Abstract
Background
We examined the prevalence of acute kidney injury (AKI) risk factors in the emergency medical unit, generated a modified risk assessment tool and tested its ability to predict AKI.
Methods
A total of 1196 patients admitted to medical admission units were assessed for patient-associated AKI risk factors. Subsequently, 898 patients were assessed for a limited number of fixed risk factors with the addition of hypotension and sepsis. This was correlated to AKI episodes.
Results
In the first cohort, the prevalence of AKI risk factors was 2.1 ± 2.0 per patient, with a positive relationship between age and the number of risk factors and a higher number of risk factors in patients ≥65 years. In the second cohort, 12.3% presented with or developed AKI. Patients with AKI were older and had a higher number of AKI risk factors. In the AKI cohort, 72% of the patients had two or more AKI risk factors compared with 43% of the cohort with no AKI. When age ≥65 years was added as an independent risk factor, 84% of those with AKI had two or more AKI risk factors compared with 55% of those with no AKI. Receiver operating characteristic analysis suggests that the use of common patient-associated known AKI risk factors performs no better than age alone as a predictor of AKI.
Conclusions
Detailed assessment of well-established patient-associated AKI risk factors may not facilitate clinicians to apportion risk. This suggests that additional work is required to develop a more sensitive validated AKI-predictive tool that would be useful in this clinical setting.
Keywords: acute tubular necrosis, AKI, chronic renal failure, chronic renal insufficiency, epidemiology
Introduction
Acute kidney injury (AKI) is a serious and common condition, which is thought to affect 3–18% of all hospitalized patients [1–4]. It is associated with adverse outcomes and high mortality [4–6] with an estimated 40 000 plus in patient deaths annually associated with AKI in the National Health Service (NHS) in UK alone [7]. It is of note that even mild cases of AKI are associated with an increase in patient mortality [8, 9]. Patients surviving the acute episode are also at increased risk of progressive chronic kidney disease (CKD) and death [4, 6, 8]. Recent data suggest that the annual cost of AKI-related inpatient care in UK amounts to £1.03 billion, which represents over 1% of the total NHS budget [7].
To date, attempts to develop specific therapies for AKI have been unsuccessful. The current emphasis is, therefore, on early detection and instigation of supportive strategies such as careful fluid balance, avoidance of nephrotoxic medication and appropriate diagnostic investigations, which have been shown to improve AKI outcome [10–12]. In 2009, the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) [13] report identified significant deficiencies in the management of AKI in hospitals in the UK. This led to the development and implementation of strategies such as the use of electronic results reporting to aid early AKI recognition [14]. In addition to errors in the recognition of patients with AKI, NCEPOD reported significant deficiencies in identifying those at risk of developing AKI [13], highlighting a sizeable proportion of patients in which AKI episodes are avoidable. Within the NHS in the UK, there is an increasing emphasis on the prevention of avoidable harm, indicating the need to develop and implement prevention strategies for AKI.
The risk factors for AKI, and the comorbidities that are more prevalent in patients with AKI, are well established [4, 15–20]. This has led to the recommendation that all emergency admissions should have a formal AKI risk assessment undertaken so that modifiable risks factors can be dealt with in a timely fashion. Acute medical admissions units (MAUs) represent the hospital entry point for most unscheduled medical admissions in the UK. Prevention and management of AKI in this setting will, therefore, go a long way to addressing the deficiencies and recommendations outlined in the NCEPOD report.
To date, there is a paucity of data that evaluate the prevalence of AKI risk factors in unselected acute hospital admissions. It is, therefore, not clear if it would be either appropriate or productive to carry out a formal complete risk assessment of AKI risk factors for all patients. Understanding the likely benefit of a comprehensive AKI risk assessment tool is critical given that there are numerous ‘risk’ assessment tools in place across a range of predictable conditions that significantly add to the work load of already over-burdened health care workers. Examples include the national early warning score of acute illness adopted in the UK, sepsis screening, venous thromboembolism/thromboprophylaxis risk assessment, falls-risk assessment and pressure ulcer risk assessment. In order to inform the debate of the merits of formal AKI risk assessment of all acute hospital admissions, this study aimed to define the prevalence of AKI-related risk factors in all patients seen and assessed at MAUs in four hospitals across South Wales. In addition, we examined the predictive value of a modified AKI risk assessment tool for the presence of or the development of AKI either at presentation or during the first week following hospital admission in an unselected patient cohort presenting to MAU.
Materials and methods
The first phase of the study was performed over a 2-week period at four MAUs in South East Wales. The contributing hospitals were located in three separate local health boards: Cwm Taf, Aneurin Bevan and Cardiff and Vale University Health Boards. The study was registered as a service evaluation project by each of the local health boards. Two of the participating hospitals were district general hospitals [Royal Gwent Hospital (770 beds), Newport and Prince Charles Hospital (430 beds)], Merthyr Tydfil, serving populations of ∼300 000 and 150 000, respectively. The two remaining hospitals were the University Hospital of Wales (1000 beds) and the University Hospital Llandough (480 beds), teaching hospitals situated in Cardiff serving a combined population of 470 000. Information was collected prospectively for all patients presenting to the MAUs. In addition to basic patient demographics, data were collected on the presence of any of comorbidities that have been highlighted previously as potential patient-associated fixed risk factors for AKI (Table 1). These were selected based on a literature review of factors shown to be increased in patients who have an AKI episode [4, 13, 16, 17, 19, 21, 22].
Table 1.
Possible risk factors for AKI that were assessed on admission
| Risk factor | Description/explanation |
|---|---|
| Age | >65 years |
| Diabetes mellitus | |
| CKD | Stage 3–5 (estimated glomerular filtration rate <60 mL/min/1.73 m2) |
| Congestive cardiac failure | History of congestive cardiac failure or current presentation consistent with acute cardiac failure |
| Ischaemic heart disease | History of previous myocardial infarction or angina |
| Peripheral vascular disease | |
| Cerebrovascular disease | History of cerebrovascular disease/stroke or transient ischaemic event |
| Peripheral vascular disease | Previous intervention for atherosclerotic vascular disease or ongoing symptoms of intermittent claudication |
| Solid organ or haematological malignancy | Active diagnosis of malignancy |
| Neurological or cognitive impairment | Residual neurological deficit following previous stroke or known diagnosis of dementia |
| Liver disease | Known history of liver disease |
| Morbid obesity | BMI > 40 |
| AIDS | Known confirmed diagnosis of AIDS under specialist care |
| ACEi/AIIRA | Current therapy at the time of admission |
| Diuretics | Current therapy at the time of admission |
| Non-steroidal anti-inflammatory medication | Current therapy at the time of admission |
| Hypotensiona | Systolic blood pressure <90 mmHg |
| Evidence of sepsisa | The presence of ≥ of the following parameters: temperature <36°C or >38°C, heart rate 90 bpm, white blood count >12 or <4106/mL, respiratory rate >20 or hyperglycaemia without diabetes |
aRisk factors included into second phase of the study only. ACEi, angiotensin converting enzyme inhibitor.
The second phase of the study was performed over a separate 2-week period in the same units. Basic patient demographics data were collected prospectively together with data on the presence of the most prevalent patient-associated fixed risk factors for AKI identified in phase 1 (hypertension, CKD, liver disease, diabetes, ischaemic heart disease and nephrotoxic medication) together with any clinical evidence of hypotension or sepsis. Subsequently for all patients, biochemical results of renal function prior to, at the point of and up to 1 week following the date of presentation at MAU were collected to identify all episodes of AKI. The definition provided by the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Guidelines for AKI was used, using creatinine (sCr) criteria for identification of AKI [23]. Baseline sCr values for patients were determined through review of all sCr values taken from the patient during the preceding 12 months. Patients with unknown baseline values had sCr values charted after AKI resolution, which further enabled approximation of baseline sCr and confirmation of true AKI. This method of baseline sCr identification is recommended in the recent KDIGO AKI guidelines [23].
Data were analysed by t-test and one-way ANOVA, and categorical data compared using a Pearson χ2 test. The discriminating value and performance of risk assessment was undertaken using receiver operating characteristic (ROC) analysis to determine the area under the ROC curve (ROC AUC).
Results
Phase 1
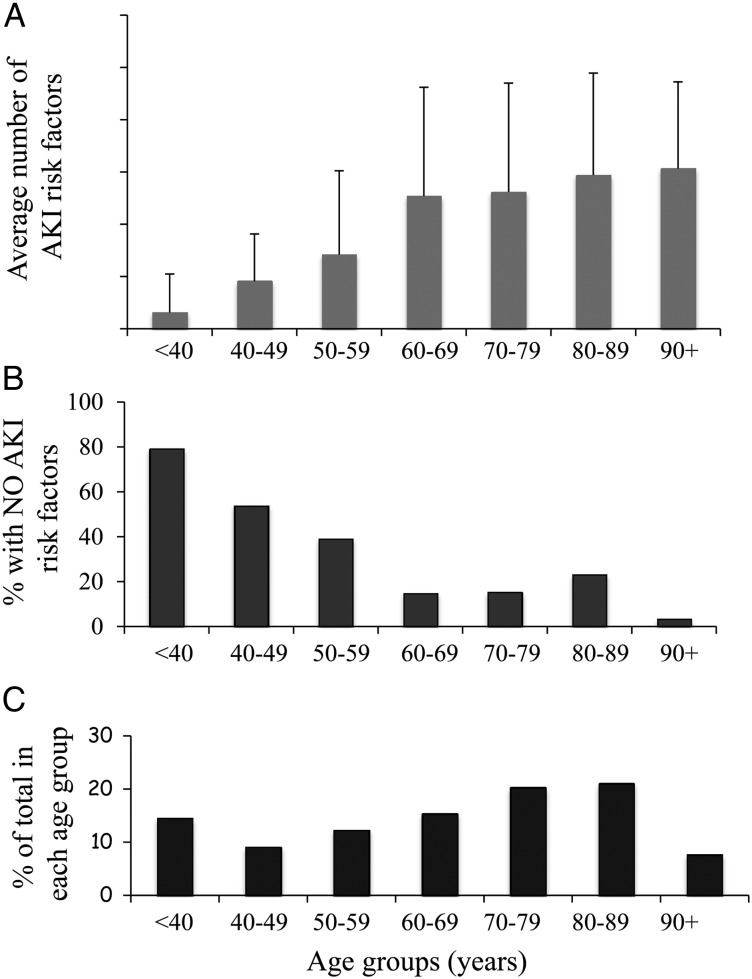
In the first phase, data were collected on a total of 1196 patients. The population of patients represents an older group of patients with a mean age of 64.8 ± 19.7 years in which 58% of the whole patient cohort was ≥65 years of age. The distribution of the proportion of patients by age is shown in Figure 1C. There was a predominance of female patients, which represented 56.7% of the whole group. There was no difference in the mean age of the female compared with the male patient cohorts (females mean age 65.6 ± 21.8 versus male mean age 65.4 ± 18.1 years, P = 0.88). The mean number of AKI risk factors per patient was 2.06 ± 1.98.
Fig. 1.
Age-dependent distribution of AKI risk factors. (A) The average number of AKI risk factors by indicated age groups with the error bars representing the standard deviation. (B) The percentage of patients in each age group with no risk factors and (C) the percentage of the total patient cohort within each age group.
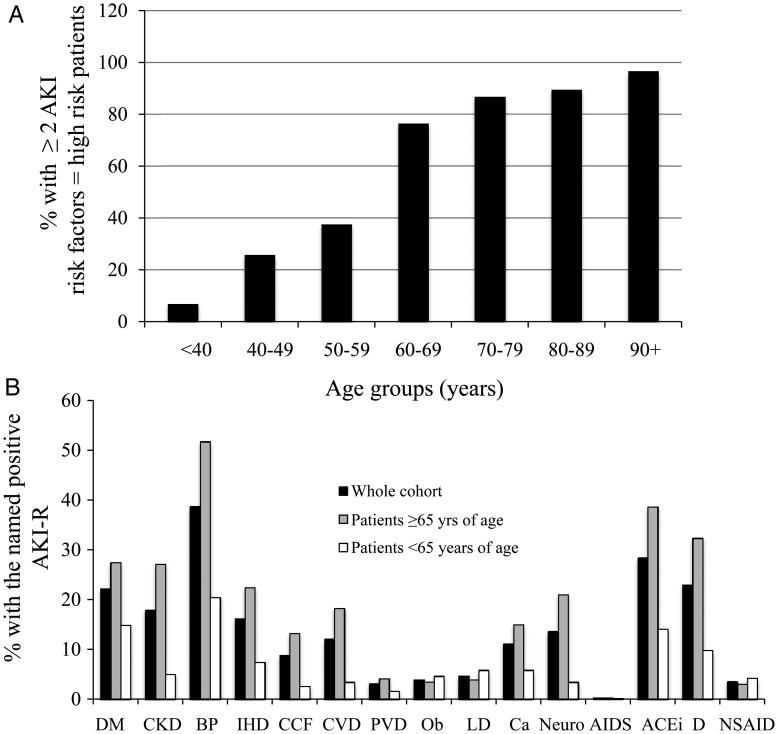
There was a clear relationship between age and the number of AKI risk factors (Figure 1A). There were a significantly higher number of risk factors in patients older than 65 years compared with those younger than 65 years of age (<65 years AKI-R 1.03 ± 1.4 versus ≥65 years 2.8 ± 2.0, P < 0.00001). Similarly, when comparing the number of patients in each age group who had no AKI risk factors, there was a clear negative association (P < 0.00001) with age (Figure 1B), with almost 80% of patients under the age of 40 having no AKI risk factors. Although when comparing the female cohorts with male cohorts, there was no difference in their ages (females 65.6 ± 21.9 versus males 65.4 ± 18.1 years, P = 0.88), overall there was a significantly lower number of AKI risk factors in the female group (1.88 ± 1.83) compared with the male cohort (2.34 ± 2.13, P = 0.0004). The presence of two or more AKI risk factors has been previously reported to predict a cohort of patients at high risk of developing AKI [24]. The percentage of patients with two or more AKI risk factors is shown in Figure 2A, demonstrating that in each of the age deciles ≥60 years of age, the vast majority of patients would be flagged as ‘high risk’.
Fig. 2.
(A) Age-dependent distribution of patients at high risk of AKI. High risk of AKI was defined as the presence of two or more fixed patient-related AKI risk factors. (B) Individual AKI risk factor (AKI-R) distribution. (B) Percentage of patients (solid black bars), patients ≥65 years of age (grey bars) or patients <65 years of age (open bars), positive for each of the individual fixed patient-related AKI risk factors. DM, diabetes mellitus; CKD, chronic kidney disease; BP, hypertension; IHD, ischaemic heart disease; CCF, congestive cardiac failure; CVD, cerebrovascular disease; PVD, peripheral vascular disease; Ob, morbid obesity; LD, liver disease; Neuro, neurological or cognitive impairment; AIDS, known diagnosis of AIDS; ACEi, angiotensin converting enzyme inhibitor; D, prescription of diuretic; NSAID, prescription of non-steroidal anti-inflammatory drugs).
The relevance of individual AKI risk factors is likely to vary according to the clinical context, which may be influenced both by geography and also by the specific clinical setting, i.e. MAU versus other clinical areas. In order to assess the likely impact of the AKI risk factors in our clinical practice, we determined the prevalence of each of the individual AKI risk factors. The percentage of patients, who had each of the individual AKI risk factors in the whole cohort and also in patients aged <65 or >65 years of age is shown in Figure 2B. In this unselected cohort of patients attending MAU, the most common AKI risk factors were as follows: hypertension (38%), treatment with angiotensin converting enzyme inhibitor (ACEi) (28%), treatment with diuretics (23%), diabetes (22%), CKD (18%) and ischaemic heart disease (16%). In the patient cohort ≥65 years of age (which represents 58% of the whole cohort), the pattern of distribution of each of the risk factors was the same although as expected the prevalence of each of the risk factors was significantly higher: hypertension (52%), treatment with ACEi (38%), treatment with diuretics (32%), diabetes (27%), CKD (27%) and ischaemic heart disease (22%).
Phase 2
In the second period of study, data were collected on 898 patients. The basic patient characteristics are given in Table 2. Of these patients, 12.25% either presented with AKI or developed AKI within 1 week of the initial presentation. The majority of AKI episodes were AKI stage 1 (82%) with AKI 2 and 3 representing only 16 and 2% of all the AKI episodes, respectively. The patients with AKI were significantly older (74.6 ± 2.3 versus 63.3 ± 20.5 years, P < 0.00001) than those without AKI and had a significantly higher number of AKI risk factors (2.3 ± 1.3 versus 1.35 ± 1.3, P < 0.0001).
Table 2.
Comparison of patients with and without documented AKI
| AKI (n = 110) | No AKI (n = 788) | |
|---|---|---|
| Age ± standard deviation (years) | 74.6 ± 2.3 | 63.3 ± 20.5 |
| Ave. number of AKI risk factors | 2.3 ± 1.3 | 1.35 ± 1.34 |
| % with ≥2 AKI risk factors | 72.7 | 40.3* |
| % ≥ AKI risk factors when age ≥65 added as RF | 83.6** | 55.3*,*** |
| % ≥ AKI risk factors when age ≥70 added as RF | 83.6** | 52.5*,*** |
| % of patients ≥65 years of age | 79.1 | 52.8*,*** |
| % of patients ≥70 years of age | 70.9 | 45.2* |
RF, risk factor.
*P < 0.001 compared to cohort with AKI.
**P < 0.05 compared with AKI % with two or more AKI risk factors.
***P < 0.001 compared with no AKI % with two or more AKI risk factors.
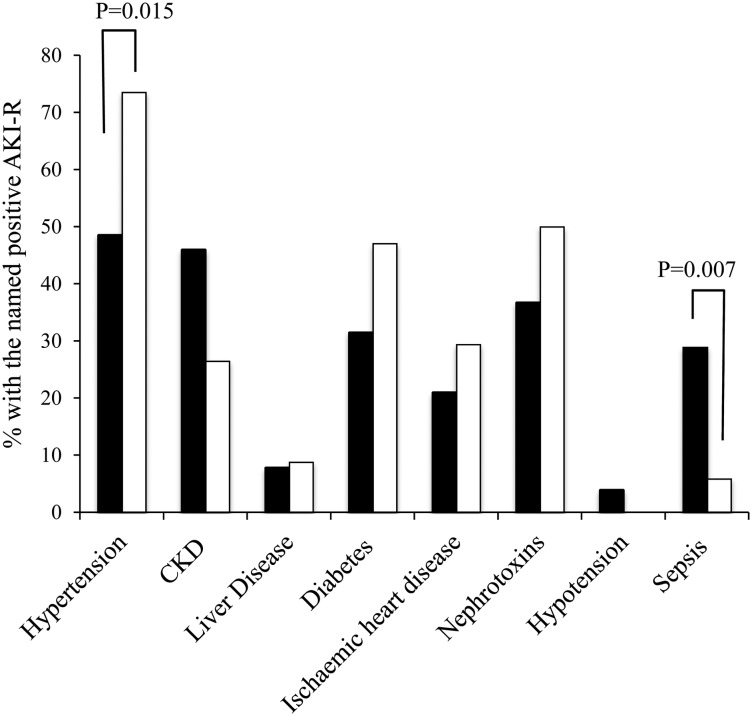
Of those patients with AKI, 69% presented with AKI (community acquired, CA-AKI) with the remaining 31% developing AKI following admission (hospital-acquired, HA-AKI). When comparing the patients with CA-AKI and those with HA-AKI, there was no significant difference in either the mean age (73.0 ± 19.6 versus 78.1 ± 112.2 years, P = 0.097) of the patient groups, nor the number of AKI risk factors (2.25 ± 1.3 versus 2.41 ± 1.44, P = 0.6). In contrast, when assessing the prevalence of individual risk factors (Figure 3) there was a higher prevalence of hypertension in the cohort of HA-AKI (P = 0.015) and a higher prevalence of sepsis in the cohort presenting with CA-AKI (P = 0.006).
Fig. 3.
Distribution of AKI risk factors (AKI-R) in HA- (solid bars) and CA-AKI (open bars). The data are expressed as a percentage of patients from either cohort. For significance, the appropriate P-value is shown. For each risk factor when no P-value is listed, the differences were not statistically significant.
To further evaluate the value of this limited simplified AKI risk assessment model, analysis was undertaken of the prevalence of AKI in those patients with two or more AKI risk factors. In the AKI cohort, 73% of the patients had two or more AKI risk factors compared with 43% of the cohorts with no AKI (P < 0.0001). Previous studies have suggested adoption of age as an independent risk factor for AKI. Subsequent analysis was made of the influence of adding either age ≥65 years or age ≥70 years as an additional risk factor. Using the age cut-off age of 65 years, 84% of those with AKI have two or more AKI risk factors compared with 73% in the model where age was not considered as risk factor (P = 0.049). Using the age cut-off of 65 years, 55% of those with no AKI had two or more AKI risk factors, which is significantly greater than the model where age was not considered as a risk factor (P < 0.0001). Increasing the cut-off age to ≥70 years had no effect on the percentage of patients with two or more AKI risk factors (84%) and led to only a small non-significant fall in those in the non-AKI cohort with two or more AKI risk factors (age ≥65 years, AKI risk ≥2 = 55%, age ≥70 years, AKI risk ≥2 = 52%, P = 0.26).
Given the clear association of AKI risk factors with age, we compared the value of the risk assessment model using numerous variables to the use of age alone as the only risk factor for AKI. In the AKI cohort, 71% of the patients were ≥70 years of age compared with 84% (P = 0.02) who had ≥ two or more AKI risk factor (RF) (including age ≥70 years as an RF). Taking a lower age cut-off, 79% of the AKI cohort were ≥65 years of age, which was not significantly less that the percentage (84%, P = 0.4) who had two or more AKI RF (including age of either ≥65 or 75 years as an RF). Using the lower age cut-off as the sole AKI risk factor, however, reduced the specificity, when compared with the use of age ≥70 years alone, as 53% of those with no AKI were in this age group compared with 45% of those aged ≥70 years (P = 0.002).
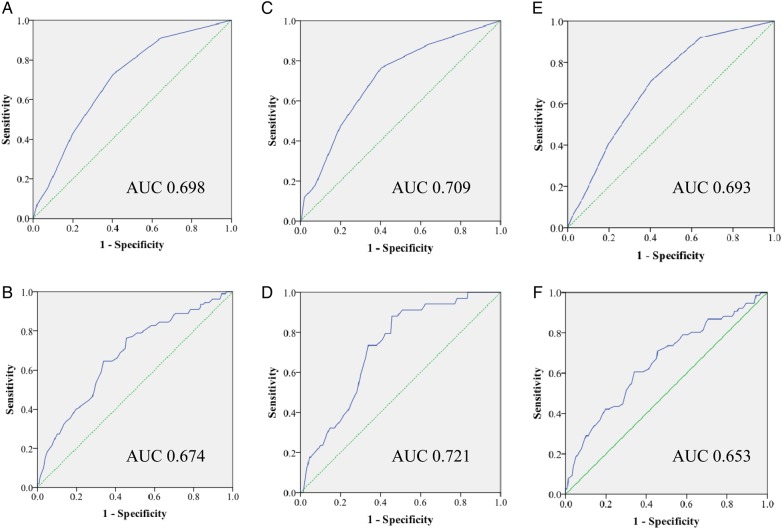
Finally, the performance of using known AKI risk factors as a tool to predict AKI was undertaken (Figure 4). For the whole cohort of patients using the AKI risk factors excluding age, the AUC ROC was 0.698. Clearly, the sensitivity and specificity of performance is dependent on the cut-off threshold applied, when a lowering of the threshold resulting in changing sensitivity and specificity in opposite directions. Using a cut-off of >2 risk factors resulted in a sensitivity of 0.427 and specificity of 0.198. This compared with an AUC ROC of 0.674 for age alone. Setting an age cut-off of ≥65 years resulted in a sensitivity of 0.791 and specificity of 0.528. This suggests either strategy of risk assessment to predict AKI is limited in their ability to discriminate by poor sensitivity and specificity.
Fig. 4.
Receiver operator characteristic curve for AKI prediction score for the whole cohort of patients using the AKI risk factors excluding age (A) or age alone (B), compared with the HA-AKI cohort using the AKI risk factors excluding age (C) or age alone (D) and the CA-AKI cohort using the AKI risk factors excluding age (E) or age alone (F). Solid line: prediction score, dashed line, reference line.
For the subgroup that developed hospital-acquired AKI to assess true predictive value, using the AKI risk factors excluding age, the AUC ROC was 0.709, compared with an AUC ROC of 0.721 for age alone. Using a cut-off of two or more risk factors resulted in a sensitivity of 0.471 and specificity of 0.198. Setting an age cut-off of ≥65 years of age resulted in a sensitivity of 0.912 and specificity of 0.528. In this subgroup, age alone therefore outperformed the formal assessment of AKI risk, although the AUC value suggests that even this is a poor discriminator.
For those who presented to hospital with CA- AKI, the prevalence of AKI RF is not truly predictive but rather but a measure of the strength of association. Using the AKI risk factors excluding age, the AUC ROC was 0.693, compared with an AUC ROC of 0.653 for age alone. Using a cut-off of >2 risk factors resulted in a sensitivity of 0.408 and specificity of 0.198. For this group setting an age cut-off of ≥65 years of age resulted in a sensitivity of 0.737 and specificity of 0.528.
Discussion
With the increasing incidence of AKI [25, 26], identifying individuals at higher risk for developing AKI has become a priority so that preventative treatment can occur in a timely manner. Numerous reports have highlighted deficiencies in the care of patients with AKI suggesting that a significant proportion of these cases are both predictable and preventable [7, 27]. In its 2009 report, NCEPOD highlighted deficiencies at all levels of patient care, and recommended ‘Initial clerking of all emergency patients should include a risk assessment for AKI’ [13].
The risk factors for AKI are well established with multiple studies comparing the prevalence of potential risk factors in populations of patients who have developed AKI compared with control groups with no AKI. Based on these studies, numerous risk assessment tools have been proposed and generated for specific clinical settings such as intensive care units [28], cardiac surgery [29], general surgery [30] or those undergoing radiological investigations involving intravenous contrast [31]. To date, few studies have evaluated the predictive value of AKI risk factors in unselected patients presenting as acute emergencies to the hospital front door. Acute medicine is the fastest growing medical specialty in the UK, and with the majority of all acute admissions now presenting through MAUs, tackling AKI in this clinical setting provides a unique opportunity to address a large proportion of the well-reported deficiencies in clinical care. Although AKI is considered to be a condition that is predominantly hospital based, we have previously demonstrated that 67% of all hospital-managed AKI is acquired in the community [4], further emphasizing the importance of initiatives aimed at tackling prevention and management at the hospital front door. Assessment of the acute admission currently involves numerous risk assessment tools to minimize patient harm. In this busy clinical environment, it is particularly important that a full assessment of the sensitivity of any new risk assessment tool is undertaken prior to any recommendation advocating its universal use. Risk factors for AKI can be divided into chronic patient-related risks and acute ‘illness- related risks [21]. In order to understand the likely benefit of a risk assessment tool based on identification of the known AKI risk factors in acute medical admissions, we initially undertook a study of their prevalence in this clinical setting.
To our knowledge, there have been no previous studies that have addressed the prevalence of AKI risk factors in all acute medical admissions. The data clearly demonstrate that the majority of patients presenting though MAUs have a significant burden of AKI risk factors. A key finding was that the burden of AKI risk factors was directly related to the age of the patients. Significantly for those aged ≥65 years, there were on average 2.8 risk factors per patient, with only 10% of this patient group having no risk factors. Although there were no differences in the ages of the male and female patient cohorts, the prevalence of AKI risk factors was significantly greater in males compared with females. These data are therefore consistent with previous studies suggesting that AKI is more common in males [6, 15, 32, 33].
Publications on the prospective predictive value of AKI risk factors are sparse. In a study of 316 patients by Finlay et al., the total number of risk factors for an individual patient was strongly predictive of AKI and patients with two or more risk factors predicting a 7.1-fold increased risk of having AKI compared with those with one or no risk factor [24]. Applying this principle, while noting that we have only assessed fixed chronic factors and therefore the model is likely to underestimate risk, would label 63% of the whole cohort as ‘high risk’ and 89% of patients aged ≥65 years. This suggests that this model of AKI risk assessment may be both time-consuming and seemingly not a sensitive discriminator of AKI risk.
Previous studies comparing the prevalence of AKI risk factors in patient cohorts who have experienced an episode of AKI have attempted to stratify the importance of risk factors. These models have defined age, diabetes and cardiovascular disease as ‘chronic major’ risk factors, while others such as hypertension, morbid obesity, liver disease, cerebrovascular disease, AIDS and cancer are classified as ‘chronic minor’ risk factors [21]. The utility of a risk factor is, in part, dependent on its prevalence in the population in which the risk is assessed. In our model, we have collected data on the prevalence of 15 known AKI risk factors. Of these, the prevalence of 5 of the 15 AKI risk factors was <5%. The clinical utility and the ease of carrying completion of AKI risk factor screening may therefore be improved with a simplified model focused on the more prevalent six risk factors (diabetes, CKD, hypertension, ischaemic heart disease, liver disease and the prescription of ‘nephrotoxic’ medications). In the second part of this study, we chose to examine the potential of using these fixed patient-associated risk factors in combination with two acute illness-related AKI risk factors, hypotension and sepsis. The incidence of AKI of 12.25% was similar to that previously reported in a similar clinical setting [4]. The true predictive value of the AKI-associated risks applies to those with HA-AKI. Using this subgroup and assuming previously published assumptions that 20–40% of cases of AKI may be predictable and potentially avoidable [13], the data suggest that in our cohort of 894 patients, between 7 and 14 patients may have avoidable AKI and therefore potentially benefit from a highly sensitive prediction tool. In this study, AKI was associated with a higher incidence of AKI risk factors. Furthermore, using this simplified model of risk assessment, the presence of two or more AKI risk factors was associated with the presence or the development of AKI. There was also a clear relationship between age and the incidence of AKI. Given the clear association between age and the number of AKI risk factors, it is therefore not surprising that using age alone produced a model that is as effective in predicting AKI as the more complex model combining comorbidities/fixed risk factors and ‘illness/acute’ risk factors. The low specificity of using either model, however, limits the clinical utility of either model. A model using age alone as an assessment tool to predict HA-AKI is predictive of outcome but with an AUC which at best can be interpreted as ‘moderate performance’. In a recent publication, Forni et al. have sought to improve the predictive value of AKI risk assessment by combining comorbidity, biochemistry and electronically monitored patient physiology to devise a practical scoring system [34]. This more detailed assessment of patients produced a tool that was predictive of outcome, although with a similar moderate AUC performance (0.72), which is in the same range as our simplified age-only ‘model’. This further reinforces that currently there are no accepted validated risk scores that can be easily applied to clinical practice.
Possible alternative approaches to improve a predictive model, although beyond the scope of the data collected in this study, might involve an assessment of the strength of association of individual factors that then might be applied to a weighted score rather than using each risk as a binary variable. Alternatively, while the inclusion of simple physiological assessment of sepsis was not particularly additive in this study, it may be that different physiological or biochemical parameters reflecting acute illness may be a direction for further evaluation. Information from such studies may subsequently allow situational risk factors to be used in combination with age (which is the best performing factor in our data)-weighted categories of risk from the condition to generate a more sensitive predictive model. Finally, alternative suggested approaches include the use of biomarkers of AKI, and several candidate molecules have been identified, particularly in the critically ill [35]. These have shown some promise although testing to date has been applied to clinical areas where AKI is common, thus limiting their application as a broad screening tool. Rather one might anticipate that biomarkers may be best used in tandem with a validated predictive model of AKI risk to increase their sensitivity and specificity.
Conclusions
In summary, risk prevention is most valuable when it enables clinicians to match the most appropriate treatment or intervention to a patient’s needs or where it allows public health systems to allocate resources effectively. Risk prevention can also be a valuable tool in the clinical research setting, facilitating epidemiological studies and patient recruitment onto trials of preventative interventions. For any risk assessment tool to be of clinical use and aid physicians in decision-making, it is also clear that the prevalence of the candidate predictive risk factors needs to be evaluated in the specific clinical setting for which its use is recommended. Although there is clear evidence to support the inclusion of each of the AKI risk factors selected for this study as valid candidate predictors, in this specific clinical setting a detailed analysis of risk factors is not helpful to the clinician in discriminating between patients at high and low risk due to the high prevalence of these validated risk factors in all patients, and particularly those aged 65 or above in whom the incidence of AKI is highest. The data collected in this study suggest a detailed assessment of the risk of AKI in all patients individually may not facilitate clinicians to apportion risk. As an alternative, while we are still awaiting an accepted validated risk assessment tool, one approach may be to focus resources towards all older patients presenting to MAU to develop prevention strategies to tackle modifiable risk factors such as the use of nephrotoxic medication and hydration, and early identification of AKI through monitoring of urine output and measurement of renal function, which therefore may impact on the incidence of HA-AKI in this subgroup.
Conflict of interest statement
None declared. The results presented in this paper have not been published previously in whole or part.
Acknowledgements
The authors thank the medical and nursing staff of the participating units. In particular, we are grateful for the support of Dr Brian Johnson and Dr Laura Rozier (University Hospital of Wales), Sister Julia Evans (University Hospital Llandough) and Dr Rana Sarafa, Miss Gina Sanki and Dr Shaun Shwana (Prince Charles Hospital).
References
- 1.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007; 18: 1292–1298 [DOI] [PubMed] [Google Scholar]
- 2.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010; 21: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 2008; 3: 844–861 [DOI] [PubMed] [Google Scholar]
- 4.Wonnacott A, Meran S, Amphlett B, et al. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 2014; 9: 1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16: 3365–3370 [DOI] [PubMed] [Google Scholar]
- 6.Talabani B, Zouwail S, Pyart RD, et al. Epidemiology and outcome of community-acquired acute kidney injury. Nephrology (Carlton) 2014; 19: 282–287 [DOI] [PubMed] [Google Scholar]
- 7.Kerr M, Bedford M, Matthews B, et al. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 2014; 29: 1362–1368 [DOI] [PubMed] [Google Scholar]
- 8.Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens 2005; 14: 265–270 [DOI] [PubMed] [Google Scholar]
- 9.Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant 2010; 25: 1833–1839 [DOI] [PubMed] [Google Scholar]
- 10.Balasubramanian G, Al-Aly Z, Moiz A, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis 2011; 57: 228–234 [DOI] [PubMed] [Google Scholar]
- 11.Harel Z, Wald R, Bargman JM, et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 2013; 83: 901–908 [DOI] [PubMed] [Google Scholar]
- 12.Meier P, Bonfils RM, Vogt B, et al. Referral patterns and outcomes in noncritically ill patients with hospital-acquired acute kidney injury. Clin J Am Soc Nephrol 2011; 6: 2215–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCEPOD. National Confidential Enquiry into Patient Outcome and Death [NCEPOD] Report Acute Kidney Injury: Adding Insult to Injury, 2009
- 14.Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 2012; 7: 533–540 [DOI] [PubMed] [Google Scholar]
- 15.Gong Y, Zhang F, Ding F, et al. Elderly patients with acute kidney injury (AKI): clinical features and risk factors for mortality. Arch Gerontol Geriatr 2012; 54: e47–e51 [DOI] [PubMed] [Google Scholar]
- 16.Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol 2009; 4: 891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu CY, Ordonez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 2008; 74: 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosla N, Soroko SB, Chertow GM, et al. Preexisting chronic kidney disease: a potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol 2009; 4: 1914–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 2009; 76: 893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley S, Diro E, Batchelor P, et al. Renal impairment among acute hospital admissions in a rural Ethiopian hospital. Nephrology (Carlton) 2013; 18: 92–96 [DOI] [PubMed] [Google Scholar]
- 21.Cruz DN, Ferrer-Nadal A, Piccinni P, et al. Utilization of small changes in serum creatinine with clinical risk factors to assess the risk of AKI in critically ill adults. Clin J Am Soc Nephrol 2014; 9: 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ftouh S, Thomas M. Acute kidney injury: summary of NICE guidance. BMJ 2013; 347: f4930. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finlay S, Bray B, Lewington AJ, et al. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med 2013; 13: 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu CY, McCulloch CE, Fan D, et al. Community-based incidence of acute renal failure. Kidney Int 2007; 72: 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu RK, McCulloch CE, Ku E, et al. Regional variation in the incidence of dialysis-requiring AKI in the United States. Clin J Am Soc Nephrol 2013; 8: 1476–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meran S, Wonnacott A, Amphlett B, et al. How good are we at managing acute kidney injury in hospital? Clin Kidney J 2014; 7: 144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Mendonca A, Vincent JL, Suter PM, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med 2000; 26: 915–921 [DOI] [PubMed] [Google Scholar]
- 29.Brown JR, Kramer RS, MacKenzie TA, et al. Determinants of acute kidney injury duration after cardiac surgery: an externally validated tool. Ann Thorac Surg 2012; 93: 570–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 2009; 110: 505–515 [DOI] [PubMed] [Google Scholar]
- 31.Wi J, Ko YG, Shin DH, et al. Prediction of contrast-induced nephropathy with persistent renal dysfunction and adverse long-term outcomes in patients with acute myocardial infarction using the mehran risk score. Clin Cardiol 2013; 36: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liangos O, Wald R, O'Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 2006; 1: 43–51 [DOI] [PubMed] [Google Scholar]
- 33.Obialo CI, Okonofua EC, Tayade AS, et al. Epidemiology of de novo acute renal failure in hospitalized African Americans: comparing community-acquired vs hospital-acquired disease. Arch Intern Med 2000; 160: 1309–1313 [DOI] [PubMed] [Google Scholar]
- 34.Forni LG, Dawes T, Sinclair H, et al. Identifying the patient at risk of acute kidney injury: a predictive scoring system for the development of acute kidney injury in acute medical patients. Nephron Clin Pract 2013; 123: 143–150 [DOI] [PubMed] [Google Scholar]
- 35.Ostermann M, Philips BJ, Forni LG. Clinical review: biomarkers of acute kidney injury: where are we now? Crit Care 2012; 16: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]