Abstract
Background. Antibody titers to influenza hemagglutinin (HA) and neuraminidase (NA) surface antigens increase in the weeks after infection or vaccination, and decrease over time thereafter. However, the rate of decline has been debated.
Methods. Healthy adults participating in a randomized placebo-controlled trial of inactivated (IIV) and live-attenuated (LAIV) influenza vaccines provided blood specimens immediately prior to vaccination and at 1, 6, 12, and 18 months postvaccination. Approximately half had also been vaccinated in the prior year. Rates of hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) titer decline in the absence of infection were estimated.
Results. HAI and NAI titers decreased slowly over 18 months; overall, a 2-fold decrease in antibody titer was estimated to take >600 days for all HA and NA targets. Rates of decline were fastest among IIV recipients, explained in part by faster declines with higher peak postvaccination titer. IIV and LAIV recipients vaccinated 2 consecutive years exhibited significantly lower HAI titers following vaccination in the second year, but rates of persistence were similar.
Conclusions. Antibody titers to influenza HA and NA antigens may persist over multiple seasons; however, antigenic drift of circulating viruses may still necessitate annual vaccination. Vaccine seroresponse may be impaired with repeated vaccination.
Keywords: antibody persistence, hemagglutinin, immune correlates, influenza, influenza vaccine, longevity of antibody, neuraminidase, serologic assays, waning
Influenza vaccines are the best tool currently available to reduce the risk of influenza infection and associated complications. These vaccines have long been evaluated based on the antibody response they elicit, particularly to the viral hemagglutinin (HA) [1, 2]. The HA and neuraminidase (NA) glycoproteins are the 2 major surface antigens of the influenza virus and facilitate infection, replication, and viral shedding [3]. Antibodies are produced against both antigens in response to infection or vaccination. HA antibody levels have been shown to correlate with protection against infection by influenza; antibodies to NA may also correlate with protection as well as reduced severity of illness [4–8].
Antibody titers to influenza HA increase in the weeks after infection or vaccination, and decrease over time thereafter; although the rate of HA titer decline has been the subject of some debate. Due to concern of rapidly declining HA titers following vaccination, there were recommendations in the United States from 1990 through 2006 to delay vaccination in the elderly until just before the influenza season [9, 10]. After this recommendation was abandoned in 2007, a review was published suggesting that vaccine-induced HA responses were maintained at high levels in persons ≥60 years of age for at least 4 months [11, 12].
Another past observation concerning HA antibody was reduced seroresponse with repeated vaccination [13–16], but with no definitive effect on vaccine effectiveness (VE) established [17, 18]. More recently, several observational studies have noted lower VE among those who were also vaccinated in the previous season [19–25], and higher VE among those with limited vaccination history compared to regular vaccinators [26]. Some studies have also demonstrated residual protection extending from vaccination in the previous season [22, 24, 25].
In part because of the dominant role of HA antibody in protection from infection, NA antibody has been less studied. Previously available assays for quantification of NA antibody were also labor intensive and used toxic reagents, making it impractical to assay large numbers of specimens [27]. However, recent collaborative efforts to standardize a lectin-based neuraminidase inhibition (NAI) assay have made quantification of NA antibody feasible [28].
Here, we estimate the rates of HA and NA antibody decline over an 18-month period following vaccination. Differences in rates are examined by history of vaccination in the prior season, vaccine type, and peak antibody titer achieved following vaccination.
METHODS
Data and Specimens
Subjects were healthy adults 18–49 years of age and participating in the second and third years of a randomized placebo-controlled trial, conducted during the 2005–2006 and 2006–2007 influenza seasons, evaluating the efficacy of the inactivated (IIV) and live-attenuated (LAIV) influenza vaccines [29]. The study was approved by the University of Michigan Medical School institutional review board. Written informed consent was obtained from all participants before enrollment. In October and November of 2005, subjects were recruited and randomized to receive IIV, LAIV, or placebo. Subjects who had participated in the previous year (2004–2005) of the trial received the same intervention to which they had previously been randomized; newly recruited subjects were randomized at enrollment. From November 2005 through April 2006, subjects were instructed to report all acute respiratory illnesses meeting a symptomatic case definition; ill subjects attended an illness visit with collection of a throat swab. Throat swabs were tested for influenza by virus isolation in cell culture and virus identification in reverse-transcription polymerase chain reaction (RT-PCR) assays. Subjects not lost to follow-up were re-enrolled prior to the 2006–2007 influenza season without revaccination and followed from November 2006 through May 2007 for acute respiratory illnesses with specimen collection and laboratory testing.
Blood specimens for serologic studies were collected immediately prior to vaccination (S1: October–November 2005), approximately 30 days after vaccination (S2: November–December 2005), approximately 6 months after vaccination following the 2005–2006 influenza season (S3: April–May 2006), approximately 12 months after vaccination prior to the 2006–2007 season (S4: October 2006), and approximately 18 months after vaccination following the 2006–2007 season (S5: April–May 2007).
Serologic Laboratory Assays
Following the 2005–2006 study year, hemagglutination-inhibition (HAI) assays were performed on a subset of complete specimen sets (S1–S3) collected that season, including all subjects reporting symptomatic illness and a random sample of those who did not report similar illness [30]. Following the 2006–2007 study year, HAI assays were performed on all complete specimen sets (S4–S5) collected that season. Because of known run-to-run variability in the HAI assay, additional HAI assays were performed on a large subset of subjects with complete specimen sets from both study years (S1–S5), including subsets of those that had previously been assayed in both the 2005–2006 and 2006–2007 sets and those that had not yet been assayed. The results of these assays, referred to as the reference set, were used to standardize the results obtained from the separately assayed 2005–2006 and 2006–2007 sets.
Prior to HAI testing, serum samples were treated overnight with receptor-destroying enzyme and heat-inactivated to prevent nonspecific inhibition; serum samples were also adsorbed with red blood cells to remove nonspecific agglutinins. Serial 2-fold dilutions (with an initial dilution of 1:8) were prepared for each serum sample set (S1–S5) in 96-well microtiter plates followed by incubation with standardized concentrations (4 HA units/25 µL) of monovalent inactivated influenza vaccine subunit material (Sanofi-Pasteur) representing the 2005–2006 A (H3N2) and B (Yamagata) vaccine virus strains (A/NewYork/55/2004 [A/California/7/2004 like], B/Jiangsu/10/2003 [B/Shanghai/361/2002 like]) [31]. Turkey red blood cells were added to wells and allowed to settle. HAI titers to each virus tested were calculated for each subject at each time point (S1–S5) as the reciprocal (eg, 160) of the highest dilution of serum (eg, 1:160) that inhibited hemagglutination. Titers below the lower limit of detection (ie, <8) were considered half the lower limit (ie, 4); titers greater than the upper limit of detection (ie, >4096) were considered twice the upper limit (ie, 8192).
NAI assays, also known as the enzyme-linked lectin assay, were performed on specimens previously assayed in the HAI reference set [28, 32]. This assay utilized a reassortant influenza virus with a mismatched HA (H6 subtype) to avoid interference by HA-specific antibodies, and the NA antigen representing the 2005–2006 A (H3N2) vaccine virus strain (kindly provided by M. Eichelberger, US Food and Drug Administration). Serum samples were heat-inactivated, and serial 2-fold dilutions (with an initial dilution of 1:10) of serum sample sets (S1–S5) were incubated with virus and then added to 96-well microtiter plates coated with fetuin. Following incubation, peroxidase-labeled peanut agglutinin (the lectin) was added, followed by peroxidase substrate to detect enzymatic cleavage of fetuin by viral NA, and the reaction optical density measured with a microplate reader. The percent inhibition of NA enzymatic activity at each serum dilution was calculated by comparison with values from virus control wells (virus but no serum); end-point NAI titers were calculated as the reciprocal of the highest dilution with at least 50% inhibition. Titers below the lower limit of detection (ie, <10) were considered half the lower limit (ie, 5); titers greater than the upper limit of detection (ie, >5120) were considered twice the upper limit (ie, 10 240).
All HAI and NAI assays were performed in the respiratory virus research laboratory at the University of Michigan, School of Public Health.
Statistical Analysis
Log base 2 (log2) transformation was applied to all HAI and NAI titers, and mean log2 titers calculated. The results of the HAI assays, run separately for the 2005–2006 and 2006–2007 specimen sets, were standardized to the results of the reference set assays. Differences in mean log2 HAI titer to each antigen were calculated for those tested both in the reference set and in the 2005–2006 set for S1–S3 specimens. Similarly, differences were calculated for S4–S5 specimens for those tested in both the reference set and in the 2006–2007 set. These antigen- and specimen-specific differences were then added as a correction factor to log2 HAI titers of individuals assayed only in the 2005–2006 and 2006–2007 sets to standardize the titer values to those of the reference set. Geometric mean titers (GMTs) at each time point were calculated as 2 to the power of the mean log2 titer. The proportions of subjects with HAI titers ≥32 or NAI titers ≥40 were calculated at each time point; an HAI titer of ≥40 has historically been used as a measure of “seroprotection” [1, 33].
Subjects were characterized by age, sex, race, and participation status in the 2004–2005 study year. Subjects were excluded from analysis if they had laboratory-confirmed influenza during the 2005–2006 or 2006–2007 season defined by RT-PCR, cell culture, or ≥4-fold HAI titer rise from preseason to postseason serum samples (S2 to S3, or S4 to S5). Participation in the 2004–2005 study year was used as a proxy for history of influenza vaccination in that season. Those enrolled in both 2004–2005 and 2005–2006 received the same intervention both years; however, history of vaccination in the 2004–2005 season was not explicitly determined for those newly enrolled in 2005–2006. Differences in characteristics across intervention groups were examined using χ2 tests for categorical variables and Kruskal–Wallis tests for continuous variables. GMTs at each time point were compared across intervention groups and by vaccination history using Wilcoxon rank-sum tests.
Rates of titer decline were estimated in linear mixed models with log2 titers as the dependent variable and time in days from the S2 (1 month postvaccination) blood draw as the independent variable. To account for correlation of titers within individuals over time, the intercept and time were modeled as random effects. Rates of titer decline were also estimated by intervention, by vaccination status in the 2004–2005 study year, and by peak (S2) log2 titers by adding respective interactions with time to the models; models with a peak log2 titer (S2) by time interaction only considered S3–S5 log2 titers in the dependent variable and did not estimate an intercept. Time in days to decrease 1 log2 titer (2-fold decrease) was calculated as the reciprocal of the model estimated rates. All statistical analyses were carried out using SAS (release 9.2, SAS Institute) software; a P value <.05 was considered to indicate statistical significance.
RESULTS
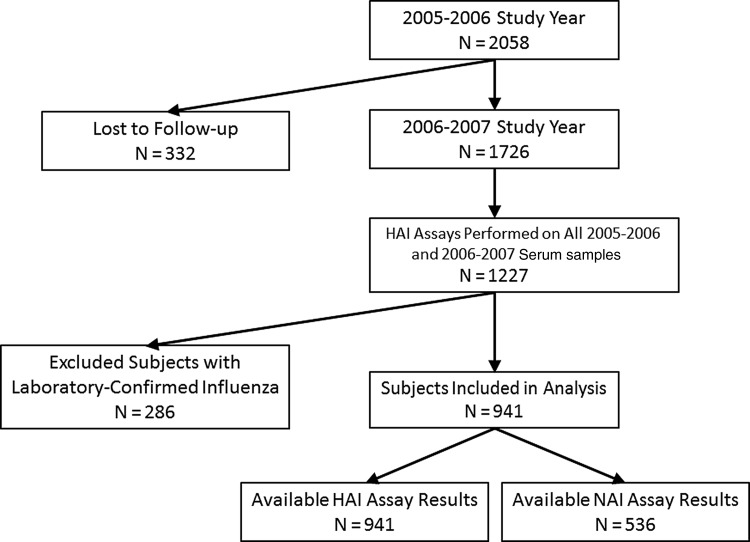
In the 2005–2006 study year, 2058 subjects enrolled and received intervention, including 972 (47%) who also participated and received the same intervention in 2004–2005 (Figure 1). Of these 2058 subjects, 1726 continued participation in 2006–2007 without revaccination. Among the 1726 subjects participating from 2005 through 2007, 1227 (71%) had HAI assays performed on all S1–S5 specimens. HAI results from 941 (77%) subjects were included in this analysis; 286 were excluded because of laboratory-confirmed influenza infection. Of the 941 subjects included in the analysis, 536 were assayed as part of the reference set and 405 standardized to the reference set. NAI assays were performed on the 536 subjects in the reference set. Included subjects did not significantly differ by age, sex, race, or participation in the 2004–2005 season from those not included due to loss to follow-up, not being tested, or laboratory-confirmed influenza infection. The characteristics of included subjects are presented in Table 1; subjects did not significantly differ across intervention groups by age, sex, race, participation in the 2004–2005 season, or proportion with standardized results.
Figure 1.
Subjects included and excluded from analyses of hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) antibody persistence.
Table 1.
Characteristics of Subjects Included in Analysis Set by Intervention
| Intervention |
P Value | Total (N = 941) | |||
|---|---|---|---|---|---|
| IIV (N = 431) | LAIV (N = 381) | Placebo (N = 129) | |||
| Percentage of participants | 45.8 | 40.5 | 13.7 | 100 | |
| Mean age, y ± SD | 25.9 ± 9.1 | 25.1 ± 8.6 | 26.1 ± 9.6 | .47a | 25.6 ± 8.9 |
| Age category, N (%) | |||||
| 18–19 y | 128 (29.7) | 121 (31.8) | 45 (34.9) | .37b | 294 (31.2) |
| 20–24 y | 166 (38.5) | 154 (40.4) | 44 (34.1) | 364 (38.7) | |
| 25–34 y | 51 (11.8) | 40 (10.5) | 9 (7.0) | 100 (10.6) | |
| 35–48 y | 86 (20.0) | 66 (17.3) | 31 (24.0) | 183 (19.5) | |
| Sex, N (%) | |||||
| Female | 271 (62.9) | 243 (63.8) | 77 (59.7) | .71b | 591 (62.8) |
| Male | 160 (37.1) | 138 (36.2) | 52 (40.3) | 350 (37.2) | |
| Race, N (%) | |||||
| White | 374 (86.8) | 333 (87.4) | 115 (89.1) | .78b | 822 (87.4) |
| Nonwhite | 57 (13.2) | 48 (12.6) | 14 (10.9) | 119 (12.6) | |
| Participated in 2004–2005, N (%)c | |||||
| Yes | 194 (45.0) | 185 (48.6) | 63 (48.8) | .54b | 442 (47.0) |
| No | 237 (55.0) | 196 (51.4) | 66 (51.2) | 499 (53.0) | |
| Standardized results, N (%) | |||||
| Yes | 187 (43.4) | 173 (45.4) | 45 (34.9) | .11b | 405 (43.0) |
| No | 244 (56.6) | 208 (54.6) | 84 (65.1) | 536 (57.0) | |
Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
a Kruskal–Wallis test.
b χ2 test.
c Participation in the 2004–2005 study year is used as a proxy for 2004–2005 vaccination status. Those enrolled in both 2004–2005 and 2005–2006 received the same intervention both years; however, history of vaccination in the 2004–2005 season was not explicitly determined for those newly enrolled in 2005–2006.
The proportions of subjects with HAI titers ≥32 or NAI titers ≥40 at each time point by intervention are presented in Table 2. Nearly all IIV recipients had HAI titers ≥32 to both influenza A (H3N2) (A/H3) and influenza B (Yamagata) (B/Y) 1 month postvaccination, and the proportion with HAI titers ≥32 remained at nearly 90% 18 months after vaccination. In contrast, 79% of IIV recipients had NAI titers ≥40 to influenza A (H3N2) (A/N2) 1 month postvaccination, but only 48% had a titer ≥40 at the 18-month follow-up. The proportions of LAIV recipients with similar titers were lower than for IIV recipients; however, patterns across time were similar.
Table 2.
Proportions of Subjects With HAI Titers ≥32 or NAI Titers ≥40 at Prevaccination, and 1, 6, 12, and 18 Month Postvaccination Time Points by Intervention
| Intervention and Antigen | Months Following Vaccination |
||||
|---|---|---|---|---|---|
| 0 | 1 | 6 | 12 | 18 | |
| IIV | |||||
| Influenza A (H3N2) HAI, % | 55.9 | 97.0 | 92.8 | 89.3 | 89.1 |
| Influenza A (H3N2) NAI, % | 37.7 | 79.1 | 59.8 | 57.0 | 48.0 |
| Influenza B HAI, % | 77.8 | 99.8 | 98.4 | 97.2 | 95.6 |
| LAIV | |||||
| Influenza A (H3N2) HAI, % | 44.9 | 68.0 | 61.7 | 62.7 | 59.3 |
| Influenza A (H3N2) NAI, % | 29.8 | 40.4 | 39.4 | 38.5 | 32.2 |
| Influenza B HAI, % | 68.8 | 92.1 | 89.8 | 84.0 | 81.9 |
| Placebo | |||||
| Influenza A (H3N2) HAI, % | 48.1 | 44.2 | 44.2 | 49.6 | 45.7 |
| Influenza A (H3N2) NAI, % | 32.1 | 36.9 | 29.8 | 32.1 | 28.6 |
| Influenza B HAI, % | 67.4 | 72.9 | 69.8 | 69.8 | 67.4 |
Abbreviations: HAI, hemagglutination inhibition; IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine; NAI, neuraminidase inhibition.
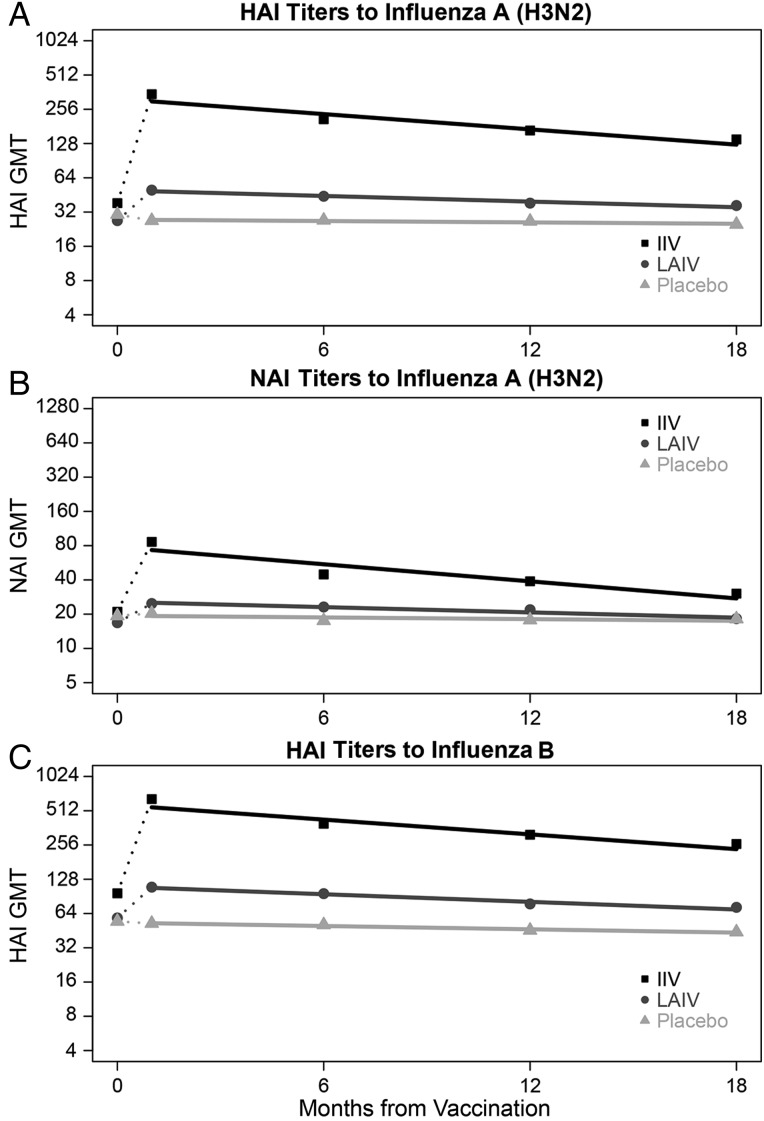
HAI GMTs were plotted by time with estimated regression lines in Figure 2A and 2C by intervention. Overall, estimated times to decrease 2-fold were 662 (95% confidence interval [CI], 588–758) days for A/H3 and 606 (95% CI, 546–685) days for B/Y. Among IIV recipients, HAI GMTs increased from prevaccination (A/H3: 38, B/Y: 96) to 1-month follow-up (A/H3: 337, B/Y: 611) before gradually decreasing through the 18-month follow-up (A/H3: 138, B/Y: 256); the estimated time to decrease 2-fold was 410 (95% CI, 369–463) days for A/H3 and 424 (95% CI, 380–476) days for B/Y. For LAIV recipients, HAI GMTs increased minimally from prevaccination (A/H3: 27, B/Y: 59) to 1-month follow-up (A/H3: 50, B/Y: 109) before gradually decreasing through the 18-month follow-up (A/H3: 37, B/Y: 72); the estimated time to decrease 2-fold was 1111 (95% CI, 840–1639) days for A/H3 and 820 (95% CI, 667–1075) days for B/Y. HAI GMTs for placebo recipients gradually decreased from preintervention (A/H3: 38, B/Y: 68) through 18-month follow-up (A/H3: 31, B/Y: 55); the estimated time to decrease 2-fold was 4545 (95% CI, 1389–3584) days for A/H3 and 1887 (95% CI, 990–25 000) days for B/Y.
Figure 2.
Geometric mean (GMT) hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) titers at prevaccination, and 1, 6, 12, and 18 month postvaccination time points, by intervention, with estimated regression lines*. *Rates of antibody change were estimated in linear mixed models with log2 titers as the dependent variable and time in days from the 1 month postvaccination blood draw as the independent variable. To account for correlation of titers within individuals over time, the intercept and time were modeled as random effects. Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
NAI GMTs were plotted by time with estimated regression lines in Figure 2B by intervention. Overall, the estimated time to decrease 2-fold was 621 (95% CI, 556–704) days. Among IIV recipients, NAI GMTs increased from 17 prevaccination to 69 at the 1-month follow-up before decreasing to 24 at the 18-month follow-up; the estimated time to decrease 2-fold was 366 (95% CI, 334–403) days. For LAIV recipients, NAI GMTs minimally increased from 13 prevaccination to 20 at the 1-month follow-up before decreasing to 15 at the 18-month follow-up; the estimated time to decrease 2-fold was 1190 (95% CI, 901–1786) days. No significant decrease in NAI GMTs among placebo recipients was observed from preintervention (A/N2: 19) through 18-month follow-up (A/N2: 18).
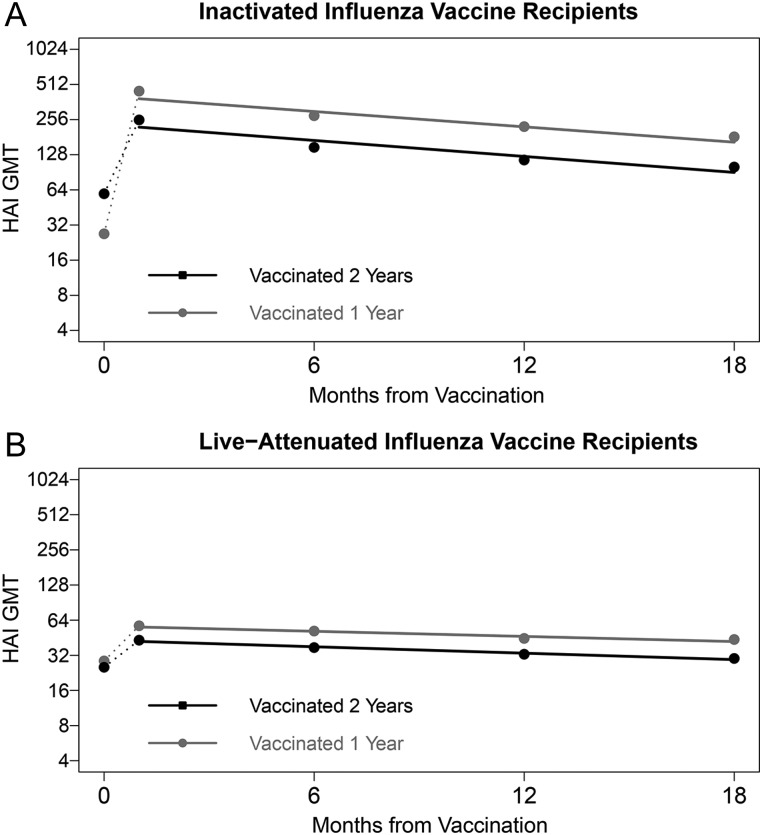
HAI GMTs to A/H3 and B/Y and NAI GMTs to A/N2 were plotted by time with estimated regression lines stratified by 2004–2005 vaccination status in Figure 3, Supplementary Figure 1, and Figure 4, respectively, for IIV and LAIV recipients. Those who received IIV in both 2004–2005 and 2005–2006 had significantly higher (P < .001) prevaccination HAI GMTs in 2005–2006 (A/H3: 59, B/Y: 226) than those not previously vaccinated (A/H3: 27, B/Y: 48). However, the GMT fold-rise following 2005–2006 vaccination was significantly lower (P < .001) for those previously vaccinated (A/H3: 4.2, B/Y: 2.1) compared to those not (A/H3: 16.0, B/Y: 15.7). HAI GMTs of previously vaccinated IIV recipients remained significantly lower (P < .001) than those not previously vaccinated at all 4 time points following 2005–2006 vaccination. Prevaccination HAI GMTs in 2005–2006 of LAIV recipients vaccinated in both 2004–2005 and 2005–2006 were not significantly different (A/H3: 25, B/Y: 57) than those not previously vaccinated (A/H3: 29, B/Y: 60). However, HAI GMTs of previously vaccinated LAIV recipients were significantly lower (P < .001) than those not previously vaccinated at all 4 time points following 2005–2006 vaccination.
Figure 3.
Geometric mean (GMT) hemagglutination inhibition (HAI) titers to influenza A (H3N2) at prevaccination, and 1, 6, 12, and 18 month postvaccination time points, by intervention and 2-year vaccination status*, with estimated regression lines**. *Participation in the 2004–2005 study year was used as a proxy for history of influenza vaccination in that season. Those enrolled in both 2004–2005 and 2005–2006 received the same intervention both years; however, history of vaccination in the 2004–2005 season was not explicitly determined for those newly enrolled in 2005–2006. **Rates of antibody change were estimated in linear mixed models with log2 titers as the dependent variable and time in days from the 1 month postvaccination blood draw as the independent variable. To account for correlation of titers within individuals over time, the intercept and time were modeled as random effects. Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
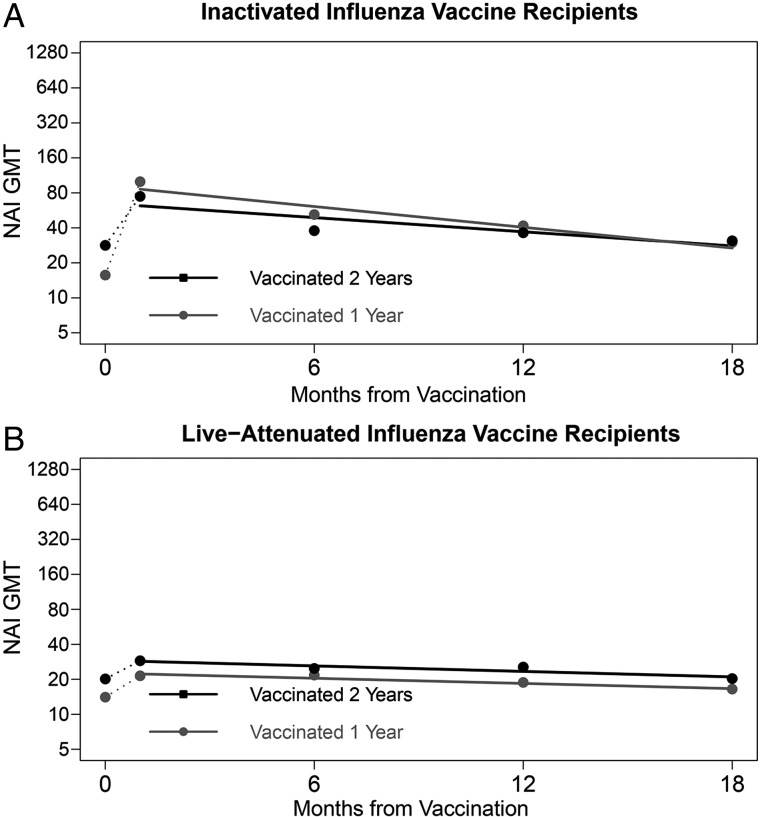
Figure 4.
Geometric mean (GMT) neuraminidase inhibition (NAI) titers to influenza A (H3N2) at prevaccination, and 1, 6, 12, and 18 month postvaccination time points, by intervention and 2-year vaccination status*, with estimated regression lines**. *Participation in the 2004–2005 study year was used as a proxy for history of influenza vaccination in that season. Those enrolled in both 2004–2005 and 2005–2006 received the same intervention both years; however, history of vaccination in the 2004–2005 season was not explicitly determined for those newly enrolled in 2005–2006. **Rates of antibody change were estimated in linear mixed models with log2 titers as the dependent variable and time in days from the 1 month postvaccination blood draw as the independent variable. To account for correlation of titers within individuals over time, the intercept and time were modeled as random effects. Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
Among IIV recipients, prevaccination NAI GMT was 28 for those previously vaccinated and 16 for those not (P < .001). However, the GMT fold-rise following 2005–2006 vaccination was significantly lower (P < .001) for those previously vaccinated (A/N2: 2.6) compared to those not (A/N2: 6.4). NAI GMTs to A/N2 following 2005–2006 vaccination were similar (P > .05) for previously vaccinated IIV recipients and those not previously vaccinated at all 4 time points. Similarly among LAIV recipients, prevaccination NAI GMT was 20 for those previously vaccinated and 14 for those not (P = .33). However, in contrast to patterns seen in IIV recipients, GMT fold-rises following 2005–2006 vaccination were similar for those previously vaccinated and those not (P = .96). NAI GMTs following 2005–2006 vaccination remained higher for previously vaccinated LAIV recipients at all 4 time points, though only significantly higher 1 month postvaccination (P = .04). HAI GMTs to both A/H3 and B/Y and NAI GMTs to A/N2 did not significantly differ by previous participation at any point among placebo recipients (data not shown).
The estimated times to decrease HAI and NAI titers by 2-fold stratified by peak postvaccination titer are presented in Table 3. For both HAI and NAI, the rate of decline was faster with higher peak titers. For example, subjects with peak HAI titers of 4096 to A/H3 were estimated to have a 2-fold reduction in titer after 374 (95% CI, 310–469) days, while a similar 2-fold reduction among subjects with peak titer of 64 was estimated to take over 4 years (1485 [95% CI, 1104–2269] days).
Table 3.
Estimates of 18-Month Postintervention HAI and NAI Titers and Times to Decrease Titer by 2-fold, Stratified by Peak Postintervention Titera
| All Subjects N (%) | Model Estimated 18-Month Titer (95% CI)b | Days to 2-fold Titer Decrease (95% CI)b,c | |
|---|---|---|---|
| Influenza A (H3N2) | |||
| Peak HAI Titera | |||
| 4 | 15 (1.6) | 5 (4 to 6) | ∞ |
| 8 | 55 (5.8) | 9 (7 to 10) | ∞ |
| 16 | 86 (9.1) | 14 (13 to 16) | 173 623 (3188 to ∞) |
| 32 | 128 (13.6) | 24 (22 to 26) | 2945 (1672 to 12 345) |
| 64 | 147 (15.6) | 40 (37 to 43) | 1485 (1104 to 2269) |
| 128 | 153 (16.3) | 67 (62 to 72) | 993 (803 to 1300) |
| 256 | 110 (11.7) | 112 (103 to 122) | 746 (620 to 936) |
| 512 | 120 (12.8) | 187 (169 to 207) | 597 (499 to 742) |
| 1024 | 67 (7.1) | 312 (275 to 354) | 498 (416 to 619) |
| 2048 | 36 (3.8) | 521 (448 to 607) | 427 (356 to 533) |
| 4096 | 24 (2.6) | 871 (728 to 1042) | 374 (310 to 469) |
| Peak NAI Titera | |||
| 5 | 63 (11.8) | 5 (4 to 6) | ∞ |
| 10 | 61 (11.4) | 8 (7 to 8) | ∞ |
| 20 | 104 (19.4) | 12 (11 to 13) | 2508 (1557 to 6445) |
| 40 | 101 (18.8) | 18 (16 to 19) | 1135 (886 to 1578) |
| 80 | 61 (11.4) | 27 (25 to 29) | 734 (603 to 935) |
| 160 | 80 (14.9) | 41 (37 to 46) | 542 (452 to 677) |
| 320 | 35 (6.5) | 63 (55 to 72) | 430 (359 to 534) |
| 640 | 19 (3.5) | 96 (82 to 114) | 356 (297 to 443) |
| 1280 | 12 (2.2) | 147 (121 to 180) | 304 (254 to 379) |
| Influenza B Yamagata | |||
| Peak HAI Titera | |||
| 4 | 15 (1.6) | 6 (5 to 8) | ∞ |
| 8 | 20 (2.1) | 10 (9 to 12) | ∞ |
| 16 | 31 (3.3) | 17 (15 to 20) | ∞ |
| 32 | 92 (9.8) | 29 (26 to 33) | 96 097 (3253 to ∞) |
| 64 | 121 (12.9) | 49 (45 to 53) | 2455 (1523 to 6337) |
| 128 | 158 (16.8) | 81 (76 to 88) | 1244 (971 to 1728) |
| 256 | 159 (16.9) | 136 (126 to 146) | 833 (698 to 1032) |
| 512 | 135 (14.3) | 227 (209 to 247) | 626 (536 to 751) |
| 1024 | 104 (11.1) | 379 (343 to 420) | 501 (432 to 598) |
| 2048 | 53 (5.6) | 634 (558 to 719) | 418 (360 to 499) |
| 4096 | 53 (5.6) | 1058 (908 to 1234) | 359 (308 to 429) |
Abbreviations: CI, confidence interval; HAI, hemagglutination inhibition; NAI, neuraminidase inhibition.
a Titer at 1-month postvaccination follow-up.
b Rates of antibody change were estimated in linear mixed models with log2 titers as the dependent variable, time in days from the 1 month postvaccination blood draw as the independent variable, and an interaction term between the peak log2 titer and time. Because peak log2 titer was included in the model as a covariate, a separate intercept was not estimated and only post-1-month log2 titers were included in the dependent variable. To account for correlation of titers within individuals over time, time was modeled as a random effect.
c Time in days to decrease 2-fold (1 log2 titer) was calculated as the reciprocal of the model estimated rates.
DISCUSSION
Minimal reductions in HAI titer over an 18-month period among participants in a randomized clinical trial were observed here. Previous studies have reported HAI titers persisting above “seroprotective” levels (HAI titer ≥40) for many months after vaccination in a high proportion of individuals, including elderly and high-risk populations [12, 34]. However, the term seroprotection can be misleading, as infections occur among individuals with HAI titers ≥40, though risk of infection decreases as titers increase [6]. This cut point was originally chosen because there was evidence that an HAI titer of 40 would protect 50% of those exposed to influenza from being infected [5, 35]. One recent study has suggested that an HAI titer of 40 may be associated with <50% protection [36]. In addition, laboratory-to-laboratory variation in the HAI assay makes it difficult to standardize interpretation of any given titer. It is, therefore, important to determine not only the proportion of subjects maintaining titers ≥40 over time, but also the absolute titer level and the rate of decline, as we have done.
NAI titer was also observed to decrease relatively slowly over the 18-month period. Past studies examining persistence of antibody to NA reported mixed observations. One study reported that NA antibodies declined to undetectable levels within 5 months following infection [37]; 2 other studies reported persistence of detectable NA antibody up to 4 years after infection [38, 39]. Evidence for the contribution of NA antibody to protection from influenza, independent of the effect of HA antibody, has been suggested by patterns of infection during the 1968 pandemic, and more recently using multivariable regression [8, 40, 41]. Given the longevity of NAI titers observed here, improving vaccine-induced response to NA might increase the practical duration of protection when HA drifts and NA does not.
IIV recipients achieved much higher postvaccination HAI and NAI titers, but experienced faster titer decline than LAIV or placebo recipients. However, titers of IIV recipients remained significantly higher than LAIV or placebo recipients after 18 months. At least part of the difference in rates of decline between IIV and LAIV recipients was explained by the higher average postvaccination titers among IIV recipients and faster rates of decline among those starting at higher titers. Further investigations into modifiers of the rate of titer decline are important for informing models of the effects of serum antibodies on protection from infection and influenza transmission dynamics.
Lower postvaccination HAI titers were observed among those vaccinated with IIV or LAIV in both 2004–2005 and 2005–2006 compared to those vaccinated only in 2005–2006. This is consistent with reports of reduced seroresponse and lower VE among those vaccinated in consecutive seasons [13–16, 19–26]. Despite the difference in initial response, there were no differences in rates of decline. Previous studies have reported slightly more rapid rates of decline and differences by previous antigenic experience [16, 42]. In this study, participation in the 2004–2005 study year was used as a proxy for history of influenza vaccination in that season. Although 2004–2005 vaccination status was not explicitly determined for those newly recruited in 2005–2006, the actual proportion vaccinated in 2004–2005 is likely to be low given the similarity of titers in the placebo group by 2004–2005 participation status.
Rates of antibody decline were presented as days to decrease 2-fold, with some estimates exceeding the study period. While these estimates are useful for comparing the magnitude of rates across groups (eg, intervention, peak titer), caution should be used in predicting titers beyond the period of observation (583 days). Subjects participating in this study were healthy adults aged 18–49 years. In contrast, those most at risk of severe outcomes of influenza infection are young children, older adults, and those with high-risk health conditions [11]. The persistence of HAI and NAI antibodies may differ in these groups. Prior studies of older and immunocompromised adults have suggested subjects initially achieving seroprotective HAI titers maintain these titers for extended periods [29, 30]. However, little is known regarding NA antibody persistence in these groups. Identification of characteristics that predict individual variation in antibody persistence is also of interest.
Although antibody titers may remain at high levels over multiple seasons, antigenic drift of circulating influenza viruses may necessitate annual vaccination [43]. Evidence presented here and elsewhere suggests that HAI response to vaccine may be impaired with repeated vaccination [13–16]. Despite this, those vaccinated in 2 consecutive seasons had higher titers than placebo recipients, even 18 months after vaccination. These findings indicate that given currently available vaccines, annual vaccination remains the best strategy for reducing risk of influenza infection and associated complications. The importance of NAI antibody, previously shown as an independent correlate of protection, is also supported by the duration of elevated titers. These results point to the need for vaccines that stimulate a greater breadth of immunity to achieve better and longer-lasting protective efficacy.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Maryna Eichelberger (CBER, FDA) for her support in establishing the NAI/ELLA assay.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (U01 AI057853 to A. S. M.); an unrestricted grant from Sanofi Pasteur.
Potential conflicts of interest. J. G. P., S. E. O., E. J., and R. T. report grant support from Sanofi Pasteur. A. S. M. reports grant support from Sanofi Pasteur and consultancy fees from Sanofi, GSK and Novavax.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Committee for Proprietary Medicinal Products. Note for guidance on harmonization of requirements for influenza vaccines. 1997. CPMP/BWP/214/96 (circular 96-0666):1–22 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf Accessed 23 March 2014.
- 2.US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. 2007. http://www.fda.gov/cber/gdlns/trifluvac.htm Accessed 23 March 2014.
- 3.Gamblin SJ, Skehel JJ. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem 2010; 285:28403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson BE, Cox MMJ. Influenza viral neuraminidase: the forgotten antigen. Expert Rev Vaccines 2011; 10:1683–95. [DOI] [PubMed] [Google Scholar]
- 5.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879–85. [DOI] [PubMed] [Google Scholar]
- 7.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133–8. [DOI] [PubMed] [Google Scholar]
- 8.Couch RB, Atmar RL, Franco LM et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis 2013; 207:974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1990; 39(RR-7):1–15. [PubMed] [Google Scholar]
- 10.Smith NM, Bresee JS, Shay DK et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55(RR-10):1–42. [PubMed] [Google Scholar]
- 11.Fiore AE, Shay DK, Haber P et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56(RR-6):1–54. [PubMed] [Google Scholar]
- 12.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502. [DOI] [PubMed] [Google Scholar]
- 13.Beyer WEP, Palache AM, Sprenger MJW et al. Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine 1996; 14:1331–9. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki S, He XS, Holmes TH et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLOS One 2008; 3:e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huijskens E, Rossen J, Mulder P et al. Immunogenicity, boostability and sustainability of the immune response after vaccination against influenza A virus (H1N1) 2009 in a healthy population. Clin Vaccine Immunol 2011; 18:1401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Künzel W, Glathe H, Engelmann H, Van Hoecke C. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine 1996; 14:1108–10. [DOI] [PubMed] [Google Scholar]
- 17.Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus ADME. Protection against influenza after annually repeated vaccination; a meta-analysis of serologic and field studies. Arch Intern Med 1999; 159:182–8. [DOI] [PubMed] [Google Scholar]
- 18.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997; 15:1114–22. [DOI] [PubMed] [Google Scholar]
- 19.Ohmit SE, Petrie JG, Malosh RE et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013; 56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmit SE, Thompson MG, Petrie JG et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis 2013; 57:474–6. [DOI] [PubMed] [Google Scholar]
- 22.Thompson MG, Li DK, Shifflett P et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis 2014; 58:449–57. [DOI] [PubMed] [Google Scholar]
- 23.Skowronski DM, Janjua NZ, De Serres G et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLOS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean HQ, Thompson MG, Sundaram ME et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohmit SE, Petrie JG, Malosh RE et al. Influenza vaccine effectiveness in households with children during the 2012–2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis 2015; 211:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean HQ, Thompson MG, Sundaram ME et al. Impact of repeated vaccination on vaccine effectiveness against influenza A (H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aymard-Henry M, Coleman MT, Dowdle WR, Laver WG, Schild GC, Webster RG. Influenza virus neuraminidase and neuraminidase inhibition test procedures. Bull WHO 1973; 48:199–202. [PMC free article] [PubMed] [Google Scholar]
- 28.Sandbulte MR, Gao J, Straight TM, Eichelberger MC. A miniaturized assay for influenza neuraminidase-inhibiting antibodies utilizing reverse genetics-derived antigens. Influenza Other Respi Viruses 2009; 3:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohmit SE, Victor JC, Teich ER et al. Prevention of symptomatic seasonal influenza in 2005–2006 by inactivated and live-attenuated vaccines. J Infect Dis 2008; 198:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Exp Rev Vacc 2011; 9:669–83. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2005–2006 influenza season. Wkly Epidemiol Rec 2005; 80:65–76. [PubMed] [Google Scholar]
- 32.Couzens L, Gao J, Westgeest K et al. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Meth 2014; 210:7–14. [DOI] [PubMed] [Google Scholar]
- 33.Beyer WE, Palache AM, Luchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res 2004; 103:125–32. [DOI] [PubMed] [Google Scholar]
- 34.Moran JJ, Rose WE, Darga AJ, Rohde KA, Hayney MS. Persistence of influenza vaccine-induced antibodies in lung transplant patients between seasons. Transpl Infect Dis 2011; 13:466–70. [DOI] [PubMed] [Google Scholar]
- 35.Cox RJ. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother 2013; 9:405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang TK, Cauchemez S, Mahen Perera RAP et al. Association between antibody titers and protection against influenza virus infection within households. J Infect Dis 2014; 210:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schild GC, Newman RW. Antibody against influenza A2 virus neuraminidase in human sera. J Hyg Camb 1969; 67:353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AJ, Davies JR. Natural infection with influenza A (H3N2). The development, persistence and effect of antibodies to the surface antigens. J Hyg Camb 1976; 77:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grilli EA, Davies JR, Smith AJ. Infection with influenza A (H1N1) 1. Production and persistence of antibody. J Hyg Camb 1986; 96:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monto AS, Kendal AP. Effect of neuraminidase antibody on Hong Kong influenza. Lancet 1973; 1:623–5. [DOI] [PubMed] [Google Scholar]
- 41.Monto AS, Petrie JG, Cross RT et al. Antibody to the influenza neuraminidase: an independent correlate of protection. J Infect Dis 2015; 212:1191–9. [DOI] [PubMed] [Google Scholar]
- 42.Cate TR, Couch RB, Parker D, Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines—1978. Rev Infect Dis 1983; 5:737–47. [DOI] [PubMed] [Google Scholar]
- 43.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine 2007; 25:6852–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.