Abstract
Peste des petits ruminants virus causes a highly infectious disease of small ruminants that is endemic across Africa, the Middle East and large regions of Asia. The virus is considered to be a major obstacle to the development of sustainable agriculture across the developing world and has recently been targeted by the World Organisation for Animal Health (OIE) and the Food and Agriculture Organisation (FAO) for eradication with the aim of global elimination of the disease by 2030. Fundamentally, the vaccines required to successfully achieve this goal are currently available, but the availability of novel vaccine preparations to also fulfill the requisite for differentiation between infected and vaccinated animals (DIVA) may reduce the time taken and the financial costs of serological surveillance in the later stages of any eradication campaign. Here, we overview what is currently known about the virus, with reference to its origin, updated global circulation, molecular evolution, diagnostic tools and vaccines currently available to combat the disease. Further, we comment on recent developments in our knowledge of various recombinant vaccines and on the potential for the development of novel multivalent vaccines for small ruminants.
Keywords: Control and eradication, Country-wise virus circulation, Live attenuated vaccine, Molecular evolution, Pathogenesis, Potential DIVA and multivalent vaccine, PPR, Reverse genetics
1. Introduction
Peste des petits ruminants (PPR) is also known as ‘goat plague’, ‘Kata’, ‘syndrome of stomatitis-pneumoenteritis’ or ‘ovine rinderpest’. It is an important infectious viral disease of domestic and wild small ruminants that threatens the food security and sustainable livelihood of farmers across Africa, the Middle East and Asia (Banyard et al., 2010). The disease is emerging in new regions of the world and is causing significant economic losses (Banyard et al., 2014). The causal agent is Peste des petits ruminants virus, which belongs to the genus Morbillivirus of the family Paramyxoviridae, Vaccines have been available to control the disease for decades with two attenuated vaccine strains, Nigeria 75/1 and Sungri 96, being regularly employed in endemic areas with great success (Sen et al., 2010). For serological diagnosis, commercially available diagnostic ELISAs with high specificity and sensitivity that detect antibodies to either the N or the H proteins of the virus, are available to assess seropositivity within a population (Balamurugan et al., 2014). However, no tools currently exist that enable serological Differentiation between Infected and Vaccinated Animals (DIVA). To this end, marker vaccines are a potential solution to the DIVA concept that may play an important role in the reduction of the disease in endemic regions and the success of any future eradication campaign.
2. Taxonomy of the causative virus
Peste des petits ruminants virus belongs to genus Morbillivirus, sub-family Paramyxovirinae, family Paramyxoviridae, and order Mononegavirales, alongside other important viral pathogens, e.g., Rinderpest virus, Measles virus, Canine distemper virus, Phocine distemper virus and the morbilliviruses of marine mammals, the cetacean morbilliviruses. The characterisation of novel morbilliviruses have recently been described, including Feline morbillivirus in cats (Woo et al., 2012) and numerous morbilli-like viruses in rodents or bats (Drexler et al., 2012). This viral order contains some of the most significant viral pathogens in the medical and veterinary fields (Table 1).
Table 1.
Classification of viruses within the order Mononegavirales.
| Order | Family | Subfamily | Genus |
|---|---|---|---|
| Mononegavirales | Bornaviridae | Bornavirus | |
| Filoviridae | Cuevavirus | ||
| Ebolavirus | |||
| Marburgvirus | |||
| Nyamiviridae | Nyavirus | ||
| Rhabdoviridae | Cytorhabdovirus | ||
| Ephemerovirus | |||
| Lyssavirus | |||
| Novirhabdovirus | |||
| Nucleorhabdovirus | |||
| Perhabdovirus | |||
| Sigmavirus | |||
| Sprivivirus | |||
| Tibrovirus | |||
| Tupavirus | |||
| Vesiculovirus | |||
| Paramyxoviridae | Pneumovirinae | Metapneumovirus | |
| Pneumovirus | |||
| Paramyxovirinae | Aquaparamyxovirus | ||
| Avulavirus | |||
| Ferlavirus | |||
| Henipavirus | |||
| Respirovirus | |||
| Rubulavirus | |||
| Morbillivirus |
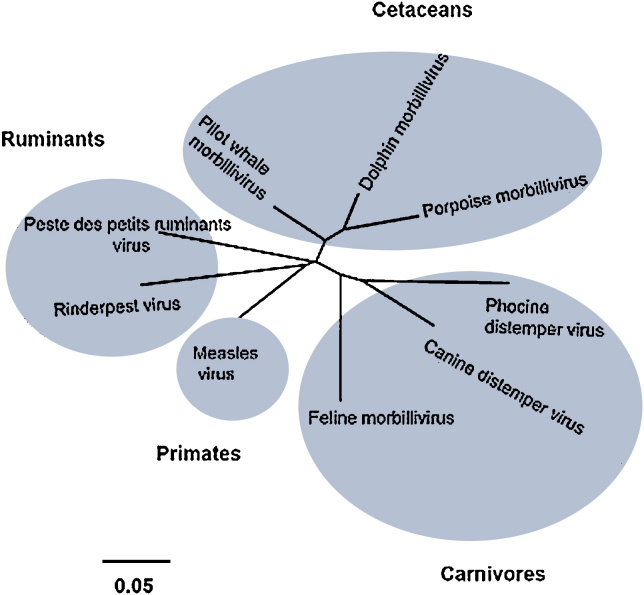
The majority of morbillivirus species can be divided into monophyletic lineages or clades following genetic analysis (Fig. 1). Due to the historical detection of cetacean morbilliviruses and the paucity of data available, for cetacean morbilliviruses several species were initially proposed, including viruses infecting dolphins (Dolphin morbillivirus), those infecting porpoise (Porpoise morbillivirus) and those infecting whales (Pilot Whale Morbillivirus). However, following further identification and genetic characterisation of these viruses, they were classified as a single monophyletic group, the cetacean morbilliviruses. Most recently, novel cetacean isolates have been described that cluster within the cetacean morbilliviruses, but are genetically divergent to those previously characterised, further extending the diversity of these viruses (Barrett et al., 1993, Taubenberger et al., 2000, Stephens et al., 2014).
Fig. 1.
Un-rooted neighbour-joining tree showing the relationships between different morbiliviruses. The phylogenetic tree was constructed using partial N gene sequences of 230 nucleotides (accession nos. NC_006383, Peste des petits ruminants virus; NC_001498, Measles virus; AB547189, Rinderpest virus; NC_001921, Canine distemper virus; KC802221, Phocine distemper virus; JQ411016, Feline morbillivirus; AY949833, Porpoise morbillivirus; NC_005283, Dolphin morbillivirus; AF200818, Pilot whale morbillivirus) with 1000 bootstrap replicates and Kimura 2-parameter model in MEGA 5.2. The scale bar indicates nucleotide substitutions per site.
In general, morbilliviruses are considered restricted in their ability to infect different species. Measles virus infections appear to occur exclusively in humans and non-human primates, rinderpest was restricted to members of the Order Artiodactyla and to date cetacean morbilliviruses have only been reported in aquatic mammals. In contrast, Peste des petits ruminants virus, whilst initially thought to be restricted to the infection of small ruminants, has recently been determined to be the cause of mass mortalities in camelids (Roger et al., 2001, El-Hakim, 2006) and has been described, on a single occasion, in felids (Balamurugan et al., 2012b), although further corroboration of this report is needed. The seemingly most promiscuous morbillivirus is Canine distemper virus. Initially thought restricted to infection of canids, the virus has been described in numerous species, including tigers, lions, hyenas, polar bears and non-human primates (Buczkowski et al., 2014). Feline Morbillivirus was initially reported in domestic cats in Hong Kong as the proposed causative agent of tubulointerstitial nephritis (Woo et al., 2012). Since this initial report, several other detections have been made in Japan (Furuya et al., 2014, Sakaguchi et al., 2014), with some isolates providing evidence of genetic recombination (Park et al., 2014).
Morbilliviruses are characterised at the molecular level most extensively through studies with the prototype virus, Measles Virus and to some extent Canine distemper virus and Rinderpest virus. Peste des petits ruminants virus remains largely uncharacterised with respect to virus replication and transcription. However, the viruses are known to be conserved across the genus with different species sharing similar characteristics. The descriptions of Peste des petits ruminants virus in this review are generalised for morbilliviruses with the inclusion of specific literature available for Peste des petits ruminants virus.
3. Characteristics of the causative virus
3.1. Virion morphology and genome structure
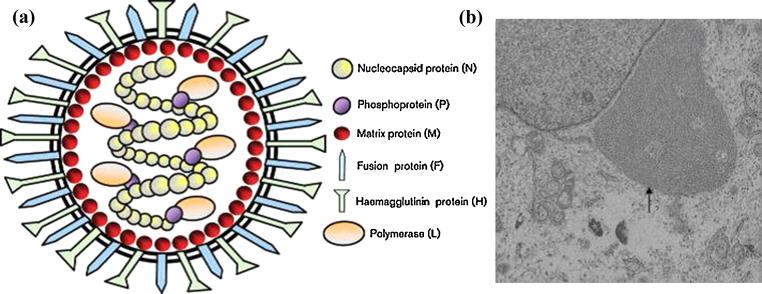
Structurally, morbilivirus virions are visualised as pleomorphic, enveloped particles as determined using negative-stain electron microscopy. The virus particle size has been determined to be between 400 and 500 nm (Gibbs et al., 1979). During virus budding, the viral envelope is derived from infected cell membrane, and is studded with glycoprotein peplomers consisting of the viral fusion (F) and haemagglutinin (H) glycoproteins (Fig. 2a). The Peste des petits ruminants virus genome consists of a non-segmented, single-stranded, negative-sense RNA molecule encapsidated by nucleoprotein (N) forming a helical nucleocapsid, in combination with the RNA-dependent RNA polymerase (L; large polymerase) and the co-factor phosphoprotein (P; polymerase complex) to form the ribonucleoprotein (RNP) complex. Ribonucleoproteins are located within the virus envelope and appear as helices with a herringbone appearance (Fig. 2b). The matrix protein (M) located on the inner surface of the envelope bridges the ribonucleoprotein and cytoplasmic tails of the membrane glycoproteins. Morbillivirus virions have been shown to be polyploid and as such can include more than one independent and functional fully encapsidated genome in the form of ribonucleoproteins (Rager et al., 2002). This polyploidy is thought to be the basis behind the virions general pleomorphy.
Fig. 2.
(a) Schematic diagram of Peste des petits ruminants virion structure (adapted from Banyard et al., 2010). The PPRV glycoproteins (F and H) are embedded within the viral envelope. The M protein lines the inner surface of virus envelope. The ribonucleoprotein complex is composed of N, P and L proteins in association with the RNA genome. (b) Electron micrograph of peste des petits ruminants nuclocapsid in the cytoplasm of an infected cell. The viral RNA, completely encapsidated in the viral N protein has a herring-bone like appearance (arrow).
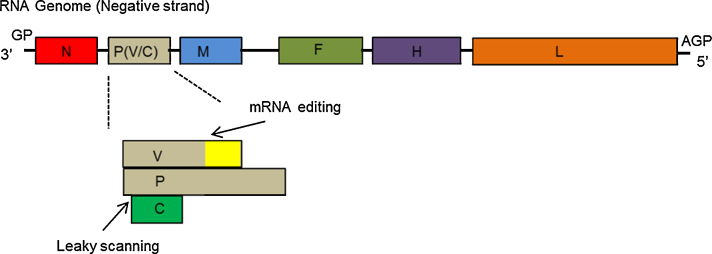
The Peste des petits ruminants virus genome is 15,948 nucleotides in length (Bailey et al., 2005) and conforms to the ‘rule of six’ (multiple of six) as reported for other paramyxoviruses (Calain and Roux, 1993), although a single virus with a hexameric nucleotide insertion in an untranslated region has recently been described (Bao et al., 2014). This strict requirement of the genome length to be divisible by six reflects on the interaction of each nucleoprotein monomer with exactly six nucleotides along the genome or anti-genome RNAs. Furthermore, the genome must be encapsidated by nucleoprotein in its entirety for efficient genome replication and virus propagation (Bailey et al., 2007). The genome organisation and genetic relationship of Peste des petits ruminants virus in comparison to other important paramyxoviruses is presented in Fig. 3.
Fig. 3.
Schematic representation of Peste des petits ruminants virus genome organisation. The PPRV genome is a non-segmented, single-stranded negative sense RNA molecule. The genome consists of six transcriptional units (encoding the nucleoprotein [N], phosphoprotein [P], matrix protein [M], fusion protein [F], haemagglutinin protein [H] and the large/polymerase [L] protein) that are flanked by a 3′ genome promoter (GP) and a 5′ anti-genome promoter (AGP) on the negative sense genome RNA. The P gene encodes for two additional non-structural proteins, namely C and V. The V protein is produced due to co-transcriptional P mRNA editing by insertion of non-template G residues at an editing site. The C protein is produced from an alternative reading frame downstream of the P initiation codon. Expression of C occurs following leaky scanning by the polymerase that reads through the first ATG and initiates at the second ATG.
As mentioned earlier, the Peste des petits ruminants virus genome consists of six transcriptional units located in the order 3′ N, P, M, F, H and L 5′ that encode for six proteins, N, P, M, F, H and L (Bailey et al., 2007). An additional two non-structural proteins, C and V, are generated from the P open reading frame through the utilisation of alternate start codons and RNA editing, respectively. (Mahapatra et al., 2003) (Fig. 3). Transcriptional units are separated from each other by conserved intergenic (IG) trinucleotides. The Peste des petits ruminants virus genome 3′ and 5′ termini sequences are complementary and conserved and from studies with other morbilliviruses play an important role as regulatory elements in replication, transcription and packaging of RNA genome during virus propagation (Banyard et al., 2005). The sizes of genes, intergenic regions, promoter lengths, open reading frames and the deduced molecular weights of the mature proteins are detailed in Table 2. The virus leader region, including the 3′ untranslated region (UTR) of the N gene, constitutes the genome promoter (GP) and, similarly, the 5′ UTR of L gene with a short trailer sequence form the anti-genome promoter (AGP). The UTR between the M and F gene ORF is unusually long (1080 nucleotides) compared to other UTRs along the virus genome and is highly rich in G and C nucleotides (68–72% GC across the region). Overall, the virus genome is relatively conserved with a maximum divergence of 12% at nucleotide and 8% at amino acid sequence level (Muniraju et al., 2014d).
Table 2.
Genome organisation of the Peste des petits ruminants virus.
| Sequence components | Position on genome | Total length | 5′UTR | ORF | 3′UTR | Intergenic region, nt | Protein size, aa | Deduced molecular weight (KDa) |
|---|---|---|---|---|---|---|---|---|
| Leader | 1–52 | 52 | NA | NA | NA | (CTT) | NA | NA |
| N | 55–1744 | 1689 | 52 | 1578 | 59 | CTT | 525 | 58 |
| P | 1748–3402 | 1655 | 59 | 1530 | 66 | CTT | 509 | 55 |
| V (P) | 1748–3402 | 1656 | 59 | 897 | 700 | CTT | 298 | 31 |
| C (P) | 1748–3402 | 1655 | 81 | 534 | 1040 | CTT | 177 | 20 |
| M | 3406–4888 | 1484 | 32 | 1008 | 444 | CTT | 335 | 38 |
| F | 4892–7302 | 2410 | 633 | 1641 | 136 | CTT | 546 | 59 |
| H | 7306–9262 | 1957 | 20 | 1830 | 107 | CTT | 609 | 69 |
| L | 9266–15908 | 6643 | 22 | 6552 | 69 | (CTA) | 2183 | 247 |
| Trailer | 15912–15948 | 37 | NA | NA | NA | NA | NA | NA |
N: nucleoprotein, P: phosphoprotein, M: matrix, F: fusion, H: haemagglutinin, L: large polymerase, UTR: untranslated region, ORF: open reading frame. NA: not applicable.
3.2. Virus replication cycle
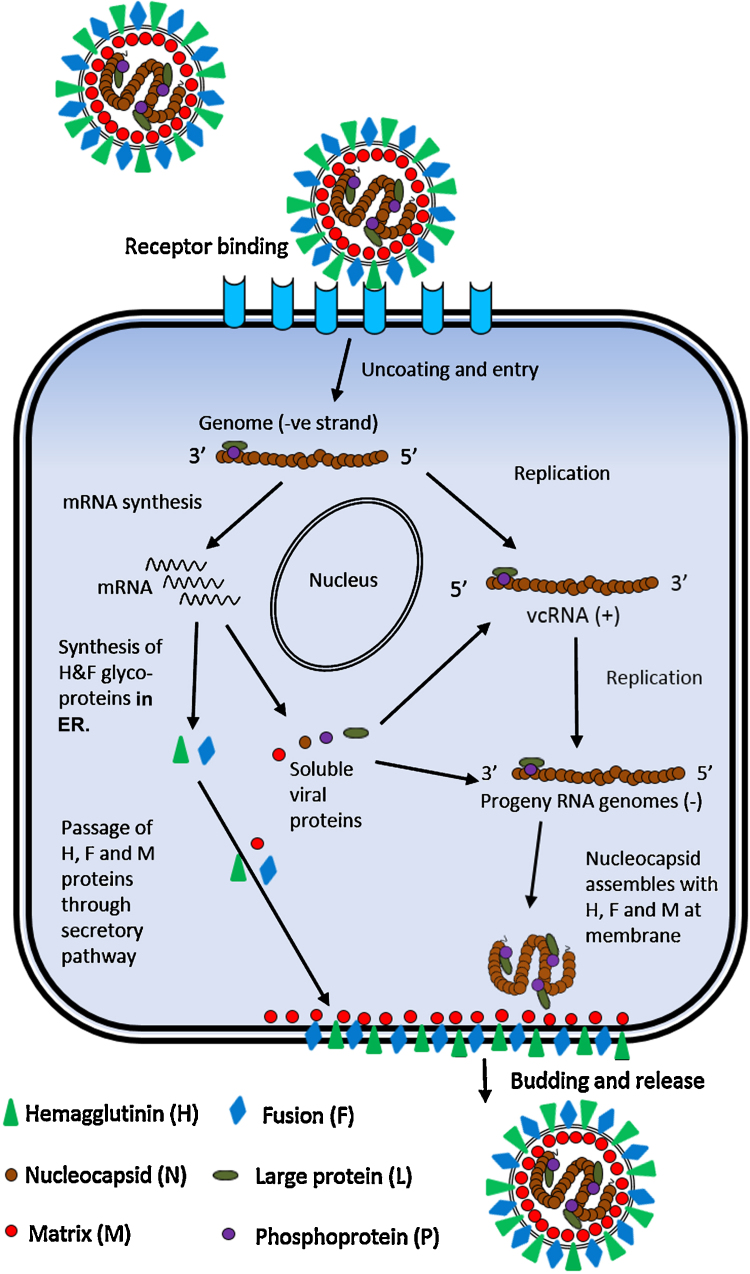
The replication cycle for different paramyxoviruses is similar and the first step is the attachment of the virus on the cell surface and membrane fusion to release a genome into the cell cytoplasm (Fig. 4) (Moss and Griffin, 2006). The H protein is responsible for the attachment of the virus to the cell surface through recognition of and binding to host cell receptor molecules, e.g., salicylic acid, immune cell marker signalling lymphocyte activation molecule (SLAM)/CD 150 (Seki et al., 2003, Adombi et al., 2011) or the epithelial cell receptor Nectin-4 (Birch et al., 2013). Attachment of the H protein to receptors activates the fusion activity of the F protein, enabling a fusion of the viral envelope with the host cell membrane and release of the viral genetic material into the cell cytoplasm.
Fig. 4.
A schematic replication of life cycle of a morbillivirus (adapted from Moss and Griffin, 2006). The first step in virus infection is the attachment of a virion to a host cell surface receptor which leads to the fusion of viral and cellular membrane. The negative sense RNA genome is released into the cell cytoplasm and transcription initiates to produce viral gene transcripts (mRNAs), which are translated using the host cell transcriptional machinery. Later, following the production of the necessary viral proteins, a switch to a replicative mode occurs that results in the production of a positive sense (+) viral complementary RNA (vcRNA +ve), a replicative intermediate which acts as a template for the generation of progeny negative sense genome RNA. The encapsidated genomes interact with the M protein and the viral glycoproteins, leading to budding of new virions at the host cell plasma membrane. ER: endoplasmic reticulum.
Morbilliviruses replicate solely in the cytoplasm of host cells. The genome of Peste des petits ruminants virus is never found as naked RNA and is fully encapsidated by the N protein to form the helical ribonucleoproteins. This ribonucleoprotein complex protects the RNA from host RNAses. The ribonucleoproteins may consist of genome sense (–ve) or antigenome sense (+ve) strands. The ribonucleoprotein complex containing the RNA encapsidated by N, in conjunction with the P and L proteins, make up the minimal replicative unit for these viruses. In the infected cell, a viral genome is released into the cytoplasm and acted upon by viral polymerase complex, which binds to the genome promoter and starts transcribing short leader RNAs. The polymerase works its way across the genome transcribing each gene in turn falling off at each intergenic region. The dissociation of the polymerase at each intergenic region across the genome leads to the build-up of a transcriptional gradient as the polymerase can only commence transcription at the genome promoter (Fig. 3). The mRNAs produced are 5′ methylated and 3′ poly-adenylated by the viral polymerase and are translated by host cell machinery. At a certain time point post-infection, the polymerase complex switches its action from the production of mRNA to the production of a full length positive sense RNA (Fig. 4). This switch is thought to be linked to the accumulation of viral proteins within the host cell, although the precise mechanism for an alteration in polymerase activity remains unclear. Following the production of a full length antigenome (+ve) RNA, the polymerase now binds to the antigenome RNA at the antigenome promoter (3′) and generates nascent full length negative sense genomes. The synthesis of viral components within the cell eventually leads to viral egress from the host cell. The M protein plays an important role in bringing the nascent RNPs and viral glycoproteins to the host cell membrane which results in the packaging, budding and release of nascent virions.
4. Epidemiological characteristics
4.1. Origin and distribution
The first report of peste des petits ruminants was published in 1942 in Côte d’Ivoire (Gargadennec and Lalanne, 1942) based on observations that the disease in small ruminants was not transmissible to cattle in contact with them. After three decades, the causative agent of the disease was defined as a distinct entity (Gibbs et al., 1979). Earlier, reports in 1871 and 1927 in Senegal and French Guinea, respectively, might have described peste des petits ruminants, but the disease was most likely considered to be rinderpest at the time (Diallo, 1988). Contrastingly, cases of rinderpest, with an absence of clinical disease in sheep and goats, are well documented in the same regions of Africa (Rossiter et al., 1982).
The molecular epidemiology of the virus, based on the sequence comparison of a small region of the F gene (322 nt; [Forsyth and Barrett, 1995]), the N gene (255 nt; [Couacy-Hymann et al., 2002]) or the H gene (298 nt; [Senthil Kumar et al., 2014]), has defined the existence of four distinct lineages (I–IV) of the virus (Shaila et al., 1996, Dhar et al., 2002, Banyard et al., 2010, Kwiatek et al., 2011a, Libeau et al., 2014). The nomenclature of lineage I and II are slightly different for analyses depending on whether the N or F gene are being considered (lineage II of F gene data is considered to be lineage I based on N gene data and vice-versa). However, recently a comparison has been made between genetic data derived from the F, N and H genes and the partial N gene sequence revealed a greater variability than the F and H genes; therefore, lineage classification according to the N gene is considered the most accurate way to type novel isolates, as this region is more divergent between lineages and between isolates within lineages (Senthil Kumar et al., 2014).
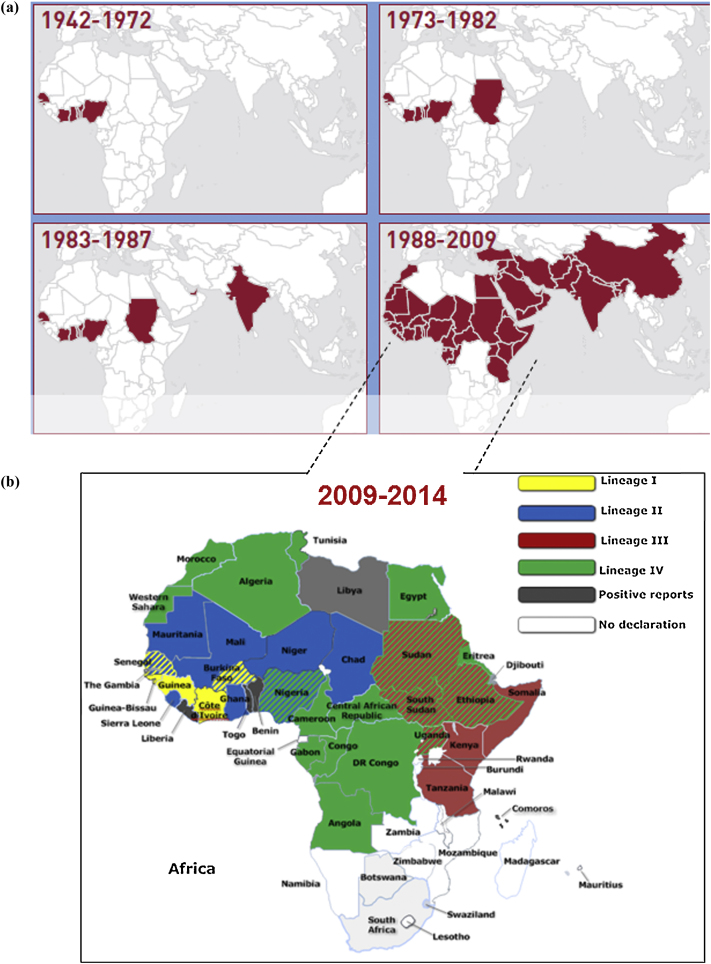
Historically, lineages I–III have been found in Africa and numbered according to the apparent spread of virus from West Africa (I and II) to East Africa (III). Lineage IV was mainly restricted to the Middle East and Asia, with few exceptions of lineage III being reported in Yemen and Oman and mixed lineages of lineage III and IV in the United Arab Emirates and Qatar. However, lineage IV has now established its presence all across the PPR endemic areas with frequent outbreaks in Africa by replacing other lineages (Kwiatek et al., 2011a, Libeau et al., 2014, Muniraju et al., 2014a). The apparent expansion of Asian lineage IV across Africa is supported by a constant increase in the incidence of disease, suggesting an increase in virulence (Libeau et al., 2014). Virus lineages circulating in Africa (Table 3) or in Asia (Table 4) are shown in Fig. 5.
Table 3.
Lineages of Peste des petits ruminants virus circulating in different countries of Africa, based on partial N/F gene sequence analysis.
NA: not available.
Lineages of isolates of Peste des petits ruminants virus were named by following the classification of lineages based on partial N gene sequence phylogenetic analysis.
Table 4.
Lineages of Peste des petits ruminants virus circulating in different countries of Asia, based on partial N/F gene sequence analysis.
| Country | Year of first report | Lineagea | Year of confirmation of outbreak through sequencing |
NCBI submission | References |
|---|---|---|---|---|---|
| Afghanistan | 1995 | NA | NA | No | Martin and Larfaoui (2003) |
| Bangladesh | 1993 | IV | 2000, 2008, 2009, 2010, 2011, 2012 | Yes | Islam et al. (2001), Rahman et al. (2011) |
| Bhutan | 2010 | IV | 2010 | Yes | Banyard et al. (2010) |
| China | 2007 | IV | 2007, 2008, 2014 | Yes | Wang et al. (2009), Banyard et al. (2014) |
| India | 1987 | IV | 1994, 1995, 1996, 1998, 1999, 2004, 2002, 2001, 2003, 2005, 2007, 2008, 2012 | Yes | Shaila et al. (1989), Nanda et al. (1996), Shaila et al. (1996), Dhar et al. (2002), Kerur et al. (2008), Balamurugan et al. (2010) |
| Iran | 1995 | IV | 1998,2010,2011,2012 | Yes | Bazarghani et al. (2006), Kwiatek et al. (2007) |
| Iraq | 1998 | IV | 2011, 2012, 2013 | Yes | Banyard et al. (2010) |
| Israel | 1993 | IV | 1993, 1995,1998, | Yes | Perl et al. (1994), Kwiatek et al. (2007) |
| Jordan | 1991 | NA | NA | No | Lefevre et al. (1991) |
| Kazakhstan | 1997 | NA | NA | No | Lundervold et al. (2004) |
| Kuwait | 1999 | IV | 1999 | Yes | Dhar et al. (2002), Banyard et al. (2010) |
| Lebanon | 2006 | NA | NA | No | Hilan et al. (2006) |
| Nepal | 1995 | IV | 1995, 2009 | Yes | Dhar et al. (2002), Banyard et al. (2010) |
| Oman | 1987 | III | 1983, 1987 | Yes | Hedger et al. (1980), Kwiatek et al. (2007), Banyard et al. (2010), Muniraju et al. (2014c) |
| Qatar | 1987 | III | 2010 | Yes | Banyard et al. (2010) |
| IV | 2010 | Yes | Banyard et al. (2010) | ||
| Pakistan | 1991 | IV | 1994, 2005, 2006, 2007, 2008, 2009, 2010, 2012, | Yes | Amjad et al. (1996) |
| Saudi Arabia | 1980 | IV | 1999, 2004 | Yes | Asmar et al. (1980), Kwiatek et al. (2007), Banyard et al. (2010) |
| Tajikistan | 2004 | IV | 2004 | Yes | Kwiatek et al. (2007) |
| Turkey | 1996 | IV | 1996,2000, 2006,2007,2008,2009,2010,2011 | Yes | Alcigir et al. (1996), Kwiatek et al. (2007), Banyard et al. (2010) |
| United Arab Emirates | 1983 | III | 1986 | Yes | Furley et al. (1987) |
| IV | NA | No | Banyard et al. (2010), Muniraju et al. (2014c) | ||
| Vietnam | 2007 | NA | NA | No | Maillard et al. (2008) |
| Yemen | 2001 | III | 2001, 2009 | Yes | Dhar et al. (2002), Banyard et al. (2010) |
NA: not available.
Lineages of isolates of Peste des petits ruminants virus were named by following the classification of lineages based on partial N gene sequence phylogenetic analysis.
Fig. 5.
Global spread of Peste des petits ruminants virus from its first detection in 1942–2014, including lineage distribution (a: adopted from Food and Agriculture Organisation (FAO, 2009), b: recent circulations of Peste des petits ruminants virus in Africa, drawn by using smart draw software).
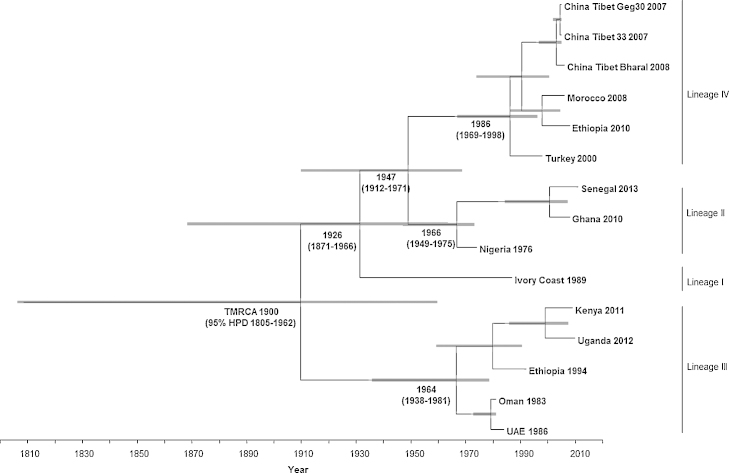
Most recently, the evolution of the virus and its relationship with Rinderpest virus has been suggested using molecular phylogenetic techniques with virus full genome sequence data. Bayesian phylogenetic studies found that Rinderpest virus is more closely related to Measles virus than to Peste des petits ruminants virus (Pomeroy et al., 2008, Furuse et al., 2010). The origin of ancestral Peste des petits ruminants virus and its relation to the other morbilliviruses have recently been estimated using Bayesian analysis of the complete virus genome sequences (Muniraju et al., 2014d). A Bayesian phylogenetic analysis of all four viral lineages mapped the time to most recent common ancestor at the beginning of the 20th century, a few decades before the first recorded description of the virus in 1942. Lineage III Peste des petits ruminants virus is proposed to have been the first to have diverged (Fig. 6). The estimated probability for the root location of an ancestral virus and individual lineages were determined as being Nigeria for Peste des petits ruminants virus, Senegal for lineage I, Nigeria/Ghana for lineage II, Sudan for lineage III and India for lineage IV (Muniraju et al., 2014d). In recent years, the disease has extended its distribution southwards in Africa, as far as southern Tanzania (2008) and the Democratic Republic of Congo and Angola (2012) (Banyard et al., 2010, Libeau et al., 2014). Disease outbreaks have also been reported across North Africa, including Tunisia (2006), Morocco (2008) and Algeria (2011). Alongside this, the European part of Turkey reported 12 confirmed outbreaks of the disease in sheep and goats in 2011–2012 (Libeau et al., 2014). In Asia, the disease has spread to Tibet (2007) and more recently, throughout China (2013–2014) (Banyard et al., 2014) and Kazakhstan (Kock et al., 2015).
Fig. 6.
Time-scaled Bayesian MCC phylogeny tree based on Peste des petits ruminants virus complete genome sequences; the tree was constructed using the UCED model and exponential tree prior (branch tips correspond to the date of collection, branch lengths reflect elapsed time—tree nodes are annotated with posterior probability values, estimated median dates of TMRCA and the corresponding 95% HPD interval values of TMRCA indicated as grey bars; the horizontal axis indicates time in years).
4.2. Host range
Sheep and goats are the primary hosts for Peste des petits ruminants virus with few reports of disease outbreak in camels (Roger et al., 2001, Saeed et al., 2004, Khalafalla et al., 2010, Kwiatek et al., 2011b). Cattle (Anderson and McKay, 1994, Lembo et al., 2013, Sen et al., 2014), buffalo (Govindarajan et al., 1997) and pigs (Nawathe and Taylor, 1979) develop subclinical infection but are not capable of excreting the virus, and thus are not considered to be important in the epidemiology of the virus. Infection of various wildlife species, mainly living under semi-free range conditions, has been reported (Table 5), although their exact role in the epidemiology of the disease needs to be studied.
Table 5.
Reported infection of wildlife species by Peste des petits ruminants virus.
| Common name | Zoological name | Diagnostic method | Country | Reference |
|---|---|---|---|---|
| Afghan Markhor goat | Capra falconeri | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| African grey duiker | Sylvicapra grimma | Serological examination | Nigeria | Ogunsanmi et al. (2003) |
| Arabian gazelle | Gazella gazella | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| Arabian mountain gazelle | Gazella gazella cora | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| Arabian oryx | Oryx leukoryx | Serological examination | Saudi Arabia, United Arab Emirates | Frolich et al. (2005) |
| Barbary sheep | Ammotragus lervia | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| Bharal | Pseudois nayaur | Clinical, serological and molecular examination | China | Bao et al. (2011) |
| Bubal hartebeests | Alcelaphus buselaphus | Serological examination | Ivory Coast | Couacy-Hymann et al. (2005) |
| Buffalo | Syncerus caffer | Serological examination | Ivory Coast | Couacy-Hymann et al. (2005) |
| Bushbuck | Tragelaphus scriptus Kinne | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| Defassa waterbuck | Kobus defassa | Serological examination | Ivory Coast | Couacy-Hymann et al. (2005) |
| Dorcas gazelle | Gazella dorcas | Clinical examination | United Arab Emirates | Furley et al. (1987) |
| Gemsbok | Oryx gazella | Clinical examination | United Arab Emirates | Furley et al. (1987) |
| Impala | Aepyceros melampus | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| Kob | Kobus kob | Serological examination | Ivory Coast | Couacy-Hymann et al. (2005) |
| Laristan sheep | Ovis gmelini | Clinical examination | United Arab Emirates | Furley et al. (1987) |
| Nubian ibex | Capra nubiana | Clinical examination | United Arab Emirates | Furley et al. (1987) |
| Persian gazelle | Gazella subgutturosa | Serological examination | Turkey | Gur and Albayrak (2010) |
| Rheem gazelle | Gazella subguttorosa marica | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| Sindh ibex | Capra aegagrus blythi | Clinical and serological examination | Pakistan | Abubakar et al. (2011) |
| Springbuck | Antidorcas marsupialis | Clinical, serological and molecular examination | United Arab Emirates | Kinne et al. (2010) |
| Thompson’s gazelle | Eudorcas thomsonii | Clinical examination | Saudi Arabia | Abu Elzein et al. (2004) |
| White-tailed deer | Odocoileus virginianus | Clinical examination | United States of America | Hamdy and Dardiri (1976) |
| Wild goat | Capra aegagrus | Clinical and serological examination | Kurdistan ( sic) | Hoffmann et al. (2012) |
Infected animals can transmit the virus to close in-contact susceptible animals through exhaled aerosol or clinical excretions (lacrimal, nasal, saliva, feces). The virus is temperature sensitive and readily inactivated in a dry environment (Rossiter and Taylor, 1994). Infected animals that recover from disease develop a life-long protective immunity and no carrier state has been identified (Hamdy et al., 1976). However, virus can circulate in animals with mild disease, leading to disease outbreaks, during which naive susceptible populations are mixed with those infected and displaying a mild form of disease (Couacy-Hymann et al., 2007b, Banyard et al., 2014). Other host factors, e.g., age, sex, breed or season, may also play a role in disease development.
5. Disease processes
5.1. Clinical signs
Although goats and sheep are the primary hosts for the virus, goats seem to be more susceptible to disease than sheep (Nanda et al., 1996), with some breeds of goat considered to be more susceptible than others (Couacy-Hymann et al., 2007a). The incubation period of the disease is typically 4–6 days, although it may range between 3 and 14 days. During the acute stage of disease, animals show pyrexia (up to 41 °C) that may last for 3–5 days and that can be accompanied by depression, anorexia and dryness of the muzzle. Watery nasal and lachrymal discharges gradually become mucopurulent with excessive salivation. Erosive lesions formed in the oral cavity may become necrotic. In severe cases of the disease, these necrotic lesions progress with the appearance of a deposit of fibrin (caseous deposit) on the tongue. In later stages of the disease, animals develop diarrhoea and coughing with labored, abdominal breathing. Finally, the animal may become dyspnoeic, suffering progressive weight loss and emaciation, ultimately leading to death. In some cases, particularly in mild infections, animals may convalesce, returning to a pre-infection health status within 10–15 days of infection. The morbidity rate can reach 100% with a high case fatality rate in the acute form of disease (Pope et al., 2013). The above described clinical signs and mortality can vary considerably depending on the virulence of the viral strain and the immunological state of the affected animal (OIE, 2012).
5.2. Pathogenesis
Disease progresses with development of lacrimal, nasal and mucosal discharges and viral material can be detected in such excretions as early as 4 days after infection. Viral antigen has also been observed by histochemical staining in the lymphoid organs, the respiratory and the gastrointestinal tract (Kumar et al., 2004, Pope et al., 2013).
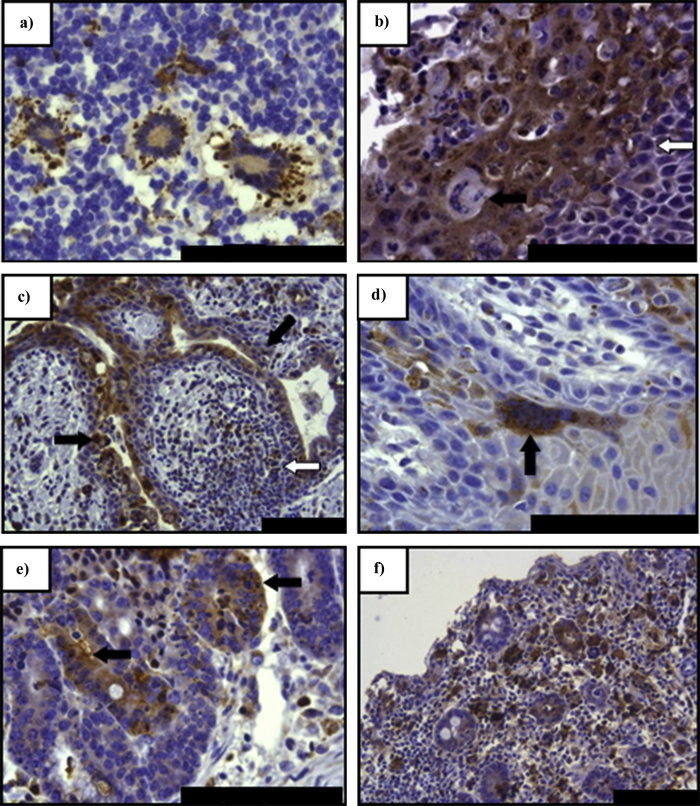
The pathogenesis of peste des petits ruminants has long been assumed to mirror that of rinderpest in cattle (Wohlsein et al., 1993, Brown and Torres, 1994). Pathogenesis studies in peste des petits ruminants have mainly been performed through experimental infection with a virulent form of the virus to develop a reliable and reproducible model (Bundza et al., 1988, El Harrak et al., 2012, Hammouchi et al., 2012, Pope et al., 2013, Baron et al., 2014b, Truong et al., 2014). Histological assessment during early infection showed an immune cell proliferation driven by the spread of the virus, similar to that caused by other morbilliviruses (Pope et al., 2013). The initial site for virus replication is observed within the tonsillar tissue and lymph nodes draining the site of inoculation. It has been proposed that the virus infected immune cells within the respiratory mucosa would migrate to the local lymphoid tissue, where primary virus amplification would occur, with the virus then entering the general circulation. Clinical signs usually develop 3 to 4 days later with established pyrexia and anorexia. Leucopoenia is observed from the fourth day post-infection with considerable reduction of CD4+ T cells (Baron et al., 2014b, Herbert et al., 2014). The viral antigens have also been observed by immunohistochemical staining in the lymphoid organs (Fig. 7a and b), the facial epithelia (Fig. 7c and d) and the gastrointestinal tract (Fig. 7e and f) (Kumar et al., 2004, Pope et al., 2013). Histopathological assessment of infected tissues revealed a large number of syncytia within the lymphoid tissue from days 5–7 post infection. In the later stage of infection erosive lesions are formed in the oral cavity that also become necrotic. The severity of clinical signs generally peaks between 6 and 8 days post infection and can continue for up to 14 days leading to death or recovery from infection. Despite the segregation of isolates of Peste des petits ruminants virus into lineages, the early stages of pathogenesis in goats infected with virulent strains of the virus had no lineage specific difference in the pathogenicity of the virus (Baron et al., 2014a).
Fig. 7.
Immunohistochemical staining of Peste des petits ruminants virus antigen in experimentally infected goats. (a) Predominately peripheral paracortical immunolabelling of syncytia within the LPSLN (7 days post-infection); dendritic-type cells present and positive for virus antigen (arrow) with an infected lymphocyte also present (open arrow). (b) Advanced epithelial infection of the pharyngeal tonsil (7 days post-infection) with syncytia formation. (c) Conjunctival mucosal epithelium (9 days post-infection); evidence of advanced epithelial and proprial infection involving a mixed population of inflammatory and epithelial cells around an exocrine gland (arrows); immunolabelling within the proprial lymphoid follicle circumscribed by this gland (open arrow). (d) Labial mucosal epithelium (9 days post-infection) with a large epithelial syncytium (arrow) seen in the lower stratum spinosum layer. (e) Severe abomasal infection (9 days post-infection) of crypt epithelial cells (arrows). (f) Marked viral infection of both glandular epithelial cells and the immune/inflammatory cells present within the caecum (7 days post-infection). Scale bars represent 100 μm. Adapted from Pope et al. (2013).
5.3. Immunosuppression
Peste des petits ruminants virus is highly lymphotropic and infection often leads to a profound immunosuppression that causes leucopoenia and reduced antibody responses (Pope et al., 2013, Rajak et al., 2005). Immunosupression by Peste des petits ruminants virus has been observed in both vaccinated and infected animals (Rajak et al., 2005). Virulent strains of the virus cause marked immunosuppression, whereas vaccination only induces a transient leucopoenia with no significant effects on the immune response (Rajak et al., 2005). Leucopoenia is observed generally from the fourth day post-infection and may revert depending on progression of disease (Pope et al., 2013, Baron et al., 2014b, Herbert et al., 2014). Blood from Peste des petits ruminants virus infected goats was tested for specific immune cell sub populations and it has been shown that no change in the proportion of WC1+ γ/δ T-cells and CD14+ monocyte/macrophage cells occurs. However, CD4+ cells were found to decrease in number from day 4 post-infection and, surprisingly, the proportion of CD8+ cells, although unchanged initially, increased by 7 days post-infection (Herbert et al., 2014).
5.4. Immunological features
Maternal antibodies against the virus can be detected in young animals and remain able to neutralise virus for three to four months enabling a level of protection in newborn animals (Libeau et al., 1992). Therefore, vaccination of new born animals is not necessary until that age (Bodjo et al., 2006). Cellular and humoral immune responses are induced upon infection or vaccination, a feature of the live attenuated vaccines available for the virus. An inflammatory response in goats infected with different strains of the virus was observed with the enhanced expression of cytokines (Atmaca and Kul, 2012, Baron et al., 2014b). Morbillivirus non-structural proteins have been shown to play a role in the blocking of type I and type II interferon action (Ohno et al., 2007, Fontana et al., 2008, Chinnakannan et al., 2013). Cell-mediated and humoral immune responses against the virus are mainly directed against the H, F and N proteins (Sinnathamby et al., 2001). However, immunisation with viral glycoproteins (H and/or F) induces protective humoral immunity, whilst immunisation with the N protein does not (Sinnathamby et al., 2001, Diallo et al., 2007). Both cytotoxic B cell and T cell epitopes have been mapped to regions on the N protein (Mitra-Kaushik et al., 2001, Choi et al., 2005) and further a B cell epitope located on the C-terminus of the N protein was demonstrated (Dechamma et al., 2006). B cell epitopes have also been mapped to the H protein of the virus (Renukaradhya et al., 2002). The CD8+ T cells, but not CD4+ cells have been demonstrated to be primed following the experimental challenge of animals vaccinated with a subunit vaccine expressing the virus H protein with a virulent virus (Herbert et al., 2014).
6. Disease diagnosis
Peste des petits ruminants can be confused with other diseases including rinderpest, bluetongue and contagious caprine pleuropneumonia, due to the similarity of these diseases in clinical signs. Diagnosis of the disease may also be complicated, as the result of secondary bacterial infections specifically caused by Mannheimia haemolytica. Therefore, in addition to clinical observations, a differential diagnosis must be confirmed by laboratory diagnostic techniques.
The laboratory tests currently available for diagnosis of the disease can be grouped into three categories: (i) those detecting virus or viral antigen (e.g., virus isolation, antigen capture ELISA, lateral flow devices), (ii) those detecting genetic material from the virus (e.g., RT-PCR, real-time PCR, LAMP PCR) and (iii) those detecting antibodies against the virus (e.g., virus neutralisation test, competitive ELISA, indirect ELISAs). The available laboratory test methods for diagnosis of the disease are summarised in Table 6. However, the efficiency of laboratory diagnosis can be greatly influenced by the integrity of the sample received, often affected by the conditions of its by collection and transportation.
Table 6.
Laboratory methods available for the diagnosis of peste des petits ruminants.
| Method | Objective |
Reference | |||||
|---|---|---|---|---|---|---|---|
| Population freedom from infection | Individual animal freedom from infection | Confirmation of clinical cases | Prevalence of infection surveillance | Immune status in individual animals or populations post-vaccination | |||
| Agar gel immunodiffusion | – | – | + | – | + | Durojaiye (1982) | |
| Competitive ELISA | ++ | ++ | – | +++ | +++ | c-H ELISA | Anderson and McKay (1994), Saliki et al. (1993) |
| c-N ELISA | Libeau et al. (1995) | ||||||
| Counter immuno-electrophoresis | – | – | + | – | – | Majiyagbe et al. (1984) | |
| Immuno-capture ELISA | – | – | +++ | – | – | Libeau et al. (1994) | |
| PCR | – | – | +++ | – | – | RT-PCR based on F gene | Forsyth and Barrett (1995) |
| RT-PCR based on N gene | Couacy-Hymann et al. (2002) | ||||||
| Multiplex RT-PCR | George et al. (2006), Saravanan et al. (2004) | ||||||
| LAMP | Li et al. (2010) | ||||||
| Real-time RT-PCR (qRT-PCR) | Bao et al. (2008), Batten et al. (2011), Kwiatek et al. (2010) | ||||||
| Virus isolation in cell culture | – | – | ++ | – | – | Cells-Vero, B95a and the vero expressing dog SLAM, CV1 expressing goat SLAM | Adombi et al. (2011) |
| Virus neutralisation | +++ | +++ | – | +++ | +++ | ||
+++: recommended method, ++: suitable method, +: may be used in some situations, but cost, reliability or other factors severely limits its application, –: not appropriate for the purpose.
Virus neutralisation is considered to be the ‘gold standard’ test for diagnosis of the disease, although it is a time-consuming method that requires tissue culture facilities. A commercially available competitive ELISA based on either H or N protein is used for the detection of antibodies and RT-PCR including real-time RT-PCR are commonly employed to detect nucleic acid. Finally, an immunochromatographic lateral flow device has been developed as a pen-side test using a monoclonal antibody, specific to the virus H protein (Bruning-Richardson et al., 2011). This test has recently been validated under field conditions for diagnosis as early as 4 days post-infection, before onset of severe clinical signs (Baron et al., 2014b).
7. Control
Preventive measures employed in disease free areas include strict restrictions on the importation of animals from disease infected regions. Disease can be efficiently controlled by isolation and slaughtering of infected animals, as well as disinfection of environmental materials and the restriction of animal movements. Immunisation can be performed with commercially available attenuated vaccines that elicit a protective immunity that has been shown to be effective for at least three years post-vaccination (Diallo et al., 2007, Sen et al., 2010). Current vaccination schedules require the immunisation of susceptible animals at least every three years (Diallo et al., 2007, Saravanan et al., 2010). Vaccination in animals aged 4–6-months is recommended (Balamurugan et al., 2012a). This is an important area in the control programmes of the disease, as introduction of unvaccinated animals into a potentially susceptible population can lead to introduction of the virus into a farm and can cause a fresh outbreak of disease. Currently available vaccines require maintenance of the cold chain to ensure maintenance of maximal virus titre for inoculation and the required serological response to vaccination.
7.1. Attenuated vaccines
Tissue culture rinderpest vaccine (TCRV) used as a heterologous vaccine against peste des petits ruminants was found to be effective, due to the antigenic relatedness between these two ruminant morbilliviruses (Mariner et al., 1993). However, use of this vaccine was prohibited in 1996, due to the lack of DIVA following vaccination and the need for extensive serological monitoring during the final stages of the rinderpest eradication campaign.
In an initial attempt to generate a homologous vaccine, Peste des petits ruminants virus was grown on primary cell line of sheep liver cells (Gilbert and Monnier, 1962), but after 65 serial passages in primary cell culture, the virus was not sufficiently attenuated (Benazet, 1984). Later, Diallo et al. (1989) successfully generated an attenuated Peste des petits ruminants virus strain, the Nigeria 75/1 strain, by serial passage in Vero cells of a virulent strain. Following 63 passages the virus was deemed suitably attenuated following extensive in vivo studies (Diallo et al., 1989, Adu et al., 1990). Similarly, at least three more vaccine strains, specifically Sungri 96, Arasur 87 and Coimbatore 97, have been developed for use as vaccines following 75 serial passages in Vero cells (Singh et al., 2010). Currently, Nigeria 75/1 (lineage II) and Sungri 96 (lineage IV) are widely used for vaccination, whereas Arasur 87 has a more limited use within India (Table 7). These attenuated vaccines are now available in freeze-dried form and include various chemical stabilisers to reduce the thermolability of the virus and reduce the need for the cold chain (Mariner et al., 1993, Worrall et al., 2000, Sen et al., 2010).
Table 7.
Characteristics of attenuated vaccines against peste des petits ruminants.
| Parametre | Vaccine strain |
||
|---|---|---|---|
| Nigeria 75/1 | Sungri 96 | Arasur 87 | |
| Passage and origin | LK-6, BK-2, Vero-63 Nigeria, sheep |
B95a-10, Vero-59 North India, goat |
Vero 75 South India, sheep |
| Complete CPE | 3–6 days | 3–6 days | 2–3 days, rapid growing |
| Safety in pregnancy | Safe | Safe | Safe |
| Lineage | II | IV | IV |
| Use | Extensively used in several countries | Extensively used in India (>20.000.000 doses annually) | Used in some areas in India |
| Virus sequence | Full genome sequenced | Full genome sequenced | Not available |
7.2. Recombinant subunit vaccines
Subunit vaccines for morbilliviruses have been developed for several species. Initial work carried out with Capripox virus strains expressing F and H proteins of Rinderpest virus were shown to protect goats against Peste des petits ruminants virus (Romero et al., 1995). Capripox virus strains expressing the homologous Peste des petits ruminants virus H (Diallo et al., 2002) or F (Berhe et al., 2003) proteins have also been shown to protect goats or sheep against infection by Peste des petits ruminants virus (Chen et al., 2010). Concerns regarding efficacy of recombinant bivalent Capripox virus and Peste des petits ruminants virus vaccine (F and H proteins) in sheep or goats with pre-existing immunity against either of the viruses have been addressed recently (Caufour et al., 2014). Animals with pre-existing immunity for either virus, achieved by immunising animals with a single-type vaccine, were inoculated with the bivalent vaccine and after four weeks were challenged with a virulent Capripox virus strain followed by a virulent Peste des petits ruminants virus strains after another three weeks. In all cases, complete protection against Capripox virus was evident; however, partial protection against Peste des petits ruminants virus was detected in animals previously immunised against Capripox virus. This indicated a limited replication of the Capripox-PPRV F and H bivalent vaccine in the presence of pre-existing antibodies against the virus, which led to a poor expression of the Peste des petits ruminants virus F and H proteins and, therefore, reduced the level of antibody response against the latter virus.
The vaccinia virus vector (Modified vaccinia Ankara) expressing Peste des petits ruminants virus F and H proteins was shown to protect goats against the disease after the administration of two doses of the vaccine (Chandran et al., 2010), which might decrease its utility as a vaccine candidate. Goats immunised with a vaccine containing recombinant fowl pox virus expressing the H or F proteins of Peste des petits ruminants virus had a poor antibody response to the heterologous proteins (Herbert et al., 2014). Recombinant adenovirus vectors expressing glycoproteins of the virus have also been developed (Qin et al., 2012, Wang et al., 2013, Herbert et al., 2014, Rojas et al., 2014). The recombinant replication-defective Human adenovirus serotype 5 incorporating H or F proteins of Peste des petits ruminants virus was shown to induce humoral and cell mediated immunity and protected goats (Herbert et al., 2014) or sheep (Rojas et al., 2014) against challenge with virulent strains of the virus. These vaccines need to be tested further in large scale studies to assess their potency and, more importantly, the duration of immunity in comparison to the conventional live attenuated vaccines, as vaccinated animals are exposed to only one or two proteins of the virus.
Subunit vaccines have also been developed based on Baculovirus expression systems expressing the H (Sinnathamby et al., 2001) or F proteins (Rahman et al., 2003) or from the H protein expressed alone in transgenic peanut plants (Khandelwal et al., 2011). However, these need to be evaluated further for safety and efficacy. Similarly, such assessments are required for DNA vaccines (Yang et al., 2013) and for virus-like particle based vaccines (Liu et al., 2014).
7.3. Improvement of existing attenuated vaccines
Many small ruminant diseases, e.g., goat pox, sheep pox, bluetongue, Rift valley fever, contagious ecthyma, border disease, overlap geographically with peste des petits ruminants and are of economic significance to small ruminant agriculture (Banyard et al., 2010, Saravanan et al., 2007, Mondal et al., 2009, Ozmen et al., 2009, Malik et al., 2011, Toplu et al., 2012). A vaccination programme with increased valency that is able to generate neutralising responses to several disease following a single administration would improve the efficiency of control programmes and reduce costs associated with such a programme. Live bivalent vaccines against goat pox and peste des petits ruminants (Rajak et al., 2005, Hosamani et al., 2006) or against sheep pox and peste des petits ruminants (Chaudhary et al., 2009) produced by combining the two antigens during the manufacturing process gave promising results, but require further standardisation and validation.
The need for the further development of vaccines against peste des petits ruminants revolves around three approaches: (i) the development of thermostable vaccine preparations to overcome the costly requirement of cold chain maintenance, (ii) the fulfilment of DIVA principles to enable effective post vaccination seromonitoring and (iii) the production of multivalent vaccines that could induce neutralising responses against several diseases alongside peste des petits ruminants. Whilst only thermostable preparations are primarily required to control the disease, vaccines that could fulfil the other two approaches would significantly reduce costs associated with vaccination, which is a significant issue in regions where the disease is endemic.
Reverse genetics techniques are being investigated to address the DIVA issue in the preparation of relevant vaccines. Reverse genetics provides a means to manipulate RNA virus genomes through DNA copies (cDNA) of the RNA genome. The new genome cDNA can then be manipulated to obtain the modified form of the virus. Use of reverse genetics techniques to negatively or positively tag vaccines (Hu et al., 2012, Muniraju et al., 2012), to modify specific epitopes (Muniraju et al., 2014b) or to insert heterologous epitopes or immunogenic antigens (Das et al., 2000, Walsh et al., 2000a, Walsh et al., 2000b, Mahapatra et al., 2006, Parida et al., 2006, Parida et al., 2007, Takeda et al., 2006, Gao et al., 2008, Ludlow et al., 2012, Yamaji and Nakayama, 2014) may lead to the development of novel vaccine preparations that fulfil the requirements described above. Recently, positively or negatively marked vaccines (Fig. 8) have been developed against peste des petits ruminants (Muniraju et al., 2014b). In a negatively marked vaccine, the epitope binding site of the C77 monoclonal antibody, the monoclonal antibody used to compete against antibodies in test sera in the competitive H ELISA, was removed by epitope deletion. This vaccine was been taken forward for in vivo assessment and was shown to be safe with a potency similar to the existing Nigeria 75/1 (Muniraju et al., 2014b). However, although the C77 mAb was unable to bind to the mutated form of H protein in in vitro tests, the mutation was not sufficient to enable DIVA under field conditions, hence further alterations to the proposed epitope within H are being investigated.
Fig. 8.
Expression of Peste des petits ruminants virus (PPRV) N and H proteins and/or GFP with C77 mAb binding activity in the virus recombinants (positive and negative marker vaccine strains) and parental virus infected cells; VDS cells were infected with viruses at an MOI of 0.01 and fixed 24 h post-infection using 4% paraformaldehyde; cells were stained separately with primary antibodies of mouse anti PPRV H (C77) and mouse anti PPRV N (C11) followed by secondary antibody Alexa Fluor 488 goat anti-mouse (red); cell nuclei were stained with DAPI (blue) and GFP autoflorescence (green); expression pattern of the virus N and H proteins were comparable between the recombinant and parental virus. The anti-PPRV H-mAb could not detect mutated H-protein in rPPRV-C77 infected cells. Autoflorescence of GFP was observed in the rescued rPPRV + GFP (an image with the autoflorescence to show GFP was not taken alongside N antibody staining). Adapted from Muniraju et al. (2014b). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
8. Concluding remarks
Peste des petits ruminants affects some of the poorest farming communities on the planet and presents a significant barrier to development and sustainability of small ruminant farming. The successful eradication of rinderpest has led the attention of the scientific community to peste des petits ruminants as a further potential target for global eradication. In a recent meeting, the World Organisation for Animal Health and the Food and Agriculture Organisation have proposed a control programme for the disease with the target year for eradication being 2030 (FAO, 2014). However, the scientific and economic infrastructures present in areas where the disease is endemic, are largely insufficient to mount a viable eradication campaign without international aid. The recent emergence of the disease across China has highlighted the ability of the virus to spread rapidly across vast areas causing significant economic losses (Banyard et al., 2014).
The vaccines currently available to protect small ruminants against the virus are sufficient to enable an eradication campaign, although there is little doubt that refinements to vaccines would reduce the costs associated with control, making eradication in areas with limited resources more readily achievable. Recent advances in the generation of recombinant versions of the virus will serve as suitable starting points for development of novel vaccine formulations (Hu et al., 2012, Muniraju et al., 2014b). The current understanding of disease pathogenesis and mechanisms of virulence require further expansion that will have ramifications on the knowledge of Peste des petits ruminants virus and related viruses of both veterinary and medical importance. Assessment of genetic data is starting to unravel elements of the evolutionary biology behind Peste des petits ruminants virus and its relationship with other morbilliviruses (Muniraju et al., 2014d). The detection and genetic characterisation of novel morbilliviruses from different species may indicate further approaches for the control and eradication of these important viral pathogens.
Conflict of interest statement
Authors have no conflict of interest.
Acknowledgements
The authors would like to acknowledge funding from BBSRC/DFID grant HH009485/1, BBSRC International Partnering AwardsBB/I026138/1 and BB/J020478/1, EU-BBSRC Anihwa grant BB/L013657/1 and DBT-BBSRC FADH grant BB/L004801/1. SP would like to thank Dr M Baron at the Pirbright Institute to review the replication figure.
References
- Abd El-Rahim I.H., Sharawi S.S., Barakat M.R., El-Nahas E.M. An outbreak of peste des petits ruminants in migratory flocks of sheep and goats in Egypt in 2006. Rev. Sci. Technol. 2010;29:655–662. doi: 10.20506/rst.29.3.2004. [DOI] [PubMed] [Google Scholar]
- Abu Elzein E.M.E., Housawi F.M.T., Bashareek Y., Gameel A.A., Al-Afaleq A.I., Anderson E. Severe PPR infection in gazelles kept under semi-free range conditions. J. Vet. Med. B. 2004;51:68–71. doi: 10.1111/j.1439-0450.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- Abubakar M., Arshed M.J., Hussain M., Ali Q. Evidence of peste des petits ruminants in serology of sheep and goats from Sindh, Pakistan. Transbound. Emerg. Dis. 2011;58:152–156. doi: 10.1111/j.1865-1682.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- Adombi C.M., Lelenta M., Lamien C.E., Shamaki D., Koffi Y.M., Traore A., Silber R., Couacy-Hymann E., Bodjo S.C., Djaman J.A., Luckins A.G., Diallo A. Monkey CV1 cell line expressing the sheep–goat SLAM protein: a highly sensitive cell line for the isolation of peste des petits ruminants virus from pathological specimens. J. Virol. Methods. 2011;173:306–313. doi: 10.1016/j.jviromet.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu F.D., Joannis T., Nwosuh E., Abegunde A. Pathogenicity of attenuated peste des petits ruminants virus in sheep and goats. Rev. Elev. Med. Vet. Pays Trop. 1990;43:23–26. [PubMed] [Google Scholar]
- Alcigir G., Atalay Vural S., Toplu N. Vol. 43. Veteriner Fakultesi Dergisi, Ankara Universitesi; 1996. pp. 181–189. (First Pathological and Immunohistological Description of Pest of Small Ruminants Virus Infection in Lambs in Turkey Turkiye’de Kuzularda Peste Des Petits Ruminants Virus Enfeksiyonunun Patomorfolojik Ve Immunhistolojik Ilk Tanimi). [Google Scholar]
- Ali B.E., Taylor W.P. Isolation of peste-des-petits-ruminants virus from the Sudan. Res. Vet. Sci. 1984;36:1–4. [PubMed] [Google Scholar]
- Amjad H., Qamar ul I., Forsyth M., Barrett T., Rossiter P.B. Peste des petits ruminants in goats in Pakistan. Vet. Rec. 1996;139:118–119. doi: 10.1136/vr.139.5.118. [DOI] [PubMed] [Google Scholar]
- Anderson J., McKay J.A. The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications to rinderpest control programmes. Epidemiol. Infect. 1994;112:225–231. doi: 10.1017/s0950268800057599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmar J.A., Radwan A.I., Abi Assi H., Rasheid A.A. University of Riyadh; Riyadh: 1980. A PPR-Like Disease of Sheep in Central Saudi Arabia: Evidence of Its Immunologic Relation to Rinderpest; Prospects for a Control Method; pp. 325–337. [Google Scholar]
- Atmaca H.T., Kul O. Examination of epithelial tissue cytokine response to natural peste des petits ruminants virus (PPRV) infection in sheep and goats by immunohistochemistry. Histol. Histopathol. 2012;27:69–78. doi: 10.14670/HH-27.69. [DOI] [PubMed] [Google Scholar]
- Bailey D., Banyard A., Dash P., Ozkul A., Barrett T. Full genome sequence of peste des petits ruminants virus, a member of the Morbillivirus genus. Virus Res. 2005;110:119–124. doi: 10.1016/j.virusres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Bailey D., Chard L.S., Dash P., Barrett T., Banyard A.C. Reverse genetics for peste-des-petits-ruminants virus (PPRV): promoter and protein specificities. Virus Res. 2007;126:250–255. doi: 10.1016/j.virusres.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Balamurugan V., Sen A., Venkatesan G., Yadav V., Bhanuprakash V., Singh R.K. Isolation and identification of virulent peste des petits ruminants viruses from PPR outbreaks in India. Trop. Anim. Health Prod. 2010;42:1043–1046. doi: 10.1007/s11250-010-9527-0. [DOI] [PubMed] [Google Scholar]
- Balamurugan V., Saravanan P., Sen A., Rajak K.K., Venkatesan G., Krishnamoorthy P., Bhanuprakash V., Singh R.K. Prevalence of peste des petits ruminants among sheep and goats in India. J. Vet. Sci. 2012;13:279–285. doi: 10.4142/jvs.2012.13.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan V., Sen A., Venkatesan G., Bhanot V., Yadav V., Bhanuprakash V., Singh R.K. Peste des petits ruminants virus detected in tissues from an Asiatic lion (Panthera leo persica) belongs to Asian lineage IV. J. Vet. Sci. 2012;13:203–206. doi: 10.4142/jvs.2012.13.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan V., Hemadri D., Gajendragad M.R., Singh R.K., Rahman H. Diagnosis and control of peste des petits ruminants: a comprehensive review. Virus Dis. 2014;25:39–56. doi: 10.1007/s13337-013-0188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyard A.C., Baron M.D., Barrett T. A role for virus promoters in determining the pathogenesis of Rinderpest virus in cattle. J. Gen. Virol. 2005;86:1083–1092. doi: 10.1099/vir.0.80752-0. [DOI] [PubMed] [Google Scholar]
- Banyard A.C., Parida S., Batten C., Oura C., Kwiatek O., Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 2010;91:2885–2897. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- Banyard A.C., Wang Z., Parida S. Peste des Petits Ruminants Virus, Eastern Asia. Emerg. Inf. Dis. 2014;20:2176–2177. doi: 10.3201/eid2012.140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J., Li L., Wang Z., Barrett T., Suo L., Zhao W., Liu Y., Liu C., Li J. Development of one-step real-time RT-PCR assay for detection and quantitation of peste des petits ruminants virus. J. Virol. Methods. 2008;148:232–236. doi: 10.1016/j.jviromet.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bao J., Wang Z., Li L., Wu X., Sang P., Wu G., Ding G., Suo L., Liu C., Wang J., Zhao W., Li J., Qi L. Detection and genetic characterization of peste des petits ruminants virus in free-living bharals (Pseuois nayaur) in Tibet, China. Res. Vet. Sci. 2011;90:238–240. doi: 10.1016/j.rvsc.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Bao J., Wang Q., Zhang Y., Liu C., Li L., Wang Z. Complete genome sequence of a novel variant strain of peste des petits ruminants virus, China/XJYL/2013. Genome Announc. 2014;2:00762–814. doi: 10.1128/genomeA.00762-14. e00762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J., Bin-Tarif A., Herbert R., Frost L., Taylor G., Baron M.D. Early changes in cytokine expression in peste des petits ruminants disease. Vet. Res. 2014;45:22. doi: 10.1186/1297-9716-45-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J., Fishbourne E., Couacy-Hyman E., Abubakar M., Jones B.A., Frost L., Herbert R., Chibssa T.R., van’t Klooster G., Afzal M., Ayebazibwe C., Toye P., Bashiruddin J., Baron M.D. Development and testing of a field diagnostic assay for peste des petits ruminants virus. Transbound. Emerg. Dis. 2014;61:390–396. doi: 10.1111/tbed.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., Visser I.K.G., Mamaev L., Goatley L., Vanbressem M.F., Osterhaus A.D.M.E. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology. 1993;193:1010–1012. doi: 10.1006/viro.1993.1217. [DOI] [PubMed] [Google Scholar]
- Batten C.A., Banyard A.C., King D.P., Henstock M.R., Edwards L., Sanders A., Buczkowski H., Oura C.C., Barrett T. A real time RT-PCR assay for the specific detection of Peste des petits ruminants virus. J. Virol. Methods. 2011;171:401–404. doi: 10.1016/j.jviromet.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Bazarghani T.T., Charkhkar S., Doroudi J., Bani Hassan E. A review on peste des petits ruminants (PPR) with special reference to PPR in Iran. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2006;53(Suppl. 1):17–18. [Google Scholar]
- Benazet B.G.H. C.f. Taylor WP (1984); 1973. La p Este Des Petits Ruminants: Etude Experimentale De La Vaccination. [Google Scholar]
- Benazet B.G.H. C.f. Taylor WP; 1984. La Peste Des Petits Ruminants: Etude Experimentale De La Vaccination. [Google Scholar]
- Berhe G., Minet C., Goff l C., Barrett T., Ngangnou A., Grillet C., Libeau G., Fleming M., Black D.N., Diallo A., le Goff C. Development of a dual recombinant vaccine to protect small ruminants against peste-des-petits-ruminants virus and capripoxvirus infections. J. Virol. 2003;77:1571–1577. doi: 10.1128/JVI.77.2.1571-1577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidjeh K., Bornarel P., Imadine M., Lancelot R. First time isolation of the PPR virus in Chad and experimental induction of the disease Premier isolement au Tchad du virus de la PPR et reproduction experimentale de la maladie. Rev. Elev. Med. Vet. Pays Trop. 1995;48:295–300. [PubMed] [Google Scholar]
- Birch J., Juleff N., Heaton M.P., Kalbfleisch T., Kijas J., Bailey D. Characterization of ovine nectin-4, a novel peste des petits ruminants virus receptor. J. Virol. 2013;87:4756–4761. doi: 10.1128/JVI.02792-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodjo S.C., Couacy-Hymann E., Koffi M.Y., Danho T. Assessment of the duration of maternal antibodies specific to the homologous peste des petits ruminant vaccine Nigeria 75/1 in Djallonké lambs. Biokemistri. 2006;18:99–103. [Google Scholar]
- Bourdin P. [Pest of small ruminants and its prophylaxis in Senegal and West Africa] La peste des petits ruminants (PPE) et sa prophylaxie au Senegal et en Afrique de l’ouest. Rev. Elev. Med. Vet. Pays Trop. 1973;26:71a–74a. [PubMed] [Google Scholar]
- Brown C.C., Torres A. Distribution of antigen in cattle infected with rinderpest virus. Vet. Pathol. 1994;31:194–200. doi: 10.1177/030098589403100206. [DOI] [PubMed] [Google Scholar]
- Bruning-Richardson A., Akerblom L., Klingeborn B., Anderson J. Improvement and development of rapid chromatographic strip-tests for the diagnosis of rinderpest and peste des petits ruminants viruses. J. Virol. Methods. 2011;174:42–46. doi: 10.1016/j.jviromet.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Buczkowski H., Muniraju M., Parida S., Banyard A.C. Morbillivirus vaccines: recent successes and future hopes. Vaccine. 2014;32:3155–3161. doi: 10.1016/j.vaccine.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundza A., Afshar A., Dukes T.W., Myers D.J., Dulac G.C., Becker S.A. Experimental peste des petits ruminants (goat plague) in goats and sheep. Can. J Vet. Res. 1988;52:46–52. [PMC free article] [PubMed] [Google Scholar]
- Calain P., Roux L. The rule of 6, a basic feature for efficient replication of sendai virus defective interfering Rna. J. Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufour P., Rufael T., Lamien C.E., Lancelot R., Kidane M., Awel D., Sertse T., Kwiatek O., Libeau G., Sahle M., Diallo A., Albina E. Protective efficacy of a single immunization with capripoxvirus-vectored recombinant peste des petits ruminants vaccines in presence of pre-existing immunity. Vaccine. 2014;32:3772–3779. doi: 10.1016/j.vaccine.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Chandran D., Reddy K.B., Vijayan S.P., Sugumar P., Rani G.S., Kumar P.S., Rajendra L., Srinivasan V.A. MVA recombinants expressing the fusion and hemagglutinin genes of PPRV protects goats against virulent challenge. Indian J. Microbiol. 2010;50:266–274. doi: 10.1007/s12088-010-0026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard L.S., Bailey D.S., Dash P., Banyard A.C., Barrett T. Full genome sequences of two virulent strains of peste-des-petits ruminants virus, the Cote d'Ivoire 1989 and Nigeria 1976 strains. Virus Res. 2008;136:192–197. doi: 10.1016/j.virusres.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Chaudhary S.S., Pandey K.D., Singh R.P., Verma P.C., Gupta P.K. A vero cell derived combined vaccine against sheep pox and Peste des Petits ruminants for sheep. Vaccine. 2009;27:2548–2553. doi: 10.1016/j.vaccine.2009.01.104. [DOI] [PubMed] [Google Scholar]
- Chen W., Hu S., Qu L., Hu Q., Zhang Q., Zhi H., Huang K., Bu Z. A goat poxvirus-vectored peste-des-petits-ruminants vaccine induces long-lasting neutralization antibody to high levels in goats and sheep. Vaccine. 2010;28:4742–4750. doi: 10.1016/j.vaccine.2010.04.102. [DOI] [PubMed] [Google Scholar]
- Chinnakannan S.K., Nanda S.K., Baron M.D. Morbillivirus V proteins exhibit multiple mechanisms to block type 1 and type 2 interferon signalling pathways. PLoS One. 2013:8. doi: 10.1371/journal.pone.0057063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Nah J., Ko Y., Kang S., Yoon K.J., Jo N., Choi K.S., Nah J.J., Ko Y.J., Kang S.Y., Jo N.I. Antigenic and immunogenic investigation of B-cell epitopes in the nucleocapsid protein of peste des petits ruminants virus. Clin. Diagn. Lab. Immunol. 2005;12:114–121. doi: 10.1128/CDLI.12.1.114-121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosseddu G.M., Pinoni C., Polci A., Sebhatu T., Lelli R., Monaco F. Characterization of peste des petits ruminants virus, Eritrea, 2002–2011. Emerg. Infect. Dis. 2013;19:160–161. doi: 10.3201/eid1901.121072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couacy-Hymann E., Roger F., Hurard C., Guillou J.P., Libeau G., Diallo A. Rapid and sensitive detection of peste des petits ruminants virus by a polymerase chain reaction assay. J. Virol. Methods. 2002;100:17–25. doi: 10.1016/s0166-0934(01)00386-x. [DOI] [PubMed] [Google Scholar]
- Couacy-Hymann E., Bodjo C., Danho T., Libeau G., Diallo A. Surveillance of wildlife as a tool for monitoring rinderpest and peste des petits ruminants in West Africa. Rev. Sci. Technol: OIE. 2005;24:869–877. [PubMed] [Google Scholar]
- Couacy-Hymann E., Bodjo S.C., Danho T., Koffi M.Y., Libeau G., Diallo A. Early detection of viral excretion from experimentally infected goats with peste-des-petits ruminants virus. Prev. Vet. Med. 2007;78:85–88. doi: 10.1016/j.prevetmed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Couacy-Hymann E., Bodjo C., Danho T., Libeau G., Diallo A. Evaluation of the virulence of some strains of peste-des-petits-ruminants virus (PPRV) in experimentally infected West African dwarf goats. Vet. J. 2007;173:178–183. doi: 10.1016/j.tvjl.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Das S.C., Baron M.D., Barrett T. Recovery and characterization of a chimeric rinderpest virus with the glycoproteins of peste-des-petits-ruminants virus: homologous F and H proteins are required for virus viability. J. Virol. 2000;74:9039–9047. doi: 10.1128/jvi.74.19.9039-9047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardi M., Lamin Saleh S.M., Batten C., Oura C., Di Nardo A., Rossi D. First evidence of peste des petits ruminants (PPR) virus circulation in Algeria (Sahrawi territories): outbreak investigation and virus lineage identification. Transbound. Emerg. Dis. 2012;59:214–222. doi: 10.1111/j.1865-1682.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- Dechamma H.J., Dighe V., Kumar C.A., Singh R.P., Jagadish M., Kumar S. Identification of T-helper and linear B epitope in the hypervariable region of nucleocapsid protein of PPRV and its use in the development of specific antibodies to detect viral antigen. Vet. Microbiol. 2006;118:201–211. doi: 10.1016/j.vetmic.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Dhar P., Sreenivasa B.P., Barrett T., Corteyn M., Singh R.P., Bandyopadhyay S.K. Recent epidemiology of peste des petits ruminants virus (PPRV) Vet. Microbiol. 2002;88:153–159. doi: 10.1016/s0378-1135(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Diallo A. Peste bovine et peste des petits ruminants. Des menaces constantes contre l’élevage dans beaucoup de pays en développement. Impact Sci. Soc. 1988;150:191–204. [Google Scholar]
- Diallo A., Taylor W.P., Lefevre P.C., Provost A. Attenuation of a strain of pest of small ruminants virus: candidate for a live homologous vaccine Attenuation d'une souche de virus de la peste des petits ruminants: candidat pour un vaccin homologue vivant. Rev. Elev. Med. Vet. Pays Trop. 1989;42:311–319. [PubMed] [Google Scholar]
- Diallo A., Barrett T., Barbron M., Meyer G., Lefevre P.C. Cloning of the nucleocapsid protein gene of peste-des-petits-ruminants virus: relationship to other morbilliviruses. J. Gen. Virol. 1994;75:233–237. doi: 10.1099/0022-1317-75-1-233. (Part 1) [DOI] [PubMed] [Google Scholar]
- Diallo A., Minet C., Berhe G., Goff l C., Black D.N., Fleming M., Barrett T., Grillet C., Libeau G., le Goff C. Goat immune response to capripox vaccine expressing the hemagglutinin protein of peste des petits ruminants. In: Gibbs E.P.J., Bokma B.H., editors. The Domestic Animal/wildlife Interface: Issues for Disease Control, Conservation, Sustainable Food Production, and Emerging Diseases. Conference and Workshop Organised by the Society for Tropical Veterinary Medicine and the Wildlife Diseases Association. Wildlife and Livestock, Disease and Sustainability: What Makes Sense? Pilanesberg National Park, South Africa, 22–27 July, 2001. <PL>New York</PL> Academy of Sciences; New York: 2002. pp. 88–91. [DOI] [PubMed] [Google Scholar]
- Diallo A., Minet C., Goff C.l., Berhe G., Albina E., Libeau G., Barrett T., le Goff C. The threat of peste des petits ruminants: progress in vaccine development for disease control. Vaccine. 2007;25:5591–5597. doi: 10.1016/j.vaccine.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Muller M.A., Maganga G.D., Vallo P., Binger T., Gloza-Rausch F., Rasche A., Yordanov S., Seebens A., Oppong S., Sarkodie Y.A., Pongombo C., Lukashev A.N., Schmidt-Chanasit J., Stocker A., Carneiro A.J.B., Erbar S., Maisner A., Fronhoffs F., Buettner R., Kalko E.K.V., Kruppa T., Franke C.R., Kallies R., Yandoko E.R.N., Herrler G., Reusken C., Hassanin A., Kruger D.H., Matthee S., Ulrich R.G., Leroy E.M., Drosten C. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012;3 doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundon W.G., Adombi C., Waqas A., Otsyina H.R., Arthur C.T., Silber R., Loitsch A., Diallo A. Full genome sequence of a peste des petits ruminants virus (PPRV) from Ghana. Virus Genes. 2014;49:497–501. doi: 10.1007/s11262-014-1109-1. [DOI] [PubMed] [Google Scholar]
- Durojaiye O.A. Precipitating antibody in sera of goats naturally affected with peste des petits ruminants. Trop. Anim. Health Prod. 1982;14:98–100. doi: 10.1007/BF02282591. [DOI] [PubMed] [Google Scholar]
- El Arbi A.S., El Mamy A.B., Salami H., Isselmou E., Kwiatek O., Libeau G., Kane Y., Lancelot R. Peste des petits ruminants virus, Mauritania. Emerg. Infect. Dis. 2014;20:334–336. doi: 10.3201/eid2002.131345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Harrak M., Touil N., Loutfi C., Hammouchi M., Parida S., Sebbar G., Chaffai N., Harif B., Messoudi N., Batten C., Oura C.A. A reliable and reproducible experimental challenge model for peste des petits ruminants virus. J. Clin. Microbiol. 2012;50:3738–3740. doi: 10.1128/JCM.01785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hakim U.A. An outbreak of peste des petits ruminants (PPR) at Aswan Province, Egypt evaluation of some novel tools for diagnosis of PPR. Assiut Vet. Med. J. 2006;52:146–157. [Google Scholar]
- FAO . FAO; 2009. Peste des Petits Ruminants (PPR): A Challenge for Small Ruminant Production. http://www.fao.org/ag/againfo/resources/documents/AH/PPR_flyer.pdf (accessed on October 2014). [Google Scholar]
- FAO . FAO; 2013. Supporting Livelihoods and Supporting Livelihoods and Peste Des Petits Ruminants (ppr) and Small Ruminant Diseases Control.http://www.fao.org/docrep/017/aq236e/aq236e.pdf [Google Scholar]
- FAO, 2014. New programme to eradicate deadly livestock disease by 2030. EMPRES, 27 October 2014.
- Farougou S., Gagara M., Mensah G.A. Prevalence of peste des petits ruminants in the arid zone in the Republic of Niger. Onderstepoort J. Vet. 2013;80 doi: 10.4102/ojvr.v80i1.544. [DOI] [PubMed] [Google Scholar]
- Fontana J.M., Bankamp B., Bellini W.J., Rota P.A. Regulation of interferon signaling by the C and V proteins from attenuated and wild-type strains of measles virus. Virology. 2008;374:71–81. doi: 10.1016/j.virol.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Forsyth M.A., Barrett T. Evaluation of polymerase chain reaction for the detection and characterisation of rinderpest and peste des petits ruminants viruses for epidemiological studies. Virus Res. 1995;39:151–163. doi: 10.1016/0168-1702(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Frolich K., Hamblin C., Jung S., Ostrowski S., Mwanzia J., Streich W.J., Anderson J., Armstrong R.M., Anajariyah S. Serologic surveillance for selected viral agents in captive and free-ranging populations of Arabian oryx (Oryx leucoryx) from Saudi Arabia and the United Arab Emirates. J. Wildl. Dis. 2005;41:67–79. doi: 10.7589/0090-3558-41.1.67. [DOI] [PubMed] [Google Scholar]
- Furley C.W., Taylor W.P., Obi T.U. An outbreak of peste des petits ruminants in a zoological collection. Vet. Rec. 1987;121:443–447. doi: 10.1136/vr.121.19.443. [DOI] [PubMed] [Google Scholar]
- Furuse Y., Suzuki A., Oshitani H. Origin of measles virus: divergence from rinderpest virus between the 11th and 12th centuries. Virol. J. 2010;7:52. doi: 10.1186/1743-422X-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T., Sassa Y., Omatsu T., Nagai M., Fukushima R., Shibutani M., Yamaguchi T., Uematsu Y., Shirota K., Mizutani T. Existence of feline morbillivirus infection in Japanese cat populations. Arch. Virol. 2014;159:371–373. doi: 10.1007/s00705-013-1813-5. [DOI] [PubMed] [Google Scholar]
- Gao Q., Park M.S., Palese P. Expression of transgenes from newcastle disease virus with a segmented genome. J. Virol. 2008;82:2692–2698. doi: 10.1128/JVI.02341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargadennec L., Lalanne A. La peste des petits ruminants Bulletin des Services Zoo Techniques et des. Epizzoties de l’Afrique Occidentale Francaise. 1942;5:16–21. [Google Scholar]
- George A., Dhar P., Sreenivasa B.P., Singh R.P., Bandyopadhyay S.K. The M and N genes-based simplex and multiplex PCRs are better than the F or H gene-based simplex PCR for Peste-des-petits-ruminants virus. Acta Virol. 2006;50:217–222. [PubMed] [Google Scholar]
- Gibbs E.P.J., Taylor W.P., Lawman M.J.P., Bryant J. Classification of peste des petits ruminants virus as the 4th member of the genus Morbillivirus. Intervirology. 1979;11:268–274. doi: 10.1159/000149044. [DOI] [PubMed] [Google Scholar]
- Gilbert Y., Monnier J. Adaptation of the virus of peste des petits ruminants to tissue cultures. Revue Elev-Med. Vet. Pays Trop. 1962;15(4):321–335. [Google Scholar]
- Govindarajan R., Koteeswaran A., Venugopalan A.T., Shyam G., Shaouna S., Shaila M.S., Ramachandran S. Isolation of pestes des petits ruminants virus from an outbreak in Indian buffalo (Bubalus bubalis) Vet. Rec. 1997;141:573–574. doi: 10.1136/vr.141.22.573. [DOI] [PubMed] [Google Scholar]
- Gur S., Albayrak H. Seroprevalance of peste des petits ruminants (PPR) in goitered gazelle (Gazella subgutturosa subgutturosa) in Turkey. J. Wildl. Dis. 2010;46:673–677. doi: 10.7589/0090-3558-46.2.673. [DOI] [PubMed] [Google Scholar]
- Hamdy F.M., Dardiri A.H. Response of white-tailed deer to infection with peste des petits ruminants virus. J. Wildl. Dis. 1976;12:516–522. doi: 10.7589/0090-3558-12.4.516. [DOI] [PubMed] [Google Scholar]
- Hamdy F.M., Dardiri A.H., Nduaka O., Breese S.S., Jr., Ihemelandu E.C. Etiology of the stomatitis pneumoenteritis complex in Nigerian dwarf goats. Can. J. Comp. Med. 1976;40:276–284. [PMC free article] [PubMed] [Google Scholar]
- Hammouchi M., Loutfi C., Sebbar G., Touil N., Chaffai N., Batten C., Harif B., Oura C., El Harrak M. Experimental infection of alpine goats with a Moroccan strain of peste des petits ruminants virus (PPRV) Vet. Microbiol. 2012;160:240–244. doi: 10.1016/j.vetmic.2012.04.043. [DOI] [PubMed] [Google Scholar]
- Hedger R.S., Barnett I.T., Gray D.F. Some virus diseases of domestic animals in the Sultanate of Oman. Trop. Anim. Health Prod. 1980;12:107–114. doi: 10.1007/BF02242618. [DOI] [PubMed] [Google Scholar]
- Herbert R., Baron J., Batten C., Baron M., Taylor G. Recombinant adenovirus expressing the haemagglutinin of peste des petits ruminants virus (PPRV) protects goats against challenge with pathogenic virus; a DIVA vaccine for PPR. Vet. Res. 2014;45:24. doi: 10.1186/1297-9716-45-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilan C., Daccache L., Khazaal K., Beaino T., Massoud E., Louis F. Sero-surveillance of “peste des petits Ruminants” PPR in Lebanon. Leban. Sci. J. 2006;7(1):9–24. [Google Scholar]
- Hoffmann B., Wiesner H., Maltzan J., Mustefa R., Eschbaumer M., Arif F.A., Beer M. Fatalities in wild goats in Kurdistan associated with peste des petits ruminants virus. Transbound. Emerg. Dis. 2012;59:173–176. doi: 10.1111/j.1865-1682.2011.01270.x. [DOI] [PubMed] [Google Scholar]
- Hosamani M., Singh S.K., Mondal B., Sen A., Bhanuprakash V., Bandyopadhyay S.K., Yadav M.P., Singh R.K. A bivalent vaccine against goat pox and Peste des Petits ruminants induces protective immune response in goats. Vaccine. 2006;24:6058–6064. doi: 10.1016/j.vaccine.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Hu Q., Chen W., Huang K., Baron M.D., Bu Z. Rescue of recombinant peste des petits ruminants virus: creation of a GFP-expressing virus and application in rapid virus neutralization test. Vet. Res. 2012;43:48. doi: 10.1186/1297-9716-43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.R., Shamsuddin M., Rahman M.A., Das P.M., Dewan M.L. An outbreak of peste des petits ruminants in Black Bengal goats in Mymensingh, Bangladesh. Bangladesh Vet. 2001;18:14–19. [Google Scholar]
- Ismail I.M., House J. Evidence of identification of peste des petits ruminants from goats in Egypt. Arch. Exp. Veterinarmed. 1990;44:471–474. [PubMed] [Google Scholar]
- Kerur N., Jhala M.K., Joshi C.G. Genetic characterization of Indian peste des petits ruminants virus (PPRV) by sequencing and phylogenetic analysis of fusion protein and nucleoprotein gene segments. Res. Vet. Sci. 2008;85:176–183. doi: 10.1016/j.rvsc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Kgotlele T., Macha E.S., Kasanga C.J., Kusiluka L.J., Karimuribo E.D., Van Doorsselaere J., Wensman J.J., Munir M., Misinzo G. Partial genetic characterization of peste des petits ruminants virus from goats in northern and eastern Tanzania. Transbound. Emerg. Dis. 2014;61(Suppl. 1):56–62. doi: 10.1111/tbed.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafalla A.I., Saeed I.K., Ali Y.H., Abdurrahman M.B., Kwiatek O., Libeau G., Obeida A.A., Abbas Z. An outbreak of peste des petits ruminants (PPR) in camels in the Sudan. Acta Trop. 2010;116:161–165. doi: 10.1016/j.actatropica.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Khandelwal A., Renukaradhya G.J., Rajasekhar M., Sita G.L., Shaila M.S. Immune responses to hemagglutinin-neuraminidase protein of peste des petits ruminants virus expressed in transgenic peanut plants in sheep. Vet. Immunol. Immunopathol. 2011;140:291–296. doi: 10.1016/j.vetimm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Kinne J., Kreutzer R., Kreutzer M., Wernery U., Wohlsein P. Peste des petits ruminants in Arabian wildlife. Epidemiol. Infect. 2010;138:1211–1214. doi: 10.1017/S0950268809991592. [DOI] [PubMed] [Google Scholar]
- Kock R.A., Orynbayev M.B., Sultankulova K.T., Strochkov V.M., Omarova Z.D., Shalgynbayev E.K., Rametov N.M., Sansyzbay A.R., Parida S. Detection and Genetic Characterization of Lineage IV Peste Des Petits Ruminant Virus in Kazakhstan. Transbound. Emerg. Dis. 2015;62(5):470–479. doi: 10.1111/tbed.12398. [DOI] [PubMed] [Google Scholar]
- Kumar P., Tripathi B.N., Sharma A.K., Kumar R., Sreenivasa B.P., Singh R.P., Dhar P., Bandyopadhyay S.K. Pathological and immunohistochemical study of experimental peste des petits ruminants virus infection in goats. J. Vet. Med. B. 2004;51:153–159. doi: 10.1111/j.1439-0450.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- Kwiatek O., Minet C., Grillet C., Hurard C., Carlsson E., Karimov B., Albina E., Diallo A., Libeau G. Peste des petits ruminants (PPR) outbreak in Tajikistan. J. Comp. Pathol. 2007;136:111–119. doi: 10.1016/j.jcpa.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Kwiatek O., Keita D., Gil P., Fernandez-Pinero J., Jimenez Clavero M.A., Albina E., Libeau G. Quantitative one-step real-time RT-PCR for the fast detection of the four genotypes of PPRV. J. Virol. Methods. 2010;165:168–177. doi: 10.1016/j.jviromet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Kwiatek O., Ali Y.H., Saeed I.K., Khalafalla A.I., Mohamed O.I., Abu Obeida A., Abdelrahman M.B., Osman H.M., Taha K.M., Abbas Z., El Harrak M., Lhor Y., Diallo A., Lancelot R., Albina E., Libeau G. Asian lineage of peste des petits ruminants virus, Africa. Emerg. Infect. Dis. 2011;17:1223–1231. doi: 10.3201/eid1707.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatek O., Ali Y.H., Saeed I.K., Khalafalla A.I., Mohamed O.I., Obeida A.A., Abdelrahman M.B., Osman H.M., Taha K.M., Abbas Z., El Harrak M., Lhor Y., Diallo A., Lancelot R., Albina E., Libeau G. Asian lineage of peste des petits ruminants virus, Africa. Emerg. Infect. Dis. 2011;17:1223–1231. doi: 10.3201/eid1707.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre P.C., Diallo A., Schenkel F., Hussein S., Staak G. Serological evidence of peste des petits ruminants in Jordan. Vet. Rec. 1991;128:110. doi: 10.1136/vr.128.5.110. [DOI] [PubMed] [Google Scholar]
- Lembo T., Oura C., Parida S., Hoare R., Frost L., Fyumagwa R., Kivaria F., Chubwa C., Kock R., Cleaveland S., Batten C. Peste des petits ruminants infection among cattle and wildlife in Northern Tanzania. Emerg. Infect. Dis. 2013;19:2037–2040. doi: 10.3201/eid1912.130973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Bao J., Wu X., Wang Z., Wang J., Gong M., Liu C., Li J. Rapid detection of peste des petits ruminants virus by a reverse transcription loop-mediated isothermal amplification assay. J. Virol. Methods. 2010;170:37–41. doi: 10.1016/j.jviromet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Libeau G., Diallo A., Calvez D., Lefevre P.C. A competitive ELISA using anti-N monoclonal antibodies for specific detection of rinderpest antibodies in cattle and small ruminants. Vet. Microbiol. 1992;31:147–160. doi: 10.1016/0378-1135(92)90073-3. [DOI] [PubMed] [Google Scholar]
- Libeau G., Diallo A., Colas F., Guerre L. Rapid differential diagnosis of rinderpest and peste des petits ruminants using an immunocapture ELISA. Vet. Rec. 1994;134:300–304. doi: 10.1136/vr.134.12.300. [DOI] [PubMed] [Google Scholar]
- Libeau G., Prehaud C., Lancelot R., Colas F., Guerre L., Bishop D.H., Diallo A. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Res. Vet. Sci. 1995;58:50–55. doi: 10.1016/0034-5288(95)90088-8. [DOI] [PubMed] [Google Scholar]
- Libeau G., Diallo A., Parida S. Evolutionary genetics underlying the spread of peste des petits ruminants virus. Anim. Front. 2014;4:14–20. [Google Scholar]
- Liu F., Wu X., Li L., Liu Z., Wang Z. Formation of peste des petits ruminants spikeless virus-like particles by co-expression of M and N proteins in insect cells. Res. Vet. Sci. 2014;96:213–216. doi: 10.1016/j.rvsc.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Ludlow M., Nguyen D.T., Silin D., Lyubomska O., de Vries R.D., von Messling V., McQuaid S., De Swart R.L., Duprex W.P. Recombinant canine distemper virus strain snyder hill expressing green or red fluorescent proteins causes meningoencephalitis in the Ferret. J. Virol. 2012;86:7508–7519. doi: 10.1128/JVI.06725-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka P.D., Erume J., Mwiine F.N., Ayebazibwe C., Shamaki D. Molecular characterization and phylogenetic study of peste des petits ruminants viruses from North central States of Nigeria. BMC Vet. Res. 2011;4:7. doi: 10.1186/1746-6148-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka P.D., Erume J., Mwiine F.N., Ayebazibwe C. Molecular characterization of peste des petits ruminants virus from the Karamoja region of Uganda (2007–2008) Arch. Virol. 2012;157:29–35. doi: 10.1007/s00705-011-1135-4. [DOI] [PubMed] [Google Scholar]
- Lundervold M., Milner-Gulland E.J., O'Callaghan C.J., Hamblin C., Corteyn A., Macmillan A. A serological survey of ruminant livestock in Kazakhstan during post-soviet transitions in farming and disease control. Acta Vet. Scand. 2004;45:211–224. doi: 10.1186/1751-0147-45-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganga G.D., Verrier D., Zerbinati R.M., Drosten C., Drexler J.F., Leroy E.M. Molecular typing of PPRV strains detected during an outbreak in sheep and goats in south-eastern Gabon in 2011. Virol. J. 2013;10 doi: 10.1186/1743-422X-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra M., Parida S., Egziabher B.G., Diallo A., Barrett T. Sequence analysis of the phosphoprotein gene of peste des petits ruminants (PPR) virus: editing of the gene transcript. Virus Res. 2003;96:85–98. doi: 10.1016/s0168-1702(03)00176-x. [DOI] [PubMed] [Google Scholar]
- Mahapatra M., Parida S., Baron M.D., Barrett T. Matrix protein and glycoproteins F and H of Peste-des-petits-ruminants virus function better as a homologous complex. J. Gen. Virol. 2006;87:2021–2029. doi: 10.1099/vir.0.81721-0. [DOI] [PubMed] [Google Scholar]
- Maillard J.C., Kiem Phan V., Tung N., Thu Nhu V., Berthouly C., Libeau G., Kwiatek O. Examples of probable host-pathogen co-adaptation/co-evolution in isolated farmed animal populations in the mountainous regions of North Vietnam. Ann. N. Y. Acad. Sci. 2008;1149:259–262. doi: 10.1196/annals.1428.086. [DOI] [PubMed] [Google Scholar]
- Majiyagbe K.A., Nawathe D.R., Abegunde A. Rapid diagnosis of peste-des-petits-ruminants (Ppr) infection, application of immunoelectroosmophoresis (Ieop) technique. Rev. Elev. Med. Vet. Pays. 1984;37:11–15. [PubMed] [Google Scholar]
- Malik Y.S., Singh D., Chandrashekar K.M., Shukla S., Sharma K., Vaid N., Chakravarti S. Occurrence of dual infection of peste-des-petits-ruminants and goatpox in Indigenous goats of Central India. Transbound. Emerg. Dis. 2011;58:268–273. doi: 10.1111/j.1865-1682.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- Mariner J.C., House J.A., Mebus C.A., Ende M.C.v.d., Van den Ende M.C. The use of thermostable Vero cell-adapted rinderpest vaccine as a heterologous vaccine against peste des petits ruminants. Res. Vet. Sci. 1993;54:212–216. doi: 10.1016/0034-5288(93)90059-o. [DOI] [PubMed] [Google Scholar]
- Martin, V., Larfaoui, F., 2003. Suspicion of foot-and-mouth disease (FMD)/peste des petits ruminants (PPR) in Afghanistan (5/05/2003) http://www.fao.org/eims/secretariat/empres/eims_search/1_dett.asp?calling=simple_s_result&publication=&webpage=&photo=&press=&lang=en&pub_id=145377 (accessed 30.08.14.).
- Mitra-Kaushik S., Nayak R., Shaila M.S. Identification of a cytotoxic T-cell epitope on the recombinant nucleocapsid proteins of rinderpest and Peste des petits ruminants viruses presented as assembled nucleocapsids. Virology. 2001;279:210–220. doi: 10.1006/viro.2000.0698. [DOI] [PubMed] [Google Scholar]
- Mondal B., Sen A., Chand K., Biswas S.K., De A., Rajak K.K., Chakravarti S. Evidence of mixed infection of peste des petits ruminants virus and bluetongue virus in a flock of goats as confirmed by detection of antigen, antibody and nucleic acid of both the viruses. Trop. Anim. Health Prod. 2009;41:1661–1667. doi: 10.1007/s11250-009-9362-3. [DOI] [PubMed] [Google Scholar]
- Mornet P., Orue J., Gillbert Y., Thiery G.S.M. La peste des petits Ruminants en Afrique occidentale française ses rapports avec la Peste Bovine. Rev. Elev. Med. Vet. Pays Trop. 1956;9:313–342. [Google Scholar]
- Moss W.J., Griffin D.E. Global measles elimination. Nat. Rev. Microbiol. 2006;4:900–908. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir M., Zohari S., Suluku R., Leblanc N., Kanu S., Sankoh F.A., Berg M., Barrie M.L., Stahl K. Genetic characterization of peste des petits ruminants virus, Sierra Leone. Emerg. Infect. Dis. 2012;18:193–195. doi: 10.3201/eid1801.111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir M., Zohari S., Berg M. Springer book; 2013. Molecular Biology and Pathogenesis of Peste Des Petits Ruminants Virus. [Google Scholar]
- Muniraju M., Buczkowski H., Banyard A.C., Parida S. Development of a reverse genetics system for Peste des petits ruminants virus: the first successful rescue of a recombinant PPRV. 1st Meeting of the Global PPRV Eradication Alliance. 2012 http://www.globalpprresearchalliance.org/downloads/gpra1.pdf [Google Scholar]
- Muniraju M., El Harrak M., Bao J., Ramasamy Parthiban A.B., Banyard A.C., Batten C., Parida S. Complete genome sequence of a peste des petits ruminants virus recovered from an Alpine goat during an outbreak in Morocco in 2008. Genome Announc. 2013;1 doi: 10.1128/genomeA.00096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniraju M., Mahapatra M., Ayelet G., Babu A., Munir M., Batten C., Banyard A.C., Parida S. Emergence of lineage IV peste des petits ruminants virus in Ethiopia: complete genome sequence of an Ethiopian isolate 2010. Trans. Emerg. Dis. 2014 doi: 10.1111/tbed.12287. [DOI] [PubMed] [Google Scholar]
- Muniraju M., Mahapatra M., Buczkowski H., Batten C., Banyard A.C., Parida S. Rescue of a vaccine strain of peste des petits ruminants virus: In vivo evaluation and comparison with standard vaccine. Vaccine. 2014;33:465–471. doi: 10.1016/j.vaccine.2014.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniraju M., Munir M., Banyard A.C., Ayebazibwe C., Wensman J., Zohari S., Berg M., Parthiban A.R., Mahapatra M., Libeau G., Batten C., Parida S. Complete genome sequences of lineage III peste des petits ruminants viruses from the Middle East and East Africa. Genome Announc. 2014;2:01023–1114. doi: 10.1128/genomeA.01023-14. e01023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniraju M., Munir M., Parthiban A.R., Banyard A.C., Bao J., Wang Z., Ayebazibwe C., Ayelet G., El-Harrak M., Mahapatra M., Libeau G., Batten C., Parida S. Molecular evolution and emergence of peste des petits ruminants virus. Emerg. Inf. Dis. 2014;20:2023–2033. doi: 10.3201/eid2012.140684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda Y.P., Chatterjee A., Purohit A.K., Diallo A., Innui K., Sharma R.N., Libeau G., Thevasagayam J.A., Bruning A., Kitching R.P., Anderson J., Barrett T., Taylor W.P. The isolation of peste des petits ruminants virus from northern India. Vet. Microbiol. 1996;51:207–216. doi: 10.1016/0378-1135(96)00025-9. [DOI] [PubMed] [Google Scholar]
- Nawathe D.R., Taylor W.P. Experimental infection of domestic pigs with the virus of peste des petits ruminants. Trop. Anim. Health Prod. 1979;11:120–122. doi: 10.1007/BF02237785. [DOI] [PubMed] [Google Scholar]
- Nyamweya M., Otunga T., Regassa G., Maloo S. ELMT Livestock Services Technical Working Group; 2009. Technical Brief of Peste Des Petits Ruminants Virus. [Google Scholar]
- OIE, 2012. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th Edition, Vol 1 and 2 (Version adopted in May 2013).
- Ogunsanmi A.O., Awe E.O., Obi T.U., Taiwo V.O. Peste des petits ruminants (PPR) virus antibodies in african grey duiker (sylvicapra grimmia) Afr. J. Biomed. Res. 2003;6:59–61. [Google Scholar]
- Ohno S., Ono N., Seki F., Takeda M., Kura S., Tsuzuki T., Yanagi Y. Measles virus infection of SLAM (CD150) knockin mice reproduces tropism and immunosuppression in human infection. J. Virol. 2007;81:1650–1659. doi: 10.1128/JVI.02134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmen O., Kale M., Haligur M., Yavru S. Pathological, serological, and virological findings in sheep infected simultaneously with Bluetongue, Peste-des-petits-ruminants, and Sheeppox viruses. Trop. Anim. Health Prod. 2009;41:951–958. doi: 10.1007/s11250-008-9284-5. [DOI] [PubMed] [Google Scholar]
- Parida S., Madhuchhanda M., Hawes P., Baron M.D., Monaghan P., Barrett T. Importance of the extracellular and cytoplasmic/transmembrane domains of the haemagglutinin protein of rinderpest virus for recovery of viable virus from cDNA copies. Virus Res. 2006;117(2):273–282. doi: 10.1016/j.virusres.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Parida S., Mahapatra M., Kumar S., Das S.C., Baron M.D., Anderson J. Rescue of a chimeric rinderpest virus with the nucleocapsid protein derived from peste-des-petits-ruminants virus: use as a marker vaccine. J. Gen. Virol. 2007;88:2019–2027. doi: 10.1099/vir.0.82913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.S., Suzuki M., Kimura M., Maruyama K., Mizutani H., Saito R., Kubota N., Furuya T., Mizutani T., Imaoka K., Morikawa S. Identification of a natural recombination in the F and H genes of feline morbillivirus. Virology. 2014;468–470C:524–531. doi: 10.1016/j.virol.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Perl S., Alexander A., Yakobson B., Nyska A., Harmelin A., Sheikhat N., Shimshony A., Davidson M., Abramson M., Rapaport E. Peste des petits ruminants (PPR) of sheep in Israel: case report. Isr. J. Vet. Med. 1994;49:59–62. [Google Scholar]
- Pomeroy L.W., Bjornstad O.N., Holmes E.C. The evolutionary and epidemiological dynamics of the paramyxoviridae. J. Mol. Evol. 2008;66(2):98–106. doi: 10.1007/s00239-007-9040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope R.A., Parida S., Bailey D., Brownlie J., Barrett T., Banyard A.C. Early events following experimental infection with peste-des-petits ruminants virus suggest immune cell targeting. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost, A., Maurice, Y., Bourdin, C., 1972. La peste des petits ruminants: existe-t-elle en Africque centrale? Paper presented at the 40th general conference of the committee of the O.I.E. Radostits OM, Gay CC, Blood DC, inchcliff KW(2000) Veterinary medicine, 9th edn. Elsevier, W. B. Saunders Co., London.
- Qin J., Huang H., Ruan Y., Hou X., Yang S., Wang C., Huang G., Wang T., Feng N., Gao Y., Xia X. A novel recombinant Peste des petits ruminants-canine adenovirus vaccine elicits long-lasting neutralizing antibody response against PPR in goats. PLoS One. 2012;7:e37170. doi: 10.1371/journal.pone.0037170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager M., Vongpunsawad S., Duprex W.P., Cattaneo R. Polyploid measles virus with hexameric genome length. EMBO J. 2002;21:2364–2372. doi: 10.1093/emboj/21.10.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.M., Shaila M.S., Gopinathan K.P. Baculovirus display of fusion protein of Peste des petits ruminants virus and hemagglutination protein of Rinderpest virus and immunogenicity of the displayed proteins in mouse model. Virology. 2003;317:36–49. doi: 10.1016/j.virol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Shadmin I., Noor M., Parvin R., Chowdhury E.H., Islam M.R. Peste des petits ruminants virus infection of goats in Bangladesh: pathological investigation, molecular detection and isolation of the virus. Bangladesh Vet. 2011;28:1–7. [Google Scholar]
- Rajak K.K., Sreenivasa B.P., Hosamani M., Singh R.P., Singh S.K., Singh R.K., Bandyopadhyay S.K. Experimental studies on immunosuppressive effects of peste des petits ruminants (PPR) virus in goats. Comp. Immunol. Microbiol. Infect. Dis. 2005;28:287–296. doi: 10.1016/j.cimid.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Renukaradhya G.J., Sinnathamby G., Seth S., Rajasekhar M., Shaila M.S. Mapping of B-cell epitopic sites and delineation of functional domains on the hemagglutinin-neuraminidase protein of peste des petits ruminants virus. Virus Res. 2002;90:171–185. doi: 10.1016/s0168-1702(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Roeder P.L., Abraham G., Kenfe G., Barrett T. Peste des petits ruminants in Ethiopian goats. Trop. Anim. Health Prod. 1994;26:69–73. doi: 10.1007/BF02239901. [DOI] [PubMed] [Google Scholar]
- Roger F., Yesus M.G., Libeau G., Diallo A., Yigezu L.M., Yilma T. Detection of antibodies of rinderpest and peste des petits ruminants viruses (Paramyxoviridae, Morbillivirus) during a new epizootic disease in Ethiopian camels (Camelus dromedarius) Rev. Med. Vet.-Toulouse. 2001;152:265–268. [Google Scholar]
- Rojas J.M., Moreno H., Valcarcel F., Pena L., Sevilla N., Martin V. Vaccination with recombinant adenoviruses expressing the peste des petits ruminants virus F or H proteins overcomes viral immunosuppression and induces protective immunity against PPRV challenge in sheep. PLoS One. 2014;9:e101226. doi: 10.1371/journal.pone.0101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C.H., Barrett T., Kitching R.P., Bostock C., Black D.N. Protection of goats against peste des petits ruminants with recombinant capripoxviruses expressing the fusion and haemagglutinin protein genes of rinderpest virus. Vaccine. 1995;13:36–40. doi: 10.1016/0264-410x(95)80008-2. [DOI] [PubMed] [Google Scholar]
- Rossiter P.B., Jessett D.M., Taylor W.P. Neutralizing antibodies to rinderpest virus in sheep and goats in Western Kenya. Vet. Rec. 1982;111(22):504–505. doi: 10.1136/vr.111.22.504. [DOI] [PubMed] [Google Scholar]
- Rossiter P.B., Taylor W.P. vol. II. Oxford University Press; Cape Town: 1994. (Peste Des Petits Ruminants. In Infectious Diseases of Livestock). [Google Scholar]
- Saeed I.K., Khalafalla A.I., El-Hassan S.M., El-Amin M.A. Peste des petits ruminants (PPR) in the Sudan: investigation of recent outbreaks, virus isolation and cell culture spectrum. J. Anim. Vet. Adv. 2004;3:361–365. [Google Scholar]
- Sakaguchi S., Nakagawa S., Yoshikawa R., Kuwahara C., Hagiwara H., Asai K., Kawakami K., Yamamoto Y., Ogawa M., Miyazawa T. Genetic diversity of feline morbilliviruses isolated in Japan. J. Gen. Virol. 2014;95:1464–1468. doi: 10.1099/vir.0.065029-0. [DOI] [PubMed] [Google Scholar]
- Salami H., Croville G., Kwiatek O., Mariette J., Klopp C., Valiere S., Guerin J.L., Lo M., Thiongane Y., Albina E., Libeau G. Complete genome sequence of a field strain of peste des petits ruminants virus isolated during 2010–2014 epidemics in Senegal. Genome Announc. 2014:00772–814. doi: 10.1128/genomeA.00772-14. e00772-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliki J.T., Libeau G., House J.A., Mebus C.A., Dubovi E.J. Monoclonal antibody-based blocking enzyme-linked immunosorbent assay for specific detection and titration of peste-des-petits-ruminants virus antibody in caprine and ovine sera. J. Clin. Microbiol. 1993;31:1075–1082. doi: 10.1128/jcm.31.5.1075-1082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan P., Singh R.P., Balamurugan V., Dhar P., Sreenivasa B.P., Muthuchelvan D. Development of a N gene-based PCR-ELISA for detection of Peste-des-petits-ruminants virus in clinical samples. Acta Virol. 2004;48(4):249–255. [PubMed] [Google Scholar]
- Saravanan P., Balamurugan V., Sen A., Sarkar J., Sahay B., Rajak K.K., Hosamani M., Yadav M.P., Singh R.K. Mixed infection of peste des petits ruminants and orf on a goat farm in Shahjahanpur, India. Vet. Rec. 2007;160:410–412. doi: 10.1136/vr.160.12.410. [DOI] [PubMed] [Google Scholar]
- Saravanan P., Balamurugan V., Sen A., Sreenivasa B.P., Singh R.P., Bandyopadhyay S.K., Singh R.K. Immune response of goats to a vero cell adapted live attenuated homologous PPR vaccine. Indian Vet. J. 2010;87:1–3. [Google Scholar]
- Seki F., Ono N., Yamaguchi R., Yanagi Y. Efficient isolation of wild strains of canine distemper virus in Vero cells expressing canine SLAM (CD150) and their adaptability to marmoset B95a cells. J. Virol. 2003;77:9943–9950. doi: 10.1128/JVI.77.18.9943-9950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Saravanan P., Balamurugan V., Rajak K.K., Sudhakar S.B., Bhanuprakash V., Parida S., Singh R.K. Vaccines against peste des petits ruminants virus. Expert Rev. Vaccines. 2010;9:785–796. doi: 10.1586/erv.10.74. [DOI] [PubMed] [Google Scholar]
- Sen A., Saravanan P., Balamurugan V., Bhanuprakash V., Venkatesan G., Sarkar J., Rajak K.K., Ahuja A., Yadav V., Sudhakar S.B., Parida S., Singh R.K. Detection of subclinical peste des petits ruminants virus infection in experimental cattle. Virus Dis. 2014;25:408–411. doi: 10.1007/s13337-014-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil Kumar K., Babu A., Sundarapandian G., Roy P., Thangavelu A., Siva Kumar K., Arumugam R., Chandran N.D., Muniraju M., Mahapatra M., Banyard A.C., Manohar B.M., Parida S. Molecular characterisation of lineage IV peste des petits ruminants virus using multi gene sequence data. Vet. Microbiol. 2014;174:39–49. doi: 10.1016/j.vetmic.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Sghaie S., Cossedu G.M., Ben Hassen S., Hammami S., Ammar H.H., Petrini A., Monaco Peste des petits ruminants virus, Tunisia, 2012–2013. Emerg. Infect. Dis. 2014;20:2184–2185. doi: 10.3201/eid2012.141116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaila M.S., Purushothaman V., Bhavasar D., Venugopal K., Venkatesan R.A. Peste des petits ruminants of sheep in India. Vet. Rec. 1989;125:602. [PubMed] [Google Scholar]
- Shaila M.S., Shamaki D., Forsyth M.A., Diallo A., Goatley L., Kitching R.P., Barrett T. Geographic distribution and epidemiology of peste des petits ruminants virus. Virus Res. 1996;43:149–153. doi: 10.1016/0168-1702(96)01312-3. [DOI] [PubMed] [Google Scholar]
- Singh R.P., De U.K., Pandey K.D. Virological and antigenic characterization of two Peste des Petits Ruminants (PPR) vaccine viruses of Indian origin. Comp. Immunol. Microb. 2010;33:343–353. doi: 10.1016/j.cimid.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Sinnathamby G., Renukaradhya G.J., Rajasekhar M., Nayak R., Shaila M.S. Immune responses in goats to recombinant hemagglutinin-neuraminidase glycoprotein of Peste des petits ruminants virus: identification of a T cell determinant. Vaccine. 2001;19:4816–4823. doi: 10.1016/s0264-410x(01)00210-9. [DOI] [PubMed] [Google Scholar]
- Soltan M.A., Abd-Eldaim M.M. Emergence of peste des petits ruminants virus lineage IV in Ismailia Province, Egypt. Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014;28C:44–47. doi: 10.1016/j.meegid.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Stephens N., Duignan P.J., Wang J.N., Bingham J., Finn H., Bejder L., Patterson I.A.P., Holyoake C. Cetacean morbillivirus in Coastal Indo-Pacific bottlenose dolphins, Western Australia. Emerg. Infect. Dis. 2014;20:666–670. doi: 10.3201/eid2004.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai E.S., Kapaga A., Kivaria F., Tinuga D., Joshua G., Sanka P. Prevalence and distribution of Peste des petits ruminants virus antibodies in various districts of Tanzania. Vet. Res. Commun. 2009;33:927–936. doi: 10.1007/s11259-009-9311-7. [DOI] [PubMed] [Google Scholar]
- Takeda M., Nakatsu Y., Ohno S., Seki F., Tahara M., Hashiguchi T., Yanagi Y. Generation of measles virus with a segmented RNA genome. J. Virol. 2006;80:4242–4248. doi: 10.1128/JVI.80.9.4242-4248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger J.K., Tsai M.M., Atkin T.J., Fanning T.G., Krafft A.E., Moeller R.B., Kodsi S.E., Mense M.G., Lipscomb T.P. Molecular genetic evidence of a novel morbillivirus in a long-finned pilot whale (Globicephalus melas) Emerg. Infect. Dis. 2000;6:42–45. doi: 10.3201/eid0601.000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplu N., Oguzoglu T.C., Albayrak H. Dual Infection of fetal and neonatal small ruminants with border disease virus and peste des petits ruminants virus (PPRV): neuronal tropism of PPRV as a novel finding. J. Comp. Pathol. 2012;146:289–297. doi: 10.1016/j.jcpa.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Truong T., Boshra H., Embury-Hyatt C., Nfon C., Gerdts V., Tikoo S., Babiuk L.A., Kara P., Chetty T., Mather A., Wallace D.B., Babiuk S. Peste des petits ruminants virus tissue tropism and pathogenesis in sheep and goats following experimental infection. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.P., Baron M.D., Anderson J., Barrett T. Development of a genetically marked recombinant rinderpest vaccine expressing green fluorescent protein. J. Gen. Virol. 2000;81:709–718. doi: 10.1099/0022-1317-81-3-709. [DOI] [PubMed] [Google Scholar]
- Walsh E.P., Baron M.D., Rennie L.F., Monaghan P., Anderson J., Barrett T. Recombinant rinderpest vaccines expressing membrane-anchored proteins as genetic markers: Evidence of exclusion of marker protein from the virus envelope. J. Virol. 2000;74:10165–10175. doi: 10.1128/jvi.74.21.10165-10175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamwayi H.M., Rossiter P.B., Kariuki D.P., Wafula J.S., Barrett T., Anderson J. Peste des petits ruminants antibodies in east Africa. Vet. Rec. 1995;136:199–200. doi: 10.1136/vr.136.8.199. [DOI] [PubMed] [Google Scholar]
- Wang Z., Bao J., Wu X., Liu Y., Li L., Liu C., Suo L., Xie Z., Zhao W., Zhang W., Yang N., Li J., Wang S., Wang J. Peste des petits ruminants virus in Tibet, China. Emerg. Infect. Dis. 2009;15:299–301. doi: 10.3201/eid1502.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu G., Chen Z., Li C., Shi L., Li W., Huang H., Tao C., Cheng C., Xu B., Li G. Recombinant adenovirus expressing F and H fusion proteins of peste des petits ruminants virus induces both humoral and cell-mediated immune responses in goats. Vet. Immunol. Immunopathol. 2013;154:1–7. doi: 10.1016/j.vetimm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Wohlsein P., Trautwein G., Harder T.C., Liess B., Barrett T. Viral-antigen distribution in organs of cattle experimentally infected with rinderpest virus. Vet. Pathol. 1993;30:544–554. doi: 10.1177/030098589303000608. [DOI] [PubMed] [Google Scholar]
- Woma T.Y., Dundon W.G., Yu D., Adombi C.M., Qasim A.M., Sabi A.A., Abraham M.N., Olaiya O.D., Bailey D., Shamaki D., Loitsch A., Quan M., Diallo A. Identification of peste-des-petits ruminants virus (PPRV) lineage IV, the Asian lineage, in Nigeria and co-circulation with PPRV lineage II. Vet. Microbiol. 2014 doi: 10.1016/j.vetmic.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Wong B.H.L., Fan R.Y.Y., Wong A.Y.P., Zhang A.J.X., Wu Y., Choi G.K.Y., Li K.S.M., Hui J., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5435–5440. doi: 10.1073/pnas.1119972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall E.E., Litamoi J.K., Seck B.M., Ayelet G. Xerovac: an ultra rapid method for the dehydration and preservation of live attenuated Rinderpest and Peste des Petits ruminants vaccines. Vaccine. 2000;19:834–839. doi: 10.1016/s0264-410x(00)00229-2. [DOI] [PubMed] [Google Scholar]
- Yamaji Y., Nakayama T. Recombinant measles viruses expressing respiratory syncytial virus proteins induced virus-specific CTL responses in cotton rats. Vaccine. 2014;32:4529–4536. doi: 10.1016/j.vaccine.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Yang X., Dou Y., Wang Q., Luo X., Zeng Q., Zhu Q., Cai X., Yang X.F., Dou Y.X., Wang Q.X., Luo X.N., Zeng Q.Y., Zhu Q.Y., Cai X.P. Construction and immunogenicity of DNA vaccine encoding F gene of peste des petits ruminants virus. Chin. Vet. Sci./Zhongguo Shouyi Kexue. 2013;43:500–504. [Google Scholar]