Abstract
Antidepressants, especially amitriptyline, are among the most frequent drug classes involved in intoxications. Despite its small molecular weight, amitriptyline is not considered to be eliminated by extracorporeal treatment methods due to its high protein binding and large volume of distribution. New high cut-off dialysers have so far not been used for removal of amitriptyline. We report two cases of amitriptyline poisoning in which we measured the amitriptyline elimination using extended high cut-off (HCO) dialysis. Despite dialyser clearances of 33 and 58 mL/min, resulting in the reduction of initial serum concentrations by ∼30%, only 211 and 920 µg of amitryptilin, respectively, (<3% of the ingested amount) could be recovered in the total collected dialysate. Hence, due to the high volume of distribution of amitriptyline, even HCO dialysis does not contribute substantially to the extracorporeal removal of amitryptilin.
Keywords: acute interstitial nephritis, antidepressants, proton pump inhibitors
Background
Antidepressants represent the sixth leading substance category causing intoxication in the USA, with tricyclic antidepressants (TCAs) being the most important category in that group. TCA intoxications led to 69 fatalities in 2012. With 6305 exposures in 2012, amitriptyline was the single most important TCA involved in intoxication [1]. Similar findings are reported all over the world: amitriptyline is the second most responsible drug for death in antidepressant poisoning in the UK, where antidepressant self-poisoning is used in 20% of all poisoning suicides [2]. TCAs were the most common suicide poison used in New Zealand between 2001 and 2005 [3]. In Tehran, Iran, amitriptyline is the most used TCA in drug intoxication, where TCAs are responsible for 16% of all admissions due to intoxication [4]. In adult out-patients, amitriptyline is normally dosed between 75 and 150 mg/day, which provides therapeutic concentrations between 80 and 250 µg/L [5]. In cases of overdose, amitriptyline may cause an alteration of consciousness, hypernatraemia, convulsive seizure, arrhythmia and respiratory depression. First-line treatment focuses on general measures as well as correction of electrolyte disorders and arrhythmia [6]. Activated charcoal is frequently administered. Extracorporeal means of toxin removal consist of charcoal haemoperfusion [7] and plasma exchange, which decreased amitriptyline blood concentrations in a few cases [8]. Due to its high plasma protein binding and volume of distribution (VOD), amitriptyline is considered to be non-dialyzable. The Extracorporeal Treatments in Poisoning (EXTRIP) workgroup therefore recommends not to use extracorporeal removal of TCA, as this approach is not likely to offer a clinical benefit [9]. We report two cases of amitriptyline poisoning in which we measured the amitriptyline elimination using a high cut-off (HCO) dialyzer.
Essential information on both patients as recommended by the EXTRIP workgroup [10] is summarized in Table 1.
Table 1.
Relevant case report characteristics recommended by the EXTRIP workgroup [10]
| Case 1 | Case 2 | |
|---|---|---|
| General information | ||
| Age (years) | 54 | 53 |
| Weight (kg) | 54 | 75 |
| Height (cm) | 168 | 157 |
| Gender | ♀ | ♀ |
| Concurrent diseases | Breast cancer, chronic pain syndrome, depression | MGUS, depression, chronic pain syndrome |
| Source providing the history of the poisoning | Paramedics | Paramedics |
| Time from ingestion to hospital admission (h) | 7.5 | 8.5 |
| Known co-medication | Lorazepam | Pantoprazole, citalopram, mirtazapine |
| Other toxins | Tilidine, ethanol | Ibuprofen, β-blockers, alcohol |
| Activated charcoal given | No | Yes |
| ICU stay (days) | 3 | 5 |
| Discharge from hospital (days) | 3 | 20 |
| Laboratory values | ||
| Albumin (g/L) | 34.8 | 42 |
| Creatinine at baseline (µmol/L)/eGFR CKD-EPI | 36/115.3 | 643/6 (AKIN III) |
| Serum amitriptyline peak concentration (µg/L) | 458 | 412 |
| Serum nortipytilin peak concentrations (µg/L) | 283 | 259 |
| Serum tilidine/nortilidin peak concentrations (µg/L) | 56/849 | NA |
| Urine excretion (mL/days) | 3100 | anuria |
| Urine amitriptyline concentration (µg/L) | 623 | NA |
| Haematocrit (%) | 40 | 36.5 |
| ECTR characteristics | ||
| Modality of ECTR | Intermitted haemodialysis | Intermitted haemodialysis |
| Indication for ECTR | Detoxification | AKI, rhabdomyolysis, detoxification |
| ECTR start after admission (h) | 2.5 | 1.5 |
| Dialyser (material/surface)a | HCO dialyser (polysulfone, 1.8 m2) | High-flux dialyser (polysulfone, 1.3 m2) HCO dialyser (polysulfone, 1.8 m2) |
| Dialysis time (min) | 295 (first treatment) 220 (second treatment) |
320 (first treatment) 775 (second treatment)/350 (HCO) |
| Blood flow (mL/min) | 300 (first treatment) 110 (second treatment) |
240 (high-flux) 240 (high-flux) |
| Dialysate flow (mL/min) | 300 (first treatment) 55 (second treatment) |
240 (high-flux) 240 (high-flux) |
| Ultrafiltration rate (mL/h) | 50 | 50 (first treatment)/50 (second treatment)/200 (HCO) |
| Anticoagulation | Heparin | Heparin |
| Amitriptyline reduction ratio (%)b | 27 | 28 (HCO) |
| ECTR amitriptyline clearance (mL/min)c | 58 | 7 (high-flux) 33 (HCO) |
| Total amount of amitriptyline in the collected dialysate (µg) | 211 | 920 (high-flux) NA (HCO) |
AKI, acute kidney injury; AKIN, acute kidney injury network; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; HPLC, high-performance liquid chromatography; MS/MS, tandem mass spectrometry.
Amitriptyline and nortriptyline were quantified (detection limit 5 µg/L) by HPLC followed by electrospray ionization and mass spectrometric detection and quantification of selected ion fragments (triple quadrupole MS/MS, API2000, PE Sciex) after simple deproteinization with acetonitrile/methanol. Tilidine and its metabolites were similarly quantified (detection limit 5 µg/L) by HPLC-MS (Waters Micromass, Quattro Micro) after fluid extraction with cyclohexane.
aAdditional membrane characteristics are reported elsewhere [11].
| (1) |
| (2) |
Case 1
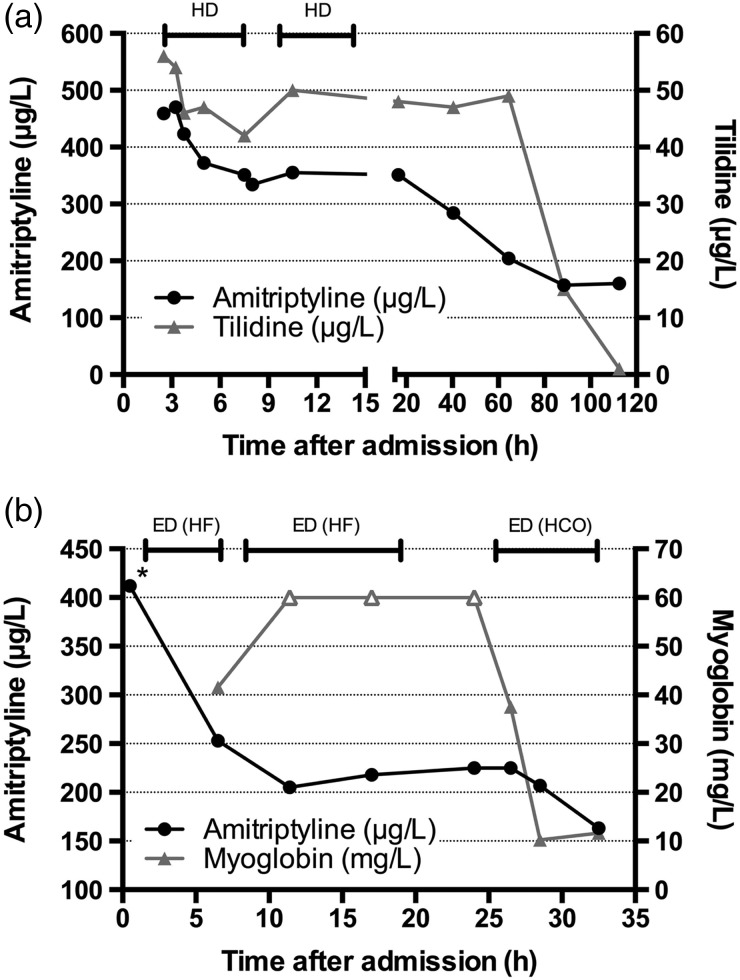
A 54-year-old Caucasian woman, who ingested an unknown amount of amitriptyline and tilidine in a suicide attempt, was admitted to our hospital deeply unconscious with a Glasgow Coma Scale score of 5. Naloxone had no effect. Blood pressure was 140/80 mmHg and heart rate was 94 bpm. Electrocardiography showed a broad QRS complex (134 ms) and prolonged QTc (517 ms), representing increased risk for ventricular arrhythmias. Based on the clinical condition, we started a 5 h dialysis using an HCO EMiC2 dialyser (polysulfone; 1.8 m2) with a dialysate and blood flow of 300 mL/min to eliminate tilidine and other potentially ingested drugs. The treatment was well tolerated, and immediately afterwards a dialysis with low dialysate and blood flow was started with the aim of performing an extended dialysis. Due to clotting of the extracorporeal circuit, this dialysis prematurely ended after 220 min. Plasma concentrations of both substances were drawn at different time points (Figure 1a). Plasma dialyser clearances for amitriptyline and tilidine were 58 and 67 mL/min, respectively, at the beginning and decreased towards the end of the first dialysis session (Supplementary Table S1). For comparison, dialyser clearances for creatinine and urea were 115 and 132 mL/min, respectively. Dialyser reduction ratios, dialyser clearance and total loss into the collected spent dialysate are listed in Supplementary Table S1.
Fig. 1.
(a) Time course of plasma concentrations for amitriptyline and tilidine. (b) Time course of amitriptyline and myoglobin concentrations during HCO dialysis treatment. Open triangles denote myoglobin values at the upper laboratory detection range. *Amitriptyline concentration measured in different laboratories.
Into the sixth hour of treatment, the patient regained consciousness and was transferred to psychiatric care after 3 days in the intensive care unit (ICU).
Case 2
A 53-year-old Caucasian female who ingested ∼1750 mg of amitriptyline and 12 g of ibuprofen in a suicide attempt was admitted to our hospital. The patient was found with a Glasgow Coma Scale score of 5. Blood pressure was 119/72 mmHg and heart rate was 96 bpm. An electrocardiogram showed a regular sinus rhythm. Serum creatinine was 634 µmol/L, sodium 137 mmol/L and creatine kinase 27 308 U/L. Amitriptyline serum concentration was 412 µg/L. The unconscious patient was transferred to our ICU, intubated and mechanically ventilated. Gastroscopy revealed unabsorbed pills in the stomach, and activated charcoal was administered after they were removed. Haemodialysis was started 2 h after admission due to anuric acute kidney injury caused by rhabdomyolysis. Dialysis using an FX60S polysulfone dialyser (Fresenius Medical Care; 1.3 m2) was performed with a blood and dialysate flow of 240 mL/min. After a quick decrease of amitriptyline to 205 µg/L at the start of the second dialysis treatment, amitriptyline concentrations did not show any remarkable decline in the following hours of treatment (Figure 1b). To enhance extracorporeal removal, we employed the HCO EMiC2 dialyser (Fresenius Medical Care, polysulfone; 1.8 m2) with a dialysate and blood flow of 240 mL/min, decreasing amitriptyline to 163 µg/L. The reduction ratio for amitryptilin was 28%. The increased removal was also confirmed by a plasma dialyser clearance of 33 mL/min for amitriptyline and 124 mL/min for myoglobin. Myoglobin serum concentrations decreased from >60 000 µg/L (upper detection limit) to 11 642 µg/L.
Five days after admission, the patient was transferred to the nephrology ward, and renal replacement therapy was stopped after 14 days of intermittent haemodialysis. On renal biopsy, eosinophil interstitial nephritis was identified as the cause of acute kidney injury. Twenty days after initial admission, the patient was transferred to a psychiatric hospital. Of note, therapy with proton pump inhibitors, a therapy the patient had been prescribed for years, can cause both rhabdomyolysis and interstitial nephritis [12].
Discussion
Very recently, the EXTIRP workgroup recommended not performing any extracorporeal treatments (ECTRs) for intoxication with TCAs [9]. This recommendation is based on the fact that conventional haemodialysis is ineffective due to the high protein binding (>90%) and large VOD (14–17 L/kg) and the lipophilic properties of TCA [13]. However, most of the haemodialysis data the workgroup based the recommendation on are from the 1960s and 1970s. Hence, data about the performance of modern membranes in TCA intoxication are limited. The potential benefit of modern means of renal replacement therapy was suggested in a report on haemodiafiltration after acute amitriptyline intoxication in which the patient showed an improved vigilance during treatment. As no amitriptyline blood concentrations were reported, establishing causality for clinical improvement is difficult [14]. Recently, extended dialysis using an HCO dialyser has been introduced to remove large quantities of free light chains in patients with multiple myeloma [11]. These dialysers are characterized by a larger pore size, which allows enhanced middle molecule removal without substantial elimination of albumin [15]. Myoglobin, accumulated in rhabdomyolysis as in one of our cases, is also dialysed by highly permeable membranes [16]. So far, there are only anecdotal reports on the use of HCO dialysers for the removal of toxins [17]. We could show that even with an increase of the amitriptyline dialyser clearance using HCO instead of high-flux dialysis, the estimated total eliminated amount of amitriptyline by HCO dialysis remains low (<0.1% of the ingested dose) and therefore may not exceed 3% of the ingested dose per HCO dialysis session, which is considered to be the lower threshold for dialysability according to EXTRIP criteria [9]. Therefore, amitriptyline can be defined as ‘non-dialysable’, even under HCO dialysis.
An additional factor influencing amitryptilin concentrations is concomitantly ingested drugs, especially those that are metabolized mainly via CYP2D6. Although these drugs, especially pantoprazole in our cases, might have an effect on metabolization, these mechanisms will most likely not affect extracorporeal removal. Notwithstanding, the inherent limitation of only two reported patients, is an important shortcoming of this article. In summary, even the elevated amitriptyline clearance by HCO membranes in the context of extended dialysis is unlikely to confer a clinical benefit, supporting a recent recommendation of EXTRIP to refrain from any ECTR in intoxication with TCAs. As a finding unrelated to the topic of extracorporeal toxin removal, we could show that HCO extended dialysis is very effective in lowering elevated myoglobin in rhabdomyolysis.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Conflicts of interest statement
None declared.
Supplementary Material
References
- 1.Mowry JB, Spyker DA, Cantilena LR, Jr, et al. 2012 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th annual report. Clin Toxicol (Phila) 2013; 51: 949–1229 [DOI] [PubMed] [Google Scholar]
- 2.Hawton K, Bergen H, Simkin S, et al. Toxicity of antidepressants: rates of suicide relative to prescribing and non-fatal overdose. Br J Psychiatry 2010; 196: 354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher LM, Kappatos D, Tisch C, et al. Suicide by poisoning in New Zealand—a toxicological analysis. N Z Med J 2012; 125: 15–25 [PubMed] [Google Scholar]
- 4.Dianat S, Zarei MR, Hassanian-Moghaddam H, et al. Tricyclic antidepressants intoxication in Tehran, Iran: epidemiology and associated factors. Hum Exp Toxicol 2011; 30: 283–288 [DOI] [PubMed] [Google Scholar]
- 5.Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 2011; 44: 195–235 [DOI] [PubMed] [Google Scholar]
- 6.Kerr GW, McGuffie AC, Wilkie S. Tricyclic antidepressant overdose: a review. Emerg Med J 2001; 18: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ash SR, Levy H, Akmal M, et al. Treatment of severe tricyclic antidepressant overdose with extracorporeal sorbent detoxification. Adv Ren Replace Ther 2002; 9: 31–41 [DOI] [PubMed] [Google Scholar]
- 8.Sari I, Turkcuer I, Erurker T, et al. Therapeutic plasma exchange in amitriptyline intoxication: case report and review of the literature. Transfus Apher Sci 2011; 45: 183–185 [DOI] [PubMed] [Google Scholar]
- 9.Yates C, Galvao T, Sowinski KM, et al. Extracorporeal treatment for tricyclic antidepressant poisoning: recommendations from the EXTRIP Workgroup. Semin Dial 2014; 27: 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavergne V, Ouellet G, Bouchard J, et al. Guidelines for reporting case studies on extracorporeal treatments in poisonings: methodology. Semin Dial 2014; 27: 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchison CA, Cockwell P, Reid S, et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol 2007; 18: 886–895 [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol 2013; 6: 443–451 [DOI] [PubMed] [Google Scholar]
- 13.Dargan PI, Colbridge MG, Jones AL. The management of tricyclic antidepressant poisoning: the role of gut decontamination, extracorporeal procedures and fab antibody fragments. Toxicol Rev 2005; 24: 187–194 [DOI] [PubMed] [Google Scholar]
- 14.Ozayar E, Degerli S, Gulec H. Hemodiafiltration: a novel approach for treating severe amitriptyline intoxication. Toxicol Int 2012; 19: 319–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt JJ, Hafer C, Clajus C, et al. New high-cutoff dialyzer allows improved middle molecule clearance without an increase in albumin loss: a clinical crossover comparison in extended dialysis. Blood Purif 2012; 34: 246–252 [DOI] [PubMed] [Google Scholar]
- 16.Sorrentino SA, Kielstein JT, Lukasz A. High permeability dialysis membrane allows effective removal of myoglobin in acute kidney injury resulting from rhabdomyolysis. Crit Care Med 2011; 39: 184–186 [DOI] [PubMed] [Google Scholar]
- 17.Baroke E, Schmidt JJ, Strunk AK, et al. Saving two lives with one dialysis treatment. Clin Nephrol 2015; 84: 104–107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.