Abstract
This narrative review evaluates translational research with respect to five important risk factors for chronic kidney disease (CKD): physical inactivity, high salt intake, smoking, diabetes and hypertension. We discuss the translational research around prevention of CKD and its complications both at the level of the general population, and at the level of those at high risk, i.e. people at increased risk for CKD or CKD complications. At the population level, all three lifestyle risk factors (physical inactivity, high salt intake and smoking) have been translated into implemented measures and clear population health improvements have been observed. At the ‘high-risk’ level, the lifestyle studies reviewed have tended to focus on the individual impact of specific interventions, and their wider implementation and impact on CKD practice are more difficult to establish. The treatment of both diabetes and hypertension appears to have improved, however the impact on CKD and CKD complications was not always clear. Future studies need to investigate the most effective translational interventions in low and middle income countries.
Keywords: CKD, diabetes mellitus, epidemiology, hypertension, physical activity
Introduction
Chronic kidney disease (CKD) is increasingly recognized as a global public health problem. People with CKD are at risk of developing end-stage renal disease (ESRD) and have a notably increased risk of cardiovascular disease (CVD) and mortality [1–4]. From 1990 to 2010 the age-adjusted death rates attributable to CKD have increased by 15% [5] and CKD is now the 19th leading cause of life years lost [3]. While some of this increase may be attributable to increased identification and coding, other factors such as demographic transition to older population profiles and rural to urban population shift in low and middle income countries must be considered [6].
Worldwide an estimated 8–16% of the general population has CKD [7]. The prevalence of CKD increases with age to about 30% in people aged over 70 years [8, 9]. Added to this is an anticipated increase in CKD prevalence as a result of the ongoing epidemics of diabetes, hypertension and obesity [8], all of which (both individually and in combination) are important risk factors for CKD [2]. In addition to the implications for morbidity and mortality, the growing prevalence of CKD has significant implications for health and social care systems, particularly considering the high cost of renal replacement therapy, the greatest burden of which may in future be felt in developing countries [3].
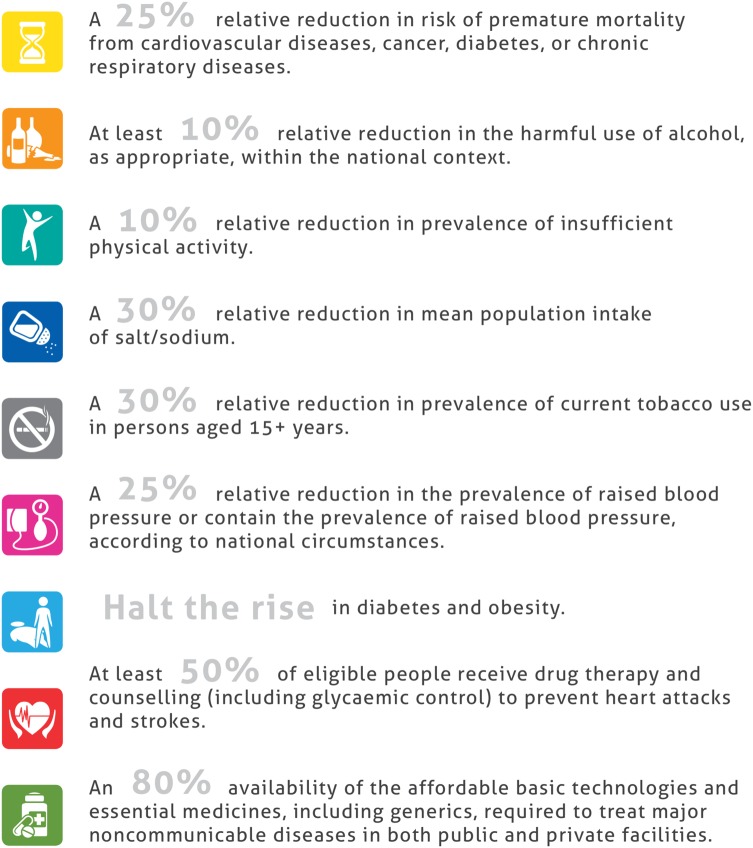
Although dependent to some extent on the causal pathophysiology, in principle, the development of CKD and its complications can be (partly) prevented or delayed [7]. In 2013 the World Health Organization's (WHO) Global Action Plan for the Prevention and Control of Non-communicable Diseases (NCDs) 2013–2020 was adopted [10]. In this plan, the WHO lists nine public health targets which will help to reduce the global burden of NCDs by targeting both lifestyle factors and specific NCDs (Figure 1) [10]. Although CKD is not a direct target in this WHO action plan, the plan does acknowledge the link between major NCDs, such as diabetes and hypertension, and CKD. Moreover, five of the WHO targets are aimed at important CKD risk factors namely: physical inactivity [11], high dietary salt intake [12], smoking [13], diabetes and hypertension [2]. In this narrative review, we will review how the available evidence on these important CKD risk factors is being translated into the implementation of measures to prevent CKD and its complications and to improve public health.
Fig. 1.
The nine global voluntary targets from the WHO Global Action Plan for the Prevention and Control of NCDs 2013–2020. Reprinted from Global action plan for the prevention and control of noncommunicable diseases 2013–2020, World Health Organization, Voluntary Global Targets, page 5, Copyright (2013).
Ideally, translational research aimed at the prevention of CKD and its complications needs to translate findings both at the level of the general population and at the level of those at high risk [14]. In the latter we will consider both people who are at increased risk of developing CKD (e.g. people with diabetes) and people with CKD who are at risk of developing complications of CKD. Focusing on these levels, this narrative review will describe translational research with respect to the five risk factors for CKD mentioned above: physical inactivity [11], high salt intake [12], smoking [13], diabetes and hypertension [2]. Thus we will consider both primary and secondary prevention strategies for CKD in a translational research framework.
Public health translational research framework
There is no clear consensus regarding the term translational research [15]. Traditionally it concerns the translation from ‘bench-to-bedside’, i.e. using basic science results to develop new treatments or diagnostics for patients [15]. In public health terms translational research is commonly interpreted as the translation of ‘research into practice’, i.e. ensuring that new research knowledge will reach the intended patients and populations and that it is implemented correctly with the prospect of improving health [15].
Both these traditional basic science and public health perspectives on translational research have been incorporated into various linear frameworks [16–18], such as the framework described by Khoury et al. [16], which distinguishes five phases of translational research (see Box 1):
Box 1.
Translational research framework as described by Khoury et al. [15]
Phase 0: description and discovery
Phase 1: from discovery to health applications
Phase 2: from health application to evidence guidelines
Phase 3: from guidelines to health practice
Phase 4: from health practice to population health outcomes
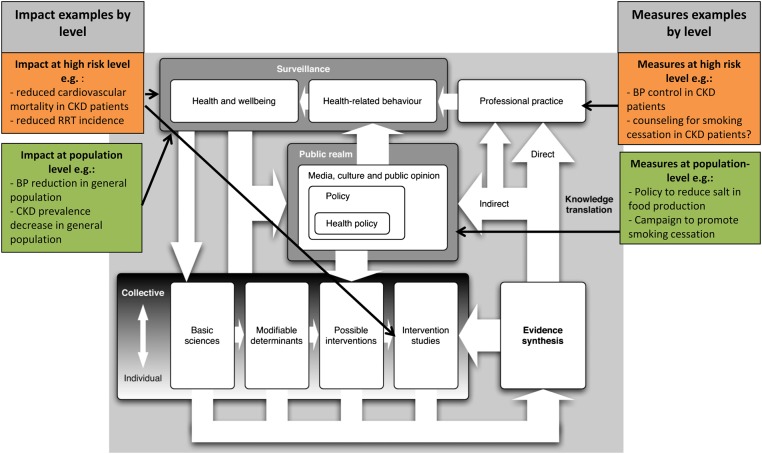
An alternative nonlinear framework has been proposed by Ogilvie et al. [19]. This framework was developed specifically for the translation of public health research [19]. They argue that translating research into improvement of public health involves a much wider scope than the translation of research by researchers alone, as the practice to be influenced is not limited to clinical practice or public health practice [19]. We have chosen to use an adaptation of this framework by Ogilvie et al. as the outline for our review, because this framework reflects the complex interactions involved in translating health research into public health improvement (Figure 2).
Fig. 2.
Ogilvie's translational research framework in the context of chronic kidney disease (adapted from a model by Ogilvie et al. [19]). RRT, renal replacement therapy.
In recent years, translational research has received increased attention from funding bodies such as the National Institute for Health Research (NIHR) in the UK [18]. However, there is evidence that the majority of funding for translational research has still historically been assigned to the translation from bench-to-bedside [20] rather than on implementation [21] or population health impact. As a consequence of this emphasis, most translational research focuses on this bench-to-bedside translation [15]. Nevertheless one may argue that the translation from ‘research to practice’, e.g. implementation into health practice, has the potential to benefit more people. It is important to remember that the greatest benefit to population health may not be achieved by simply targeting those at highest risk. To cite Rose ‘A large number of people at a small risk may give rise to more cases of disease than the small number who are at high risk’ [22]. We will therefore consider population-level measures and their impact as well as ‘high-risk’ level measures and their impact.
Lifestyle factors
Physical inactivity
Population-level measures
Many observational studies have shown that in healthy subjects regular physical activity is associated with reduced morbidity and all-cause and cardiovascular mortality, leading to the development of general population physical activity guidelines in many countries [11, 23, 24]. Increasing physical activity at population level is a complex topic encompassing many different strategies including policies, advocacy, environmental/infrastructure changes, and awareness and education [23]. These strategies fit in the ‘Public realm’ box of the Ogilvie model (Figure 2). Several governments have implemented campaigns to increase physical activity of their population, such as the ‘exercise 30 min a day’ and ‘10 000 steps a day’ campaigns in the Netherlands and Belgium, respectively (http://www.10000stappen.be/; http://www.30minutenbewegen.nl/home-ik-wil-bewegen.html). In line with this, the WHO Global Action Plan for the Prevention and Control of Non-communicable Diseases aims for a ‘10% relative reduction in the prevalence of physical inactivity’ [10].
There is a wide variety of methods used to increase physical activity levels of the population, from changing infrastructure of urban areas to methods of influencing behavioural change. It falls outside the scope of this review to describe all of these in detail; however, they are of great relevance for both the general population and for people at the high-risk level. One specific example is the recent development of the increasing availability and use of mobile health applications (‘apps’) and other electronic devices to monitor and adjust physical activity and other health-related behaviour. This has prompted the US Food and Drug Administration (FDA) to publish guidance on mobile medical apps for the industry in 2013 [25]. This guidance clarifies which apps are considered to be subject to the FDA authority and the regulatory requirements that will apply to such apps. A report by Research2guidance predicted that by 2015, 500 million people will be using mobile healthcare applications [26]. The development and use of apps to monitor physical activity may likely influence public health awareness and perhaps modify health-related behaviour of both the general population and patients. Yet the effectiveness of these apps in increasing physical activity needs further study.
Population-level impact
In 2002, 31% of the adult European population reported regularly undertaking sufficient physical activity, i.e. 30 min of moderate physical activity ≥5 times a week [27]. Several European countries have published physical activity trend data, which fits into the box ‘Health-related behaviour’ of the Ogilvie model (Figure 2). In the Netherlands, the percentage of the population fulfilling the minimum physical activity level criteria has increased from 52 to 62% in the period from 2001 to 2011 [28]. In the UK, from 1997 to 2012, the percentage of men and women meeting the physical activity criteria increased from 32 to 43% and 21 to 32%, respectively [29]. Despite the increased physical activity seen in both the Netherlands [30] and the UK [31], obesity prevalence is still increasing in both countries. In the UK the obesity prevalence increased between 1993 and 2012 from 13.2 to 24.4% and 16.4 to 25.1% in males and females, respectively [31]. These data suggest that interventions to increase physical activity are effective in increasing the physical activity level, but not in decreasing the obesity prevalence in the general population [32]. However, increased physical activity is related to various other health benefits such as improved glycaemic control, reduced blood pressure and reduced cardiovascular risk [33].
In terms of interventions, a recent systematic review by Laine et al. [34] concluded that community rail trails, pedometers and school health education programmes were the most cost-effective measures to increase physical activity on a population level. Prior to this, Roux et al. [35] had modelled the cost-effectiveness of seven community-based physical activity interventions, such as community-wide campaigns and the creation of physical activity information and opportunities, and concluded that all seven strategies considered were cost effective at reducing the incidence of chronic diseases such as coronary heart disease, ischaemic stroke and type 2 diabetes.
Additional high-risk level measures
Although multiple studies report beneficial impact of physical activity in people with CKD [36, 37], evidence-based guidelines with specific exercise recommendations for people with CKD are lacking [38, 39]. Nonetheless, multiple guidelines include an exercise recommendation for people with CKD based on low-grade evidence [1, 40, 41], which fit into the box ‘Professional practice’ of the Ogilvie model (Figure 2). For example, Kidney Disease: Improving Global Outcomes (KDIGO) recommends encouraging people with CKD to undertake physical activity compatible with cardiovascular health and tolerance five times a week for at least 30 min [1]. Few studies examined exercise adherence by people with CKD [39]. In a randomized controlled trial in which people with CKD received 8 weeks of supervised training followed by 10 months of home-based training, self-reported adherence dropped from 70% during the supervised training to 53% at the end of the 10 months home-based training period [42].
High-risk level impact
In people with CKD exercise training is associated with reduced body mass index (BMI) and improved physical functioning [42], such studies fit into the box ‘Intervention studies’ (Figure 2). Importantly, many people with CKD are older and promoting physical activity in older subjects is influenced by specific challenges. As expected, individuals aged 65 years or older often report perceived poor health and symptoms of physical disabilities as major barriers to physical activity [43]. Despite these additional barriers for elderly patients, a review by Brawley et al. [43] has shown that physical activity interventions may still increase the amount of physical activity in older patients. In patients with established CVD, randomized trials have shown that regular physical activity is effective in reducing CVD incidents and in improving life expectancy [11]. In conclusion, there is good evidence that increasing physical activity is effective in the secondary prevention of CVD in general, including some evidence specifically in CKD.
High dietary salt intake
Population-level measures
The WHO Global Action Plan has set the target of ‘a 30% relative reduction in mean population salt intake’ [10]. The recommended absolute salt intake by the WHO in 2012 is a dietary intake of <5 g/day [44]. It is worth noting that recommended targets vary across individual European countries, from specific recommendations such as <9 g/day in the Netherlands to more general advice such as ‘to avoid salt and food rich in salt’ in Greece and Hungary [45].
Since an estimated 70% of dietary salt intake in Western countries is obtained through bread and processed foods [46], only salt restriction in bread and processed foods will substantially reduce dietary salt intake and (possibly) achieve improvement in public health outcomes. This restriction needs to be implemented by policy makers or the food industry. In 2014, Webster et al. [47] identified 83 countries with national initiatives to reduce dietary salt intake. Fifty-nine countries, of which 32 European countries, reported collaboration with the food industry to reduce salt content of food [47]. These initiatives include both voluntary salt reduction targets, which are non-binding agreements with the food industry, and mandatory salt reduction targets, which are agreements enforced by legislation or penalty for non-compliance. Overall, the majority of identified initiatives related to voluntary targets implemented parallel to programmes directed at changing consumer attitudes and behaviour relating to salt [47]. All of these recommendations fit into the ‘Public realm’ box of the Ogilvie model (Figure 2).
Population-level impact
In an overview of national initiatives to encourage the food industry to reduce salt, Webster et al. [47] identified 17 countries that reported a reduction of salt levels in one or multiple products, nine of which were in Europe. All nine European countries reduced the salt content of bread, ranging from a 6% reduction in Belgium to 29% reduction in Ireland [47]. Finland started salt reduction efforts as early as 1978 including mandatory warning labels for food products high in salt [48]. By 2002 the Finnish average salt intake had reduced from 12 to 9 g per day [48]. These studies on salt intake fit within the box ‘Health-related behaviour’ (Figure 2).
Bibbins-Domingo et al. [49] projected that even minor reductions in dietary salt intake (e.g. 1 g a day) through population-wide salt reduction strategies would be cost-effective to reduce cardiovascular events and lower medical costs. From 1970 to 1995 the cardiovascular mortality decreased by 65% [50] in Finland. According to Vartiainen et al. [51] 32 and 38% of the stroke mortality reduction was explained by a decrease in diastolic blood pressure for males and females, respectively. The reduction in mortality from ischaemic heart disease could be explained by a decrease in diastolic blood pressure in 15 and 31% of the males and females, respectively [52]. Within the general population, dietary salt reduction reduces the risk of cardiovascular events and possibly all-cause mortality [53].
Additional high-risk level measures
Although evidence-based clinical practice guidelines for people with CKD generally recommend reduction in dietary salt intake [54], the various guidelines report different salt targets. KDIGO recommends a salt intake of <2 g/day [55] and the Canadian Society of Nephrology recommends sodium targets depending on hypertensive status [40]. These guidelines can be considered to be in the ‘Professional practice’ box of the Ogilvie model (Figure 2). Restricting dietary salt intake can be challenging and, in clinical practice, recommended salt targets are often not achieved in people with CKD [56, 57]. This may be influenced by insufficient emphasis on salt reduction by care providers [12], yet is likely also influenced by patient non-adherence [57]. Dietary salt recommendations can be confusing for patients and the general population alike, and adhering to recommended targets is difficult for individuals. Therefore interventions at the population level, such as legislation influencing food manufacturers to reduce salt content, are the most likely to succeed in reducing dietary salt intake of both the general population and people with CKD.
High-risk level impact
In people with CKD, salt reduction is a low risk and cost-effective strategy to reduce blood pressure as compared with blood pressure-reducing drugs [54]. Salt restriction reduces both hypertension and proteinuria in people with CKD [54, 58]. However a recent post hoc analysis of the ONTARGET and TRANSCEND trial found no association between low sodium diet and risk of ESRD [59]. Unfortunately there are no studies on the effect of salt reduction on mortality [54].
Smoking
Population-level measures
Smoking is associated with an increased risk of CKD in the general population [60]. The WHO target is ‘a 30% relative reduction in prevalence of current tobacco use in person aged 15+ years’ [10]. In Europe there are various, ‘Public realm’, measures against smoking, such as specific excise taxes on tobacco [61] and the ban on tobacco advertising in the entire European Union (EU) [61]. Currently, 17 EU countries have smoke free laws in place, such as a ban on smoking in public spaces and in the workspace [62]. Additionally, the EU has developed multiple anti-smoking campaigns [63].
Population-level impact
There is some conflicting evidence on the effectiveness of mass media campaigns in reducing smoking uptake and prevalence [64]. While the vast majority are shown to be effective [64, 65], there is a need to be careful about the campaign message and mode of delivery in order to be effective [64]. Policies which ban smoking in public places and the workspace have been shown to be effective in reducing tobacco consumption [66, 67]. However, according to The Lancet Commissions, specific excise taxes are the most effective intervention against tobacco use and related non-communicable diseases at population level [68]. The WHO estimated that doubling prices on tobacco by raising specific excise taxes would lead to an increase in tobacco tax revenues of $100 billion [69], despite reducing tobacco consumption by one third [61]. In contrast, reducing tobacco consumption by a third through other measures would actually lead to an estimated decrease in tobacco tax revenue of $100 billion [69]. Tobacco-specific excise taxes are therefore highly cost effective.
Recently Bilano et al. [70] reported the global trends for tobacco use. From 2000 to 2010, tobacco use decreased in men in all 31 European high-income countries, but in women tobacco use decreased only in 27 of these countries [70]. Smoking cessation appears to improve kidney function, yet established proteinuria seems to be irreversible [71, 72]. The increase in life expectancy after smoking cessation in the general population has been well documented [61].
Additional high-risk level measures
Several studies have documented that smoking in people with CKD and people at risk for CKD is related to both progression of CKD and cardiovascular mortality [13]. Hence smoking cessation is recommended for people with CKD [1]. There are few studies which investigate how often smoking cessation counselling is recommended by physicians. A study performed in hospitalized CKD patients with heart failure found that smoking cessation counselling is less often promoted as kidney function declines [73]. Another study performed in patients at-risk for CVD, including 923 CKD patients, found that in Canadian primary care about 50% of smokers received smoking cessation counselling [74]. Plenty of residual opportunity therefore remains to promote smoking cessation in clinical contexts.
High-risk level impact
To our knowledge no studies are available on the effectiveness of smoking cessation strategies in people with CKD [13]. Although only few studies have investigated the effect of smoking cessation on renal function in people with CKD, all studies found a positive effect [13].
Non-communicable diseases as risk factors
Diabetes
CKD screening in diabetic patients
Screening for kidney disease in people with diabetes is now generally recommended [75]. Moreover, several studies have suggested that CKD screening is cost effective in this context [76, 77]. Nonetheless, adherence to CKD screening guidelines varies markedly across countries and between physicians [78, 79].
Treatment of diabetes
Multiple studies have shown that the development and progression of albuminuria in diabetic subjects can be prevented through strict glycaemic control and the use of angiotensin converting enzyme inhibitors (ACEi) [80–82]. Accordingly, the use of glycaemic control medication and ACEi in diabetic subjects has increased over the years [83, 84]. Golan et al. [77] have proposed that treating all middle-aged type 2 diabetics with ACEi is the most cost-effective strategy to slow progression to end-stage renal disease (ESRD).
The impact of this improved treatment of diabetes on CKD and its complications is unclear. Some studies have described the trends in diabetic kidney disease prevalence, yet these are influenced by both incidence and survival [83, 84]. There are some reports on the change of renal replacement therapy (RRT) incidence for ESRD due to diabetes. From 1996 to 2006 the incidence of RRT for ESRD in diabetics decreased in the USA [85]. This is in line with results from Europe, in which the incidence of RRT for ESRD due to type 1 and 2 diabetes in the general population decreased between 1998 and 2011 [86]. Although this decline may be due to slower progression of CKD caused by improved treatments and earlier detection, it may also be partly explained by a change in renal replacement initiation practices.
Hypertension
CKD screening in hypertensive patients
Almost all guidelines recommend screening for CKD in hypertensive subjects [87, 88]. Boulware et al. [89] have shown that screening in hypertensive subjects is cost-effective irrespective of age.
Treatment of hypertension
Although hypertension increases the risk of developing CKD, there is no clear evidence that blood pressure reduction lowers that risk [90, 91]. Importantly, hypertension is among the biggest single disease risk factors for global disease burden [3] and therefore hypertension treatment should be a high priority at the population level even if evidence of its impact on CKD incidence is limited. In CKD patients, treatment of hypertension is recommended to reduce CKD progression and lower cardiovascular disease risk [1]. Medication adherence to antihypertensive agents within CKD patients has been estimated to be around 67% [92].
Using data from the Health Survey for England, Aitken et al. [84] observed an increase in the use of antihypertensive drugs in hypertensive subjects in England between 2003 and 2008. In this same period, the systolic and diastolic blood pressure decreased in the hypertensive population while the CKD prevalence within the hypertensive population stayed approximately the same [84].
In CKD patients there is evidence that more could be done to improve blood pressure control. Several studies have identified that recommended blood pressure targets are only achieved in <50% of patients [56, 93]. A study in CKD patients in primary care found that older age, greater albuminuria levels and diabetes were all associated with poorer blood pressure control [93].
Conclusion
In this narrative review we have discussed five important CKD risk factors that are targeted by the WHO Action Plan for the Prevention and Control of NCDs: physical inactivity, high dietary salt intake, smoking, diabetes and hypertension. Considering the framework proposed by Ogilvie et al. we have described measures involving various stakeholders, such as policy makers, health practitioners and the food industry. Thus highlighting the importance of seeing the ‘big picture’ when reviewing the translation of research in the context of public health.
Lifestyle factors
At the population level, all three lifestyle risk factors were translated to implemented measures, varying from campaigns promoting physical activity and reducing dietary salt intake to specific excise taxes on tobacco. The implemented lifestyle measures appeared to have a positive impact at the population level, as physical inactivity, dietary salt intake and smoking were all reduced after implementation of population-wide measures. The population health impact was mostly shown in studies focusing on cardiovascular outcomes and mortality reductions. Only few studies have described the impact of lifestyle improvements on kidney outcomes, which were limited to proteinuria and kidney function.
At the ‘high-risk’ level, the discussed lifestyle studies focused on the individual impact of interventions. We were unable to find studies investigating to what extent successful measures were implemented in CKD health practice.
NCDs as risk factors for CKD
The treatment of both diabetes and hypertension appears to have improved with increased prescription rates for glycaemic control medication, ACEi and antihypertensive medication. However, studies on blood pressure control suggest that blood pressure control remains poor in CKD patients, especially in diabetic CKD patients. As with other conditions, the uncontrolled roll out of treatment improvements to the high-risk patients makes the impact on development of CKD and CKD complications difficult to establish.
Limitations
The importance of risk factors may vary per region [7] and we have only described studies performed in Western developed countries. As CKD and its influence on public health are influenced by many factors, we could not provide a comprehensive overview of all relevant factors. We recognize, for example, that other factors, such as obesity, have an important impact on kidney function (either directly or indirectly via influencing blood pressure or other mediators) [2]. We chose to focus on a selected group of major determinants of global morbidity and mortality, and described five factors specifically highlighted by the WHO Action Plan for the Prevention and Control of NCDs. However, even within this selected group, we did not perform a systematic search and consequently we may have failed to include relevant studies. Nonetheless we believe that the topics discussed describe important exemplars of translational research and achievements with regard to CKD prevention and public health.
Recommendations for future research
There is a need for population-level studies focusing on the impact of lifestyle measures on hard renal outcomes such as start of RRT. Moreover, longitudinal studies are needed to prove causation between implemented measures and associated outcomes.
With regard to high-risk level measures, studies are needed to investigate the implementation of recommended measures in CKD health practice. Importantly, to fully assess the impact of implemented measures on the CKD population at large, one needs either registries or studies that repeatedly collect data on both measures and associated outcomes in CKD populations.
Of note, we have discussed five important risk factors for CKD, but there are multiple other important factors and measures relevant to the prevention of CKD and its complications, such as acute kidney injury [94], alcohol use [95] and (dietary) interventions for obesity [7]. Since in low- and middle-income countries the burden of CKD and related non-communicable diseases is increasing rapidly [3], there is an urgent need for understanding the most effective translational interventions in these countries.
Conflict of interest statement
None declared.
Acknowledgements
Joost Daams (Collection manager, medical information specialist, Medical Library Academic Medical Center, Amsterdam).
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 2.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease study (GBD) 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385: 117–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez AD, Williams TN, Levin A, et al. Remembering the forgotten non-communicable diseases. BMC Med 2014; 12: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. [Erratum appears in Lancet 2013; 381: 628. Lancet 2012; 380: 2095–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Global Health and Aging Bethesda, MD: WHO, 2011 [Google Scholar]
- 7.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272 [DOI] [PubMed] [Google Scholar]
- 8.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 9.Roth M, Roderick P, Mindell J. Kidney Disease and Renal Function. Leeds: NHS, 2011 [Google Scholar]
- 10.WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: WHO, 2013
- 11.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006; 174: 801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thijssen S, Kitzler TM, Levin NW. Salt: its role in chronic kidney disease. J Ren Nutr 2008; 18: 18–26 [DOI] [PubMed] [Google Scholar]
- 13.Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 14.Ortiz A. Translational nephrology: what translational research is and a bird's-eye view on translational research in nephrology. Clin Kidney J 2015; 8: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf SH. The meaning of translational research and why it matters. JAMA 2008; 299: 211–213 [DOI] [PubMed] [Google Scholar]
- 16.Khoury MJ, Gwinn M, Ioannidis JP. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol 2010; 172: 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westfall JM, Mold J, Fagnan L. Practice-based research—‘Blue Highways’ on the NIH roadmap. JAMA 2007; 297: 403–406 [DOI] [PubMed] [Google Scholar]
- 18.Cooksey SD. A review of UK health research funding. London, UK: Crown Copyright, 2006, pp. 1–120.
- 19.Ogilvie D, Craig P, Griffin S, et al. A translational framework for public health research. BMC Public Health 2009; 9: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moses H, III, Dorsey ER, Matheson DH, et al. Financial anatomy of biomedical research. JAMA 2005; 294: 1333–1342 [DOI] [PubMed] [Google Scholar]
- 21.May C. Towards a general theory of implementation. Implement Sci 2013; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose G. Sick individuals and sick populations. Int J Epidemiol 1985; 14: 32–38 [DOI] [PubMed] [Google Scholar]
- 23.WHO. A Guide for Population-Based Approaches to Increasing Levels of Physical Activity: Implementation of the WHO Global Strategy on Diet, Physical Activity and Health. Switzerland: WHO, 2007 [Google Scholar]
- 24.NICE. NICE Guidance Physical Activity. London: NICE, 2012
- 25.Food and Drug Administration. Mobile Medical Applications; Guidance for Industry and Food and Drug Administration Staff. 2015. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM263366.pdf (9 February 2015, date last accessed)
- 26.Jahns R-G. Global Mobile Health Trends and Figures Market Report 2013–2017. Research2Guidance, 2010: 1–31 [Google Scholar]
- 27.Cavill N, Kahlmeier S, Racioppi F. (eds). Physical Activity and Health in Europe: Evidence for Action. Denmark: World Health Organisation, 2006 [Google Scholar]
- 28.Centraal Bureau voor de Statistiek. Gezondheidsenquete. The Hague: CBS, 2012 [Google Scholar]
- 29.Scholes S, Mindell J. Chapter 2: Physical activity in adults. In Health Survey England 2012. 2013; 1–49 [Google Scholar]
- 30.Milieuhygiene RvVe. http://www.nationaalkompas.nl/gezondheidsdeterminanten/persoonsgebonden/overgewicht/trend/ 2014. (22 April 2015, date last accessed). Available from: http://www.nationaalkompas.nl/gezondheidsdeterminanten/persoonsgebonden/overgewicht/trend/
- 31.Lifestyles Statistics Team, Health and Social Care Information Centre. Statistics on Obesity, Physical Activity and Diet. Leeds: Health and Social Care Information Centre, 2014 [Google Scholar]
- 32.Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med 2015; 49: 967–968 [DOI] [PubMed] [Google Scholar]
- 33.Kujala UM. Evidence on the effects of exercise therapy in the treatment of chronic disease. Br J Sports Med 2009; 43: 550–555 [DOI] [PubMed] [Google Scholar]
- 34.Laine J, Kuvaja-Kollner V, Pietila E, et al. Cost-effectiveness of population-level physical activity interventions: a systematic review. Am J Health Promot 2014; 29: 71–80 [DOI] [PubMed] [Google Scholar]
- 35.Roux L, Pratt M, Tengs TO, et al. Cost effectiveness of community-based physical activity interventions. Am J Prev Med 2008; 35: 578–588 [DOI] [PubMed] [Google Scholar]
- 36.Chen IR, Wang SM, Liang CC, et al. Association of walking with survival and RRT among patients with CKD stages 3–5. Clin J Am Soc Nephrol 2014; 9: 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sietsema KE, Amato A, Adler SG, et al. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 2004; 65: 719–724 [DOI] [PubMed] [Google Scholar]
- 38.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis 2012; 59: 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; Cd003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. CMAJ 2008; 179: 1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant 2015; 30 (Suppl 2): ii1–142 [DOI] [PubMed] [Google Scholar]
- 42.Howden EJ, Coombes JS, Strand H, et al. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis 2015; 65: 583–591 [DOI] [PubMed] [Google Scholar]
- 43.Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med 2003; 25 (3 Suppl 2): 172–183 [DOI] [PubMed] [Google Scholar]
- 44.WHO. Guideline: Sodium Intake for Adults and Children. Geneva: World Health Organization, 2012 [PubMed] [Google Scholar]
- 45.WHO. Reducing Salt Intake in Populations: Report of a WHO Forum and Technical Meeting. Paris, France: WHO, 2006 [Google Scholar]
- 46.Sanchez-Castillo CP, Warrender S, Whitehead TP, et al. An assessment of the sources of dietary salt in a British population. Clin Sci (Colch) 1987; 72: 95–102 [DOI] [PubMed] [Google Scholar]
- 47.Webster J, Trieu K, Dunford E, et al. Target salt 2025: a global overview of national programs to encourage the food industry to reduce salt in foods. Nutrients 2014; 6: 3274–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webster JL, Dunford EK, Hawkes C, et al. Salt reduction initiatives around the world. J Hypertens 2011; 29: 1043–1050 [DOI] [PubMed] [Google Scholar]
- 49.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362: 590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puska P, Vartiainen E, Tuomilehto J, et al. Changes in premature deaths in Finland: successful long-term prevention of cardiovascular diseases. Bull World Health Organ 1998; 76: 419–425 [PMC free article] [PubMed] [Google Scholar]
- 51.Vartiainen E, Sarti C, Tuomilehto J, et al. Do changes in cardiovascular risk factors explain changes in mortality from stroke in Finland? BMJ (Clinical Research Ed) 1995; 310: 901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vartiainen E, Puska P, Pekkanen J, et al. Changes in risk factors explain changes in mortality from ischaemic heart disease in Finland. BMJ (Clinical Research Ed) 1994; 309: 23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet 2011; 378: 380–382 [DOI] [PubMed] [Google Scholar]
- 54.McMahon EJ, Campbell KL, Bauer JD, et al. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev 2015; 2: Cd010070. [DOI] [PubMed] [Google Scholar]
- 55.KDIGO. Clinical Practice Guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2: 85 [Google Scholar]
- 56.Leonardis D, Mallamaci F, Enia G, et al. The MAURO study: baseline characteristics and compliance with guidelines targets. J Nephrol 2012; 25: 1081–1090 [DOI] [PubMed] [Google Scholar]
- 57.McMahon EJ, Campbell KL, Mudge DW, et al. Achieving salt restriction in chronic kidney disease. Int J Nephrol 2012; 2012: 720429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogt L, Waanders F, Boomsma F, et al. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19: 999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smyth A, Dunkler D, Gao P, et al. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int 2014; 86: 1205–1212 [DOI] [PubMed] [Google Scholar]
- 60.Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int 2007; 71: 159–166 [DOI] [PubMed] [Google Scholar]
- 61.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014; 370: 60–68 [DOI] [PubMed] [Google Scholar]
- 62. http://ec.europa.eu/health/tobacco/smoke-free_environments/index_en.htm. (19 March 2015, date last accessed)
- 63. http://ec.europa.eu/health/tobacco/ex_smokers_are_unstoppable/index_en.htm. (19 March 2015, date last accessed)
- 64.Durkin S, Brennan E, Wakefield M. Mass media campaigns to promote smoking cessation among adults: an integrative review. Tob Control 2012; 21: 127–138 [DOI] [PubMed] [Google Scholar]
- 65.Bala MM, Strzeszynski L, Topor-Madry R, et al. Mass media interventions for smoking cessation in adults. Cochrane Database Syst Rev 2013; 6: Cd004704. [DOI] [PubMed] [Google Scholar]
- 66.Callinan JE, Clarke A, Doherty K, et al. Legislative smoking bans for reducing secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev 2010; Cd005992. [DOI] [PubMed] [Google Scholar]
- 67.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. BMJ (Clinical Research Ed) 2002; 325: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jamison DT, Summers LH, Alleyne G, et al. Global health 2035: a world converging within a generation. Lancet 2013; 382: 1898–1955 [DOI] [PubMed] [Google Scholar]
- 69.WHO. WHO technical manual on tobacco tax administration, 2010. Geneva: WHO, 2010.
- 70.Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet 2015; 385: 966–976 [DOI] [PubMed] [Google Scholar]
- 71.Halimi JM, Giraudeau B, Vol S, et al. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int 2000; 58: 1285–1292 [DOI] [PubMed] [Google Scholar]
- 72.Athyros VG, Katsiki N, Doumas M, et al. Effect of tobacco smoking and smoking cessation on plasma lipoproteins and associated major cardiovascular risk factors: a narrative review. Curr Med Res Opin 2013; 29: 1263–1274 [DOI] [PubMed] [Google Scholar]
- 73.Patel UD, Hernandez AF, Liang L, et al. Quality of care and outcomes among patients with heart failure and chronic kidney disease: A Get With the Guidelines-Heart Failure Program study. Am Heart J 2008; 156: 674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naicker K, Liddy C, Singh J, et al. Quality of cardiovascular disease care in Ontario's primary care practices: a cross sectional study examining differences in guideline adherence by patient sex. BMC Fam Pract 2014; 15: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.American Diabetes Association. Standards of Medical Care in Diabetes—2010. Diabetes Care 2010; 33 (Suppl 1): S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ (Clinical Research Ed) 1995; 311: 1595–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golan L, Birkmeyer JD, Welch HG. The cost-effectiveness of treating all patients with type 2 diabetes with angiotensin-converting enzyme inhibitors. Ann Intern Med 1999; 131: 660–667 [DOI] [PubMed] [Google Scholar]
- 78.Renard LM, Bocquet V, Vidal-Trecan G, et al. Adherence to international follow-up guidelines in type 2 diabetes: a longitudinal cohort study in Luxembourg. PLoS One 2013; 8: 1–9: e80162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sloan FA, Bethel MA, Lee PP, et al. Adherence to guidelines and its effects on hospitalizations with complications of type 2 diabetes. Rev Diabet Stud 2004; 1: 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Remuzzi G, Macia M, Ruggenenti P. Prevention and treatment of diabetic renal disease in type 2 diabetes: The BENEDICT study. J Am Soc Nephrol 2006; 17 (Suppl 2): S90–SS7 [DOI] [PubMed] [Google Scholar]
- 81.Shamoon H, Duffy H, Fleischer N, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 82.Turner R. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 83.de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305: 2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aitken GR, Roderick PJ, Fraser S, et al. Change in prevalence of chronic kidney disease in England over time: comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open 2014; 4: e005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010; 33: 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pippias M, Jager KJ, Kramer A, et al. The changing trends and outcomes in renal replacement therapy: results from the ERA-EDTA Registry. Nephrol Dial Transplant 2015; doi:10.1093/ndt/gfv327 [DOI] [PubMed] [Google Scholar]
- 87.Nederlands Huisartsen Genootschap (NHG), Cardiovasculair risicomanagement (Tweede herziening). Huisarts Wet 2012; 55: 14–28. Available from https://www.nhg.org/standaarden/volledig/cardiovasculair-risicomanagement (April 2015, date last accessed) [Google Scholar]
- 88.Lopez-Vargas PA, Tong A, Sureshkumar P, et al. Prevention, detection and management of early chronic kidney disease: a systematic review of clinical practice guidelines. Nephrology 2013; 18: 592–604 [DOI] [PubMed] [Google Scholar]
- 89.Boulware LE, Jaar BG, Tarver-Carr ME, et al. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA 2003; 290: 3101–3114 [DOI] [PubMed] [Google Scholar]
- 90.Levey AS, Schoolwerth AC, Burrows NR, et al. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis 2009; 53: 522–535 [DOI] [PubMed] [Google Scholar]
- 91.Turner G, Wiggins K, Johnson D. Cari Guidelines: Primary prevention of chronic kidney disease: blood pressure targets Westmead: Kidney Health Australia, 2012 [Google Scholar]
- 92.Schmitt KE, Edie CF, Laflam P, et al. Adherence to antihypertensive agents and blood pressure control in chronic kidney disease. Am J Nephrol 2010; 32: 541–548 [DOI] [PubMed] [Google Scholar]
- 93.Fraser SD, Roderick PJ, McIntyre NJ, et al. Suboptimal blood pressure control in chronic kidney disease stage 3: baseline data from a cohort study in primary care. BMC Fam Pract 2013; 14: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 2015; 385: 2616–2643 [DOI] [PubMed] [Google Scholar]
- 95.White SL, Polkinghorne KR, Cass A, et al. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dial Transplant 2009; 24: 2464–2472 [DOI] [PubMed] [Google Scholar]