Abstract
COL4A5 mutations are a known cause of Alport syndrome, which typically manifests with haematuria, hearing loss and ocular symptoms. Here we report on a 16-year-old male patient with a negative family history who presented with proteinuria, progressive renal failure and haemolysis, but without overt haematuria or hearing loss. A renal biopsy revealed features of atypical IgA nephropathy, while a second biopsy a year later showed features of focal segmental glomerulosclerosis, but was finally diagnosed as chronic thrombotic microangiopathy. Targeted sequencing of candidate genes for steroid-resistant nephrotic syndrome and congenital thrombotic microangiopathy was negative. Despite all therapeutic efforts, including angiotensin-converting enzyme inhibition, immunosuppressive therapy, plasma exchanges and rituximab, the patient progressed to end-stage renal disease. When a male cousin presented with nephrotic syndrome years later, whole-exome sequencing identified a shared disruptive COL4A5 mutation (p.F222C) that showed X-linked segregation. Thus, mutations in COL4A5 give rise to a broader spectrum of clinical presentation than commonly suspected, highlighting the benefits of comprehensive rather than candidate genetic testing in young patients with otherwise unexplained glomerular disease. Our results are in line with an increasing number of atypical presentations of single-gene disorders identified through genome-wide sequencing.
Keywords: Alport, FSGS, IgA nephropathy, nephrotic syndrome, thrombotic microangiopathy
Introduction
Mutations in COL4A5, a gene on chromosome X encoding collagen type IV, can cause Alport syndrome, which usually manifests with the triad of haematuria, hearing loss and ocular symptoms (lenticonus, retinal flecks). The disease-causing mutations decrease the mechanical stability of the glomerular basement membrane (GBM). While light microscopy studies typically show focal segmental glomerulosclerosis (FSGS) over the timecourse, specific anomalies observed by electron microscopy include thickening, splitting and fragmentation of the lamina densa [1].
Therapeutic options are limited and include renin–angiotensin–aldosterone system (RAAS) blockade to slow progression [2, 3]. Nevertheless, Alport syndrome ultimately leads to end-stage renal disease [4]. The prognosis of both renal and extrarenal manifestations varies by genotype [5]. Here, we present a patient with an X-linked COL4A5 mutation, who presented with proteinuria, but otherwise without typical signs of Alport syndrome.
Case report
A 16-year-old Caucasian male presented to the hospital with a 3-week history of lower extremity and periorbital oedema. He also reported short-lasting episodes (few minutes) of blurred vision. Other than sports injuries he had been healthy. He had taken Isotretinoin 20 mg QD for ∼1.5 years for acne, but had stopped the medication. Parents and the extended family were healthy, and there was no history of hereditary kidney disease.
On presentation he had a blood pressure of 138/86 mmHg. The physical examination showed periorbital and lower extremity oedema, but was otherwise unremarkable. Laboratory results showed potassium 5.2 mmol/L, creatinine 0.9 mg/dL and mild haemolysis [haemoglobin 12.3 g/dL, platelets 315 000 G/L, haptoglobin not detectable, lactate dehydrogenase (LDH) 236 U/L]. Albumin was decreased (2.9 g/dL), and a spot urine revealed a proteinuria of 3.4 g/g creatinine. The dipstick was positive for blood and the urinary sediment showed few dysmorphic erythrocytes but no casts. Serology for ANA, ANCA, hepatitis, HIV and phospholipid antibodies was negative. Complement factors (C3, C4) were normal. The patient was referred to ophthalmology for short episodes of blurred vision, but neither the eye examination, MRI imaging studies of the head, nor a spinal tap showed any abnormalities. Fundus hypertonicus and lenticonus were ruled out. The patient did not report any hearing problems.
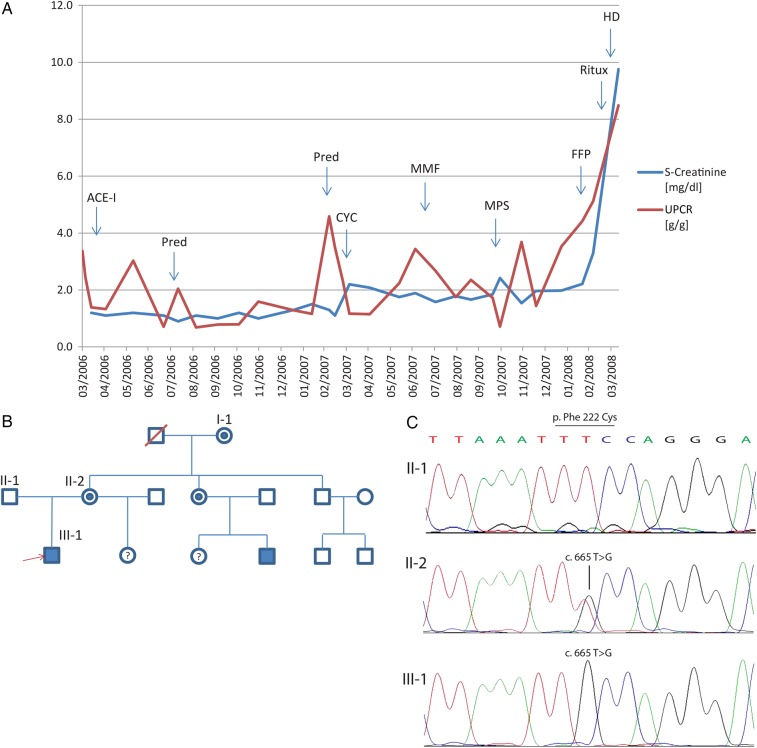
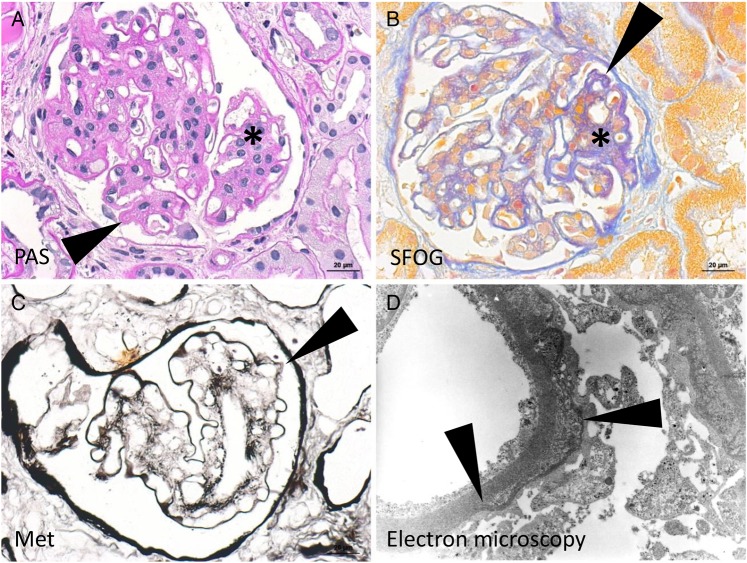
A renal biopsy was performed and showed a broadened and hypercellular mesangial field as well as a focal thickening and double contours of the peripheral basement membranes (Supplementary Figure S1A and B). Immunofluorescence microscopy showed mesangial and peripheral C3 and IgA deposits. Basement membrane lamellation was present in the electron microscopy (Figure 1). A presumptive diagnosis of IgA nephropathy with atypical features was made, and ramipril and furosemide were started (Figure 2A). Ramipril was replaced with candesartan, but proteinuria and serum creatinine continued to increase with a urinary protein-creatinine ratio (UPCR) of 5.4 g/g and a serum creatinine of 1.2 mg/dL. Steroids were started, and the UPCR dropped to 1.1 g/g. Since the patient developed Cushingoid features, steroids were replaced with IV cyclophosphamide. Due to recurrent neutropaenia, however, cyclophosphamide was substituted with mycophenolat mofetil; creatinine remained relatively stable at 1.6 mg/dL with an UPCR of 2.4 g/g.
Fig. 1.
Clinical course, pedigree and chromatograms. (A) Serum creatinine and urinary protein-creatinine ratio (UPCR) over time. Therapeutic interventions in response to creatinine/proteinuria rises are indicated as follows: ACE-I, angiotensin-converting enzyme inhibitor; Pred, prednisone; CYC, cyclophosphamide; MMF, mycophenolat-mofetil; MPS, plasma exchange (membrane plasma separator); FFP, fresh-frozen plasma; Ritux, rituximab; HD, haemodialysis. (B) Pedigree demonstrating a classical X-linked inheritance. Both the grandmother and the patient's mother are mutation carriers, as is the aunt of the index patient. The index patient (marked by red arrowhead) and his cousin are affected by the disease. (C) Exemplary chromatograms for the index patient and his parents demonstrating the confirmation of the whole-exome sequencing findings by Sanger sequencing.
Fig. 2.
Histology of kidney biopsy 2006. (A) Broadened and hypercellular mesangial field (asterisk) as well as focal thickening of peripheral basement membranes (arrowhead, exemplary) is displayed in the PAS staining. (B) The acid fuchsin-Orange G staining displays collagen fibres in blue, which shows double contours of peripheral basement membranes (arrowhead, exemplary) and collagenous matrix in the mesangium (asterisk). (C) Focal double contours are also highlighted in the Methenamine silver stain (arrowhead). (D) Basement membrane lamellation is displayed in the electron microscopy. Endothelial cells and podocytes are slightly autolytic.
When the serum creatinine increased to 2.2 mg/dL, a second kidney biopsy was performed 12 months after the first presentation, revealing isolated double contours and prominent FSGS with hyalinosis. The electron microscopy showed distorted capillary walls and expansion of the mesangium. The case was reviewed by several pathologists, and classified as membrane-proliferative glomerulonephritis with features of chronic thrombotic microangiopathy. However, candidate gene sequencing did not reveal mutations in F2, F5, CFH, CFI, MCP, ADAMTS13 and NPHS2.
In light of the pathological features of chronic thrombotic microangiopathy and persistent proteinuria, plasma exchanges with fresh frozen plasma were performed. This therapy led to transient normalization of haemolysis and a nearly 3-fold reduction of proteinuria. However, creatinine and proteinuria began to rise again, and rituximab 375 mg/m2 was administered. Finally, end-stage renal disease developed, and dialysis was initiated. As the family history for kidney disease was negative at that time, 3 years later, the patient received a living related donor transplant from his mother. During the next 4 years of follow-up, the patient developed neither haematuria, nor proteinuria. LDH returned to the reference range, while haptoglobin remained suppressed. A post-transplant biopsy 3 years after transplantation did not provide evidence of splitting of the GBM in the donor organ, nor of GBM thinning (309 ± 74 nm, reference value 320 ± 50 nm; Supplementary Figure S1C and D).
Seven years after the first contact, a maternal cousin of the patient presented at the age of 18 years with nephrotic-range proteinuria (4.8 g/g creatinine) as an incidental finding. Renal function was normal, and there were no features of haemolysis. The cousin did not report any eye or hearing problems. The kidney biopsy showed a mesangio-proliferative glomerulonephritis with mesangial sclerosis with IgM, IgG, C3 and C1q deposits. Therapy with an ACE inhibitor was initiated, while a trial of therapy with prednisolone had no effect.
Because of the co-occurrence, whole-exome sequencing was performed on selected family members (Figure 2B). We found a thymine-to-guanine substitution at nucleotide 665 (c.T665G, rs281874761) in exon 5 of the COL4A5 gene on the X chromosome, resulting in a phenylalanine-to-cysteine missense mutation at codon 222 (p.F222C). The mutation, confirmed by Sanger sequencing (Figure 2C), disrupts an amino acid sequence highly conserved down to zebrafish, and is not found in data from large-scale sequencing projects (1000 Genomes Project, Exome Sequencing Project, ExAC Browser). It is rated as damaging by several prediction softwares (PolyPhen2, SIFT, CADD). So far, the mutation has been identified in one previous report of a family with end-stage renal disease without signs of Alport syndrome [6]. This report did not contain any information on the presence of thrombotic microangiopathy. Whole-exome sequencing did not reveal any additional known or novel and potentially disease-causing mutations (defined as stop, splice, missense or frameshift mutations) of frequency <1% in large-scale sequencing data and predicted pathogenicity in COL4A5 or other genes causing known monogenic kidney diseases including complement factors (Supplementary Table S1).
Discussion
We report on a patient with a COL4A5 mutation with glomerular disease and signs of chronic thrombotic microangiopathy.
It is important to note that our patient did not exhibit all typical clinical symptoms of Alport syndrome. While toddlers usually present without (or just low-grade) proteinuria, adolescent patients may present with proteinuria. Remarkably, our patient as well as his maternal cousin presented with significant nephrotic-range proteinuria as their leading symptom, while both ocular symptoms and hearing loss were absent. Approximately 96% of female COL4A5 carriers show haematuria [7], but the patient's mother did not reveal any haematuria on repeated testing. Because she also did not show any other signs of chronic kidney disease, she was accepted as transplant donor, years before the correct diagnosis was established.
Another unusual feature of the index case was the haemolysis, leading to the diagnosis of ‘chronic microangiopathy’. Curiously, the patient's cousin, bearing the same p.F222C mutation, did not show any signs of haemolysis (haemoglobin, LDH within normal range), suggesting that this manifestation could be due to the continued presence of an independent, as yet unidentified genetic or environmental cause in the index patient. An extended search for potentially pathogenic mutations in known candidate genes for thrombotic microangiopathy did not yield any results. While possible explanations for the haemolysis also include hypertension or medication use, these factors were ruled out in the beginning: medications had been stopped prior to presentation, and haemolysis persisted under anti-hypertensive treatment. Thus, while a genetically or environmentally induced auto-immune aetiology seems the most probable explanation for the haemolysis, only functional studies could rule out conclusively whether the p.F222C mutation leads to mild or intermittent haemolysis.
Becknell et al. [6] previously characterized a large family with male carriers of the p.F222C COL4A5 variant, suffering from rapid deterioration of kidney function and heavy proteinuria, but not exhibiting the classical clinical and biopsy findings associated with Alport syndrome. The renal prognosis was also less favourable with the mean age of reaching end-stage renal disease before the age of 18 (range 9–22 years) as opposed to 25 years (range 7–39 years) [4] for classic Alport syndrome. No signs of haemolysis or thrombotic microangiopathy have been reported in this family. No functional studies have been performed proving the variant to be disease-causing.
A recent study identified seven families with hereditary FSGS and nephrotic-range proteinuria [8] classified as hereditary FSGS. In fact, variants in the COL4A3 and COL4A4 genes causing the kidney disease were subsequently identified in these families. Another study reports on 8 heterozygous COL4A3/COL4A4 mutations in 16 families with Thin Basement Membrane Nephropathy and FSGS [2]. Therefore, phenotypic presentations deviating from the classical Alport syndrome not only exist for the COL4A5 gene, but also for the more commonly affected genes COL4A3 and COL4A4.
There are several reasons why the family history may be unrevealing in cases such as our patient. In Western countries, families are often small and the family history may not be informative. Moreover, mutations may arise de novo, as illustrated in a recent large exome sequencing study, where as many as 83% of the autosomal dominant and 40% of the X-linked mutant alleles of patients with a molecular diagnosis arose de novo [9]. Our case illustrates the pitfalls of taking a candidate gene approach to evaluate genetic causes of early-onset renal disease. Whole-exome sequencing in the meantime has become a feasible alternative to identify established mutations as a cause of hereditary renal disease, and to identify mutations that expand the phenotypic spectrum of single-gene disorders. A timely genetic diagnosis would have prevented unnecessary therapies in our patient and helped with transplant donor selection.
In conclusion, this case demonstrates that COL4A5 mutations need to be considered in young patients with otherwise unexplained glomerular disease, even in the absence of haematuria, deafness or ocular symptoms. Although we cannot rule out additional mutations or environmental effects, the reported case suggests that COL4A5 mutations may present with proteinuria and in our case also with haemolysis.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Informed consent
All patients and family members provided written informed consent.
Conflict of interest statement
The authors declare no conflict of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Supplementary Material
References
- 1.Noel LH. Renal pathology and ultrastructural findings in Alport's syndrome. Ren Fail 2000; 22: 751–758 [DOI] [PubMed] [Google Scholar]
- 2.Gross O, Licht C, Anders HJ, et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 2012; 81: 494–501 [DOI] [PubMed] [Google Scholar]
- 3.Savige J, Gregory M, Gross O, et al. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol 2013; 24: 364–375 [DOI] [PubMed] [Google Scholar]
- 4.Kruegel J, Rubel D, Gross O. Alport syndrome—insights from basic and clinical research. Nat Rev Nephrol 2013; 9: 170–178 [DOI] [PubMed] [Google Scholar]
- 5.Gross O, Netzer KO, Lambrecht R, et al. Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant 2002; 17: 1218–1227 [DOI] [PubMed] [Google Scholar]
- 6.Becknell B, Zender GA, Houston R, et al. Novel X-linked glomerulopathy is associated with a COL4A5 missense mutation in a non-collagenous interruption. Kidney Int 2011; 79: 120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jais JP, Knebelmann B, Giatras I, et al. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a ‘European Community Alport Syndrome Concerted Action’ study. J Am Soc Nephrol 2003; 14: 2603–2610 [DOI] [PubMed] [Google Scholar]
- 8.Malone AF, Phelan PJ, Hall G, et al. Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int 2014; 86: 1253–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Muzny DM, Reid JG, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med 2013; 369: 1502–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.