ABSTRACT
Purpose
The SVOne is a portable Hartmann-Shack wavefront aberrometer that can be attached to a smartphone to determine the refractive error of the eye objectively. The aim of the present study was to compare the findings of the SVOne with retinoscopy, subjective refraction, and two commercially available autorefractors (Topcon KR-1W and Righton Retinomax-3).
Methods
Refractive error was assessed both with and without cycloplegia in 50 visually normal, young adults using the five techniques described above. Further, to assess repeatability of the instruments, the entire procedure was repeated in a subgroup of 10 subjects. All data were analyzed in terms of power vectors (M, J0, and J45).
Results
No significant difference was observed between the mean values of M (spherical equivalent) for the different techniques. However, a significantly higher mean value of precyclopegic J0 was recorded for the SVOne, which also had the highest limits of agreement for both the J0 and J45 astigmatic components. Retinoscopy and subjective refraction showed the best repeatability (in terms of M values) for precycloplegic and postcycloplegic measurements, respectively. High and significant linear correlations were observed between the subjective findings and the other four techniques.
Conclusions
The results indicate that the SVOne handheld aberrometer provides measurements of refractive error in normal, young individuals that are not significantly different from other subjective and objective procedures. This instrument is valuable for vision screenings, as well as examinations taking place outside the clinical office. It may also serve as an adjunct in the standard optometric examination.
Key Words: aberrometer, ametropia, astigmatism, autorefractor, refraction, refractive error, smartphone
The SVOne (Smart Vision Labs, New York, NY) is a portable Hartmann-Shack wavefront aberrometer1,2 that measures aberrations of the eye using wavefront sensors. After the device has acquired measurement images, it uses Zernike decomposition to separate the low-order refractive errors from high-frequency aberrations.3 The device converts Zernike defocus and astigmatism terms into the conventional sphere, cylinder, and axis format. The instrument connects easily to a smartphone for alignment purposes, as well as downloading and storage of data. The dimensions of the overall device are 82 mm × 130 mm × 140 mm, with a weight of 402 g (including the smartphone). The manufacturer states that it can measure refractive errors within a range of ±10 diopters (D) for the spherical component and ±5 D for the cylinder. Refractive power and astigmatic axis are recorded in 0.25-D and 1-degree increments, respectively. The instrument measures and averages five readings over a 5-second period. The device is shown in Figs. 1 and 2, and further details can be found in the US patent for the aberrometer.4 In addition to the measurement of ocular aberrations, it may also be used as an objective autorefractor. Given its portability, this presents a significant advantage over larger autorefractors, which are typically table mounted and therefore not easy to remove from the clinical setting either for vision screenings or when examining housebound individuals. A further advantage of this small device occurs when examining a wheelchair user or obese patient who may have difficulty positioning themselves onto the chin and forehead rest of a table-mounted autorefractor.
FIGURE 1.

The SVOne aberrometer/autorefractor.
FIGURE 2.
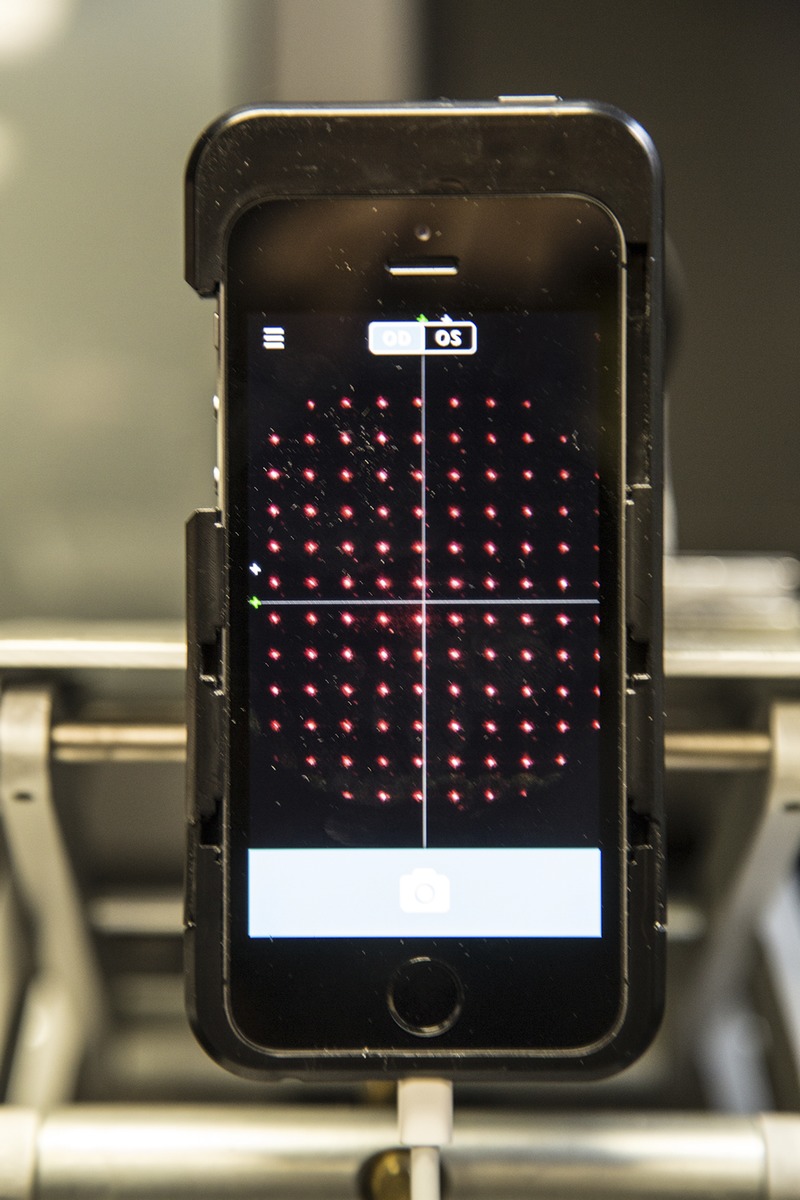
The array of Hartmann-Shack images are clearly visible on the smartphone screen attached to the SVOne. A color version of this figure is available online at www.optvissci.com.
Although the use of aberrometers in the clinical setting has expanded rapidly in recent years, particularly in relation to refractive surgery5,6 and the design of customized lenses,7,8 this article will be restricted to the use of the device for the objective measurement of refractive error. In assessing any new autorefractor, especially one that represents a marked departure from currently available instruments, it is important to evaluate its accuracy and repeatability. Accordingly, the aim of the present investigation was to compare the findings of the SVOne with retinoscopy, subjective refraction, and two other commercially available autorefractors, namely, the Topcon KR-1W wavefront analyzer (Topcon Corp, Tokyo, Japan) and the Righton Retinomax-3 handheld autorefractor (Righton Ophthalmic Instruments, Tokyo, Japan). Assessment of refractive error was undertaken both before and after instillation of a cycloplegic agent.
METHODS
The study was performed on 50 subjects (34 female, 16 male) between 18 and 31 years (mean age, 24.8 years; SEM, 0.35 years). They were drawn from the students and staff at the SUNY (State University of New York) College of Optometry. All had corrected visual acuity of at least 6/6 (20/20) in each eye and a pupil diameter between 3 and 8 mm under the testing conditions adopted (subdued clinic room lighting). Any subject with contraindications to the use of cycloplegia, such as a diagnosis of glaucoma or ocular hypertension or any suspicion of such a diagnosis, with an intraocular pressure greater than 24 mm Hg, or taking any topical or systemic medication that may interact adversely with the cycloplegic agent was excluded from the study. The methodology followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects after an explanation of the nature and possible consequences of the study. The protocol was approved by the institutional review board at the SUNY College of Optometry. The study was conducted in the Clinical Vision Research Center at the College.
Before all measurements, an abbreviated case history, corrected monocular and binocular distance visual acuity, tonometry (using a Marco Nidek NT-2000 automatic noncontact tonometer; Nidek Co Ltd, Aichi, Japan), pupil diameter (using the Neuroptics VIP-200 Pupillometer; NeurOptics, Inc, Irvine, CA), and assessment of the posterior pole with a direct ophthalmoscope (undilated) were carried out to ensure subjects met the inclusion and exclusion criteria described above. Subsequently, the refractive error of the subject’s right eye was assessed using the following techniques: (1) streak retinoscopy, (2) subjective refraction, (3) objective autorefraction using the SmartVision SVOne, (4) objective autorefraction using the Topcon KR-1W wavefront analyzer, and (5) objective autorefraction using the Retinomax-3 autorefractor. To minimize the possibility of experimenter bias from knowledge of the autorefractor findings, retinoscopy was always performed first (with the left eye fogged during the procedure), followed by subjective refraction using the Jackson cross cylinder and sphere refinement, to achieve an endpoint of maximum plus (or minimum minus) to best visual acuity.9 While performing retinoscopy before the subjective refraction is the conventional clinical protocol, one could argue that knowledge of the retinoscopy result could have affected the subjective findings.9 During both procedures, subjects viewed a projected, calibrated Snellen visual acuity chart at a distance of 5 m. After subjective refraction, the refractive error was quantified using each of the three automated devices. The order of testing of these instruments was counterbalanced across subjects to prevent order effects. Subjects were instructed to fixate a single optotype within the 20/25 line of the distance visual acuity chart, and five readings were recorded for each device and subsequently averaged. The left eye remained open and unfogged during the autorefractor procedures.
When recording refractive error with the SVOne, alignment was achieved by having the subject look into the instrument, and they were instructed to position the device with their hand so that the diffuse red light emitted from the aberrometer was visible and centered in their field of view. The diffuse light contains a bright, defocused red dot, about 1 mm in diameter, surrounded by a red speckled annulus (similar in appearance to the speckle pattern of a laser optometer10). The annulus subtended an angle of about 15 degrees at the eye. Once the device had been positioned as described, the examiner was able to see the array of Hartmann-Shack images on the smartphone screen (see Fig. 2). The subject was then directed to fixate the distant acuity chart, and the examiner readjusted the position of the instrument so that the image array was centered and maintained on the smartphone screen. Because of this change of fixation, a slight rotation of the device away from the principal axis could have occurred. The effects of tilting the device are discussed later in this article. The instrument was triggered by depressing the camera button on the phone, whereupon five readings were automatically recorded and downloaded to the smartphone (Apple iPhone 5s; Apple Inc, Cupertino, CA).
After the precycloplegic measurements of refractive error were completed, cycloplegia was induced by instilling one drop of the topical anesthetic proparacaine hydrochloride (0.5%), followed 5 minutes later by one drop of tropicamide (1%) into each eye. Adequate cycloplegia was defined as having a subjective amplitude of accommodation of less than 2 D.11 This was assessed by measuring the push-up amplitude of accommodation12 with a supplementary +2.00-D lens added to the distance refractive correction. The endpoint for the push-up procedure was taken as the report of first, slight, sustained blur.12 In the event that the amplitude of accommodation still exceeded 2 D 30 minutes after the cycloplegic had been instilled, a second drop of tropicamide (1%) was instilled into each eye. Once adequate cycloplegia had been achieved (i.e., amplitude of accommodation less than 2 D), then the refractive measurements were repeated using the methodology described above.
Additionally, to assess repeatability of the instruments, the entire procedure was repeated in a subgroup of 10 subjects, with the second session being scheduled between 3 and 14 days after their initial testing. This subgroup comprised the first 10 subjects examined who were willing to return for a second trial. All results were converted into power vectors (M, J0, and J45) as described by Thibos et al.13 Statistical analysis was performed using StatistiXL software (www.statistixl.com; Broadway-Nedlands, Western Australia).
Two laboratory trials were also conducted using the SVOne. The first assessed the ability of the instrument to detect known changes in refractive error, whereas the second examined the effect of tilt on the measured refractive state. Both laboratory trials were conducted using a schematic eye (Bernell Corp model BC2174; Bernell Corp, Mishawaka, IN), with the SVOne being positioned immediately adjacent to the front of the schematic eye on a stable platform. For each condition, five readings of the refractive state were recorded, converted to vector format, and averaged. The resulting mean was converted back to the standard nomenclature of sphere, cylinder, and axis.
To evaluate the ability of the SVOne to detect known changes in refractive error, spherical trial lenses from +6.00 to −6.00 D (in 1-D steps) were placed in front of the schematic eye, and the resulting refractive error was measured. Although ±6 D does not cover the total measuring range of the device, it will encompass more than 90% of the normal adult population.14,15 To evaluate the effect of tilt, the schematic eye was rotated 5, 10, or 15 degrees about a horizontal axis (both up and down), while the SVOne was mounted in a horizontal position. The effect of tilt was examined both without any trial lenses present, as well as with the addition of a −3.00 trial lens cylinder, axis 135 degrees.
RESULTS
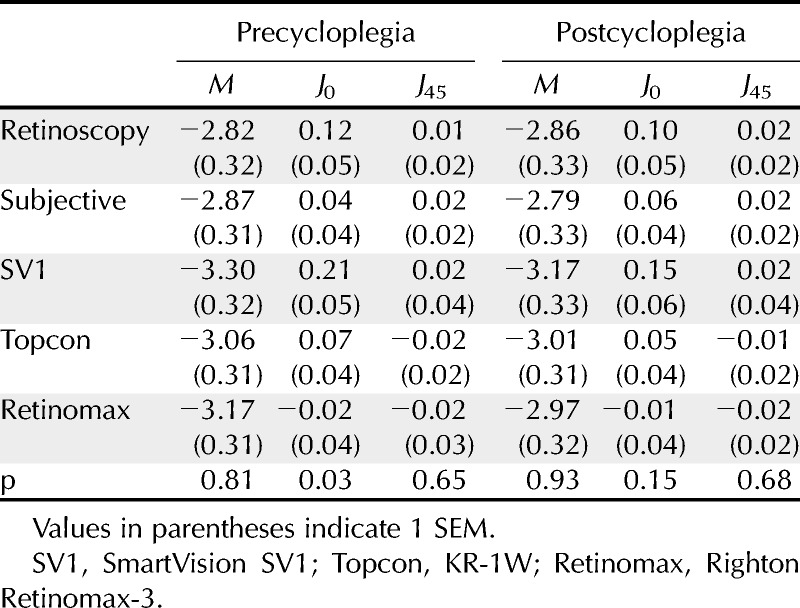
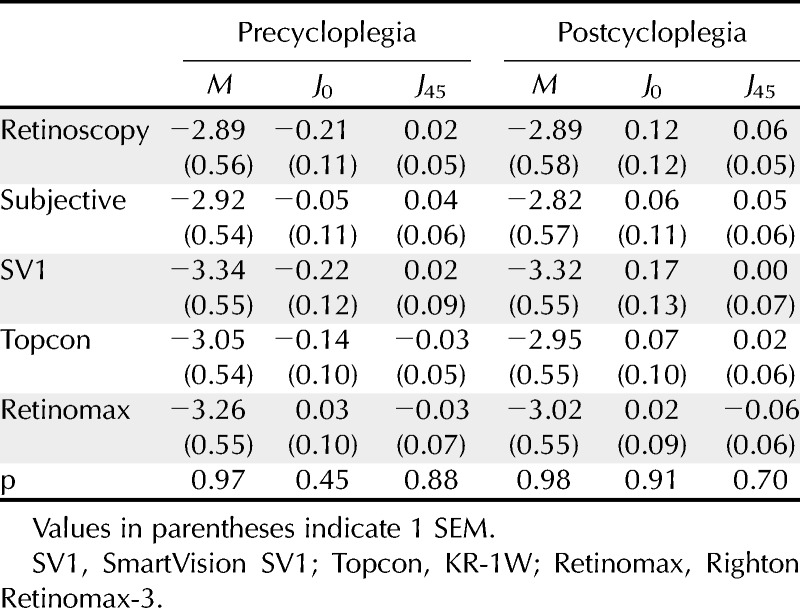
Based on the cycloplegic subjective refraction results, the mean spherical equivalent refractive error was −2.79 D (SEM, ±0.33; range, +2.75 to −6.63 D). Mean astigmatism was −0.52 D (SEM, ±0.08; range, 0 to 2.50 D). Mean values of M, J0, and J45 for the five methods of measurement are shown in Table 1. A one-way analysis of variance indicated that the only significant difference between the techniques was for the precycloplegic, J0 parameter (F4,249 = 4.16; p = 0.003). Post hoc analysis using the Tukey test revealed that the J0 finding for the SVOne was significantly higher than the value for both subjective refraction (p = 0.045) and the Retinomax instrument (p = 0.001) by 0.17 and 0.23 D, respectively. None of the other post hoc comparisons for this parameter were significant (p > 0.05).
TABLE 1.
Mean values of M, J0, and J45 vectors (in diopters) measured both before and after instillation of a cycloplegic agent using each of the five measurement techniques
To compare the difference between each of the findings and subjective refraction, the 95% limits of agreement (LOA) was quantified using the technique described by Bland and Altman.16 Here, the difference between each vector measurement with respect to the subjective finding was determined, and the LOA was calculated as 1.96 multiplied by the SD of the differences. These values are shown in Table 2, with lower values representing better agreement. For all astigmatic conditions (i.e., both J0 and J45 for precycloplegia and postcycloplegia), the SVOne had the largest LOA. The difference between the SVOne and the subjective refraction findings (in terms of M, J0, and J45), with respect to the mean of these two values, is shown in Fig. 3.
TABLE 2.
Ninety-five percent LOA (in diopters) between each technique and subjective refraction (calculated as 1.96 multiplied by the SD of the differences)
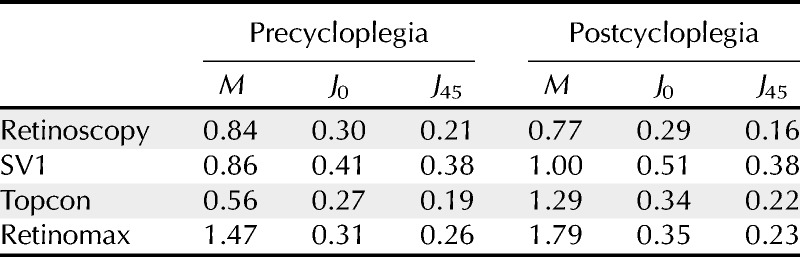
FIGURE 3.
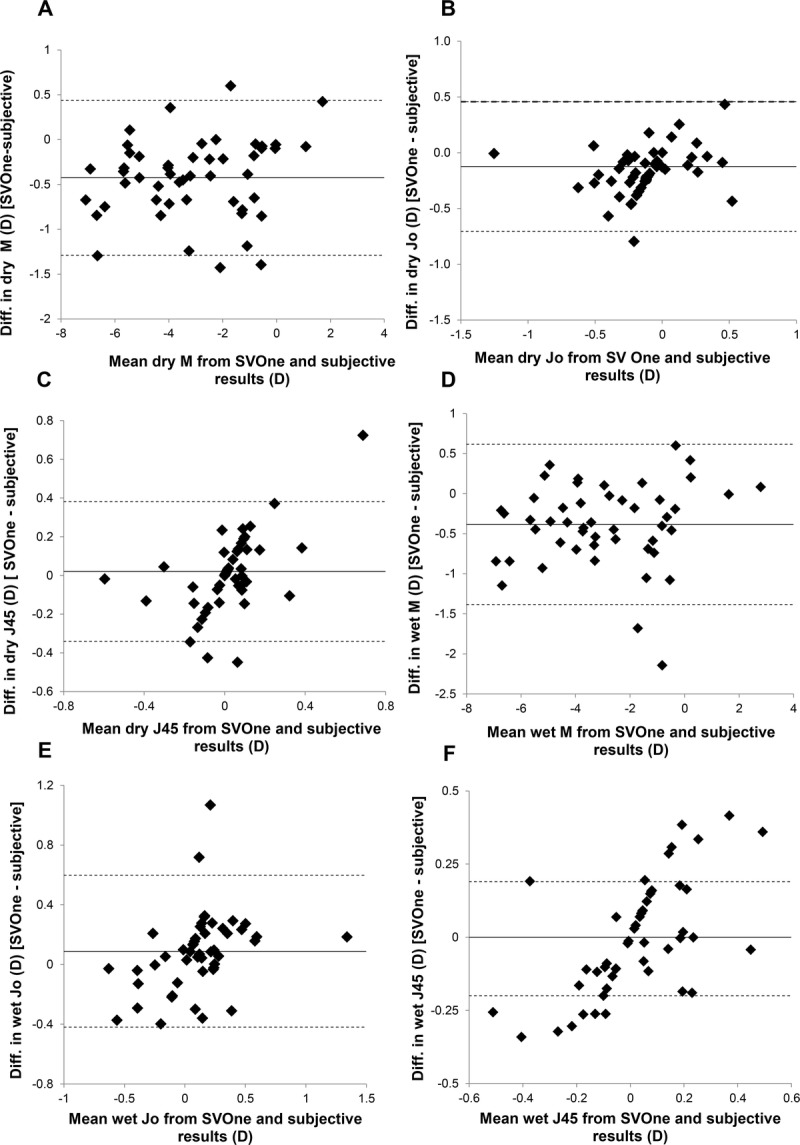
Plots showing the difference between the SVOne and subjective refraction findings, with respect to the mean of these two results for 50 subjects. A to C show the findings for M, J0, and J45, respectively, without cycloplegia. D to F show the findings for M, J0, and J45, respectively, under cycloplegia. In all figures, the solid line represents the mean difference, whereas the upper and lower dashed lines indicate the 95% LOA (calculated as 1.96 times the SD of the differences).
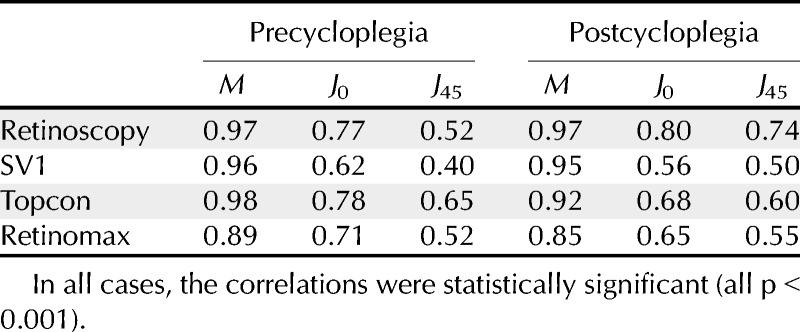
Additionally, linear regression analysis was used to compare each of the parameters with the findings from subjective refraction. Correlation coefficients are listed in Table 3. All were high and statistically significant.
TABLE 3.
Square of the linear correlation coefficient (r2) between each technique and subjective refraction
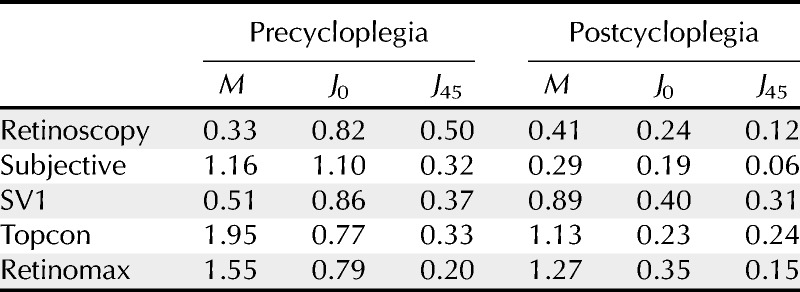
Analysis of the postcycloplegic subjective refraction findings showed that the mean amount of astigmatism was only 0.52 D (SEM, 0.08; range, 0 to 2.50 D). Accordingly, to examine the results for subjects with higher amounts of astigmatism, the data were reanalyzed for a subgroup of 18 subjects who had at least 0.75 D of astigmatism based on the postcycloplegic subjective results (mean, 0.97 D; SEM, 0.10 D). Mean values of M, J0, and J45 for the five methods of measurement for this subgroup are shown in Table 4. No significant difference between the means for the five methods of measurement was observed for this subgroup (p > 0.05).
TABLE 4.
Mean values of M, J0, and J45 vectors (in diopters) measured both before and after the instillation of a cycloplegic agent using each of the five measurement techniques in a subgroup of 18 subjects who all had at least 0.75 D of astigmatism
Repeatability measurements for the subgroup of 10 subjects are shown in Table 5. The best repeatability of M (smallest LOA) for the precycloplegic and postcycloplegic measurements was found using retinoscopy and subjective refraction, respectively.
TABLE 5.
Ninety-five percent LOA (in diopters) calculated as 1.96 multiplied by the SD of the differences when each procedure was repeated on a subgroup of 10 subjects
Laboratory Trials
Mean values of refractive error after the introduction of spherical trial lenses are shown in Fig. 4. An almost perfect linear correlation was observed (r2 = 0.998). The vertical offset of the regression line indicates that the schematic eye had a small (0.62 D) hyperopic refractive error.
FIGURE 4.
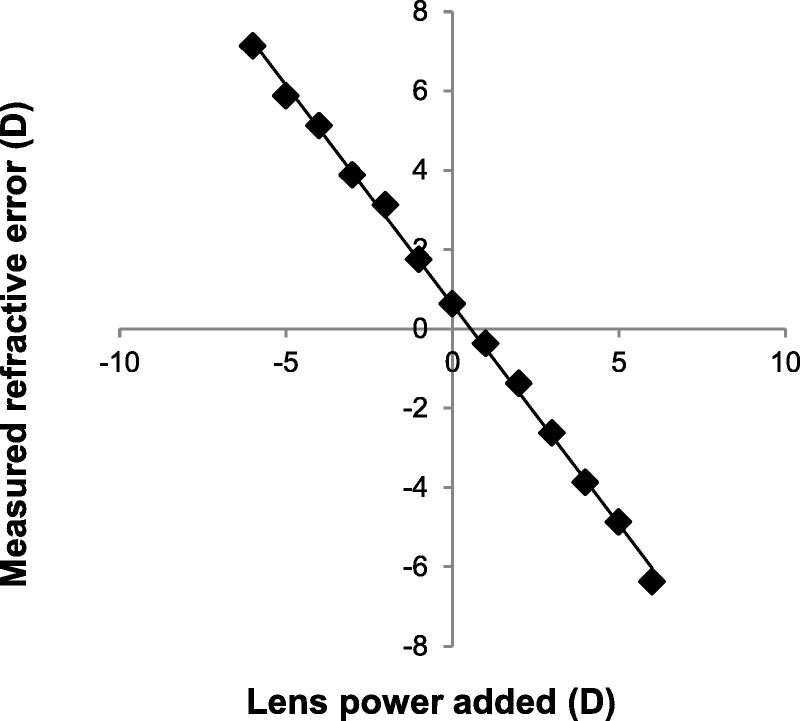
Mean values of refractive error measured from a schematic eye using the SVOne after the introduction of spherical trial lenses. An almost perfect linear correlation was observed (r2 = 0.998). The equation of the regression line was y = −1.11x + 0.62.
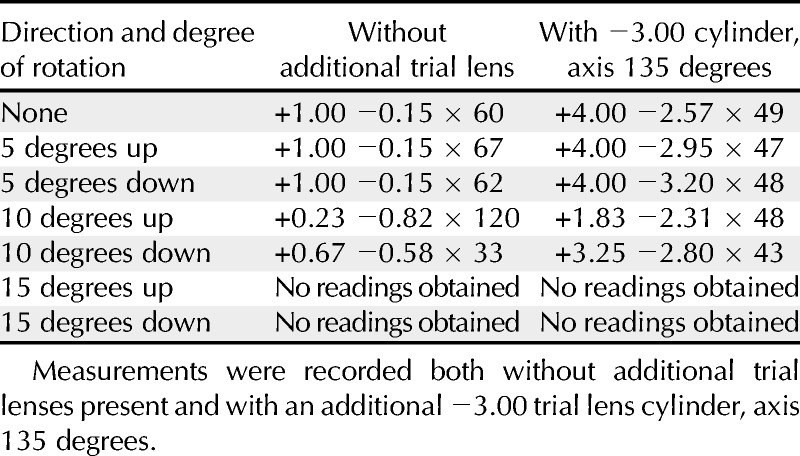
Mean values of refractive error obtained using the SVOne when the schematic eye was tilted about its horizontal axis are shown in Table 6. Readings could not be obtained when the eye was tilted by 15 degrees or more.
TABLE 6.
Mean refractive error (in diopters) measured from a schematic eye using the SVOne when the eye was rotated about its horizontal axis
DISCUSSION
The results indicate that in terms of determining M (i.e., the spherical equivalent refractive error), the findings for the SVOne handheld device were not significantly different from the other techniques examined here, both with and without cycloplegia. Interestingly, for both the precycloplegic and postcycloplegic findings, the mean value of M for the SVOne showed 0.43 and 0.38 D more myopia, respectively, when compared with the subjective results (Table 1). However, these differences were not statistically significant.
In considering the 95% LOA between subjective refraction and the other measurement techniques for values of M, the Topcon autorefractor and retinoscopy showed the best agreement under precycloplegic and postcycloplegic conditions, respectively (see Table 2). In contrast, the Retinomax device showed the poorest agreement for both test conditions. However, the SVOne showed the highest LOA values for J0 and J45 both with and without cycloplegia. This poorer agreement with regard to the cylinder may be attributed to difficulties with instrument alignment and orientation. However, the data presented in Table 6 indicate that tilting the instrument by 5 degrees has little impact on the refractive measurements when minimal astigmatism is present, whereas no readings could be obtained when the device was tilted by 15 degrees or more. It should also be noted that when the device is tilted by 10 degrees, almost half of the array of Hartmann-Shack images are no longer visible. This is shown in Fig. 5. Accordingly, it will be obvious to the operator that the device has been tilted away from the proper alignment. As expected, the effect of tilt on an eye having a larger amount of astigmatism is more marked (Table 6). For these cases, the lack of precision in alignment of the device may account for some of the variation in the measurements of astigmatism.
FIGURE 5.

Appearance of the smartphone screen when measuring the refractive error of a schematic eye that has been tilted 10 degrees about its horizontal axis. Almost half of the array of Hartmann-Shack images are no longer visible. Accordingly, it is obvious to the operator that the device has been tilted away from the proper alignment. A color version of this figure is available online at www.optvissci.com.
In considering the repeatability of each technique based on the difference between two examinations spaced up to 14 days apart (Table 5), retinoscopy and subjective refraction showed the highest repeatability of M (smallest LOA) for the precycloplegic and postcycloplegic measurements, respectively. The highest LOA were found for the Topcon and Retinomax instruments for both cycloplegic conditions. Interestingly, in terms of M, the SVOne showed better repeatability than both the Topcon and Retinomax devices (precycloplegia and postcycloplegia). However, the SVOne showed the highest LOA for J0 and J45 under cycloplegia, thus reflecting probable alignment and orientation issues, especially with the dilated pupil.
It should be pointed out that this initial study was purposely conducted on healthy young adults under optimal test conditions to obtain an assessment of the device. The mean spherical refractive error was −2.57 D, with a range from +2.75 to −6.75 D. However, only four of the subjects were hyperopic, and nine had spherical refractive errors between plano and −0.50. As noted previously, 32 of the 50 subjects had astigmatism of less than 0.75 D. Therefore, we would recommend that the study be repeated on subjects having a wider range of refractive errors, as well as in children (the most likely group to participate in a vision screening process), because the results may differ in these populations (e.g., greater variability would be expected). In particular, it is important that subjects with high astigmatic errors be evaluated, because the ability of the device to determine the cylinder axis and power accurately and repeatably in these individuals is more critical. Examination of the variability when testing elderly patients with less transparent ocular media and ocular disease would also be valuable. In addition, it is of interest to note that these data show high correlations and small (mostly nonsignificant) differences between the five methods of measurement tested. This would indicate that in the population tested, the effect of higher aberrations on the measured refractive error is small.
Finally, either retinoscopy or autorefraction should only be considered as the first step in determining a patient’s refractive correction. Although automated refraction may, on occasions, be more repeatable than subjective refraction,17 Bullimore et al.18 compared patient’s acceptance of spectacle prescriptions determined by either autorefractor or subjective refraction. Two pairs of spectacles were fabricated with each refractive correction, and the patients (n = 195) wore the two prescriptions in a double-masked, crossover design, with each pair being worn for at least 3 weeks. When asked which pair they would prefer to keep, 100 patients (51%) preferred those determined by subjective refraction, 56 (29%) preferred the autorefractor finding, and the remainder (39 patients, 20%) considered both pairs equally acceptable. These authors concluded that overall patient acceptance and satisfaction was better for prescriptions determined by subjective refraction. Accordingly, although both retinoscopy and autorefraction play a useful role at the start of the vision examination, it cannot be assumed that they will provide a prescription that will necessarily satisfy the patient’s visual requirements.
Kenneth J. Ciuffreda
SUNY College of Optometry
33 W 42nd St
New York
NY 10036
e-mail: kciuffreda@sunyopt.edu
ACKNOWLEDGMENT
This research was funded by a grant from Smart Vision Labs and the Pilot Health Technology Initiative of New York City. Neither of the authors have a financial interest in any of the products tested.
REFERENCES
- 1. Shack RV, Platt BC. Production and use of a lenticular Hartmann screen. J Opt Soc Am 1971; 61: 656. [Google Scholar]
- 2. Thibos LN, Hong X. Clinical applications of the Shack-Hartmann aberrometer. Optom Vis Sci 1999; 76: 817– 25. [DOI] [PubMed] [Google Scholar]
- 3. Guirao A, Williams DR. A method to predict refractive errors from wave aberration data. Optom Vis Sci 2003; 80: 36– 42. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y. Portable wavefront aberrometer. US Patent US9066683 B2, June 30, 2015.
- 5. Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci 2001; 42: 1396– 403. [PubMed] [Google Scholar]
- 6. Marcos S. Aberrations and visual performance following standard laser vision correction. J Refract Surg 2001; 17: S596– 601. [DOI] [PubMed] [Google Scholar]
- 7. Piers PA, Weeber HA, Artal P, Norrby S. Theoretical comparison of aberration-correcting customized and aspherical intraocular lenses. J Refract Surg 2007; 23: 374– 84. [DOI] [PubMed] [Google Scholar]
- 8. Dietze HH, Cox MJ. Correcting ocular spherical aberration with soft contact lenses. J Opt Soc Am A 2004; 21: 473– 85. [DOI] [PubMed] [Google Scholar]
- 9. Rosenfield M. Subjective refraction. In: Rosenfield M, Logan N, eds. Optometry: Science, Techniques and Clinical Management, 2nd ed Philadelphia, PA: Butterworth Heinemann; 2008: 209– 28. [Google Scholar]
- 10. Hennessy RT, Leibowitz HW. Laser optometer incorporating the Badal principle. Behav Res Methods Instrum 1972; 4: 237– 9. [Google Scholar]
- 11. Milder B. Tropicamide as a cycloplegic agent. Arch Ophthalmol 1961; 66: 70– 2. [DOI] [PubMed] [Google Scholar]
- 12. Rosenfield M. Accommodation. In: Zadnik K, ed. The Ocular Examination. Measurements and Findings. Philadelphia, PA: Saunders; 1997: 87– 121. [Google Scholar]
- 13. Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci 1997; 74: 367– 75. [DOI] [PubMed] [Google Scholar]
- 14. Stenstrom S. Investigation of the variation and correlation of the optical elements of human eyes (translated by Woolf D). Am J Optom Arch Am Acad Optom 1948; 48: 218– 32, 286–299, 340–350, 388–397, 438–449, 496–504. [PubMed] [Google Scholar]
- 15. Rosenfield M. Refractive status of the eye. In: Benjamin WJ, Borish IM, eds. Borish’s Clinical Refraction, 2nd ed St. Louis, MO: Butterworth-Heinemann; 2006: 3– 34. [Google Scholar]
- 16. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical assessment. Lancet 1986; 1: 307– 10. [PubMed] [Google Scholar]
- 17. Bullimore MA, Fusaro RE, Adams CW. The repeatability of automated and clinician refraction. Optom Vis Sci 1998; 75: 617– 22. [DOI] [PubMed] [Google Scholar]
- 18. Bullimore MA, Adams CW, Fusaro RE, Bauman M, Cotteral RM, Sarver JN, Twelker JD, Graham AD. Acceptance of auto-refractor and clinician prescriptions: a randomized clinical trial. Visual Science and its Applications. Technical Digest Series, vol. 1 Washington, DC: Optical Society of America; 1996: 194– 7. [Google Scholar]


