Abstract
Background
Extended periods of haemodialysis (HD) can improve patient outcomes. In-centre nocturnal haemodialysis (INHD) should be explored as a method of offering extended periods of HD to patients unsuitable for or unable to perform home therapy.
Methods
Ten self-selecting, prevalent HD patients started an INHD programme to assess feasibility and patient satisfaction. Quality-of-life (QOL) measures were evaluated at enrolment and after 4 months of INHD using the EQ-5D, the Hospital Anxiety and Depression Scale (HADS) and the SF-12 questionnaires. Demographic, biochemical and haematological data and data on dialysis adequacy were collected before starting INHD and after 4 months.
Results
Three of the 10 patients failed to complete the 2-week run-in period. Seven patients completed the 4-month programme, with mean dialysis time of 355 ± 43.92 min throughout the period. The EQ-5D visual analogue score improved from 48 ± 16.89 to 72 ± 13.2 (P = 0.003) and the HADS anxiety score decreased from 9 ± 5.83 to 3.57 ± 3.04 (P = 0.029). The urea reduction ratio improved from 71.57 ± 2.29% to 80.43 ± 3.101% (P < 0.001), with improvements in phosphate control, reducing to within the target range from 1.73 ± 0.6 to 1.2 ± 0.2 (P = 0.08). Ultrafiltration (UF) volumes increased during the study from 2000 ± 510 to 2606 ± 343 mL (P = 0.015); there was a significant reduction in mean UF rate adjusted for body weight from 6.47 ± 1.71 to 4.61 ± 1.59 mL/kg/h (P = 0.032). Sensitivity analyses confirmed the significance of these results.
Conclusions
This single-centre study showed a 4-month programme of extended hours INHD is safe and associated with improvements in QOL measures, decreased UF rates and measures of dialysis adequacy. These data have been used to expand our service and inform the design of future randomized controlled trials to examine medical endpoints.
Keywords: dialysis, in-centre, nocturnal, QOL, ultrafiltration rate
Introduction
The most recent UK renal registry data show the incidence of new patients starting on haemodialysis (HD) increased by 1.2% in 2013 [1]. The morbidity and mortality for patients on maintenance haemodialysis (HD) remains high despite advances in dialysis technologies, with cardiovascular-related deaths the leading cause of death in this patient group [2]. The symptom burden related to dialysis is high [3, 4], including fatigue [5], disordered sleep and pain [6] and depression [7]. Many people on dialysis are not able to continue in paid employment, and patient-reported quality of life is low [8]. Home HD is an excellent way of offering increased flexibility in the way maintenance HD patients dialyse, and currently 1113 patients (4.7% of HD patients) are on a home HD programme in the UK [9]. There are, however, significant barriers that mean home HD is not possible for many patients, including physician-related factors (e.g. lack of knowledge and expertise) and patient-related factors (e.g. lack of awareness, fears and anxieties about dialysing away from medical care, perceived care-giver burden and specific fears for some about self-cannulation) [10].
The large numbers of patients currently undertaking unit-based HD programmes in the UK means there is less and less flexibility in the way dialysis can be offered, with rigid programmes of 4 h three times a week in designated time slots that are difficult to manipulate or alter. This has significant implications for patient quality of life and satisfaction arising from dialysis therapy. In this time of austerity, finding alternative ways of delivering effective dialysis programmes that use existing resources and facilities can only be of benefit to our patients.
In-centre nocturnal HD (INHD) offers patients the opportunity to dialyse overnight while asleep for extended periods of time and in a number of small studies has been shown to improve a wide range of clinical parameters, including phosphate and anaemia control [11–14], blood pressure [15–17], left ventricular mass [18–20] and other cardiovascular measures [21–23]. Furthermore, INHD has been reported to significantly improve or maintain aspects of quality of life and cognition [11, 19, 24, 25]. One aspect widely reported to improve is sleep quality, which has been shown by a number of groups [25–28]. There is good evidence that these benefits are derived from more than just an increased dose of dialysis, as simply increasing urea Kt/V during standard HD to >1.3 does not improve outcomes in the same way. Moreover, the benefits of increasing dialysis duration are indicated by the reduced rates of mortality seen in Tassin, France, where 8-h sessions thrice weekly are common [29, 30]. As this may suggest, there is evidence that INHD may specifically lead to a reduction in all-cause mortality [11, 16, 31].
In response to patient feedback, we set up a pilot project to explore the possibility of delivering an INHD programme at the Leicester General Hospital Outpatient Dialysis Unit. Currently we are the only NHS centre to run an INHD programme in the UK. We aimed to set up a service that was cost-effective, deliverable, sustainable, safe, improved patient satisfaction and was acceptable to patients and staff. It is hoped that the outcomes from this service evaluation will support the expansion of this programme and inform the need for research into the outcomes of patients who embark on a programme of INHD.
Methods
In the summer of 2014, 10 prevalent HD patients switched from standard daytime in-centre HD of 4 h three times per week to INHD. All patients arrived for dialysis late in the evening (10 pm onwards) and dialysed in hospital beds on the dialysis unit adjacent to the hospital as outpatients. Initially patients were dialysed for 360–390 min, and the period of dialysis was extended thereafter with patient agreement to a maximum of 480 min. This was in response to our annual dialysis patient satisfaction survey, where many patients expressed a desire for increased flexibility around dialysis schedules. After cost analysis and the logistics of providing dialysis staff were worked out, 10 patients who were all established on HD for >3 months were switched to a nocturnal programme. These patients volunteered for the programme and the switch was made electively. Patients were given a 2-week run-in period where their daytime slots were held for them in case they wanted to revert to daytime dialysis in the event they found INHD unsuitable. Staffing levels overnight were maintained at the same ratios as if patients were dialysing during the day.
Quality-of-life assessments
At the time of switching to INHD, patient-reported quality-of-life outcomes were recorded using three validated questionnaires:
The EQ-5D is a standardized measure of health status developed by the EuroQol Group that provides a simple measure of health for clinical and economic appraisal [32]. The EQ-5D consists of two pages—the EQ-5D descriptive system and the EQ visual analogue scale (EQ VAS). The descriptive system comprises five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The EQ VAS records the respondent's self-rated health on a vertical, visual analogue scale where the endpoints are labelled ‘Best imaginable health state’ and ‘Worst imaginable health state’. This is a quantitative measure of health outcome as judged by the individual respondents [32, 33].
The Hospital Anxiety and Depression Scale (HADS) is a reliable and reproducible instrument for detecting states of depression and anxiety [34]. It contains both depression (HADSd) and anxiety components (HADSa), and the subscales are valid measures of the severity of emotional disorder.
The 12-item Short-Form Health Survey (SF-12) is a generic health-related quality of life (HRQoL) questionnaire that consists of 12 questions related to physical and mental health status, providing a physical component summary (PCS) and a mental component summary [35].
Patients completed the same questionnaires after 4 months of INHD and were also asked to record their thoughts and feelings about switching to INHD, particularly any anxieties or hopes before starting and any positive and negative feelings or events encountered during the 4-month period on INHD. They were asked to self-assess whether their anxieties were misplaced, their hopes and expectations were met and to critique their positive and negative experiences as well as give an overall impression of their experience of INHD and how it had changed their daily lives. These were explored and recorded from individual informal conversations between patients and the clinic staff.
Biochemical data, haematological data and measures of dialysis adequacy
Biochemical and haematological data and data on dialysis duration and adequacy were collected, as well as information on pre-dialysis blood pressures and ultrafiltration (UF) rates/volumes. These were compared with results after 4 months. Demographic data and medications information were also collected at the time of changing to INHD and after 4 months.
Statistics
Statistical analysis with SPSS version 22 was used for analysis. Paired t-tests were used to assess differences between patient questionnaire scores at the start and end of the study and to assess for differences between biochemical and haematological results, as well as between measures of dialysis adequacy. Missing data were assumed to be ‘missing at random’ (MAR), with the potential to bias the results. Sensitivity analyses were undertaken to investigate the impact of MAR data from patients who did not complete the 4-month programme. Missing data were handled using the mean imputation method [36].
Results
Patient demographic data
The initial 10 patients included 2 females and 8 males with a mean age of 51.9 years (range 21–75). The baseline demographic data of the initial 10 patients are shown in Table 1. Three of the 10 patients failed to complete the 2-week run-in period and reverted to their daytime dialysis slots. Nine of the 10 patients dialysed via native arteriovenous fistulae and one with a synthetic graft, and all were needled with the rope ladder technique.
Table 1.
Baseline characteristics of all 10 study participants initially enrolled in the study
| Parameter | Mean (±SD) |
|---|---|
| Age (years) | 51.9 (±17.37) |
| Men/women | 8/2 |
| Dialysis vintage (months) | 30.9 (±32.73) |
| Initial systolic blood pressure (mmHg) | 144.7 (±19.52) |
| Initial diastolic blood pressure (mmHg) | 76.1 (±12.31) |
| Initial calcium (mmol/L) | 2.34 (±0.12) |
| Initial phosphate (mmol/L) | 1.76 (±0.52) |
| URR (%) | 71.8 (±2.39) |
| Haemoglobin (g/L) | 105.9 (±18.38) |
| Dry weight (kg) | 81.14 (±16.74) |
Quality-of-life scores
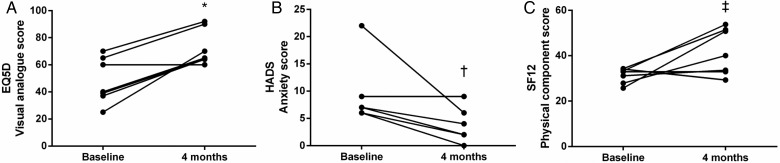
There were improvements in all quality-of-life scores, though some did not meet statistical significance. These are shown in Table 2. The mean EQ-5D visual analogue score improved from 48 ± 16.89 to 72 ± 13.2 (P < 0.01), with the mean HADS anxiety score decreasing from 9 ± 5.83 to 3.57 ± 3.04 (P = 0.029). There was also a trend towards significant improvement in mean SF-12 PCS scores from 31.31 ± 3.32 to 41.69 ± 10.19 (P = 0.052) (see Figure 1). Sensitivity analyses did not alter the significance of the results (see Table 3).
Table 2.
Change between start and end of study period for selected outcomes measured
| Variable | Pre-INHD (mean ± SD) | After 4 months of INHD (mean ± SD) | P-value (paired t-test) |
|---|---|---|---|
| EQ-5D descriptive score | 0.473 (±0.397) | 0.763 (±0.152) | 0.096 |
| EQ-5D visual analogue score | 48 (±16.89) | 72 (±13.2) | <0.01* |
| HADS anxiety score | 9 (±5.83) | 3.57 (±3.04) | 0.029* |
| HADS depression score | 8.29 (±4.19) | 4 (±2.94) | 0.065 |
| SF-12 physical component score | 31.31 (±3.32) | 41.69 (±10.19) | 0.052 |
| SF-12 mental component score | 41.1 (±14.85) | 50.9 (±8.09) | 0.152 |
| URR (%) | 71.57 (±2.3) | 80.43 (±3.1) | <0.001* |
| Phosphate (mmol/L) | 1.73 (±0.6) | 1.2 (±0.2) | 0.08 |
| Adjusted calcium (mmol/L) | 2.33 (±0.1) | 2.30 (±0.1) | 0.637 |
| Haemoglobin (g/dL) | 104.7 (±20.9) | 108.0 (±16.6) | 0.582 |
| Pre-HD systolic BP (mmHg) | 143.3 (±22.2) | 140.0 (±11.5) | 0.644 |
| Pre-HD diastolic BP (mmHg) | 73.6 (±11.4) | 74.3 (±9.3) | 0.875 |
| Absolute UF rate (mL/h) | 513.12 (±121.43) | 355.79 (±66.37) | 0.026* |
| Relative UF rate (mL/kg/h) | 6.47 (±1.71) | 4.61 (±1.59) | 0.032* |
| Total UF volume (mL) | 2000 (±510) | 2606 (±343) | 0.015* |
Patients who did not complete the 4-month programme were excluded from analysis.
*Statistically significant differences on paired t-test.
Fig. 1.
Improvements in quality-of-life scores: (A) EQ-5D visual analogue score, *P < 0.01; (B) HADS anxiety score, †P = 0.029; (C) SF-12 physical component score, ‡P = 0.052.
Table 3.
Sensitivity analysis: comparison of standard and adjusted data to account for potential bias from non-completing subjects and missing data
| Variable | MD (95% CI), P-value [standard analysis] | MD (95% CI), P-value [adjusted analysis] |
|---|---|---|
| EQ-5D visual analogue score | 24 (12.0, 36.3), P < 0.01 | 24.1 (16.4, 31.8), P < 0.001 |
| HADS anxiety score | −5.43 (−10.1, −0.78), P = 0.029 | −5.3 (−8.2, −2.4), P < 0.01 |
| SF-12 physical component score | 10.37 (0.1, 20.8), P = 0.052 | 10.4 (2.8, 17.9), P = 0.01 |
| URR (%) | 8.86 (6.98, 10.74), P < 0.001 | 8.62 (7.02, 10.22), P < 0.001 |
| Absolute UF rate (mL/h) | −157.33 (−288.1, −26.6), P = 0.026 | −157.53 (−240.1, −74.9), P = 0.02 |
| Relative UF rate (mL/kg/h) | −1.86 (−3.5, −0.2), P = 0.032 | −1.86 (−2.9, −0.8), P = 0.03 |
| Total UF volume (mL) | 606 (165, 1045), P = 0.015 | 607 (329, 885), P = 0.01 |
Results expressed as mean difference (MD) and 95% confidence interval (CI) with P-values for standard and adjusted analyses. There were no observed differences in significance.
Dialysis duration, pump speed and UF rates
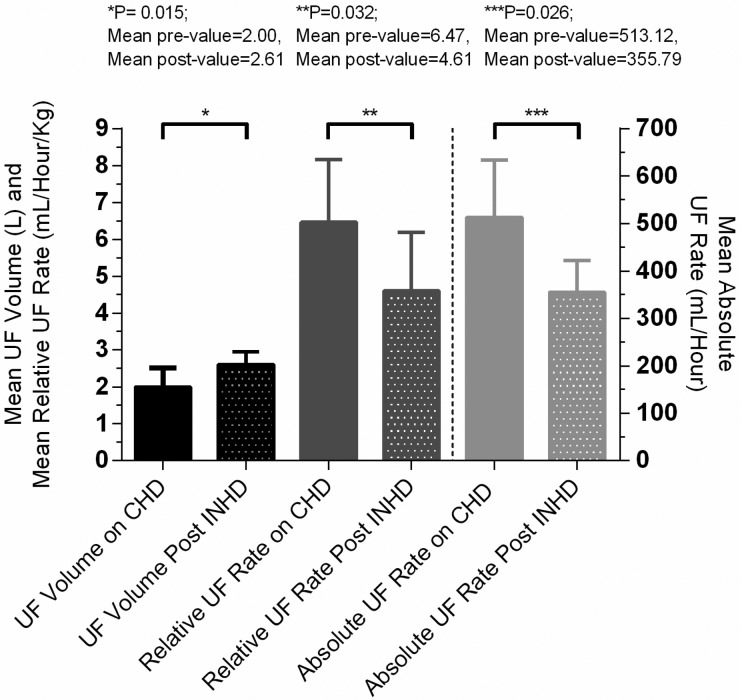
The mean dialysis duration was 355 min (SD ± 43.92); all patients were dialysed with a blood pump speed of 300 mL/min and a dialysate flow rate of 500 mL/min. The mean UF rates (mL/h) and mean UF rates adjusted for body weight (mL/kg/h) before starting INHD (when patients were still on conventional therapy) and at the end of the 4-month study period were compared. Total UF volumes (mL) were recorded between the start and the end of the study period by taking the average UF volume of the last 10 dialysis sessions before starting INHD and the last 10 sessions at the end of the 4-month period of INHD (Table 2). As anticipated, there was a significant reduction in the mean absolute UF rates from 513.12 mL/h (SD ± 121.43) on conventional daytime dialysis to 355.79 mL/h (SD ± 66.37) on INHD (P = 0.026). There was also a significant decrease in the mean relative UF rate (adjusted for body weight) from 6.47 (SD ± 1.71) to 4.61 mL/kg/h (SD ± 1.59) (P = 0.032). These two findings occurred despite the mean total UF volume increasing from 2000 (SD ± 510) to 2606 mL (SD ± 343) (P = 0.015) (Figure 2). The validity of these results was again confirmed with sensitivity analyses (see Table 3).
Fig. 2.
Mean changes in UF volumes (L), relative UF rates (mL/h/kg) and absolute UF rates (mL/h) between prior to starting INHD and after 4 months on INHD. CHD, conventional haemodialysis.
Dialysis adequacy, biochemical and haematological changes
Dialysis ‘adequacy’ was assessed by small molecule clearance, and urea reduction ratio (URR) was used as a surrogate marker for this as well as pre-dialysis systolic and diastolic blood pressures. Biochemical and haematological changes and changes in dialysis adequacy are summarized in Table 2.
Dialysis adequacy as measured by URR improved significantly from 71.57 ± 2.29 to 80.43 ± 3.101% (P < 0.001; see Table 3). There was a trend towards improved phosphate control, with the mean value reducing to within current therapeutic targets from 1.73 ± 0.6 to 1.2 ± 0.2 (P = 0.08), with no change in doses, numbers, or type of oral phosphate binders. There were no observed differences in mean haemoglobin or erythropoietin dose, mean plasma calcium or mean pre-HD systolic or diastolic BP, but this was a pilot study and not powered to detect such differences.
Patients' experience
From the comments collected from patients throughout the study period, the patient experience has been extremely good. Positive changes reported by the seven patients who completed 4 months of INHD included
increased energy levels in the daytime,
having more interest in day-to-day activities,
having more time in the daytime for social and leisure activities,
helping them feel like they have a more ‘normal life’,
finding it easier to get jobs done in the daytime,
not having to schedule all life events around dialysis.
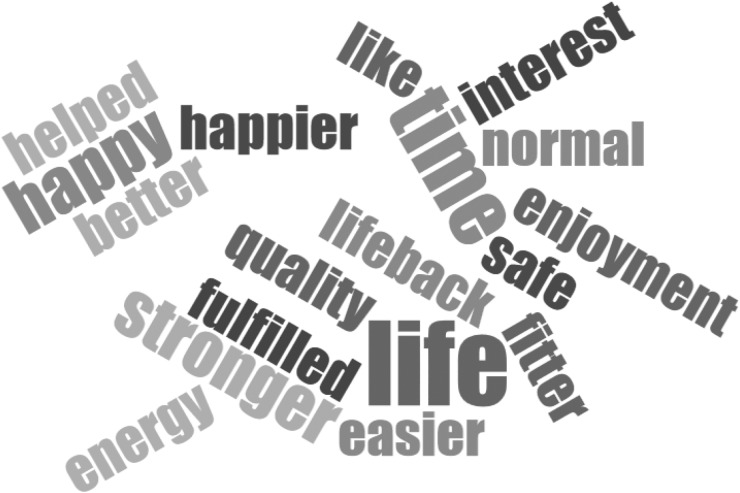
Several patients said that they felt like they had 3 more days of life, and the increased energy levels meant they were more productive in this time. Several also reported that it was the closest they would ever get to ‘having a transplant’. All but one patient said that, given the option, they would never want to return to daytime dialysis again. A word cloud illustrating the key positive themes extracted from patient feedback is shown in Figure 3. There were very few negative comments about the INHD experience from the patients who completed the 4-month programme. One patient reported not being able to sleep well while on dialysis and waited until HD had finished before sleeping fully. A different patient reported being occasionally disturbed from sleep by machine alarms or other patients, but they considered these to be minor problems and they did not deter them from wanting to continue the programme. All seven patients have continued on INHD after the study finished.
Fig. 3.
Key positive themes extracted from informal patient feedback.
Patients who stopped INHD
Three patients abandoned INHD before the end of the 2-week run-in period. The reasons given for changing back to conventional HD were poor quality of sleep, still feeling anxious and unable to relax while asleep for fear of needle dislodgement and did not like spending 3 nights a week away from family members.
There were no adverse events reported during the study period. One patient was admitted to hospital from the dialysis unit for treatment of pneumonia but was discharged 3 days later straight back to their nocturnal slots.
Discussion
Our pilot data show that INHD is deliverable, safe and has a positive impact on patient-related quality-of-life measures, including anxiety and self-reported health and well-being. Although it was not originally intended to look at dialysis-related outcome measures, we observed an improvement in phosphate control, with a mean reduction to within current guideline targets. This finding is consistent with the improvements in serum phosphate reported in other studies of INHD [11, 14, 17, 23]. Although the literature suggests improvements in phosphate that accompany extended periods of HD are also accompanied by a reduction in phosphate binders [13, 15, 25], our sample size was small, the time scale was relatively short and this finding was not corroborated.
Patient quality of life is a relatively under-researched and -reported outcome in dialysis patients. Several studies have looked at the effects of INHD and quality-of-life measures. Ok et al. [11] found that INHD preserved quality of life compared with conventional unit-based HD, whereas Bugeja et al. [25] and Van Eps et al. [37] found significant improvements in a number of domains, including frequency of dialysis-associated symptoms, sleep quality and energy levels. INHD has been shown to restore melatonin rhythm [26] and reduce sleep apnoea by increasing pharyngeal size [28]. In the only study of its kind, Jassal et al. [24] reported that INHD was associated with significant improvements in multiple aspects of cognition, although this study included only 12 patients.
Previous studies looking at determinants of dialysis-induced cardiac injury found that larger UF volumes (>2.1 L) were strongly associated with myocardial stunning as assessed by new regional wall abnormalities on echocardiogram [38], but a subsequent study looking at the effects of frequent HD on dialysis-induced cardiac injury suggested a strongly positive relationship between rate of UF and myocardial stunning [39]. Although we observed an increase in the total UF volumes between the start and the end of the study period, the significant reduction in UF rates may negate the increase in total volume and this hypothesis needs testing in future patients undergoing INHD.
Typically, HD patients have significantly reduced aerobic capacities compared with age-matched controls [40, 41]. Due to the time commitments associated with conventional unit-based dialysis, it seems logical that if treatment is conducted at night, then people may be more active during the day, thus preventing the severe decline of aerobic capacity. Chan et al. [14] investigated aerobic capacity in frequent home HD patients and found that after switching to nocturnal dialysis, patients significantly increased aerobic capacity as assessed by graded ergometer testing. This solitary observation is very promising, although, again, it was conducted with a relatively small cohort.
Two case–control trials investigated mortality and survival as a primary outcome in INHD patients, whereas one randomized controlled trial (RCT) investigated death rates in frequent home nocturnal HD patients. Evidence from the two case–control studies suggests that INHD leads to significant reductions in mortality rates, reporting hazard ratios of 0.28 [11] and 0.7 [16] versus conventional unit-based HD patients. The Frequent Haemodialysis Network (FHN) RCT did not show a significant reduction in mortality; however, their nocturnal dialysis study group was relatively small, with only 45 patients [17]. As well as reduced mortality, another case–control study highlighted a significant reduction in hospitalization rates, even when corrected for the type of vascular access [16].
There have been a number of studies suggesting that patients on INHD have significant improvements in blood pressure, and in most cases with a reduced number of blood pressure medications [11, 12, 14–17, 23, 25]. In addition to this, a meta-analysis of four studies on the effects of extended HD duration on left ventricular mass showed significant reductions after switching regime [42], which is supported by further recent studies [43, 44]. In addition to improving blood pressure and reducing left ventricular hypertrophy, one study also found a reduction in myocardial fibrosis [23]. The reductions we observed in UF rates intuitively suggest this should cause less myocardial stunning, which is related to UF volume and rate in dialysis patients [38] and is thought to contribute to myocardial fibrosis.
It is reported that haemoglobin, like phosphate, is consistently better in patients on INHD compared with patients on standard unit-based HD [11, 13–16], and there are studies reporting a reduction in the use of erythropoietin stimulating agents to maintain target haemoglobin [11, 23, 25]. There are also studies that suggest iron stores are maintained closer to the target range [12, 13, 25], but it must be noted that the patients included in many of these studies are not representative of the general dialysis population. Our study found no differences in haemoglobin or phosphate, but this is most likely due to the small number of patients.
As expected, and consistent with our results, longer periods of dialysis have led to increasing ‘quality’ of dialysis as measured by small molecule clearance. Several studies have shown significant improvements in Kt/V [14, 15, 25], and one study used URR as a surrogate marker for this [13]. A study by Ok et al. [11] showed that while the levels of β2 microglobulin increased in patients on conventional unit-based HD, it was unchanged in patients on INHD [11] and van Eps et al. actually showed improvements in levels of β2 microglobulin in patients on INHD [37]. These improvements come despite the reductions in blood pump speeds and in some cases dialysate flow rates.
One potential drawback of nocturnal dialysis, which was highlighted in the FHN study, is a trend of increased fistula complications [17]. The study found that people in the nocturnal arm of their trial had an increased incidence of complications. This could, however, be related to frequency of use rather than duration, as no other study reported this finding and their protocol used frequent nocturnal dialysis with patients dialysing 5 days per week.
There are important limitations to this study. This study was entirely observational in nature, designed to assess the tolerability of a programme of INHD and to ensure the service was sustainable and deliverable. It is a single-centre experience of just 10 self-selected patients and changes were assessed over a 4-month period only. This study was not specifically powered to detect changes in medical and dialysis related outcomes. Nevertheless, we have shown that INHD leads to significant improvements in a number of domains that are consistent with other published data. Moreover, sensitivity analysis has demonstrated that our results remain significant after adjusting for the impact of the missing data from the three patients who did not complete the programme.
Conclusion
This experience shows, once again, that the best dialysis modality is the one chosen by the patient, in line with their needs and expectations. INHD should be offered alongside all other traditional dialysis schedules on the basis that, for some, it will enhance the patient experience.
Further controlled trials are needed to fully elucidate the effect of INHD on both medical, psychological and quality-of-life outcomes.
Authors’ roles
M.G.B. drafted the manuscript and was involved in data collection, statistical analysis and final preparation. D.C. drafted the manuscript and was involved in statistical analysis. A.S. contributed to manuscript revision. R.B. contributed to the conceptual design and manuscript revision. J.B. contributed to the conceptual design and final approval of the manuscript.
Funding
This study was supported by the National Institute for Health Research Leicester Cardiovascular Biomedical Research Unit based at University Hospitals of Leicester and the University of Leicester.
Conflicts of interests
None declared.
Acknowledgements
This study was granted approval by the clinical audit standards and effectiveness team at the University Hospitals of Leicester (Project number 7479). We acknowledge the hard work by all the nursing and management staff at University Hospitals Leicester NHS trust in helping to set up and ensure the ongoing success of this programme. This work is original and has not been previously published elsewhere.
References
- 1.Gilg J, Pruthi R, Fogarty D. UK Renal Registry 17th Annual Report: Chapter 1 UK renal replacement therapy incidence in 2013: national and centre-specific analyses. Nephron Physiol 2015; 129(Suppl 1): 1–29 [DOI] [PubMed] [Google Scholar]
- 2.Steenkamp R, Rao A, Roderick P. UK Renal Registry 17th Annual Report: Chapter 5 survival and cause of death in UK adult patients on renal replacement therapy in 2013: national and centre-specific analyses. Nephron Physiol 2015; 129(Suppl 1): 99–129 [DOI] [PubMed] [Google Scholar]
- 3.Davison S, Jhangri G, Johnson J. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int 2006; 69: 1621–1625 [DOI] [PubMed] [Google Scholar]
- 4.Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage 2010; 39: 477–485 [DOI] [PubMed] [Google Scholar]
- 5.Jhamb M, Argyropoulos C, Steel JL, et al. Correlates and outcomes of fatigue among incident dialysis patients. Clin J Am Soc Nephrol 2009; 4: 1779–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unruh ML, Sanders MH, Redline S, et al. Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis 2008; 52: 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedayati S, Bosworth H, Kuchibhatla M, et al. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int 2006; 69: 1662–1668 [DOI] [PubMed] [Google Scholar]
- 8.Cruz MC, Andrade C, Urrutia M, et al. Quality of life in patients with chronic kidney disease. Clinics 2011; 66: 991–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao A, Casula A, Castledine C. UK Renal Registry 17th Annual Report: Chapter 2 UK renal replacement therapy prevalence in 2013: national and centre-specific analyses. Nephron Physiol 2015; 129(Suppl 1): 31–56 [DOI] [PubMed] [Google Scholar]
- 10.Tennankore KK, Chan CT, Curran SP. Intensive home haemodialysis: benefits and barriers. Nat Rev Nephrol 2012; 8: 515–522 [DOI] [PubMed] [Google Scholar]
- 11.Ok E, Duman S, Asci G, et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dial Transplant 2011; 26: 1287–1296 [DOI] [PubMed] [Google Scholar]
- 12.David S, Kuempers P, Eisenbach GM, et al. Prospective evaluation of an in-centre conversion from conventional haemodialysis to an intensified nocturnal strategy. Nephrol Dial Transplant 2009; 24: 2232–2240 [DOI] [PubMed] [Google Scholar]
- 13.Powell JR, Oluwaseun O, Woo YM, et al. Ten years experience of in-center thrice weekly long overnight hemodialysis. Clin J Am Soc Nephrol 2009; 4: 1097–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CT, Notarius CF, Merlocco AC, et al. Improvement in exercise duration and capacity after conversion to nocturnal home haemodialysis. Nephrol Dial Transplant 2007; 22: 3285–3291 [DOI] [PubMed] [Google Scholar]
- 15.Alloatti S, Molino A, Manes M, et al. Long nocturnal dialysis. Blood Purif 2002; 20: 525–530 [DOI] [PubMed] [Google Scholar]
- 16.Lacson E, Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol 2012; 23: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocco MV, Lockridge RS, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int 2011; 80: 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol 2001; 12: 2759–2767 [DOI] [PubMed] [Google Scholar]
- 19.Walsh M, Culleton B, Tonelli M, et al. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int 2005; 67: 1500–1508 [DOI] [PubMed] [Google Scholar]
- 20.Fagugli RM, Pasini P, Quintaliani G, et al. Association between extracellular water, left ventricular mass and hypertension in haemodialysis patients. Nephrol Dial Transplant 2003; 18: 2332–2338 [DOI] [PubMed] [Google Scholar]
- 21.Barraclough N, Mooney D, Mullins K, et al. Improved cardiac structure and function in a patient transitioned to in-centre nocturnal haemodialysis. Nephrology 2012; 17(Suppl. 2): 89–9621854501 [Google Scholar]
- 22.Chan C, Floras J, Miller J, et al. Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant 2002; 17: 1518–1521 [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Rong S, Mei C, et al. Effects of thrice-weekly in-center nocturnal vs. conventional hemodialysis on integrated backscatter of myocardial tissue. Hemodial Int 2011; 15: 200–210 [DOI] [PubMed] [Google Scholar]
- 24.Jassal S, Devins G, Chan C, et al. Improvements in cognition in patients converting from thrice weekly hemodialysis to nocturnal hemodialysis: a longitudinal pilot study. Kidney Int 2006; 70: 956–962 [DOI] [PubMed] [Google Scholar]
- 25.Bugeja A, Dacouris N, Thomas A, et al. In-center nocturnal hemodialysis: another option in the management of chronic kidney disease. Clin J Am Soc Nephrol 2009; 4: 778–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch BC, Hagen EC, Nagtegaal JE, et al. Effects of nocturnal hemodialysis on melatonin rhythm and sleep-wake behavior: an uncontrolled trial. Am J Kidney Dis 2009; 53: 658–664 [DOI] [PubMed] [Google Scholar]
- 27.Koch BC, van der Putten K, Van Someren EJ, et al. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study). Nephrol Dial Transplant 2010; 25: 513–519 [DOI] [PubMed] [Google Scholar]
- 28.Beecroft JM, Hoffstein V, Pierratos A, et al. Nocturnal haemodialysis increases pharyngeal size in patients with sleep apnoea and end-stage renal disease. Nephrol Dial Transplant 2008; 23: 673–679 [DOI] [PubMed] [Google Scholar]
- 29.Charra B, Chazot C, Jean G, et al. Long 3×8 hr dialysis: a three-decade summary. J Nephrol 2003; 16(Suppl 7): S64–S69 [PubMed] [Google Scholar]
- 30.Laurent G, Charra B. The results of an 8 h thrice weekly haemodialysis schedule. Nephrol Dial Transplant 1998; 13(Suppl 6): 125–131 [DOI] [PubMed] [Google Scholar]
- 31.Lacson E, Jr, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol 2010; 5: 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group TE. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208 [DOI] [PubMed] [Google Scholar]
- 33.Brooks R, EuroQol Group EuroQol: the current state of play. Health Policy 1996; 37: 53–72 [DOI] [PubMed] [Google Scholar]
- 34.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370 [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Gnanalingham K, Casey A, et al. Quality of life assessment using the Short Form-12 (SF-12) questionnaire in patients with cervical spondylotic myelopathy: comparison with SF-36. Spine (Phila Pa 1976) 2006; 31: 639–643 [DOI] [PubMed] [Google Scholar]
- 36.Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol 2013; 13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Eps CL, Jeffries JK, Johnson DW, et al. Quality of life and alternate nightly nocturnal home hemodialysis. Hemodial Int 2010; 14: 29–38 [DOI] [PubMed] [Google Scholar]
- 38.Burton JO, Jefferies HJ, Selby NM, et al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009; 4: 914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jefferies HJ, Virk B, Schiller B, et al. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol 2011; 6: 1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smart N, Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology 2011; 16: 626–632 [DOI] [PubMed] [Google Scholar]
- 41.Smart N, McFarlane J, Cornelissen V. The effect of exercise therapy on physical function, biochemistry and dialysis adequacy in haemodialysis patients: a systematic review and meta-analysis. Open J Nephrol 2013; 3: 25 [Google Scholar]
- 42.Susantitaphong P, Koulouridis I, Balk EM, et al. Effect of frequent or extended hemodialysis on cardiovascular parameters: a meta-analysis. Am J Kidney Dis 2012; 59: 689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wald R, Yan AT, Perl J, et al. Regression of left ventricular mass following conversion from conventional hemodialysis to thrice weekly in-centre nocturnal hemodialysis. BMC Nephrol 2012; 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knap B, Večerić-Haler Ž, Benedik M, et al. Fibroblast growth factor 23 and left ventricular mass index in maintenance hemodialysis patients: standard versus long nocturnal hemodialysis. Ther Apher Dial 2013; 17: 407–411 [DOI] [PubMed] [Google Scholar]