112.4±34.6 | 38.4±20.7 | 8.98 | 47 | <0.001* |
| MCH (pg) | 29.8±5.3 | 13.8±5.5 | 9.04 | 47 | <0.001* |
| MCHC (%) | 27.5±5.9 | 39.1±8.9 | 2.48 | 47 | 0.09 |
| WBC (x103) | 11.3±3.7 | 11.6±4.8 | 0.94 | 47 | 0.39 |
| Band neutrophil (%) | 8.4±3.8 | 13.7±8.2 | 1.10 | 47 | 0.33 |
| Mature neutrophil (%) | 49.4±17.2 | 40.4±15.4 | 1.31 | 47 | 0.27 |
| Monocyte (%) | 2.8±1.6 | 3.7±3.2 | 0.21 | 47 | 0.80 |
| Lymphocyte (%) | 46.4±22.9 | 43.7±15.9 | 0.23 | 47 | 0.79 |
| Eosinophil (%) | 5.6±3.2 | 5.0±2.0 | 0.06 | 47 | 0.93 |
| Basophil (%) | 1.6±0.5 | 1.0±1.0 | 2.37 | 47 | 0.10 |
| Total protein (mg/dl) | 87.4±10.6 | 116.8±45.9 | 6.17 | 47 | 0.004* |
| Albumin (mg/dl) | 26.9±2.7 | 27.6±5.4 | 0.88 | 47 | 0.42 |
| Glucose (mg/dl) | 86.5±48.2 | 47.2±19.7 | 3.92 | 47 | 0.02* |
| Cholesterol (mmol/l) | 87.4±22.4 | 69.6±4.9 | 2.67 | 47 | 0.07 |
| Phosphorus (mg/dl) | 2.2±0.5 | 4.0±0.8 | 1.02 | 47 | 0.35 |
| Potassium (mmol/l) | 3.4±1.0 | 4.5±0.8 | 1.89 | 47 | 0.16 |
There were no significant differences for hematology and serum chemistry among different age groups except for RBC and eosinophil counts. RBC count was higher in adults (6.3±3.3 x106 cells/µl) in comparison to juveniles (5.8±1.9x106 cells/µl) and puppies (5.7±2.3x106 cells/µl) (p<0.001), and eosinophil count was higher in adults (5.9±2.8) and juveniles (4.5±2.4) than in puppies (1.6±2.8) (p<0.01) (Table 4).
Table 4.
Hematology and serum chemistry values of stray dogs in different age groups
| Hematology/serum biochemistry | Adult (n=29) | Juvenile (n=18) | Puppy (n=3) | F | DF | p |
|---|---|---|---|---|---|---|
| RBC (×106 cells/µl) | 6.3±3.3 | 5.8±1.9 | 5.7±2.3 | 0.18 | 47 | 0.001* |
| Hb (g/dl) | 12.4±1.3 | 13.1±2.4 | 8.6±1.3 | 7.52 | 47 | 0.27 |
| PCV (%) | 41.4±10.4 | 39.5±10.0 | 31.6±5.5 | 1.33 | 47 | 0.51 |
| ESR (% in first hour) | 3.4±0.3 | 3.5±0.3 | 3.4±0.3 | 0.68 | 47 | 0.66 |
| MCV(fl) | 82.1±43.4 | 73.3±20.7 | 70.4±23.0 | 0.40 | 47 | 0.35 |
| MCH (pg) | 23.4±8.3 | 24.4±7.5 | 17.0±7.8 | 1.07 | 47 | 0.35 |
| MCHC (%) | 31.9±8.6 | 35.1±10.8 | 28.0±8.0 | 1.06 | 47 | 0.83 |
| WBC (x103) | 10.6±3.6 | 10.4±3.6 | 6.1±3.1 | 2.10 | 47 | 0.13 |
| Band neutrophil (%) | 11.5±5.8 | 15.2±9.5 | 15.3±4.5 | 1.56 | 47 | 0.22 |
| Mature neutrophil (%) | 43.2±13.2 | 33.3±15.5 | 33.0±32.1 | 2.54 | 47 | 0.09 |
| Monocyte (%) | 3.1±3.4 | 2.6±1.6 | 4.6±3.0 | 0.67 | 47 | 0.51 |
| Lymphocyte (%) | 45.8±14.8 | 48.8±10.2 | 47.0±26.9 | 0.24 | 47 | 0.78 |
| Eosinophil (%) | 5.9±2.8 | 4.5±2.4 | 1.6±2.8 | 4.29 | 47 | 0.01* |
| Basophil (%) | 0.9±0.9 | 0.8±0.6 | 0.6±1.1 | 0.26 | 47 | 0.76 |
| Total protein (mg/dl) | 98.1±24.7 | 83.6±14.0 | 92.7±5.8 | 2.68 | 47 | 0.07 |
| Albumin (mg/dl) | 25.6±3.8 | 26.5±4.4 | 26.6±2.4 | 0.34 | 47 | 0.71 |
| Glucose (mg/dl) | 55.5±27.5 | 57.2±31.2 | 51.5±5.4 | 0.05 | 47 | 0.94 |
| Cholesterol (mmol/l) | 78.9±14.9 | 71.5±10.2 | 77.2±15.5 | 1.68 | 47 | 0.19 |
| Phosphorus (mg/dl) | 3.1±1.1 | 3.8±3.4 | 2.8±0.7 | 0.65 | 47 | 0.52 |
| Potassium (mmol/l) | 4.3±1.1 | 3.7±0.6 | 3.8±0.3 | 2.85 | 47 | 0.06 |
Dogs with poor body condition showed significantly higher RBC count (14.8±0.0 x106 cells/µl) compared with dogs with fair (5.5±1.8 x106 cells/µl) and dogs with good body condition (5.5±1.7 x106 cells/µl). There was also a significant impact for body condition on values of MCV (20.1±0.0 fl) and MCH (8.7±0.0 pg) (p<0.001) (Table 5). Other hematology and serum chemistry parameters were more or less similar in dogs with differing body conditions (Table 5).
Table 5.
Hematology and serum chemistry values of stray dogs with different body conditions
| Hematology/serum biochemistry | Good (n=21) | Fair (n=26) | Poor (n=3) | F | DF | p |
|---|---|---|---|---|---|---|
| RBC (×106 cells/µl) | 5.5±1.7 | 5.5±1.8 | 14.8±0.0 | 39.10 | 47 | 0.001* |
| Hb (g/dl) | 12.9±1.6 | 12.0±2.4 | 13.0±0.0 | 1.17 | 47 | 0.31 |
| PCV (%) | 40.4±8.9 | 41.1±11.2 | 30.0±0.0 | 1.65 | 47 | 0.20 |
| ESR (% in first hour) | 3.5±0.2 | 3.4±0.3 | 3.9±0.0 | 3.21 | 47 | 0.04* |
| MCV(fl) | 75.5±27.7 | 87.2±37.2 | 20.1±0.0 | 5.80 | 47 | 0.001* |
| MCH (pg) | 24.9±6.9 | 23.9±7.8 | 8.7±0.0 | 6.56 | 47 | 0.001* |
| MCHC (%) | 33.3±7.7 | 31.3±10.6 | 43.3±0.0 | 2.32 | 47 | 0.10 |
| WBC (x103) | 10.0±3.7 | 10.1±3.6 | 12.9±4.5 | 0.84 | 47 | 0.43 |
| Band neutrophil (%) | 10.3±4.3 | 14.7±8.3 | 17.6±11.5 | 2.88 | 47 | 0.06 |
| Mature neutrophil (%) | 40.6±14.1 | 36.8±17.7 | 47.0±6.0 | 0.72 | 47 | 0.49 |
| Monocyte (%) | 4.0±3.7 | 2.3±2.0 | 2.0±0.0 | 2.08 | 47 | 0.13 |
| Lymphocyte (%) | 42.4±13.7 | 50.0±14.2 | 52.0±0.0 | 2.00 | 47 | 0.14 |
| Eosinophil (%) | 6.0±2.8 | 4.3±2.8 | 7.0±0.0 | 2.81 | 47 | 0.07 |
| Basophil (%) | 0.8±0.8 | 0.8±0.7 | 2.0±0.0 | 3.19 | 47 | 0.05* |
| Total protein (mg/dl) | 100.5±28.3 | 87.3±13.3 | 83.0±6.0 | 2.65 | 47 | 0.08 |
| Albumin (mg/dl) | 26.1±3.6 | 25.4±3.6 | 30.7±7.2 | 2.54 | 47 | 0.08 |
| Glucose (mg/dl) | 60.8±29.4 | 54.1±27.2 | 36.9±15.1 | 1.07 | 47 | 0.35 |
| Cholesterol (mmol/l) | 77.1±15.3 | 75.7±13.2 | 73.1±0.4 | 0.13 | 47 | 0.87 |
| Phosphorus (mg/dl) | 4.2±3.1 | 2.7±0.7 | 3.3±0.3 | 2.99 | 47 | 0.05* |
| Potassium (mmol/l) | 4.3±1.0 | 3.9±0.9 | 3.8±1.0 | 0.80 | 47 | 0.45 |
Discussion
Glucose and cholesterol in stray dogs were significantly lower than those observed in the reference range, which may indicate starvation or at least poor food intake. Starvation and mal-absorption are considered to be the main causes of low blood sugar in dogs, but other factors such as a hepatic problem, insulin treatment, hypothyroidism, increased excretion in the urine (renal glucosuria) and idiopathic conditions (unknown cause) in some toy breeds of dogs are also associated with glucose problems. Hypoglycemia demonstrated in microfilariaemic dogs suggested liver dysfunction secondary to circulatory disturbance.
In addition, the hypoglycemia was attributed to glucose consumption by the Dipetalonema viteae and Brugia pahangi parasites (Court et al., 1986). Increased total Protein on the other hand can indicate contact with tick-transmitted diseases such as ehrlichiosis and babesiosis.
Protein profile of serum samples showed an increase in total protein and globulin concentration with a decrease in the albumin values in dogs infested with microfilariae as compared to non-infested dogs. Observed hyperproteinemia can be attributed to an increase in the γ-globulin concentration in response to the parasitic antigens or to release of hemoglobin from destructed erythrocytes (Moustafa et al., 1991). The obtained hypoalbuminemia likely corresponds to degenerative changes in the haemoparasitized organs (mainly liver). Similar results have been previously reported (Safwat and El-Abdin, 1982; Kitagawa et al., 1998). The large standard deviation in the hematology and serum chemistry may be due to different groups of animals within 50 dogs such as different stage of estrous cycle (proestrous, estrous, diestrous and anestrous), different age group (adult, juvenile, and puppy) and different body conditions as well as nutritional stage of individual stray dogs.
Age
In the study, RBC count was significantly higher in puppies as was noted in previous literature. Erythrocyte numbers were high at birth, but fell rapidly as puppies began nursing.
Reduction of TEC values continued during the first month of life.
These changes were related to increased destruction of fetal erythrocytes as well as the rapid growth of the puppy. Circulating red cell mass was significantly reduced (Lee et al., 1976).
In the second month of a puppy’s life, a gradual increase in RBC takes place and continues until adult levels are attained at about one year of age (Anderson and Gee, 1958).
A number of studies done on German Shepherds revealed no significant difference in RBC, WBC, Hb, MCV, MCH, MCHC and differential leucocytes counts between adults and juveniles (Konrad et al., 1980).
Young canids tend to have lower RBC, hemoglobin and hematocrit values than mature adults. In beagles, there were increases with age in the hematocrit, hemoglobin and RBC values, and maximum values were reached only between 13 and 24 months of age (Bulgin et al., 1970).
Other studies in dogs have shown that the hemoglobin and hematocrit increased until 18 months of age (Weiner and Bradley, 1972). Blood loss due to infestations with external and internal parasites could also lead to anemia.
Sex
Differences in ESR between the sexes were of little practical value. Sedimentation of erythrocytes in blood has been studied extensively in the past and it is known that this property of the blood is influenced by red cell and plasma characteristics.
This is reflected in species differences seen in the ESR in health and changes in ESR during disease. Several technical factors are also known to affect the ESR (Ham and Curtis, 1938; Lloyd, 1958; Miale, 1967; Whitby and Britton, 1969; Williams and Trainer, 1971).
It has been found that ESR increases with the length of the tube and height of the blood column, and that it decreases when the bore size of the tube is less than 2.5 mm (Ham and Curtis, 1938). In our present study the ESR values were higher in male dogs than in females.
This finding was in contrast with results of previous studies. The extent of ESR variation is affected by a variety of factors. In a study involving 382 male and 382 female beagles between 8 and 16 months of age, age-related changes in RBC, Hb and PCV were seen but no sex influence was noted (Brunk and Becker-Berger, 1980).
Some investigators have reported higher RBC, hematocrit and hemoglobin values in male dogs, but others have observed no differences between the sexes (Anderson and Gee 1958, Michaelson et al., 1966; Brunk and Becker-Berger 1980). No significant differences between the sexes were found in the blood values of conditioned wild coyotes or pen-raised coyotes (Jain, 1986).
Reproductive stage
RBC count was higher in pregnant bitches. Erythrocyte production increases during pregnancy while erythrocyte mass per unit of body weight remains constant throughout the entire pregnancy and hemoglobin and hematocrit progressively decrease into the third trimester (Lund and Donovan, 1967; Peck and Arias, 1979; Heilmann, 1987).
Physiological anemia is solely due to a dilution decrease in hemoglobin concentration. There is increased plasma volume of about 50% and red cell mass of about 18-25%, which was very much consistent with our findings. Moreover, PCV decreases in pregnant bitches due to a shorter life span of erythrocyte and hemo-dilatation (McFee, 1973; Lurie, 1993; Cavill, 1995).
The MCV provides an indication of the status or size of erythrocytes and epitomizes either normal or abnormal cell division during erythropoiesis (Nussey et al., 1995). The MCV values were lower in pregnant bitches due to higher RBC number during pregnancy periods in canine populations. The different stages of estrous cycle in stray dogs had frequently influenced the progesterone and estrogen concentration, which can determine the serum chemistry profile in these semi-wild animals.
Body condition
In this study, dogs with poor body condition displayed high RBC counts in comparison with fair and good body conditioned dogs. This is seen in dehydrated animals as their blood becomes more concentrated. This is also noted in other conditions, such as some cases of shock, response to high altitudes (the air is ’thinner,’ containing less oxygen, so more RBC’s are put into circulation), diseases of the lungs, etc. Conditions decreasing the amount of oxygen reaching the tissues of the body will cause higher numbers of RBCs to be found in the complete blood cell count (CBC).
MCV and MCH values are inversely related to RBC count or hematocrit value. Erythrocytosis, also called polycythemia, is defined by an increase in total RBC number, PCV, and Hb concentration above reference intervals.
Erythrocytosis occurs frequently in dogs and can arise due to a number of causes. Erythrocytosis may be relative, due to a decrease in total plasma fluid volume, or absolute, due to an increase in RBC production (Sharma and Joshi, 2002).
Conclusion
Stray dogs live closely with humans in Bangladesh. In recent years, the sources of emerging and reemerging diseases were mainly of animal origin, especially originating from wildlife.
Chances of disease emergence from stray dogs are of great concern in Bangladesh. These findings provide important baseline data with which to examine the health status of stray dogs in south and south-east Asia. The study examined the effect of sex, pregnancy status, age, and body condition score on blood cell counts and biochemistry values.
This data may help to understand the emergence of new diseases from this semi-wild animal including the deadly rabies virus.
Acknowledgment
Funding for this study was provided, in part, by the Eppley Foundation for Research and the Rockefeller Foundation
References
- Ali W, Khan F.K, Doulah S, Majumdar J.U. Surveillance of rabies in Dacca, Bangladesh. Med. Res. Co. Bull. 1977;3(2):117–123. [PubMed] [Google Scholar]
- Anderson A.C, Gee W. Normal blood values in the Beagle. Vet. Med. 1958;53:135–138. [Google Scholar]
- Brunk R, Becker-Berger S. Statistical examination of age and sex specific differences in blood parameters in English beagle dogs. Berl Munch Tierarztl Wochenschr. 1980;93:128–132. [PubMed] [Google Scholar]
- Bulgin M.S, Munn S.L, Gee W. Hematologic changes to 4 and one-half years of age in clinically normal beagles. J. Am. Vet. Med. Assoc. 1970;157:1064–1070. [PubMed] [Google Scholar]
- Campbell T.W. 2nd Ed. Ames: Iowa state Uni. Press; 1995. Avian haematology and cytology. [Google Scholar]
- Cavill I. Iron and erythropoisis in normal subjects and in pregnancy. J. Perinat. Med. 1995;23:47–50. doi: 10.1515/jpme.1995.23.1-2.47. [DOI] [PubMed] [Google Scholar]
- Court J.P, Martin-Short M, Lees G.M. A comparison of the response of Dipetalonema viteae and Brugia pahangi adult worms to antifilarial agents. Trop. Med. Parasitol. 1986;37(4):375–380. [PubMed] [Google Scholar]
- Fowler M.E, Miller R.E. 5th ed. St. Louis, MO: Saunders; 2003. Zoo and Wild Animal Medicine; pp. 485–486. [Google Scholar]
- Ham T.H, Curtis F.C. Plasma fibrinogen response in man. Influence of the nutritional state, induced hyperpyrexia, infectious diseases and liver damage. Med. 1938;17:413–445. [Google Scholar]
- Heilmann L. Blood theology and pregnancy. Bailliere Clin. Haem. 1987;1(3):777–799. doi: 10.1016/s0950-3536(87)80024-0. [DOI] [PubMed] [Google Scholar]
- Howlett J.C, Bailey T.A, Samour J.H, Naldo J.L, D’aloia M. Age-related hematologic changes in captive-reared houbara, white-bellied, and rufouscrested bustards. J. Wildlife Dis. 2002;38:804–816. doi: 10.7589/0090-3558-38.4.804. [DOI] [PubMed] [Google Scholar]
- Jain N.C. Schalm’s Veterinary Hematology. 4th ed. N. C. Jain. Philadelphia: Lea & Febiger; 1986. Normal values in blood of laboratory, fur-bearing, and miscellaneous zoo, domestic, and wild animals; p. 337. [Google Scholar]
- Kaneko J.J, Harvey J.W, Bruss M.L. 6th ed. Vol. 493. San Diego, CA: Academic Press; 2008. Clinical Biochemistry of Domestic Animals; pp. 889–895. [Google Scholar]
- Kitagawa H, Kitoh K, Ohba Y. Comparison of laboratory test results before and after surgical removal of heartworms in dogs with vena caval syndrome. J. Am. Vet. Med. Assoc. 1998;213(8):1134–1136. [PubMed] [Google Scholar]
- Konrad J, Kupak M, Husak S. Hematology of the clinically healthy dog. Vet. Med. (Praha) 1980;25(7):405–412. [PubMed] [Google Scholar]
- Lee P, Brown M.E, Hutzler P.T. Blood volume changes and production and destruction of Erythrocytes in Newborn Dogs. Am. J. Vet. Res. 1976;37(5):561–575. [PubMed] [Google Scholar]
- Lloyd H.E. Estimation of the erythrocyte sedimentation rate of capillary blood;description of a new method. Ann. Rheum. Dis. 1958;17:234–239. doi: 10.1136/ard.17.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C.J, Donovan J.C. Blood volume during pregnancy: significance of plasma and red cell volumes. Am J. Obstet. Gynaecol. 1967;98:393–403. [PubMed] [Google Scholar]
- Lurie S. Changes in age distribution of erythrocytes during pregnancy, A longitudinal study. Gynaecol. Obstet. Invest. 1993;36:141–144. doi: 10.1159/000292613. [DOI] [PubMed] [Google Scholar]
- Mcfee J.G. Anemia in pregnancy a reappraisal. Obstet. Gynaecol. Surv. 1973;28:769–793. doi: 10.1097/00006254-197311000-00001. [DOI] [PubMed] [Google Scholar]
- Miale B. 3rd ed. St. Louis: The C. V. Mosby Company; 1967. Laboratory Medicine-Hematology. [Google Scholar]
- Michaelson S.M, Scherr K, Gilt S. The blood of the normal beagle. J. Am. Vet. Med. Assoc. 1966;148:532–534. [PubMed] [Google Scholar]
- Moustafa A.M, Agag B, Esmat M, Selim A.M. Studies on filariasis in Egyptian buffaloes. III. Clinical observations and electrophoretic patterns in sera of naturally infested buffaloes with microfilaria before and after treatment with stipophon. Zagazig Vet. J. 1991;19:583–595. [Google Scholar]
- Nussey G, Van Vuren J.H.J, Du Preez H.H. Effect of copper on the haematology and osmoregulation of the Mozambique tilapia Oreochromis mossambicus (Cichlidae) Comp. Biochem. Phys. 1995;(111A):369–380. doi: 10.1016/0742-8413(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Peck T.M, Arias F. Hematologic changes associated with pregnancy. Clin. Obstet. Gynaecol. 1979;22:485–498. doi: 10.1097/00003081-197912000-00002. [DOI] [PubMed] [Google Scholar]
- Safwat M.S, El-Abdin Y.Z. Some biochemical studies on the serum of infested and non-infested camels with Dipetalonema evansi. Egyptian J. Vet. Sci. 1982;19:141–145. [Google Scholar]
- Sharma M, Joshi C. Serum mineral and haemato-biochemical profile of microfilariae infested cattle in India: Its effects on production and therapy. Asian-Australasian J. Anim. 2002;15(3):357–365. [Google Scholar]
- Smith S.A. Specific species appropriate hematology. In: Feldman B.F, Zinkl J.G, Jain N.C, editors. Schalm’s Veterinary Hematology. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000. pp. 1055–1224. [Google Scholar]
- Weiner D.J, Bradley R.E. The hemogram and certain serum protein fractions in normal beagle dogs. Vet. Med. Sm. Anim. Clin. 1972;67:393–398. [PubMed] [Google Scholar]
- Whitby L, Britton C.J.C. 10th ed. London: Churchill; 1969. Disorders of the Blood; p. 85. [Google Scholar]
- WHO. New Delhi: 1998. Report. Informal consultation. Regional strategies for elimination of Rabies (WHO, South-East Asia Regional Office) p. 7. [Google Scholar]
- Williams J.L, Trainer D.O. A hematological study of snow, blue and Canada geese. J. Wildlife Dis. 1971;7:258–265. [PubMed] [Google Scholar]


