Abstract
A six years eight months pregnant lioness at the Dulahajara Safari Park, Chakoria, Cox’s Bazar, Bangladesh, was presented with dystocia. This paper described the pre-, intra- and postoperative procedures including anesthetic protocol carried out and performing a caesarean section to remove dead fetuses and the successful recovery of the lioness without complications.
Introduction
The lion (Panthera leo) is a member of the family Felidae and one of four big cats in the genus Panthera. Currently, wild lions exist in Sub-Saharan Africa and in Asia with a critically endangered remnant population. Until the late Pleistocene, approximately 10,000 years ago, the lion was the second most widespread large land mammal, found in most of Africa, across Eurasia from Western Europe to India, and in the Americas from the Yukon to Peru (Harington, 1969).
The life span of a lion is approximately 10-14 years in the wild, while in captivity they can live up to 20 years or more (Smuts, 1982). Lions are polyestrous, with heat lasting 4 days. Litters can consist of 1-9 cubs with the average being 3-4. Gestation periods last from 100 to 119 days and the weight of newborns is approximately 1.3 kgs. Lions become sexually mature at 3-4 years of age.
Cesarean section is common in small felidae and canidae practice, especially in practices dedicated to reproductive or emergency and critical care. In one study, 58% of caesarean sections were performed on an emergency basis (Moon et al., 1998). Surgical intervention is required in approximately 60–80% of dystocia cases in the bitch and queen (Gilson, 2003). Timely and appropriate interventions for dystocia, either medical or surgical, are crucial for both maternal and fetal survival. Determination of surgical necessity is based primarily on the condition of the dam, progression of labor, and fetal heart rate. This paper describes the caesarean section, anesthetic procedures and preoperative management for the lioness with dystocia.
Case History
A six years eight months pregnant lioness presenting vaginal discharge and lack of appetite was observed in the Dulahajara Safari Park, Chakoria, Cox’s Bazar, Bangladesh. The lioness conceived on 1st August 2008. Minor clinical manifestations were noted 7th December 2008. On 14th December the lioness was administered Dinoprost® (Prostaglandin, PGF2α at the dose of 5 cc I/M) to initiate uterine musculature contractions and open the uterus in order to facilitate delivery. By 16th December, the lioness was still unable to give birth on her own and it was determined a caesarean section was necessary. Prior to surgery, a full physical examination was completed. The lioness was weak, her blood pressure and heart rate were normal.
Caesarean Operation and anesthetic procedure
A blow pipe with 15 cc anesthetic dart was used to immobilize the lioness while after she had been moved to a small enclosure (100 ft length, 60 width and 16 ft height) (Fig. 1).
Fig. 1.

Anesthesia of lion
The anesthetic agents Ketamil (Katamine Hcl-100 mg/ml at the dose of 10.1 ml), Romazine (Xylazine Hcl-100 mg/ml at the dose of 2.7 ml) and Atropine sulphate (at the dose of 3 ml) were injected intra muscularly. The site of the incision was right of the body just 4 cm above midline (Fig. 2).
Fig. 2.
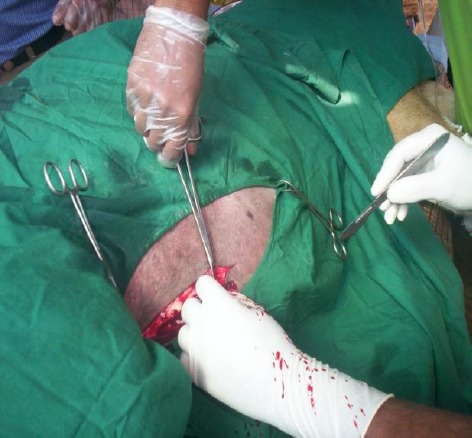
Skin Incision over the midline for caesarean section
To ensure sterilization of the operation site, providone iodine (povisep®) was used. During the surgery three fetuses were expelled from the uterus, all deceased (Fig. 3). After removing the fetuses, 3000 ml I/V Hartman’s solution and saline were used to clean the uterus and internal organs.
Fig. 3.
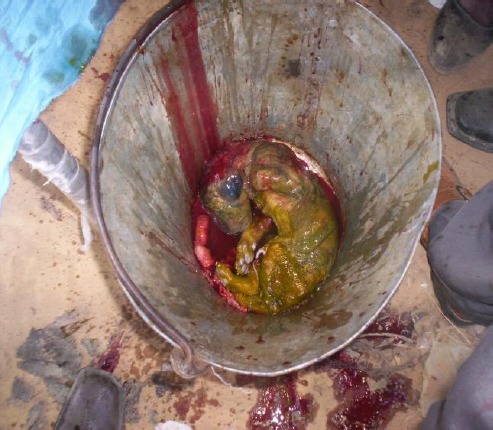
Dead fetus
Suturing was completed for endometrium (size-1 catgut- Peritonium 2/0) (Fig. 4), for muscle (2/0 catgut) and for skin (silk/nylon).
Fig. 4.
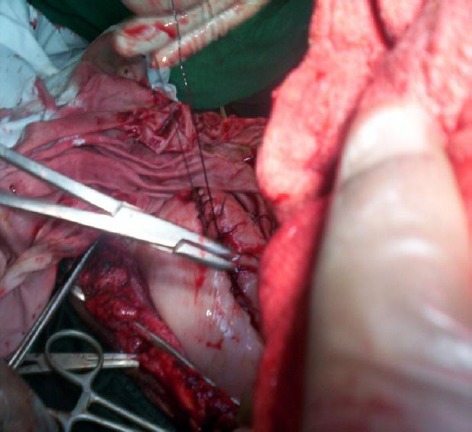
Suturing over the uterus
Postoperative Management
For postoperative care, Inj. Cfeftron® (ceftriaxone- 2 mg I/M for 7 days), Inj. Ketovet® (Ketopropen-5cc I/M for 5 days), Inj. Genacin® (Gentamycin-10 I/M for 7 days), Inj. Histavet® (Antihistaminic- 10 cc I/M for 7 days) were administered. The lioness’ living quarters were regularly sanitized with chlorinated based antiseptic Timsen®. The operative site gradually recovered and the lioness began feeding on 18th December, 2008.
Discussion
During the surgical procedure, the lioness was carefully observed to measure the depth of anesthesia. If any signs of alertness were noted, additional Xylazine with Ketamine were administered. Immobilized lions must be approached with great caution. It is critical to ensure that the animal is appropriately sedated and immobilized and if any movement or alertness is noted, then additional drugs are administered (Nielsen, 1999). The lioness was kept in a lateral recumbent position and was monitored frequently with great caution. Immobilized lions should be kept in lateral recumbency with extended head and neck with the tongue pulled forward to maintain open airways (Lewis, 1994). The principles of anesthetic monitoring in lions are the same as for domestic cats. Following immobilization, depth of anesthesia should be assessed before any procedures commence. As in domestic cats, the animal’s vital signs (heart rate, respiratory rate, color of mucous membranes and capillary refill time) should be recorded at 5 minutes intervals during the immobilization period (Thurmon et al., 1996; Nielsen, 1999; Morris, 2001). The entire operative procedure was conducted in an enclosed and protected area to avoid outside disturbances. Eyes were protected from direct sunlight to avoid retinal damage which can occur in ketamine-anesthetized animals (Lewis, 1994; Lin, 1996). Prophylactic fluid therapy, using lactated Ringer’s solution (5-10 ml/kg/hr) was used and is desirable to maintain extracellular fluid volume during prolonged anesthesia (Thurmon et al., 1996).
In postoperative care analgesia should be provided whenever invasive painful surgeries are performed. Various opiates (butorphanol, buprenorphine and morphine) and non-steroidal anti-inflammatory drugs (flunixin, meglumine, phenylbutazone and ketoprofen) can be used to alleviate acute postoperative pain in lions. The lion referenced in this study received this therapy as well as antibiotic treatment for 7 days to prevent any secondary microbial infection. Some studies show that Flunixin (1 mg/kg, daily) and Phenylbutazone (10 mg/kg, daily, and oral) have been used in lions for up to 7 days with no adverse effects (Thurmon et al., 1996).
Conclusion
Dystocia is commonly handled by caesarean section in small felidae. However, caesarean section in large felidae is a difficult and uncommon practice in Bangladesh especially with zoo and wildlife in which facilities are very limited. Although the lioness in this study survived, her cubs did not. The Dulahajra Safari Park provides a great opportunity in Bangladesh for the improvement of facilities and veterinary care for the country’s captive wildlife. In addition, the development of a rehabilitation centre and wild animal clinic is recommended to better serve Bangladesh’s wild animal populations.
References
- Gilson S.D. Cesarean section. In: Slatter D, editor. Textbook of small animal surgery. Philadelphia, PA: Saunders; 2003. pp. 1517–20. [Google Scholar]
- Harington C.R. “Pleistocene remains of the lion-like cat (Panthera atrox) from the Yukon Territory and northern Alaska”. Can J. Earth Sci. 1969;6(5):1277–1288. [Google Scholar]
- Lewis J.C.M. Anesthesia of non-domestic cats. In: Hall LW, Taylor PM, editors. Anaesthesia of the cat. London: Bailliere Tindall; 1994. pp. 310–349. [Google Scholar]
- Lin H.C. Dissociative anesthetics. In: Thurmon JC, Tranquilli WT, Benson GJ, editors. Lumb ‘Jones’ Veterinary anesthesia. 3rd ed. Baltimore: Williams & Wilkins; 1996. pp. 241–296. [Google Scholar]
- Moon P.F, Erb H.N, Ludders J.W. Perioperative management and mortality rates of dogs undergoing Cesarean section in the United States and Canada. JAm Vet Med Assoc. 1998;213:365–369. [PubMed] [Google Scholar]
- Morris P.J. Chemical immobilization of felids, ursids, and small ungulates. Vet. Clin. North Am. Exot. Anim. Pract. 2001;4:267–298. doi: 10.1016/s1094-9194(17)30060-9. [DOI] [PubMed] [Google Scholar]
- Nielsen L. Iowa: Iowa State University Press; 1999. Chemical immobilization of wild and exotic animals; pp. 227–281. [Google Scholar]
- Smuts G.L. Johannesburg: Macmillian South Africa (Publishers) (Pty.) Ltd; 1982. Lion; p. 231. ISBN 0-86954-122-6. [Google Scholar]
- Thurmon J.C, Tranquilli W.T, Benson G.J. Anesthesia of wild, exotic, and laboratory animals. In: Thurmon JC, Tranquilli WT, Benson GJ, editors. Lumb ‘Jones’ Veterinary anesthesia. 3rd ed. Baltimore: Williams & Wilkins; 1996. pp. 183–209. 448-478, 686-735. [Google Scholar]


