Graphical abstract
Keywords: Taenia solium, Cysticercosis, Pig, Cyst distribution, Diagnosis, Control
Highlights
-
•
Taenia solium cyst distribution was determined in the carcass musculature of 209 naturally exposed pigs.
-
•
81% of the infected animals had cysts in the tongue, masticatory muscles and/or heart muscles.
-
•
Dissection of a limited proportion of the muscles of pigs could be used to assess the outcomes of cysticercosis control.
Abstract
Many interventions against Taenia solium are evaluated by assessing changes in the prevalence of porcine cysticercosis ascertained by carcass dissection. Financial and logistical difficulties often prohibit dissection of entire pig carcasses. We assessed 209 pigs from rural areas of Cameroon and Peru for the presence of T. solium cysticerci and determined the distribution of parasites within the musculature of infected animals. Considering the presence of cysts in the tongue, masticatory muscles and heart, 31 of the 38 (81%) naturally infected animals were identified as having cysts. Dissection of only the tongue, masticatory muscles and heart provides a relatively sensitive and highly specific method for diagnosis of porcine cysticercosis.
Taenia solium is the etiological agent of neurocysticercosis in humans. In countries where T. solium is endemic, it is considered to be the most frequent preventable cause of seizure disorders, being associated with 29% of people with epilepsy (Ndimubanzi et al., 2010). The Food and Agriculture Organization ranks T. solium as the most important foodborne parasitic infection from a global perspective (Robertson et al., 2013) and infection with T. solium is recognised by the World Health Organization as a Neglected Tropical Disease (World Health Organization, 2015).
Humans act as the obligate definitive host for T. solium and harbour the adult tapeworm stage in the small intestine. Pigs become infected with the larval (cysticercus) stage which encysts in the striated muscles and brain after eating faeces of a person harbouring the adult tapeworm, or items contaminated with eggs from the faeces of a tapeworm carrier. The life cycle is completed when poorly cooked pork meat containing cysticerci is eaten by a person, leading to the development of a tapeworm. The medical significance of T. solium arises because if a person accidentally ingests items contaminated with tapeworm eggs, the parasite is able to establish infection with the larval stage in humans, often encysting in the brain and other neural tissue, causing neurocysticercosis.
The life cycle of T. solium provides several opportunities for interventions to be attempted to prevent the parasite’s transmission. Public education, sanitation, chemotherapy of taeniasis, chemotherapy of porcine cysticercosis and vaccination against porcine cysticercosis, among other measures, have been assessed in various combinations in attempts to effect control of disease transmission (Murrell, 2005, Garcia et al., 2007, Lightowlers, 2013). An important issue for T. solium control initiatives is the choice of which parameters are measured in order to evaluate the outcomes (Lightowlers et al., 2015). The prevalence of clinically proven cysticercosis, or taeniasis, in humans is relatively low in many regions where the disease is endemic (Mahanty et al., 2010). The sample sizes that would be required to be evaluated in order to achieve statistically significant impacts on human cysticercosis or taeniasis may be prohibitive. Porcine cysticercosis has been the measure that has been most commonly evaluated as part of T. solium control trials. A decrease in porcine cysticercosis, caused by either reducing human taeniasis or due to direct interventions in pigs, could be expected to affect the subsequent incidence of human T. solium taeniasis and, indirectly, the incidence of neurocysticercosis.
There are two general methodologies available for assessment of porcine cysticercosis – direct assessment of infection in pigs or serological methods. Serology has been used frequently to assess the prevalence of porcine cysticercosis. However, it has become increasingly clear that most serologically positive pigs are not infected with any cysticerci and hence serology is not a specific method that can be used to assess the prevalence of porcine cysticercosis (Devleesschauwer et al., 2013, Gavidia et al., 2013, Jayashi et al., 2014). Tongue inspection for the detection of cysts in live animals provides a valuable but insensitive direct measure of T. solium infection in pigs (Phiri et al., 2006). Estimates of the sensitivity of tongue palpation vary widely, from as low as 16% in some instances (Phiri et al., 2006) to 70% (Gonzalez et al., 1990). Slicing of all carcass meat provides the most definitive measure of porcine cysticercosis prevalence and intensity, however the cost of purchasing animals, the time required for slicing muscle tissue to identify and count cysts, and the requirement of trained staff to conduct the procedure, are major disincentives to its use. It is possible that the slicing of a proportion of the meat, rather than all the striated muscles of an entire carcass, may provide an adequately sensitive measure of infection. Some studies of porcine cysticercosis have sliced selected sites (heart, tongue, diaphragm) as well as half the remaining carcass musculature (Huerta et al., 2001, Boa et al., 2002, Dorny et al., 2004, Assana et al., 2010), however slicing even half the carcass constitutes an onerous task.
The distribution of cysticercosis in naturally or experimentally infected pigs has been described (Vargas Mendez et al., 1986, Sciutto et al., 1998, Boa et al., 2002). In the studies that have assessed naturally infected animals, all have used pigs that were pre-selected as likely to be infected (e.g. by tongue palpation), hence the infection intensities were generally very high. Here we present data concerning the distribution of T. solium cysticerci among certain muscle groups in unselected, naturally infected pigs from Cameroon and Peru, and determine the sensitivity of T. solium diagnosis through slicing of only the heart, tongue and masticatory muscles.
The location and number of T. solium cysticerci were determined in 102 pigs approximately 12–13 months of age from the Mayo–Danay administrative department of the Far North region of Cameroon (Assana et al., 2010) and in 107, 9–11 month-old pigs from the Morropon district in the province of Morropon, Piura, Peru (Jayashi et al., 2012). The pigs were non-vaccinated control animals involved in field trials of vaccines against T. solium (Assana et al., 2010, Jayashi et al., 2012). In Cameroon, T. solium infection was assessed at necropsy by slicing the brain, tongue, heart, head muscles and musculature from half of the carcass split longitudinally, while in Peru the striated musculature from the entire carcass and brain were dissected. The number of cysticerci was assessed by slicing the tissues using scalpels at a thickness of approximately 3 mm. An estimate of cyst numbers for the whole carcass of the Cameroonian pigs was made by doubling the number initially identified by slicing half the carcass. In cases of heavy infection (many hundreds) among the pigs in Cameroon, the number of cysts in 1 kg of carcass musculature was counted and the number in the remaining carcass meat estimated on a weight basis. Parts of several skeletal muscle groups were chosen to comprise the 1 kg sample, representing a representative sample rather than muscle from any particular muscle group. In Peru, the striated musculature from the entire carcass was sliced and cyst numbers were assessed. In both Cameroon and Peru the muscles of the head were assessed together and included the masseter, pterygoid and temporal muscles. In Cameroon the triceps were assessed specifically, while in Peru the muscles of the foreleg were assessed together.
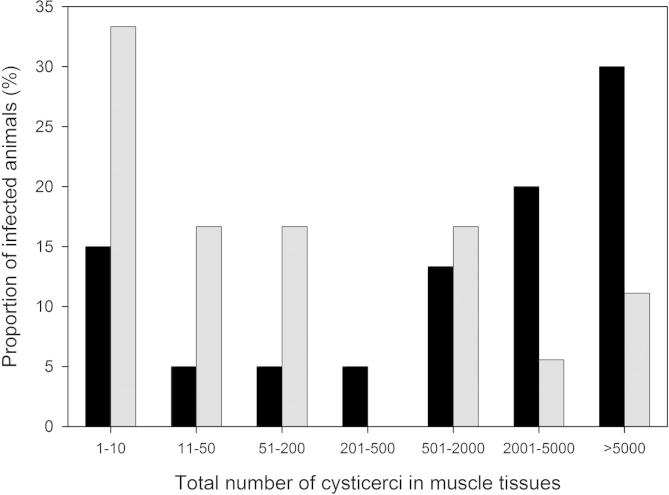
In Cameroon, 20 of the 102 animals examined were detected as having T. solium cysticerci. In Peru, there were 18 infected pigs among the 107 animals assessed. The distribution of cysticerci in the various carcass sites of the infected animals is shown in Table 1 and the intensities of infection summarised in Fig. 1. The intensity of infections in Cameroon was greater than in Peru. In Cameroon, four of the 20 (20%) infected pigs had relatively light infections (<50 cysts throughout the carcass) whereas in Peru nine of the 18 (50%) infected animals had <50 cysts. Exceptionally heavy infections (>10,000 cysts) amounted to 30% (six out of 20) in Cameroon and 5% (one of 18) in Peru. Considering only the heart, tongue and masticatory muscles, 18 of the 20 infected pigs (90%) in Cameroon had cysts and in the animals from Peru 13 of 18 animals (72%) were found to have cysts. In total, 31 of the 38 pigs (81%) that had been proved to be infected through extensive carcass dissection were detected as being infected by dissection of the heart, tongue and masticatory muscles alone. All the infected animals not found to have any cysts in the heart, tongue or masticatory muscles had relatively light infections (<50 cysts, mean = 15, median = 7). Among the pigs from Cameroon, inclusion of the triceps muscles from one foreleg (Table 1), together with the heart, tongue and masticatory muscles would have identified all infected pigs, achieving a diagnostic sensitivity of 100%. Among the pigs from Peru, inclusion of the muscles from the right or left forelegs (data not shown), together with the heart, tongue and masticatory muscles, would have identified 15 or 16 of the 18 infected animals, respectively, raising the diagnostic sensitivity to 83–88% among that group of animals.
Table 1.
Numbers of muscle cysticerci in unselected pigs naturally infected with Taenia solium.
| Cameroon |
Peru |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal number | Total muscle cysts | Tongue |
Masticatory muscles |
Heart |
TMH total |
Tricepsc |
Animal number | Total muscle cysts | Tongue |
Masticatory muscles |
Heart |
TMH total |
|||||||||
| No.a | %b | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| 1 | 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 66.7 | 1 | 1 | 0 | 0.0 | 0 | 0.0 | 1 | 100 | 1 | 100 |
| 2 | 6 | 0 | 0.0 | 0 | 0.0 | 2 | 33.3 | 2 | 33.3 | 1 | 16.7 | 2 | 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 3 | 10 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 40.0 | 3 | 1 | 0 | 0.0 | 1 | 100 | 0 | 0.0 | 1 | 100 |
| 4 | 14 | 0 | 0.0 | 1 | 7.1 | 0 | 0.0 | 1 | 7.1 | 3 | 21.4 | 4 | 4 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 1 | 25.0 |
| 5 | 100 | 6 | 6.0 | 17 | 17.0 | 0 | 0.0 | 23 | 23.0 | 23 | 23.0 | 5 | 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 6 | 479 | 11 | 2.3 | 27 | 5.6 | 11 | 2.3 | 49 | 10.2 | 96 | 20.0 | 6 | 7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 7 | 781 | 8 | 1.0 | 18 | 2.3 | 8 | 1.0 | 34 | 4.3 | 77 | 9.9 | 7 | 27 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 8 | 987 | 17 | 1.7 | 48 | 4.9 | 23 | 2.3 | 88 | 8.9 | 106 | 10.7 | 8 | 36 | 1 | 2.8 | 0 | 0.0 | 0 | 0.0 | 1 | 2.8 |
| 9 | 1491 | 11 | 0.7 | 25 | 1.7 | 4 | 0.3 | 40 | 2.7 | 107 | 7.2 | 9 | 49 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 10 | 1527 | 14 | 0.9 | 32 | 2.1 | 31 | 2.0 | 77 | 5.0 | 146 | 9.6 | 10 | 75 | 2 | 2.7 | 2 | 2.7 | 0 | 0.0 | 4 | 5.3 |
| 11 | 2041 | 23 | 1.1 | 132 | 6.5 | 7 | 0.3 | 162 | 7.9 | 166 | 8.1 | 11 | 167 | 6 | 3.6 | 5 | 3.0 | 3 | 1.8 | 14 | 8.4 |
| 12 | 2116 | 63 | 3.0 | 40 | 1.9 | 1 | 0.0 | 104 | 4.9 | 116 | 5.5 | 12 | 200 | 5 | 2.5 | 5 | 2.5 | 1 | 0.5 | 11 | 5.5 |
| 13 | 3812 | 43 | 1.1 | 286 | 7.5 | 62 | 1.6 | 391 | 10.3 | 312 | 8.2 | 13 | 1012 | 10 | 1.0 | 26 | 2.6 | 18 | 1.8 | 54 | 5.3 |
| 14 | 3985 | 49 | 1.2 | 321 | 8.1 | 33 | 0.8 | 403 | 3.8 | 403 | 10.1 | 14 | 1084 | 14 | 1.3 | 19 | 1.8 | 0 | 0.0 | 33 | 3.0 |
| 15 | 10,543 | 166 | 1.6 | 803 | 7.6 | 143 | 1.4 | 1112 | 7.4 | 1007 | 9.6 | 15 | 1084 | 20 | 1.8 | 68 | 6.3 | 6 | 0.6 | 94 | 8.7 |
| 16 | 14,955 | 321 | 2.1 | 926 | 6.2 | 11 | 0.1 | 1258 | 8.4 | 1125 | 7.5 | 16 | 3436 | 139 | 4.0 | 57 | 1.7 | 29 | 0.8 | 225 | 6.5 |
| 17 | 14,981 | 206 | 1.4 | 1023 | 6.8 | 92 | 0.6 | 1321 | 7.2 | 1207 | 8.1 | 17 | 8234 | 128 | 1.6 | 188 | 2.3 | 115 | 1.4 | 431 | 5.2 |
| 18 | 18,343 | 207 | 1.1 | 1183 | 6.4 | 122 | 0.7 | 1512 | 4.7 | 891 | 4.9 | 18 | 18,566 | 317 | 1.7 | 523 | 2.8 | 328 | 1.8 | 1168 | 6.3 |
| 19 | 31,978 | 432 | 1.4 | 1644 | 5.1 | 151 | 0.5 | 2227 | 6.0 | 1829 | 5.7 | ||||||||||
| 20 | 37,080 | 743 | 2.0 | 2403 | 6.5 | 193 | 0.5 | 3339 | 9.0 | 3708 | 10.0 | ||||||||||
TMH, tongue, masticatory muscles and heart.
Number of cysticerci.
Percentage of total muscle cysts.
Triceps muscles from one foreleg.
Fig. 1.
Intensity of infection with Taenia solium cysticerci in naturally infected pigs from Cameroon (black bars) and Peru (grey bars). Cyst numbers per carcass musculature were grouped by range for 20 infected pigs from Cameroon and 18 infected animals from Peru.
Dissection of the entire carcass musculature and brain would provide the most sensitive method for diagnosis of porcine cysticercosis, although even this may fail to detect infections involving only a very small number of cysts. A limitation of the study undertaken in Cameroon was that musculature from only half the carcass was dissected, potentially missing some cases of light infections should all cysts have been localised to one side of the carcass. It is unclear whether the intensity of infections in pigs would be affected by the introduction of T. solium control measures. Many cases of porcine cysticercosis cluster around the households of tapeworm carriers (Sarti-Gutierrez et al., 1988, Lescano et al., 2007, O’Neal et al., 2012), and the frequent occurrence of very heavy infections in pigs suggests that transmission occurs relatively often by the animals’ direct consumption of human faeces. This transmission pattern would not be affected by implementation of several of the available intervention measures for T. solium.
In previous studies involving pre-selected or experimentally infected animals, Vargas Mendez et al. (1986) identified the predilection sites for T. solium infection in pigs to be masseter, pterygoid and the triceps whereas Sciutto et al. (1998) found the higher density of cysts in the intercostal and shoulder muscles and tongue. Boa et al. (2002) found the predilection sites for T. solium to be the psoas, masseters and triceps. The anatomical sites examined by these authors, and in the unselected, naturally infected pigs described here, differed and do not allow a direct comparison of cyst intensities for many areas of the carcass. However, our findings of infections commonly occurring in the masticatory muscles and tongue, even in many lightly infected animals, tend to confirm others’ observations of these as predilection sites. These are convenient sites to assess, along with the heart, because they are relatively easily obtained from a carcass and their removal does not greatly affect carcass value.
We conclude that porcine cysticercosis can be diagnosed in naturally infected pigs with a sensitivity of approximately 80%, including correct diagnosis in many lightly infected animals, by dissecting only the heart, tongue and masticatory muscles. This is suggested as a feasible, highly specific and relatively low cost method for diagnosis of porcine cysticercosis that can be used for assessment of T. solium control initiatives. Where feasible, sensitivity for diagnosis would be improved by slicing a larger proportion of the carcass musculature, particularly the muscles of one or both forelimbs.
Acknowledgements
Funding is acknowledged from The Wellcome Trust, Animal Health in the Developing World Grant 075818, UK and the Australian National Health and Medical Research Council Grants 1003546 and 1043327. The authors thank the Cysticercosis Working Group in Tumbes, Peru for their support during animal necropsies.
References
- Assana E., Kyngdon C.T., Gauci C.G., Geerts S., Dorny P., De Deken R., Anderson G.A., Zoli A.P., Lightowlers M.W. Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int. J. Parasitol. 2010;40:515–519. doi: 10.1016/j.ijpara.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boa M.E., Kassuku A.A., Willingham A.L., 3rd, Keyyu J.D., Phiri I.K., Nansen P. Distribution and density of cysticerci of Taenia solium by muscle groups and organs in naturally infected local finished pigs in Tanzania. Vet. Parasitol. 2002;106:155–164. doi: 10.1016/s0304-4017(02)00037-7. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B., Aryal A., Tharmalingam J., Joshi D.D., Rijal S., Speybroeck N., Gabriel S., Victor B., Dorny P. Complexities in using sentinel pigs to study Taenia solium transmission dynamics under field conditions. Vet. Parasitol. 2013;193:172–178. doi: 10.1016/j.vetpar.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Dorny P., Phiri I.K., Vercruysse J., Gabriel S., Willingham A.L., III, Brandt J., Victor B., Speybroeck N., Berkvens D. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int. J. Parasitol. 2004;34:569–576. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Garcia H.H., Gonzalez A.E., Del Brutto O.H., Tsang V.C., Llanos-Zavalaga F., Gonzalvez G., Romero J., Gilman R.H. Strategies for the elimination of taeniasis/cysticercosis. J. Neurol. Sci. 2007;262:153–157. doi: 10.1016/j.jns.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Gavidia C.M., Verastegui M.R., Garcia H.H., Lopez-Urbina T., Tsang V.C., Pan W., Gilman R.H., Gonzalez A.E., Cysticercosis Working Group in Peru Relationship between serum antibodies and Taenia solium larvae burden in pigs raised in field conditions. PLoS Negl. Trop. Dis. 2013;7:e2192. doi: 10.1371/journal.pntd.0002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A.E., Cama V., Gilman R.H., Tsang V.C., Pilcher J.B., Chavera A., Castro M., Montenegro T., Verastegui M., Miranda E., Balazar H. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am. J. Trop. Med. Hyg. 1990;43:194–199. doi: 10.4269/ajtmh.1990.43.194. [DOI] [PubMed] [Google Scholar]
- Huerta M., de Aluja A.S., Fragoso G., Toledo A., Villalobos N., Hernandez M., Gevorkian G., Acero G., Diaz A., Alvarez I., Avila R., Beltran C., Garcia G., Martinez J.J., Larralde C., Sciutto E. Synthetic peptide vaccine against Taenia solium pig cysticercosis: successful vaccination in a controlled field trial in rural Mexico. Vaccine. 2001;20:262–266. doi: 10.1016/s0264-410x(01)00249-3. [DOI] [PubMed] [Google Scholar]
- Jayashi C.M., Kyngdon C.T., Gauci C.G., Gonzalez A.E., Lightowlers M.W. Successful immunization of naturally reared pigs against porcine cysticercosis with a recombinant oncosphere antigen vaccine. Vet. Parasitol. 2012;188:261–267. doi: 10.1016/j.vetpar.2012.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayashi C.M., Gonzalez A.E., Neyra R.C., Rodriguez S., Garcia H.H., Lightowlers M.W. Validity of the Enzyme-linked Immunoelectrotransfer Blot (EITB) for naturally acquired porcine cysticercosis. Vet. Parasitol. 2014;199:42–49. doi: 10.1016/j.vetpar.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescano A.G., Garcia H.H., Gilman R.H., Guezala M.C., Tsang V.C., Gavidia C.M., Rodriguez S., Moulton L.H., Green J.A., Gonzalez A.E., Cysticercosis Working Group in Peru Swine cysticercosis hotspots surrounding Taenia solium tapeworm carriers. Am. J. Trop. Med. Hyg. 2007;76:376–383. [PubMed] [Google Scholar]
- Lightowlers M.W. Control of Taenia solium taeniasis/cysticercosis: past practices and new possibilities. Parasitology. 2013;140:1566–1577. doi: 10.1017/S0031182013001005. [DOI] [PubMed] [Google Scholar]
- Lightowlers M.W., Garcia H.H., Gauci C.G., Donadeu M., Abela-Ridder B. Monitoring the outcomes of interventions against Taenia solium: options and suggestions. Parasite Immunol. 2015 doi: 10.1111/pim.12291. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S., Garcia H.H., Cysticercosis Working Group in Peru Cysticercosis and neurocysticercosis as pathogens affecting the nervous system. Prog. Neurobiol. 2010;91:172–184. doi: 10.1016/j.pneurobio.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Murrell K.D.E. OIE/WHO/FAO; Paris, France: 2005. WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniasis/cysticercosis. [Google Scholar]
- Ndimubanzi P.C., Carabin H., Budke C.M., Nguyen H., Qian Y.J., Rainwater E., Dickey M., Reynolds S., Stoner J.A. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl. Trop. Dis. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal S.E., Moyano L.M., Ayvar V., Gonzalvez G., Diaz A., Rodriguez S., Wilkins P.P., Tsang V.C., Gilman R.H., Garcia H.H., Gonzalez A.E., Cysticercosis Working Group in Peru Geographic correlation between tapeworm carriers and heavily infected cysticercotic pigs. PLoS Negl. Trop. Dis. 2012;6:e1953. doi: 10.1371/journal.pntd.0001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiri I.K., Dorny P., Gabriel S., Willingham A.L., 3rd, Sikasunge C., Siziya S., Vercruysse J. Assessment of routine inspection methods for porcine cysticercosis in Zambian village pigs. J. Helminthol. 2006;80:69–72. doi: 10.1079/joh2005314. [DOI] [PubMed] [Google Scholar]
- Robertson L.J., van der Giessen J.W., Batz M.B., Kojima M., Cahill S. Have foodborne parasites finally become a global concern? Trends Parasitol. 2013;29:101–103. doi: 10.1016/j.pt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Sarti-Gutierrez E.J., Schantz P.M., Lara-Aguilera R., Gomez Dandoy H., Flisser A. Taenia solium taeniasis and cysticercosis in a Mexican village. Trop. Med. Parasitol. 1988;39:194–198. [PubMed] [Google Scholar]
- Sciutto E., Martinez J.J., Villalobos N.M., Hernandez M., Jose M.V., Beltran C., Rodarte F., Flores I., Bobadilla J.R., Fragoso G., Parkhouse M.E., Harrison L.J., de Aluja A.S. Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs. Vet. Parasitol. 1998;79:299–313. doi: 10.1016/s0304-4017(98)00180-0. [DOI] [PubMed] [Google Scholar]
- Vargas Mendez G.D., Saldierna U., Navarro Fierro R., Acevedo Hernandez A., Flisser de M.A., Aluja A.S.D. Localizacion del cisticerco de la Taenia solium en diferentes regiones musculares del cerdo y su importancia para la inspeccion sanitaria. Localization of Taenia solium cysticerci in different muscular regions of swine and its significance in meat inspectionVet. Mexico. 1986;17:275–279. [Google Scholar]
- World Health Organization, 2015. Investing to overcome the global impact of Neglected Tropical Diseases. Third WHO Report on Neglected Tropical Diseases. WHO/HTM/NTD/2015.1, Geneva, Switzerland.