Abstract
Background. We evaluated the immunogenicity of trivalent inactivated influenza vaccine (IIV3) in pregnant women with and those without human immunodeficiency virus (HIV) infection and the persistence of hemagglutination-inhibiting antibodies in mothers and infants.
Methods. Antibodies were measured before vaccination, 1 month after vaccination, at delivery, and at postpartum week 24 in mothers and within 1 week of birth and at 8, 16, and 24 weeks of age in infants.
Results. We enrolled 98 HIV-uninfected and 100 HIV-infected pregnant women, including 93% with a CD4+ T-cell count of ≥200 cells/µL. Compared with HIV-uninfected women, HIV-infected women had lower seroconversion rates (ranging from 63%–92% vs 36%–40%), lower antibody titers through postpartum week 24, and overlapping antibody half-lives (ranging from 106–121 vs 87–153 days). Infant titers were lower than the maternal titers within 1 week of delivery, regardless of vaccine strain and HIV exposure status. Compared with HIV-unexposed infants, HIV-exposed infants had a similar transplacental influenza virus antibody transfer ratio, lower titers, and a lower frequency of titers ≥1:40 (ranging from 82%–95% vs 43%–79%) at birth and higher antibody half-lives (ranging from 43–45 vs 56–65 days).
Conclusions. Compared with HIV-uninfected pregnant women, HIV-infected pregnant women had lower antibody responses and persistence. Compared with HIV-unexposed infants, HIV-exposed infants had lower antibody levels at birth but similar antibody levels after 8 weeks of life. Early IIV3 administration during pregnancy did not decrease antibody titers among infants at birth.
Keywords: influenza vaccine, HIV, immunogenicity, pregnancy, transplacental antibody transfer
Pregnant women, young infants, elderly persons, and people with certain medical conditions, such as human immunodeficiency virus (HIV) infection, have increased incidence and morbidity of influenza [1–4]. Annual influenza vaccination is recommended for these groups, except for infants <6 months of age [5]. We recently reported that immunization of HIV-uninfected and HIV-infected pregnant women with trivalent inactivated influenza vaccine (IIV3) was safe and immunogenic and partially protected the women and their HIV-unexposed infants up until 6-months of age against laboratory-confirmed influenza [6]. The most likely mechanism of protection of the infants is transplacental transfer of maternal antibodies [7]. Thus, vaccination during pregnancy increases the level of maternal hemagglutination-inhibiting (HAI) antibodies, which are subsequently transferred to the fetus and confer passive immunity [6, 8–12].
The immunosuppressive effect of HIV infection may further compound the pregnancy-associated changes, resulting in higher influenza-related complications and attenuation of immune responses to vaccines [13–15]. During the 2009 pandemic due to influenza A(H1N1) (A[H1N1]pdm09), pregnant women accounted for 56% of all A(H1N1)pdm09-associated deaths in South Africa, among whom the prevalence of HIV infection was 71%, compared with 33% among women attending antenatal care [16]. The immunogenicity of influenza vaccines in HIV-infected adults generally correlates with CD4+ T-cell count and inversely correlates with HIV load [17]. However, even with the use of antiretroviral therapy (ART), HIV-infected individuals have poor immune responses to influenza vaccination [18, 19]. There are very few studies on the immunogenicity of influenza vaccines in HIV-infected pregnant women and only 1 head-to-head comparison between HIV-infected and HIV-uninfected pregnant women [18].
The aim of this study was to describe and compare the immunogenicity of IIV3 administered during pregnancy between HIV-uninfected and HIV-infected women and to evaluate the persistence of HAI antibodies in their infants until 24 weeks of age. This analysis reports on the coprimary objective of a previously published study of the safety, immunogenicity, and efficacy of IIV3 in women-infant dyads (clinical trial registration NCT01306682, NCT01306669).
SUBJECTS AND METHODS
Study Design
The 2 randomized, double-blinded, placebo-controlled trials of IIV3 in HIV-uninfected and HIV-infected pregnant women are described elsewhere [6]. In brief, pregnant women with a documented HIV infection status were randomized (1:1) to receive IIV3 or placebo in 2 parallel cohort studies. Enrollment occurred between 3 March and 2 June 2011. Women and their infants were followed up to postpartum week 24.
Vaccine
The study used the influenza vaccine recommended by the World Health Organization for the southern hemisphere in 2011 (A/California/7/2009 [A{H1N1}pdm09], A/Victoria/210/2009 [A{H3N2}], and B/Brisbane/60/2008-like virus [B/Victoria lineage]; VAXIGRIP; Sanofi-Pasteur, Lyon, France) [20, 21].
Immunogenicity
Maternal blood specimens were collected immediately before vaccination, 1 month (28–35 days) after vaccination, within 7 days of delivery, and at postpartum week 24 (168–175 days). Among infants, blood specimens were obtained within 7 days of birth, at 8 weeks (56–63 days) of age, at 16 weeks (111–119 days) of age, and at 24 weeks (168–175 days) of age; HAI titers were measured as previously described [6, 22]. HAI titers of <1:10 were considered to be seronegative, and titers of ≥1:40 were considered to be seroprotective. Seroconversion was defined as a ≥4-fold increase in HAI titer from before to after IIV3 receipt, with an HAI titer of ≥1:40 after IIV3 receipt. The results for the first 2 maternal time points and the birth titers among infants have been previously described [6], but no comparison of IIV3 immunogenicity between the HIV-uninfected and HIV-infected cohorts was previously undertaken. In this analysis, we compare the immunogenicity end points of the 2 cohorts who received IIV3 and were enrolled in parallel during 2011, and we analyze the effect of maternal HIV infection on the kinetics of HAI antibodies in women and their infants until postpartum week 24.
Statistical Analysis
The sample size was based on detecting a ≥20% difference, with 80% power and an α of < 0.05, in the proportion of HIV-exposed infants with HAI titers of ≥1:40 at birth to individual IIV3 strains, compared with an anticipated proportion of 75% among infants of HIV-uninfected women having such titers. This required a sample size of 180 women (1:1), which allowed for up to 10% nonevaluable subjects. The targeted sample size provided 90% power to detect a ≥67% difference in the coprimary end point of differences in seroconversion rates between HIV-infected women who received IIV3 and those who received placebo. Included in the analysis were infants with blood specimens collected within the protocol-defined periods and who were born ≥28 days after their mothers had been vaccinated and were delivered at term (ie, those with a gestational age of ≥37 weeks at birth or a birth weight of ≥2500 g), unless otherwise stated. Participants who had a PCR-confirmed influenza episode or serological evidence of influenza virus infection (defined as a ≥4-fold increase in HAI titers at delivery or postpartum week 24, compared with titers in the previous specimen, for mothers, and as a ≥4-fold titer increase between birth and any of the subsequent visits, for infants) during the follow-up period were excluded from the analyses of the putative strain after the diagnosis of infection. Supplementary Figures 1 and 2 show the number of participants who contributed data at each visit.
Proportions were compared by χ2 or Fisher exact tests, and demographic continuous variables were compared by the Student t test or Mann–Whitney U test. Logistic and linear regression analyses were performed to assess predictors of seroconversion, HAI titers in the infants, and transplacental antibody transfer. Geometric mean titers (GMTs), transplacental transfer, and the corresponding 95% confidence intervals (CIs) were estimated using logarithmic transformation and compared between cohorts by using the Student t test. Comparisons between cohorts were adjusted for baseline characteristics that were significantly different. A P value of <.05 was considered statistically significant.
On the assumption that antibody concentrations (Ab) follow an exponential decay model (in which Ab = Ab0e−βt), where Ab0 is defined as the antibody concentration at time (t) 0, then the antibody decay half-life is calculated as –ln(2)/β. To obtain estimates for β, a linear mixed model was fit to the natural log of the maternal and infant antibody concentrations, with participant as a random effect. The half-life was calculated using the above relationship and the estimated coefficient for time from 1 month after IIV3 receipt (mothers) or time since birth (infants); models were adjusted for Ab0.
Data were collected and managed using Research Electronic Data Capture (REDCap) [23]. Analyses were performed using STATA, version 12.1 (College Station, Texas), and R (R Foundation for Statistical Computing; Vienna, Austria).
Ethical Considerations
Studies were approved by the Human Research Ethics Committee of the University of the Witwatersrand (101 106 and 101 107) and conducted in accordance with good clinical practice guidelines. Mothers provided written informed consent for themselves and their infants.
RESULTS
Study Population
The baseline characteristics of the 98 HIV-uninfected and 100 HIV-infected pregnant women were similar, except that a higher percentage of HIV-uninfected women (30.6% vs 18.2%; P = .042) were primigravidae (Table 1). Sixty-nine HIV-unexposed and 76 HIV-exposed infants born to IIV3 recipients fulfilled the per-protocol definitions and had at least 1 blood specimen collected within the protocol-defined periods. Babies were born at a mean of 81 days (range, 28–175 days) after their mothers’ vaccination. Of the 76 HIV-exposed infants, 2 died and 1 was lost to follow-up before an HIV test was performed, 7 did not undergo HIV-specific PCR, and 66 had nonreactive results of HIV-specific PCR.
Table 1.
Characteristics of Mothers Who Received Seasonal Trivalent Influenza Vaccine (IIV3), by Human Immunodeficiency Virus (HIV) Status, and Their Infants
| Characteristica | HIV-Uninfected Cohort |
HIV-Infected Cohort |
||
|---|---|---|---|---|
| Subjects, No. | Value | Subjects, No. | Value | |
| Mothers | ||||
| Age, y | 98 | 26.4 ± 5.2 | 100 | 27.2 ± 4.9 |
| Body mass indexb | 57 | 29.1 ± 4.6 | 81 | 29.2 ± 5.0 |
| Gestational age, wk | 98 | 26.8 ± 4.6 | 100 | 27.6 ± 3.9 |
| Primigravidc | 98 | 30 (30.6) | 99 | 18 (18.2) |
| Time after vaccination, d | ||||
| Of first postvaccination immunogenicity visitd | 60 | 30.6 ± 2.2 | 70 | 30.1 ± 2.0 |
| Of delivery immunogenicity visitd | 43 | 87.9 ± 37.6 | 61 | 78.7 ± 32.0 |
| Of postpartum wk 24 immunogenicity visitd | 50 | 248.6 ± 40.2 | 60 | 243.9 ± 32.1 |
| CD4+ T-cell count | ||||
| <200 cells/µL | NA | NA | 99 | 7 (7.1) |
| 200–350 cells/µL | NA | NA | 99 | 29 (29.3) |
| 351–500 cells/µL | NA | NA | 99 | 33 (33.3) |
| >500 cells/µL | NA | NA | 99 | 30 (30.3) |
| HIV load ≤40 copies/mL | NA | NA | 98 | 18 (18.4) |
| Receiving antiretroviral therapye | NA | NA | 100 | 80 (80.0) |
| Infants | ||||
| Births <370/7 | 69 | 1 (1.5) | 76 | 5 (6.6) |
| Birth weight, kg | 69 | 3.2 (2.3–4.0) | 75 | 3.0 (2.1–4.3) |
| Low birth weight | 69 | 2 (2.9) | 76 | 3 (4.0) |
| Time between vaccination and birth, d | 69 | 84.4 ± 36.6 | 76 | 77.5 ± 30.2 |
| Age, d | ||||
| At first infant antibody measurementd | 39 | 5 (3–7) | 56 | 5 (3–7) |
| At 8-wk antibody measurementd | 50 | 56 (56–63) | 56 | 57 (56–63) |
| At 16-wk antibody measurementc | 49 | 112 (112–119) | 57 | 113 (112–119) |
| At 24-wk antibody measurementd | 44 | 169 (168–175) | 53 | 169 (168–175) |
Data are mean ± SD, no. (%) of women, or median (range).
Abbreviation: NA, not applicable.
a Data for mothers were recorded at the time of IIV3 receipt, unless otherwise indicated.
b Body mass index is defined as the weight in kilograms divided by the height in meters squared.
c P = .042.
d Only participants who had their schedule visits within the study periods were included in the immunogenicity analyses.
e Includes participants receiving antiretroviral therapy for prevention of mother-to-child HIV transmission and those receiving highly active antiretroviral treatment.
IIV3 Immunogenicity in Pregnant Women
At baseline, HIV-uninfected women had higher A(H1N1)pdm09 and A(H3N2) GMTs than HIV-infected women and similar B/Victoria GMTs (Figure 1). One month after IIV3 receipt, a higher percentage of HIV-uninfected women than HIV-infected women seroconverted to each of the vaccine strains (70.0% vs 40.3% for A[H1N1]pdm09 [P = .001], 63.3% vs 35.7% for A[H3N2] [P = .002], and 91.7% vs 40.0% for B/Victoria [P < .001]), ≥1 strain (96.7% vs 58.6%; P < .001), at least 2 strains (81.7% vs 37.1%; P < .001), and 3 strains (46.7% vs 18.6%; P = .001). The proportions of HIV-uninfected and HIV-infected women who achieved HAI titers of ≥1:40 were 96.7% versus 67.2% for A(H1N1)pdm09, 85.0% versus 48.6% for A(H3N2), and 98.3% versus 65.7% for B/Victoria (P < .001 for all comparisons). Comparison of immune responses did not change after adjustment for having been pregnant previously.
Figure 1.
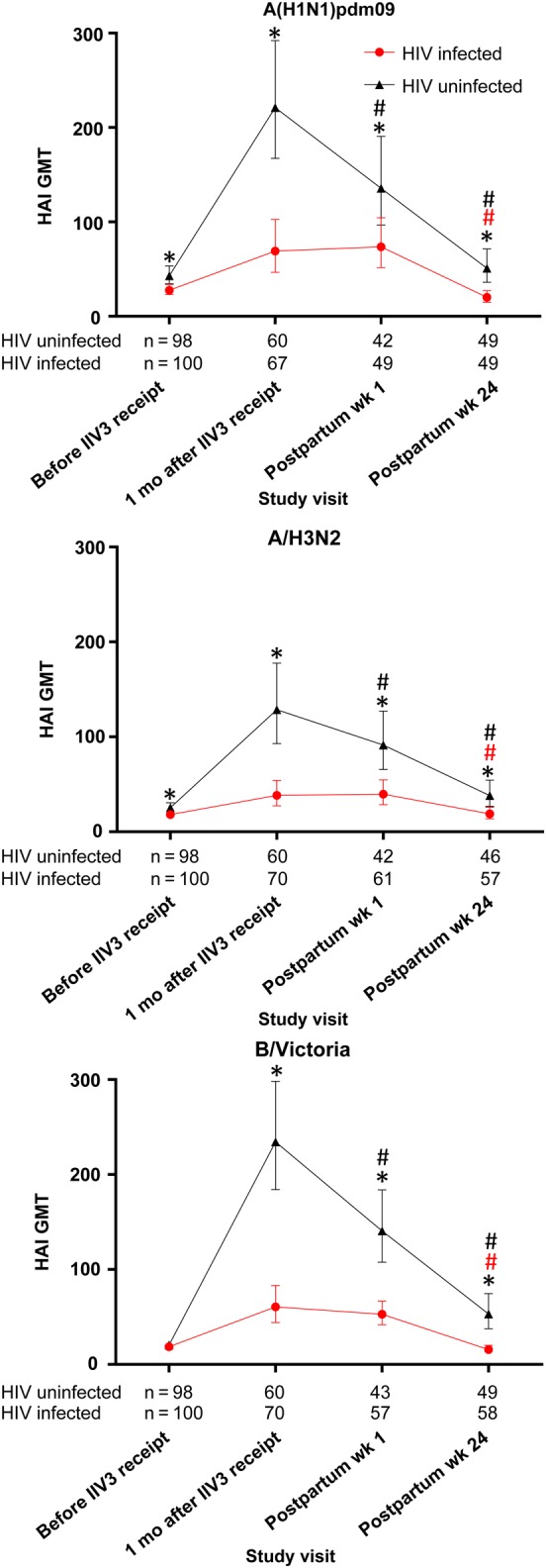
Geometric mean titers (GMT) of hemagglutination-inhibiting antibodies (HAIs) in pregnant women who received trivalent inactivated influenza vaccine (IIV3) during pregnancy. GMTs were measured before IIV3 receipt, 1 month following IIV3 receipt, within 1 week of delivery, and at postpartum week 24. *P < .05 for the comparison between human immunodeficiency virus (HIV)-infected and HIV-uninfected mothers; #P < .05 for the comparison with the visit 1 month after IIV3 receipt. Vertical bars indicate 95% confidence intervals.
HIV-uninfected women who seroconverted to A(H1N1)pdm09 had lower baseline GMTs (30.7; 95% CI, 24.7–38.2) than women who did not seroconvert (77.0; 95% CI, 39.7–149.4; P = .006), with a similar trend observed for the other strains. In HIV-uninfected women, gravidity (primigravid vs multigravid), age (<25 vs ≥25 years), body mass index, and gestational age at vaccination had no effect on seroconversion rates (Table 2).
Table 2.
Predictors of Seroconversion Against Influenza Virus Strains Among Pregnant Women 1 Month After Receipt of Trivalent Inactivated Influenza Vaccine (IIV3)
| Predictor | Nonseroconverters | Seroconverters | OR (95% CI)a | P Value |
|---|---|---|---|---|
| HIV-uninfected women | ||||
| HAI titer at vaccination, by strain | ||||
| A(H1N1)pdm09 | 77.0 (39.7–149.4) | 30.7 (24.7–38.2) | 0.09 (.015–.50) | .006 |
| A(H3N2) | 33.1 (20.8, 52.8) | 20.7 (16.0, 26.9) | 0.26 (.06, 1.07) | .1 |
| B/Victoria | 30.3 (14.0, 65.5) | 19.7 (17.3, 22.5) | 0.02 (.0002, 1.75) | .1 |
| Age at vaccination, by strain | ||||
| A(H1N1)pdm09 | ||||
| <25 y | 11/18 (61.1) | 20/42 (47.6) | Reference | |
| ≥25 y | 7/18 (38.9) | 22/42 (52.4) | 1.73 (.56, 5.32) | .3 |
| A(H3N2) | ||||
| <25 y | 12/22 (54.6) | 19/38 (50.0) | Reference | |
| ≥25 y | 10/22 (45.5) | 19/38 (50.0) | 1.20 (.42, 3.44) | .7 |
| B/Victoria | ||||
| <25 y | 4/5 (80.0) | 27/55 (49.1) | Reference | |
| ≥25 y | 1/5 (20.0) | 28/55 (50.9) | 4.15 (.44, 39.52) | .2 |
| ≥1 strain | ||||
| <25 y | 2/2 (100) | 29/68 (50.0) | Reference | |
| ≥25 y | 0/2 (0) | 29/68 (50.0) | 1 | |
| ≥2 strains | ||||
| <25 y | 8/11 (72.7) | 23/49 (46.9) | Reference | |
| ≥25 y | 3/11 (27.3) | 26/49 (53.1) | 3.01 (.71, 12.73) | .1 |
| 3 strains | ||||
| <25 y | 17/32 (53.1) | 14/28 (50.0) | Reference | |
| ≥25 y | 15/32 (46.9) | 14/28 (50.0) | 1.13 (.41, 3.13) | .8 |
| HIV-infected women | ||||
| HAI titer at vaccination, by strain | ||||
| A(H1N1)pdm09 | 23.8 (16.4, 34.5) | 30.9 (24.0, 39.9) | 1.89 (.59, 6.08) | .3 |
| A(H3N2) | 13.6 (10.7, 17.3) | 21.7 (16.3, 29.0) | 5.97 (1.28, 27.79) | .023 |
| B/Victoria | 18.4 (15.5, 21.9) | 19.0 (16.2, 22.4) | 1.36 (.15, 12.39) | .8 |
| Age at vaccination, by strain | ||||
| A(H1N1)pdm09 | ||||
| <25 y | 11/40 (27.5) | 15/27 (55.6) | Reference | |
| ≥25 y | 29/40 (72.5) | 12/27 (44.4) | 0.30 (.11, .85) | .023 |
| A(H3N2) | ||||
| <25 y | 14/45 (31.1) | 12/25 (48.0) | Reference | |
| ≥25 y | 31/45 (68.9) | 13/25 (52.0) | 0.49 (.18, 1.34) | .2 |
| B/Victoria | ||||
| <25 y | 13/42 (31.0) | 13/28 (46.4) | Reference | |
| ≥25 y | 29/42 (69.1) | 15/28 (53.6) | 0.52 (.19, 1.39) | .2 |
| ≥1 strain | ||||
| <25 y | 6/29 (20.7) | 20/41 (48.8) | Reference | |
| ≥25 y | 23/29 (79.3) | 21/41 (51.2) | 0.27 (.09, .81) | .02 |
| ≥2 strains | ||||
| <25 y | 11/44 (25.0) | 15/26 (57.7) | Reference | |
| ≥25 y | 33/44 (75.0) | 11/26 (42.3) | 0.24 (.09, .69) | .008 |
| 3 strains | ||||
| <25 y | 21/57 (36.8) | 5/13 (38.5) | Reference | |
| ≥25 y | 36/57 (63.2) | 8/13 (61.5) | 0.93 (.27, 3.23) | .9 |
| CD4+ T-cell count at vaccination, by strain | ||||
| A(H1N1)pdm09 | ||||
| <350 cells/µL | 21/39 (53.9) | 8/27 (29.6) | Reference | |
| ≥350 cells/µL | 18/39 (46.2) | 19/27 (70.4) | 2.77 (.98, 7.83) | .05 |
| A(H3N2) | ||||
| <350 cells/µL | 21/44 (47.7) | 8/25 (32.0) | Reference | |
| ≥350 cells/µL | 23/44 (52.3) | 17/25 (68.0) | 1.94 (.69, 5.42) | .2 |
| B/Victoria | ||||
| <350 cells/µL | 20/41 (48.8) | 9/28 (21.1) | Reference | |
| ≥350 cells/µL | 21/41 (51.2) | 19/28 (67.9) | 2.01 (.74, 5.48) | .2 |
| ≥1 strain | ||||
| <350 cells/µL | 16/28 (57.1) | 13/41 (31.7) | Reference | |
| ≥350 cells/µL | 12/28 (42.9) | 28/41 (68.3) | 2.87 (1.06, 7.78) | .038 |
| ≥2 strains | ||||
| <350 cells/µL | 21/43 (48.8) | 8/26 (30.8) | Reference | |
| ≥350 cells/µL | 22/43 (51.2) | 18/26 (69.2) | 2.15 (.77, 5.99) | .1 |
| 3 strains | ||||
| <350 cells/µL | 25/56 (44.6) | 4/13 (30.8) | Reference | |
| ≥350 cells/µL | 31/56 (55.4) | 9/13 (69.2) | 1.81 (.50, 6.49) | .4 |
Data are geometric mean titer (95% confidence interval) or proportion of subjects (%). Seroconversion was defined as a ≥4-fold increase in hemagglutination-inhibiting antibody titers from baseline to 1 month after vaccination and a titer of ≥1:40 after vaccination. Other variables analyzed that were not significantly associated with seroconversion 1 month after IIV3 administration during pregnancy included maternal body mass index, gestational age at vaccination, primigravid versus multigravid status, and, in HIV-infected women, CD4+ T-cell count as a continuous variable, plasma HIV load, and use of antiretroviral therapy at the time of vaccination.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
a Data were calculated by regression analysis. An OR of >1 corresponds to an increased rate of seroprotection, and an OR of <1 corresponds to a decreased rate of seroprotection in comparison to the reference value.
HIV-infected women who seroconverted to A(H3N2) had higher baseline GMTs (21.7; 95% CI, 16.3–29.0) than women who did not seroconvert (13.6; 95% CI, 10.7–17.3; P = .023). Furthermore, HIV-infected women aged <25 years had higher seroconversion rates to ≥1 or ≥2 strains and to A(H1N1)pdm09, compared with women aged ≥25 years. Women with a CD4+ T-cell count of >350 cells/µL also had higher seroconversion rates to ≥1 strain. HIV load, ART use at the time of vaccination, gravidity, body mass index, and gestational age at vaccination did not affect seroconversion rates in HIV-infected women (Table 2). After stratification of women into a group receiving ART at baseline, a group that started ART after IIV3 receipt, and a group that never initiated ART, analysis revealed that GMTs at delivery and at postpartum week 24 did not differ between these 3 groups. Furthermore, HIV load decreased from baseline to after delivery within these 3 groups, although the difference was significant only among the women who were receiving ART at enrollment (median, 651 copies/mL [interquartile range, 51–12 469 copies/mL] vs 165 copies/mL [interquartile range, 54–1655 copies/mL]; P = .007).
Influenza Virus Antibody Kinetics After IIV3 Receipt
The HAI antibody titers in HIV-uninfected women decreased from 1 month after IIV3 receipt to postpartum week 24 (mean, 248.6 days after IIV3 receipt). However, GMTs at postpartum week 24 were still higher than those before IIV3 receipt for A(H3N2) (38.2 vs 25.4; P = .027) and B/Victoria (53.1 vs 20.3; P < .001; Figure 1).
HIV-infected women had lower HAI GMTs to all vaccine strains at all visits, compared with HIV-uninfected women, except for B/Victoria at baseline (Figure 1). Among HIV-infected women at postpartum week 24 (mean, 243.9 days after IIV3 receipt), GMTs returned to levels before IIV3 receipt for all strains (Figure 1). At postpartum week 24, the percentage of HIV-uninfected and HIV-infected women who retained HAI titers of ≥1:40 were 71.4% versus 44.9% for A(H1N1)pdm09, 69.6% versus 31.6% for A(H3N2), and 75.5% versus 25.9% for B/Victoria (P < .001 for all comparisons).
The half-life of antibody titers to A(H1N1)pdm09 was longer in HIV-uninfected (mean, 121 days; 95% CI, 100–141), compared with HIV-infected women (87 days; 95% CI, 76–98; P = .002). Titers to A(H3N2) declined, however, more quickly in HIV-uninfected women, compared with HIV-infected women (half-life, 118 days [95% CI, 97–138] vs 153 days [95% CI, 123–184]; P = .045; Table 3 and Supplementary Figure 3). After adjustment for titers at 1 month after IIV3 receipt, differences in antibody decay remained.
Table 3.
Half-life of Hemagglutination-Inhibiting Antibody (HAI) Titers to 3 Influenza Virus Strains Among Mothers Who Received Trivalent Inactivated Influenza Vaccine (IIV3) and Their Infants, by Human Immunodeficiency Virus (HIV) Status
| Population, Strain | Unadjusted Half-life, d (95% CI) |
Adjusted Half-life, d (95% CI)a |
||||
|---|---|---|---|---|---|---|
| HIV-Uninfected Cohort (n=70) | HIV-Infected Cohort (n=83) | P Value | HIV-Uninfected Cohort (n=70) | HIV-Infected Cohort (n=83) | P Value | |
| Mothersb | ||||||
| A(H1N1)pdm09 | 120.6 (100.0–141.2) | 86.8 (76.0–97.6) | .002 | 121.5 (100.8–142.2) | 89.6 (78.2–100.9) | .004 |
| A(H3N2) | 117.5 (97.2, 137.8) | 153.4 (123.3, 183.5) | .045 | 119.4 (98.9, 140.0) | 160.2 (127.9, 192.5) | .029 |
| B/Victoria | 105.8 (89.8, 121.8) | 98.6 (85.6, 111.6) | .5 | 106.7 (90.8, 122.7) | 101.7 (88.1, 115.2) | .6 |
| Infantsc | HIV-Unexposed Cohort (n=59) | HIV-Exposed Cohort (n=73) | HIV-Unexposed Cohort (n=59) | HIV-Exposed Cohort (n=73) | ||
| A(H1N1)pdm09 | 44.6 (40.6, 48.6) | 56.2 (50.5, 61.8) | .001 | 46.1 (41.9, 50.3) | 56.4 (50.8, 62.1) | .004 |
| A(H3N2) | 43.6 (39.7, 47.5) | 64.9 (57.5, 72.3) | <.001 | 44.4 (40.4, 48.3) | 65.1 (57.7, 72.5) | <.001 |
| B/Victoria | 42.8 (39.0, 46.6) | 63.3 (55.8, 70.7) | <.001 | 43.9 (40.0, 47.8) | 63.7 (56.2, 71.1) | <.001 |
Abbreviation: CI, confidence interval.
a Data were adjusted for initial HAI titers.
b All samples collected from 28 days after IIV3 receipt to postpartum day 175 were included even if collected outside of the other protocol-defined study periods.
c All samples collected from birth to 175 days of age were included even if collected outside of the other protocol-defined study periods.
Transplacental Antibody Transfer in HIV-Exposed and HIV-Unexposed Infants
Infants GMTs at birth were consistently lower than the GMTs in the mothers. Infant GMTs correlated with maternal GMTs at delivery in both HIV-uninfected women (rho = 0.890 for A[H1N1]pdm09, 0.639 for A[H3N2], and 0.663 for B/Victoria; P < .001 for all comparisons) and HIV-infected women (rho =0.816 for A[H1N1]pdm09, 0.823 for A[H3N2], and 0.653 for B/Victoria; P < .001 for all comparisons). The infant/maternal antibody ratios were similar in the 2 cohorts, except for the ratio for A(H1N1)pdm09, which was higher in the HIV-uninfected cohort (0.90 [95% CI, .74–1.08] vs 0.66 [95% CI, .54–.82]; P = .046). Ratios in the HIV-uninfected and HIV-infected cohorts for A(H3N2) were 0.82 (95% CI, .60–1.11) and 0.92 (95% CI, .76–1.11), respectively (P = .5), and for B/Victoria 0.96 (95% CI, .77–1.21) and 0.96 (95% CI, .80–1.17), respectively (P = .9). Significantly higher A(H1N1)pdm09 transplacental antibody transfer in the HIV-uninfected cohort was only detected in primigravidae.
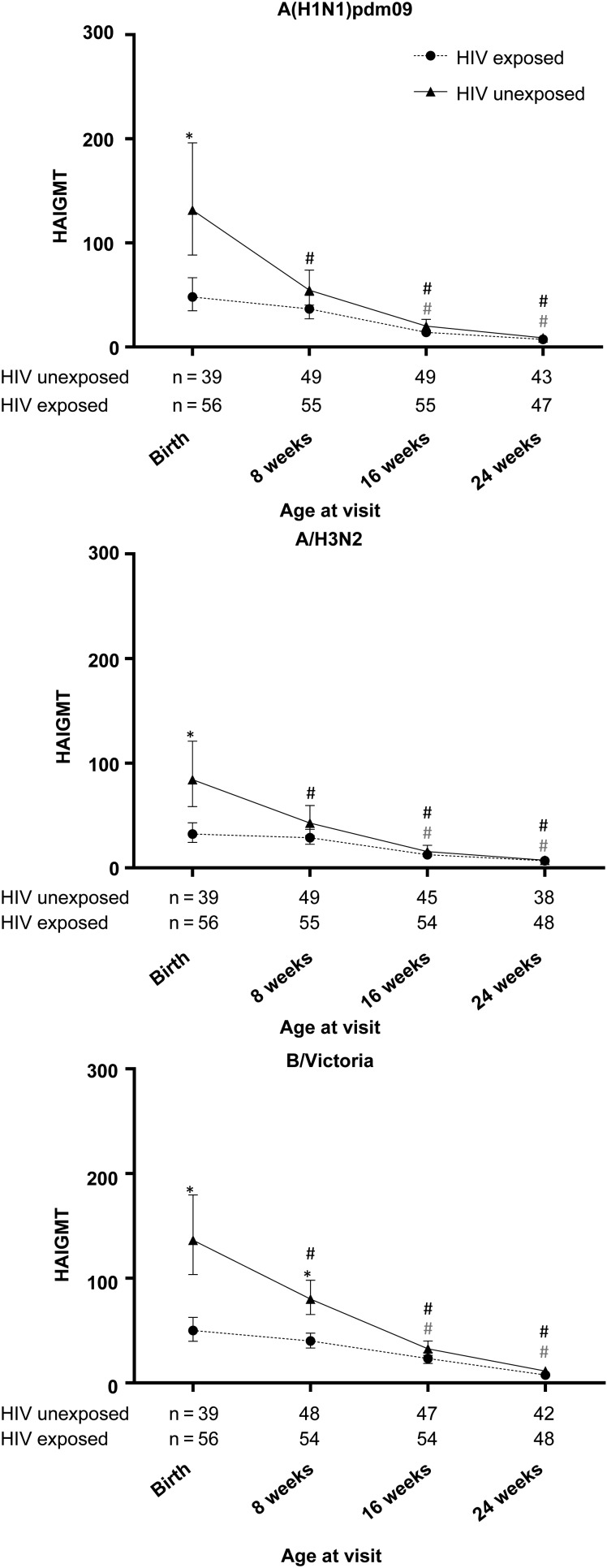
At birth, a higher proportion of HIV-unexposed infants than HIV-exposed infants had HAI titers of ≥1:40 for all strains (89.7% vs 60.7% for A[H1N1]pdm09 [P = .002], 82.1% vs 42.9% for A[H3N2] [P < .001], and 94.9% vs 78.6% for B/Victoria [P = .027]). Similarly, GMTs at birth were higher in HIV-unexposed infants, compared with HIV-exposed infants (Figure 2).
Figure 2.
Geometric mean titers (GMT) of hemagglutination-inhibiting antibodies (HAIs) in infants born to mothers who received trivalent inactivated influenza vaccine during pregnancy. GMTs were measured within 1 week of birth and at 8, 16, and 24 weeks of age. *P < .05 for the comparison between human immunodeficiency virus (HIV)-exposed and HIV-unexposed infants. #P < .05 for the comparison with the birth visit. Vertical bars indicate 95% confidence intervals.
The analysis of factors that affected the transplacental transfer in HIV-uninfected women showed higher transfer for A(H1N1)pdm09 and A(H3N2), with longer time between vaccination and delivery. Lower transfer and birth HAI titers for ≥2 of the vaccine strains were observed in infants born at <37 weeks of gestation, compared with infants born at ≥37 weeks of gestation; in infants born to women aged ≥25 years at vaccination, compared with those born to younger women; and infants born to women who had been pregnant before, compared with those born to women who had not been pregnant before (Table 4).
Table 4.
Factors Influencing Transplacental Antibody Transfer Ratio and Hemagglutination-Inhibiting Antibody (HAI) Titers at Birth in Infants Born to Human Immunodeficiency Virus (HIV)–Uninfected Mothers Who Received Trivalent Inactivated Influenza Vaccine (IIV3) During Pregnancy
| Factor | A/(H1N1)pdm09 |
A(H3N2) |
B/Victoria |
|||
|---|---|---|---|---|---|---|
| Rho (95% CI) | P Value | Rho (95% CI) | P Value | Rho (95% CI) | P Value | |
| Interval between vaccination and delivery (per 1-d increase) | ||||||
| Per-protocol group | ||||||
| Log infant antibody titer | ||||||
| Unadjusted | 0.0007 (−.005 to .006) | .8 | −0.0004 (−.005 to .004) | .9 | −0.0002 (−.004 to .003) | .9 |
| Adjusted | 0.003 (−.0003 to .006) | .08 | 0.001 (−.004 to .007) | .6 | −0.0001 (−.005 to .003) | .6 |
| Infant:mother antibody ratio | ||||||
| Unadjusted | 0.002 (−0.0001, 0.005) | .05 | 0.004 (0.0002, 0.008) | .041 | 0.001 (−0.002, 0.004) | .5 |
| Adjusted | 0.004 (0.001, 0.006) | .004 | 0.005 (0.0008, 0.009) | .022 | 0.0009 (−0.003, 0.005) | .6 |
| Non–per-protocol groupa | ||||||
| Log infant antibody titer | ||||||
| Unadjusted | 0.001 (−0.004, 0.006) | .6 | 0.001 (−0.003, 0.005) | .6 | 0.0001 (−0.003, 0.003) | .9 |
| Adjusted | 0.002 (−0.001, 0.006) | .1 | 0.001 (−0.004, 0.006) | .6 | −0.001 (−0.005, 0.002) | .5 |
| Infant:mother antibody ratio | ||||||
| Unadjusted | 0.002 (0.0001, 0.004) | .041 | 0.004 (0.001, 0.008) | .016 | 0.001 (−0.002, 0.004) | .5 |
| Adjusted | 0.004 (0.001, 0.006) | .004 | 0.005 (0.0008, 0.009) | .022 | 0.0009 (−0.003, 0.005) | .6 |
| Gestational age at birthb | ||||||
| ≥37 wk | Reference | Reference | Reference | |||
| <37 wk | ||||||
| Log infant antibody titer | ||||||
| Unadjusted | −0.74 (−1.31, −0.17) | .012 | −0.93 (−1.43, −0.42) | .001 | −0.16 (−0.53, 0.22) | .4 |
| Adjusted | −0.60 (−1.06, −0.14) | .012 | −0.91 (−1.70, −0.12) | .025 | −0.13 (−0.64, 0.37) | .6 |
| Infant:mother antibody ratio | ||||||
| Unadjusted | −0.33 (−0.60, −0.06) | .017 | −0.44 (−0.86, -0.02) | .040 | −0.02 (−0.31, 0.34) | .9 |
| Adjusted | −0.57 (−0.94, −0.20) | .004 | −0.85 (−1.50, −0.19) | .013 | −0.05 (−0.55, 0.46) | .8 |
| Maternal age at vaccination | ||||||
| <25 y | Reference | Reference | Reference | |||
| ≥25 y | ||||||
| Log antibody titer | ||||||
| Unadjusted | −0.51 (−0.81, −0.20) | .002 | −0.11 (−0.43, 0.21) | .5 | −0.32 (−0.54, −0.10) | .005 |
| Adjusted | −0.34 (−0.53, −0.15) | .001 | −0.24 (−0.57, 0.08) | .1 | −0.32 (−0.53, −0.12) | .003 |
| Infant:mother antibody ratio | ||||||
| Unadjusted | −0.19 (−0.34, −0.03) | .019 | −0.20 (−0.46, 0.07) | .1 | −0.22 (−0.41, −0.03) | .024 |
| Adjusted | −0.23 (−0.40, −0.06) | .010 | −0.33 (−0.61, −0.05) | .023 | −0.28 (−0.49, −0.06) | .013 |
| Gravidity | ||||||
| Primigravida | Reference | Reference | Reference | |||
| Multigravida | ||||||
| Log antibody titer | ||||||
| Unadjusted | −0.47 (−0.80, −0.14) | .007 | −0.27 (−0.59, 0.05) | .1 | −0.34 (−0.57, −0.12) | .004 |
| Adjusted | −0.36 (−0.54, −0.17) | .001 | −0.38 (−0.71, 0.06) | .023 | −0.37 (−0.57, −0.16) | .001 |
| Infant:mother antibody ratio | ||||||
| Unadjusted | −0.21 (−0.37, −0.06) | .009 | −0.17 (−0.45, 0.10) | .2 | −0.23 (−0.43, −0.03) | .023 |
| Adjusted | −0.26 (−0.43, −0.10) | .003 | −0.34 (−0.64, −0.04) | .026 | −0.28 (−0.51, −0.06) | .014 |
| Gestational age at vaccination | ||||||
| <27 wk | Reference | Reference | Reference | |||
| ≥27 wk | ||||||
| Log antibody titer | ||||||
| Unadjusted | 0.03 (−0.32, 0.39) | .8 | 0.02 (−0.30, 0.34) | .9 | 0.04 (−0.21, 0.28) | .8 |
| Adjusted | −0.08 (−0.30, 0.14) | .5 | −0.05 (−0.39, 0.28) | .7 | 0.14 (−0.08, 0.37) | .2 |
| Infant:mother antibody ratio | ||||||
| Unadjusted | −0.04 (−0.20, 0.13) | .7 | 0.09 (−0.36, 0.18) | .5 | 0.06 (−0.14, 0.26) | .5 |
| Adjusted | −0.08 (−0.27, 0.10) | .4 | −0.13 (−0.43, 0.18) | .4 | 0.08 (−0.15, 0.32) | .5 |
Titer and ratio data are for 39 and 38 individuals, respectively, unless otherwise indicated. A positive value corresponds to an increase in the log titer or ratio, compared with the reference value. Adjusted values were adjusted for maternal HAI titer 1 month after IIV3 receipt, unless otherwise indicated.
Abbreviation: CI, confidence interval.
a Titer and ratio data are for 41 and 40 infants, respectively. Included are 2 infants who were excluded from the per-protocol analyses because they were born <28 days after maternal IIV3 receipt. Preterm infants are excluded.
b Titer and ratio data are for 43 and 42 infants, respectively. Included are 4 infants who were excluded from the per-protocol analyses; 2 were born <28 days after maternal IIV3 receipt, and 2 were born at <37 weeks gestation with a birth weight of <2500 g. Adjusted values were adjusted for maternal HAI titers 1 month after maternal IIV3 receipt and the interval between vaccination and delivery.
In the HIV-infected cohort, an inverse relationship was observed between birth HAI titers and time from vaccination to delivery and mother's age; after adjustment for maternal titers at 1 month after IIV3 receipt, the associations were no longer detected (Supplementary Table).
HAI Antibody Decay in Infants
Infant HAI antibodies waned after birth (Figure 2). By 8 weeks of age, even though HIV-unexposed infants still had higher GMTs than HIV-exposed infants, the difference was only significant for B/Victoria (Figure 2). The rate of antibody decay was faster in HIV-unexposed infants, compared with HIV-exposed infants. The estimated mean antibody half-life in HIV-unexposed infants, compared with HIV-exposed infants, was 45 days (95% CI, 41–49) versus 56 days (95% CI, 51–62) for A(H1N1)pdm09, 44 days (95% CI, 40–48) versus 65 days (95% CI, 58–72) for A(H3N2), and 43 days (95% CI, 39–47) versus 63 days (95% CI, 56–71) for B/Victoria (P ≤ .001 for all comparisons; Table 3 and Supplementary Figure 4). Similar results were obtained after adjustment for birth titers (Table 3).
DISCUSSION
HIV-infected pregnant women generated less robust humoral responses to all 3 influenza virus strains in the 2011 southern hemisphere seasonal IIV3, compared with HIV-uninfected women. One month after IIV3 receipt, <41% of HIV-infected women seroconverted to at least 1 strain, compared with 92% of HIV-uninfected women. In HIV-uninfected and HIV-infected cohorts, although the efficiency of transplacental antibody transfer was similar, lower amounts of antibodies were transferred to HIV-exposed infants, owing to lower titers in their mothers.
It is noticeable that the low antibody response to IIV3 in HIV-infected women did not affect the vaccine efficacy against PCR-confirmed influenza, as previously reported for 57.7% of HIV-infected women and 50.4% of HIV-uninfected women [6], while we found that only 49%–67% of the HIV-infected women achieved HAI titers of ≥1:40. The immunogenicity and efficacy of IIV3 in HIV-uninfected women are in agreement with HAI titers of ≥1:40 being predictive of 50% protection against influenza in healthy adults [24]. However, the data suggest that IIV3 may confer protection to HIV-infected women by additional mechanisms and that the use of HAI titers as a correlate of protection needs to be validated in this population. This is in agreement with our previously observations in HIV-infected nonpregnant adults [19]. The value of birth HAI titers as a predictor of protection against influenza virus infection in infants is unknown. It is possible that protection of infants may be mediated by transplacental transfer of maternal antibody, in which case the lower level of antibody titers among the HIV-exposed infants at birth may place them at greater risk of developing influenza and warrants evaluation of other, more-immunogenic formulations of IIV in HIV-infected women. However, IIV3 receipt during pregnancy may also protect the infants through cocooning [25], in which case the amount of transferred antibodies may not be critical.
Our findings that IIV3 generates low antibody levels in HIV-infected women are in agreement with previous studies that showed that HIV-infected adults generate lower responses to vaccination with seasonal IIV3 and monovalent A(H1N1)pdm09, compared with healthy adults [14, 18, 26]. Some studies reported that high CD4+ T-cell counts, low HIV levels, current ART use, and younger age correlated with improved immune responses to vaccines in HIV-infected individuals [26–29]. We did not observe a relationship between immunologic or virologic status at vaccination and the immunogenicity of IIV3. This may be because the majority of participants in our study had a CD4+ T-cell count of >350 cells/µL and because 80% were receiving ART. Younger age (<25 years) was associated with higher rates of seroconversion to A(H1N1)pdm09 in HIV-infected women. Previous studies in HIV-uninfected and HIV-infected adults also reported an association of high antibody responses to monovalent A(H1N1)pdm09 with younger age [26, 30], suggesting that robust thymic function may contribute to the magnitude of the responses to vaccination. However, in HIV-infected adults, younger age may also reflect a shorter duration of HIV infection and better immune preservation. Interestingly, we did not find an association between age and immunogenicity in HIV-uninfected women, supporting the latter explanation for the association between age and immunogenicity in the HIV-infected group.
In HIV-uninfected women, high baseline HAI titers correlated with lower seroconversion rates, which is consistent with previous studies. In contrast, high baseline titers correlated with improved seroconversion rates in HIV-infected women, suggesting that HIV-infected participants may primarily rely on their immunologic memory to mount adequate responses to IIV3; studies have shown that this is true for responses to other vaccines in HIV-infected individuals [31].
By approximately 8 months after IIV3 receipt (ie, postpartum week 24), 70% of HIV-uninfected women maintained HAI titers of ≥1:40 to at least 1 strain, compared with only 26% of HIV-infected women. However, the limited persistence of HAI titers of ≥1:40 in HIV-infected women was not consistently related to a quicker decay in antibody titers, compared with findings for HIV-uninfected women, but was due to lower titers after IIV3 receipt. This waning of antibodies may influence the influenza virus infection rates late during influenza season and may need to be taken into consideration when designing optimal immunization schedules for HIV-infected women. Nevertheless, as indicated above, HAI titers are not necessarily an adequate correlate for protection in HIV-infected individuals.
We found equally efficient transplacental transfer between HIV-uninfected mothers and HIV-infected mothers. However, because of the strong correlation between infant titers at birth and maternal titers at delivery, birth titers were ≥1:40 in 82%–95% of HIV-unexposed infants and 43%–79% of HIV-exposed infants. We found infant titers to be consistently lower than maternal titers, regardless of HIV infection status. Previous studies reported a wide range of ratios of infant GMTs to maternal GMTs, with some studies reporting ratios >1, particularly in cord blood [9, 32–34]. Maternal antibody levels declined rapidly in infants, with a half-life of 43–45 days in HIV-unexposed infants, which was in agreement with a study conducted in Bangladesh (half-life, 42–50 days; 95% CI, 37–56) [10]. The impact of the decreasing antibody concentration on protection against influenza virus infection in young infants, for whom there is no definitive correlate of protection, needs to be further evaluated. Antibody half-life was longer in HIV-exposed infants; exploratory analysis showed that the antibody half-life was not associated with HAI titers at birth. A potential explanation for this finding is that different isotypes composed the HAI response to vaccination in HIV-infected and HIV-uninfected women. The response to influenza vaccine in immunocompetent adults is predominantly immunoglobulin G1 (IgG1), with a half-life of 21 days, and a smaller IgG3 component, with a half-life of 7 days [35]. The IgG3 component presumably accounts for the rapid initial antibody decay observed after IIV3 receipt, and IgG1 likely accounts for the subsequent gradual decay. In HIV-infected women, the curve of antibody decay lacks a rapid decay phase, suggesting a decreased level or absence of the IgG3 component. In this scenario, levels of the transplacentally transferred IgG1 would continue to decay slowly in HIV-exposed infants. It is unclear whether enough IgG3 is present at delivery in HIV-uninfected women and whether the transfer is sufficient to account for the rapid antibody decay in their infants. Further studies are needed to confirm or refute this hypothesis. Another speculative explanation for the slower antibody decay in HIV-exposed infants is that vaccine antigens crossed the placenta and that some of the antibodies were made by the infant, thereby prolonging their half-life. Although particle exchange across the placenta and immune memory to antigens to which the mother is exposed during pregnancy have been demonstrated in the infants [36], this has not been shown specifically for influenza vaccines. Additional studies are required to test this hypothesis.
The amount of maternal antibodies transferred to the infant is influenced by the interval between immunization and delivery [34, 37]. In the HIV-uninfected cohort, we found that a higher level of transplacental transfer was associated with a longer interval between vaccination and delivery. The programmatic distribution of influenza vaccine to pregnant women can be challenging because of the difficult in reconciling the time of vaccine availability with the best time to vaccinate during pregnancy to optimize protection of the women and the infants. Even though the participants in our study only received vaccine at >20 weeks of gestational age, our results suggest that pregnant women should be immunized as soon as vaccine is available, to protect themselves as early as possible during the influenza season and to increase the cumulative level of antibodies transferred to their fetus. A recent study showed that vaccinating mothers <15 days before delivery did not increase antibody titers in the newborn [37]. Similarly, our analysis also suggests that transplacental transfer was less efficient if birth occurred within 28 days after IIV3 receipt, but the number of participants in this category was very limited. As expected, preterm birth was associated with a lower level of transplacental transfer.
While our study had the advantage of simultaneously evaluating relatively large groups of HIV-infected and HIV-uninfected women with similar baseline demographic characteristics, a potential limitation was the inclusion of only a small number of HIV-infected participants with a CD4+ T-cell count of <200 cells/µL. Although this precluded a precise assessment of the impact of severe immunosuppression on IIV3-associated immune responses, it accurately represented the current landscape of HIV infection, in which infected individuals are treated earlier than in the past and have better-preserved CD4+ T-cell counts. Our study was also limited to only 1 influenza season, a single IIV3 formulation, and a single site. At the birth immunogenicity visit, only 39 of 69 eligible HIV-unexposed infants and 56 of 75 HIV-exposed infants were available for analysis; nevertheless, there were no differences in demographic characteristics between those for whom samples were available and those for whom samples were not available (data not shown). Furthermore, since the sample size calculation assumed that a lower percentage of HIV-unexposed infants (75%) would achieve titers of ≥1:40, compared with the actual percentage (>82%), the study had enough power to detect a difference between HIV-exposed and HIV-unexposed infants, even for the least immunogenic strain, with the reduced number of participants analyzed. The immunogenicity analyses excluded participants who experienced influenza virus infections confirmed by PCR or identified serologically on the basis of a ≥4-fold increase in titers at consecutive visits. Although serological analysis is not 100% sensitive for detecting influenza virus infection, we used this criterion to avoid inflating the antibody response, especially in the HIV-infected cohort, which experienced a much higher influenza attack rate.
STUDY GROUP MEMBERS
Members of the Maternal Flu Trial Team are as follows: Peter V. Adrian (Vaccine Preventable Diseases Unit, Department of Science and Technology, National Research Foundation, and Respiratory and Meningeal Pathogens Research Unit, Medical Research Council, University of the Witwatersrand, Johannesburg, South Africa), Keith P. Klugman (Hubert Department of Global Health, Rollins School of Public Health, and Division of Infectious Diseases, School of Medicine, Emory University, Atlanta, Georgia), Kathleen M. Neuzil and Justin R. Oritz (Vaccine Access and Delivery Global Program, PATH, Seattle, Washington), Eric A. F. Simões (Department of Pediatrics, Medicine and Pathology, University of Colorado School of Medicine, and Center for Global Health, Department of Epidemiology, Colorado School of Public Health, Aurora, Colorado), Florette Treurnicht and Marietjie Venter (National Institute for Communicable Diseases, National Health Laboratory Service, Centre for Vaccines and Immunology, Johannesburg), and Avy Violari (Perinatal HIV Research Unit, University of the Witwatersrand).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Niteen Wairagkar, program officer acting on behalf of the Bill and Melinda Gates Foundation; the study participants; Nirvashni Dwarka and the staff of the Departments of Obstetrics, Neonatology, and Paediatrics at Chris Hani Baragwanath Academic Hospital, Soweto, South Africa, for their dedication to their patients, including our trial participants; the study midwives, nurses, laboratory staff, counselors, and data capturers; Richard Madimabe, Julie Patterson, and David Claypool; the District Research Committee, Johannesburg Health District, Department of Health; and the Gauteng Department of Health and Social Development, Policy, Planning, and Research.
Disclaimer. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of their institutions or organizations or of the sponsors. The funders did not participate in any aspect of the study, including study conduct, data collection, data analysis, or manuscript writing.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant OPP1002747); the Colorado Clinical and Translational Sciences Institute, National Center for Advancing Translational Sciences, National Institutes of Health (award UL1 TR000154 to REDCAP); the Department of Science and Technology, South African Research Chairs Initiative; the Vaccine Preventable Diseases Unit, National Research Foundation; and the Respiratory and Meningeal Pathogens Research Unit, Medical Research Council.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Matflu Team, Peter V. Adrian, Keith P. Klugman, Kathleen M. Neuzil, Justin R. Oritz, Eric A. F. Simões, Florette Treurnicht, Marietjie Venter, and Avy Violari
References
- 1.Neuzil KM, Coffey CS, Mitchel EF Jr et al. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr 2003; 34:304–7. [DOI] [PubMed] [Google Scholar]
- 2.Neuzil KM, Reed GW, Mitchel EF et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998; 148:1094–102. [DOI] [PubMed] [Google Scholar]
- 3.Radwan HM, Cheeseman SH, Lai KK et al. Influenza in human immunodeficiency virus-infected patients during the 1997–1998 influenza season. Clin Infect Dis 2000; 31:604–6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–40. [DOI] [PubMed] [Google Scholar]
- 5.Meeting of the Strategic Advisory Group of Experts on Immunization, April 2012 - conclusions and recommendations. Wkly Epidemiol Rec 2012:201–16. [PubMed] [Google Scholar]
- 6.Madhi SA, Cutland CL, Kuwanda L et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371:918–31. [DOI] [PubMed] [Google Scholar]
- 7.Englund JA. The influence of maternal immunization on infant immune responses. J Comp Pathol 2007; 137(suppl 1):S16–9. [DOI] [PubMed] [Google Scholar]
- 8.Puck JM, Glezen WP, Frank AL et al. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis 1980; 142:844–9. [DOI] [PubMed] [Google Scholar]
- 9.Steinhoff MC, Omer SB, Roy E et al. Influenza immunization in pregnancy--antibody responses in mothers and infants. N Engl J Med 2010; 362:1644–6. [DOI] [PubMed] [Google Scholar]
- 10.Zaman K, Roy E, Arifeen SE et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–64. [DOI] [PubMed] [Google Scholar]
- 11.Zuccotti G, Pogliani L, Pariani E et al. Transplacental antibody transfer following maternal immunization with a pandemic 2009 influenza A(H1N1) MF59-adjuvanted vaccine. JAMA 2010; 304:2360–1. [DOI] [PubMed] [Google Scholar]
- 12.Abzug MJ, Nachman SA, Muresan P et al. Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women. Clin Infect Dis 2013; 56:1488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroon FP, van Dissel JT, de Jong JC et al. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 2000; 18:3040–9. [DOI] [PubMed] [Google Scholar]
- 14.Miotti PG, Nelson KE, Dallabetta GA et al. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA 1989; 262:779–83. [PubMed] [Google Scholar]
- 15.Nelson KE, Clements ML, Miotti P et al. The influence of human immunodeficiency virus (HIV) infection on antibody responses to influenza vaccines. Ann Intern Med 1988; 109:383–8. [DOI] [PubMed] [Google Scholar]
- 16.Archer B, Cohen C, Naidoo D et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveill 2009; 14:pii:19369. [DOI] [PubMed] [Google Scholar]
- 17.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis 2009; 9:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson K, Weinberg A. Reduced immunogenicity of influenza vaccines in HIV-infected compared with uninfected pregnant women is associated with regulatory T cells. AIDS 2011; 25:595–602. [DOI] [PubMed] [Google Scholar]
- 19.Madhi SA, Maskew M, Koen A et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis 2011; 52:128–37. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Recommended viruses for influenza vaccines for use in the 2011 southern hemisphere influenza season. http://www.who.int/influenza/vaccines/virus/recommendations/201009_Recommendation.pdf?ua=1. Accessed 31 January 2014.
- 21.Sanofi-pasteur. VAXIGRIP®. Inactivated influenza vaccine (Split Virion). http://products.sanofi.com.au/vaccines/VAXIGRIP_NZ_PI.pdf. Accessed 31 January 2014.
- 22.Weinberg A, Song LY, Walker R et al. Anti-influenza serum and mucosal antibody responses after administration of live attenuated or inactivated influenza vaccines to HIV-infected children. J Acquir Immune Defic Syndr 2010; 55:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobson D, Curry RL, Beare AS et al. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maltezou HC, Fotiou A, Antonakopoulos N et al. Impact of postpartum influenza vaccination of mothers and household contacts in preventing febrile episodes, influenza-like illness, healthcare seeking, and administration of antibiotics in young infants during the 2012–2013 influenza season. Clin Infect Dis 2013; 57:1520–6. [DOI] [PubMed] [Google Scholar]
- 26.Crum-Cianflone NF, Eberly LE, Duplessis C et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis 2011; 52:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamanaka H, Teruya K, Tanaka M et al. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr 2005; 39:167–73. [PubMed] [Google Scholar]
- 28.Fuller JD, Craven DE, Steger KA et al. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis 1999; 28:541–7. [DOI] [PubMed] [Google Scholar]
- 29.Evison J, Farese S, Seitz M et al. Randomized, double-blind comparative trial of subunit and virosomal influenza vaccines for immunocompromised patients. Clin Infect Dis 2009; 48:1402–12. [DOI] [PubMed] [Google Scholar]
- 30.Zhu FC, Wang H, Fang HH et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 2009; 361:2414–23. [DOI] [PubMed] [Google Scholar]
- 31.Abu Raya B, Srugo I, Kessel A et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels - a prospective study. Vaccine 2014; 32:5787–93. [DOI] [PubMed] [Google Scholar]
- 32.Tsatsaris V, Capitant C, Schmitz T et al. Maternal immune response and neonatal seroprotection from a single dose of a monovalent nonadjuvanted 2009 influenza A(H1N1) vaccine: a single-group trial. Ann Intern Med 2011; 155:733–41. [DOI] [PubMed] [Google Scholar]
- 33.Lin SY, Wu ET, Lin CH et al. The safety and immunogenicity of trivalent inactivated influenza vaccination: a study of maternal-cord blood pairs in Taiwan. PLoS One 2013; 8:e62983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson LA, Patel SM, Swamy GK et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis 2011; 204:854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frasca D, Diaz A, Romero M et al. Effects of age on H1N1-specific serum IgG1 and IgG3 levels evaluated during the 2011–2012 influenza vaccine season. Immun Ageing 2013; 10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legrand FA, Nixon DF, Loo CP et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One 2006; 1:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanchard-Rohner G, Meier S, Bel M et al. Influenza vaccination given at least 2 weeks before delivery to pregnant women facilitates transmission of seroprotective influenza-specific antibodies to the newborn. Pediatr Infect Dis J 2013; 32:1374–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.