Abstract
Structural and functional brain connectivity, synaptic activity and information processing require highly coordinated signal transduction between different cell types within the neurovascular unit and intact blood-brain barrier (BBB) functions. Here, we examine the mechanisms regulating the formation and maintenance of the BBB and functions of BBB-associated cell types. Furthermore, we discuss the growing evidence associating BBB breakdown with the pathogenesis of inherited monogenic neurological disorders and complex multifactorial diseases including Alzheimer’s disease.
Introduction
Blood vessels in the brain are organized with surprising precision supporting the major brain circuits tasked with sensation, memory and motion (Andreone et al., 2015; Blinder et al., 2013; Zlokovic, 2011). Proper structural and functional brain connectivity, synaptic activity and information processing all require precise regulation of cerebral blood flow (CBF), oxygen delivery and energy metabolite supply (Attwell et al., 2010; Iadecola, 2013). These key central nervous system (CNS) functions are maintained by the highly coordinated activity of multiple cell types within the neurovascular unit (NVU), including vascular cells (endothelial cells, pericytes, smooth muscle cells), glia (astrocytes, oligodendroglia, microglia) and neurons (Figure 1) (Zlokovic, 2011).
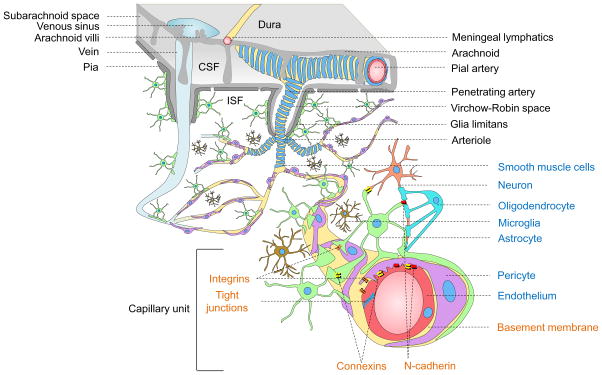
Figure 1. Neurovascular unit.
Vessels in the Subarachnoid space: The subarachnoid space contains cerebrospinal fluid (CSF) that drains via arachnoid villi into the venous sinuses. Cerebral arteries branch into smaller pial arteries. Cerebral veins empty into dural venous sinuses. Meningeal lymphatic vessels carry CSF and immune cells to deep cervical lymph nodes.
Intracerebral vessels: Pial arteries give rise to the penetrating arteries that branch into arterioles all covered by vascular smooth muscle cells (blue). The penetrating arteries are separated from brain parenchyma by the glia limitans, an astrocytic endfeet layer that forms the outer wall of the Virchow-Robin spaces containing brain interstitial fluid (ISF, white). Arterioles branch off into capillaries and the vessels enlarge, as they become venules and veins.
Brain capillary unit: Endothelial cells (red) connected by tight junctions form the blood-brain barrier. Pericytes (purple) and endothelium share a common basement membrane (yellow) and connect with each other with several transmembrane junctional proteins including N-cadherin and connexins. Astrocytes (green) connect with pericytes, endothelial cells and neurons (peach). Microglia (brown) regulate immune responses. Oligodendro-cytes (aqua) support neurons with axonal myelin sheath. Integrins make connections between cellular and matrix components.
Within the NVU, the endothelial cells form the blood-brain barrier (BBB) that limits entry of potentially neurotoxic plasma components, blood cells and pathogens into the brain (Winkler et al., 2011). Importantly, these endothelial cells express multiple substrate-specific transport systems that control transport of nutrients, energy metabolites and other essential molecules from blood into the brain and the transport of metabolic waste products from brain’s interstitial fluid (ISF) into the blood. The meningeal lymphatic vessels contain cerebrospinal fluid (CSF) and immune cells and drain into the deep cervical lymph nodes (Aspelund et al., 2015; Louveau et al., 2015). Thus, the BBB serves as a key homeostatic site of the nervous system connecting CNS, systemic circulation, and major systems in the body such as respiratory, renal, hepatic and immune systems.
In this review, first we examine the cellular and molecular mechanisms regulating the formation and maintenance of the BBB. We then look into how major NVU cell types contribute to BBB functions and how molecular alterations and aberrant signal transduction within the NVU leads to BBB breakdown that is associated with secondary neuronal injury and neurodegeneration. In particular, we discuss the role of NVU and BBB breakdown in the etiology and pathogenesis of inherited monogenic neurological disorders and complex neurodegenerative disorders such as Alzheimer’s disease (AD). Finally, we discuss key questions in the field for future investigation.
BBB development
Different stages of BBB formation are illustrated in Figure 2A. The neural microenvironment provides initial cues for CNS angiogenesis and induction of the BBB properties (Obermeier et al., 2013). At embryonic day E10 in mice, the angioblasts of the perineural vascular plexus penetrate the neuroectoderm guided by neuroectoderm-secreted vascular endothelial growth factor (VEGF), which results in formation of the nascent ‘leaky’ blood vessels (Potente et al., 2011). Wnt ligands secreted by neural cells elicit canonical Wnt signaling in the endothelium by binding to the Frizzled receptors (Wang et al., 2012b) and co-receptors low-density lipoprotein receptor-related protein (LRP) 5 and 6, which in turn activates β-catenin-dependent pathways (Daneman et al., 2009; Liebner et al., 2008; Zhou et al., 2014) (Figure 2B). Activation of Wnt/β-catenin signaling leads to induction of genes critical for the BBB formation, such as glucose transporter Glut1 (Stenman et al., 2008), and death receptors DR6 and TROY (Tam et al., 2012). In addition, an orphan G-protein coupled receptor, Gpr124 acts as a specific co-activator of Wnt/β-catenin signaling at the BBB (Kuhnert et al., 2010; Zhou and Nathans, 2014).
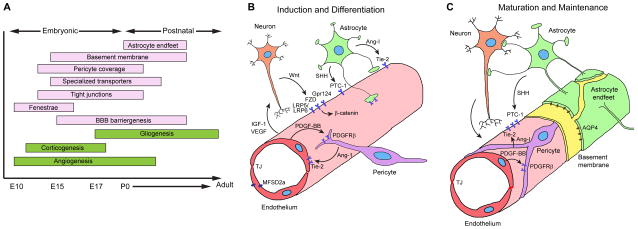
Figure 2. Blood-brain barrier development in the murine central nervous system.
A. Developmental timeline. Restriction of paracellular and transcellular transport of solutes is accomplished by elimination of endothelial fenestrae and pinocytosis, formation of a continuous endothelial monolayer connected with the tight junctions, creation of highly selective endothelial transport systems, and establishment of specialized perivascular structures, including the basement membrane and the coverage of the endothelial capillary wall by pericytes and astrocytic endfeet. E, embryonic days; P, postnatal days.
B. Induction and differentiation. Wnt ligands (Wnt7a/7b) secreted by neural cells bind to endothelial Frizzled receptors (FZD) and co-receptors low-density lipoprotein receptor-related protein (LRP) 5 and 6, which activate β-catenin signaling, leading to the induction of BBB specific genes. G-protein coupled receptor 124 (Gpr124) co-activates Wnt/β-catenin signaling. Endothelial cells secrete platelet-derived growth factor BB (PDGF-BB), which interacts with platelet derived growth factor receptor-β (PDGFR-β) in pericytes, inducing pericyte recruitment. Pericytes and astrocytes secrete angiopoietin-1 (Ang-1) that acts on endothelial Tie-2 receptor leading to microvascular maturation and highly stable and impermeable BBB. Pericytes are required for the expression of endothelial major facilitator superfamily domain-containing protein 2a (MFSD2a) that is critical for the BBB formation and maintenance. Astrocytes secrete sonic hedgehog (SHH) that acts on endothelial patched homolog 1 (PTC-1) receptor eliciting signaling which contributes to the BBB formation. Endothelial cells secrete vascular growth factor (VEGF) and insulin growth factor (IGF-1) contributing to proper neurovascular patterning. Additional signal transduction pathways may also participate in BBB formation. TJ, tight junction.
C. Maturation and maintenance. Postnatally, brain capillaries are covered by mature pericytes sharing the basement membrane with endothelium. Astrocytic endfeet form the outer layer of the mature capillaries. Peri-cytes and astrocytes continue secreting matrix proteins (yellow) of the basement membrane. Signaling pathways mediating BBB induction and differentiation likely continue to play a role in BBB maturation and maintenance and their dysregulation may lead to BBB breakdown causing different central nervous system pathologies. AQP4, aquaporin-4 water channel.
The primitive BBB is formed at embryonic day E15 in mice (Ben-Zvi et al., 2014; Daneman et al., 2010), but the exact timing is species-dependent and varies regionally. It is debatable, whether humans and/or other mammals are born with a fully functional BBB (Saunders et al., 2013). Recruitment of pericytes to the developing endothelial capillary wall is critical for the formation and maintenance of the BBB (Armulik et al., 2010; Bell et al., 2010; Daneman et al., 2010). While some pathways have been implicated, it remains unclear exactly which signals are involved in the pericyte-mediated induction and regulation of the BBB (Figure 2B). Astrocytes recruited at a later stage further assist endothelium in acquiring BBB characteristics, barrier properties and CNS immune quiescence (Alvarez et al., 2011) (Figure 2B).
The BBB functions continue to mature after birth, but the exact time window remains elusive and is likely species-dependent (Hagan and Ben-Zvi, 2015; Saunders et al., 2013). At a mature stage, the mammalian BBB is stabilized by highly specialized perivascular structures (Figure 2C).
Cellular Components of the BBB
Endothelial Cells and Cellular Junctions
A number of factors contribute to the physical barrier of BBB, including endothelial tight junction (TJ) and adherens junction (AJ) proteins, inhibition of non-selective fenestrae, pinocytosis and bulk flow transcytosis, as well as suppression of leukocyte adhesion molecules (Obermeier et al., 2013). Endothelium allows rapid free diffusion of oxygen from blood to brain and carbon dioxide from brain to blood, which is essential for normal brain metabolism and regulation of pH in the brain ISF, neurons and other NVU cells. Small lipophilic molecules and drugs, with a molecular weight of < 400 Da and form of <8 hydrogen bonds, can cross the BBB (Pardridge, 2015).
The major endothelial transport systems and cellular junction molecules are briefly discussed below, and described in detail elsewhere (Daneman and Prat, 2015; Hagan and Ben-Zvi, 2015; Tietz and Engelhardt, 2015; Zlokovic, 2008, 2011). A preliminary molecular atlas of the BBB based on manual collection of available data on protein and RNA expression as well as physiological measurements from different published investigations is provided in Table 1. It is noteworthy that some of the listed components are not confirmed in recent RNAseq data (see e.g. http://web.stanford.edu/group/barres_lab/brain_rnaseq.html). Since expression may be species, strain, disease or context dependent, we provide Table 1 as an all-inclusive data source and encourage the readers to explore and critically examine the specifics in existing literature.
Table 1.
Molecular atlas of the blood-brain barrier: transport systems and cellular junctions.
| Transport systems | Transporters | Abbreviation | Gene name | Substrates | Direction of transport |
|---|---|---|---|---|---|
| Active efflux | ABC-transporters | MDR1/P-gp | ABCB1 | Drugs, xenobiotics | Endothelium to blood |
| ABCA2 | ABCA2 | --- | |||
| BCRP | ABCG2 | Drugs, xenobiotics | |||
| MRP1 | ABCC1 | Drugs, drug conjugates | |||
| MRP2 | ABCC2 | ||||
| MRP3 | ABCC3 | ||||
| MRP4 | ABCC4 | Nucleosides | Bidirectional | ||
| MRP5 | ABCC5 | Endothelium to blood | |||
| Solute carrier-mediated transport | Carbohydrate transporters | GLUT1 | SLC2A1 | Glucose | Bidirectional |
| SGLT1 | SLC5A1 | Blood to brain | |||
| HMIT | SLC2A13 | Myoinositol | Blood to brain | ||
| SMIT | SLC5A3 | ||||
| Amino acid transporters | CAT1 | SLC7A1 | Cationic L-amino acids (y+) (e.g. lysine, arginine) | Bidirectional | |
| CAT3 | SLC7A3 | ||||
| LAT1 | SLC7A5 | Large neutral amino acids (L) (e.g. tryptophan, tyrosine) | Bidirectional | ||
| LAT2 | SLC7A6 | ||||
| SNAT1 | SLC38A1 | Glutamine and Small neutral amino acids (A) | Brain to blood | ||
| SNAT2 | SLC38A2 | ||||
| SNAT 3 | SLC38A3 | Glutamine (N) | |||
| SNAT 5 | SLC38A5 | Bidirectional | |||
| ASCT1 | SLC1A4 | Neutral amino acids (ASC) | Brain to blood | ||
| ASCT2 | SLC1A5 | ||||
| EAAT1 | SLC1A3 | Excitatory amino acids (EAAT) (e.g. glutamate, aspartate) | |||
| EAAT2 | SLC1A2 | ||||
| EAAT3 | SLC1A1 | ||||
| GLYT1 | SLC6A9 | Glycine | |||
| TAUT | SLC6A6 | Taurine, GABA | |||
| Monocarboxylic acid transporter | MCT1 | SLC16A1 | Lactate, ketone bodies | Bidirectional | |
| MCT2 | SLC16A7 | Lactate (Proton exchanger) | Brain to blood | ||
| Hormone transporter | MCT8 | SLC16A2 | T3 thyroid hormone | Blood to brain | |
| Fatty acids | FATP-1 | SLC27A1 | Fatty acids | Blood to brain | |
| FATP-4 | SLC27A4 | ||||
| Nucleotide transporters | CNT2 | SLC28A2 | Nucleotides, nucleobases | Brain to blood | |
| ENT1 | SLC29A1 | Bidirectional | |||
| ENT2 | SLC29A2 | ||||
| Organic anion and cation transporters | OAT3 | SLC22A8 | Organic anions (e.g. indoxyl sulfate, benzylpenicillin aminohippuric acid) | Brain to blood | |
| OATP1A4 | SLCO1A4 | Bidirectional | |||
| OATP2B1 | SLCO2B1 | ||||
| OATP1C1 | SLCO1C1 | Thyroxine | |||
| OCT1 | SLC22A1 | Organic cations (e.g. morphine, MPTP, creatinine) | Blood to brain | ||
| OCT2 | SLC22A2 | ||||
| OCT3 | SLC22A3 | ||||
| OCTN2 | SLC22A5 | Organic cations, carnitine | Bidirectional | ||
| Amine transporter | PMAT | SLC29A4 | Organic cations, MPP+ | Brain to blood | |
| Choline transporter | CTL1 | SLC44A1 | Choline | Bidirectional | |
| Vitamin transporters | SMVT | SLC5A6 | Multivitamins | Blood to brain | |
| Receptor-mediated transport | Protein ligands | V1 | AVPR1A | Arginine-vasopressin | Bidirectional |
| TfR | TFRC, TFR2 | Transferrin | Blood to brain | ||
| LEP-R | LEPR | Leptin | |||
| IR | INSR | Insulin | |||
| LRP1 | LRP1 | Apolipoproteins, Amyloid-β, etc. | Brain to blood | ||
| LRP2 | LRP2 | ||||
| RAGE | AGER | Glycosylated proteins, Aβ, S-100, etc. | Blood to brain | ||
| Major facilitators | Fatty acids | MFSD2a | MFSD2a | Docosahexaenoic acid | Blood to brain |
| Cellular junctions | Components | Abbreviation | Genes | Functions | |
| Tight junctions | Occludin | Occludin | OCLN | Not required for TJ formation | |
| Claudins | Claudin-1 | CLDN1 | Sealing BBB | ||
| Claudin-3 | CLDN3 | Unknown | |||
| Claudin-5 | CLDN5 | Size-selective barrier to small molecules (<800 D) | |||
| Claudin-12 | CLDN12 | Unknown | |||
| Membrane-associated guanylate kinases | ZO-1 | TJP1 | Multi-domain scaffolding proteins, cytoskeleton anchorage for TJ proteins | ||
| ZO-2 | TJP2 | ||||
| ZO-3 | TJP3 | ||||
| Adherens junctions | Adherens junctions | Cadherins | CDH5 | Cytoskeleton link, modulating receptor signaling, regulating transendothelial migration of lymphocytes | |
| PECAM-1 | PECAM1 | ||||
| Other Junctional molecules | JAMs | JAM-A | F11R | Modulating junctional tightness, regulating transendothelial migration of lymphocytes | |
| JAM-B | JAM2 | ||||
| JAM-C | JAM3 | ||||
| ESAM | ESAM | ESAM | |||
| Gap Junctions | Connexin hemichannels | CX30 | GJB6 | Cell-cell communication, required for BBB | |
| CX43 | GJA1 | ||||
| Cytoskeleton | Dystrophin | Dystrophin | DMD | Organization of actin cytoskeleton | |
Active efflux
Multiple ATP-binding cassette (ABC) proteins are expressed on the luminal, blood-facing endothelial plasma membrane of the BBB, which restricts the permeability of large number of toxins including therapeutic agents (Miller, 2015). ABC transporters are ATP-driven efflux pumps for xenobiotics and endogenous metabolites. Their high expression at the BBB contributes to CNS pharmacoresistance (Table 1). Decreased expression and/or functional activity of ABC BBB transporters were reported in patients with Alzheimer’s disease (AD) and Parkinson’s’ disease (PD) (Zlokovic, 2011) and were shown to lead to accumulation of amyloid β-peptide (Aβ) in the brain in an animal model of AD (Cirrito et al., 2005). The clinical potential of targeting ABC transporters for disease management and drug delivery improvement, however, remains elusive.
Carrier-mediated transport (CMT)
CMT systems are expressed by genes within the Solute Carrier (SLC) Transporter Gene Family, including > 300 transporter genes encoding membrane-bound proteins that facilitate the transport of a wide array of substrates across biological membranes (Lin et al., 2015). At the BBB, the SLC proteins facilitate the transcellular transport of a variety of molecules, including carbohydrates, amino acids, monocarboxylic acids, hormones, fatty acids, nucleotides, organic anions, amines, choline and vitamins (Table 1) (Daneman and Prat, 2015; Pardridge, 2015; Zlokovic, 2008). Human genetic studies have provided important insight into the roles of the more recently characterized SLC transporters in both rare and common diseases. As discussed below, genetic alterations in brain endothelial SLC2A1 (Glut1) that transports glucose into the brain and maintains the BBB integrity (Winkler et al., 2015) and in SLC16A2 that transports T3 thyroid hormone into the brain have been implicated in the development of neurological disorders (Benarroch, 2014; Kersseboom et al., 2013). Screening for BBB-penetrating small molecules that can use the existing CMT systems at the BBB as surrogate ligands has been recently proposed as a new model for CNS discovery programs (Pardridge, 2015).
Receptor-mediated transport (RMT)
Peptide bond prevents larger peptides and proteins from using the amino acid CMT systems to cross the BBB (Zlokovic et al., 1985). However, certain neuroactive peptides (Zlokovic, 1995), regulatory proteins, hormones and growth factors can use RMT systems to slowly cross the BBB (Pardridge, 2015; Zlokovic, 2008) (Table 1). For example, Aβ can cross the BBB to enter the circulation through LRP1 (Deane et al., 2004) or the other way around via the receptor for advanced glycation end products (RAGE) on RAGE-expressing endothelium, particularly under pathological conditions (Deane et al., 2003, 2012). Moreover, RMT systems, for example the transferrin receptor (TfR), have been utilized for the CNS drug delivery (Bray, 2015).
Major facilitator superfamily
Docosahexaenoic acid (DHA), an essential omega-3 fatty acid, is transported into the brain by the endothelial major facilitator superfamily domain-containing protein 2a (MFSD2a) (Nguyen et al., 2014). Mice lacking Mfsd2a show brain DHA deficits and develop BBB breakdown (Ben-Zvi et al., 2014), suggesting that MFSD2a has the dual function of transporting fatty acids into the brain and maintaining BBB integrity (Betsholtz, 2014; Zhao and Zlokovic, 2014).
TJ proteins seal brain endothelia, contributing to the anatomical barrier (Table 1). Mutations in and loss of some TJ proteins lead to BBB breakdown and are associated with neurological disorders, as discussed below (Tietz and Engelhardt, 2015; Zlokovic, 2008). TJ proteins are connected to cortical actin cytoskeleton via multi-domain scaffolding proteins of the peripheral membrane-associated guanylate kinase (MAGUK) family, i.e. ZO-1, ZO-2 and ZO-3 (Tietz and Engelhardt, 2015). ZO-1 deficiency disrupts TJs, and reduced ZO-1 levels are associated with BBB breakdown in many neurological disorders (Zlokovic, 2011) (Figure 3). AJ proteins such as cadherins and platelet endothelial cell adhesion molecule-1 also contribute to barrier properties, and the space between these proteins is approximately 20 nm, which is smaller compared to that between TJs (Tietz and Engelhardt, 2015) (Table 1; Figure 3). Brain endothelial cells are anchored to the basement membrane through integrins, which interact with extracellular matrix (ECM) proteins, such as laminin, collagen, and perlecan, and mediate signaling by activating ECM ligands, growth factors and growth factor receptors (Baeten and Akasso-glou, 2011). Mice lacking β1-integrins in endothelial cells develop aberrant cadherin signaling, loss of junctional claudin-5 and immature BBB (Yamamoto et al., 2015). Similarly, mice lacking astrocyte-secreted laminin exhibit BBB breakdown (Yao et al., 2014).
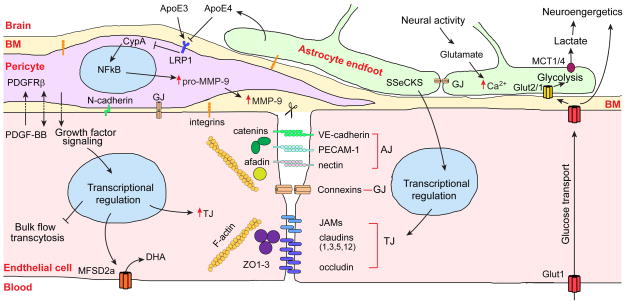
Figure 3. The vascular triad of the blood-brain barrier.
Endothelial cells are connected with each other through the tight junction (TJ), adherens junction (AJ) and gap junction (GJ) proteins. In the TJs, occludin, claudins and junctional adhesion molecules (JAMs) form a impermeable barrier to fluids and are connected to F-actin filaments by the zonula occludens ZO-1, ZO-2 and ZO-3 multi-domain scaffolding proteins of the membrane-associated guanylate kinase family. GJs formed by connexin hemichannels are specialized for direct intercellular communications. AJs are formed by homotypic binding of VE-cadherin, platelet endothelial cell adhesion molecule-1 (PECAM-1) and Nectin. Catenins link VE-cadherin to F-actin, while nectin is secured to F-actin by afadin.
Pericytes communicate with the endothelial cells via growth factor-mediated signaling (unidirectional or bidirectional), adhesion via N-cadherin homotypic binding and GJs. Pericyte can modulate BBB permeability by regulating gene expression in the endothelial cells resulting in upregulation of TJ proteins, inhibition of bulk flow transcytosis and upregulation of brain endothelial specific docosahexaenoic acid (DHA) transporter, a major facilitator domain-containing protein 2A (MFSD2a). Both endothelial cells and pericytes are embedded in the basement membrane (BM) and anchored to BM via integrins. PDGF-BB, platelet-derived growth factor BB; PDGFRβ, platelet-derived growth factor receptor-β.
Astrocytes regulate expression of matrix metallo-proteinase-9 (MMP-9) in pericytes by secreting apolipoprotein E (ApoE). ApoE3, but not ApoE4, binds to the low density lipoprotein receptor-related protein 1 (LRP1) in peri-cytes, which suppresses the proinflammatory cyclophilin A (CypA)-nuclear factor-κB (NFκB)-MMP-9 pathway and degradation of TJ and BM proteins causing BBB breakdown. Astrocytes signal endothelial cells by Src-suppressed C-kinase substrate (SSeCKS) to increase TJ protein expression. Neural activity-dependent glutamate release increases [Ca2+] in the astrocytic endfeet, which regulates vascular tone. The GJs connect the adjacent astrocytic endfeet. Glucose gets into the brain via the endothelial Glut1 transporter and is taken up by neurons via Glut3. Glucose is taken up by astrocytes mainly by Glut2 and is metabolized to lactate, which is exported to neurons by the monocarboxylic MCT1 and MCT4 transporters.
Pericytes
Pericytes share a basement membrane with endothelial cells and form direct synaptic-like peg-socket focal contacts with endothelium through N-cadherin and connexins, allowing exchanges of ions, metabolites, second messengers and ribonucleic acids between the two cell types (Armulik et al., 2011). Pericytes play important roles in maintaining BBB integrity, aiding in angiogenesis and microvascular stability (Armulik et al., 2011; Winkler et al., 2011), regulating capillary diameter and CBF (Hall et al., 2014; Peppiatt et al., 2006), and phagocytosing toxic metabolites (Sagare et al., 2013). They have also been reported to have multipotent stem cell capabilities (Nakagomi et al., 2015). Pericyte degeneration and injury occur in many neurological diseases including AD (Baloyannis and Baloyannis, 2012; Farkas and Luiten, 2001; Halliday et al., 2015; Sengillo et al., 2013), mild dementia (Montagne et al., 2015), amyotrophic lateral sclerosis (ALS) (Winkler et al., 2013), and stroke (Hall et al., 2014).
Much of the insight into pericyte biology stems from the analysis of pericyte-deficient mice with disrupted platelet-derived growth factor BB (PDGF-BB)/platelet derived growth factor receptor-β (PDGFR-β) signaling (Armulik et al., 2011). PDGF-BB secreted by endothelial cells binds to heparan sulphate porteoglycans in the basement membrane, and its concentration gradient regulates pericyte proliferation, migration and recruitment to the vessel wall through PDGFRβ receptor in pericytes. PDGF-B or PDGFRβ null mice have a complete loss of pericytes, resulting in rupture of CNS microvessels, microaneurysms and embryonic lethality (Lindahl et al., 1997; Tallquist et al., 2003). Pericytes are essential for maintaining BBB integrity in the adult and aging CNS (Armulik et al., 2010; Bell et al., 2010; Daneman et al., 2010) (Figure 3). Implications of disrupted PDGF-B/PDFGRβ signaling for human neurological disorders are discussed below. Multiple signaling pathways in pericytes contribute to CNS vascular stability as examined in detail elsewhere (Armulik et al., 2011; Winkler et al., 2011). Moreover, signaling between astrocytes and pericytes exerts significant impact on BBB integrity. On one hand, studies in transgenic apolipoprotein E (APOE) mice have shown that APOE4, a major genetic risk factor for AD (Zlokovic, 2013), leads to disruption of BBB integrity by activating the proinflammatory cyclophilin-A (CypA)-nuclear factor-κB-matrix-metalloproteinase-9 (MMP-9) pathway in pericytes, which in turn leads to degradation of the basement membrane and TJ proteins causing chronic BBB breakdown followed by neuronal dysfunction and secondary neurodegenerative changes (Bell et al., 2012). In contrast, apoE3 and apoE2, which confer a lower risk for AD, suppress the CypA-MMP-9 pathway through LRP1 on pericytes, supporting the maintenance of BBB functions (Figure 3). On the other hand, pericyte loss leads to the loss of astrocyte-derived components from the endfeet (Armulik et al., 2010), but the pericyte-derived signal(s) in this case are unknown.
Astrocytes
Astrocytes contribute to a variety of dynamic regulations in the neural system and play a vital role in CNS inflammation in neurodegenerative diseases (Clarke and Barres, 2013; Sofroniew, 2015). However, it remains unclear whether astrocytes are essential for BBB maintenance. The glial limitans ensheathing the penetrating arterial blood vessels, and the outer layer of mature capillaries are formed by astrocytic endfeet (Figure 3). Yet, genetic lineage tracing and ablation studies showed that regional ablation had no effect on BBB permeability (Tsai et al., 2012). In contrast, others have shown that Src-suppressed C-kinase substrate (SSeCKS) in astrocyte progenitors regulates angiogenesis and formation of TJs at the BBB by modulating VEGF and Ang-1 expression (Lee et al., 2003). Future studies should provide more definitive answers as to whether astrocytes play a role in BBB maintenance in the adult and aging brain.
BBB and Monogenic Neurological Disorders
Several genetic diseases appear to originate in individual cell types of the NVU and link to specific roles in BBB development, function and regulation. Such diseases, while rare, offer insights into causal pathogenic links and chains of events. Below we briefly discuss some of these diseases, the relevant cell types (Table 2) and the potential pathogenic role of BBB dysfunction.
Table 2.
Human monogenic inherited diseases with blood-brain barrier dysfunction.
| Disease | Symptoms | Cell types | Subcellular components | Proteins | Genes |
|---|---|---|---|---|---|
| Band-like calcification with simplified gyration and polymicrogyria (BLC-PMG) | Early-onset seizures, severe microcephaly, developmental delay with bilateral polymicrogyria and a band of gray matter calcification on brain imaging. | Tight junctions | Occludin | OCLN | |
| Hemorrhagic destruction of the brain, subependymal calcification and congenital cataracts | Brain hemorrhage, subependymal calcification, congenital cataracts, spasticity, exaggerated deep-tendon reflexes, and seizures | JAM-C | JAM3 | ||
| Familial cerebral cavernous malformations (CCM) | Seizures, headaches, intracerebral hemorrhages, focal neurological deficits, and gait ataxia. | Endothelial cells | Cytoplasm and nucleus | CCM1 | KRIT1 |
| CCM2 | CCM2 | ||||
| CCM3 | PDCD10 | ||||
| Cerebral small vessel disease | Lacunar ischemic strokes, deep intracerebral hemorrhages, white matter hyperintensities | Basement membrane | COL4A1 | COL4A1 | |
| COL4A2 | COL4A2 | ||||
| GLUT1 deficiency syndrome (De Vivo syndrome) | Early-onset seizures, microcephaly, mild movement disorder and developmental delay. | Transporters | GLUT1 | SLC2A1 | |
| Microcephaly | Lethal or non-lethal microcephaly, intellectual disability, spasticity and absent speech. | MFSD2a | MFSD2a | ||
| Allan-Herndon-Dudley syndrome (AHDS) | Severe psychomotor retardation and altered serum thyroid parameters. | MCT8 | SLC16A2 | ||
| Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) | Migraines, progressive dementia, mood disorders and minor strokes. | Vascular smooth muscle cells | Receptor | CADASIL | NOTCH3 |
| Primary familial brain calcification (Idiopathic basal ganglia calcification, Fahr’s disease) | Calcifications in the basal ganglia, cerebellum, thalamus and brainstem lead to motor, cognitive and psychiatric symptoms. | Pericytes | Growth factor Receptor Transporter Receptor | PDGF-BB | PDGFB |
| PDGFRβ | PDGFRB | ||||
| PIT2 | SLC20A2 | ||||
| SYG1 | XPR1 | ||||
| Alexander disease | Infantile form: megalencephaly, seizures and impaired physical and mental development, early death. Later-onset: bulbar signs including difficulties with coordination, speech and swallowing. | Astrocytes | Cytoskeleton | GFAP | GFAP |
| Megalencephalic leukoencephalopathy with subcortical cysts (MLC) | Macrocephaly, cerebral white matter swelling and subcortical cysts, increased water content in the brain, and myelin and astrocyte vacuolation. | Membrane protein | MLC1 | MLC1 | |
| GLIALCAM | HEPACAM | ||||
| Congenital muscular dystrophy 1A (MDC1A) | Muscular weakness, raised creatine phosphokinase, cerebral white matter abnormalities, respiratory insufficiency, early death. | Basement membrane | LAMM | LAMA2 | |
| Nasu-Hakkola disease (NHD) | Progressive senile dementia characterized by extensive demyelination, accumulation of axonal spheroids and multifocal bone cysts. | Microglia | Membrane protein | DAP12 | TYROBP |
| TREM2 | TREM2 |
Endothelial cells
Several inherited CNS diseases are caused by mutations in genes that play pivotal roles in endothelial cells or in the endothelium-derived ECM. These genes encode proteins that are either structural components or regulators of the endothelial cell-cell junctions, the vascular basement membrane, or transporters critically involved in BBB maintenance. For instance, mutations in the genes encoding the TJ proteins occludin (O’Driscoll et al., 2010) and junctional adhesion molecule-C (JAM-C) (Woodfin et al., 2011) lead to severe problems in brain growth, hemorrhage and calcification (Table 2). These pathologies may result from uncontrolled leakage of solutes and plasma proteins across the endothelial junctions and/or neuroinflammatory changes due to increased transendothelial migration of leukocytes, a process inhibited by JAM-C. Another example is familial cerebral cavernous malformations (CCM), occurring in hereditary or sporadic forms and together affecting approximately 0.5% of the population. CCM is caused by mutations in three genes, CCM1-3, leading to similar thin-walled, leaky vascular lesions of venous origin (Fischer et al., 2013). The CCM proteins likely act together in a complex that maintains endothelial junctional organization and polarization and inhibits endothelial-to-mesenchymal transition (Maddaluno et al., 2013). Collagen COL4A1 and COL4A2 are abundant in all basement membranes and expressed by many cell types including vascular endothelial cells. Mutations in these genes are associated with a diverse range of problems in several organs, including the brain, where it is associated with cerebral hemorrhage and small vessel disease (Gould et al., 2006). Work in animal models with Col4a1 deficiency suggests that increased vessel fragility could make the animal susceptible to hemorrhage either upon mild trauma or due to the anatomy of the vessel that is particularly sensitive to increased hemodynamic stress (Kuo et al., 2012).
Among transporters mutated in brain disorders, two striking examples of BBB endothelial proteins are GLUT1 and MFSD2a. GLUt1, the major glucose transporter at the BBB, is mutated and functionally inactivated in human GLUT1 deficiency syndrome, a disease associated with early onset seizures and microcephaly (Wang et al., 2000). This is consistent with the importance of sufficient GLUT1 levels and glucose transport across the BBB for brain function and its role in maintaining the BBB integrity (Winkler et al., 2015). Similar to GLUT1, MFSD2a is highly expressed on brain endothelial cells. It transports lipids in the form of lysophoshatidylcholine coupled to certain long fatty acyl chains and is critical for the maintenance of the BBB integrity (Ben-Zvi et al., 2014; Nguyen et al., 2014). Microcephaly syndrome was recently shown to be caused by inactivating mutations in MFSD2A, the severity of the syndrome correlating with the degree of functional inactivation of the MFSD2A protein (Alakbarzade et al., 2015; Guemez-Gamboa et al., 2015). These studies provide insight into the cause of a rare disease, but perhaps more importantly, are an illustration to how basic physiological knowledge may come from human and mouse genetics and offer a fundamental insight into the mechanisms by which brain transports lipids across the BBB (Betsholtz, 2015) and maintains BBB integrity (Betsholtz, 2014; Zhao and Zlokovic, 2014). A third example of a brain disease associated with a BBB transporter is Allan-Herndon-Dudley syndrome, a psychomotor retardation syndrome caused by inactivating mutations in the triiodothyronin (T3) transporter SLC16A2 (MCT8) (Dumitrescu et al., 2004; Friesema et al., 2004). It is thought that the severe intellectual disability and movement problems observed in these patients are due to deficient transport of T3 from the blood to the brain, resulting in impairment of neuronal development and function. Indeed, different degrees of SLC16A2 inactivation correlate with the phenotypic consequences in patients (Capri et al., 2013).
Vascular mural cells
CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is a relatively common (2–4/100,000 individuals) autosomal dominant stroke syndrome caused by mutations in NOTCH3, a gene known to be specifically expressed in vascular mural cells (Chabriat et al., 2009). Although, the precise pathogenic mechanisms of CADASIL remain unresolved, recent studies of Notch 3 null mice demonstrated focal disruption of the BBB with tracer leakage and perivascular fibrin deposits in the CNS (Henshall et al., 2015). Primary familial brain calcification (PFBC, a.k.a idiopathic basal ganglia calcification (IBGC) or Fahr’s disease) is characterized by early onset microvascular calcification occurring in certain deep brain regions, most notably the basal ganglia. Disease symptoms include motoric and cognitive problems suggestive of significant neuronal dysfunction. The recent description of loss-of-function mutations in PDGFB and PDGFRB genes in PFBC (Keller et al., 2013; Nicolas et al., 2013) suggests a role for pericytes in this disease. In different mouse models based on mutations in Pdgfb that led to variable levels of defect in PDGF-B/PDGFRβ signaling, a correlation was noted among the extent of pericyte loss, BBB deficiency and brain calcification (Keller et al., 2013). This is suggestive of a role for BBB dysfunction in PFBC, possibly involving changes in phosphate transport, since mutations in the phosphate transporters SLC20A2 and XPR1 also cause PFBC (Legati et al., 2015; Wang et al., 2012a).
Astrocytes and Microglia
Mutations in astrocyte-specific genes are associated with various neurological disorders, such as glial fibrillay acidic protein (GFAP) in Alexander disease, and MLC1 and HEPACAM in megalecenphalic leukoenceph-alopathy with subcortical cysts. These mutations may affect BBB integrity. However, the involvement of brain vascular dysfunction in these diseases remains to be established. Laminin α2 chains (LAMA2), an astrocyte gene encoding the basement membrane protein LAMA2, is mutated in congenital muscular dystrophy, a disease that affects the brain in some patients in ways that are suggestive of a defective BBB (e.g., brain edema) (Alkan et al., 2007). Reactive microglia are commonly found in association with leaky brain vessels. Mutations in the microglia-specific genes DAP12 and TREM2 cause Nasu-Hakkola disease (Sasaki et al., 2015), a neurodegenerative disorder with unclear pathogenesis. It remains to be determined if Nasu-Hakkola disease is associated with a dysfunctional BBB.
BBB and Multifactorial Neurodegenerative Diseases
Vascular contributions to dementia and AD (Iadecola, 2013; Montagne et al., 2015; Montine et al., 2014; Snyder et al., 2015; Sweeney et al., 2015) and other neurodegenerative disorders such as ALS (Winkler et al., 2013), PD (Korczyn, 2015) and Huntington disease (HD) (Drouin-Ouellet et al., 2015) are increasingly recognized. A number of vascular dysfunctions are frequently associated with neurodegeneration, including hypertension, cerebrovascular disorder, BBB breakdown, etc (Iadecola, 2013; Montagne et al., 2015; Snyder et al., 2015). Experimental studies in murine transgenic models with a chronic BBB breakdown due to aberrant endothelial-pericyte and/or astrocyte-pericyte signaling have shown that accumulation in the CNS, particularly in neurons, of blood-derived neurotoxic proteins including fibrinogen, thrombin, red blood cell-derived hemoglobin, iron-containing hemosiderin, free iron and/or plasmin can initiate and/or contribute to neurodegeneration (Armulik et al., 2010; Bell et al., 2010, 2012; Daneman et al., 2010; Davalos et al., 2012). ApoE knockout mice with dysfunctional BBB, but not wild type mice with normal BBB, develop psychotic behavioral impairment when injected with N-methyl-D-aspartate receptor autoantibody–positive serum, suggesting that seroprevalence in neuropsychiatric diseases could be related to an insult to BBB integrity (Hammer et al., 2014). The model we propose in Figure 4 may apply to various types of vascular-mediated neurodegeneration.
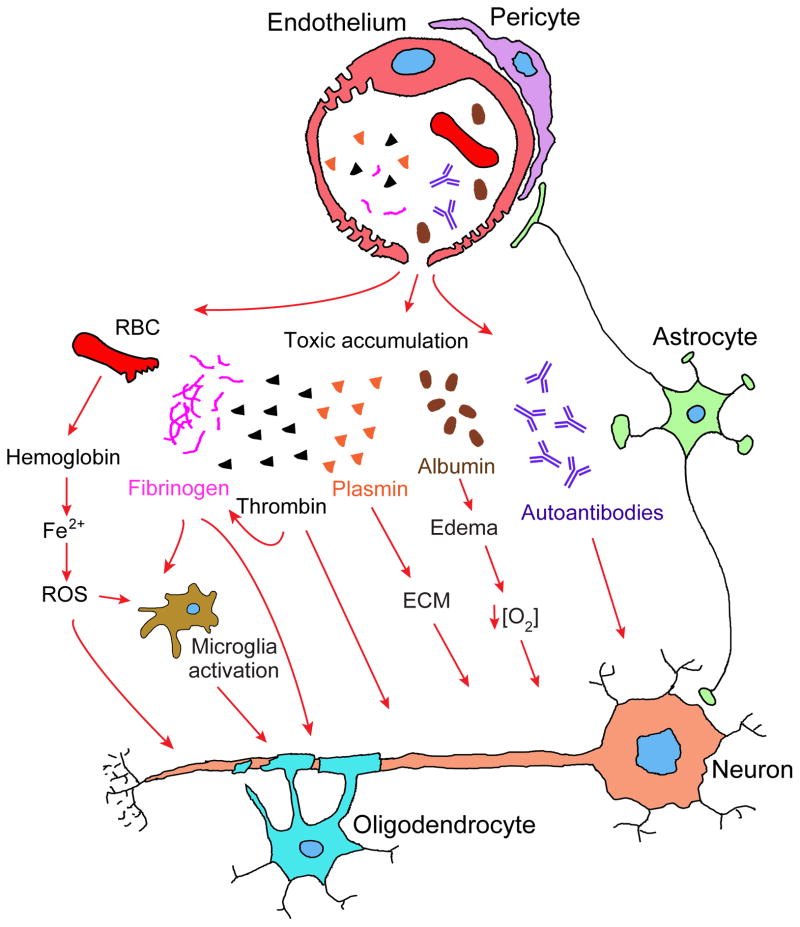
Figure 4. Vascular-mediated neurodegeneration.
Aberrant pericyte-endothelial or astrocyte-pericyte signal transduction leads to BBB breakdown resulting in brain accumulation of 1) red blood cell (RBC)-derived neurotoxic hemoglobin and iron (Fe2+) causing production of reactive oxygen species (ROS) and oxidant stress to neurons; 2) neuronal toxic blood-derived proteins such as fibrinogen, thrombin, and plasminogen, which could be converted into plasmin that in turn degrades neuronal extracellular matrix (ECM) and leads to detachment of neurons and cell death; 3) fibrinogen that activates microglia, promotes neuroinflammation and demyelination, and prevents myelination by oligodendrocyte progenitor cells; 4) albumin that contributes to the development of vasogenic edema, capillary hypoperfusion and hypoxia. BBB breakdown can also lead to the loss of immune privilege resulting in development of anti-brain antibodies against different axonal and membrane components of neurons.
Accumulations of blood-derived proteins in the hippocampus and cortex (e.g., immunoglobulins, albumin, fibrinogen, and thrombin) were observed in post-mortem human studies, indicating BBB damage in AD (Halliday et al., 2015; Hultman et al., 2013; Sweeney et al., 2015; Zipser et al., 2007) and ALS (Winkler et al., 2013), both associated with degeneration of pericytes (Baloyannis and Baloyannis, 2012; Farkas and Luiten, 2001; Halliday et al., 2015; Sengillo et al., 2013; Winkler et al., 2013). BBB impairments were also found in other neurological disorders including multiple sclerosis, PD and HD (Drouin-Ouellet et al., 2015; Korczyn, 2015; Zlokovic, 2011). Moreover, microbleeds and accumulation of iron were observed in the brains of patients with preclinical and clinical AD symptoms (Yates et al., 2014; Zonneveld et al., 2014), particularly in the hippocampus (Raven et al., 2013). It has also been shown that age-dependent early BBB breakdown is accelerated in individuals with mild dementia (Montagne et al., 2015). Some studies using the CSF to plasma ratio of blood-derived albumin, also reported BBB damage in AD particularly associated with vascular risk factors or in individuals at a genetic risk for AD (Sweeney et al., 2015). Thus, findings in complex human brain diseases support, at least in part, the proposed model of vascular-mediated neurodegeneration (Figure 4). Below, we discuss AD in more detail.
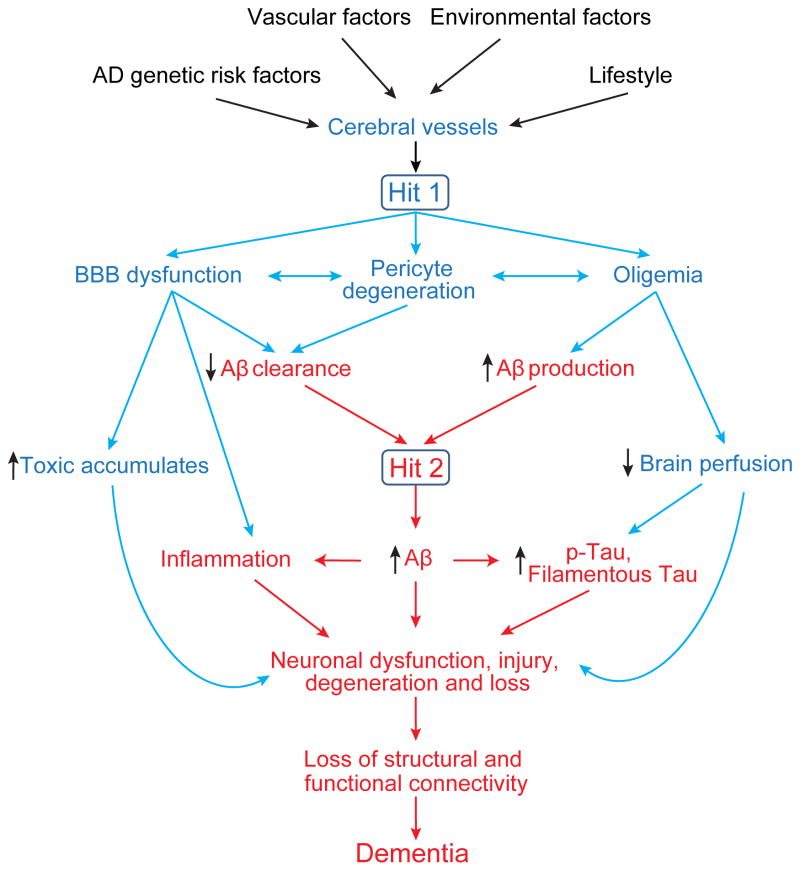
AD patients develop an early neurovascular dysfunction, progressive neurodegeneration, selective loss of neurons, and accumulation in the brain of Aβ pathology and neurofibrillary tangles composed of aggregated hyperphosphorylated Tau (Querfurth and LaFerla, 2010; Zlokovic, 2011). Strikingly, AD affects all cell types of the NVU depending on the disease stage, including endothelial and mural cells, glia, and neurons (Sweeney et al., 2015). The neurovascular hypothesis of AD proposes that cerebrovascular dysfunction and disruption in the neurovascular integrity contributes to the onset and progression of cognitive decline, and that cerebral blood vessels are the converging point of pathogenic events leading to dementia (Zlokovic, 2011). Vascular damage can be triggered by genetics, vascular risk factors, environmental factors, and lifestyle (Sagare et al., 2013; Sweeney et al., 2015; Zlokovic, 2011). Primary damage of the cerebrovasculature leads to brain accumulation of blood-derived neurotoxins, and the decrease in brain perfusion can cause neuronal injury. Vascular damage also influences neurodegeneration pathway mediated by Aβ. In combination, vascular damage and elevated Aβ have a striking synergistic effect on neuronal Tau phosphorylation and pathology, leading to accelerated loss of neurons (Sagare et al., 2013) (Figure 5).
Figure 5. The neurovascular hypothesis for Alzheimer’s disease.
AD genetics, vascular factors, environment and lifestyle can independently and/or synergistically lead to cere-brovascular injuries including BBB dysfunction, pericyte degeneration and cerebral blood flow reductions (oli-gemia), initiating a cascade of events that can either 1) directly cause neuronal injury and damage independently of Aβ (Hit 1, blue), and/or 2) accelerate the Aβ-dependent neurodegeneration (Hit 2, red). In the Aβ-dependent pathway, BBB dysfunction leads to faulty clearance of Aβ from brain, whereas reduced brain perfusion increases Aβ production, both causing Aβ accumulation in the brain. Reduced brain perfusion (Hit 1) and elevated levels of Aβ (Hit 2) can independently and/or synergistically lead to Tau hyperphosphorylation (p-Tau) and formation of filamentous Tau pathology. Additionally, the two hits can exacerbate neuroinflammation.
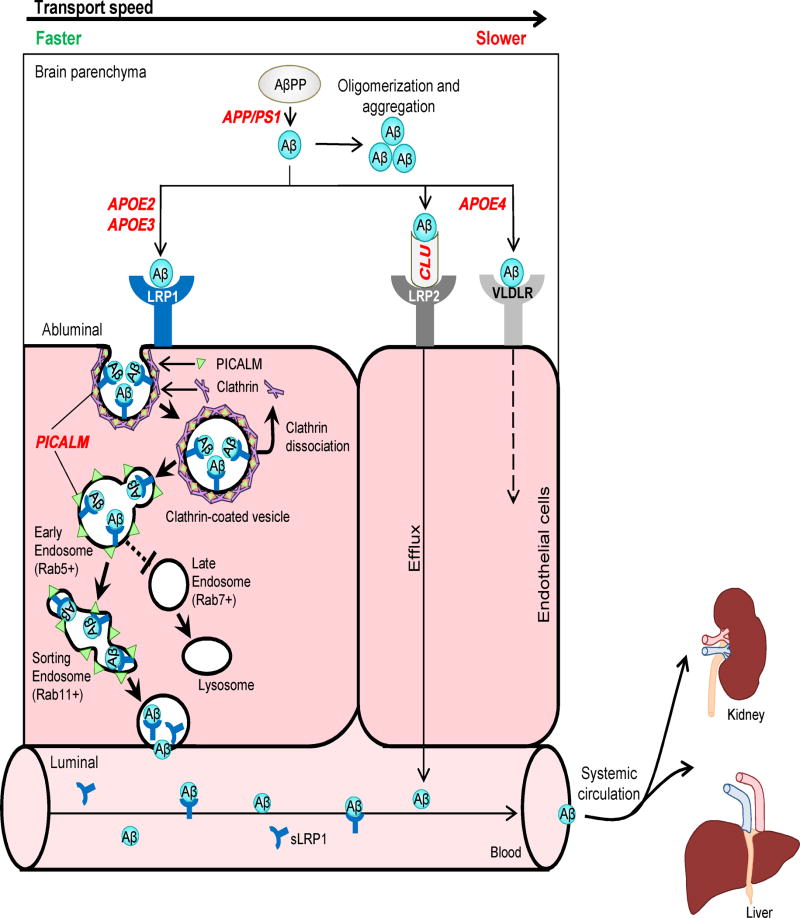
Faulty Aβ clearance from the brain leads to elevated Aβ in patients with sporadic AD (Mawuenyega et al., 2010). Experimental studies have shown that in various animal models, Aβ is cleared from the brain primarily by transvascular clearance across the BBB (70–85%), whereas a minor portion is removed by the ISF flow (Bading et al., 2002; Deane et al., 2004; Tarasoff-Conway et al., 2015). The molecular mechanism for Aβ clearance across the BBB has been recently elucidated in greater detail (Figure 6). Briefly, Aβ produced in the brain binds to LRP1 at the abluminal side of the BBB, causing its rapid internalization into endothelial cells and clearance through the blood. Phosphatidylinositol binding clathrin assembly protein (PICALM) is critical for clathrin/PICALM-mediated internalization of LRP1-Aβ complexes by the endothelium and guides intracellular trafficking of Aβ-containing endocytic vesicles across endothelium by sequential fusion with Rab5-positive early endosomes and Rab11-positive sorting endosomes for exocytosis at the luminal side of the BBB, which completes Aβ transcytosis cycle across the BBB (Zhao et al., 2015).
Figure 6. Alzheimer’s amyloid β-peptide clearance across the blood-brain barrier.
Transvascular Aβ clearance. LRP1 binds Aβ at the abluminal side of endothelium, which recruits PICALM, resulting in PICALM/clathrin-dependent endocytosis of LRP1-Aβ complexes. Next, PICALM guides the trafficking of Aβ-LRP1 endocytic vesicles to Rab5+ early endosomes and then to Rab11+ sorting endosomes for exo-cytosis at the luminal side of the BBB, resulting in Aβ transcytosis. PICALM guides Aβ away from Rab7+ late endosomes and lysosomes. Apolipoprotein J (apoJ; CLU) facilitates Aβ42 clearance across the BBB via LRP2.
Systemic Aβ clearance. Aβ binds to soluble LRP1 (sLRP1) in plasma. Circulating sLRP1-Aβ complexes are transported to liver and kidney for elimination from the body.
Genetic risk factors. APOE2 and APOE3 carry lower risk for AD compared to APOE4. CLU variants influence risk for sporadic AD, but their effects on Aβ clearance are presently unknown. Some protective PICALM variants lead to increased PICALM expression and enhanced Aβ clearance across the BBB. PSEN1 mutations causing early autosomal dominant AD lead to increased production of Aβ, particularly Aβ42, which increases Aβ load for clearance across the BBB.
In plasma, soluble form of LRP1 (sLRP1) generated by the proteolytic cleavage of LRP1 by β-secretase binds and sequesters free Aβ40 and Aβ42 (Sagare et al., 2007), mediating Aβ transport via blood to the excretory organs (Zlokovic et al., 2010) (Figure 6). Aβ42 can bind to Clusterin (CLU or apoJ) in the brain ISF, and apoJ-Aβ complexes are transported across the BBB into circulation by LRP2 (Bell et al., 2007). Enzymatic degradation of Aβ by neprilysin, insulin-degrading enzyme, matrix metalloproteinases, plasmin and tissue plasminogen activator contributes to its clearance from brain (Querfurth and LaFerla, 2010).
Several frequently studied genes, of which the mutations are associated with high risk for sporadic and familial AD, play a role in the cerebrovascular system. For instance, APOE4 is the strongest genetic risk factor for late-onset AD that exerts direct toxic cerebrovascular effects (Zlokovic, 2013). Compared to the other two APOE isoforms, i.e., APOE3 or APOE2, APOE4 increases BBB damage, cerebral amyloid angiopathy and fibrinogen and iron deposition in the brain of AD patients (Halliday et al., 2013, 2015; Hultman et al., 2013; Sweeney et al., 2015; Zipser et al., 2007; Zonneveld et al., 2014). Consistently, findings in APOE4 transgenic mice suggest that vascular changes may precede neuronal changes and behavioral deficits (Bell et al., 2012). ApoE can bind to Aβ. While ApoE2-Aβ and ApoE3-Aβ complexes are rapidly cleared across the BBB via LRP1, the removal rate of ApoE4-Aβ complexes is lower, mediated by slow internalization and transcytosis via very low-density lipoprotein receptor (VLDLR) (Bell et al., 2007; Deane et al., 2008) (Figure 6).
Another example is PICALM, a validated genetic risk factor for AD (Harold et al., 2009; Lambert et al., 2009). PICALM mediates endocytosis and internalization of cell receptors and intracellular trafficking of endocytic proteins (Treusch et al., 2011; Zhao et al., 2015). It is highly expressed in brain endothelium of the BBB (Parikh et al., 2014; Zhao et al., 2015) and plays a central role in Aβ clearance across the BBB (Figure 6). A recent study suggested that compared to the protective allele, the non-protective allele of the rs3851179 PICALM variant leads to decreased PICALM expression in endothelial cells and substantially lower Aβ clearance in an in vitro model of the BBB (Zhao et al., 2015), suggesting that PICALM variants may affect AD pathogenesis, potentially through a vascular mechanism.
Similarly, mutations in Clusterin (CLU, apoJ) are associated with sporadic AD (Harold et al., 2009; Lambert et al., 2009). CLU binds to several different proteins including Aβ, and has been shown to prevent aggregation and promote clearance of Aβ peptides across the BBB (Bell et al., 2007) (Figure 6). Presenilin (PSEN) mutations cause autosomal dominant AD and increase Aβ production in the brain (Querfurth and LaFerla, 2010). PSEN1 mutations also lead to major cerebrovascular pathology in humans including degeneration of pericytes and mural cells, BBB breakdown, and Aβ deposits in small cerebral vessels (Sweeney et al., 2015). Similar cerebrovascular pathology was found in transgenic PSEN1 mice (Gama Sosa et al., 2010), although it is worth noting that how vascular pathology relates to the increased Aβ production during disease progression remains elusive.
Conclusions and future directions
Recent advances in human genetics and the corresponding transgenic models indicate that almost every non-neuronal cell type of the NVU could be affected by some monogenic inherited disorders. This association can provide insights into the potential pathogenic links among BBB dysfunction, neuronal injury, neurodegeneration and neurological disorders caused by NVU disruption and BBB breakdown. On the other hand, the relationship among neurovascular integrity, brain structural and functional connectivity, cognitive function and neurological symptomatology in complex disorders such as AD still awaits to be directly explored in the most relevant in vivo context, which has only recently became possible with the development of novel state-of-the art neuroimaging and molecular biomarker approaches. Experimental studies combining genetic, environmental and lifestyle factors hold promise to further advance our knowledge of multifactorial CNS disorders and to establish the concept that the loss of healthy cerebral blood vessels and BBB integrity influences the course and clinical phenotype of neurological disorders in a region-specific manner.
Some important questions remain to be addressed. First, it is still unclear whether in the living human brain, cerebrovascular changes and BBB breakdown can drive the initial pathogenic events that lead to neuronal injury, disrupted structural and functional brain connectivity and early neurological symptoms, such as cognitive decline in AD and motor changes in ALS, PD or HD. Second, further studies are warranted to test whether the underlying molecular mechanisms of NVU disruption and BBB breakdown might point to new targets for therapeutic development to prevent and/or treat neurodegenerative disorders. Third, technological advance is required to determine if neurovascular dysfunction and BBB breakdown are detectable in the living human brain, prior to the development of the full spectrum of neurological symptoms. Last but not least, future investigations need to address whether molecular and imaging biomarkers of neurovascular dysfunction can serve as reliable prognostic and/or diagnostic tools to predict the development of neurodegenerative disorders.
From the basic science side, pushing the envelope further to generate a comprehensive proteomics and RNAseq molecular atlas of the BBB in animals and humans would provide a valuable resource for discovering and studying new targets and signaling pathways mediating the crosstalk among different cell types within the NVU. This may lead to the development of new transgenic animal models, pluripotent stem cell models of the BBB, NVU and different neurological disorders, serving as valuable platforms for drug discovery and for testing novel drug delivery approaches.
Acknowledgments
Drs. Zhen Zhao and Amy R. Nelson contributed equally in preparing some sections of this review. The work of B.V.Z. is supported by the National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, R01AG039452 and Cure Alzheimer’s Fund. The work of C.B. is supported by the European Research Council (ERC advanced grant No 294556 BBBARRIER), the Knut and Alice Wallenberg Foundation, Leducq Foundation (Sphingonet), Swedish Cancer Foundation, the Swedish Science Council, and Uppsala University. We apologize to authors whose work we could not cite because of the limit on the number of references and therefore in some instances we mostly cited the overview articles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alakbarzade V, Hameed A, Quek DQY, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47:814–817. doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- Alkan A, Sigirci A, Kutlu R, Aslan M, Doganay S, Yakinci C. Merosin-negative congenital muscular dystrophy: diffusion-weighted imaging findings of brain. J Child Neurol. 2007;22:655–659. doi: 10.1177/0883073807303219. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bour-bonnière L, Bernard M, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Andreone BJ, Lacoste B, Gu C. Neuronal and Vascular Interactions. Annu Rev Neurosci. 2015;38:25–46. doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, Ghiso J, Frangione B, Zlokovic BV. Brain clearance of Alzheimer’s amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target. 2002;10:359–368. doi: 10.1080/10611860290031831. [DOI] [PubMed] [Google Scholar]
- Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. 2011;71:1018–1039. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J Neurol Sci. 2012;322:117–121. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Brain glucose transporters: implications for neurologic disease. Neurology. 2014;82:1374–1379. doi: 10.1212/WNL.0000000000000328. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C. Physiology: Double function at the blood-brain barrier. Nature. 2014;509:432–433. doi: 10.1038/nature13339. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Lipid transport and human brain development. Nat Genet. 2015;47:699–701. doi: 10.1038/ng.3348. [DOI] [PubMed] [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N. Biologics: Transferrin’ bispecific antibodies across the blood-brain barrier. Nat Rev Drug Discov. 2015;14:14–15. doi: 10.1038/nrd4522. [DOI] [PubMed] [Google Scholar]
- Capri Y, Friesema ECH, Kersseboom S, Touraine R, Monnier A, Eymard-Pierre E, Des Portes V, De Michele G, Brady AF, Boespflug-Tanguy O, et al. Relevance of different cellular models in determining the effects of mutations on SLC16A2/MCT8 thyroid hormone transporter function and genotype-phenotype correlation. Hum Mutat. 2013;34:1018–1025. doi: 10.1002/humu.22331. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagacé M, Kuan WL, Saint-Pierre M, Dury RJ, Alata W, St-Amour I, Mason SL, et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann Neurol. 2015;78:160–177. doi: 10.1002/ana.24406. [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med. 2013;19:302–308. doi: 10.1016/j.molmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Friesema ECH, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MHA, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet Lond Engl. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- Gama Sosa MA, Gasperi RD, Rocher AB, Wang ACJ, Janssen WGM, Flores T, Perez GM, Schmeidler J, Dickstein DL, Hof PR, et al. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am J Pathol. 2010;176:353–368. doi: 10.2353/ajpath.2010.090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, et al. Role of COL4A1 in Small-Vessel Disease and Hemorrhagic Stroke. N Engl J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan N, Ben-Zvi A. The molecular, cellular, and morphological components of blood-brain barrier development during embryogenesis. Semin Cell Dev Biol. 2015;38:7–15. doi: 10.1016/j.semcdb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, Sirén AL, Pardo LA, Sper-ling S, Mohd Jofrry S, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2014;19:1143–1149. doi: 10.1038/mp.2013.110. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall TL, Keller A, He L, Johansson BR, Wallgard E, Raschperger E, Mäe MA, Jin S, Betsholtz C, Lendahl U. Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler Thromb Vasc Biol. 2015;35:409–420. doi: 10.1161/ATVBAHA.114.304849. [DOI] [PubMed] [Google Scholar]
- Hultman K, Strickland S, Norris EH. The APOE ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Westenberger A, Sobrido MJ, García-Murias M, Domingo A, Sears RL, Lemos RR, Ordoñez-Ugalde A, Nicolas G, da Cunha JEG, et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet. 2013;45:1077–1082. doi: 10.1038/ng.2723. [DOI] [PubMed] [Google Scholar]
- Kersseboom S, Kremers GJ, Friesema ECH, Visser WE, Klootwijk W, Peeters RP, Visser TJ. Mutations in MCT8 in patients with Allan-Herndon-Dudley-syndrome affecting its cellular distribution. Mol Endocrinol Baltim Md. 2013;27:801–813. doi: 10.1210/me.2012-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczyn AD. Vascular parkinsonism-characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11:319–326. doi: 10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet. 2012;21:R97–R110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Legati A, Giovannini D, Nicolas G, López-Sánchez U, Quintáns B, Oliveira JRM, Sears RL, Ramos EM, Spiteri E, Sobrido MJ, et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat Genet. 2015;47:579–581. doi: 10.1038/ng.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14:543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–496. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bate-man RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. Regulation of ABC transporters blood-brain barrier: the good, the bad, and the ugly. Adv Cancer Res. 2015;125:43–70. doi: 10.1016/bs.acr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Koroshetz WJ, Babcock D, Dickson DW, Galpern WR, Glymour MM, Greenberg SM, Hutton ML, Knopman DS, Kuzmichev AN, et al. Recommendations of the Alzheimer’s disease-related dementias conference. Neurology. 2014;83:851–860. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A, Matsu-yama T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells Dayt Ohio. 2015;33:1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Pottier C, Maltête D, Coutant S, Rovelet-Lecrux A, Legallic S, Rousseau S, Vaschalde Y, Guyant-Maréchal L, Augustin J, et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology. 2013;80:181–187. doi: 10.1212/WNL.0b013e31827ccf34. [DOI] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll MC, Daly SB, Urquhart JE, Black GCM, Pilz DT, Brockmann K, McEntagart M, Abdel-Salam G, Zaki M, Wolf NI, et al. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am J Hum Genet. 2010;87:354–364. doi: 10.1016/j.ajhg.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets. 2015;19:1059–1072. doi: 10.1517/14728222.2015.1042364. [DOI] [PubMed] [Google Scholar]
- Parikh I, Fardo DW, Estus S. Genetics of PICALM expression and Alzheimer’s disease. PloS One. 2014;9:e91242. doi: 10.1371/journal.pone.0091242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J Alzheimers Dis JAD. 2013;37:127–136. doi: 10.3233/JAD-130209. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sasaki A, Kakita A, Yoshida K, Konno T, Ikeuchi T, Hayashi S, Matsuo H, Shioda K. Variable expression of microglial DAP12 and TREM2 genes in Nasu-Hakola disease. Neurogenetics. 2015:1–12. doi: 10.1007/s10048-015-0451-3. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA. Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med. 2013;34:742–752. doi: 10.1016/j.mam.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol Zurich Switz. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:1055–1068. doi: 10.1038/jcbfm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:E52. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, Cao TC, Weimer RM, Carano RAD, van Bruggen N, Watts RJ. Death receptors DR6 and TROY regulate brain vascular development. Dev Cell. 2012;22:403–417. doi: 10.1016/j.devcel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz S, Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treusch S, Hamamichi S, Goodman JL, Matlack KES, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, et al. Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast. Science. 2011;334:1241–1245. doi: 10.1126/science.1213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SPJ, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li Y, Shi L, Ren J, Patti M, Wang T, de Oliveira JRM, Sobrido MJ, Quintáns B, Baquero M, et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate home-ostasis. Nat Genet. 2012a;44:254–256. doi: 10.1038/ng.1077. [DOI] [PubMed] [Google Scholar]
- Wang D, Kranz-Eble P, De Vivo DC. Mutational analysis of GLUT1 (SLC2A1) in Glut-1 deficiency syndrome. Hum Mutat. 2000;16:224–231. doi: 10.1002/1098-1004(200009)16:3<224::AID-HUMU5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012b;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D, Adams RH. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat Commun. 2015;6:6429. doi: 10.1038/ncomms7429. [DOI] [PubMed] [Google Scholar]
- Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–1273. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zlokovic BV. Blood-brain barrier: a dual life of MFSD2A? Neuron. 2014;82:728–730. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest. 2014;124:3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neuro-biol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12:1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low density lipoprotein receptor related protein-1: A serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkeph-alin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- Zonneveld HI, Goos JDC, Wattjes MP, Prins ND, Scheltens P, van der Flier WM, Kuijer JPA, Muller M, Barkhof F. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology. 2014;82:698–704. doi: 10.1212/WNL.0000000000000150. [DOI] [PubMed] [Google Scholar]