Abstract
De Quervain’s stenosing tenosynovitis (DQST) treatments include corticosteroid injection around the tendon sheath; however there is some ambiguity concerning the efficacy of this treatment. The aim of this systematic review and meta-analysis is to examine the totality of evidence relating to the use of corticosteroid injection in DQST when compared to placebo or other active treatments. A systematic literature search was conducted in July 2014. Only randomized control trials (RCTs) were included. Outcome measures included impairment, activity limitation and participation restriction. Five RCTs were identified with 165 patients, 88 in the treatment group and 77 in the control group.
Patients who received corticosteroid injection (n=142) had a higher rate of resolution of symptoms [RR 2.59, 95% CI: 1.25 to 5.37, p=0.05, I2=62%]. This group reported greater pain relief as assessed by Visual Analogue Scale (VAS) at first assessment [mean difference -2.51, 95% CI: -3.11 to -1.90, p=0.0003, I2=65%] and demonstrated a statistically significant improvement in function (n=78) as measured by the DASH score and Dutch AIMS-HFF score [SMD -0.83, 95% CI: -1.54 to -0.12, p=0.02, I2=48]. This review confirms that corticosteroid injection results in a statistically significant increase in resolution of symptoms, pain relief and increased function in the treatment of DQST.
Keywords: De Quervain, corticosteroid injection, meta-analysis, stenosing tenosynovitis, review, systematic
1. BACKGROUND
De Quervains Stenosing Tenosynovitis (DQST) is a condition characterised by thickening of and by the accumulation of mucopolysaccharide in the sheath of the abductor pollicis longus and extensor pollicis brevis tendons, which cross under the extensor retinaculum in the first dorsal compartment of the wrist [1]. The extensor retinaculum is a fibrous band attached to the underlying radius which prevents bowstringing of the extensor tendons off the dorsum of the wrist. The condition takes its name from the Swiss physician de Quervain who first described a case series of five patients in 1895. Prevalence is estimated at 0.5% among men and 1.3% among women [2].
Risk factors include repetitive or forceful manual work and pregnancy [3]. DQST is commonly considered as a work-related musculoskeletal disorder of the upper limb [4], which in total costs the US economy an estimated $13 to $20 billion annually [5]. The differential diagnosis includes osteoarthritis of the first carpometacarpal (CMC) joint, ganglia, infectious tenosynovitis, Wartenberg’s syndrome and intersection syndrome [6]. The patient typically complains of pain over the radial styloid process which is likely caused by mechanical friction as the tendon passes through its narrowed compartment. On clinical examination, there is tenderness over the radial side of the wrist and symptoms can be elicited clinically by means of Finklestein’s test.
Nonsurgical modalities are the first line of treatment and include rest, ice, non-steroidal anti-inflammatory drugs, therapeutic exercise and splinting [7]. Corticosteroid injection is then the mainstay of treatment for those patients who do not respond to the above. Other described treatments include: acupuncture [8], ozone oxygen and hyaluronic acid injections [9], ultrasound-guided percutaneous needle tenotomy and platelet-rich plasma (PRP) injection [10], and prolotherapy [11]. Surgery is reserved for failure of conservative modalities and involves release of the first dorsal compartment.
However there exists controversy as to the efficacy of steroid injection as a treatment for DQST. Two previous systematic reviews support the use of corticosteroid injection however the quality of studies included in the reviews was low. A pooled quantitative analysis by Richie et al. [12] in 2003 did not include any RCTs. A Cochrane systematic review in 2009 [13] identified only one RCT—consisting of 18 pregnant or lactating women randomized to either steroid injection or thumb splinting—which found steroid injection to be the most effective form of conservative treatment. However the trial was of poor methodological quality with evidence of a spectrum of bias and low patient numbers.
A number of RCTs have been published in recent years that explore the effectiveness of steroid injection as a treatment for DQST. Therefore the aim of this systematic review and meta-analysis is to examine the totality of evidence relating to the use of corticosteroid injection in DQST when compared to placebo or other active treatments.
2. METHODS
2.1. Definitions and Study Identification
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct this review [14]. A number of operational definitions were defined using the methods recommended by the Cochrane Handbook for Systematic Reviews of Interventions [15]. The ‘population of interest’ is adults with a clinical diagnosis of DQST, the ‘intervention’ is corticosteroid injection and the ‘comparisons’ are other conservative treatments or placebo injection. Outcomes of interest are those of impairment, activity limitation or participation restriction and include measures of self reported pain, functional status and global measures of improvement. Only randomised controlled trials (RCTs) are included in this review.
2.2. Literature Search
We performed a systematic search in the following databases: PubMed, EMBASE, AMED, CINAHL Science Direct, CENTRAL, and Cochrane Library. Keywords and MeSH terms included (abductor pollicis longus OR extensor pollicis brevis OR de Quervain’s disease OR de Quervain tenosynovitis OR repetitive strain injury) AND (Corticosteroid OR steroid OR Betamethasone OR Hydrocortisone OR Methylprednisolone OR Triamcinolone OR injection). This search was supplemented by hand searching references of retrieved articles and searching Google Scholar. All searches were completed in July 2014 with no language or date restrictions placed on the search strings.
2.3. Study Selection
One review author (PR) identified and screened the titles and abstracts of articles retrieved through electronic searches. Two reviewers (PR and RG) independently assessed the full-text articles to identify the eligible studies for inclusion. Any disagreements were resolved through discussion between the two reviewers.
2.4. Data Extraction
Information including the authors, study setting, study population, treatment type, mode of delivery, frequency and duration of the intervention, outcome measures and follow-up periods were all extracted and documented for each included study. For the purposes of the meta-analysis, outcomes at baseline, post-intervention and follow-up time points were recorded. In studies with multiple comparison groups, the most relevant comparison group was chosen for analysis. The authors were contacted if further information was needed.
2.5. Methodological Quality Assessment
The methodological quality of the included studies were evaluated using the Cochrane risk of bias tool under the headings of random sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessors), incomplete outcome data, selective reporting and other potential threats of validity. Two review authors (PR and NP) independently assessed the methodological quality for each included study. A study was considered to have a low risk of bias if all the criteria were met and if one or more of the criteria were not met or partially met, then the study was considered to have an unclear risk of bias. A study was considered to have a high risk of bias if one or more of the criteria were not met.
2.6. Data Synthesis and Statistical Analysis
The statistical analysis was conducted using Review Manager 5 (RevMan) [Version 5.1.7 Cochrane Collaboration 2012]. Outcomes of interest for the meta-analysis were impairment (Visual Analogue Scale [VAS]), activity limitation (i.e. Disabilities of Arm, Shoulder and Hand questionnaire [DASH] or DUTCH Arthritis Impact Measurement Scale). The mean difference (MD) in outcomes between the control and the steroid group post-intervention and at follow-up time points was used as the mode of analysis. We addressed the impact of sample size by estimating a weighting factor for each study and assigning larger effect-weights in studies with larger samples.
In cases where the studies used different scales or instruments to assess the same outcome (i.e. activity limitation or participation restriction), the standardised mean difference (SMD) with 95% confidence interval (CI) was used as the mode of analysis. Statistical heterogeneity was measured using the I2 statistic. We used an I2 statistic of ≤50% as the cut-off point for acceptable heterogeneity and applied the fixed-effects model below this point. In cases where the I2 statistic >50%, we reported the more conservative random effects model.
3. RESULTS
3.1. Study Identification and Selection
The process of identifying, screening and selecting relevant RCTs is displayed in the PRISMA diagram in Fig. (1). Based on the titles and abstracts screening process, six studies were selected for full-text review by the author (PR). Upon review of the full-text articles, one study was excluded as it was not a RCT and the remaining five studies were included in the review. In total, all five studies were included in the meta-analysis.
Fig. (1).
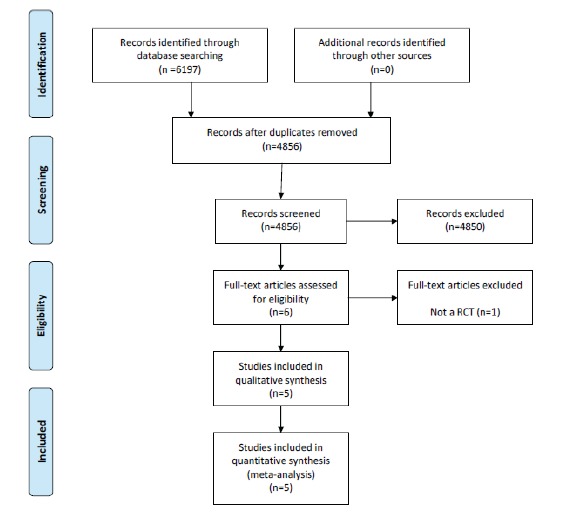
PRISMA flow diagram of studies in the review.
3.2. Study characteristics
Table 1 provides a summary of all the studies included in the systematic review. The included studies were published between 2002 and 2013. Studies included patients that ranged in age from 20 to 76 years. Three studies took place in orthopaedic outpatient clinic, one in a physical medicine and rehabilitation clinic and one in a general practice setting.
Table 1.
Descriptive characteristics of studies included in the review.
| Author & Country | Study Setting | Treatment Numbers | Control Numbers | Sex (Female/Male) | Mean Age Treatment/ Control | Intervention Details | Comparison | Outcome Measures |
|---|---|---|---|---|---|---|---|---|
| Avci et al., 2002 Turkey | Orthopaedic clinic | 10(9) | 9 | All female | 28 | Injection 26-gauge tuberculin needle 0.5 mL of 0.5% bupivacaine & Methylprednisolone (10 mg, 0.25 mL) | Thumb spica splint | ‘total pain relief and a negative Finkelstein’s test result’ |
| Peters-Veluthamaningal et al., 2009 The Netherlands | General practice | 9 | 12 | 10/2 placebo 3/6 control | 51.2/52.3 | One or two local injections of 1 ml of triamcino-lonacetonide 10 mg/ml | 1 ml of NaCl 0.9% injection (placebo) | Main outcomes were immediate treatment response, severity of pain, improvement as perceived by participant and functional disability using sub items hand and finger function of the Dutch Arthritis Impact Measurement Scale (Dutch AIMS-2-HFF) |
| Mehdinasab et al., 2010 Iran | Orthopaedic clinic | 37 | 36 | 32/5 Injection and cast 32/4 Cast alone | 32.83/29.61 | Methylprednisolone 1 mL (40 mg) acetate injection plus thumb spica cast | Wrist thumb spica cast alone | Treatment was considered successful if wrist pain, tenderness and Finkelstein test resolved and patient had a 90% reduction in pain score |
| Hadianfard et al., 2013 Iran | Physical medicine and rehabilitation clinic | 15 | 15 | 11/4 Injection 13/2 Acupuncture | 39.47/41.93 | One injection of 1 mL of (40 mg) methylprednisolone acetate and 1 mL of 2% lidocaine | The acupuncture group received five acupuncture sessions of 30 minutes duration | The degree of disability and pain was evaluated by using the Quick Disabilities of the Arm, Shoulder, and Hand (Q-DASH) scale and the Visual Analogue Scale (VAS) |
| Makarawung et al., 2013 USA | Outpatient clinic of a tertiary care hospital | 17 | 5 | Single injection with 1 mL dexamethasone (4 mg dexamethasone sodium phosphate per milliliter saline; American Region Laboratories Inc., Shirley, NY) mixed with 1 mL 1% lidocaine (10 mg lidocaine hydrochloric acid per milliliter saline; Abbott Labs, N. Chicago, IL) | Single placebo injection of 2 mL 1% lidocaine | The primary outcome was arm-specific disability as measured with the DASH questionnaire, also recorded were the 10 cm Visual Analogue Scale (VAS) to measure pain intensity, the DASH, the Center for Epidemiologic Studies-Depression Scale (CES-D) to measure symptoms of depression, the Pain Catastrophizing Scale (PCS) to evaluate pain catastrophizing |
There were 165 patients included in the review, 88 in the treatment group and 77 in the control group. Two studies compared corticosteroid injection to a thumb spica splint alone, one study compared corticosteroid injection to placebo injection of lidocaine, one study compared corticosteroid injection to a placebo injection of NaCl and one study compared corticosteroid injection to acupuncture. Follow-up of participants ranged from 1 to 17 months. Outcome measures ranged from pain assessment using the VAS or another categorical scale, clinical examination including Finklestein’s test, assessment of function using the Dutch AIMS-HFF score, DASH or other criterion and assessment of treatment response using a combination of patients percieved improvement in symptoms and clinical examination including Finklestein’s test.
3.3. Methodological Quality
Table 2 provides an overview of the methodological quality assessment of the included studies. Overall the selected papers were of low methodological quality. There were three studies with a high risk of bias and two studies with an unclear risk of bias, no studies had a low risk of bias. Only one study clearly described random sequence generation and allocation concealment. Three of five studies failed to clearly describe blinding of participants and personnel, and blinding of outcome assessment. There was evidence for incomplete outcome reporting in three studies and selective outcome reporting in two studies respectively.
Table 2.
Methodological quality assessment of the included studies
| Authors | Selection bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | Overall Risk of Bias | |
|---|---|---|---|---|---|---|---|---|
| Random Sequence Generation | Allocation Concealment | Blinding of Participants & Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Source of Bias | Overall Risk of Bias | |
| Avci et al., 2002 | No | No | Unclear | Unclear | Yes | Yes | Unclear | High Risk of Bias |
| Peters-Veluthamaningal et al., 2009 | Yes | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear Risk of Bias |
| Mehdinasab et al., 2010 | No | No | No | No | Unclear | Yes | Unclear | High Risk of Bias |
| Hadianfard et al., 2013 | Unclear | Unclear | No | No | No | Yes | Unclear | High Risk of Bias |
| Makarawung et al., 2013 | Unclear | Unclear | Yes | Yes | Yes | Unclear | Unclear | Unclear Risk of Bias |
4. META-ANALYSIS
4.1. Proportion of Participants Who Demonstrated Full Recovery
Four of the five studies recorded the proportion of participants who demonstrated full recovery post treatment. , as shown in Fig. (2). Avci et al. [16] described full recovery as total pain relief and a negative Finklestein’s test. Mehdinasab et al. [17] considered treatment successful if wrist pain, tenderness and Finkelstein’s test resolved and the patient had a 90% reduction in pain score. Peters et al. [18] used a four point scale to describe direct treatment response based on consensus between physician and participant and Hadianfard et al. [19] assessed recovery according to the percentage of improvement in disability and pain status over baseline with nearly complete success of treatment described as 80% or more improvement. Pooled data from these four studies (n=142) using a random effects model indicates a significant increase in the resolution of symptoms in patients treated with corticosteroid injection [RR 2.59, 95% CI: 1.25 to 5.37, p=0.05, I2=62%]. However the variability of the various outcome assessments and control treatments used will contribute to the significant heterogeneity between studies.
Fig. (2).
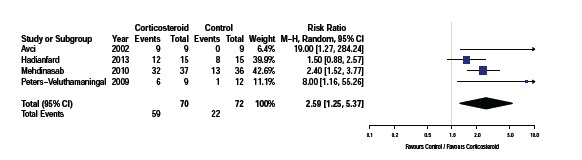
Proportion of participants who demonstrated full recovery following trial.
4.2. Differences in Pain Between the Groups at First Assessment Post-Injection
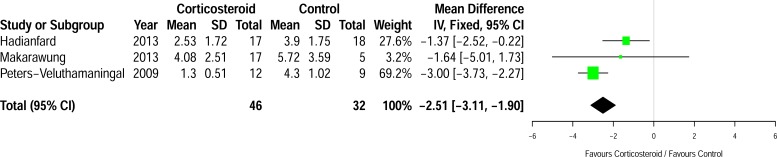
Three studies (n=78) assessed mean differences in pain at first assessment post injection, as shown in Fig. (3). Two of the studies assessed pain using the VAS [19, 20] and one measured severity of pain using a numerical rating scale where zero equals no pain and ten correlates with severe pain [18]. Follow-up time ranged from one week [18] to two weeks [19]. There was a significant reduction in pain with corticosteroid injection when compared to the control group with a mean difference of 2.51 cm [95% CI: -3.11 to -1.90, p=0.0003, I2=65%] in favour of the corticosteroid group.
Fig. (3).
Differences in mean pain between the groups at first assessment post-injection.
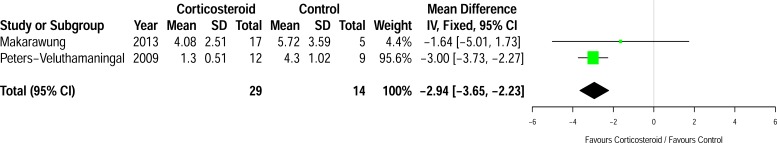
A further planned subgroup analysis using a fixed effects model of the two studies [18, 20] that compared corticosteroid injection to placebo injection (see Fig. 4) also showed a reduction in pain score at first assessment post injection (n=43) [mean difference -2.94 cm, 95% CI (-3.65 – -2.23 cm), p=0.0003, I2=0%].
Fig. (4).
Subgroup analysis of the differences in mean pain between the groups at first assessment post-injection in trials that used placebo injection.
4.3. Differences in Disability Between the Groups at First Assessment Post-Injection
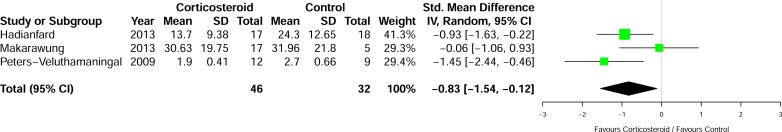
Three studies measured activity limitations and participation restrictions in the groups using the DASH questionnaire [19, 20] and the AIMS-HFF score [18] as shown in Fig. (5). The results showed a statistically significant improvement with corticosteroid over control [SMD -0.83, 95% CI: -1.54 to 0.12, p=0.02, I2=48%].
Fig. (5).
Differences in mean disability between the groups at first assessment post-injection.
5. DISCUSSION
5.1. Statement of Principal Findings
This meta-analysis suggests that patients who received corticosteroid injection for the treatment of DQST were statistically significantly more likely to have full resolution of their symptoms during the follow-up period [range: 6 weeks [19] to 17 months [16]. The corticosteroid group also had statistically significantly less pain and activity limitation at first follow-up post injection [range: 1 week [18] and 1 month [20] than their counterparts who received placebo injection, thumb spica splint or acupuncture.
5.2. Results in the Context of the Current Literature
The results of this meta-analysis are in keeping with the current view of the effectiveness of corticosteroid injection in the treatment of DQST. A previous review by Richie et al. [12] pooled the results of seven observational studies and reported a 83% symptom resolution rate with injection alone. These findings were significantly higher than any other treatment modality with resolution rates of 61% for injection and splint, 14% for splint alone, 0% for rest or non-steroidal anti-inflammatory drugs. A Cochrane review in 2009 [13] concluded that there was silver-level evidence for the superiority of corticosteroid injection over splintage for pain relief in DQST but also reported that this conclusion was supported by only one small RCT of poor methodological quality by Avci et al. [16] in 2002. Since then a number of other RCTs have been performed.
A later meta-analysis [21] comparing splinting to corticosteroid injection in two trials and the results also favoured corticosteroid injection. Mardani-Kivi et al. also studied the efficacy of corticosteroid injection alone when compared to corticosteroid injection (methylprednisolone) with thumb spica casting and reported that a combination of both were better than injection alone as regards treatment success and functional outcomes, citing success rates of 69% and 93% respectively [22]. Hajder et al. studied the efficacy of ultrasound guided injection of triamcinolone citing a success rate of 91% after up to two injections, highlighting the potential role of ultrasound in increasing injection accuracy [23].
Goldfarb et al. studied the effect of steroid and local anaesthetic injection with and without bicarbonate finding an acidic injection preparation had no effect on the incidence of flare reaction compared to the neutral formula with bicarbonate [24]. To study the effect of an oral non-steroidal anti-inflammatory on the efficacy of steroid injection, Jirarattanaphochai et al. randomly assigned 160 patients to steroid alone or to steroid with concurrent administration of oral nimesulide, showing no benefit of oral NSAID administration [25].
5.3. Strengths and Weaknesses of the Study
This review evaluated the evidence in relation to the effectiveness of corticosteroid injection for DQST. Data was pooled from a broad range of studies with comparable baseline characteristics of the treatment and control groups both within and between studies (with the exception of Avci et al. [16] who had pregnant or lactating female participants only) enhancing the generalisability of the findings. Two authors independently evaluated the methodological quality of each RCT using a validated method for assessing the quality of such studies. In addition, sensitivity analyses examined the effect of important methodological variables.
However, the results of the review should be interpreted in the context of the study limitations. None of the five RCTs included in the review met all criteria of methodological quality recommended by the Cochrane Handbook. Most studies failed to adequately describe the randomization process, blind participants, personnel or outcome assessors. This affects the internal validity of the RCTs and weakens the interpretation of the findings in the broader clinical context. Additionally, there was substantial heterogeneity because of different methodologies, control groups, types of injection administered, duration of treatment, outcome measures, and follow-up time. In particular the type and dosage of steroid used in the injection varied between studies.
One study used 0.25 mL of 40 mg/mL (10 mg) methylprednisolone [16], two studies utilized 1 mL of 40 mg/mL of methylprednisolone [17, 19], one study used 1 mL of 10 mg/mL (10 mg) triamcinolonacetonide [18] and another study used 1 mL of 4 mg/mL (4 mg) dexamethasone [20]. Local anaesthetic was also injected with the corticosteroid in three of the studies and in two RCTs the treatment group also had concurrent splinting. The treatment received by the control group also varied with two groups receiving placebo injection of 1 mL of 0.9% NaCl [18] or 2 mL of 1% lidocaine [20], two groups treated in thumb spica splint [16, 17] and one study using acupuncture [19]. The follow-up time also varied greatly between studies ranging from one week [18] to 17 months [16]. Therefore, the findings should be considered in the context of these limitations.
5.4. Clinical Implications and Areas for Future Research
The results of this meta-analysis highlight the efficacy of corticosteroid injection in terms of pain relief and function. Despite its limitations the results obtained from this analysis are more easily generalizable to the population as a whole than previous studies and should provide clinicians and patients alike with more confidence in choosing corticosteroid injection as the first line treatment in severe cases of DQST. Other conservative treatments can be considered in early or mild cases with surgery reserved for cases that the fail to resolve following injection.
Further larger, multi-centre, methodologically robust RCTs are needed to examine the optimum dose of different injection constituents in terms of steroid type, strength of dose, types and doses of added local anaesthetic agents and combinations of injection with or without concurrent splinting. Standardized diagnostic criteria, such as those in the de Quervains screening tool developed by Batteson et al. [26], would also serve to aid recruitment of a homogenous patient cohort to future studies. Uniform outcome measures across future studies would also aid analysis including measures of function improvement. Longer term follow-up of patients in studies and a description of the natural history of DQST would also increase the validity of future studies [13].
CONCLUSION
This review confirms that corticosteroid injection results in an increase in the resolution of symptoms, increased pain relief and increase in function when compared to other active and placebo controls. Further larger, multi-centre RCTs are required to examine the optimal type and dose of steroid in such injections.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Clarke M.T., Lyall H.A., Grant J.W., Matthewson M.H. The histopathology of de Quervain ?(tm)s disease. J. Hand Surg. [Br.] 1998;23(6):732–734. doi: 10.1016/S0266-7681(98)80085-5. [DOI] [PubMed] [Google Scholar]
- 2.Walker-Bone K., Palmer K.T., Reading I., Coggon D., Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. 2004;51(4):642–651. doi: 10.1002/art.20535. [DOI] [PubMed] [Google Scholar]
- 3.Schned E.S. DeQuervain tenosynovitis in pregnant and postpartum women. Obstet. Gynecol. 1986;68(3):411–414. doi: 10.1097/00006250-198609000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Sluiter J.K., Rest K.M., Frings-Dresen M.H. Criteria document for evaluating the work-relatedness of upper-extremity musculoskeletal disorders. Scand. J. Work Environ. Health. 2001;27(Suppl. 1):1–102. doi: 10.5271/sjweh.637. [DOI] [PubMed] [Google Scholar]
- 5.Aptel M., Aublet-Cuvelier A., Cnockaert J.C. Work-related musculoskeletal disorders of the upper limb. Joint Bone Spine. 2002;69(6):546–555. doi: 10.1016/S1297-319X(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 6.Shiraj S., Winalski C.S., Delzell P., Sundaram M. Radiologic case study. Intersection syndrome of the wrist. Orthopedics. 2013;36(3):165–, 225-227. doi: 10.3928/01477447-20130222-01. [DOI] [PubMed] [Google Scholar]
- 7.Ilyas A.M. Nonsurgical treatment for de Quervain ?(tm)s tenosynovitis. J. Hand Surg. Am. 2009;34(5):928–929. doi: 10.1016/j.jhsa.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 8.da Silva J.B., BatigA lia F. Acupuncture in De Quervain ?(tm)s disease: a treatment proposal. Acupunct. Med. 2014;32(1):70–72. doi: 10.1136/acupmed-2013-010486. [DOI] [PubMed] [Google Scholar]
- 9.Moretti M. Effectiveness of oxygen-ozone and hyaluronic acid injections in De Quervain's syndrome. Int J Ozone Ther. 2012;11(1):31–33. [Google Scholar]
- 10.Peck E., Ely E. Successful treatment of de Quervain tenosynovitis with ultrasound-guided percutaneous needle tenotomy and platelet-rich plasma injection: a case presentation. PM R. 2013;5(5):438–441. doi: 10.1016/j.pmrj.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Tseng V., Ibrahim V., Yokel N.R. Prolotherapy for dequervain's tenosynovitis/tendonosis: a case report. PM R. 2012;4(10):S275. [Google Scholar]
- 12.Richie C.A., III, Briner W.W., Jr Corticosteroid injection for treatment of de Quervain ?(tm)s tenosynovitis: a pooled quantitative literature evaluation. J. Am. Board Fam. Pract. 2003;16(2):102–106. doi: 10.3122/jabfm.16.2.102. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Veluthamaningal C., van der Windt D.A., Winters J.C., Meyboom-de Jong B. Corticosteroid injection for de Quervain ?(tm)s tenosynovitis. Cochrane Database Syst. Rev. 2009;(3):CD005616. doi: 10.1002/14651858.CD005616.pub2/abstract. [Internet]. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration Version 5.1.0, 2011. Available from: http://handbook.co chrane.org/front_ page.htm [Accessed on 13 June, 2014]. 2011.
- 16.Avci S., Yilmaz C., Sayli U. Comparison of nonsurgical treatment measures for de Quervain ?(tm)s disease of pregnancy and lactation. J. Hand Surg. Am. 2002;27(2):322–324. doi: 10.1053/jhsu.2002.32084. [DOI] [PubMed] [Google Scholar]
- 17.Mehdinasab S.A., Alemohammad S.A. Methylprednisolone acetate injection plus casting versus casting alone for the treatment of de Quervain ?(tm)s tenosynovitis. Arch. Iran Med. 2010;13(4):270–274. [PubMed] [Google Scholar]
- 18.Peters-Veluthamaningal C., Winters J.C., Groenier K.H., Meyboom-DeJong B. Randomised controlled trial of local corticosteroid injections for de Quervain ?(tm)s tenosynovitis in general practice. BMC Musculoskelet. Disord. 2009;10:131. doi: 10.1186/1471-2474-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadianfard M., Ashraf A., Fakheri M., Nasiri A. Efficacy of acupuncture versus local methylprednisolone acetate injection in De Quervain ?(tm)s tenosynovitis: a randomized controlled trial. J. Acupunct. Meridian Stud. 2014;7(3):115–121. doi: 10.1016/j.jams.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Makarawung D.J., Becker S.J., Bekkers S., Ring D. Disability and pain after cortisone versus placebo injection for trapeziometacarpal arthrosis and de Quervain syndrome. Hand (NY) 2013;8(4):375–381. doi: 10.1007/s11552-013-9529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashraf M.O., Devadoss V.G. Systematic review and meta-analysis on steroid injection therapy for de Quervain ?(tm)s tenosynovitis in adults. Eur. J. Orthop. Surg. Traumatol. 2014;24(2):149–157. doi: 10.1007/s00590-012-1164-z. [DOI] [PubMed] [Google Scholar]
- 22.Mardani-Kivi M., Karimi Mobarakeh M., Bahrami F., Hashemi-Motlagh K., Saheb-Ekhtiari K., Akhoondzadeh N. Corticosteroid injection with or without thumb spica cast for de Quervain tenosynovitis. J. Hand Surg. Am. 2014;39(1):37–41. doi: 10.1016/j.jhsa.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Hajder E., de Jonge M.C., van der Horst C.M., Obdeijn M.C. The role of ultrasound-guided triamcinolone injection in the treatment of de Quervain ?(tm)s disease: treatment and a diagnostic tool? Chir. Main. 2013;32(6):403–407. doi: 10.1016/j.main.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Goldfarb C.A., Gelberman R.H., McKeon K., Chia B., Boyer M.I. Extra-articular steroid injection: early patient response and the incidence of flare reaction. J. Hand Surg. Am. 2007;32(10):1513–1520. doi: 10.1016/j.jhsa.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, Jianmongkol S, Chatuparisute P, Jung S. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am . 2004;86-a(12):2700–6. doi: 10.2106/00004623-200412000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Batteson R., Hammond A., Burke F., Sinha S. The de Quervain ?(tm)s screening tool: validity and reliability of a measure to support clinical diagnosis and management. Musculoskelet. Care. 2008;6(3):168–180. doi: 10.1002/msc.129. [DOI] [PubMed] [Google Scholar]