Abstract
An experiment was conducted to study chlorpyrifos chronic toxicity in broilers and the protective effect of vitamin C. Oral administration of 0.8 mg/kg body weight (bw) (1/50 LD50) chlorpyrifos (Radar®), produced mild diarrhea and gross lesions comprised of paleness, flaccid consistency and slightly enlargement of liver. Histopathologically, chlorpyrifos produced degenerative changes in various organs. Oral administration of 100 mg/kg bw vitamin C partially ameliorated the degenerative changes in kidney and heart. There was insignificant alteration in biochemical and haematological profiles. It is concluded that supplementation of vitamin C reduced the severity of lesions induced by chronic chlorpyrifos toxicity in broilers.
Keywords: chlorpyrifos, chronic toxicity, clinicopathology, vitamin C, broilers
Introduction
Chlorpyrifos as an organophosphate, is one of the most widely used insecticides in agriculture worldwide to control wide range of insect and arthropod pests in agriculture and turf (Giesy et al., 1999). Chlorpyrifos is also applied to the soil surrounding or beneath buildings as protection against termites (Barron and Woodburn, 1995) including chicken houses (Leidy et al., 1991). The indiscriminate use of insecticides has led to a widespread concern over the potential adverse effects of these chemicals on human and animal health. The exposure to low levels of chlorpyrifos over a long period of time would have more serious impacts on human and animal health.
Numerous studies have examined chronic toxicity of chlorpyrifos to birds, which reported adverse effects on fertility, hatchability and embryo deformities including twisted necks and shortened/indented backs in bobwhites and adult chickens (Schom et al., 1973) and reduction in body weight, egg production, eggshell thickness, egg weight and hatchling weight (Gile and Meyers, 1986). Significant decline in the humoral immunity was also reported in broilers due to chlorpyrifos chronic toxicity and supplementation of vitamin C has significantly enhanced the immune response (Kammon et al., 2010b). Ascorbic acid (vitamin C) protects cells from oxidative stress by scavenging free radicals.
Most studies concerning such supplementation have focused on the preventive and curative properties in diseases of poultry (Panda and Rao, 1994), mycotoxin toxicity (Hoehler and Marquardt, 1996) and heat stress (Panda et al, 2007). However, scanty reports are available on the clinicopathological implications of chlorpyrifos and the ameliorating effect of vitamin C against its chronic toxicity. Therefore, the present study was conducted to investigate the chronic clinicopathological implications of chlorpyrifos in broilers and the ability of vitamin C to modulate these implications.
Materials and Methods
Experimental design
Broiler chicks (n = 90) of day-old were randomly segregated into three groups of 30 chicks each and were kept in separate pens. Chicks were acclimatized to the place for nine days before the treatment.
On day 10, chicks in group I were administered 0.8 mg/kg bw (1/50 LD50) chlorpyrifos (Radar®) orally using micropipette. Chicks in group II were given chlorpyrifos at 0.8 mg/kg bw plus vitamin C at 100 mg/kg bw (Sisco Research Laboratories, Pvt. Ltd., Bombay, India) by the same route as in group I, while group III was given similar volume of distilled water (DW) orally and served as a control for both groups. The insecticide and vitamin C doses were calculated on weekly body weight basis and were administered until day 45.
On day 24, eight chickens were randomly selected from each group and humanely euthanized for collection of blood and tissue samples. On day 45, remaining chickens were humanely euthanized and representative blood and tissue samples were collected.
Blood samples were collected in heparinized and non-heparinized vials for biochemical and haematological analysis. The experiment was performed as per approval of IAEC, GADVASU and proper attention and care was given to the chickens used in this experiment.
Biochemical analysis
Blood samples were collected in non-heparinized vials from selected chickens of all groups on day 24 and 45 by cardiac puncture. Samples were centrifuged at 1000 rpm for 15 minutes. Sera were then used for analysis of various biochemical parameters viz. acetylcholinesterase (AChE), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AKP), creatine kinase (CK), uric acid, creatinine, glucose, cholesterol, total protein and albumin using Bayer Diagnostics India Ltd kits.
Haematological parameters
Haemoglobin: Haemoglobin was measured by cyanomethemoglobin method using Drabkin’s solution (BTL, India) as per the method described by Benjamin (1978).
Total leukocyte count: Blood samples were collected in heparinized vials (0.2 ml of 1% heparin/5 ml of blood). Total leukocyte counts were determined with the aid of an improved Neubauer counting chamber using Natt and Herrick’s diluent in a ratio of 1:200 (Natt and Herrick, 1952) in which 20 µl of blood was added to 4 ml of the Natt and Herrick’s solution. The leukocytes stained dark blue and the total leukocyte concentration was obtained by counting all the leukocytes in the nine large squares in the ruled area of the counting chamber using the following equation:
TLC/mm3 = [total cells in 9 squares + 10% of total cells] × 200 (Campbell and Ellis 2007).
Differential leukocyte count: A fresh (without anticoagulant) drop from each blood sample was smeared on clean glass slide and dried in air before staining with Wright-Giemsa stain. One hundred white blood cells were counted under oil immersion and the results were expressed in percentage.
Gross and histopathological examination
The detailed post mortem of chickens was conducted on day 24 and 45 and the representative tissue samples were collected in 10% neutral formalin. After overnight washing in running water and dehydration in ascending grades of alcohol, the tissues were embedded in paraffin and 5 μm thick sections were cut and stained with haematoxylin and eosin (H&E) as per the method of Luna (1968). For comparative histopathology of chickens in the three different groups, the severity of lesions of all stained sections were examined by light microscopy and were scored as no change (–), mild (+), moderate (++) or severe (+++). The description of lesions recorded for scoring of various tissues is given in Table 1.
Table 1.
Description of lesions recorded for scoring of various tissues
| Tissue | Lesions |
|---|---|
| Proventriculus | Degenerative changes of the tips of the mucosal papillae, necrosis of glandular cells and accumulation of exfoliated cells in the lumen of the glands. |
| Intestine | Increased number of goblet cells (mucous degeneration) and necrosis associated with sloughing of the epithelial cells. |
| Liver | Dilation of hepatic sinusoids, vacuolar degeneration and fatty changes, coagulative necrosis and proliferation of bile duct cells. |
| Pancreas | Degeneration and necrosis of glandular acini, degeneration and necrosis of beta cells and proliferation of interlobular ducts. |
| Kidney | Congestion and haemorrhage, vacuolar degeneration of tubular epithelial cells, coagulative necrosis, sloughing of tubular cells, necrosis of glomeruli and proliferative glomerulitis. |
| Brain | Swelling of capillary endothelial cells, vacuolization of neurons, neuronal degeneration and necrosis, congestion and haemorrhages in cerebellum and degeneration of Purkinje cells. |
| Heart | Granular degeneration and infiltration of inflammatory cells. |
Statistical analysis
Analysis of variance (ANOVA), with Tukey HSD post hoc test was used for multiple comparisons of biochemical and haematological parameters. Mann-Whitney U nonparametric test was used for the differences between lesion scores.
Results and Discussion
There were no significant changes in biochemical parameters on day 24 (Table 2) and day 45 (Table 3).
Table 2.
Effect of chronic oral administration of chlorpyrifos on various serum biochemical parameters in broiler chickens on day 24
| Biochemical parameters | Groups | ||
|---|---|---|---|
| C | CH | CHC | |
| AKP (IU/L) | 4057±348 | 4791±185 | 4252±564 |
| ALT (IU/L) | 4.7±2.3 | 5.6±3.8 | 4.7±2.5 |
| AST (IU/L) | 217±26 | 224±14 | 211±23 |
| AChE (IU/L) | 1073±101 | 1148±100 | 1270±71 |
| Glucose (mg/dl) | 259±16 | 291±10 | 278±10 |
| Cholesterol (mg/dl) | 110±4 | 108±4 | 105±3.5 |
| Uric acid (mg/dl) | 7.3±0.43 | 6.7±0.37 | 6.8±0.39 |
| Creatine kinase (IU/L) | 3085±499 | 2311±459 | 2238±88 |
| Creatinine (mg/dl) | 0.65±0.05 | 0.67±0.03 | 0.65±0.04 |
| Total protein (g/dl) | 2.8±0.11 | 2.8±0.13 | 2.6±0.16 |
| Albumin (g/dl) | 1.53±0.05 | 1.56±0.06 | 1.54±0.07 |
C= Control group treated with distilled water (n=8).
CH= Chlorpyrifos group (0.8 mg/kg bw) (n=8).
CHC= Chlorpyrifos + Vitamin C group (0.8 mg/kg bw + 100 mg/kg bw) (n=8).
Values indicate mean ± S.E.
The difference between values is not significant at P<0.05.
Table 3.
Effect of chronic oral administration of chlorpyrifos on various serum biochemical parameters in broiler chickens on day 45
| Biochemical parameters | Groups | ||
|---|---|---|---|
| C | CH | CHC | |
| AKP (IU/L) | 991±32 | 971±17 | 933±44 |
| ALT (IU/L) | 5±0.5 | 6.5±1.1 | 5.8±0.5 |
| AST (IU/L) | 228±13 | 240±9 | 227±8 |
| AChE (IU/L) | 1181±154 | 905±47 | 987±84 |
| Glucose (mg/dl) | 216±5 | 233±7 | 222±8 |
| Cholesterol (mg/dl) | 90±3 | 89±2 | 91±4 |
| Uric acid (mg/dl) | 5.15±0.37 | 5.64±0.28 | 5.51±0.23 |
| Creatine kinase (IU/L) | 3999±211 | 4284±398 | 3957±406 |
| Creatinine (mg/dl) | 0.65±0.02 | 0.67±0.03 | 0.66±0.04 |
| Total protein (g/dl) | 3.55±0.17 | 3.35±0.13 | 3.37±0.12 |
| Albumin (g/dl) | 2.0±0.06 | 2.1±0.04 | 2.0±0.03 |
C= Control group treated with distilled water (n=22).
CH= Chlorpyrifos group (0.8 mg/kg bw) (n=22).
CHC= Chlorpyrifos + Vitamin C group (0.8 mg/kg bw +100 mg/kg bw) (n=22).
Values indicate mean ± S.E.
The difference between values is not significant at P<0.05.
The activity of serum AChE in broiler chickens administered chlorpyrifos remained unaltered. The activity of serum AST and ALT were slightly elevated in chlorpyrifos-treated group and chlorpyrifos plus vitamin C group as compared to control. In contrast, administration of chlorpyrifos to cockerels aged 4-6 weeks for 16 days significantly decreased activity of ALT and increased activity of AST (Obaineh and Matthew, 2009). However, earlier findings revealed that several days following injury, ALT levels might be spuriously low (Turk and Casteel, 1997). In acute hepatic injury caused by chlorpyrifos, ALT and AST were significantly elevated (Kammon et al., 2010a) whereas in chronic disease ALT and AST may be normal or only slightly elevated (Cornelius, 1989). Moreover, in chronic liver disease with fibrosis and a reduction in the number of functional hepatocytes, plasma activities of liver enzymes may be within normal limits despite the presence of severe liver fibrosis (Lumeij, 1997). In the present study, AST serum activity increased with age, which is consistent with Sandhu et al. (1998). This is possibly due to muscle development that is usually happening during this period of age, as observed in turkey by Szabo et al. (2005). Chlorpyrifos did not significantly influence the activity of serum AKP, but this enzyme was slightly elevated in chlorpyrifos-treated group compared to control on day 45 of age. AKP serum activity was higher on day 24 due to a higher bone development as compared to its activity on day 45, which is consistent with the observations of Silva et al. (2007). Chlorpyrifos did not produce significant changes in serum levels of glucose, cholesterol, creatinine, total protein, albumin, uric acid and activity of CK.
The administration of chlorpyrifos at 0.8 mg/kg bw did not produce any significant changes in the concentration of haemoglobin, TLC and DLC in broiler chickens on day 24 (Table 4) and day 45 (Table 5). However, no change in haemoglobin, TLC and DLC was observed when White Leghorn cockerels fed Ekalux (Quinalphos) insecticide for 20 days (Mohiuddin and Ahmed, 1986). In another study, Thaker (1988) reported no significant changes in haemoglobin and TLC in White Leghorn chicks orally administered malathion and endosulfan. Krishna and Ramachandran (2009) reported no treatment related or interactive effects on haematological parameters in Wistar rats following exposure to chlorpyrifos for a period of 14 days. The findings of the present study suggest that chronic exposure of broilers to chlorpyrifos at 0.8 mg/kg bw has no significant toxic effects on haemopoietic system.
Table 4.
Effect of chronic oral administration of chlorpyrifos on various haematological parameters in broiler chickens on day 24 and protective effect of vitamin C
| Groups | Hb (g/dl) | TLC (103/mm3) | DLC | |||||
|---|---|---|---|---|---|---|---|---|
| H | L | M | E | B | H:L | |||
| Control (DW) (n=8) | 8.8±1.6 | 12.348±0.6 | 34±2.6 | 60±2.3 | 4.8±0.9 | 1.8±0.3 | 0±0 | 0.55±0.07 |
| Chlorpyrifos (0.8 mg/kg bw) (n=8) | 8.3±2 | 11.384±0.4 | 34±1.7 | 59±1.6 | 4.8±0.7 | 1.8±0.3 | 0±0 | 0.56±0.04 |
| Chlorpyrifos + Vitamin C (0.8 mg/kg bw + 100 mg/kg bw) (n=8) | 8.7±2.1 | 11.743±0.4 | 35±2.7 | 58±2.9 | 5±0.7 | 1.6±0.2 | 0±0 | 0.62±0.09 |
Hb= Haemoglobin; TLC= Total leukocyte count; DLC= Differential leukocyte count; H= Heterophils; L= Lymphocytes; M=Monocytes; E=Eosinophils; B=Basophils; H:L=Heterophils/Lymphocytes ratio.
Values indicate mean ± S.E.
The difference between values is not significant at P<0.05.
Table 5.
Effect of oral administration of chlorpyrifos on various haematological parameters in broiler chickens on day 45 and protective effect of vitamin C
| Groups | Hb (g/dl) | TLC (103/mm3) | DLC | |||||
|---|---|---|---|---|---|---|---|---|
| H | L | M | E | B | H:L | |||
| Control (DW) (n=22) | 9.76±5 | 13.728±1.1 | 32.8±2.8 | 60±2.6 | 5±0.6 | 1.6±0.4 | 0±0 | 0.58±0.08 |
| Chlorpyrifos (0.8 mg/kg bw) (n=22) | 6.87±2 | 13.970±1 | 33.4±1.7 | 61±1.8 | 4±0.7 | 1.2±0.3 | 0.47±0.3 | 0.56±0.04 |
| Chlorpyrifos + Vitamin C (0.8 mg/kg bw + 100 mg/kg bw) (n=22) | 7.74±4 | 13.774±0.8 | 32.1±1.4 | 60±2.1 | 5±0.9 | 2.1±0.5 | 0.2±0.2 | 0.57±0.04 |
Hb= Haemoglobin; TLC= Total leukocyte count; DLC= Differential leukocyte count; H= Heterophils; L= Lymphocytes; M=Monocytes; E=Eosinophils; B=Basophils; H:L=Heterophils/Lymphocytes ratio.
Values indicate mean ± S.E.
The difference between values is not significant at P<0.05.
The main gross lesion observed in chickens administered chlorpyrifos was paleness and flaccid consistency of slightly enlarged liver. Histopathologically, proventriculus of chickens administered chlorpyrifos showed mild necrosis of glandular cells and accumulation of exfoliated cells in the lumen of proventricular glands on day 24. There was mild degeneration of the tips of the mucosal papillae, moderate necrosis of glandular cells and accumulation of desquamated cells in the lumen of the glands on day 45.
Mild mucous degeneration and mild necrosis associated with sloughing of epithelial cells were observed in the intestine on day 24. Lesions observed on day 45 comprised mild mucous degeneration and moderate necrosis of villi associated with sloughing of the epithelial cells. The observed lesions in the intestine suggested that chlorpyrifos has some irritant effects on the epithelial membrane. Enteritis has been observed in a dog with oral ingestion of dichlorvos insecticide (Snow, 1973). Chlorpyrifos produced mild dilation of hepatic sinusoids, moderate vacuolar degeneration and fatty changes and moderate coagulative necrosis on day 24. In addition to lesions observed on day 24, chlorpyrifos produced mild proliferation of bile duct epithelium on day 45. Some of the lesions observed in liver in present study are consistent with several workers who reported congestion, vacuolar degeneration and fatty changes, focal to extensive necrosis, hyperplasia of kupffer cells, dilation of sinusoids, nuclear aberrations, cytoplasmic degranulation and pyknotic nuclei (Gupta and Chandra, 1977; Varshneya et al., 1986; Chaudhary et al., 2003). Histopathological examinations showed focal areas of necrosis in liver and proliferation and fibrosis of bile duct in broiler chicks treated orally with fenvalerate for 28 days (Majumder et al., 1994). Goel et al. (2005) described marked alterations of liver after 8 weeks of treatment in chlorpyrifos-intoxicated rats. The hepatic cords were disrupted at most of the places. A few hepatocytes were vacuolated and had lost the usual polyhedral shape. Vacuolization along with hepatocytes ballooning was more severe near the portal tracts with a resultant widening of the sinusoidal spaces and some degree of hepatic hypertrophy.
In pancreas, chlorpyrifos produced mild degeneration of glandular acini, mild necrosis of glandular cells and mild degeneration of beta islets on day 24. In addition to those lesions, chlorpyrifos produced mild proliferation of interlobular duct of pancreas on day 45.
Chronic oral administration of chlorpyrifos produced mild congestion and haemorrhage, mild vacuolar degeneration of tubular epithelial cells and mild focal coagulative necrosis and sloughing of tubular cell in chickens examined on day 24. On day 45, chlorpyrifos produced moderate severity of similar lesions to that seen on day 24 in addition to moderate necrosis of glomeruli (Fig. 1) and mild proliferative glomerulitis. There was significant difference (p=0.043) between lesions (focal coagulative necrosis and sloughing of tubular cells) observed in kidney of chickens administered chlorpyrifos only and chickens administered chlorpyrifos plus vitamin C on day 45 (Table 6) suggesting partial ameliorating effect of vitamin C to reverse pathological changes produced by chlorpyrifos chronic toxicity. These lesions are similar to earlier studies, which revealed marked congestion and focal degenerative changes in the lining of distal convoluted and collecting duct leading to nephrosis in kidney of rats after repeated oral administration of endosulfan (Gupta and Chandra, 1977). Majumder et al. (1994) observed larger size of glomeruli, glomerular and tubular necrosis in broiler chicks following subacute toxicity of fenvalerate. This is reasonable since the renal tubules are particularly sensitive to toxic influences, in part because they have high oxygen consumption and vulnerable enzyme systems, and in part, because they have complicated transport mechanisms that may be used for transport of toxins and may be damaged by such toxins. In addition, the tubules are exposed to toxic chemicals during their excretion and elimination by the kidneys (Tisher and Brenner, 1989). The presence of necrosis may be related to the depletion of ATP, which finally leads to the death of the cells (Shimizu et al., 1996). One possible mechanism for the tubular lesions observed was the direct toxic effect on the cell function. Other possible mechanisms for the tubular lesions may involve reactive free radicals or oxidative stress, or both (Alden and Frith, 1992). Biologically reactive free radicals are electron-deficient compounds (electrophiles) that bind to cellular electron-rich compounds such as proteins and lipids (Goldstein and Schellmann, 1995). Mixed-function oxidases catalyze the formation of these toxic radicals. Reactive free radicals bind covalently to critical cellular macromolecules and interfere with normal biological activity. Oxidative stress is induced by increasing production of reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide and hydroxyl radicals (Goldstein and Schellmann, 1995). ROS can induce lipid peroxidation, inactivate cellular enzymes, depolymerize polysaccharides, and induce deoxyribonucleic acid breaks and chromosome breakage.
Fig. 1.
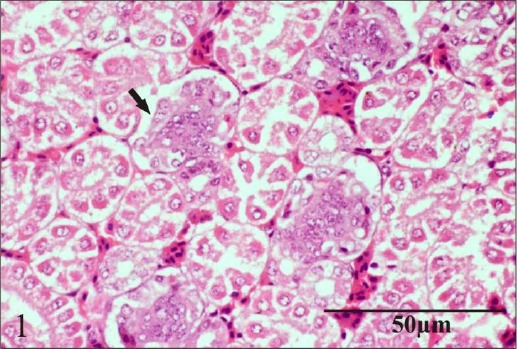
Section of kidney showing moderate necrosis of glomeruli.
Table 6.
Comparative histopathology of kidney and heart in chickens following chronic oral administration of chlorpyrifos on day 24 and 45
| Lesions scored | Groups | P-value | ||||
|---|---|---|---|---|---|---|
| Day 24 (n=5) | Day 45 (n=5) | |||||
| CH | CHC | CH | CHC | Day 24 | Day 45 | |
| Kidney: | ||||||
| Congestion and haemorrhage | + | + | ++ | + | NS | NS |
| Vacuolar degeneration of tubular epithelial cells | + | + | ++ | + | NS | NS |
| Focal coagulative necrosis and sloughing of tubular cells | + | + | ++ | + | NS | 0.043 |
| Necrosis of glomeruli | – | – | ++ | + | NS | NS |
| Proliferative glomerulitis | – | – | + | – | NS | NS |
| Heart: | ||||||
| Granular degeneration | + | – | ++ | + | NS | NS |
| Infiltration of inflammatory cells | – | – | ++ | – | NS | 0.034 |
CH= Chlorpyrifos group (0.8 mg/kg bw).
CHC= Chlorpyrifos + Vitamin C group (0.8 mg/kg bw + 100 mg/kg bw).
– = No change; + = mild; ++ = moderate; +++ = severe; mean of lesion score.
Statistical difference between CH group and CHC group was determined by Mann-Whitney U test.
NS= Not significant
Significance assumed at P < 0.05.
Brain of chickens administered chlorpyrifos showed mild neuronal degeneration and vacuolation and mild degeneration in Purkinje cells on day 24 and 45.
In the heart, there was mild granular degeneration of myocytes on day 24. On day 45, moderate granular degeneration and loss of striations (Fig. 2) besides moderate infiltration of mixed inflammatory cells composed mainly of mononuclear cells and heterophils (Fig. 3) were observed.
Fig. 2.
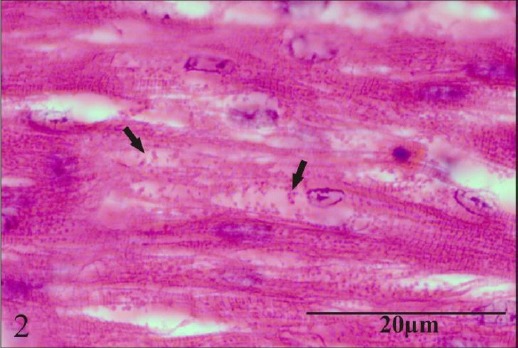
Section of heart showing moderate granular degeneration and loss of striations.
Fig. 3.
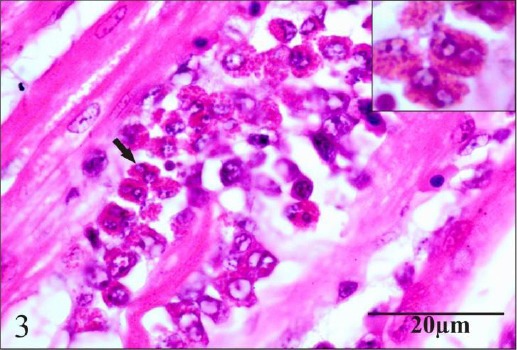
Section of heart showing moderate infiltration of mixed inflammatory cells composed mainly of mononuclear cells and heterophils.
The moderate infiltration of inflammatory cells observed in heart of chlorpyrifostreated group on day 45 was significantly different (p=0.034) from lesions in heart of chlorpyrifos plus vitamin C group indicating protective effect of vitamin C (Table 6).
Following administration of endosulfan in poultry, Bhattacharya et al. (1993) reported coagulative necrosis and fragmentation in cardiac muscle, coronary vessels filled with RBCs and the presence of mononuclear cells in the spaces between the myofibrils.
Chronic oral toxicity of chlorpyrifos produced lesions in other organs viz. lung, gizzard and thyroid gland. Lesions observed were congestion and edema in lungs and mild edema in muscular layer of gizzard. Thyroid gland showed proliferation of follicular cells (Fig. 4).
Fig. 4.
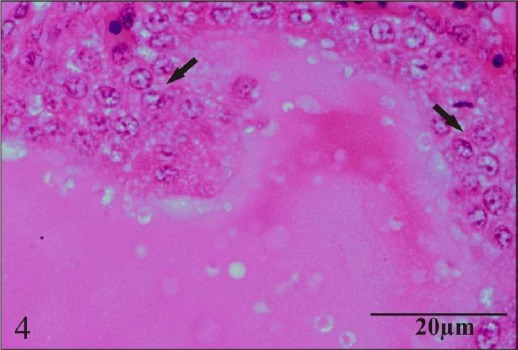
Section of thyroid gland showing proliferation of follicular cells.
Pulmonary edema was a common lesion in most animals following organophosphate pesticides poisoning. The histological picture is generally characterized by edema of the lungs, bronchoconstriction, congestion and intra-alveolar hemorrhage (Abdelsalam, 1987).
The results of present study suggest that chronic exposure to chlorpyrifos at 0.8 mg/kg bw produced microscopic lesions in various organs of broiler chickens indicating cellular toxicity in these organs, which was partially ameliorated by oral administration of vitamin C at 100 mg/kg bw.
References
- Abdelsalam E.B. Organophosphorus compounds I. Toxicity in domestic animals. Vet. Res. Commun. 1987;11(3):212–219. doi: 10.1007/BF00570918. [DOI] [PubMed] [Google Scholar]
- Alden C.L, Frith C.H. Urinary System 1992. In: Hashek W M, Rousseaux C.G, editors. Handbook of Toxicologic Pathology. San Diego, CA: Academic Press; 1992. pp. 316–379. [Google Scholar]
- Barron M, Woodburn B. Ecotoxicology of chlorpyrifos. Rev. Environ. Contam. Toxicol. 1995;144:1–92. doi: 10.1007/978-1-4612-2550-8_1. [DOI] [PubMed] [Google Scholar]
- Benjamin M.M. 3rd Edn. Ames, Iowa, USA: The Iowa State University Press; 1978. Outline of Veterinary Clinical Pathology. [Google Scholar]
- Bhattacharya S, Ghosh R.K, Mondal T.K, Chakrabarty A.K, Basak D.K. Some histopathological changes in chronic endosulfan (ThionalR) toxicity in poultry. Indian J. Anim. Health. 1993;32:9–11. [Google Scholar]
- Campbell T.W, Ellis C.K. 3rd Edn. UK: Blackwell Publishing Ltd; 2007. Avian and Exotic Animal Hematology and Cytology. [Google Scholar]
- Chaudhary N, Sharma M, Verma P, Joshi S.C. Hepato and nephrotoxicity in rat exposed to endosulfan. J. Environ. Biol. 2003;24(3):305–308. [PubMed] [Google Scholar]
- Cornelius C.E. Serum enzyme activities and other markers for detecting hepatic necrosis, cholestasis or cocarcinoma 1989. In: Kaneko J.J, Harvey J.W, Bruss L.M, editors. Clinical biochemical of domestic animals. Academy Press; 1989. pp. 381–386. [Google Scholar]
- Giesy J.P, Solomon K.R, Coats J.R, Dixon K.R, Giddings J.M, Kenaga E.E. Chlorpyrifos: Ecological Risk Assessment in North American Aquatic Environments. Rev. Environ. Contam. Toxicol. 1999;160:1–129. doi: 10.1007/978-1-4612-1498-4_1. [DOI] [PubMed] [Google Scholar]
- Gile J.D, Meyers S.M. Effect of adult mallard age on avian reproductive tests. Arch Environ. Contam. Toxicol. 1986;15:751–756. doi: 10.1007/BF01054922. [DOI] [PubMed] [Google Scholar]
- Goel A, Dani V, Dhawan D.K. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem. Biol. Inter. 2005;156:131–140. doi: 10.1016/j.cbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Gupta P.K, Chandra S.V. Toxicity of Endosulfan after repeated oral administration to rats. Bull. Environ. Contam. Toxicol. (Historical Archieve) 1977;18:378–384. doi: 10.1007/BF01683436. [DOI] [PubMed] [Google Scholar]
- Goldstein R.S, Schellmann R.G. Toxic responses of the kidney 1995. In: Klaassen C D, editor. Casarett and Doull’s Toxicology: The Basic Science of Poisons. New York, NY: McGraw-Hill companies Inc; 1995. pp. 417–442. [Google Scholar]
- Hoehler D, Marquardt R.R. Influence of vitamin E and C on the toxic effects of ochratoxin A and T-2 toxin in chicks. Poult. Sci. 1996;75:1508–1515. doi: 10.3382/ps.0751508. [DOI] [PubMed] [Google Scholar]
- Kammon A.M, Brar R.S, Banga H.S, Sodhi S. Patho-biochemical studies on hepatotoxicity and nephrotoxicity on exposure to chlorpyrifos and imidacloprid in layer chickens. Vet. Arhiv. 2010a;80(5):663–672. [Google Scholar]
- Kammon A.M, Brar R.S, Sodhi S, Banga H.S, Nagra N.S, Singh J. Ameliorating effect of vitamin C on immunological implications induced by chronic chlorpyrifos toxicity in broilers. Libyan Vet. Med. J. 2010b;1(1):164–180. [Google Scholar]
- Krishna H, Ramachandran A.V. Biochemical alterations induced by the acute exposure to combination of chlorpyrifos and lead in Wistar rats. Biol. Med. 2009;1(2):1–6. [Google Scholar]
- Leidy R.B, Wright C.G, Dupree H.E. Applicator exposure to airborne concentrations of a termiticide formulation of chlorpyrifos. Bull. Environ. Contam. Toxicol. 1991;40:177–183. doi: 10.1007/BF01688637. [DOI] [PubMed] [Google Scholar]
- Lumeij J.T. Avian clinical biochemistry 1997. In: Kaneko J.J, Harvey J.W, Bruss L.M, editors. Clinical Biochemistry of Domestic Animals. Academic Press; 1997. pp. 857–883. [Google Scholar]
- Luna L.G. 3rd Edn. New York: McGraw Hill book Co; 1968. Manual of Histological Staining Methods of Armed Forces Institute of Pathology. [Google Scholar]
- Majumder S, Chakraborty A.K, Mandal T.K, Bhattacharya A, Basak D.K. Subacute toxicity of fenavalerate in broiler chicks: concentration, cytotoxicity and biochemical profiles. Indian J. Exp. Biol. 1994;32(10):752–756. [PubMed] [Google Scholar]
- Mohiuddin S.M, Ahmed M.N. Effect of feeding ekalux (Quinalphos) pesticide in poultry. Indian Vet. J. 1986;63:796–798. [Google Scholar]
- Natt M.P, Herrick C.A. A new diluent for counting the erythrocytes and leukocytes of the chicken. Poult. Sci. 1952;31:735–737. [Google Scholar]
- Obaineh O.M, Matthew A.O. Toxicological effects of chlorpyrifos and methidathion in young chickens. African J. Biochem. Res. 2009;3:48–51. [Google Scholar]
- Panda S.K, Rao A.T. Effect of vitamin E selenium combination on chickens infected with infectious bursa of Fabricius disease virus. Vet. Rec. 1994;134:242–243. doi: 10.1136/vr.134.10.242. [DOI] [PubMed] [Google Scholar]
- Panda A.K, Ramarao S.V, Raju M.V.L.N. Effect of vitamin C supplementation on performance, immune response and antioxidant status of heat stressed White Leghorn layers. Indian J. Poult. Sci. 2007;42(2):169–173. [Google Scholar]
- Sandhu B.S, Singh B, Brar R.S. Haematological and biochemical studies in broiler chickens fed ochratoxin and inoculated with inclusion body hepatitis virus, singly and in concurrence. Vet. Res. Commun. 1998;22:335–346. doi: 10.1023/a:1006177222023. [DOI] [PubMed] [Google Scholar]
- Schom C.B, Abbott U.K, Walker N. Organophosphorus pesticide effects on domestic and game bird species. Dursban. Poult. Sci. 1973;52:2083. [Google Scholar]
- Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Retardation of chemical hypoxiainduced necrotic cell death by Bcl-2 and ICE inhibitors: Possible involvement of common mediators in apoptotic and necrotic signal transductions. Oncogene. 1996;12:2045–2050. [PubMed] [Google Scholar]
- Snow D.H. The acute toxicity of dichlorvos in the dog II Pathology. Australian Vet. J. 1973;49:120–125. doi: 10.1111/j.1751-0813.1973.tb06757.x. [DOI] [PubMed] [Google Scholar]
- Silva P.R.L, Freitas Neto O.C, Laurentiz A.C, Junqueira O.M, Fagliari J.J. Blood serum components and serum protein test of Hybro-PG broilers of different ages. Brazilian J. Poult. Sci. 2007;9(4):229–232. [Google Scholar]
- Szabo A, Mezes M, Horn P, Suto Z, Bazar G, Romvari R. Developmental dynamics of some blood biochemical parameters in the growing turkey (Meleagris Gallopavo) Acta Vet. Hungary. 2005;53(4):397–409. doi: 10.1556/AVet.53.2005.4.1. [DOI] [PubMed] [Google Scholar]
- Thaker A.M. Hisar: Ph.D. Thesis submitted to Haryana Agriculture University; 1988. Toxicological and immunological studies on long term exposure to malathion and endosulphan in WLH chicks. [Google Scholar]
- Tisher C.C, Brenner B.M. Vol. 1. Philadelphia: J.B. Lippincott company; 1989. Renal Pathology with Clinical and Functional Correlation. [Google Scholar]
- Turk J.R, Casteel S.W. Clinical Biochemistry in Toxicology 1997. In: Kaneko J.J, Harvey J.W, Bruss M.L, editors. Clinical Biochemistry of Domestic Animals. California: Academic Press; 1997. pp. 829–835. [Google Scholar]
- Varshneya C, Bahga H.S, Sharma L.D. Toxicological evaluation of dietary lindane in cockerels. Indian J. Poult. Sci. 1986;21:312–315. [Google Scholar]


