Abstract
A 12-year-old female Shih Tzu dog was referred with diarrhea. Hematological examination indicated severe non-regenerative anemia. Bone marrow aspiration smears and core biopsy specimens revealed normal bone marrow. Based on those results, non-regenerative immune-mediated anemia was diagnosed. The dog was initially treated using prednisolone and cyclosporine. However, this treatment regimen did not prove effective. Nevertheless, the patient achieved a good hematological response after the administration of a combination therapy of human immune globulin and mycophenolate mofetil. Such a combination therapy may prove effective against non-regenerative immune-mediated anemia.
Keywords: Dog, Human immune globulin, Mycophenolate mofetil, Non-regenerative immune-mediated anemia
Introduction
Non-regenerative immune-mediated anemia (NRIMA) characterized by reticulocytopenia is considered uncommon in dogs (Scott-Moncrieff et al., 1995). NRIMA forms of immune-mediated hemolytic anemia have been hypothesized to be due to similar immunopathologic processes, in which an antibody is directed against red blood cell precursor antigens and mature red blood cell membrane antigens in their non-regenerative form (Jonas et al., 1987). Anti-erythrocyte antibody production may be a primary or secondary effect of viral infection, drug administration, vaccination, neoplasia, or blood transfusion (Duval and Giger, 1996). Treatment for NRIMA in dogs relies on corticosteroids and immunosuppressive drugs such as cyclophosphamide and azathioprine (Scott-Moncrieff et al., 1995; Stokol et al., 2000). In addition, some dogs that failed to respond to the above treatment have responded to other drug combinations, including cyclosporine and human intravenous immune globulin (hIVIG) (Scott-Moncrieff et al., 1995; Stokol et al., 2000).
Mycophenolate mofetil (MMF) is an immunosuppressive drug proven effective in reducing allograft rejection in human renal transplantation (Halloran, 1997). In recent clinical reports in humans, MMF has been shown to be useful in the treatment of many hematological disorders (Howard et al., 2002; Lin et al., 2002; Alba et al., 2003; Hou et al., 2003; Gan et al., 2005; Zhang et al., 2005).
In this case report, a dog with NRIMA was presented in which a good hematological response was achieved after an administration of MMF with hIVIG, and such a response suggests a possible role for this agent in the disease.
Case Details
A 12-year-old female Shih Tzu dog was referred with a three-day history of diarrhea. The dog had been vaccinated one week earlier with a combination vaccine (Duramune MX8, Kyoritsuseiyaku, Tokyo, Japan), and had been vaccinated every year so far. There were no special medical history, and no abnormal value was observed in a previous blood analysis.
A physical examination revealed a pale membrane. No additional abnormality was detected during the physical examination. Laboratory data at the time of admission indicated severe non-regenerative anemia (packed cell volume (PCV) 18%; reference range, 37 to 55%) with low reticulocyte counts (9.28×109/L; reference range, > 60×109/L), a moderate decrease in platelet counts (100×109/L; reference range, 200 to 500×109/L), mild elevations of both aspartate aminotransferase (97 U/L; reference range, 23-89 U/L) and blood urea nitrogen (25.4 mg/dL; reference range, 9.0-23.0 mg/dL), as well as increased levels of C-reactive protein (100 mg/L; reference range, 0-10 mg/L).
Mean cell volume and mean corpuscular hemoglobin concentration were normal, indicating normocytic normochromic anemia. The appropriate coagulation tests proved normal. Autoagglutination, a Coombs’ test and an antinuclear antibody test yielded negative results; urinalysis was normal. Abdominal radiography and ultrasonography revealed mild splenomegaly, characterized by homogeneous echogenicity.
Treatment every 12 h with ampicillin (20 mg/kg, SC) and prednisolone (1 mg/kg, SC) began on day 1. On day 3, non-regenerative anemia (PCV 14%, reticulocyte count were 5.73×109/L) had not improved (Figure 1). Bone marrow aspirate smears revealed a small number of erythrophagocytosis cases, but core biopsy specimens revealed normal bone marrow. The erythropoietin levels (66.4 mIU/mL; reference range, 7-37 mIU/mL) and serum iron levels (246 μg/dL; reference range, 30-180μg/dL) were increased. On the basis of those findings, NRIMA was diagnosed. Administrations of oral cyclosporine (5 mg/kg, PO, every 12 h) commenced.
Fig. 1.
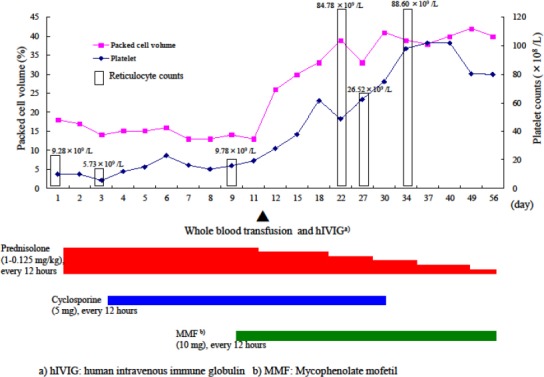
Clinical course of treatment with prednisolone, cyclosporine, human intravenous immune globulin and mycophenolate mofetil in a dog with non-regenerative immune-mediated anemia.
On day 10, there was still no improvement in the non-regenerative anemia (PCV 12%, in either the reticulocyte count of 9.78×109/L) or the platelet count of 167 × 109/L (Figure 1). Administration of MMF (10 mg/kg, PO, every 12 h) began. On day 12, a whole blood transfusion and hIVIG (0.5 g/kg, IV, as a 12-h infusion) were administered. By day 13, PCV had increased to 26%. On day 22, PCV (39%) and the reticulocyte count (84.78 ×109/L) continued to recover (Figure 1).
Due to the elevation of both hepatic enzyme activities and alanine aminotransferase (274 U/L; reference range, 29-84 U/L) and the alkaline phosphatase (1808 U/L; reference range, 62-150 U/L), the dose of prednisolone was gradually decreased. On day 27, however, both PCV (33%) and the reticulocyte count (26.52×109/L) had again declined (Figure 1), but PCV had recovered to 39% by day 34. PCV, hepatic enzyme activities and alkaline phosphatase had returned to normal by day 56 (Figure 1).
Discussion
In the present case, thrombocytopenia, which is commonly observed together with immune-mediated hemolytic anemia in dogs (Klag et al., 1993; Mitchell and Kruth, 2010), was detected in conjunction with NRIMA. In one retrospective study, 26% of the dogs with a diagnosis of immune-mediated hemolytic anemia had been vaccinated within four weeks of diagnosis (Duval and Giger, 1996).
This case had been vaccinated one week earlier with a combination vaccine. However, this case was diagnosed as NRIMA. Thus, considering the half-life of erythrocytes in dogs, the case seems unrelated to a vaccine.
Hypercellularity, erythroid hyperplasia and myelofibrosis are usually observed in the bone marrow of NRIMA patients (Scott-Moncrieff et al., 1995; Stokol et al., 2000; Weiss, 2006).
On the other hand, there is no pathognomonic finding on bone marrow biopsy. Bone marrow plasma cell hyperplasia or lymphocytosis supports the diagnosis, but its absence does not rule it out (Abrams-Ogg, 2010).
No clear abnormality was observed by marrow aspirate smears in this case. However, the diagnosis was NRIMA because of the small number of erythrophagocytosis cases observed by bone marrow aspirate smears.
hIVIG has been shown to be of some benefit in immune-mediated hemolytic anemia in human patients who are refractory to conventional treatment (Flores et al., 1993).
A blockade of Fc receptors on mononuclear phagocytic cells has been proposed as the most likely mechanism accounting for the rapid, early response to hIVIG treatment (Scott-Moncrieff et al., 1995). It has long-term effects, since the half-life of hIVIG in humans is 18 to 32 days. However, in dogs, it is much shorter (seven to nine days (Scott-Moncrieff et al., 1997)).
MMF is metabolized to mycophenolic acid, which inhibits 5’-inosine monophosphate dehydrogenase, a critical enzyme in the de novo synthesis of purine nucleotides. MMF suppresses both B- and T-lymphocyte proliferation, autoantibody production, and the glycosylation of adhesion molecules (Allison and Eugui, 1996; Hood and Zarembski, 1997). In recent clinical reports in humans, MMF has been shown to be useful in the treatment of many hematological disorders, such as autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura and aplastic anemia (Howard et al., 2002; Lin et al., 2002; Alba et al., 2003; Hou et al., 2003; Gan et al., 2005; Zhang et al., 2005). Moreover, its use for the treatment of aplastic anemia has also been tried in veterinary medicine (Yuki et al., 2007).
The major adverse effects of MMF in humans include diarrhea, vomiting, dysuria, leucopenia and anemia. Since such effects are usually reversible and transient (Hood and Zarembski, 1997), they did not appear in the present case. In a clinical report of a human patient with autoimmune hemolytic anemia treated using MMF, the recovery of hemoglobin levels took about one month, indicating that MMF is a slow-acting drug (Lin et al., 2002). In addition, in a prior case of aplastic anemia (Yuki et al., 2007), it took MMF about three weeks to take effect, as with azathioprine (Jergens, 1999).
Our present case was initially treated with prednisolone and cyclosporine, a regimen that failed to prove effective. Next, a medication with the slow-acting drug mycophenolate mofetil was planned, but a transitional medicine was first needed until the effect of the mycophenolate mofetil was revealed. As a result, when human immune globulin was administered, it combined smoothly with the mycophenolate mofetil.
It seemed that a decrease in PCV on day 27 had negated the effect of hIVIG, which indicated that prednisolone and cyclosporine were ineffective. However, since this was a period during which MMF begins to take effect, PCV increased again, and complete remission was obtained.
In conclusion, MMF may prove effective against NRIMA, and hIVIG medication appeared effective until MMF took effect.
References
- Abrams-Ogg A. Nonregenerative anemia. In: Ettinger S. J, Feldman E. C, editors. Textbook of veterinary internal medicine. Philadelphia: W.B. Saunders; 2010. pp. 788–797. [Google Scholar]
- Alba P, Karim M.Y, Hunt B.J. Mycophenolate mofetil as a treatment for autoimmune haemolytic anemia in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus. 2003;12:633–635. doi: 10.1191/0961203303lu419cr. [DOI] [PubMed] [Google Scholar]
- Allison A.C, Eugui E.M. Purine metabolism and immunosuppressive effects of mycophenolate mofetil. Clin. Transplant. 1996;10:77–84. [PubMed] [Google Scholar]
- Duval D, Giger U. Vaccine-associated immune-mediated hemolytic anemia in the dog. J. Vet. Intern. Med. 1996;10:290–295. doi: 10.1111/j.1939-1676.1996.tb02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Cunningham-Rundles C, Newland A.C, Bussel J.B. Efficacy of intravenous immunoglobulin in the treatment of autoimmune hemolytic anemia: results in 73 patients. Am. J. Hematol. 1993;44:237–242. doi: 10.1002/ajh.2830440404. [DOI] [PubMed] [Google Scholar]
- Gan G.G, Leong C.F, Sangkar J.V, Teh A, Goh K.Y, Cheong S.K. The use of mycophenolate mofetil in treating patients with non responding aplastic anemia. Med. J. Malaysia. 2005;60:311–313. [PubMed] [Google Scholar]
- Halloran P. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The international mycophenolate mofetil renal transplant study groups. Transplantation. 1997;63:39–47. doi: 10.1097/00007890-199701150-00008. [DOI] [PubMed] [Google Scholar]
- Hood K.A, Zarembski D.G. Mycophenolate mofetil: a unique immunosuppressive agent. Am. J. Health Syst. Pharm. 1997;54:285–294. doi: 10.1093/ajhp/54.3.285. [DOI] [PubMed] [Google Scholar]
- Hou M, Peng J, Shi Y, Zhang C, Qin P, Zhao C, Ji X, Wang X, Zhang M. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2003;70:353–357. doi: 10.1034/j.1600-0609.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- Howard J, Hoffbrand A.V, Prentice H.G, Mehta A. Mycophenolate mofetil for the treatment of refractory auto-immune haemolytic anemia and auto-immune thrombocytopenia purpura. Br. J. Haematol. 2002;117:712–715. doi: 10.1046/j.1365-2141.2002.03430.x. [DOI] [PubMed] [Google Scholar]
- Jergens A.E. Inflammatory bowel disease. Vet. Clin. North Am. Small Anim. Pract. 1999;29:501–521. [PubMed] [Google Scholar]
- Jonas L.D, Thrall M.A, Weiser M.G. Nonregenerative form of immune-mediated hemolytic anemia in dogs. J. Am. Anim. Hosp. Assoc. 1987;23:201–204. [Google Scholar]
- Klag A.R, Giger U, Shofer F.S. Idiopathic immune-mediated hemolytic anemia in dogs:42 cases (1986-1990) J. Am. Vet. Med. Assoc. 1993;23:783–788. [PubMed] [Google Scholar]
- Lin J.T, Wang W.S, Yen C.C, Chiou T.J, Liu J.H, Hsiao L.T, Yang M.H, Chao T.C, Tai C.J, Chen P.M. Myelodysplastic syndrome complicated by autoimmune hemolytic anemia: remission of refractory anemia following mycophenolate mofetil. Ann. Hematol. 2002;81:723–726. doi: 10.1007/s00277-002-0539-3. [DOI] [PubMed] [Google Scholar]
- Mitchell K, Kruth S. Immune-mediated hemolytic anemia and other regenerative anemias. In: Ettinger S. J, Feldman E. C, editors. In Textbook of veterinary internal medicine. Philadelphia: W.B. Saunders; 2010. pp. 761–772. [Google Scholar]
- Scott-Moncrieff J.C, Reagan W.J, Glickman L.T, DeNicola D.B, Harrington D. Treatment of nonregenerative anemia with human gamma-globulin in dogs. J. Am. Vet. Med. Assoc. 1995;206:1895–1900. [PubMed] [Google Scholar]
- Scott-Moncrieff J.C, Reagan W.J, Snyder P.W, Glickman L.T. Intravenous administration of human immune globulin in dogs with immune-mediated hemolytic anemia. J. Am. Vet. Med. Assoc. 1997;210:1623–1627. [PubMed] [Google Scholar]
- Stokol T, Blue J.T, French T.W. Idiopathic pure red cell aplasia and nonregenerative immune-mediated anemia in dogs: 43 cases (1988-1999) J. Am. Vet. Med. Assoc. 2000;216:1429–1436. doi: 10.2460/javma.2000.216.1429. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996-2004) J. Vet. Intern. Med. 2006;20:955–961. doi: 10.1892/0891-6640(2006)20[955:arsoti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yuki M, Sugimoto N, Otsuka H, Tanahashi S, Katoh M, Hirano T, Nishii N, Suzuki K. Recovery of a dog from aplastic anaemia after treatment with mycophenolate mofetil. Aust. Vet. J. 2007;85:495–497. doi: 10.1111/j.1751-0813.2007.00201.x. [DOI] [PubMed] [Google Scholar]
- Zhang W.G, Ji L, Cao X.M, Chen Y.X, He A.L, Liu J, Zhao W.H, Zou S.P. Mycophenolate mofetil as a treatment for refractory idiopathic thrombocytopenic purpura. Acta Pharmacol. Sin. 2005;26:598–602. doi: 10.1111/j.1745-7254.2005.00088.x. [DOI] [PubMed] [Google Scholar]


