Abstract
Background. Tenofovir is a potent anti-human immunodeficiency virus (HIV) agent that decreased risk of herpes simplex virus type 2 (HSV-2) acquisition in HIV pre-exposure prophylaxis trials. Whether tenofovir has utility in established HSV-2 disease is unclear.
Methods. We randomized immunocompetent women with symptomatic HSV-2 infection to oral tenofovir disoproxil fumarate (TDF)/placebo vaginal gel, oral placebo/tenofovir (TFV) vaginal gel, or double placebo (ratio 2:2:1) in a one-way cross-over trial. Women collected genital swabs twice daily for HSV PCR during 4-week lead-in and 5-week treatment phases. The primary intent-to-treat end point was within-person comparison of genital HSV shedding and lesion rates.
Results. 64 women completed the lead-in phase and were randomized. Neither TDF nor TFV gel decreased overall shedding or lesion rate in the primary analysis; TFV gel decreased quantity of HSV DNA by −0.50 (−0.86–0.13) log10 copies/mL. In the per-protocol analysis, TDF reduced shedding (relative risk [RR] = 0.74, P = .006) and lesion rates (RR = 0.75, P = .032); quantity of virus shed decreased by 0.41 log10 copies/mL.
Conclusions. Oral TDF modestly decreased HSV shedding and lesion rate, and quantity of virus shed when used consistently. Vaginal TFV gel decreased quantity of virus shed by 60%. In contrast to effects on HSV-2 acquisition, tenofovir is unlikely to provide clinically meaningful reductions in the frequency of HSV shedding or genital lesions.
Clinical Trials Registration. NCT01448616
Keywords: herpes simplex virus-2 (HSV-2), tenofovir, vaginal microbicide, HSV-2 transmission, HIV acquisition, genital herpes
An estimated 417 million people worldwide aged 15–49 years are infected with herpes simplex virus type 2 (HSV-2), and an additional 19.2 million new infections occur annually [1]. HSV-2 is the leading cause of genital ulcer disease worldwide, and disseminated infections cause morbidity and mortality in both adults and neonates. In addition, HSV-2 seropositivity increases the risk of human immunodeficiency virus type 1 (HIV-1) transmission by 2–3-fold [2–4]. As such, up to 40%–60% of new HIV-1 acquisitions are attributable to genital HSV coinfection [3]. Classic nucleoside analogs, such as acyclovir and valacyclovir, are effective at treating and preventing genital herpes recurrences, but they fail to completely suppress viral shedding and sexual transmission [5–7]. No vaccine for HSV currently exists; novel therapies are needed.
Unexpectedly, several HIV preexposure prophylaxis (PrEP) trials of the nucleotide analog reverse transcriptase inhibitor (NRTI) tenofovir disoproxil fumarate (TDF) and tenofovir (TFV) vaginal gel have reduced acquisition of both HSV-2 and HIV-1. The CAPRISA 004 study, in which women used pericoital TFV gel, demonstrated 51% reduction in HSV-2 acquisition and 39% reduction in HIV-1 acquisition [8, 9]. Among women with detectable plasma levels of TFV, the VOICE trial, evaluating daily use of TFV vaginal gel, demonstrated a 46% reduction in HSV-2 seroconversion [10]. A subgroup analysis of the Partners PrEP study revealed that, among couples discordant for HIV-1 and HSV-2 infection, the risk of HSV-2 acquisition was decreased by 33% among HIV-1/HSV-2–seronegative persons receiving TDF or TDF/emtricitabine (TDF/FTC), compared with those receiving placebo [11]. Conversely, the iPREX study did not demonstrate a decreased risk of HSV-2 seroconversion among HSV-2/HIV-1–negative men receiving TDF/FTC for PrEP, despite reducing the risk of HIV acquisition by 44% [12].
With growing evidence that tenofovir may have anti-HSV activity, the question remains whether either oral or topical formulation can reduce genital viral shedding and herpes lesions in persons already infected with HSV-2. To evaluate the impact of tenofovir on established HSV-2 infection in the absence of HIV-1 infection, we undertook a randomized, double-blind, cross-over trial of oral and vaginal tenofovir in HIV-uninfected women with symptomatic HSV-2 infection.
METHODS
Study Participants
HIV-1–seronegative and HSV-2–seropositive women aged 18–50 years were recruited at the University of Washington Virology Research Clinic (Seattle, Washington). We enrolled women who had symptomatic genital herpes diagnosed at least 1 year previously, had ≥2 genital recurrences during the last year (or during the year prior to suppressive antiviral therapy), and were willing to not use antiviral therapy and to use effective contraception during the study. We excluded women who had hepatitis B virus infection or were at immediate risk for hepatitis B virus or HIV acquisition, women who were immunosuppressed, and women who had significant medical comorbidities, elevated hepatic transaminase levels, abnormal pelvic examination findings, or contraindications to taking tenofovir, including renal insufficiency.
Study Design and Oversight
The study was designed and implemented by the University of Washington investigators. The UW Human Subjects Review Committee approved the protocol, and all participants provided written informed consent. The study was sponsored by Gilead Sciences. Gilead Sciences also provided TDF tablets, and CONRAD provided TFV vaginal gel. Randomization codes were generated by a biostatistician not otherwise involved with study procedures. All authors contributed to the work and take responsibility for the accuracy of data presented. The sponsors reviewed the manuscript but did not participate in the analysis or decision to submit the manuscript for publication.
Study Drugs
The manufacturer provided TDF as a single 300-mg tablet with matching placebo. The 1% TFV gel is a clear pH-buffered gel provided in a single-use applicator that delivers 4 mL of gel (40 mg of tenofovir) intravaginally. The matching universal placebo gel has been tested in vitro and was verified to have no anti-HSV properties; both products were used in the VOICE and CAPRISA trials [13, 14]. Adherence to the study regimen was calculated by recording the fraction of returned pills among those dispensed between study visits and the proportion of gel applicators returned unused. Participants also recorded use of study products as part of daily diary entries.
Study Procedures
The participants obtained mixed anogenital swabs twice daily and completed daily diaries in which they noted genital symptoms and genital lesions [7, 15, 16]. Women who returned ≥90% of swabs during the 4-week lead-in period were randomly assigned at a ratio 2:2:1 to one of 3 arms: oral TDF tablet and placebo vaginal gel, oral placebo tablet and TFV vaginal gel, or double placebo. Women took both investigational products once daily and continued genital swabbing and daily diaries for an additional 35 days. Participants completed surveys on the acceptability of the vaginal gel at the midpoint and end of the study.
Laboratory Studies
HSV-2 infection was confirmed with the University of Washington Western blot [17]. DNA was extracted from swabs containing genital secretions and analyzed with real-time quantitative polymerase chain reaction (PCR) assay (TaqMan) specific to HSV glycoprotein B, as previously described [18]. Swabs were considered positive when ≥150 HSV DNA copies/mL were observed [19]. As a proxy for human cellular exposure and therefore as evidence of swab use (A. H. Mujugira, S. Selke, L. Drolette, A. S. Magaret, A. Wald, L. Corey, personal communication), we measured β-globin DNA detection during lead-in and treatment phases from participants randomized to vaginal placebo gel. Comparison of these detection quantities served to evaluate both adherence to sample collection procedures and the sensitivity of detecting HSV DNA after vaginal gel application.
Safety Evaluation
Adverse events were graded using the Adverse Events Table of the National Institute of Allergy and Infectious Diseases [20]. Participant report, physical examination, and laboratory investigation contributed to the safety assessment. Laboratory safety assessments were performed at baseline and after completion of the treatment phase.
Statistical Methods
With a sample size of 22 in each active arm, we had 80% power to detect a 50% reduction in HSV-2 shedding with an α of 0.05, assuming a baseline viral shedding rate of 20% [21]. We included the double-placebo arm to demonstrate stability in shedding rates between lead-in and treatment phases in the absence of active investigational product.
Shedding rate was calculated as the proportion of swabs with detectable HSV DNA among those collected by all participants within each phase in each arm. The asymptomatic shedding rate was the proportion of swabs testing positive for HSV on days during which no lesions were reported. We determined the proportion of lesion-days by self-report in individual participants and overall for each phase in each arm. Shedding episodes were defined as discrete periods of HSV–positive swabs bounded by 2 HSV–negative swabs before and after [22]. Adherence to the study regimen was calculated by recording the fraction of returned pills among those dispensed between study visits and proportion of gel applicators returned unused. Participants also recorded use of study products as part of daily diary entries.
To allow time for therapeutic effect, we excluded swabs collected during the first 7 days of the treatment phase from analysis. Thus, our intent-to-treat analysis included all participants who returned at least 1 swab in the lead-in phase and 1 swab after day 7 in the treatment phase. We performed Poisson generalized linear mixed-effects models with an offset for individual exposure duration to evaluate within-arm differences in shedding rate between the lead-in and treatment phases. The models were run separately for each arm, and no inter-arm comparisons were performed. We evaluated secondary outcomes of asymptomatic shedding, number of shedding episodes, and frequency of lesions, using similar Poisson models. Evaluation of within-arm HSV log10 copies per milliliter quantity change was performed using a linear mixed-effects model, including only HSV–positive samples. We also described the vaginal gel acceptability survey results.
Given the variability of adherence to the study drugs and small sample size, we performed a per-protocol analysis, defined post hoc as a treatment phase of ≥33 days with ≥90% adherence to the active drug (or to the oral tablet, in the double-placebo arm), based on counting of the returned product. We selected these criteria to achieve a presumptive 30-day duration of biologic exposure to the active drug.
RESULTS
Study Population
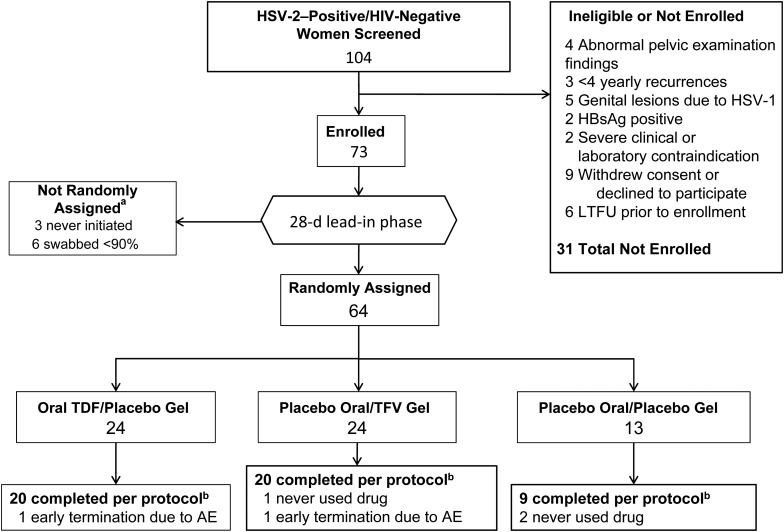
A total of 104 women were screened for this study; 16 did not meet the inclusion criteria and were not enrolled, and 15 declined entry to the study (Figure 1). Seventy-three women began the 4-week lead-in phase, and the 64 women who returned at least 90% of swabs were randomly assigned to one of 3 treatment arms. Most patients were white (37 [58%]) or African American (17 [27%]). The median age was 37.3 years, the median duration of clinical genital herpes was 9.3 years, and the median number of annual clinical recurrences was 5 (Table 1). Of 64 randomly assigned women included in the intent-to-treat analysis, 49 met the per-protocol criteria.
Figure 1.
Flow of subjects through the study. aAll enrolled participants performed twice-daily genital swabbing and kept daily diaries for 28 days during the lead-in phase. Only participants completing ≥90% of swabs during the lead-in phase were randomly assigned at a ratio of 2:2:1 to a treatment arm. bPer protocol is defined as use of study drug for a minimum of 33 days and ≥90% adherence to the regimen, as determined by returned pill or applicator counts. Abbreviations: AE, adverse event; HBsAg, hepatitis B virus surface antigen; HIV, human immunodeficiency virus; HSV-1/2, herpes simplex virus 1/2; LTFU, lost to follow-up; TDF, tenofovir disoproxil fumarate; TFV, tenofovir.
Table 1.
Baseline Characteristics of Herpes Simplex Virus Type 2 (HSV-2)–Seropositive Immunocompetent Women Randomly Assigned to Receive Oral or Vaginal Tenofovir or Double-Placebo to Evaluate the Effect of Treatment on HSV-2
| Characteristic | Oral TDF/Placebo Gel (n = 24) | Oral Placebo/TFV Gel (n = 27) | Double Placebo (n = 13) |
|---|---|---|---|
| Age, y | 39.1 (20.8–50.8) | 36.7 (21.1–50.7) | 41.0 (19.2–48.7) |
| Race | |||
| White | 14 (58.3) | 14 (51.9) | 9 (69.2) |
| Black | 5 (20.8) | 10 (37.0) | 2 (15.4) |
| Asian/Pacific Islander | 1 (4.2) | 0 | 1 (7.7) |
| Other/multiple | 4 (16.7) | 3 (11.1) | 1 (7.7) |
| Education level | |||
| Less than high school | 1 (4.2) | 0 | 1 (7.7) |
| High school graduate | 16 (66.7) | 20 (74.1) | 6 (46.2) |
| College graduate or higher | 7 (29.2) | 7 (25.9) | 6 (46.2) |
| Recurrences in prior year, no. | 5 (2–24) | 4 (0–6a) | 6 (2–12) |
| Time since HSV-2 diagnosis, y | 15.3 (1.4–29.7) | 10.0 (1.1–31.8) | 7.1 (1.0–32.5) |
Data are median (range) or no. (%) of subjects.
Abbreviations: TDF, tenofovir disoproxil fumarate; TFV, tenofovir.
a Two participants had no genital herpes recurrences during the immediate year prior to enrollment while receiving acyclovir but reported >2 annual recurrences prior to initiating HSV suppressive therapy.
The median treatment duration was 35.5 days (range, 13–39 days) in the oral TDF arm, 36 days (range, 13–39 days) in the TFV gel arm, and 35 days (range, 2–37 days) in the double-placebo arm. Median adherence, measured by returned product count, was 97.1% (interquartile range [IQR], 91.0%–100.0%) to the tablet in the oral TDF arm, 97.3% (IQR, 94.3%–100.0%) to the gel in the TFV gel arm, and 97.1% (IQR, 94.4%–100.0%) to both placebo products in that arm.
Effect of Oral TFV on HSV Shedding and Lesions
Overall, 2587 swabs were collected in the oral TDF arm, and HSV DNA was detected at least once in 66.7% of women. The HSV shedding rate was 22.9% during the lead-in phase and 19.5% during oral tenofovir administration (Table 2); the relative risk (RR) was 0.86 (95% confidence interval [CI], .72–1.01 (P = .086; Table 3). The asymptomatic shedding rate decreased from 17.5% during the lead-in phase to 13.7% during treatment (RR, 0.74; 95% CI, .59–.92; P = .010). The median quantity of HSV DNA in positive swabs was 4.02 log10 copies/mL (IQR, 3.21–5.29 log10 copies/mL) in the lead-in phase, with a change of −0.16 log10 copies/mL (95% CI, −.40 to .07 log10 copies/mL; P = .18) in the treatment phase. Women reported genital lesions on 11.8% of days in the lead-in phase and on 11.6% of days during oral tenofovir therapy (Table 2). The number of participants experiencing a decrease in shedding rate appeared to be similar to that experiencing an increase in shedding rate between the lead-in and treatment phases.
Table 2.
Genital Herpes Simplex Virus Type 2 (HSV-2) Shedding and Lesions in Immunocompetent HSV-2–Seropositive Women, by Randomization Arm
| Variable | Oral TDF/Placebo Gel | Oral Placebo/TFV Gel | Double Placebo |
|---|---|---|---|
| Swabs, total no. | 2587 | 2841 | 1223 |
| Shedding ratea | |||
| Lead-in phase | 295/1291 (22.9) | 204/1477 (16.0) | 151/708 (21.3) |
| Treatment phase | 253/1296 (19.5) | 164/1364 (12.0) | 105/515 (20.4) |
| Asymptomatic shedding rateb | |||
| Lead-in phase | 200/1140 (17.5) | 102/1345 (7.6) | 88/611 (14.4) |
| Treatment phase | 157/1145 (13.7) | 104/1266 (8.2) | 53/438 (12.1) |
| Shedding episodes, total no. | |||
| Lead-in phase | 34 | 36 | 27 |
| Treatment phase | 32 | 36 | 16 |
| Log-quantity shed on positive swabs, median (IQR) | |||
| Lead-in phase | 4.02 (3.21–5.29) | 4.47 (2.92–6.24) | 3.71 (2.78–5.54) |
| Treatment phase | 4.11 (3.23–5.39) | 4.40 (3.37–5.13) | 4.22 (3.35–5.45) |
| Lesion ratec | |||
| Lead-in phase | 79/667 (11.8) | 68/784 (8.7) | 50/368 (13.6) |
| Treatment phase | 78/670 (11.6) | 50/709 (7.1) | 39/265 (14.7) |
Abbreviations: TDF, tenofovir disoproxil fumarate; TFV, tenofovir; IQR, interquartile range.
a Data are no. of positive swabs/total no. of swabs collected (%).
b Data are no. of positive swabs on lesion-free days/total no. of swabs collected on lesion-free days (%).
c Data are no. of lesion-positive days/total no. of study days (%).
Table 3.
Intention-to-Treat and Per Protocol Analyses of the Risk of Herpes Simplex Virus (HSV) Shedding and Genital Lesions With Oral Tenofovir Disoproxil Fumarate (TDF) and Vaginal Tenofovir (TFV) Gel
| Variable | Intent-to-Treata |
Per Protocolb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral TDF/Placebo Gel |
Oral Placebo/TFV Gel |
Double Placebo |
Oral TDF/Placebo Gel |
Oral Placebo/TFV Gel |
Double Placebo |
|||||||
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95%CI) | P Value | RR (95%CI) | P Value | RR (95%CI) | P Value | |
| Shedding | 0.86 (.72–1.02) | .086 | 0.94 (.75–1.16) | .54 | 0.90 (.67–1.22) | .47 | 0.74 (.60–.91) | .006 | 0.95 (.74–1.22) | .69 | 0.78 (.57–1.07) | .11 |
| Asymptomatic shedding | 0.74 (.59–.92) | .010 | 1.30 (.96–1.76) | .090 | 0.75 (.49–1.14) | .15 | 0.69 (.54–.89) | .006 | 1.38 (.95–1.99) | .085 | 0.73 (.48–1.11) | .12 |
| Shedding episodes | 0.94 (.56–1.57) | .81 | 1.17 (.71–1.92) | .52 | 0.84 (.41–1.71) | .58 | 0.83 (.47–1.47) | .50 | 1.25 (.71–2.20) | .41 | 0.73 (.33–1.58) | .37 |
| Lesions | 0.98 (.70–1.37) | .90 | 0.80 (.54–1.18) | .25 | 0.95 (.57–1.57) | .82 | 0.75 (.57–.97) | .032 | 0.81 (.61–1.09) | .15 | 0.75 (.51–1.08) | .11 |
| Log10 copies/mL, Mean (95% CI) | P Value | Log10 copies/mL, Mean (95% CI) | P Value | Log10 copies/mL, Mean (95% CI) | P Value | Log10 copies/mL, Mean (95% CI) | P Value | Log10 copies/mL, Mean (95% CI) | P Value | Log10 copies/mL, Mean (95% CI) | P Value | |
| Change in HSV quantityc | −0.16 (−.40 to .07) | .18 | −0.50 (−.86 to −.13) | .008 | 0.16 (−.27 to .60) | .45 | −0.41 (−.70 to −.13) | .003 | −0.60 (−.98 to −.21) | .003 | 0.18 (−.27 to .64) | .42 |
Abbreviations: CI, confidence interval; RR, relative risk.
a Analysis includes all participants randomized to each arm.
b Analysis includes participants with ≥33 days of drug use and ≥90% adherence to active drug or, in the double-placebo arm, the higher of the 2 study products.
c Between the lead-in and treatment periods.
In the per-protocol analysis, the risk for HSV-2 shedding, lesions, asymptomatic shedding, and quantity of shedding were statistically significantly reduced with treatment (Table 3). The RR for shedding was 0.74 (95% CI, .60–.91; P = .006), the RR for asymptomatic shedding was 0.69 (95% CI, .54–.89; P = .006), and the RR for lesions was 0.75 (95% CI, .57–.97; P = .032). The HSV quantity on days when shedding was detected was decreased by 0.41 log10 copies (P = .004).
Effect of Vaginal Tenofovir on HSV Shedding and Lesions
Women in the TFV gel arm collected 2841 swabs, and 63.0% of women had HSV DNA detected at least once. During the lead-in phase, shedding was present on 16.0% of days, compared with 12.0% of days during the treatment phase (RR, 0.94; 95% CI, .75–1.16; P = .54). Similarly, there was no significant change observed in asymptomatic shedding (Table 3). The median HSV DNA load was 4.47 log10 copies/mL (IQR, 2.92–6.24 log10 copies/mL) during the lead-in phase, and use of TFV gel was associated with a change of −0.50 log10 copies/mL (95% CI, −.86 to −.13 log10 copies/mL; P = .008). Women had lesions on 8.7% of days in the lead-in phase and 7.1% of days during gel use. The per-protocol analysis similarly showed no significant decrease with treatment in HSV-2 shedding, asymptomatic shedding, lesions, or number of shedding episodes. However, the quantity of HSV DNA detected changed by −0.60 log10 copies during treatment (95% CI, −.98 to −.21 log10 copies; P = .003; Table 3).
Shedding in the Double-Placebo arm
A total of 1223 swabs were collected in the double-placebo arm, and HSV DNA was detected in 84.6% of women at least once. During the lead-in phase, the shedding rate was 21.3% and was not different in the treatment phase (Tables 2 and 3). The median HSV DNA load on swabs with detectable DNA was 3.71 log10 copies/mL (IQR, 2.78–5.54 log10 copies/mL), with no difference in quantity between the lead-in and treatment phases (Table 2). There were no significant changes in outcomes in either the intent-to-treat or per-protocol analyses in the double-placebo arm (Tables 3).
To evaluate whether the presence of gel interfered with the collection of samples for PCR analysis, we performed a β-globin DNA PCR assay on 68 paired samples from the 34 participants from the 2 arms receiving vaginal placebo gel. β-globin was detected on all swabs, with a mean quantity of 6.56 log10 copies/mL (range, 5.80–7.60 log10 copies/mL) in the lead-in phase and 6.29 log10 copies/mL (range, 4.88–7.22 log10 copies/mL) in the treatment phase. The mean change in DNA load was −0.27 log10 copies/mL (95% CI, −.11 to −.33 log10 copies/mL; P = .001). In vitro, the presence of gel did not interfere with the HSV DNA PCR assay (data not shown).
Adverse Events
Eleven people in the oral TDF arm, 7 in the TFV gel arm, and 3 in the double placebo arm reported 64 total adverse events considered possibly or definitely related to an investigational product; 60 events were grade 1 or 2. Four grade 3 events were reported; no grade 4 or severe adverse events were observed. Two grade 3 events occurred in the same participant, with severe abdominal pain and diarrhea, beginning as moderate pain on the second day of oral TDF use and escalating over 2 weeks. The second participant experienced severe diarrhea, beginning on the third day of oral TDF use. Both participants reported spontaneous resolution of symptoms after 15 and 22 days respectively and did not discontinue product use. Another participant in the oral TDF arm terminated the study drugs on day 13 because of grade 2 diarrhea and abdominal cramping. The last grade 3 event was an increase in the frequency of chronic migraines in a participant randomly assigned to receive TFV gel who had stopped using the gel 8 days earlier because of a urinary tract infection and was only receiving oral placebo at time of migraine onset. These last 2 participants terminated the study early owing to adverse events. The most commonly reported symptoms in the oral TDF arm were diarrhea (16.7%), nausea (12.5%), and abdominal pain (12.5%; Supplementary Table 4). In the TFV gel arm, 11.1% experienced vaginal burning, itching, or discomfort, and 11.1% experienced headache; genital symptoms were reported with similar frequency across all 3 arms. Abnormal values of posttreatment chemistry tests, liver function tests (aspartate transaminase, alanine transaminase, alkaline phosphatase, and total bilirubin levels), and complete blood count were rare and appeared in all 3 arms at similar rates. There were no abnormalities in serum creatinine levels before or after the study in any participant.
Acceptability of Vaginal Gel Product
Sixty-one women completed vaginal gel acceptability questionnaires at the study midpoint, of whom 54 completed a second questionnaire at study completion. Overall, 26.3% of women “liked a lot” or “somewhat liked” the gel product, 30.2% were neutral, and the remainder “somewhat” (23.8%) or “very much” (21.4%) disliked the product. Most women described the gel as easy to use, although less so during menses. Most reported they would use the gel if it were proven to reduce either their own signs and symptoms (69.7%) or risk of transmitting genital herpes (67.4%). However, few women (19.3%) preferred to use the gel if an oral option was equally effective. Women reported that the gel was noticeable to themselves and partners during intercourse, and thus use could not be easily concealed. Most reported that the gel did not change the experience of sexual intercourse, while several women found the lubrication beneficial. Women frequently noted that mess from the gel was problematic as it leaked out from the vagina after application, soiling undergarments and bedclothes. Responses were similar between those who were randomly assigned to TFV or placebo gel arms.
DISCUSSION
We tested the efficacy of oral and vaginal tenofovir to prevent genital HSV-2 shedding and genital lesions in a randomized, controlled trial of immunocompetent women with symptomatic genital herpes. In the intent-to-treat analysis, neither tenofovir preparation reduced HSV shedding or genital lesions. However, among women with especially high adherence, oral TDF but not vaginal TFV reduced HSV shedding, asymptomatic shedding, and lesions by 25%–30%. Both tenofovir products reduced the quantity of DNA shed by half a log. These findings suggest that tenofovir has some biological activity against HSV-2 in vivo, but either the level is insufficient or the potency is too low to affect HSV-2 replication in the genital tract to a clinically meaningful degree.
Our findings are consistent with the known pathophysiology of established HSV-2 infection. Following primary infection, HSV-2 becomes latent in sacral ganglia. Periodic reactivation of latent infection then reaches skin and mucosa via sensory nerve roots, where infection propagates in the epithelial cells, leading to both formation of lesions and viral shedding [23, 24]. It is likely that high levels of an antiviral agent must be delivered to central and peripheral nervous system to achieve sufficient concentrations to prevent reactivation. The 50% inhibitory concentration (IC50) of tenofovir against HSV-2 is 50–100 times greater than for HIV-1 [25–27]. HIV-targeted dosing of oral tenofovir will therefore likely not reach sufficient concentrations in this privileged anatomic site, while increased toxicity precludes higher systemic doses. Furthermore, analysis of genital mucosa samples and cervical lavage in prior studies demonstrated that oral TDF easily achieves inhibitory concentrations for HIV-1 but not HSV-2 and exemplifies why, even with excellent adherence, oral TDF has limited usefulness in preventing lesion formation in established HSV-2 infection [25–27].
Because topical vaginal administration results in 56-fold lower serum concentrations than oral administration, no effect in the peripheral or central nervous system would be expected. However, vaginal TFV gel results in much higher local concentration of the drug within local tissue than achieved with systemic administration [28] and therefore may be better able to inhibit propagation of the infection once it reaches the epithelium. Conversely, and consistent with known pharmacodynamic data, TFV gel appeared to produce minimally larger reductions in the quantity of virus shed relative to oral TDF but failed to reduce the shedding rate, shedding episode frequency, or lesions.
Prior to our study, only 1 study has directly evaluated the effect of oral tenofovir on shedding in persons with established HSV-2 infection. In a cohort study involving 30 HSV-2/HIV–coinfected persons, Tan et al found no difference in HSV shedding in persons receiving antiretroviral therapy with and without TDF in the regimen [29]. However, only persons who were asymptomatic or minimally symptomatic while already receiving TDF or an alternative NRTI were enrolled, thus leaving little room to observe differences in shedding rate. In the iPREX trial, a subgroup analysis of HSV-2–infected, HIV-uninfected men who have sex with men demonstrated a 49% decrease in reported grade ≥2 genital ulcer adverse events among those receiving TDF for PrEP; genital HSV shedding was not assessed in that study [12]. Our finding of a modest benefit of oral TDF on genital HSV shedding and genital lesions demonstrates that there is a biologic effect of tenofovir in established HSV-2 infection, but it is not large enough to affect the clinical course of infection. This small effect is unlikely to reduce the risk of sexual transmission of HSV-2. Conversely, acyclovir and valacyclovir reduce the genital shedding rate by 80%–82% and reduce genital lesions by 86%–87% [6], but reduce HSV-2 transmission to partners by approximately 50% [7].
Additionally, because there was a small decrease in the quantity of β-globin detected in the treatment phase, there may have been a decrease in the sensitivity of detecting HSV DNA in the treatment phase, even in participants randomly assigned to the placebo gel arm. This may explain why we found a trend toward decreased shedding in the double-placebo arm and may have exaggerated the already small effect of the intervention. Future studies involving use of vaginal gels should consider use of placebo gel during the lead-in phase, to exclude this possibility.
Limitations of our study include its small size and limited person-time to detect differences in genital lesion recurrences. Additionally, we used proxy measures of adherence to the study regimen, rather than a biologic measure of exposure, such as tenofovir levels in serum or vaginal fluid. However, given excellent swabbing adherence, it is unlikely that our highly motivated study participants had significant drug adherence issues; we believe it unlikely that a participant would obtain swabs twice daily but not use study product. The expected high proportion of women returned swabs with HSV DNA present; and swab numbers and pill and gel applicator counts were well correlated. Further evidence of consistent swabbing is evident by the fact that all randomly sampled swabs in this study contained a high quantity of human DNA, as measured by β-globin PCR. Strengths of this study include the use of rigorous and well-documented methods for measurement of genital HSV shedding.
This is the first study to evaluate the effect of oral and vaginal tenofovir on viral shedding and lesion-days in HIV-seronegative women with genital HSV-2 infection. Despite their potential to achieve high local tissue concentrations and prevent HSV acquisition, neither oral nor vaginal tenofovir inhibited HSV-2 reactivation events in HSV-2–infected persons. However, oral and vaginal tenofovir products remain promising for prevention of HSV-2 and HIV-1 acquisition in persons who are seronegative for both pathogens [30, 31]. Persons who are infected with HSV-2 and uninfected with HIV should consider a tenofovir product to prevent HIV acquisition, together with valacyclovir or acyclovir to prevent HSV-2 recurrences and reduce transmission risk.
In summary, our study showed no effect of vaginal TFV and a minimal effect of oral TDF on established HSV-2 infection in HIV-1–seronegative women. Additionally, few women in this setting were enthusiastic about using a vaginal gel to decrease genital herpes symptoms or transmission unless it was proven to be more efficacious than a pill.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and staff at the Virology Research Clinic at University of Washington.
Financial support. This work was supported by Gilead Sciences and CONRAD.
Potential conflicts of interest. A. W. received grants from the National Institutes of Health (NIH), Agenus, Genentech, Genocea, Gilead, and Vical during this study; received royalties from UptoDate; and has been a consultant for Aicuris, Eisai, and Amgen. C. J. received grants from Gilead, Aicuris, Agenus, Genocea, and Vical and royalties from UptoDate. J. M. received royalties from UptoDate and grants from the NIH. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 3.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 2002; 185:45–52. [DOI] [PubMed] [Google Scholar]
- 4.Serwadda D, Gray RH, Sewankambo NK et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis 2003; 188:1492–7. [DOI] [PubMed] [Google Scholar]
- 5.Hobson A, Wald A, Wright N, Corey L. Evaluation of a quantitative competitive PCR assay for measuring of HSV DNA in genital tract secretions. J Clin Microbiol 1997; 35:548–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Wald A, Krantz E et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis 2004; 190:1374–81. [DOI] [PubMed] [Google Scholar]
- 7.Corey L, Wald A, Patel R et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350:11–20. [DOI] [PubMed] [Google Scholar]
- 8.Abdool Karim Q, Abdool Karim SS, Frohlich JA et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim SSA. Results of the CAPRISA 004 trial of tenofovir gel. Presented at: XVIII International AIDS Conference, Vienna, Austria, 18–23 July 2010. [Google Scholar]
- 10.Marrazzo J, Rabe L, Kelly C et al. Association of tenofovir (TFV) detection with reduced risk of herpes simplex virus type-2 (HSV-2) acquisition in the VOICE (MTN 003) study. AIDS Res Hum Retroviruses 2014; 30:A31. [Google Scholar]
- 11.Celum C, Morrow RA, Donnell D et al. Daily oral tenofovir and emtricitabine-tenofovir preexposure prophylaxis reduces herpes simplex virus type 2 acquisition among heterosexual HIV-1-uninfected men and women: a subgroup analysis of a randomized trial. Ann Intern Med 2014; 161:11–9. [DOI] [PubMed] [Google Scholar]
- 12.Marcus JL, Glidden DV, McMahan V et al. Daily oral emtricitabine/tenofovir preexposure prophylaxis and herpes simplex virus type 2 among men who have sex with men. PLoS One 2014; 9:e91513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrazzo J, Ramjee G, Nair G et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN 003). Presented at: 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, USA, 3–6 April 2013. [Google Scholar]
- 14.Schwartz JL, Rountree W, Kashuba AD et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One 2011; 6:e25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston C, Saracino M, Kuntz S et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 2012; 379:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wald A, Zeh JE, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996; 124:8–15. [DOI] [PubMed] [Google Scholar]
- 17.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (Immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988; 26:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 2003; 188:1345–51. [DOI] [PubMed] [Google Scholar]
- 19.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol 2007; 45:1618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institutes of Health. Divison of AIDS table for grading the severity of adult and pediatric adverse events.
- 21.Tronstein E, Johnston C, Huang ML et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011; 305:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mark KE, Wald A, Magaret AS et al. Rapidly cleared episodes of oral and anogenital herpes simplex virus shedding in HIV-infected adults. J Acquir Immune Defic Syndr 2010; 54:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffer JT, Abu-Raddad L, Mark KE et al. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med 2009; 1:7ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffer JT, Swan D, Al Sallaq R et al. Rapid localized spread and immunologic containment define Herpes simplex virus-2 reactivation in the human genital tract. eLife 2013; 2:e00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrei G, Lisco A, Vanpouille C et al. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe 2011; 10:379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold BC, Dezzutti CS, Richardson BA et al. Antiviral activity of genital tract secretions after oral or topical tenofovir pre-exposure prophylaxis for HIV-1. J Acquir Immune Defic Syndr 2014; 66:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nixon B, Jandl T, Teller RS et al. Vaginally delivered tenofovir disoproxil fumarate provides greater protection than tenofovir against genital herpes in a murine model of efficacy and safety. Antimicrob Agents Chemother 2014; 58:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendrix CW, Chen BA, Guddera V et al. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan DH, Kaul R, Raboud JM, Walmsley SL. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS 2011; 25:207–10. [DOI] [PubMed] [Google Scholar]
- 30.Mesquita PM, Rastogi R, Segarra TJ et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother 2012; 67:1730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar GN, Alt C. Prophylactic treatment with a novel bioadhesive gel formulation containing aciclovir and tenofovir protects from HSV-2 infection. J Antimicrob Chemother 2014; 69:3282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.