Abstract
Chronic kidney disease (CKD) is a public health problem. Although physical activity is essential for the prevention and treatment of most chronic diseases, exercise is rarely prescribed for CKD patients. The objective of the study was to search for and appraise evidence on the effectiveness of exercise interventions on health endpoints in CKD patients. A systematic review was performed of randomized clinical trials (RCTs) designed to compare exercise with usual care regarding effects on the health of CKD patients. MEDLINE, EMBASE, Cochrane Central, Clinical Trials registry, and proceedings of major nephrology conference databases were searched, using terms defined according to the PICO (Patient, Intervention, Comparison and Outcome) methodology. RCTs were independently evaluated by two reviewers. A total of 5489 studies were assessed for eligibility, of which 59 fulfilled inclusion criteria. Most of them included small samples, lasted from 8 to 24 weeks and applied aerobic exercises. Three studies included only kidney transplant patients, and nine included pre-dialysis patients. The remaining RCTs allocated hemodialysis patients. The outcome measures included quality of life, physical fitness, muscular strength, heart rate variability, inflammatory and nutritional markers and progression of CKD. Most of the trials had high risk of bias. The strongest evidence is for the effects of aerobic exercise on improving physical fitness, muscular strength and quality of life in dialysis patients. The benefits of exercise in dialysis patients are well established, supporting the prescription of physical activity in their regular treatment. RCTs including patients in earlier stages of CKD and after kidney transplantation are urgently required, as well as studies assessing long-term outcomes. The best exercise protocol for CKD patients also remains to be established.
Keywords: chronic kidney disease, dialysis, exercise, physical activity
Introduction
Chronic kidney disease (CKD) is a current public health problem associated with progression to end-stage renal disease (ESRD), cardiovascular disease and increased mortality rates. The disease has a progressive course, and it is estimated that for every patient on renal replacement therapy (RRT) there are 20–25 patients with milder kidney damage [1].
The risk of cardiovascular events increases proportionally with the decline of glomerular filtration, reaching rates 10–20 times higher than in the general population among ESRD patients [1]. The mortality rate of CKD patients is 15–30 times higher than that of healthy individuals. The disease is also associated with greater health expenditures [2] and lower health-related quality of life (HRQOL) [3].
Physical activity is one of the key elements for the prevention of chronic diseases. Among the general population, physical activity reduces the risk of complex chronic diseases, particularly ischemic heart disease, contributes to blood pressure and glucose control and improves the HRQOL [3].
The prescription of exercise for CKD patients is less usual than for other chronic diseases. This is noteworthy, considering that physical activity levels among CKD patients are significantly lower than among healthy individuals [4]. Moreover, low aerobic capacity, a physical fitness marker that can be improved by exercise, has been pointed to as the strongest predictor of mortality among ESRD patients [5]. Assuming that the benefits of exercise could also apply to CKD patients, physical activity deserves to be considered as a major component of treatment in all stages of the disease [4].
In order to critically appraise the evidence currently available on the issue, we conducted a systematic literature review on the effectiveness of exercise interventions among CKD patients.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement for the conduct of meta-analyses of intervention studies was followed [6].
Eligibility criteria were randomized controlled trials (RCT) evaluating any type of exercise intervention, including advising for physical activity practice, in CKD patients, regardless of their disease stage. The studies based on the same sample, but with different outcomes were included. Only studies with adults (≥18 years) were selected.
Studies on the acute effects of exercise (intervention lasting <8 weeks) and/or quasi-experimental studies were excluded.
Literature search
A search for articles up to and including June 2015 was made from MEDLINE (accessed via PubMed) and EMBASE; we combined these search results with searches of the Cochrane Central Register, and clinical trials registry databases. Conference proceedings abstracts also were hand searched (American Society of Nephrology from 2003 to 2014, European Renal Association–European Dialysis and Transplant Association from 2002 to 2014 and World Congress of Nephrology from 2001 to 2012).
The initial search included terms such as ‘exercise’, ‘physical activity’, ‘chronic renal disease’ and related entry terms associated with a high-sensitivity strategy search. Most of the eligible studies were found on the PubMed database. By the specific search strategy used for the PubMed database, we used the following terms:
((((((((((exertion, physical[MeSH Terms]) OR exercise[MeSH Terms]) OR ‘exercise therapy’) OR physical activity[MeSH Terms]) OR physical fitness[MeSH Terms]) OR resistance training[MeSH Terms]) OR aerobic exercise[MeSH Terms]) OR exercise[MeSH Terms])) OR (((((((((((((((((exercise) OR ‘exercise training’) OR ‘physical activity’) OR ‘aerobic exercise’) OR ‘aerobic training’) OR ‘resistance program’) OR ‘resistance exercise’) OR ‘resistance training’) OR ‘aerobic program’) OR ‘endurance exercise’) OR ‘endurance training’) OR ‘endurance program’) OR ‘physical activity’) OR ‘physical activities’) OR ‘exercise therapy’) OR ‘exercise test’) OR ‘physical rehabilitation’))).
(((((((((((((renal dialysis[MeSH Terms]) OR uremia[MeSH Terms]) OR renal replacement therapy[MeSH Terms]) OR hemodialysis[MeSH Terms]) OR hemodialyses[MeSH Terms]) OR dialysis[MeSH Terms]) OR chronic kidney failure[MeSH Terms]) OR renal insufficiency[MeSH Terms]) OR kidney failure[MeSH Terms]) OR kidney transplantation[MeSH Terms]) OR dialyses[MeSH Terms])) OR ((((((((((((uremia) OR ‘renal replacement therapy’) OR hemodialysis) OR hemodialyses) OR dialysis) OR dialyses) OR ‘chronic kidney failure’) OR ‘chronic kidney disease’) OR ‘renal insufficiency’) OR ‘kidney failure’) OR ‘renal disease’) OR ‘kidney transplantation’)).
Data extraction
The articles identified in the literature search were screened by two independent extractors (F.C.B and M.B) who were blinded to authorship. The initial screening was based on only titles and abstracts. After that, the full text of potentially eligible articles was evaluated. Data extraction of selected RCTs was performed by two independent reviewers (F.C.B and M.B). Discrepancies between the two extractors were discussed until consensus was reached.
Outcome measures
This review focused on clinically relevant outcomes, measured using physiological and psychological variables associated with progression and complications of CKD.
Primary outcomes:
Physical fitness: aerobic capacity, muscular strength;
Health-related quality of life (measured through well-established, reliable and validated instruments);
Cardiovascular dimensions: heart rate variability (HRV) index, mean RR, mean standard deviation of normal-to-normal intervals (SDNN), pulse wave velocity (PWV) and arterial stiffness;
Nutritional measures: body composition (visceral fat, waist circumference and leg lean mass), body mass index, waist circumference);
Depression;
Systemic inflammation: interleukin 6, C-reactive protein.
Secondary outcomes:
Blood lipids: total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides;
Progression of CKD: determined as glomerular filtration from serum creatinine and/or cystatin C and/or radioisotope tracing.
Assessment of risk of bias
Reviewers (F.C.B and M.B) independently assessed the risk of bias of included studies using the Cochrane Collaboration's tool [7]. The quality of RCTs was judged by selection bias (method of recruitment, proper method of randomization at baseline, concealment of treatment allocation, similarity of groups at baseline and provision of eligibility criteria), detection bias (use of masked outcome assessment, blinded administrator and blinded patients) and attrition bias (level of adherence to the intervention, completeness of follow-up and use of intention-to-treat analysis). Any disagreement concerning data extraction and/or quality was resolved in a consensus meeting.
Each item was rated by assigning a judgment of high, low or unclear risk of material bias. We define material bias as bias of sufficient magnitude to have a notable effect on the results or conclusions of the trial, recognizing the subjectivity of any such judgment.
Results
Literature search
We retrieved 5489 articles in searches from inception through June 2015 from MEDLINE, EMBASE, PubMed, Cochrane Central, clinical trials registries, and nephrology conference proceedings. Initially 486 duplicated articles were excluded. Of the 5003 articles examined for eligibility, 4861 were excluded based on the title or abstract. The full texts of 142 potentially eligible studies were evaluated. Of these, 59 fulfilled inclusion criteria and were included in the review (Figure 1).
Fig. 1.
Exercise interventions in chronic renal disease patients: literature search results.
Selected trials
Table 1 describes the studies in terms of sample size, type and duration of intervention, CKD stage, main outcome measures and results. Fifty-nine studies, randomizing 2858 participants, were identified and selected for this review. The number of participants was usually small, ranging from 11 [23] to 297 [42] subjects, the last being a multicenter study. In 38 studies (67%), the sample size was <50 participants [8–10, 12–19, 21–26, 28–30, 35, 37–41, 45–47, 49, 50, 52, 53, 55–58, 60]. Only three studies used a healthy control group [61, 51, 62] in addition to a CKD control group. Most interventions lasted from 8 to 24 weeks; six trials lasted ≥1 year [26, 31–33, 43, 63]. Only one study compared exercise advice and rehabilitation counseling for pre-dialysis and dialysis patients [22].
Table 1.
Description of the studies on exercise interventions in CKD patients
| Author, year | Groups (n) | Intervention | Time (weeks) | CKD stage | Outcome variables | Main results (refers to I group compared with C group) |
|---|---|---|---|---|---|---|
| Afshar et al. (2010) [8] | Ia = 7 Ir = 7 C = 7 |
Ia: aerobic training Ir: resistance training C: usual care |
8 | HD | Blood chemistry (urea, creatinine, lipids, CRP), Kt/V and anthropometric measure | CRP and creatinine reduction in aerobic exercise (P = 0.005) and resistance (P = 0.036), no effect on weight, urea, lipids or Kt/V |
| Akiba et al. (1995) [9] | I = 10 C = 10 |
I: exercise training C: usual care |
12 | HD | Aerobic capacity (VO2 max, VO2AT) | VO2 max (P < 0.05) and VO2AT (P < 0.05) were decreased in C group and unchanged in I group |
| Baria et al. (2014) [10] | Ic = 10 Ih = 8 C = 9 |
Ic: center aerobic Ih: home aerobic C: usual care |
12 | Obese PH | Body composition, abdominal distribution of fat | Visceral fat and waist circumference decreased 6.4 ± 6.4 mm (P < 0.01) and 2.0 ± 2.3 cm (P = 0.03) and leg lean mass increased 0.5 ± 0.4 kg (P < 0.01) |
| Bohm et al. (2014) [11] | Ic = 30 Ip = 30 |
Ic: cycle ergometer Ip: home-based walking |
24 | HD | Capacity aerobic, strength lower, flexibility, accelerometer and HRQL | No significant differences in any outcomes were identified between interventions groups |
| Carmack et al. (1995) [12] | I = 23 I = 25 |
I: aerobic training C: attention wait-list |
10 | HD | VO2 peak, depression | Significant improvement in aerobic capacity. There were no significant changes between groups on measures of depression |
| Castaneda et al. (2001) [13] | I = 14 C = 12 |
I: low protein diet + resistance training C: low protein diet only |
12 | P-HD | TBP, muscle fibers type I and II, GFR | TBP, I and II fibers increased 4 ± 8%, 24 ± 31%, 22%; strength: I: 32 ± 14%; C: −13 ± 20% (P < 0.001); ΔGFR I: 1.18; C: −1.62 (P = 0.048) |
| Castaneda et al. (2004) [14] | I = 14 C = 12 |
I: low protein diet + resistance training C: low protein diet only |
12 | P-HD | CRP, IL-6, CSA of muscle fibers, muscle strength | CRP (−1.7 mg/L; P = 0.01), IL-6 (−4.2 pg/mL; P = 0.01) decreased, type I (24 ± 31%), type II (22 ± 41%) and strength (28 ± 14%; P = 0.001) increased |
| Cheema et al. (2007) [15] | I = 24 C = 25 |
I: intense resistance training C: usual care |
12 | HD | Muscle CSA, lipid content and strength, CRP and quality of life | Muscle strength (RR = 0.59; P = 0.04), body weight (RR = 0.62; P = 0.06) and CRP (RR = −0.63; 95% CI −0.54–0.00) improved; no change in muscle CSA |
| Chen (2010) [11] | I = 25 C = 25 |
I: intradialytic low-intensity strength training C: stretching |
24 | HD | SPPB, lower body strength, body composition and quality of life | SPPB improved 21.1% (43.1%) in I versus 0.2% (38.4%) in C (P = 0.03); sensitivity analysis: SPPB correlated to knee extensor strength (r = 0.33) |
| Deligiannis et al. (1999) [16] | IHd = 30 CHd = 30 CS = 30 |
IHd: supervised training 3×/w non-dialysis CHd and Cs: usual care |
28 | HD | HRV, SDNN, VO2 max | HRV increased from 22 ± 7 to 28 ± 9 (P < 0.05), SDNN from 0.11 ± 0.03 to 0.13 ± 0.04 (P < 0.05), VO2 max by 41% and exercise testing duration by 33% |
| Deligiannis (1999) [17] | Ia = 16 Ib = 10 Chd = 12 Cs = 15 |
Ia: aerobic HD Ib: aerobic at home Chd: HD controls Cs: healthy controls |
28 | HD | Spiroergometric echocardiographic HRpeak, VO max, pulmonary ventilation |
Ia and Ib: increased exercise time 33%/17% and VO2 max 43%/14%; Ia: increase in FE 5% and SVI 14%; unchanged in Chd |
| DePaul et al. (2002) [18] | I = 20 C = 18 |
I: resistance and aerobic C: range-of-motion exercises |
12 | HD | Submaximal workload, muscle strength, 6MWT, QOL, symptoms scores | Increased on the submaximal exercise test and muscle strength, but not in 6MWT, symptoms questionnaire or quality of life |
| Dobsak et al. (2012) [19] | Iet = 11 Iems = 11 C = 10 |
Iet: aerobic training Iems: electrostimulation C: usual care |
20 | HD | Wpeak, 6MWT, muscle power (Fmax), urea clearance and HRQOL | Significant improvement of Wpeak, Fmax and 6MWT in ET and EMS. No difference between ET and EMS groups |
| Dong et al. (2011) [20] | I = 33 C = 33 |
I: Resistance training plus nutrition C: nutrition alone |
24 | HD | Body composition, muscle strength, biochemical parameters, recall dietary | No difference in lean body mass. Weight and strength increased in I group |
| Eidemak et al. (1997) [21] | I = 15 C = 15 |
I: aerobic training C: usual care |
24 | P-HD | VO2 max, BP, HR, serum lipids, GFR | Maximal work capacity increased in the exercise group. No difference in ΔGFR |
| Fitts (1999) [22] | PR = 9 PC = 9 DR = 9 DC = 9 |
R: exercise coaching C: usual lifestyle P: pre-dialysis D: hemodialysis. |
24 | P-HD and HD | 6MWT, HRQL, resting HR | PR walked more. Hematocrit increased in R. Quality of life was stable or improved in PR, but declined in PC. PR benefited more than DR |
| Frey (1999) [23] | I = 6 C = 5 |
I: cycle 60–80% of maximal heart rate C: usual care |
12 | HD | Dietary recalls, prealbumin, transferrin and pre-dialysis and post-dialysis albumin | No increased visceral proteins |
| Giannaki et al. (2013) [24] | I = 12 C = 12 |
I: aerobic training C: usual care |
26 | HD (RSL) | Severity of RLS, functional capacity, sleep quality, depression levels | RLS severity decreased (P = 0.017), depression score (P = 0.002) and daily sleepiness (P = 0.05) improved |
| Giannaki et al. (2013) [25] | IE = 16 Ida = 8 C = 8 |
IE: aerobic training Ida: dopamine agonist C: usual care |
26 | HD (RSL) | Severity of RLS, functional capacity, muscle quality, depression, sleep quality | RLS improved in groups exercise and dopamine agonist (P = 0.03) and only agonist group improved sleep score (P = 0.016) |
| Goldberg et al. (1983) [26] | I = 14 C = 11 |
I: aerobic training 3 to 5 times weekly C: usual care |
52 ± 4 | HD | Aerobic capacity, BP, lipids, Ht, weight, fasting plasma insulin | Increased aerobic capacity 21%, exercise stress test 19%, decrease in BP, plasma insulin 20%, TG 33%. Increase in HDL, Ht |
| Gordon et al. (2012) [27] | I = 33 C = 33 |
I: Hatha yoga exercise C: usual care |
16 | HD | Serum total cholesterol, LDL, HDL and TG | Decrease in TG, LDL and total cholesterol/HDL ratio |
| Gregory et al. (2011) [28] | I = 14 C = 11 |
I: supervised exercise and dietary counseling C: usual care. |
48 | P-HD | Treadmill testing, IGF-I, IGF-II, IGFBP-1 | No difference in the IGF system. Interaction between group and time for VO2 and total treadmill time |
| Headley et al. (2012) [29] | I = 14 C = 11 |
I: personal training and dietary counseling C: usual care |
48 | P-HD | VO2 peak, eGFR, resting and ambulatory HR, lipids, CRP and IL-6 | Increase in VO2 peak from 18.1 ± 7.8 to 20.1 ± 7.3, reductions of HR, increases in LDL and TG, no effect in eGFR |
| Headley et al. (2014) [30] | I = 25 C = 21 |
I: aerobic training C: usual care |
16 | P-HD | Arterial stiffness, aerobic capacity, endothelin1, nitrate/nitrite, CRP, HRQL | No change in arterial stiffness (PWV). 8.2% increase in VO2 peak, physical function, vitality, bodily pain |
| Howden et al. (2013) [31] | I = 41 C = 42 |
I: lifestyle and aerobic and resistance training C: usual care |
52 | P-HD | Peak VO2, left ventricular function, arterial stiffness, anthropometric measures | Improved peak VO2 (P = 0.004), weight loss (P = 0.02), diastolic function (P = 0.001), arterial elastance (P = 0.01). No change in BP |
| Johansen, (2006) [32] | Iex = 20 Iex/nd = 20 Ind = 19 P = 20 |
Iex: resistance training Iex/nd: exercisee + nd Ind: nandrolone P: placebo |
12 | HD | Body composition (LBM), muscle size and strength, physical performance and activity | LBM: nandrolone increased (P < 0.0001), ex no effect. Quadriceps CSA increased in ex (P = 0.01) and nd (P < 0.0001). Ex increased physical functioning (P = 0.04) |
| Koh (2010) [33] | IHd = 27 IHo = 21 C = 22 |
IHd: intradialytic cycle IHo: home-based walking C: usual care |
24 | HD | 6MWT, PWV, augmentation index, physical activity, physical functioning. | No differences between Δ6MWT (intra Hd +14%, home +11%, usual care +5%), PWV, or any secondary outcome measure |
| Konstantinou (2002) [10] | IOhd = 21 IHd = 12 IHo = 12 CHd = 13 Cs = 15 |
IOhd: outpatient training IHd: during HD training IHo: non-supervised home CHd: HD controls Cs: healthy controls |
24 | HD | VO2 peak, VO2AT, exercise time, dropout rate | IOhd: higher dropout; VO2 peak increased 43%, VO2 AT 37%, exercise time 33% IHd: 24, 18 and 22%; IHo: 17, 8, 14%; respectively. Intense exercise outpatient is the most effective training |
| Kopple et al. (2007) [34] | Icve = 10 Ires = 15 Imix = 12 C = 14 |
Icve: aerobic training Ires: resistance training Imix: combined training C: usual care |
20 | HD | Mean body and fat mass, mid-thigh CSA, BMI, mRNA levels of growth factors genes in muscle | mRNA increased for IGF-IEa, IGF-IEc, IGF-IR, IGF-II, IGFBP-2, and IGFBP-3. No change in CRP, TNF, and IL-6 concentrations |
| Koufaki (2002) [35] | I = 18 C = 15 |
I: aerobic cycle C: usual care |
12 | HD/CAPD | Functional capacity, 6MWT, VO2 peak, VO2 at ventilatory threshold | Significantly improved peak exercise capacity 21.2 ± 7.2 to 26.9 ± 6.2 and C = 23.7 ± 6.8 to 24.1 ± 7.2 |
| Kouidi (2009) [36] | I = 30 C = 29 |
I: combined training C: usual care |
42 | HD | VO2 peak, FE, HR variability | VO2 peak from 16.4 ± 5.4 to 21.4 ± 6.8 mL/kg/min and HRV increased 12.6 ± 16.3 (P < 0.001) |
| Kouidi et al. (2010) [37] | I = 25 C = 25 |
I: HD cycling C: usual care |
52 | HD | VO2 peak, VCO2/VO2, depression (BDI and HADS), HRV (SDNN, LF/HF) | VO2 peak increased 24%, exercise time 61.4%, LH/HF 17% and SDNN 59%. Decreased BDI 34.5% and HADS 23.5% |
| Kouidi et al. (2013) [38] | I = 12 C = 12 |
I: aerobic training C: usual care |
24 | TX | HRV, arterial baroreflex sensitivity | VO2 peak increased by 15.8% (P < 0.05) and all depressed HRV and BRS indices were improved after training |
| Leehey et al. (2009) [39] | I = 7 C = 6 |
I: aerobic 6 weeks + 18 weeks home supervised C: usual care |
24 | P-HD + DM2 | VO2 max, exercise duration, GFR, Hb, HbA1, lipids, CRP and 24-h proteinuria | Increase in exercise duration. No difference in GFR, Hb, HbA1, lipids, CRP or 24-h proteinuria |
| de Lima et al. (2013) [40] | Ia = 10 Is = 11 C = 11 |
Ia: aerobic, bicycle Is: training load ankle C: usual care |
8 | HD | Respiratory strength, lung function, functional capacity, biochemistry, HRQOL | Improvement (P < 0.05) in the maximal inspiratory pressure, number of steps achieved, and quality of life |
| Makhlough et al. (2012) [41] | I = 25 C = 23 |
I: aerobic training C: usual care |
8 | HD | Calcium, phosphate, potassium, Hb | Decrease in serum phosphate (by 1.84 mg/dL) and potassium (0.69 mg/dL) |
| Mallamaci et al. (2014) [42] | I = 151 C = 146 |
I: home exercise program C: usual care |
24 | HD | 6MWT and Sit-to-Stand test | Increased 6MWT, 369 ± 113 to 324 ± 116 (P < 0.001) and sit-to-stand 18.3 ± 19.7 ± 6.7 s between groups |
| Matsumoto et al. (2007) [43] | I = 22 C = 33 |
I: exercise training C: usual care |
52 | HD | Albumin, HRQOL, creatinine generation (CGR) | Serum albumin, CGR and HRQOL increased in the I group |
| Mohseni et al. (2013) [44] | I = 25 C = 25 |
I: aerobic training C: usual care |
8 | HD | Dialysis efficacy (Kt/v and URR) | Increased intragroup URR (P = 0.003) and Kt/v (P = 0.001), between groups not stated |
| Molsted et al. (2004) [45] | I = 22 C = 21 |
I: aerobic training 2× week C: usual care |
20 | HD | Aerobic capacity, 2-min stair climbing, squat test, SF-36, BP and lipids | Increase in aerobic capacity and Physical Summary Score (SF36) |
| Mortazavi et al. (2013) [46] | I = 13 C = 13 |
I: aerobic training 3× week C: usual care |
16 | HD RLS |
Severity of RLS, HRQL (SF-63) | Decreased scores of RLS and HRQL no difference between groups |
| Orcy et al. (2012) [38] | I = 13 C = 13 |
I: resistance and aerobic C: resistance only |
10 | HD | Functional performance (6MWT) |
6MWT changed 39.7 ± 61.4 m in I group and −19.2 ± 53.9 m in C group (P = 0.02) |
| Ouzouni et al. (2009) [47] | I = 20 C = 15 |
I: resistance and aerobic C: usual care |
40 | HD | VO2 peak, HRQOL, personality parameters | Increased VO2 peak (21.1%) and physical HRQOL, decreased depression |
| Painter et al. (2002a) [48] | IHtU = 10 IHtN = 12 CHtU = 14 CHtN = 12 |
I: aerobic training C: usual care HtU: Ht 30–33% HtN: Ht 40–42% |
20 | HD | Treadmill, VO2 peak HRQL (SF-36) |
I: increased VO2 peak (P = 0.03), physical functioning (P = 0.01); HtN: increased general health (P = 0.03) |
| Painter et al. (2002b) [32] | I = 54 C = 43 |
I: exercise at home C: usual care |
52 | Tx | Symptom-limited exercise, VO2 peak, isokinetic testing, body composition, SF-36 | Increased VO2 (24.0 ± 7.5 to 30.1 ± 10.3 mL/kg/min) and muscle strength. No differences in body composition or HRQL |
| Painter (2003) [33] | I = 51 C = 45 |
I: aerobic training C: usual care |
52 | Tx | Maximal exercise testing, risk factors, Framingham equations | Increase in total cholesterol, HDL-C, and body mass index over time. No differences between groups |
| Parsons (2004) [49] | I = 6 C = 7 |
I: aerobic cycle C: usual care |
08 | HD | SF-36, KtV, 2-h DUC, BP, and maximal work capacity | Only DUC in the first 2-h was higher in I group |
| Pellizzaro et al. (2013) [50] | RMT = 11 PMT = 14 C = 14 |
RMT: inspiratory muscles PMT: knee extensor muscle C: usual care |
10 | HD | Respiratory strength, functional capacity, HRQOL, inflammatory state | ΔPI and ΔPE increased in RMT; Δ6MWT increased in RMT and PMT, CRP reduced and HRQOL increased in RMT and PMT |
| Petraki et al. (2008) [51] | I = 22 CHD = 21 CS = 20 |
I: aerobic during HD CHD: HD controls Cs: healthy controls |
28 | HD | Arterial baroreflex sensitivity, spiroergometric study | Improvement in VO2 peak, exercise time and arterial baroreflex sensitivity |
| Reboredo et al. (2010) [52] | I = 11 C = 11 |
I: aerobic during HD C: usual care |
12 | HD | HRV and LVF by Holter and echocardiography | No differences in HRV or LVF between the groups |
| Reboredo (2011) [53] | I = 14 C = 14 |
I: aerobic during HD C: usual care |
12 | HD | VO2 peak and time to exercise intolerance (Tlim) | Training improved 50 to 200% in Tlim and VO2 peak in 15–20% |
| Rossi et al. (2014) [54] | I = 59 C-48 |
I: treadmill cardiovascular and weight training+usual C: usual care | 12 | P-HD | 6 MWT, sit-to-stand test | Intervention significant: 6-MWT 19% improvement (P < 0.001), sit-to-stand test 29% improvement (P < 0.001) |
| Segura-Ortí (2009) [55] | IRT = 19 ILA = 8 |
IRT: resistance during HD. ILA: aerobic |
24 | HD | Aerobic capacity, muscle strength, HRQOL | IRT improved right knee extensor muscles strength. No difference in physical tests |
| Song and Sohng (2012) [56] | I = 20 C = 20 |
I: resistance training C: usual care |
12 | HD | Body composition, physical fitness, HRQOL, lipid profile | Muscle strength and HRQOL increased, cholesterol and triglyceride decreased |
| Toussaint (2008) [57] | I = 9 C = 10 |
I: aerobic cycle (cross-over) C = usual care |
12 | HD | PWV, measurements of BNP | PWPV improved, BNP decreased |
| Tsuyuki et al. (2003) [58] | I = 17 C = 12 |
I: aerobic exercise HD C: usual care |
20 | HD | VO2 peak, BP, oxygen uptake efficiency slope (OUES) | OUES increased in physical training group, no change in control group |
| van Vilsteren (2005) [59] | I = 60 C = 43 |
I: resistance before and aerobic during HD C: usual care |
12 | HD | Kt/V, Ht, cholesterol, BP, weight, physical fitness, SF-36, behavior | Improvement in behavior, reaction time, lower extremity muscle strength, KtV and quality of life |
| Wilund et al. (2010) [60] | I = 8 C = 9 |
I: aerobic training C: usual care |
16 | HD | Walk test, cholesterol, OS, CRP, IL-6, K, P, Ca, ALP, urea, albumin, heart function | Walk increased 17%, OS and epicardial fat reduced. No change in CRP, IL-6 or other variables |
| Yurtkuran (2007) [17] | I = 19 C = 18 |
I: yoga-based exercises C: usual care |
12 | HD | Visual analogue scale (pain, fatigue, sleep), grip strength, biochemical variables | Improvement in pain −37%, fatigue −55%, sleep disturbance −25%, strength +15%, Ht +13%, creatinine −14%, cholesterol −15% |
I, intervention group; C, control group; P-HD, pre-HD; HD, hemodialysis; Tx, renal transplantation; HRV, heart rate variability; SDNN, standard deviation of normal-to-normal intervals; HF, marker of vagal activity; LF, parameter that includes both sympathetic and vagal influences; ratio LF/HF, marker of sympathovagal balance; RPE, rating of perceived exertion; CRP, C-reactive protein; 6MWT, 6-minute walk test; CSA, cross-sectional area; VO2AT, anerobic threshold; VO2 max, maximal oxygen consumption; eGFR, estimated glomerular filtration rate; TBP, total body potassium; SPPB, Short Physical Performance Battery; RLS, restless leg syndrome; LBM, lean body mass; PWV, pulse wave velocity; RMT, respiratory muscle training; PMT, peripheral muscle training; PI(max), maximal inspiratory pressure; PE(max), maximal expiratory pressure; FVC, forced vital capacity; URR, urea reduction ratio; STS, sit-to-stand; Wpeak, peak workload; DUC, dialysate urea clearance; OS, oxidative stress; IL-6, interleukin 6; K, potassium; P, phosphorus; Ca, calcium; ALP, alkaline phosphatase.
Twenty-eight of the 59 studies were published after 2009 (Table 1). Despite the fact that diabetes and hypertension are the main comorbidities associated to CKD, only one study was restricted to diabetic CKD [39] and one to obese pre-dialysis patients [10].
Assessment of the quality of studies
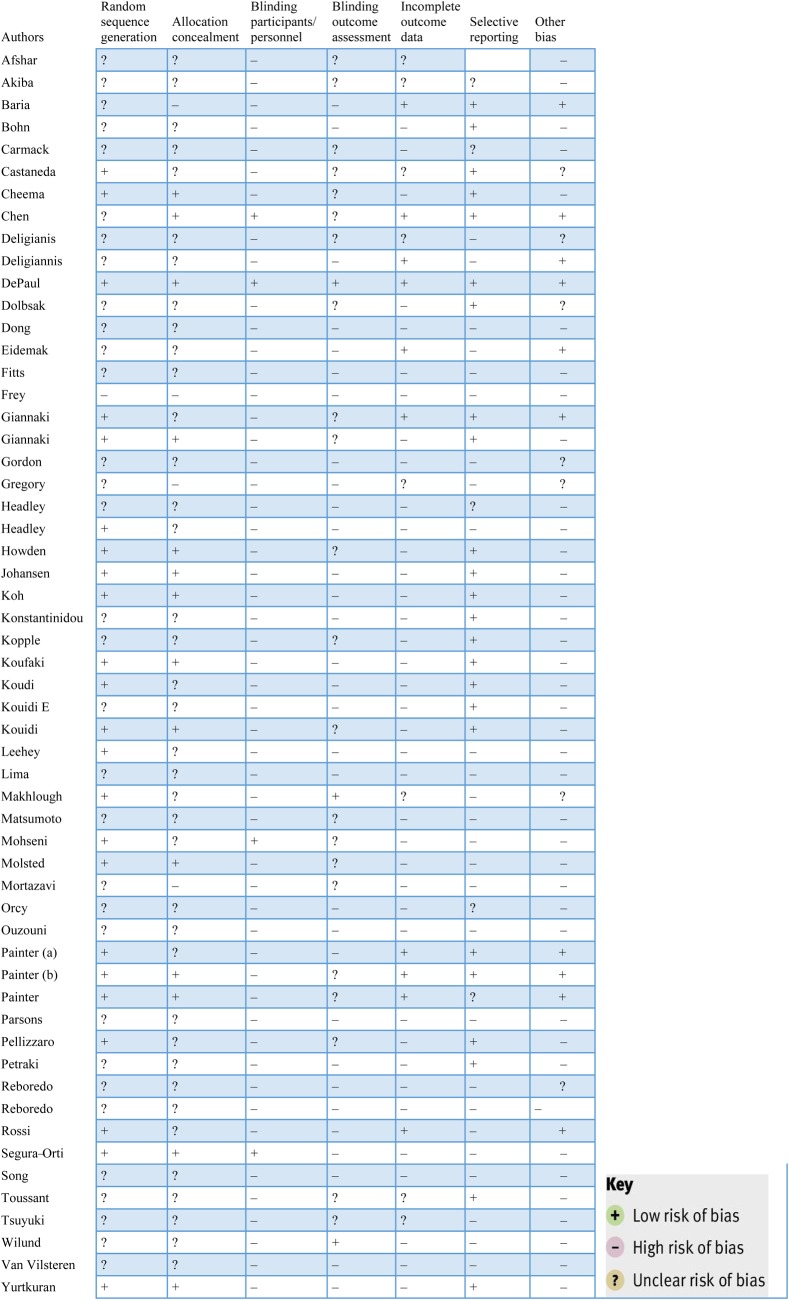
Results of the risk of bias assessment are presented in Figure 2. Risks of bias due to blinding and incomplete outcome data were assessed across all outcomes within each included study.
Fig. 2.
Risk of bias assessments of RCTs on the effectiveness of exercise interventions among CKD patients.
Selection bias
Allocation: The most frequently detected shortcomings were related to randomization and generation of the allocation sequence. Only 23 studies had adequate randomization [13, 15, 17, 18, 24, 25, 31–33, 35–37, 39, 44, 45, 48, 50, 54, 55, 64–67] and concealment of the allocation sequence was described in only 14 RCTs [11, 15, 17, 18, 25, 31–33, 35–37, 45, 55, 65].
Detection bias
Blinding: The blinding process of participants, care providers and assessors was described in six studies [18, 32, 33, 45, 55, 59]. Only three studies used blinding of the outcomes [18, 44, 60].
Attrition bias
Dropout rates: Forty-one of 59 studies reported dropout rates. Thirty-two had a dropout rate between 0 and 30% [10, 11, 13, 14, 16–18, 20–25, 29, 32, 33, 36, 39, 43, 47, 49, 55, 57, 58, 61–66, 68]. One study [12] had a dropout rate >70%.
Intention-to-treat analysis: The analysis was conducted according to the intention-to-treat principle in only 10 studies [10, 13, 16, 18, 21, 24, 32, 33, 54, 67].
Adherence to interventions: Only nine RCTs reported on compliance, which was computed as the number of sessions attended out of the total possible sessions, expressed as a percentage [11, 15, 31, 38, 56, 64, 65, 67, 69]. The compliance ranged from 70 [31] to 89% [11, 65].
Other quality indicators
Sample size calculations were not consistently presented in the articles, making it difficult to interpret whether non-significant findings were due to insufficient study power in eight studies [16, 18, 21, 24, 32, 33, 48, 67].
Types and durations of interventions
The intervention was based on aerobic exercise in 44 studies [9–12, 16, 17, 21–24, 26, 29, 30, 32, 33, 35–37, 39, 42–46, 48–51, 53, 56–58, 60–63, 65, 66], lasting from 30 to 90 min per session, with intensities ranging from 60 to 80% of maximal oxygen consumption (VO2 max). Resistance exercises were used in nine studies [8, 13–15, 20, 55, 56, 64], two were associated with nutrition [13, 20] and nine combined aerobic and resistance exercises [18, 31, 34, 36, 38, 47, 50, 54, 59]. One study applied as intervention intradialytic electromyostimulation of leg extensors [19]. Two RCTs [25, 64] compared drug treatment with exercise, with one of them including only CKD patients with restless legs [25]. Gordon et al. [27] and Yurtkuran et al. [17] used yoga-based exercise. Most studies used intradialytic exercise two to three times a week. Supervised exercise interventions were used in the most studies; one study used physical activity advice alone [22].
Outcomes measures
Health-related quality of life (HRQOL): HRQOL was analyzed in 21 studies [18, 19, 22, 32, 40, 43, 45–50, 55, 59, 64, 67, 70]. Five studies found improvements in only the Physical Component Score [32, 45, 47, 48, 64], with ∼10% higher scores in the exercise groups. The 36-item Short Form Health Survey (SF-36) [12, 15, 18, 23, 32, 40, 43, 67] was the instrument most frequently used. Only two studies used a disease-specific instrument, the Kidney Disease Quality of Life (KDQOL) [40, 50]. The Quality of Life Index [47], Scale of Life Satisfaction [63] and Sleep Quality [24] were each used once.
Physical fitness: Measures of physical fitness, such as oxygen peak consumption (VO2 peak) were assessed in 20 aerobic interventions [9, 12, 16, 21, 29–32, 35–37, 39, 47, 48, 51, 53, 58, 61–63]. On average, aerobic exercise lasting from 8 weeks to 6 months improved VO2 peak ∼20%, from 8.2% [30] to 43% [61, 62].
Heart rate variability (HRV): Seven studies evaluated the effect of exercise on HRV [16, 29, 36, 37, 51, 52, 63]: five in hemodialysis, one in kidney transplant patients [37] and one pre-dialysis [29]. Only the study by Reboredo et al. [52] found no difference in HRV. When HRV was assessed in the time domain, the outcome measures were SDNN and HRV index.
Lipid profile: Most studies including lipid profiles [8, 15, 17, 20, 21, 26, 29, 39, 45, 56, 59, 60] as an outcome measure found no effect of the exercise intervention on LDL cholesterol, HDL cholesterol or triglycerides, with the exception of one study that used yoga as the intervention, which reported a 15% decrease in lipids after a 12-week follow-up [17].
Inflammatory markers: Inflammatory biomarkers such as C-reactive protein (CRP) and interleukins were evaluated in 10 studies [8, 13–15, 29, 30, 34, 39, 50, 60]; in general, a positive effect of exercise interventions was detected for such outcomes. Compared with the control group, a significant reduction of CRP (P = 0.005 and P = 0.036) was demonstrated in aerobic and resistance training groups in the Afshar et al. study [8]. In another study [15], high-intensity resistance training decreased CRP, with a risk reduction (RR) of −0.63 (95% CI −0.54–0.00). Castaneda et al. [14] also applied resistance training, which decreased CRP 1.7 mg/L (P = 0.01), with no change in the control group. Kopple et al. [34] compared the effect of different forms of exercise training (endurance, strength or combination), and found no change in serum CRP with exercise training or in the control group.
Muscular strength: Nine RCTs whose intervention was resistance training [8, 13–15, 20, 32, 55, 56, 67] or aerobic and resistance training [8, 54, 59] measured muscular strength as one of their outcome measures, two in pre-dialysis patients [11, 14], another in kidney transplant patients [32] and the remaining in hemodialysis patients. One study using aerobic and muscle electrostimulation [19] and an other with yoga [17] also measured the effects on muscular strength. All of these found an increase in strength after intervention. Mallamaci et al. [42] assessed lower limb strength in patients on hemodialysis, and scores on the sit-to-stand test increased from 18.3 ± 5.1 to 19.7 ± 6.7 (P = 0.009) in the intervention group compared with the control group. In pre-dialysis patients [11, 14, 54], the intervention group improved ∼28% (P < 0.001) for muscular strength, as in kidney transplant patients [32]. Dobsak et al. [19] applied aerobic exercise (AT) or muscle electrostimulation (EMS) and muscle power increased with EMS to 222.2 ± 36.6 (P = 0.046) and with AT to 230.3 ± 31.1 (P = 0.033), while the control group remained at 187.8 ± 29.7. The yoga-based study found an improvement of 15% in muscular strength [17].
Body composition: Six studies analyzed the effects of resistance exercise on body composition. Four studied hemodialysis patients [15, 20, 64, 67] and one analyzed kidney transplant patients [32]. Resistance exercise did not change body composition. One RCT analyzed the effect of exercise in obese pre-dialysis patients [10] and found a visceral fat and waist circumference decrease of 6.4 ± 6.4 mm (P < 0.01) and 2.0 ± 2.3 cm (P = 0.03) and leg lean mass increased 0.5 ± 0.4 kg (P < 0.01).
CKD progression: The effect of aerobic [21, 29–31, 39] or resistance [13, 31] training on CKD progression was measured in four RCTs. A slower decline in the rate of change in glomerular filtration was found in one study in patients randomized to a low protein diet and resistance training [13].
PWV and arterial stiffness: Two studies measured PWV in pre-dialysis [30, 31] patients and found no effect of exercise. Toussant et al. [57] studied hemodialysis patients and found a positive effect of aerobic exercise (9.04±0.59 versus 10.16±0.74, P = 0.008), but Koh et al. [65] did not find a difference between intradialytic exercise or usual care.
Depression: Depression was analyzed in four RCTs, three of them using aerobic exercise [12, 24, 63] and one aerobic plus resistance exercise [47]. Exercise decreased depression scores. The Beck Depression Index decreased 34.5% in the Kouidi et al. trial [63] and 39.4% in Ozouni et al. trial [47], as occurred in the study by Ginakki et al. [24] (P = 0.002). Only Carmack et al. [12] was unable to find a benefit of exercise on depression.
CKD stage and type of treatment: Forty-five [8, 9, 11, 12, 15–20, 23–27, 34–38, 40, 41, 43–53, 55–62, 64, 67, 70] of 59 studies were carried out in ESRD patients on hemodialysis. Only one study included both hemodialysis and peritoneal dialysis patients [35]. Five of these studies had CRP as an outcome measure; in three of them CRP was inversely correlated with physical activity [8, 15, 50] and another found no change [34, 39, 60]. Fourteen studies assessed VO2 peak and all of them found a significant increase with aerobic exercise [29–31, 36, 37, 47, 48, 51, 53, 62, 63]. Strength was measured in nine resistance studies [15, 18, 20, 32, 55, 56, 59, 67], all of them with a positive finding. HRQOL was assessed in 18 studies [15, 18, 19, 22, 40, 43, 45, 47–50, 55, 56, 59, 64, 67, 70]; 11 of them found increases in HRQOL in the exercise groups, both in aerobic and resistance training [22, 40, 43, 45, 47, 48, 50, 56, 59, 64]. However, four of these studies found improvements only in the physical component of the HRQOL [45, 47, 48, 64].
Pre-dialysis: Eleven studies [10, 13, 14, 21, 22, 28–31, 39, 54] included pre-dialysis patients; one study enrolled patients on dialysis and pre-dialysis [39]. The studies that included pre-dialysis patients actually reported findings from eight different interventions, because one study generated two different publications [13, 14]. Three studies used CRP as an outcome measure [14, 30, 39], of which only one found a positive association between exercise and decreased CRP [14]. Six studies [13, 21, 29–31,39] evaluated the progression of CKD, and only Castaneda et al. [13] found a significant positive effect of the exercise intervention on this outcome. Physical capacity was assessed through VO2 peak in five studies [21, 28–31], all of them with positive results. Three publications [13, 14, 31] used resistance training, two of them using the same study [13, 14], and in another [30] the training was associated with lifestyle interventions. HRQOL was measured in two studies [22, 30], one of them [22] included pre-dialysis and dialysis patients, and both found improved HRQOL after exercise.
Kidney transplantation: Three RCTs [32, 33, 37] assessing exercise in kidney transplant patients were found, all of them using aerobic training interventions. The outcome measures were VO2 peak, analyzed in two studies [32, 37], which increased 16–20%. HRV was analyzed in Koudi et al. [37], with positive findings as well. One of them found no difference in HRQOL with exercise [32] and another found increases in HDL cholesterol in the exercise group [33].
Discussion
This systematic review gathered consistent evidence of the positive effects of aerobic exercise on physical fitness, muscular strength and quality of life in ESRD patients. The evidence regarding exercise effects on other health outcomes and/or in earlier stages of CKD are weaker and heterogeneous.
The improvement in fitness through exercise in ESRD patients is a noteworthy finding. Functional capacity is usually impaired in CKD patients, reaching ∼60–65% of the age-predicted value [71]. This low level of fitness is worrisome considering its association with poorer HRQOL [32, 48] and higher mortality in CKD patients [72].
Exercise requires the integrated function of multiple vital organs. Low exercise capacity is also an independent predictor of mortality in other chronic disease populations. Since physical training can improve functional capacity, maybe it can also increase survival. Although there is no RCT up to now confirming this hypothesis, the Dialysis Morbidity and Mortality Study, a cohort study, found that dialysis patients engaged in more frequent exercise presented a significantly reduced mortality rate versus less active peers [73].
Patients on dialysis are also weaker when compared with healthy sedentary subjects, and weakness may contribute to their poor physical functioning. Muscular strength has been described as a significant predictor of gait speed [73] and VO2 peak [73] in dialysis patients.
The findings of our systematic review are in accordance with those of Heiwe and Jacobsen, whose meta-analysis for the Cochrane Collaboration was published in 2011 [74] and updated in 2014 [75]. They found significant beneficial effects of various exercise interventions in CKD patients on physical fitness, muscular functioning, walking capacity, cardiovascular function and HRQOL, with stronger evidence for dialysis patients and aerobic exercise programs.
Our finding about HRV is also interesting. Although there are few RCTs on the issue, six of the seven studies assessing HRV included in this review found significant improvement in this variable after exercise [16, 29, 36, 37, 51, 63]. The association between CKD and low HRV is consistent across multiple stages of the disease, including micro- and macro-albuminuria, decreased estimated glomerular filtration rate (eGFR) and ESRD [76]. In addition to CKD, other risk factors for low HRV include older age, obesity, diabetes, sedentary lifestyle, low HDL cholesterol, high insulin levels, elevated CRP and high systolic blood pressure [76]. Some studies [76] have shown that elevated resting heart rate and low HRV are associated with an increased risk of ESRD, CKD-related hospitalization and arrhythmias.
The Cochrane meta-analysis also found that the HRV index significantly improved after 6 months of mixed aerobic and resistance training in hemodialysis patients [74, 75]. The United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK-Heart) [77] recently found that the SDNN, an HRV index, <100 ms was associated with increased mortality. The impact of HRV on mortality might be explained by the sympathetic/parasympathetic balance, with sympathetic nervous system overactivation in patients with lower HRV, which led to an increased susceptibility to malignant arrhythmia [78].
Considering that exercise improves HRV in hemodialysis patients, and that arrhythmia is one of the major causes of CV mortality in this subset of patients [79], we might expect a positive effect of exercise on mortality in hemodialysis patients. However, to date, only one study analyzed the effect of high-intensity mixed aerobic and resistance training in hemodialysis patients on arrhythmias and found no difference between the intervention and control groups [36].
Despite the auspicious findings, this systematic review has some limitations. The RCTs included present moderate to low quality and high risk of bias. Assessment of the quality of trial randomization, the avoidance of exclusions after trial entry and blinding have been proposed as the most important methodological components of controlled trials [80].
First, during the preparation of this review, a large number of exercise trials were excluded because no randomization of participants had been done. Allocation concealment also seems to be a significant issue. Inadequate concealment can lead to bias in many ways, sometimes as the result of deliberate subversions (usually well intentioned), or as the result of subconscious actions. Trials that reported either inadequate or unclear concealment methods yielded estimates of odds ratios that were exaggerated by an average of 41 or 30%, respectively, compared with estimates of odds ratios derived from trials that apparently had taken adequate steps to conceal treatment allocation [81]. Only 14 studies included in this review reported allocation concealment [11, 15, 17, 18, 25, 31–33, 35–37, 45, 55, 65]. Furthermore, most of them did not analyze data according to the intention-to-treat principle, thus increasing the risk of selection bias. Not using intention-to-treat analysis might have inflated the apparent results. Moreover, there were deficiencies in the reporting of methodological information and findings, such as method of randomization, dropout rate, adherence to the intervention and controls. Readers of the present study should be aware that it is possible that a trial could have been classified as having lower quality than it truly had because data and/or information were missing. Despite the availability of guidelines aimed at standardizing the reporting of clinical trials, publications often omit essential methodological details.
In addition to methodological and reporting problems with the current literature about the effects of exercise on health of ESRD, we detected the scarcity of trials including patients in earlier stages of CKD.
From a public health standpoint, the fact that most studies included only hemodialysis patients represents a point of concern, because patients as well as health systems would most benefit from primary prevention or from interventions that increase survival and/or delay the need for RRT in earlier stages of CKD, whose population is ∼20 times greater than the ESRD population. The need for RCTs in pre-dialysis patients is amplified by positive findings from observational studies in CKD stages II–IV showing the association of higher physical activity with slower rates of glomerular filtration decline [82] and higher survival rates [72]. If could be confirmed that exercise interventions are effective in delaying CKD progression and/or decrease mortality, the potential impact of such interventions for public health would be enormous.
Despite the lack of RCT-based evidence about the effect of exercise on mortality, the already documented effects of exercise on physical function, strength, quality of life and cardiovascular index, such as HRV, are enough to support the recommendation of moderate-intensity physical activity for CKD patients. Exercise interventions need to be progressively included in the regimens of hemodialysis centers, aiming for inclusion in all dialysis centers, even though the best exercise protocol for dialysis patients remains to be established.
Conflict of interest statement
All the authors declared no competing interests. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG RG, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011; 80: 1258–1270 [DOI] [PubMed] [Google Scholar]
- 3.Lee I-M, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012; 380: 219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen K, Painter P. Exercise for patients with CKD: what more is needed? Adv Chronic Kidney Dis 2009; 16: 407–409 [DOI] [PubMed] [Google Scholar]
- 5.Cheema BSB. Review article: Tackling the survival issue in end-stage renal disease: time to get physical on haemodialysis. Nephrology 2008; 13: 560–569 [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W-65–W-94 [DOI] [PubMed] [Google Scholar]
- 7.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008, chap. 8. Available from www cochrane-handbook org
- 8.Afshar R, Shegarfy L, Shavandi N, et al. Effects of aerobic exercise and resistance training on lipid profiles and inflammation status in patients on maintenance hemodialysis. Indian J Nephrol 2010; 20: 185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiba T, Matsui N, Shinohara S, et al. Effects of recombinant human erythropoietin and exercise training on exercise capacity in hemodialysis patients. Artif Organs 1995; 19: 1262–1268 [DOI] [PubMed] [Google Scholar]
- 10.Baria F, Kamimura MA, Aoike DT, et al. Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol Dial Transplant 2014; 29: 857–864 [DOI] [PubMed] [Google Scholar]
- 11.Bohm C, Stewart K, Onyskie-Marcus J, et al. Effects of intradialytic cycling compared with pedometry on physical function in chronic outpatient hemodialysis: a prospective randomized trial. Nephrol Dial Transplant 2014; 29: 1947–1955 [DOI] [PubMed] [Google Scholar]
- 12.Carmack CL, Amaral-Melendez M, Boudreaux E, et al. Exercise as a component of the physical and psychological rehabilitation of hemodialysis patients. Int J Rehabil Health 1995; 1: 13–23 [Google Scholar]
- 13.Castaneda C, Gordon PL, Uhlin KL, et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med 2001; 135: 965–976 [DOI] [PubMed] [Google Scholar]
- 14.Castaneda C, Gordon PL, Parker RC, et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 2004; 43: 607–616 [DOI] [PubMed] [Google Scholar]
- 15.Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol 2007; 18: 1594–1601 [DOI] [PubMed] [Google Scholar]
- 16.Deligiannis A, Kouidi E, Tourkantonis A. Effects of physical training on heart rate variability in patients on hemodialysis. Am J Cardiol 1999; 84: 197–202 [DOI] [PubMed] [Google Scholar]
- 17.Yurtkuran M, Alp A, Dilek K. A modified yoga-based exercise program in hemodialysis patients: a randomized controlled study. Complement Ther Med 2007; 15: 164–171 [DOI] [PubMed] [Google Scholar]
- 18.DePaul V, Moreland J, Eager T, et al. The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: a randomized controlled trial. Am J Kidney Dis 2002; 40: 1219–1229 [DOI] [PubMed] [Google Scholar]
- 19.Dobsak P, Homolka P, Svojanovsky J, et al. Intra-dialytic electrostimulation of leg extensors may improve exercise tolerance and quality of life in hemodialyzed patients. Artif Organs 2012; 36: 71–78 [DOI] [PubMed] [Google Scholar]
- 20.Dong J, Sundell MB, Pupim LB, et al. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr 2011; 21: 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eidemak I, Haaber AB, Feldt-Rasmussen B, et al. Exercise training and the progression of chronic renal failure. Nephron 1997; 75: 36–40 [DOI] [PubMed] [Google Scholar]
- 22.Fitts SS, Guthrie MR, Blagg CR. Exercise coaching and rehabilitation counseling improve quality of life for predialysis and dialysis patients. Nephron 1999; 82: 115–121 [DOI] [PubMed] [Google Scholar]
- 23.Frey S, Mir AR, Lucas M. Visceral protein status and caloric intake in exercising versus nonexercising individuals with end-stage renal disease. J Ren Nutr 1999; 9: 71–77 [DOI] [PubMed] [Google Scholar]
- 24.Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, et al. A single-blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome. Nephrol Dial Transplant 2013; 28: 2834–2840 [DOI] [PubMed] [Google Scholar]
- 25.Giannaki CD, Sakkas GK, Karatzaferi C, et al. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol 2013; 14: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg A, Geltman E, Hagberg J, et al. Therapeutic benefits of exercise training for hemodialysis patients. Kidney Int Suppl 1983; 16: S303–S309 [PubMed] [Google Scholar]
- 27.Gordon L, McGrowder DA, Pena YT, et al. Effect of exercise therapy on lipid parameters in patients with end-stage renal disease on hemodialysis. J Lab Physicians 2012; 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory SM, Headley SA, Germain M, et al. Lack of circulating bioactive and immunoreactive IGF-I changes despite improved fitness in chronic kidney disease patients following 48 weeks of physical training. Growth Horm IGF Res 2011; 21: 51–56 [DOI] [PubMed] [Google Scholar]
- 29.Headley S, Germain M, Milch C, et al. Exercise training improves HR responses and V·O2peak in predialysis kidney patients. Med Sci Sports Exerc 2012; 44: 2392–2399 [DOI] [PubMed] [Google Scholar]
- 30.Headley S, Germain M, Wood R, et al. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis 2014; 64: 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howden EJ, Leano R, Petchey W, et al. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 2013; 8: 1494–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Painter PL, Hector L, Ray K, et al. A randomized trial of exercise training after renal transplantation. Transplantation 2002; 74: 42–48 [DOI] [PubMed] [Google Scholar]
- 33.Painter PL, Hector L, Ray K, et al. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis 2003; 42: 362–369 [DOI] [PubMed] [Google Scholar]
- 34.Kopple JD, Wang H, Casaburi R, et al. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol 2007; 18: 2975–2986 [DOI] [PubMed] [Google Scholar]
- 35.Koufaki P, Mercer TH, Naish PF. Effects of exercise training on aerobic and functional capacity of end-stage renal disease patients. Clin Physiol Funct Imaging 2002; 22: 115–124 [DOI] [PubMed] [Google Scholar]
- 36.Kouidi EJ, Grekas DM, Deligiannis AP. Effects of exercise training on noninvasive cardiac measures in patients undergoing long-term hemodialysis: a randomized controlled trial. Am J Kidney Dis 2009; 54: 511–521 [DOI] [PubMed] [Google Scholar]
- 37.Kouidi E, Vergoulas G, Anifanti M, et al. A randomized controlled trial of exercise training on cardiovascular and autonomic function among renal transplant recipients. Nephrol Dial Transplant 2013; 28: 1294–1305 [DOI] [PubMed] [Google Scholar]
- 38.Orcy RB, Dias PS, Seus TL, et al. Combined resistance and aerobic exercise is better than resistance training alone to improve functional performance of haemodialysis patients—results of a randomized controlled trial. Physiother Res Int 2012; 17: 235–243 [DOI] [PubMed] [Google Scholar]
- 39.Leehey DJ, Moinuddin I, Bast JP, et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol 2009; 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Lima MC, Cicotoste CdL, Cardoso KdS, et al. Effect of exercise performed during hemodialysis: strength versus aerobic. Ren Fail 2013; 35: 697–704 [DOI] [PubMed] [Google Scholar]
- 41.Makhlough A, Ilali E, Mohseni R, et al. Effect of intradialytic aerobic exercise on serum electrolytes levels in hemodialysis patients. Iran J Kidney Dis 2012; 6: 119–123 [PubMed] [Google Scholar]
- 42.Mallamaci F, Manfredini M, Bolignano D, et al. A personalized, low-intensity, easy to implement, home exercise program improves physical performance in dialysis patients: the Exercise Introduction to Enhance Performance in Dialysis (EXCITE) Trial. Dialysis: Identifying Risk Factors and Improving Noncardiovascular Outcomes. J Am Soc Nephrol 2014; 25: 57 A). [Google Scholar]
- 43.Matsumoto Y, Furuta A, Furuta S, et al. The impact of pre-dialytic endurance training on nutritional status and quality of life in stable hemodialysis patients (Sawada study). Ren Fail 2007; 29: 587–593 [DOI] [PubMed] [Google Scholar]
- 44.Mohseni R EZA, Ilali E, Adib-Hajbaghery M, et al. The effect of intradialytic aerobic exercise on dialysis efficacy in hemodialysis patients: a randomized controlled trial. Oman Med J 2013; 28: 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molsted S, Eidemak I, Sorensen HT, et al. Five months of physical exercise in hemodialysis patients: effects on aerobic capacity, physical function and self-rated health. Nephron Clin Pract 2004; 96: c76–c81 [DOI] [PubMed] [Google Scholar]
- 46.Mortazavi M, Vahdatpour B, Ghasempour A, et al. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis. ScientificWorldJournal 2013; 2013: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouzouni S, Kouidi E, Sioulis A, et al. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil 2009; 23: 53–63 [DOI] [PubMed] [Google Scholar]
- 48.Painter P, Moore G, Carlson L, et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis 2002; 39: 257–265 [DOI] [PubMed] [Google Scholar]
- 49.Parsons TL, Toffelmire EB, King-VanVlack CE. The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol 2004; 61: 261–274 [DOI] [PubMed] [Google Scholar]
- 50.Pellizzaro CO, Thomé FS, Veronese FV. Effect of peripheral and respiratory muscle training on the functional capacity of hemodialysis patients. Ren Fail 2013; 35: 189–197 [DOI] [PubMed] [Google Scholar]
- 51.Petraki M, Kouidi E, Grekas D, et al. Effects of exercise training during hemodialysis on cardiac baroreflex sensitivity. Clin Nephrol 2008; 70: 210–219 [DOI] [PubMed] [Google Scholar]
- 52.Reboredo Mde M, Pinheiro Bdo V, Neder JA, et al. Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J Bras Nefrol 2010; 32: 367–373 [PubMed] [Google Scholar]
- 53.Reboredo MM, Neder JA, Pinheiro BV, et al. Constant work-rate test to assess the effects of intradialytic aerobic training in mildly impaired patients with end-stage renal disease: a randomized controlled trial. Arch Phys Med Rehabil 2011; 92: 2018–2024 [DOI] [PubMed] [Google Scholar]
- 54.Rossi AP, Burris DD, Lucas FL, et al. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol 2014; 9: 2052–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segura-Orti E, Kouidi E, Lison JF. Effect of resistance exercise during hemodialysis on physical function and quality of life: randomized controlled trial. Clin Nephrol 2009; 71: 527–537 [DOI] [PubMed] [Google Scholar]
- 56.Song W-J, Sohng K-Y. Effects of progressive resistance training on body composition, physical fitness and quality of life of patients on hemodialysis. J Korean Acad Nurs 2012; 42: 947–956 [DOI] [PubMed] [Google Scholar]
- 57.Toussaint ND, Polkinghorne KR, Kerr PG. Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial Int 2008; 12: 254–263 [DOI] [PubMed] [Google Scholar]
- 58.Tsuyuki K, Kimura Y, Chiashi K, et al. Oxygen uptake efficiency slope as monitoring tool for physical training in chronic hemodialysis patients. Ther Apher Dial 2003; 7: 461–467 [DOI] [PubMed] [Google Scholar]
- 59.van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant 2005; 20: 141–146 [DOI] [PubMed] [Google Scholar]
- 60.Wilund KR, Tomayko EJ, Wu P-T, et al. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant 2010; 25: 2695–2701 [DOI] [PubMed] [Google Scholar]
- 61.Deligiannis A, Kouidi E, Tassoulas E, et al. Cardiac effects of exercise rehabilitation in hemodialysis patients. Int J Cardiol 1999; 70: 253–266 [DOI] [PubMed] [Google Scholar]
- 62.Konstantinidou E, Koukouvou G, Kouidi E, et al. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med 2002; 34: 40–45 [DOI] [PubMed] [Google Scholar]
- 63.Kouidi E, Karagiannis V, Grekas D, et al. Depression, heart rate variability, and exercise training in dialysis patients. Eur J Cardiovasc Prev Rehabil 2010; 17: 160–167 [DOI] [PubMed] [Google Scholar]
- 64.Johansen KL, Painter PL, Sakkas GK, et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol 2006; 17: 2307–2314 [DOI] [PubMed] [Google Scholar]
- 65.Koh KP, Fassett RG, Sharman JE, et al. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis 2010; 55: 88–99 [DOI] [PubMed] [Google Scholar]
- 66.Makhlough A, Ilali E, Mohseni R, et al. Effect of intradialytic aerobic exercise on serum electrolytes levels in hemodialysis patients. Iran J Kidney Dis 2012; 6: 119–123 [PubMed] [Google Scholar]
- 67.Chen JL, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant 2010; 25: 1936–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reboredo Mde M, Pinheiro Bdo V, Neder JA, et al. Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J Bras Nefrol 2010; 32: 367–373 [PubMed] [Google Scholar]
- 69.Afshar R, Shegarfy L, Shavandi N, et al. Effects of aerobic exercise and resistance training on lipid profiles and inflammation status in patients on maintenance hemodialysis. Indian J Nephrol 2010; 20: 185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koh KP, Fassett RG, Sharman JE, et al. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis 2010; 55: 88–99 [DOI] [PubMed] [Google Scholar]
- 71.Painter P, Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens 2013; 22: 615–623 [DOI] [PubMed] [Google Scholar]
- 72.Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stack AG, Molony DA, Rives T, et al. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis 2005; 45: 690–701 [DOI] [PubMed] [Google Scholar]
- 74.Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; 10: CD003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2014; 64: 383–393 [DOI] [PubMed] [Google Scholar]
- 76.Drawz PE, Babineau DC, Brecklin C, et al. Heart rate variability is a predictor of mortality in chronic kidney disease: a report from the CRIC Study. Am J Nephrol 2013; 38: 517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-Heart). Circulation 1998; 98: 1510–1516 [DOI] [PubMed] [Google Scholar]
- 78.Otterstad JE, Ford I. The effect of carvedilol in patients with impaired left ventricular systolic function following an acute myocardial infarction. Eur J Heart Fail 2002; 4: 501–506 [DOI] [PubMed] [Google Scholar]
- 79.U.S. Renal Data System, USRDS 2013. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013. [Google Scholar]
- 80.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995; 273: 408–412 [DOI] [PubMed] [Google Scholar]
- 81.Hewitt C, Hahn S, Torgerson DJ, et al. Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ 2005; 330: 1057–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol 2014; 25: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]