Abstract
Background
Longer nephrology care before end-stage renal disease (ESRD) has been linked with better outcomes.
Methods
We investigated whether longer pre-end-stage renal disease (ESRD) nephrology care was associated with lower mortality at both the patient and state levels among 443 761 incident ESRD patients identified in the USA between 2006 and 2010.
Results
Overall, 33% of new ESRD patients had received no prior nephrology care, while 28% had received care for >12 months. At the patient level, predictors of >12 months of nephrology care included having health insurance, white race, younger age, diabetes, hypertension and US region. Longer pre-ESRD nephrology care was associated with lower first-year mortality (adjusted hazard ratio = 0.58 for >12 months versus no care; 95% confidence interval 0.57–0.59), higher albumin and hemoglobin, choice of peritoneal dialysis and native fistula and discussion of transplantation options. Living in a state with a 10% higher proportion of patients receiving >12 months of pre-ESRD care was associated with a 9.3% lower relative mortality rate, standardized for case mix (R2 = 0.47; P < 0.001).
Conclusions
This study represents the largest cohort of incident ESRD patients to date. Although we did not follow patients before ESRD onset, our findings, both at the individual patient and state levels, reflect the importance of early nephrology care among those with chronic kidney disease.
Keywords: dialysis, glomerular filtration rate, kidney transplantation, nephrology referral, vascular access
Introduction
In 2012, a total of 114 893 individuals in the USA reached end-stage renal disease (ESRD) [1]. For those approaching ESRD, clinical practice guidelines cover multiple care domains, such as optimal management of blood pressure, diabetes and anemia, as well as preparation for ESRD, including timely vascular access placement or referral for kidney transplantation [2]. The involvement of a nephrologist throughout the more advanced stages of chronic kidney disease (CKD), preferably in collaboration with a multidisciplinary team, has been strongly recommended [3]. Earlier referral provides the nephrologist time to identify and manage reversible conditions, ensure avoidance of nephrotoxic agents, administer specific therapies, recommend dietary and lifestyle changes to slow the progression of kidney decline, manage comorbidities and complications and institute regular follow-up, education and activation of social support. Even if progression to ESRD is inevitable, earlier nephrology care can optimally prepare the patient for renal replacement therapy, both physically and mentally.
Early studies considering the impact of nephrologist involvement prior to ESRD generally assessed only a few months of care (e.g. 1–4 months prior to ESRD onset). More recent publications have considered periods longer than the Kidney Disease Outcomes Quality Initiative (KDOQI)–recommended minimum of 6 months of pre-ESRD care. Results from the national Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study suggested that increased nephrologist involvement was associated with improvements in patient preparation and outcomes [4]. Other studies have indicated that the involvement of a nephrologist prior to initiating dialysis improves a patient's readiness for dialysis [5–7], access to transplant waiting lists [8] and first-year survival after the start of dialysis [9–15]. These studies reported on selected populations, with data collected prior to 2008.
We analyzed more recent and nationwide US ESRD data from 2006 through 2010 to explore variations in pre-ESRD nephrology care among incident ESRD patients and its association with mortality at both the patient and state levels. We hypothesized that a longer duration of pre-ESRD care would be associated with improved outcomes at, and following, the onset of ESRD. Analyses were performed at both the usual patient level and the region/state level, which have implications for policymakers for improving population health.
Materials and methods
Study design
The U.S. Department of Health and Human Services' Centers for Medicare and Medicaid Services (CMS) provides federally managed health insurance for those with ESRD. Data from the CMS Medical Evidence Form 2728 are required to be submitted prospectively for all incident ESRD patients in the USA [16]. Questions added to Form 2728 in 2005 included ‘Prior to ESRD therapy: was the patient under care of a nephrologist (yes, no, unknown)? If yes, was the duration 6–12 months or >12 months?’ An answer of ‘yes’ to the first question but no answer to the second question was considered to be a duration of <6 months. Other questions requested information on the type of vascular access used on first outpatient dialysis, and whether the patient was informed of transplant options.
All 537 940 incident cases of ESRD occurring between 1 January 2006 and 31 December 2010 with information from Form 2728 were eligible for inclusion in these analyses. Individuals were excluded if their pre-ESRD nephrology care status was unknown (n = 69 789; 13%) or if their ESRD was likely due to acute kidney injury (n= 24 390; 5%; based on the International Classification of Diseases, Ninth Revision, Clinical Modification codes for primary cause of ESRD’ 282.60, 282.69, 283.11, 572.4, 580.0, 580.89, 583.4, 583.6, 593.81, 593.83, 646.20, 866.0). The final dataset included 443 761 (82%) individuals (dialysis or transplant recipients) beginning ESRD therapy; 11 590 (2.6%) of these were transplants.
Predictors and outcomes
Additional data collected from Form 2728 included patient demographics, comorbid conditions, health insurance coverage and recent (within 45 days) laboratory results. Mortality data were identified via linkage with CROWNWeb (Consolidated Renal Operations in a Web-enabled Network), the CMS Renal Management Information System (REMIS) and CMS Form 2746 (ESRD Death Notification). When multiple mortality dates were recorded (n = 18), the median was used for analyses; most differences were of just a few days. State of residence was as reported at the time of ESRD onset; states were grouped by US region (I–X), as defined by the CMS.
We chose to examine first-year mortality after the onset of ESRD among dialysis patients because first-year mortality is particularly high and is most likely affected by pre-ESRD exposures [17]. Outcome indicators of health and ESRD preparedness were serum albumin >3.5 versus ≤3.5 g/dL, hemoglobin >11 versus ≤11 g/dL, choice of peritoneal dialysis (PD) versus hemodialysis (HD) in dialysis patients, availability of a mature graft or fistula versus catheter and whether informed of transplant options versus not informed among dialysis patients.
Statistical methods
Separate analyses were conducted with each of the following types of outcomes: (i) indicators of health and ESRD preparedness at the start of ESRD (data from 2006 to 10) and (ii) mortality in the first year of dialysis at both the individual and state levels (data from 2007 to 10).
All indicators of health and ESRD preparedness outcomes, dichotomized into more versus less desirable categories, were assessed for their association with the duration of pre-ESRD care (four categories) using logistic regression. In addition to the duration of pre-ESRD care, other covariates included in the models were age, gender, race, ethnicity, body mass index (BMI) at ESRD incidence, diabetes as the primary cause of ESRD, comorbidities at incidence (congestive heart failure, atherosclerotic heart disease, cerebrovascular disease, other cardiac diseases, chronic obstructive pulmonary disease, peripheral vascular disease, diabetes, cancer, tobacco use, alcohol dependence, drug dependence, inability to ambulate and inability to transfer), nursing home status and year of incidence. Associations of the duration of pre-ESRD care with each of these outcome indicators are presented as adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
Time to death within the first year of dialysis was modeled using Cox regression censored at the earlier of transplant or Day 365 since first dialysis. The duration of pre-ESRD care was the exposure of primary interest, with adjustment for the same covariates listed in the previous paragraph. Unadjusted and partially adjusted models were also fit to assess possible confounding. Hazard ratios (HRs) and 95% CIs are presented. For some analyses, states were grouped by region (I–X) as defined by the CMS.
In addition to the individual-level analyses described above, a state-level ecologic analysis was performed in the dialysis population to assess the association between the proportion of patients with >12 months of pre-ESRD nephrology care and first-year mortality, where both the predictor and outcome were adjusted for the same covariates (age, sex, race, ethnicity, diabetes, nursing home status, BMI and comorbidities at ESRD onset and state age-adjusted race-specific death rate). The outcome was the average of the 2007–9 year-specific standardized mortality ratios (SMRs) for each state compared with the US dialysis population. These SMRs were obtained from the CMS Dialysis Facility Reports Technical Notes on the Standardized Mortality Ratio for the Dialysis Facility Reports [18]. The predictor was the estimated proportion of new ESRD patients in each state with >12 months of pre-ESRD nephrology care, standardized for the same covariates as the SMR (except the state death rate). Each standardized proportion was obtained by using logistic regression to estimate the predicted probability of >12 months pre-ESRD nephrology care for each patient, then averaging those probabilities across all patients in the state. Linear regression was used to estimate the ecologic association between the standardized proportion of patients with >12 months pre-ESRD nephrology care and the SMR. Data were analyzed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Of the 443 761 incident cases of ESRD in the USA included in this study, 33% had received no nephrology care prior to ESRD onset, 14% had received <6 months, 25% had received ≥6 but ≤12 months and 28% had received >12 months of care prior to ESRD onset (Table 1). Almost half (49%) were ≥65 years of age, 38% were age 45–64 and <1% were of ≤18 years of age. Diabetes was listed as the primary cause of ESRD for 59% of these individuals, 86% had hypertension, 7% had no health insurance and <1% were nursing home residents.
Table 1.
Frequency distribution (row percents) of prior duration of nephrology care among all incident ESRD patients (2006–10) in the USA by selected variables
| Overall | Duration of nephrology carea |
||||
|---|---|---|---|---|---|
| No care | <6 months | 6–12 months | >12 months | ||
| 100% (n = 443 761) | 33% (n = 147 746) | 14% (n = 61 086) | 25% (n = 110 339) | 28% (n = 124 590) | |
| Year | |||||
| 2006 | 88 186 | 33.4 | 12.6 | 27.7 | 26.3 |
| 2007 | 87 366 | 33.9 | 12.3 | 26.7 | 27.1 |
| 2008 | 87 897 | 33.6 | 13.6 | 24.3 | 28.5 |
| 2009 | 90 129 | 33.0 | 14.9 | 23.4 | 28.7 |
| 2010 | 90 183 | 32.6 | 15.5 | 22.3 | 29.6 |
| Age (years) | |||||
| <18 | 3472 | 30.1 | 17.6 | 21.2 | 31.2 |
| 18–44 | 53 960 | 41.5 | 12.5 | 23.5 | 22.6 |
| 45–64 | 168 731 | 34.8 | 13.4 | 25.3 | 26.6 |
| 65–74 | 104 276 | 29.6 | 14.1 | 25.6 | 30.7 |
| ≥75 | 113 322 | 30.7 | 14.5 | 24.3 | 30.5 |
| Sex | |||||
| Female | 195 943 | 32.7 | 13.8 | 25.2 | 28.3 |
| Male | 247 818 | 33.8 | 13.8 | 24.6 | 27.9 |
| Race | |||||
| White | 290 578 | 31.4 | 13.9 | 24.8 | 29.9 |
| African American | 127 243 | 37.8 | 13.2 | 24.7 | 24.3 |
| Asian | 18 944 | 31.4 | 15.7 | 26.3 | 26.6 |
| Native American | 4943 | 35.9 | 11.5 | 26.2 | 26.3 |
| Others | 2053 | 37.2 | 12.4 | 23.7 | 26.7 |
| Ethnicity | |||||
| Hispanic | 59 521 | 40.4 | 10.7 | 26.8 | 22.0 |
| Non-Hispanic | 382 240 | 32.2 | 14.2 | 24.6 | 29.0 |
| BMI (kg/m2) | |||||
| <15 | 2136 | 38.2 | 17.0 | 23.3 | 21.6 |
| 15–19 | 32 041 | 39.6 | 13.3 | 23.7 | 23.5 |
| 20–24 | 115 583 | 35.9 | 13.5 | 24.4 | 26.2 |
| 25–29 | 124 150 | 32.8 | 13.9 | 24.9 | 28.5 |
| ≥30 | 165 668 | 30.6 | 13.9 | 25.4 | 30.2 |
| Primary cause of ESRD | |||||
| Diabetes | 262 499 | 31.0 | 14.0 | 26.6 | 28.4 |
| Other | 181 262 | 36.7 | 13.4 | 22.3 | 27.7 |
| Other comorbidity | |||||
| Hypertension | 382 399 | 32.1 | 13.5 | 25.2 | 29.2 |
| Cardiac failure | 147 019 | 34.4 | 13.3 | 24.8 | 27.5 |
| ASHD | 98 165 | 29.1 | 13.1 | 25.1 | 32.8 |
| CVA | 42 924 | 32.9 | 12.6 | 25.0 | 29.5 |
| COPD | 40 503 | 34.6 | 13.0 | 23.6 | 28.8 |
| Cancer | 32 910 | 34.3 | 12.7 | 22.6 | 30.4 |
| Health insurance | |||||
| Any | 411 589 | 31.2 | 14.2 | 25.4 | 29.3 |
| None | 32 172 | 59.7 | 8.8 | 18.5 | 13.0 |
| Nursing home | |||||
| Resident | 209 | 31.6 | 16.3 | 22.0 | 30.1 |
| Nonresident | 443 552 | 33.3 | 13.8 | 24.9 | 28.1 |
| Region of residenceb | |||||
| I | 15 562 | 20.6 | 8.1 | 29.2 | 42.2 |
| II | 49 465 | 37.5 | 8.4 | 26.1 | 28.1 |
| III | 46 445 | 31.7 | 20.4 | 22.2 | 25.7 |
| IV | 98 738 | 32.9 | 15.1 | 23.8 | 28.2 |
| V | 72 560 | 32.0 | 17.1 | 21.8 | 29.1 |
| VI | 59 740 | 39.0 | 4.4 | 28.9 | 27.7 |
| VII | 17 638 | 31.1 | 12.5 | 23.2 | 33.2 |
| VIII | 9109 | 29.5 | 11.9 | 23.5 | 35.0 |
| IX | 61 289 | 33.7 | 19.4 | 26.8 | 20.2 |
| X | 13 079 | 26.0 | 8.1 | 25.6 | 40.3 |
ASHD, atherosclerotic heart disease; BMI, body mass index; CVA, cerebrovascular atherosclerosis; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease.
aDuration of nephrology care prior to ESRD onset as reported on CMS Form 2728.
bRegions are as follows—I: CT, MA, ME, NH, RI and VT; II: NJ, NY, VI and PR; III: DE, MD, PA, VA, WV and DC; IV: AL, FL, GA, KY, MS, NC, SC and TN; V: IL, IN, MI, MN, OH and WI; VI: AR, LA, NM, OK, TX; VII: IA, KS, MO and NE; VIII: CO, MT, ND, SD, UT and WY; IX: AZ, CA, HI, NV, AS, GU and MP; X: AK, ID, OR and WA. For state abbreviations and map, see http://www.stateabbreviations.us.
Any nephrology care prior to ESRD onset (versus no care) was associated with better health status and preparedness at the start of ESRD (Table 2). In these adjusted analyses, pre-ESRD nephrology care was positively associated with initial serum albumin >3.5 g/dL, hemoglobin >11 g/dL and having been informed of transplant options. Pre-ESRD nephrology care was strongly and positively associated with initial PD versus HD [OR for >12 months versus no care = 5.50 (95% CI 5.29–5.73)] and initial graft or fistula versus catheter access [OR = 11.3 (95% CI 11.0–11.5)]. Although not formally analyzed due to small numbers, preemptive transplants were received by only three patients who had no pre-ESRD nephrology care, but by 13, 15 and 47 patients who had <6, 6–12 and >12 months of pre-ESRD nephrology care, respectively.
Table 2.
Adjusteda odds ratios (95% confidence intervals) for health and end stage renal disease (ESRD) preparedness at the time of ESRD, by duration of nephrology care (<6, 6–12 and >12 months versus no care)
| Outcome | No care | <6 months | 6–12 months | >12 months |
|---|---|---|---|---|
| Favorable laboratory values | ||||
| Albumin >3.5 versus ≤3.5 g/dL | 1 | 1.96 (1.91, 2.00) | 1.91 (1.88, 1.95) | 2.50 (2.45, 2.54) |
| Hemoglobin >11 versus ≤11 g/dL | 1 | 1.54 (1.51, 1.58) | 1.51 (1.48, 1.54) | 1.76 (1.73, 1.80) |
| Optimal dialysis methods | ||||
| Starting with peritoneal or other versus hemodialysis | 1 | 4.57 (4.37, 4.78) | 4.23 (4.06, 4.41) | 5.51 (5.29, 5.73) |
| First vascular access with graft or fistula versus catheter | 1 | 6.77 (6.59, 6.96) | 6.45 (6.29, 6.62) | 11.26 (10.99, 11.53) |
| Access to transplantation | ||||
| Informed of transplantation option versus not informed | 1 | 1.43 (1.39, 1.46) | 1.39 (1.36, 1.41) | 1.51 (1.49, 1.54) |
| Number of patients: total | n = 147 746 | n = 61 086 | n = 110 339 | n = 124 590 |
| Albumin >3.5 g/dL | n = 63 624 | n = 32 782 | n = 57 148 | n = 71 450 |
| Hemoglobin >11 g/dL | n = 39 520 | n = 20 674 | n = 36 715 | n = 45,934 |
| Starting with peritoneal or other dialysis type | n = 3454 | n = 5857 | n = 9431 | n = 13 784 |
| First access with graft or fistula | n = 9240 | n = 19 232 | n = 32 941 | n = 53 234 |
| Informed of transplant options | n = 43 846 | n = 14 317 | n = 26 547 | n = 28 600 |
aAdjusted for age, gender, race, ethnicity, insurance status, body mass index, diabetes as a cause of ESRD, comorbidities, year of incidence and nursing home residence. For all odds ratios presented, P-values were <0.0001.
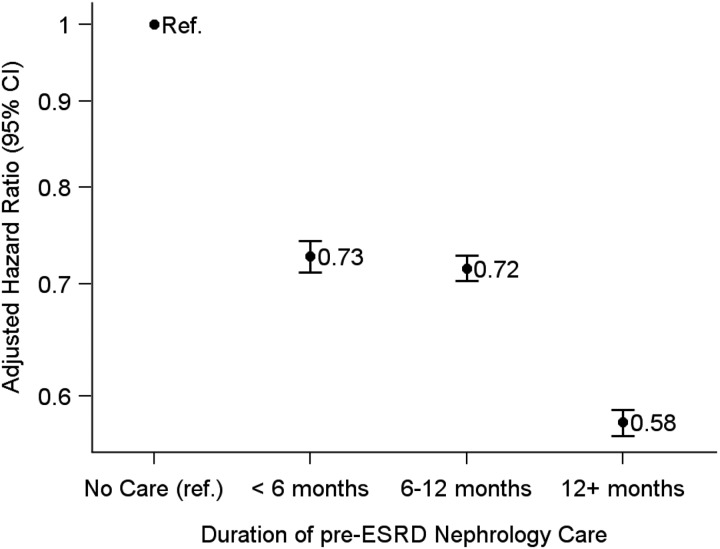
There was an inverse dose–response association between the duration of pre-ESRD nephrology care and the mortality rate in the year after dialysis onset (Figure 1). Compared with patients with no prior nephrology care, the adjusted HR was 0.73 (95% CI 0.71–0.74) for patients with <6 months of care, 0.72 (95% CI 0.70–0.73) for patients with 6–12 months of care and 0.58 (95% CI 0.57–0.59) for patients with >12 months of care.
Fig. 1.
Adjusted hazard ratios (and 95% confidence intervals) for mortality during the first year of dialysis, by duration of pre-ESRD nephrology care (<6, 6–12 and >12 months versus no care). Models were adjusted for age, gender, race, ethnicity, insurance, body mass index, diabetes as a cause of ESRD, comorbidities, year of incidence and nursing home residence. ESRD, end stage renal disease; Ref, reference category.
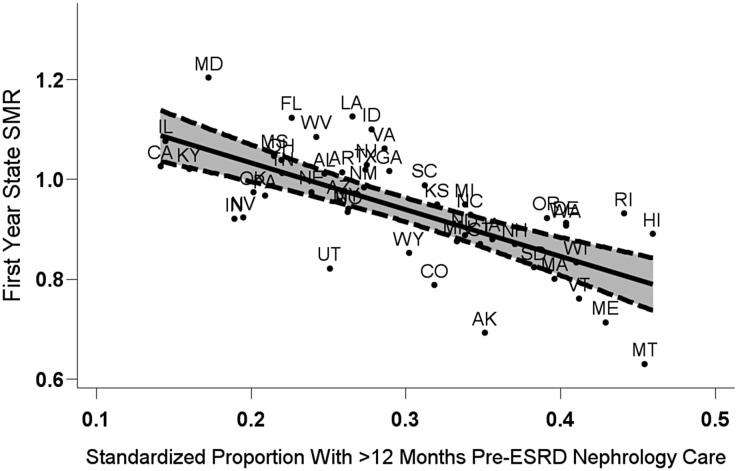
Figure 2 shows the ecologic association between the standardized proportion of new ESRD patients with >12 months of pre-ESRD nephrology care (X-axis) and the SMR for first-year mortality (Y-axis) among all US states. States with a 10% higher standardized proportion of patients receiving >12 months of pre-ESRD care had, on average, a 9.3% lower SMR (R2 = 0.47; P < 0.001).
Fig. 2.
First-year state-specific standardized mortality ratio (SMR) by adjusted state-specific probability of >12 months of pre-ESRD nephrology care among US dialysis patients, 2007–10. The fitted regression line (; R2 = 0.47, P < 0.001) is given with pointwise 95% confidence intervals. Each state SMR is based on an adjusted comparison of first-year mortality in the dialysis populations of that state versus the USA, standardized to the US mean covariate values. Both the SMR and proportion with >12 months of pre-ESRD nephrology care were adjusted for the same covariates: patient's age, sex, race, ethnicity, body mass index, comorbidities at incidence, diabetes as the listed cause of ESRD, duration of ESRD, nursing home status and population death rates (SMR only). ESRD, end stage renal disease.
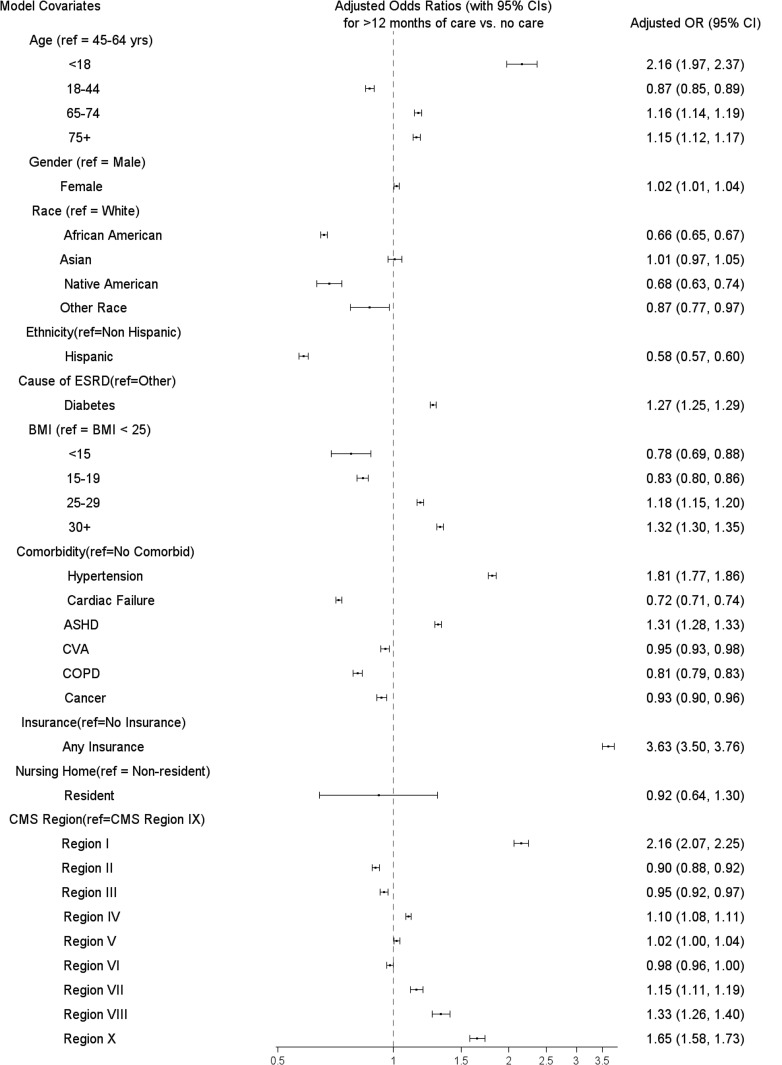
Figure 3 summarizes adjusted associations between patient characteristics and >12 months of pre-ESRD nephrology care versus no care. The strongest predictor of this outcome was insurance status [OR for insured versus uninsured = 3.6 (95% CI 3.5–3.8)]. There was also a positive monotonic association between BMI and the duration of pre-ESRD nephrology care. Other groups with more pre-ESRD care included age <18 years (versus older) and hypertension (versus no comorbidity listed in Figure 3). More than 12 months of pre-ESRD care was less frequent in African Americans and Native Americans (versus whites) and Hispanics (versus non-Hispanics). There was considerable variability in pre-ESRD nephrology care across the nine CMS regions. More than 12 months of pre-ESRD care was most common in Regions I (New England states), X (Alaska, Idaho, Oregon, Washington) and VIII (Colorado, Montana, North Dakota, South Dakota, Utah, Wyoming).
Fig. 3.
Adjusted1 OR (95% CI) for >12 months of pre-ESRD nephrology care versus no care by category of selected covariates. ASHD, atherosclerotic heart disease; BMI, body mass index; CI, confidence interval; CMS, Centers for Medicare and Medicaid Services; CVA, cerebrovascular atherosclerosis; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; OR, odds ratio. 1Adjusted for age, sex, race, ethnicity, BMI, diabetes as a cause of ESRD, comorbidities (hypertension, cardiac failure, ASHD, CVA, COPD, cancer), insurance status, nursing home residence, year of incidence and CMS region. Regions are as follows—I: CT, MA, ME, NH, RI and VT; II: NJ, NY, VI and PR; III: DE, MD, PA, VA, WV and DC; IV: AL, FL, GA, KY, MS, NC, SC and TN; V: IL, IN, MI, MN, OH and WI; VI: AR, LA, NM, OK and TX; VII: IA, KS, MO and NE; VIII: CO, MT, ND, SD, UT and WY; IX: AZ, CA, HI, NV, AS, GU and MP; X: AK, ID, OR and WA. For state abbreviations, see http://www.stateabbreviations.us.
Discussion
Although a number of studies have examined the link between pre-ESRD care and patient outcomes after ESRD onset, this is not only the largest study of its kind to date, including >400 000 incident ESRD patients throughout the USA during a recent 5-year period (2006–10), but it is also unique in that it reports the results of an analysis at the state level. Results indicate that US states that demonstrate a greater proportion of patients receiving longer nephrology care prior to ESRD have lower SMRs at their dialysis facilities. While this result may simply be due to the contextual effects of living in a state that has other factors/policies that improve access to care or health status, it raises the possibility that if states/regions were to make concerted policy efforts to ensure a longer duration of pre-ESRD nephrology care, the mortality rates at their dialysis facilities could potentially improve.
Consistent with previous reports from many smaller US studies [4–14], as well as international studies [19–23], our results indicate that a longer duration of pre-ESRD nephrology care is associated with both better patient readiness for ESRD and greater 1-year survival after the start of dialysis. Patients receiving at least 12 months of nephrology care were more likely to be better informed of their ESRD options including PD and transplant, more likely to have a preemptive transplant or have a functional graft or fistula in place for dialysis, have more favorable laboratory values at the start of ESRD and have a higher probability of surviving the critical first year of dialysis treatment. In spite of these benefits, one-third of new ESRD patients were found to have had no pre-ESRD nephrology care, and such care varied appreciably across US regions. Many who initiated ESRD therapy were from expected high-risk populations (i.e. older, diagnosed with diabetes and/or hypertension, with disproportionate representation of minorities). However, nearly half of this incident population was <65 years of age, a third were of normal or low BMI and nearly 10% were uninsured, all factors that may make them less likely to be identified by the healthcare system or to see a nephrologist for care.
Results linking individual-level nephrologist care and patient survival during the first year of dialysis were also seen at the state level, even when adjusting for individual patient characteristics. These results may reflect the contextual effect of living in a state with more nephrology care, controlling for individual pre-ESRD nephrology-care status. Alternatively, they may reflect the individual-level effect of receiving nephrology care earlier in the course of CKD (as suggested by our findings in Figure 1) or ecologic bias resulting from missing data in the state-level analysis; those two effects are confounded in our ecologic analysis [24]. Furthermore, the ecologic findings may also be confounded by unmeasured patient-level or state-level factors such as healthcare policies or programs affecting population awareness and access. Given the strong association we observed between health insurance coverage and longer duration of pre-ESRD nephrology care, we might expect the Affordable Care Act to have a noticeable impact on CKD nephrology care and early ESRD mortality.
Results presented here show that the majority of US ESRD patients do not receive at least 12 months of pre-ESRD care, with wide variation in the length of care across the country. Although 12 months of pre-ESRD care was found to be optimal in our study, Bradbury et al. [25] found that any predialysis nephrology care improved postdialysis mortality, with the greatest benefit found during the first 4 months of dialysis. Barriers to pre-ESRD care arise from a variety of sources, with a major factor being healthcare access. As our results show, pre-ESRD care was related to patient characteristics, including demographics, insurance status and health indicators. These results are consistent with the findings of McClellan et al. [15], who emphasized patients' socioeconomic status as a major predictor of access to nephrology care, and related geographic variation in care to zip codes of residence associated with poverty. Crews et al. [26] found that low socioeconomic status was associated with a much larger association with kidney disease in African Americans than in whites. In addition, there is a growing body of literature detailing, at both the individual and healthcare-system levels, a lack of awareness of declining kidney function [27–30]. National Institutes of Health-based initiatives, such as the National Kidney Disease Education Program, aim to increase awareness of CKD at both the patient and provider levels, which may lead to corresponding increases in nephrologist care [31]. Another potential barrier to care is the availability of practicing nephrologists to meet the needs of a growing population with CKD [32, 33]. Delay of primary care physicians in referring patients to nephrologists represents another barrier to optimization of care [34] that might explain the significant proportion of ‘acute’ discoveries of ESRD. Advancements in healthcare information technology [e.g. computer-based decision support systems and automated reporting of estimated glomerular filtration rate (eGFR)], as well as the development of organized CKD clinics and CKD registries, may alleviate some of these deficiencies by reminding clinicians from both primary and specialty care to test, manage and appropriately refer individuals with established kidney disease as well as those with identified kidney disease risk factors [35, 36]. In this context, research into system changes that could reduce barriers to nephrology care, improve patient outcomes and reduce health disparities is needed.
Limitations of this study include a lack of data to adjust for potential confounders such as a patient's socioeconomic status, social support network, awareness of disease, general access to care, symptom severity or prior rate of CKD progression. A second limitation is that ‘<6 months’ on the Form 2728 question on the duration of pre-ESRD care could only be selected by nonendorsement of the other two categories; this choice was indistinguishable from nonendorsement because the duration of nephrologist care was unknown, possibly leading to misclassification. A third limitation is the nonavailability of a patient's eGFR at their first nephrology visit, as internationally recognized guidelines such as those from the KDOQI and Kidney Disease: Improving Global Outcomes base nephrology referral on a patient's eGFR or CKD stage. Similarly, the number and quality of nephrology visits within the indicated duration of care were unavailable. This additional information could be addressed prospectively by ongoing studies such as the Chronic Renal Insufficiency Cohort study [37]. Perhaps the major limitation of this study is that the study population was restricted to new ESRD patients. Thus, it does not allow us to directly address the fundamental question of interest for guiding clinical practice: at what point in the course of CKD should individuals initiate nephrology care? Recommending referral at least 12 months before ESRD requires inaccessible knowledge of the future. To address this fundamental clinical question, we would need to follow patients with CKD from earlier stages to the start of ESRD or death, using an observational or randomized design.
Because the duration of pre-ESRD nephrology care from the Medical Evidence Report (Form 2728) is not strongly correlated with that variable from Medicare claims [38], we recognize that our variable is likely measured with error, which could have biased our results. The direction of such bias, however, would probably be toward the null value of HR = 1, unless the amount of misclassification was strongly and monotonically associated with mortality, conditional on measured covariates, which seems unlikely. Thus, the strong associations we observed were not likely due to misclassification bias. Furthermore, our estimates of the relative hazard of mortality with and without prior nephrology care are consistent with those of Bradbury et al. [25].
In conclusion, we found that a large proportion of new ESRD patients in the USA had not received pre-ESRD nephrology care, and the average duration of such care varied widely across the country. Our findings suggest that early nephrology care of CKD patients may, if the disease progresses to ESRD, reduce first-year mortality after ESRD onset, presumably by improving the patient's health and readiness for renal replacement therapy. Our state-level analysis suggests significant variation in this pattern of care among US states and indicates the potential benefits of policy-driven, earlier CKD care for ESRD patients living in regions with lower rates and with longer duration nephrology care. In this context, the proportion of CKD patients receiving timely nephrology care is likely to improve with greater availability of health insurance under the Affordable Care Act, which may yield health benefits to persons with CKD/ESRD through improved access to care as well as general health promotion efforts.
The Department of Health and Human Services' Healthy People 2010 (HP2010) objectives to improve pre-ESRD care (particularly nephrology referral and arteriovenous fistula placement) have been retained in the proposed HP2020 objectives [39]. These national objectives, as well as the ongoing multipronged response to CKD that US federal agencies have developed [40], should continue to help policymakers focus efforts and allocate resources appropriately. The results provided here should stimulate thoughtful discussion on developing public health strategies to encourage earlier referral to a nephrologist among all socio-economic groups. Such strategies have the potential to improve outcomes for those with CKD and ESRD.
Authors' contributions
The authors contributed to this article as follows: R.S. developed the original investigation concept; E.H., B.W.G., H.M., A.T., N.S. and T.S. took part in guiding or executing the data analysis; A.T. created all figures; manuscript preparation was led by B.W.G., H.M., E.H. and R.S.; all authors contributed to interpretation of data and writing the manuscript. R.S., N.S., and T.S. had full access to all study data; the corresponding author assumes final responsibility for the decision to submit for publication. Technical writing and editorial assistance were provided by Janet Kavanagh, MS.
Conflicts of interest statement
All authors declare no competing interests. The funder had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Acknowledgements
This study was supported through grants from the Centers for Medicare & Medicaid Services, CMS contract numbers HHSM-500-2006-00042C and HHSM-500-2011-00091C. Support for this work was also provided under a cooperative agreement from the Centers for Disease Control and Prevention (CDC) through grant numbers 5U36CD319276 and 5U58DP003836-04. Publication and report contents are solely the responsibility of the authors and do not necessarily represent the official views of the CMS or the CDC.
References
- 1.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: USRDS, 2012 [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 3.Thanamayooran S, Rose C, Hirsch DJ. Effectiveness of a multidisciplinary kidney disease clinic in achieving treatment guideline targets. Nephrol Dial Transplant 2005; 20: 2385–2393 [DOI] [PubMed] [Google Scholar]
- 4.Astor BC, Eustace JA, Powe NR, et al. Timing of nephrologist referral and arteriovenous access use: the choice study. Am J Kidney Dis 2001; 38: 494–501 [DOI] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Glynn RJ, Levin R, et al. Late referral and modality choice in end-stage renal disease. Kidney Int 2001; 60: 1547–1554 [DOI] [PubMed] [Google Scholar]
- 6.Avorn J, Winkelmayer WC, Bohn RL, et al. Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 2002; 55: 711–716 [DOI] [PubMed] [Google Scholar]
- 7.Winkelmayer WC, Glynn RJ, Levin R, et al. Late nephrologist referral and access to renal transplantation. Transplantation 2002; 73: 1918–1923 [DOI] [PubMed] [Google Scholar]
- 8.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137: 479–486 [DOI] [PubMed] [Google Scholar]
- 9.Avorn J, Bohn RL, Levy E, et al. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med 2002; 162: 2002–2006 [DOI] [PubMed] [Google Scholar]
- 10.Stack AG. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 2003; 41: 310–318 [DOI] [PubMed] [Google Scholar]
- 11.Khan SS, Xue JL, Kazmi WH, et al. Does predialysis nephrology care influence patient survival after initiation of dialysis? Kidney Int 2005; 67: 1038–1046 [DOI] [PubMed] [Google Scholar]
- 12.Chan MR, Dall AT, Fletcher KE, et al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med 2007; 120: 1063–1070 [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa T, Bragg-Gresham JL, Yamazaki S, et al. Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin J Am Soc Nephrol 2009; 4: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006; 48(Suppl 1): S248–S273 [DOI] [PubMed] [Google Scholar]
- 15.McClellan WM, Wasse H, McClellan AC, et al. Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol 2009; 20: 1078–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dialysis Facility Reports. Ann Arbor, MI: University of Michigan Kidney Epidemiology and Cost Center, 2014. https://www.dialysisdata.org/ (15 February 2010, date last accessed) [Google Scholar]
- 17.Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 2013; 85: 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Technical Notes on the Standardized Mortality Ratio (SMR) for the Dialysis Facility Reports. Ann Arbor, MI: University of Michigan Kidney Epidemiology and Cost Center, 2014. https://www.dialysisdata.org/sites/default/files/content/Methodology/SMR%20Documentation.pdf (19 January 2015, date last accessed) [Google Scholar]
- 19.Jones C, Roderick P, Harris S, et al. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant 2006; 21: 2133–2143 [DOI] [PubMed] [Google Scholar]
- 20.Dogan E, Erkoc R, Sayarlioglu H, et al. Effects of late referral to a nephrologist in patients with chronic renal failure. Nephrology 2005; 10: 516–519 [DOI] [PubMed] [Google Scholar]
- 21.Gorriz J, Sancho A, Pallardo L, et al. [Prognostic significance of programmed dialysis in patients who initiate renal substitutive treatment. Multicenter study in Spain]. Nefrologia 2001; 22: 49–59 [PubMed] [Google Scholar]
- 22.Mendelssohn DC, Malmberg C, Hamandi B. An integrated review of ‘unplanned’ dialysis initiation: reframing the terminology to ‘suboptimal’ initiation. BMC Nephrol 2009; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan S, Amedia CA. Economic burden of chronic kidney disease. J Eval Clin Pract 2008; 14: 422–434 [DOI] [PubMed] [Google Scholar]
- 24.Morgenstern H. Ecologic studies. In: Rothman KJ, Greenland S, Lash TL. (eds). Modern Epidemiology. 3rd edn Philadelphia: Lippincott Williams & Wilkins, 2008, pp. 511–531 [Google Scholar]
- 25.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89–99 [DOI] [PubMed] [Google Scholar]
- 26.Crews DC, Charles RF, Evans MK, et al. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis 2010; 55: 992–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whaley-Connell A, Sowers JR, McCullough PA, et al. Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 2009; 53: S11–S21 [DOI] [PubMed] [Google Scholar]
- 28.Ferris M, Shoham DA, Pierre-Louis M, et al. High prevalence of unlabeled chronic kidney disease among inpatients at a tertiary-care hospital. Am J Med Sci 2009; 337: 93–97 [DOI] [PubMed] [Google Scholar]
- 29.Flessner MF, Wyatt SB, Akylbekova EL, et al. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis 2009; 53: 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med 2008; 168: 2268–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narva AS, Briggs M. The National Kidney Disease Education Program: improving understanding, detection, and management of CKD. Am J Kidney Dis 2009; 53: S115–S120 [DOI] [PubMed] [Google Scholar]
- 32.Kohan DE, Rosenberg ME. The chronic kidney disease epidemic: a challenge for nephrology training programs. In: Levin A and Himmelfarb J (eds). Seminars in Nephrology. Amsterdam: Elsevier Science, Abstract 29, 2009, pp. 539–547. [DOI] [PubMed] [Google Scholar]
- 33.Pogue VA, Norris KC, Dillard MG. Kidney disease physician workforce: where is the emerging pipeline? J Natl Med Assoc 2002; 94: 39S. [PMC free article] [PubMed] [Google Scholar]
- 34.Levin A. Consequences of late referral on patient outcomes. Nephrol Dial Transplant 2000; 15: 8–13 [DOI] [PubMed] [Google Scholar]
- 35.Patel TG, Pogach LM, Barth RH. CKD screening and management in the Veterans Health Administration: the impact of system organization and an innovative electronic record. Am J Kidney Dis 2009; 53: S78–S85 [DOI] [PubMed] [Google Scholar]
- 36.den Hartog JR, Reese PP, Cizman B, et al. The costs and benefits of automatic estimated glomerular filtration rate reporting. Clin J Am Soc Nephrol 2009; 4: 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol 2003; 14: S148–S153 [DOI] [PubMed] [Google Scholar]
- 38.Kim JP, Desai M, Chertow GM, et al. Validation of reported predialysis nephrology care of older patients initiating dialysis. J Am Soc Nephrol 2012; 23: 1078–1085 [DOI] [PubMed] [Google Scholar]
- 39.US Department of Health and Human Services. Healthy People 2020 Topics & Objectives. Washington, DC: US Department of Health and Human Services, 2010. http://www.healthypeople.gov/2020/topicsobjectives2020/default (19 January 2015, date last accessed) [Google Scholar]
- 40.Narva AS, Briggs M, Jordan R, et al. Toward a more collaborative federal response to chronic kidney disease. Adv Chronic Kidney Dis 2010; 17: 282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]