Abstract
Background
Sclerostin is a soluble inhibitor of the Wnt signalling pathway and has been shown to be associated with decreased bone turnover and vascular and/or valvular calcification in patients with chronic kidney disease. Common carotid artery intima-media thickness (CIMT) assessment and common carotid artery (CCA) plaque identification with ultrasound imaging are well-recognized tools for the identification and monitoring of atherosclerosis. The aim of the present study was to investigate whether the circulating levels of sclerostin might be associated with carotid artery atherosclerosis in prevalent haemodialysis patients.
Methods
In this cross-sectional study, serum sclerostin concentrations were measured using a commercially available enzyme-linked immunosorbent assay kit. CIMT was measured and carotid plaques were identified by B-mode and Doppler ultrasound imaging.
Results
One hundred and twenty-two prevalent haemodialysis patients were involved in the study. Serum sclerostin levels were higher in patients with plaques in CCA than patients free of plaques (227 ± 166 versus 117 ± 91 pmol/L, P = 0.016). A significant correlation was recorded between serum sclerostin levels and CIMT (r = 0.459, P < 0.0001). In the multiple regression analysis, sclerostin concentrations were one of the independent factors that remained significantly associated with CIMT.
Conclusion
Sclerostin is independently associated with CIMT although further studies are needed.
Keywords: carotid artery intima-media thickness, haemodialysis, sclerostin
Introduction
A growing body of evidence indicates that abnormalities of bone and mineral metabolism in chronic kidney disease (CKD) may contribute to the development of cardiovascular disease (CVD) and increased cardiovascular mortality, with the most likely link being the development of vascular calcification [1, 2]. The signalling pathways involved in these processes remain incompletely understood [3, 4] and accumulating data have raised interest in understanding bone and mineral metabolism regulation and its consequences in patients with CKD.
Sclerostin is a soluble inhibitor of the Wnt/b-catenin (canonical) signalling pathway [5]. The main action of sclerostin is a decrease in bone formation via inhibiting osteoblast proliferation and differentiation, and promoting osteoblast apoptosis [6]. Recently, increased levels of sclerostin were shown to be associated with decreased bone turnover and osteoblast number in dialysis patients [7]. On the other hand, sclerostin has been demonstrated to be upregulated during vascular smooth muscle cell (SMC) calcification in vitro [8]. More recently, it was shown that high serum sclerostin was associated with the extent of aortic valve calcification and that in aortic valve tissue, sclerostin strongly co-localized with areas of calcification in dialysis patients [9].
Since CKD mineral and bone disorder influences cardiovascular event rate and mortality in CKD populations, the relationship between serum sclerostin levels and the future outcome was analysed in CKD cohorts. Recent studies in haemodialysis patients [10, 11] and in non-dialysed CKD patients [12] revealed the association of high levels of serum sclerostin with higher mortality in dialysis patients. In contrast, low serum sclerostin levels were shown to be associated with increased mortality risk in haemodialysis patients [13, 14]. These studies were performed in subjects with different clinical characteristics and with different sclerostin assays, yielding more questions as to whether sclerostin will be used as a biomarker for both cardiovascular and bone health statuses in CKD.
Wnt signalling activity in general and sclerostin activity in particular are associated with ectopic and vascular calcification processes beyond bone mineralization [15]. Further evidence is needed to answer the question regarding the effects of sclerostin on arteriosclerosis (pro- or anti-calcific). Therefore, the aim of the present study was to investigate the association of circulating concentrations of sclerostin with carotid artery atherosclerosis in prevalent haemodialysis patients, due to limited data in this area.
Materials and methods
Patients
The study was conducted at the RFM Dialysis Center, Ankara, Turkey in August 2011. One hundred and fifty-seven haemodialysis patients were screened based on the following inclusion/exclusion criteria: 18 years or older, stably treated with haemodialysis for at least 1 year, Kt/V > 1.2 during the previous 6 months, and no signs of liver disease, clinically evident active infection, autoimmune disease or known malignancy. Overall, participants were comprised of 122 prevalent haemodialysis patients (64 women and 58 men, mean age 55 ± 13 years, mean haemodialysis vintage: 58 ± 20 months, on haemodialysis three times a week). The patients were suffering from end-stage renal disease (ESRD) due to diabetic nephropathy (n = 38), hypertensive nephrosclerosis (n = 32), chronic glomerulonephritis (n = 25), chronic pyelonephritis (n = 12) and polycystic disease (n = 8). The renal diagnosis was unknown in seven patients. The presence of residual renal function (RRF) was defined as residual glomerular filtration rate ≥1 mL/min/1.73 m2. The mean RRF was 0.7 ± 1.1 mL/min/1.73 m2. Further detail about hemodialysis therapy is included in the section “Supplementary Data”.
Angiotensin-converting enzyme inhibitors (n = 46), angiotensin receptor blockers (n = 62), beta-blockers (n = 76) and calcium channel blockers (n = 70) were given for hypertension management. Patients were prescribed treatments including CaCO3 (30%), sevelamer-HCl (26%), Ca acetate (44%), calcitriol (69%), anti-platelet agents (71%), warfarin (4%) and erythropoietin (59%). The mean erythropoietin dose was 145 U/kg/week, achieving a mean haemoglobin (Hb) serum level of 11.2 g/dL; <10% of patients had serum Hb <10 g/dL.
None of the patients received glucocorticoid, statin, bisphosphonates or denosumab. Calcimimetics were not used in this cohort.
The study protocol was performed according to the Declaration of Helsinki and was approved by the local ethics committee of Yuksek Ihtisas Training and Research Hospital, Ankara, Turkey and all the patients provided their written informed consent before entering the study.
Clinical parameters and biochemical assays
Information regarding baseline demographic characteristics, the aetiology of ESRD and the presence of diabetes mellitus (DM) was collected by reviewing medical records. Cardiovascular history was defined as history of myocardial infarction, percutaneous coronary artery intervention, cardiac by-pass or valvular surgery, peripheral artery disease or stroke.
Venous blood samples were drawn after an overnight fasting period. The blood sample was obtained directly through an arteriovenous fistula on a mid-week non-dialysis day. Biochemical serum parameters (creatinine, blood urea nitrogen, glucose, electrolytes, albumin and complete blood count calcium, phosphate, lipids, protein, cholesterol and triglycerides) were performed via the standard laboratory procedure, using an automated analyser. Serum C-reactive protein (CRP) level was detected by rate nephelometry (IMAGE). The spKt/V value was calculated according to the Daugirdas second-generation formula [16]. The normalized protein catabolic rate (nPCR) was calculated as a measure of the daily protein intake of patients [17].
Outcome, exposures and covariates
The primary outcome was the association of circulating concentrations of sclerostin with carotid artery atherosclerosis in prevalent haemodialysis patients. The primary exposure variable was the baseline serum sclerostin level, measured at the initiation of outpatient haemodialysis. The serum sclerostin level was measured via a commercially available enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions and as used in previous studies by Cejka et al. [7, 18]. The intra-assay and inter-assay coefficients of variation were 7.5 and 6.3%, respectively. The serum intact parathormone and alkaline phosphatase levels were studied by means of a computerized auto-analyser (Hitachi 717; Boehringer-Mannheim). The intact parathormone level was measured by the electrochemiluminescence immunoassay [Roche PTH (Intact PTH)]. The serum was available for the radioimmunoassay of 25-dihydroxy vitamin D3 levels (DiaSorin Corporation, Stillwater, MN) in all patients.
Common carotid B-mode and Doppler ultrasound imaging
A high-resolution B-mode ultrasound of the common carotid arteries (CCAs) with scanning on the longitudinal axis until the bifurcation and on the transversal axis was performed using an instrument generating a wide-band ultrasonic pulse with a middle frequency of 7.5 MHz (Siemens Elegra Ultrasonography Systems, Tokyo, Japan). For each carotid artery, two longitudinal measurements were obtained by rotating (1801 increments) the vessels along the axis. All patients were blindly examined by one experienced operator. Carotid artery intima media thickness (CIMT) is measured at 1 cm proximal to the bifurcation on each side as previously described [19].
Statistical analysis
Data analysis was performed by using SPSS for Windows, version 11.5 (SPSS, Inc., Chicago, IL). Whether the distributions of continuous variables were normal or not was determined with the use of the Shapiro Wilk test. Normally distributed continuous variables are presented as mean ± standard deviation, and non-normally distributed continuous variables are presented as median with 25th and 75th percentiles. Categorical data are presented as percentages.
Differences in clinical and biochemical parameters were compared using the analysis of variance for continuous variables and the χ2 test for categorical variables, whereas the non-parametric Kruskal–Wallis test was used for non-normally distributed continuous variables.
Spearman correlation coefficients were obtained for all potential predictor variables to look for confounding variables. A P-value of <0.05 was considered statistically significant. Linear regression analyses were performed to examine relationships between CIMT and clinical variables. Multiple regression analysis was performed to assess the combined effects of clinical variables on CIMT.
Results
Association of baseline characteristics and sclerostin
The demographic and clinical characteristics of the study patients are depicted in Table 1. The mean serum sclerostin level in haemodialysis patients was higher when compared with age- and gender-matched healthy controls (152 ± 137 versus 19 ± 8 pmol/L, P < 0.0001). Sclerostin levels were not significantly different in patients with a positive history of hypertension (172 ± 128 versus 153 ± 132 pmol/L, P = 0.50), dyslipidaemia (149 ± 112 versus 159 ± 127 pmol/L, P = 0.73) and smoking (162 ± 135 versus 144 ± 128 pmol/L, P = 0.32) compared with those without. However, male patients had significantly higher serum sclerostin levels than female patients (203 ± 112 versus 131 ± 105 pmol/L, P = 0.04). Moreover, patients with a positive cardiovascular history had higher serum levels than patients that did not have such history (198 ± 127 versus 133 ± 119 pmol/L, P = 0.048). Furthermore, patients with type 2 DM tended to have higher sclerostin levels than patients without DM (198 ± 116 versus 154 ± 129 pmol/L, P = 0.09). No interaction between the use of calcitriol and serum sclerostin levels was found.
Table 1.
Baseline demographic and clinical characteristics of patients
| Parameter | All patients (n = 122) | Patients with plaques in CCA (n = 90) | Patients with no plaques in CCA (n = 32) | P-value |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 55 ± 13 | 62 ± 10 | 50 ± 14 | 0.03 |
| Female/male (n/n) | 64/58 | 44/46 | 20/12 | 0.32 |
| Haemodialysis vintage (months) (mean ± SD) | 58 ± 20 | 66 ± 19 | 55 ± 29 | 0.13 |
| Diabetes (n, %) | 38 (31) | 32 (35.5) | 6 (18.8) | 0.04 |
| Cardiovascular history (n, %) | 49 (40) | 39 (43.3) | 10 (31.2) | 0.20 |
| Smoking (n, %) | 27 (22.1) | 24 (26.6) | 3 (9.3) | 0.16 |
| RRF (mL/min/1.73 m2) (mean ± SD) | 0.70 ± 1.10 | 0.72 ± 1.00 | 0.70 ± 0.90 | 0.48 |
| Ultrafiltration (kg/dialysis session) (mean ± SD) | 2.70 ± 1.20 | 2.90 ± 1.00 | 2.60 ± 1.20 | 0.78 |
| AV fistula flow (mL/min) (mean ± SD) | 295 ± 64 | 300 ± 58 | 294 ± 60 | 0.56 |
| BMI (kg/m2) (mean ± SD) | 21.9 ± 3.0 | 22.3 ± 3.5 | 21.9 ± 2.7 | 0.36 |
| Systolic BP (mmHg) (mean ± SD) | 123 ± 28 | 126 ± 19 | 118 ± 28 | 0.03 |
| Diastolic BP (mmHg) (mean ± SD) | 69 ± 10 | 69 ± 4 | 66 ± 12 | 0.42 |
| Hb (g/dL) (mean ± SD) | 11.1 ± 1.0 | 11.0 ± 1.5 | 10.6 ± 1.3 | 0.33 |
| Calcium (mg/dL) (mean ± SD) | 8.9 ± 0.7 | 9.2 ± 0.4 | 8.9 ± 0.4 | 0.45 |
| Phosphate (mg/dL) (mean ± SD) | 5.2 ± 1.0 | 5.3 ± 0.6 | 4.99 ± 0.67 | 0.13 |
| Alkaline phosphatase (IU/L) (mean ± SD) | 136 ± 102 | 139 ± 104 | 132 ± 108 | 0.95 |
| 25-OH Vitamin D3 (nmol/L) (mean ± SD) | 14 ± 9 | 17 ± 12 | 13 ± 9 | 0.39 |
| Log iPTH (pg/mL) (mean ± SD) | 2.20 ± 0.42 | 2.10 ± 0.40 | 2.28 ± 0.41 | 0.003 |
| Sclerostin (pmol/L) (mean ± SD) | 152 ± 137 | 227 ± 166 | 117 ± 91 | 0.016 |
| LDL-C (mg/dL) (mean ± SD) | 100 ± 37 | 90 ± 44 | 104 ± 37 | 0.04 |
| HDL-C (mg/dL) (mean ± SD) | 39 ± 11 | 37 ± 9 | 40 ± 12 | 0.24 |
| Triglycerides (mg/dL) (mean ± SD) | 144 (102; 312) | 139 (122; 301) | 146 (102; 312) | 0.48 |
| Uric acid (mg/dL) (mean ± SD) | 6.5 ± 0.9 | 6.4 ± 0.8 | 6.9 ± 1.0 | 0.09 |
| CRP (mg/L) (mean ± SD) | 11 (0.6; 36) | 19 (1; 36) | 9 (0.6; 17) | 0.02 |
| Albumin (g/L) (mean ± SD) | 3.95 ± 0.37 | 3.80 ± 0.34 | 4.09 ± 0.57 | 0.04 |
| nPCR (g/kg/d) (mean ± SD) | 1.1 (0.92; 1.29) | 1.0 (0.92; 1.25) | 1.1 (0.98; 1.29) | 0.88 |
| Kt/Vurea (mean ± SD) | 1.65 ± 0.26 | 1.64 ± 0.19 | 1.68 ± 0.12 | 0.62 |
| ESA use (n, %) | 72 (59) | 53 (58.8) | 19 (59.3) | 0.95 |
| Calcitriol use (n, %) | 84 (69) | 61 (67.7) | 23 (71.8) | 0.82 |
| Calcimimetic use (n, %) | 0 | |||
| Statin use (%) | 0 | |||
| Anti-hypertensive agents use (n, %) | 68 (55.7) | 50 (55.5) | 18 (56.2) | 0.89 |
| Aspirin/anti-platelet agents use (n, %) | 92 (75) | 67 (74.4) | 25 (78) | 0.77 |
| Treatment with calcium-based phosphate binders (n, %) | 83 (74) | 67 (74.4) | 16 (50) | 0.20 |
AV, arteriovenous; BP, blood pressure; BMI, body mass index; ESA, erythropoiesis-stimulating agent; CRP, C-reactive protein; Log iPTH, logarithm of serum intact parat hormone; LDL-C, low-density lipoprotein cholesterol; nPCR, normalized protein catabolic rate; HDL-C, high-density lipoprotein cholesterol; statins, HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase inhibitors.
No significant correlations were observed between serum sclerostin levels and age, haemodialysis vintage, systolic and diastolic blood pressure (BP), low-density lipoprotein (LDL)- and high-density lipoprotein (HDL)-cholesterol, triglycerides, Hb, serum albumin, calcium, phosphate, alkaline phosphatase, uric acid and CRP levels. However, sclerostin levels were negatively correlated with log serum intact parathormone levels (r = −0.222, P = 0.005).
Association of baseline characteristics and presence of atherosclerotic plaques in carotid artery
Table 1 shows that patients with atherosclerotic plaques in carotid artery were older and had higher systolic BP, serum levels of sclerostin and CRP levels and also a higher prevalence of DM. Moreover, these patients had lower log serum intact parathormone levels, LDL-cholesterol and albumin concentrations when compared with patients free of plaques (Table 1).
Risk factors for increased CIMT
CIMT significantly correlated with age (r = 0.29, P = 0.05), smoking (r = 0.08, P = 0.045), haemodialysis vintage (r = −0.377, P = 0.048), systolic BP (r = 0.41, P = 0.05), serum albumin (r = −0.35, P = 0.01), CRP (r = 0.32, P = 0.016), phosphate (r = 0.403, P = 0.0001) and log intact parathormone levels (r = 0.29, P = 0.028).
Serum sclerostin levels and CIMT
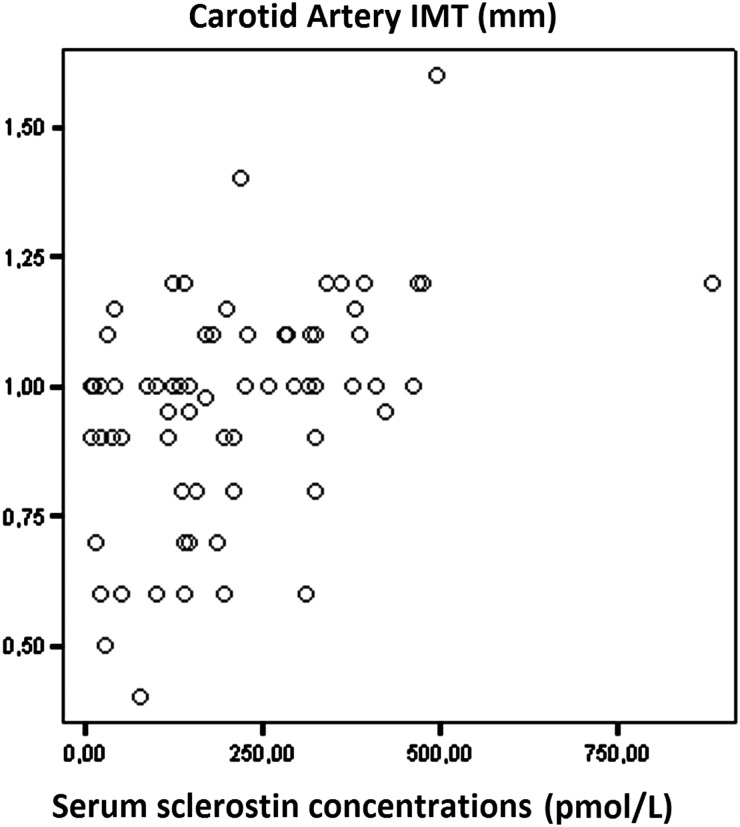
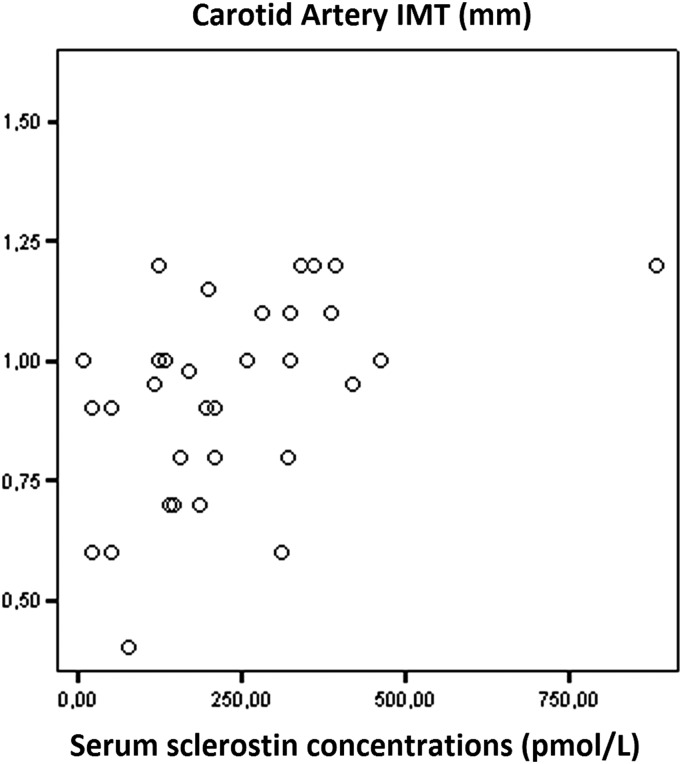
Significant correlations were recorded between serum sclerostin levels and CIMT (r = 0.459, P < 0.0001; Figure 1). Serum sclerostin levels were positively correlated with CIMT in both diabetic (r = 0.676, P = 0.008; Figure 2) and non-diabetic haemodialysis patients (r = 0.504, P = 0.003; Figure 3).
Fig. 1.
Correlation between serum sclerostin levels and carotid artery intima-media thickness (CIMT).
Fig. 2.
Correlation between serum sclerostin levels and carotid artery intima-media thickness (CIMT) in diabetic haemodialysis patients (r = 0.676, P = 0.008).
Fig. 3.
Correlation between serum sclerostin levels and carotid artery intima-media thickness (CIMT) in non-diabetic haemodialysis (r = 0.504, P = 0.003).
Linear regression analysis
Independent variables included in the multivariable model were the variables found to correlate significantly with CIMT (age, smoking, systolic BP, serum albumin, CRP, phosphate, sclerostin and log intact parathormone levels) and the variables found to have statistical significance in the univariate analysis (haemodialysis vintage, presence of DM, positive cardiovascular history and treatment with calcium-based phosphate binders). Moreover, LDL-cholesterol levels were also involved in the final analysis because of the related P-value of ≤0.2 in the univariate analysis.
Among these, age, log intact parathormone levels, serum CRP and sclerostin concentrations were the only parameters that remained significantly associated with CIMT (Table 2). Levels of phosphate in the highest tertile showed an increased prevalence for the presence of atheromatous plaque in the carotid artery when compared with the lowest tertile, although this was not significant (Table 2). The results did not differ significantly when further adjusted for vitamin D and oral phosphate binder use (data not shown). Similarly, no interaction has been found with oral phosphate binders.
Table 2.
Univariate and multivariate logistic regression analysis for CIMT
| Parameter | HR; 95 CI% | P-value | HR; 95 CI% | P-value |
|---|---|---|---|---|
| Age | 1.09 (0.99, 1.13) | 0.040 | 1.02 (0.94–1.20) | 0.050 |
| Men | 1.24 (1.08–1.36) | 0.760 | ||
| Dialysis vintage | 1.42 (1.26–1.55) | 0.010 | 1.14 (0.99–1.29) | 0.306 |
| Smoking | 1.16 (1.06–1.32) | 0.050 | 1.06 (1.01–1.14) | 0.270 |
| Presence of type 2 DM | 6.32 (3.00–18.44) | 0.001 | 4.19 (1.44–8.60) | 0.380 |
| Positive cardiovascular history | 3.40 (1.17–9.56) | 0.029 | 2.36 (1.17–8.22) | 0.120 |
| Treatment with calcium-based phosphate binders | 2.43 (1.36–3.99) | 0.025 | 1.30 (1.19–2.41) | 0.150 |
| BMI | 0.98 (0.76–1.11) | 0.890 | ||
| Systolic BP | 1.08 (1.03–1.17) | 0.040 | 0.98 (0.95–1.01) | 0.478 |
| Diastolic BP | 0.98 (0.92–1.8) | 0.910 | ||
| LDL-cholesterol | 1.17 (1.00–1.29) | 0.192 | 1.05 (1.00–1.09) | 0.321 |
| HDL-cholesterol | 0.92 (0.86–1.01) | 0.340 | ||
| Triglycerides | 1.00 (0.98–1.02) | 0.870 | ||
| Hb | 1.17 (1.14–1.59) | 0.419 | ||
| Albumin | 0.64 (0.59–0.82) | 0.020 | 0.77 (0.62–0.88) | 0.326 |
| Calcium | 1.03 (0.98–3.01) | 0.898 | ||
| Phosphate (reference) T1 | 1.00 ( | 0.91 | ||
| T2 versus T1 | 1.08 ( | 0.73 | ||
| T3 versusT1 | 1.23 ( | 0.63 | ||
| Log serum intact PTH | 1.26 (1.10–1.62) | 0.044 | 1.13 (1.08–1.47) | 0.050 |
| 25-hydroxy vitamin D3 | 0.77 (0.72–0.91) | 0.660 | ||
| Sclerostin | 0.47 (0.33–0.66) | 0.006 | 0.61 (0.44–0.78) | 0.030 |
| Uric acid | 0.84 (0.69–1.09) | 0.510 | ||
| CRP | 1.22 (0.97–1.37) | 0.010 | 1.19 (0.99–1.28) | 0.019 |
| Alkaline phosphatase | 1.02 (0.99–1.22) | 0.472 |
DM, diabetes mellitus; BMI, body mass index; BP, blood pressure; Log iPTH, logarithm of serum intact parat hormone; CRP, C-reactive protein; T1, phosphate tertile 1 (<4.57 mg/dL); T2, phosphate tertile 2 (4.57, 5.26 mg/dL); T3, phosphate tertile 3 (>5.26 mg/dL).
Discussion
The present study depicts independent associations between serum sclerostin levels, abnormal CIMT and carotid plaques in maintenance haemodialysis patients. The results raise the possibility of a specific pathophysiologic effect of sclerostin on atherosclerosis, distinct from its effects on bone metabolism. Increased SMC proliferation and migration entirely change the composition and structure of the blood vessel wall, leading to atherosclerosis [20]. Canonical Wnt signalling and accompanying β-catenin activation have been shown to be pro-proliferative in arterial and venous SMCs, both in vitro and in vivo [17]. Sclerostin slows the canonical Wnt signalling pathway and inhibits osteoblast activity and bone formation by sequestering LRP5 and LRP6 [21]. Moreover, missense mutations in LRP6 have been shown to be associated with premature coronary artery disease [22]. Retarding the Wnt signalling pathway by using a dominant-negative LRP has been depicted to significantly reduce arterial SMC proliferation [23]. Furthermore, FrzA/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, delayed arterial SMC entry into the S-phase [24]. In addition to SMC proliferation, animal models of intimal thickening have revealed increased β-catenin levels, which suggest the involvement of the Wnt-β-catenin pathway also in SMC migration [25, 26]. Moreover, Wnt proteins are also known to promote the migration of various cell types, including monocytes and endothelial cells [20, 27, 28]. Furthermore, the Wnt pathway has been described to play an important role in the regulation of endothelial inflammation, vascular calcification and mesenchymal stem cell differentiation [29]. As a result, considering the fact that atherosclerosis is both an actively regulated and progressive process, we might speculate that high sclerostin levels might be indicative of a sort of defensive mechanism that may attenuate the upregulation of the canonical Wnt pathway [30, 31] and lead to the restoration of quiescent Wnt signalling observed under healthy conditions.
Our data also reveal increased sclerostin levels in patients with carotid plaques compared with patients without. Sclerostin has been demonstrated to be upregulated in vascular SMC, which previously transformed into osteocytic phenotype under calcifying circumstances [8]. Recently, it has been suggested that increased circulating sclerostin levels might protect dialysis patients from cardiovascular calcification, and that low bone-specific alkaline phosphatase activity may be the causal pathway [32]. Sclerostin is a potent inhibitor of alkaline phosphatase activity, which inactivates the potent calcification inhibitor, the inorganic pyrophosphate [13]. In line with previous work, accumulating data suggest that Wnt signalling pathway inhibitors overexpression in calcifying vasculature (advanced carotid plaques and calcified aortas) might even be vasculoprotective and anti-calcific, most probably by closing a feedback loop that aims to retard further mineralization [3, 8]. A more recent experimental rat model supports this idea by showing the overexpression of secreted Frizzled-related proteins (another group of Wnt pathway inhibitors) in the late but not the early stages of vascular calcification [3]. Furthermore, our previous observation on arteriovenous fistula calcification [15] also seems in line with the above-mentioned reports which showed that in patients with CKD, higher circulating sclerostin levels were independently associated with a lower risk for vascular calcification. The association between CIMT and serum sclerostin in the present study is somewhat in contrast to the findings of a recent work that showed a lack of correlation between sclerostin and a surrogate marker of arterial stiffness in CKD patients [33]. However, a negative association was seen between circulating concentrations of other secreted Wnt signalling inhibitor (DKK1) and arterial stiffness marker [33]. Different demographic and clinical characteristics of the study patients (haemodialysis vintage, percentage of diabetic patients, etc.) cohort size, preanalytical biomarker stability and assay characteristics of the investigated biomarkers might influence such associative investigations [9].
In this work, male haemodialysis patients had significantly higher serum sclerostin levels than female patients. Larger bone mass in males, hormonal effect (a role of oestrogen in reducing sclerostin levels), skeletal remodelling and imbalances in vascular remodelling with aging in males might be responsible for the observed differences [34–36]. Furthermore, diabetic patients tended to have higher sclerostin levels in this study. Type 2 DM is frequently associated with increased sclerostin concentrations due to, particularly in the presence of atherosclerotic disease [37], decreased serum iPTH levels and low bone turnover [35] as well as increased fat mass [38] and bone mineral density [39]. However, some more recent studies revealed no significant difference in sclerostin levels regarding DM [11, 32, 40]. Contradictory results might originate from different patient characteristics, preanalytical sclerostin stability and assay characteristics [9].
The positive correlation between CIMT and sclerostin levels in diabetic haemodialysis patients in the present work contrasts with the general diabetic population [30, 41]. Different kits were used to measure sclerostin level in this study and therefore, the preanalytical biomarker stability and assay characteristics of the sclerostin might explain the differences [9]. Furthermore, the presence of uraemia may contribute to both increased sclerostin levels and atherosclerosis, and in this situation, the influence of sclerostin levels could be more pronounced. Moreover, the same controversy might be observed in the nature of the association between sclerostin and CIMT, as seen previously in the survival studies of CKD populations [10–14].
Despite commonly observed negative association between haemodialysis vintage and surrogate markers of atherosclerosis [42–44] in dialysis patients, statistical significance was lost when adjustments were performed with stronger clinical and laboratory parameters. This is somewhat in contrast to other studies revealing haemodialysis vintage as an independent predictor in CVD surrogate parameters [45, 46]. These studies emphasize that vascular damage caused by uraemia is time sensitive, and atherosclerotic disease is a complex and multifactorial process that still requires more basic research and clinical data.
The limitations of the present work include the cross-sectional nature of both sclerostin and CIMT measurements, the relatively small sample size and the lack of bone histomorphometric data and bone biomarkers. Finally, we are not able to establish the causative nature of the associations between sclerostin and CIMT.
In conclusion, additional investigations are needed to elucidate whether changes in sclerostin levels (or the Wnt-β-catenin pathway) play a significant role in the pathogenesis of atherosclerotic disease in patients with CKD.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1.Raggi P, Kleerekoper M. Contribution of bone and mineral abnormalities to cardiovascular disease in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3: 836–843 [DOI] [PubMed] [Google Scholar]
- 2.Moe S, Drüeke T, Cunningham J, et al. Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69: 1945–1953 [DOI] [PubMed] [Google Scholar]
- 3.Román-García P, Carrillo-López N, Fernández-Martín JL, et al. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone 2010; 46: 121–128 [DOI] [PubMed] [Google Scholar]
- 4.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol 2012; 8: 529–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 2006; 116: 1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drüeke TB, Lafage-Proust MH. Sclerostin: just one more player in renal bone disease? Clin J Am Soc Nephrol 2011; 6: 700–703 [DOI] [PubMed] [Google Scholar]
- 7.Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 2011; 6: 877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu D, Mackenzie NC, Millán JL, et al. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One 2011; 6: e19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandenburg VM, Kramann R, Koos R, et al. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol 2013; 14: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins L, Liabeuf S, Oliveira RB, et al. On behalf of the European Uremic Toxin Work G (2014) Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol Ther 2014; 10: 463–470 [DOI] [PubMed] [Google Scholar]
- 11.Goncalves FL, Elias RM, Dos Reis LM, et al. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol 2014; 15: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanbay M, Siriopol D, Saglam M, et al. Serum sclerostin and adverse outcomes in non-dialyzed chronic kidney disease patients. J Clin Endocrinol Metab 2014; 99: E1854–E1861 [DOI] [PubMed] [Google Scholar]
- 13.Viaene L, Behets GJ, Claes K, et al. Sclerostin: another bone related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant 2013; 28: 3024–3030 [DOI] [PubMed] [Google Scholar]
- 14.Drechsler C, Evenepoel P, Vervloet MG, et al. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 2015; 30: 288–293 [DOI] [PubMed] [Google Scholar]
- 15.Brandenburg VM, D'Haese P, Deck A, et al. From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol 2015; doi:10.1007/s00467-015-3069-7 [DOI] [PubMed] [Google Scholar]
- 16.Daugirdas JT. Second generation logarithmic estimates of single pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol 1993; 4: 1205–1213 [DOI] [PubMed] [Google Scholar]
- 17.Depner TA, Daugirdas JT. Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol 1996; 7: 780–785 [DOI] [PubMed] [Google Scholar]
- 18.Cejka D, Jäger-Lansky A, Kieweg H, et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant 2012; 27: 226–230 [DOI] [PubMed] [Google Scholar]
- 19.Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74: 1399–1406 [DOI] [PubMed] [Google Scholar]
- 20.Mill C, George SJ. Wnt signalling in smooth muscle cells and its role in cardiovascular disorders. Cardiovasc Res 2012; 95: 233–240 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Liu P, Liu W, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet 2005; 37: 945–952 [DOI] [PubMed] [Google Scholar]
- 22.Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 2007; 315: 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Adhikari N, Li Q, et al. The LDL receptor related protein LRP6 regulates proliferation and survival through the Wnt cascade in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 2004; 287: H2376–H2383 [DOI] [PubMed] [Google Scholar]
- 24.Ezan J, Leroux L, Barandon L, et al. FrzA/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovasc Res 2004; 63: 731–738 [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Xiao Y, Mou Y, et al. A role for the b-catenin/ T-cell factor signaling cascade in vascular remodeling. Circ Res 2002; 90: 340–347 [DOI] [PubMed] [Google Scholar]
- 26.Slater SC, Koutsouki E, Jackson CL, et al. R-cadherin:b-catenin complex and its association with vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol 2004; 24: 1204–1210 [DOI] [PubMed] [Google Scholar]
- 27.Tickenbrock L, Schwable J, Strey A, et al. Wnt signaling regulates transendothelial migration of monocytes. J Leukoc Biol 2006; 79: 1306–1313 [DOI] [PubMed] [Google Scholar]
- 28.Cheng CW, Yeh JC, Fan TP, et al. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Comm 2007; 365: 285–290 [DOI] [PubMed] [Google Scholar]
- 29.Askevold ET, Gullestad L, Aakhus S, et al. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc 2012; 1: e002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaudio A, Privitera F, Pulvirenti I, et al. The relationship between inhibitors of the Wnt signalling pathway (sclerostin and Dickkopf-1) and carotid intima-media thickness in postmenopausal women with type 2 diabetes mellitus. Diab Vasc Dis Res 2014; 11: 48–52 [DOI] [PubMed] [Google Scholar]
- 31.Funato Y, Michiue T, Asashima M, et al. The thioredoxin related redox-regulating protein nucleoredoxin inhibits Wnt beta-catenin signalling through dishevelled. Nat Cell Biol 2006; 8: 501–508 [DOI] [PubMed] [Google Scholar]
- 32.Claes KJ, Viaene L, Heye S, et al. Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab 2013; 98: 3221–3228 [DOI] [PubMed] [Google Scholar]
- 33.Thambiah S, Roplekar R, Manghat P, et al. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int 2012; 90: 473–480 [DOI] [PubMed] [Google Scholar]
- 34.Mödder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 2011; 26: 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jean G, Chazot C. Sclerostin in CKD-MBD: one more paradoxical bone protein? Nephrol Dial Transplant 2013; 28: 2932–2935 [DOI] [PubMed] [Google Scholar]
- 36.Kirmani S, Amin S, McCready LK, et al. Sclerostin levels during growth in children. Osteoporos Int 2012; 23: 1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Martin A, Rozas-Moreno P, Reyes- García R, et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2012; 97: 234–241 [DOI] [PubMed] [Google Scholar]
- 38.Urano T, Shiraki M, Ouchi Y, et al. Association of circulating sclerostin levels with fat mass and metabolic disease—related markers in Japanese postmenopausal women. J Clin Endocrinol Metab 2012; 97: E1473–E1477 [DOI] [PubMed] [Google Scholar]
- 39.Garnero P, Sornay-Rendu E, Munoz F, et al. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int 2013; 24: 489–494 [DOI] [PubMed] [Google Scholar]
- 40.Newby AC, George SJ. Proliferation, migration, matrix turnover and death of smooth muscle cells in native coronary and vein graft atherosclerosis. Curr Opin Cardiol 1996; 11: 574–582 [DOI] [PubMed] [Google Scholar]
- 41.Morales-Santana S, García-Fontana B, García-Martín A, et al. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013; 36: 1667–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiraki N, Nakashima A, Doi S, et al. Low serum testosterone is associated with atherosclerosis in postmenopausal women undergoing hemodialysis. Clin Exp Nephrol 2014; 18: 499–506 [DOI] [PubMed] [Google Scholar]
- 43.Collado S, Coll E, Deulofeu R, et al. Prevalence of cardiovascular disease in uraemia and relevance of cardiovascular risk factors. Nefrologia 2010; 30: 342–348 [DOI] [PubMed] [Google Scholar]
- 44.Utescu MS, Couture V, Mac-Way F, et al. Determinants of progression of aortic stiffness in hemodialysis patients: a prospective longitudinal study. Hypertension 2013; 62: 154–160 [DOI] [PubMed] [Google Scholar]
- 45.Damjanovic T, Djuric Z, Markovic N, et al. Screening of vascular calcifications in patients with end-stage renal diseases. Gen Physiol Biophys 2009; 28: 277–283 [PubMed] [Google Scholar]
- 46.Schlieper G, Brandenburg V, Djuric Z, et al. Risk factors for cardiovascular calcifications in non-diabetic Caucasian haemodialysis patients. Kidney Blood Press Res 2009; 32: 161–168 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.