Abstract
Importance
Advantages of using efavirenz as part of treatment for HIV-infected children include once-daily dosing, simplification of co-treatment for tuberculosis, preserving ritonavir-boosted lopinavir for second-line treatment, and harmonization of adult and pediatric treatment regimens. However, there have been concerns about possible reduced viral efficacy of efavirenz in children exposed to nevirapine for prevention of mother-to-child transmission (PMTCT).
Objective
To evaluate whether nevirapine-exposed children, initially virally-suppressed on ritonavir-boosted lopinavir-based therapy, can transition to efavirenz-based therapy without risk of viral failure.
Design, Setting, Participants
Randomized, open-label, non-inferiority trial conducted at Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa, June 2010 to December 2013. Three hundred HIV-infected children exposed to nevirapine for PMTCT, ≥ 3 years of age, and with plasma HIV RNA <50 copies/ml on ritonavir-boosted lopinavir-based therapy were enrolled; 298 were randomized, and 292 (98%) were followed to 48 weeks post-randomization.
Intervention
Switch to efavirenz-based therapy (n=150) or continue on ritonavir-boosted lopinavir-based therapy (n=148).
Main Outcomes and Measures
Risk difference (delta) between groups in (1) viral rebound; i.e., one or more HIV RNA >50 copies/ml, and (2) viral failure; i.e., confirmed HIV RNA >1000 copies/ml; with a non-inferiority bound for the delta of −0.10. Immunologic and clinical responses were secondary endpoints.
Results
The Kaplan-Meier probability of viral rebound by 48 weeks was 0.176 (n=26) in the efavirenz group and 0.284 (n=42) in the ritonavir-boosted lopinavir group. Probabilities of viral failure were 0.027 (n=4) in the efavirenz and 0.020 (n=3) in the ritonavir-boosted lopinavir group. The risk difference of viral rebound was 0.107 (1-sided 95% CI: 0.028,∞) and −0.007 (1-sided 95% CI: −0.036, ∞) for viral failure. We rejected the null hypothesis that efavirenz is inferior to ritonavir-boosted lopinavir (p<.001) for both endpoints. By 48 weeks, CD4 percentage was 2.88 (95% CI: 1.26, 4.49) units higher in the efavirenz than in the ritonavir-boosted lopinavir group.
Conclusions and Relevance
Among HIV-infected children exposed to nevirapine for PMTCT and initially virally-suppressed on ritonavir-boosted lopinavir-based therapy, switching to efavirenz-based therapy compared with continuing ritonavir-boosted lopinavir-based therapy did not result in significantly higher rates of viral rebound or viral failure. This therapeutic approach may offer advantages in children such as these.
Introduction
Implementation of pediatric antiretroviral treatment (ART) programs in sub-Saharan Africa has resulted in significant reductions in morbidity and mortality among HIV-infected children, changing a rapidly fatal disease into a chronic condition.1 The success of ART programs in low resource settings has been attributed to a public health approach, whereby standardized, population guidelines facilitate individual patient management.2 For infants and young children, ritonavir-boosted lopinavir-based therapy is recommended as first-line ART.3 Initially, ritonavir-boosted lopinavir was recommended only for infants exposed to nevirapine for prevention of mother-to-child transmission (PMTCT); but later, was shown to also have better virological efficacy in unexposed infants and young children.4, 5 In adults and older children, efavirenz is recommended as part of first-line ART.3
For HIV-infected children older than three years, efavirenz has advantages for long-term maintenance therapy. Recommending efavirenz for older children would harmonize their regimen with adult guidelines and reduce the cost of national programs. Efavirenz may avoid some of the metabolic toxicities associated with ritonavir-boosted lopinavir and simplifies co-treatment for tuberculosis.6 Ritonavir-boosted lopinavir has an unpleasant taste, posing major adherence challenges for parents administering this drug in syrup form to their children, still too young to swallow tablets.6 Efavirenz has the advantage of once-daily dosing, which has been shown to improve adherence and virologic outcome.7
Non-nucleoside reverse transcriptase inhibitors (NNRTI) continue to be recommended for PMTCT. This includes efavirenz or nevirapine as part of maternal therapy and infant nevirapine prophylaxis which is recommended regardless of maternal regimen.3, 8 With improved PMTCT coverage, the majority of the, albeit shrinking number of, children who acquire HIV infection have NNRTI resistance prior to starting therapy.9 We previously evaluated whether children initially started on ritonavir-boosted lopinavir-based therapy could safely transition to nevirapine-based therapy soon after achieving viral load suppression. Our results supported the clinical utility of this strategy with some caveats. Resistance selected during PMTCT led to a higher rate of virologic failure in the group transitioning to nevirapine.10-12 In the new trial presented here, we evaluate whether a switch to efavirenz can overcome this limitation. Specifically, we tested, among children perinatally-exposed to nevirapine as part of PMTCT, whether those whose viral load was initially suppressed on ritonavir-boosted lopinavir-based therapy can transition to efavirenz-based therapy without increased risk of viral failure.
Methods
Study design
We conducted a randomized, open-label, non-inferiority trial between June 2010 and December 2013 at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa. Children were randomized to switch to efavirenz-based therapy or to continue on ritonavir-boosted lopinavir-based therapy and were followed for 48 weeks after randomization. The non-inferiority design was chosen as efavirenz was not expected to have better virologic outcomes than the standard regimen. The study was approved by the Institutional Review Boards of Columbia University and the University of the Witwatersrand. The child’s mother or legal guardian provided signed informed consent.
Children were eligible for enrollment if they had nevirapine exposure as part of PMTCT, were currently receiving ritonavir-boosted lopinavir-based therapy started <36 months of age provided for at least one year, and had an HIV RNA test <50 copies/ml. All children in the control arm of our prior trial10,11 still in follow-up were screened for eligibility. In addition, clinicians responsible for the care of HIV-infected children at other clinics in the area were approached about referring children meeting our eligibility criteria. Random assignments were generated by the study statistician using a permuted block design with block sizes between 8 and 12 and were concealed in opaque envelopes opened on-site at the time of randomization. Children were followed at 4, 8, 16, 24, 32, 40, and 48 weeks after randomization.
Drug regimens
Efavirenz was prescribed once-daily in the evening at 200mg and 300mg for weight-bands 10-13.9 kg and 14-24.9 kg, respectively. Efavirenz was available in 50mg and 200mg capsules. If children were unable to swallow capsules, caregivers were shown how to open the capsules and dissolve the contents in water. Ritonavir-boosted lopinavir syrup was given twice-per-day at 230mg/m2per dose. Children able to swallow tablets were given one tablet twice-per-day (200mg lopinavir/50mg ritonavir) if body surface area <0.9 or two tablets twice-per-day if >0.9. Both groups received adherence counseling at the time of randomization and at each study visit.
At the time the study was undertaken, local guidelines advised against the use of stavudine for new patients, but provided no guidance for those already on it. At enrollment, children who were receiving stavudine were screened for eligibility for a sub-study of pre-emptive switching to abacavir compared to remaining on stavudine. Children not eligible for the sub-study remained on the other two antiretroviral drugs that they were already receiving. Stavudine was given at 1mg/kg (twice daily) and abacavir at 8mg/kg (twice daily). Some children were receiving zidovudine (180mg/m2 twice daily). Lamivudine (4mg/kg twice daily) was used as the third drug for all children. All medications were dose-adjusted at every visit based on growth.
Study endpoints
HIV RNA quantity in plasma was measured at 4, 8, 16, 24, and 48 weeks. Based on the results of our prior trial,10,11 we identified two primary virologic endpoints: (1) viral rebound, defined as one or more HIV-1 RNA measurements >50 copies/ml, and (2) viral failure, defined as confirmed (i.e., two or more) HIV-1 RNA measurements >1000 copies/ml by 48 weeks after randomization. All children with >50 copies/ml at a scheduled study visit were recalled for a repeat test.
CD4 cell count and CD4 percentage were measured at baseline, 24, and 48 weeks. Complete blood count was performed at baseline and 24 weeks, and alanine transaminase (ALT) at baseline and 32 weeks. Fasting lipid panel was performed at baseline and 40 weeks. Weight and height, concomitant medications, and other clinical conditions were recorded at each visit. The Strengths and Difficulties Questionnaire (SDQ), a validated standardized screening questionnaire of emotional/behavioral problems,13 was administered at baseline and 40 weeks. Caregivers were asked to return all unused medications at each study visit. These were reconciled by the pharmacist with the expected usage of each drug as a measure of adherence.
Plasma HIV RNA (AmpliPrep/COBAS® TaqMan® HIV-1 Test, version 2.0, Roche, Branchburg, NJ) measurements, CD4 cell determinations, blood counts, and liver function tests were conducted by Clinical Laboratory Services in Johannesburg and reported directly to the site for use in clinical management. The quantification range of the HIV RNA assay was 20-10,000,000 copies/ml. All samples with >1000 copies/ml were tested at the National Institute for Communicable Diseases for drug resistance using population sequencing as previously described.14
Statistical analysis
The sample size of 300 was selected to detect a non-inferiority bound of −0.15 around the risk difference for viral rebound (endpoint 1) and bound of −0.11 for viral failure (endpoint 2). Our prior trial10,11 had observed the risk of viral rebound to be 0.55, which means that based on our non-inferiority bound we were prepared to tolerate a risk ≤ 0.70 in the intervention group. At a Data Safety and Monitoring Board (DSMB) review of a planned interim analysis, it was noted that virologic endpoints were less common than anticipated and that, for endpoint 2, the stopping criteria had been attained; i.e., non-inferiority at the pre-specified bound had been confirmed. The DSMB requested re-calculation of expected non-inferiority bounds based on the interim results. These were re-calculated to be −0.10 for both endpoints. The DSMB advised completion of the study with this narrower non-inferiority bound which would allow for stronger conclusions to be drawn from the non-inferiority analysis.
Intent-to-treat analyses were conducted utilizing all available follow-up data. The cumulative probabilities of virologic endpoints were calculated using Kaplan-Meier methods. Follow-up time for the children not followed to 48 weeks was censored at their last follow-up visit. To address the non-inferiority design, the risk difference (delta) for each endpoint was calculated as the difference in Kaplan-Meier probabilities. The standard errors of the difference were calculated with the delta method. P-values for the non-inferiority analysis were calculated from one-sided t-test and tested the null hypothesis that delta (probability in control minus probability in efavirenz group) was ≤ −0.10. The threshold to define significance was p=0.05. Differences in outcomes between the randomized groups by variation in the other antiretroviral drugs contained in the regimens were investigated in stratified analyses. Other outcomes were compared across groups using t-tests for continuous variables and Chi-squared or Fisher’s exact tests for categorical variables. For comparison of adherence outcomes between the two groups, generalized estimating equation (GEE) models were used. All p-values, other than the non-inferiority analysis, were two-sided and considered a p-value of <0.05 as statistically significant. Weight- and height-for-age Z-scores were calculated using WHO software. Analyses were done using SAS version 9.1.3 (Cary, NC).
Results
Study population
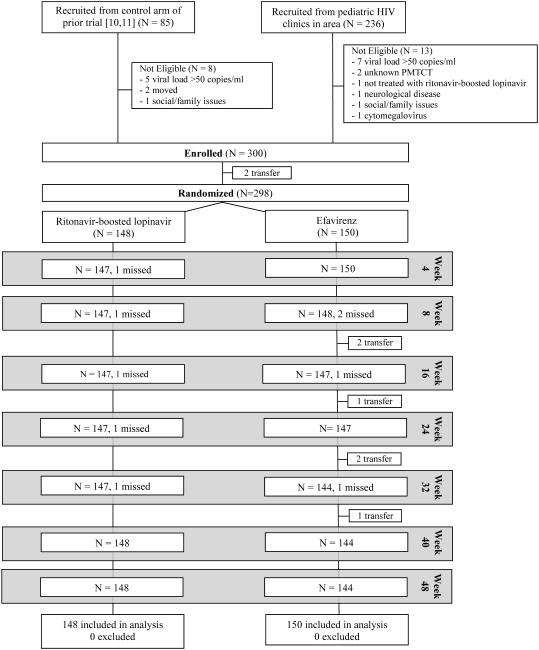
A total of 300 children were enrolled in the trial, 223 of 236 children referred from pediatric HIV clinics in the area and 77 of 85 children from the control arm of our prior trial.10, 11 Two children discontinued the study prior to randomization, resulting in 150 children randomized to switch to efavirenz-based therapy and 148 to remain on ritonavir-boosted lopinavir-based therapy (Figure 1).
Figure 1.
Disposition of study participants from screening, enrollment, randomization and follow-up post-randomization
Note: “Transfer” indicates children whose care was transferred to other facilities due to relocation.
At randomization, children had started ART at an average of 9.3 months (range 3 weeks to 32 months), had been on ART for an average of 3.5 years, and were an average of 4.3 years of age. Most (73.5%) had been exposed to both maternal and infant nevirapine for PMTCT, the remainder to either maternal or infant nevirapine. Just over half (53%) of the study population was female. Other characteristics are shown in Table 1. No children died in the 48 weeks after randomization, and retention in the study was excellent with 292/298 (98%) followed through 48 weeks. All six children not retained through the end of the study were in the efavirenz group. All of these were due to relocations out of the area with no apparent link to randomized group.
Table 1.
Characteristics at baseline of 298 HIV-infected children randomized to remain on a ritonavir-boosted lopinavir-based regimen or to switch to an efavirenz-based regimen
| Characteristic | All | Ritonavir- boosted Lopinavir |
Efavirenz |
|---|---|---|---|
| N | 298 | 148 | 150 |
| Source Population, N (%) Control group in prior trial New recruits |
76 (25.5) 222 (74.5) |
37 (25.0) 111 (75.0) |
39 (26.0) 111 (74.0) |
| Sex, N (%) Male Female |
140 (47.0) 158 (53.0) |
68 (46.0) 80 (54.0) |
72 (48.0) 78 (52.0) |
| Age (years), N (%) 3-3.9 4-4.9 5-5.9 ≥6 Mean (95% CI) |
139 (46.6) 103 (34.6) 34 (11.4) 22 (7.4) 4.27 (4.16, 4.38) |
71 (48.0) 50 (33.8) 15 (10.1) 12 (8.1) 4.26 (4.10, 4.41) |
68 (45.3) 53 (35.3) 19 (12.7) 10 (6.7) 4.28 (4.13, 4.43) |
| Duration on ART (years), N (%) 1-1.9 2-2.9 3-3.9 ≥4 Mean (95% CI) |
15 (5.0) 67 (22.5) 135 (45.3) 81 (27.2) 3.5 (3.40, 3.61) |
9 (6.1) 35 (23.6) 65 (43.9) 39 (26.4) 3.47 (3.32, 3.63) |
6 (4.0) 32 (21.3) 70 (46.7) 42 (28.0) 3.53 (3.38, 3.67) |
| Other two antiretrovirals, N (%) Abacavir/lamivudine Stavudine/lamivudine Zidovudine/lamivudine Abacavir/zidovudine |
173 (58.1) 113 (37.9) 11 (3.7) 1 (0.3) |
85 (57.4) 55 (37.2) 7 (4.7) 1 (0.7) |
88 (58.7) 58 (38.7) 4 (2.7) 0 (0.0) |
| PMTCT history, N (%) Maternal ART + Infant nevirapine Maternal zidovudine and/or nevirapine + Infant nevirapine Maternal zidovudine and/or nevirapine only Infant nevirapine only |
13 (4.4) 206 (69.1) 62 (20.8) 17 (5.7) |
6 (4.0) 105 (71.0) 29 (19.6) 8 (5.4) |
7 (4.7) 101 (67.3) 33 (22.0) 9 (6.0) |
| Age at ART initiation (months), N (%) <3 months 3-5.9 months 6-11.9 months 12-23.9 months 24-36 months Mean (95% CI) |
44 (14.8) 92 (30.9) 79 (26.5) 73 (24.5) 10 (3.4) 9.25 (8.47, 10.04) |
21 (14.2) 42 (28.4) 42 (28.4) 36 (24.3) 7 (4.7) 9.44 (8.32, 10.55) |
23 (15.3) 50 (33.3) 37 (24.7) 37 (24.7) 3 (2.0) 9.07 (7.97, 10.18) |
| Baseline CD4 %, N (%) <25 >25 Mean (95% CI) Missing |
18 (6.6) 257 (93.5) 34.7 (33.9, 35.5) 23 |
9 (6.6) 128 (93.4) 34.4 (33.3, 35.5) 11 |
9 (6.5) 129 (93.5) 35.0 (33.7, 36.2) 12 |
| Mother born in South Africa, N (%) Yes No Missing |
267 (89.9) 30 (10.1) 1 |
137 (92.6) 11 (7.4) 0 |
130 (87.3) 19 (12.8) 1 |
| Highest grade completed by caregiver, N (%) 1-7 (any primary school) 8-9 10-11 12 Any post high school education Missing |
27(9.2) 28(9.5) 110 (37.3) 124 (42.0) 6 (2.0) 3 |
12 (8.2) 14 (9.5) 61 (41.5) 59 (40.1) 1 (0.7) 1 |
15 (10.1) 14 (9.5) 49 (33.1) 65 (43.9) 5 (3.4) 2 |
| Caregiver has paid job, N (%) Yes No Missing |
135 (45.5) 162 (54.5) 1 |
65 (43.9) 83 (56.1) 0 |
70 (47.0) 79 (53.0) 1 |
| Inside tap in child’s home, N (%) Yes No Missing |
150 (50.5) 147 (49.5) 1 |
71 (48.0) 77 (52.0) 0 |
79 (53.0) 70 (47.0) 1 |
| Inside toilet in child’s home, N (%) Yes No Missing |
142 (47.8) 155 (52.2) 1 |
70 (47.3) 78 (52.7) 0 |
72 (48.3) 77 (51.7) 1 |
| Electricity in child’s home, N (%) Yes No Missing |
270 (90.9) 27 (9.1) 1 |
136 (91.9) 12 (8.1) 0 |
134 (89.9) 15 (10.1) 1 |
| Fridge in child’s home, N (%) Yes No Missing |
249 (83.8) 48 (16.2) 1 |
128 (86.5) 20 (13.5) 0 |
121 (81.2) 28 (18.8) 1 |
| Radio in child’s home, N (%) Yes No Missing |
263 (88.6) 34 (11.4) 1 |
134 (90.5) 14 (9.5) 0 |
129 (86.6) 20 (13.4) 1 |
| TV in child’s home, N (%) Yes No Missing |
267 (89.9) 30 (10.1) 1 |
133 (89.9) 15 (10.1) 0 |
134 (89.9) 15 (10.1) 1 |
| Does child have to go hungry, N (%) Yes No Missing |
45 (15.2) 252 (84.8) 1 |
17 (11.5) 131 (88.5) 0 |
28 (18.8) 121 (81.2) 1 |
Categorical variables are presented as percentages and continuous variables as means and their 95% confidence intervals. All denominators are shown.
Abbreviations: Antiretroviral therapy (ART), Confidence Interval (CI), Prevention of mother-to-child HIV transmission (PMTCT).
Primary endpoints
The Kaplan-Meier probability of viral rebound >50 copies/ml by 48 weeks was 0.176 (n=26) in the efavirenz group and 0.284 (n=42) in the ritonavir-boosted lopinavir group. Probabilities of viral failure (i.e., confirmed HIV RNA > 1000 copies/ml) were 0.027 (n=4) in the efavirenz group and 0.020 (n=3) in the ritonavir-boosted lopinavir group (Table 2). For viral rebound >50 copies/ml, the risk difference was 0.107 (1-sided 95% CI: 0.028,∞), and for viral failure, −0.007 (1-sided 95% CI: −0.036, ∞). The lower bounds of both of these 1-sided 95% CI exceeded the −0.10 pre-specified non-inferiority bound. We rejected the null hypothesis that efavirenz is inferior to ritonavir-boosted lopinavir (p<.001) for both endpoints, accepting the alternative hypothesis that efavirenz, relative to a standard approach using ritonavir-boosted lopinavir, is non-inferior for both primary endpoints.
Table 2.
Cumulative probabilities of primary endpoints among 148 HIV-infected children remaining on a ritonavir-boosted lopinavir-based regimen and 150 switched to an efavirenz-based regimen and tests of non-inferiority.
| Ritonavir- boosted lopinavir |
Efavirenz | Non-inferiority p-value* |
|
|---|---|---|---|
| Viral rebound (one or more HIV-1 RNA measurement >50 copies/ml) |
|||
| N Viral rebound | 42 | 26 | |
| Probability (95% CI)# | 0.284 (0.211, 0.357) | 0.176 (0.115, 0.238) | <.001 |
| Delta§ (1-sided 95% CI) | 0.107 (0.028, ∞) | ||
| Viral failure (two or more HIV-1 RNA measurements >1000 copies/ml) |
|||
| N Viral failure | 3 | 4 | |
| Probability (95% CI) | 0.020 (0.002, 0.043) | 0.027 (0.001, 0.054) | <.001 |
| Delta§ (1-sided 95% CI) | −0.007 (−0.036, ∞) |
p-value is the probability of rejecting the null hypothesis H0: Delta≤−0.10 in favor of the alternative hypothesis Ha : Delta>−0.10 when H0 is true.
Delta = Probability of endpoint in ritonavir-boosted lopinavir group minus probability of endpoint in efavirenz group.
Cumulative probabilities were calculated using Kaplan-Meier methods. There were no exclusions. Six children not followed through 48 weeks were censored at their last follow-up visit.
Details on the seven children with viral failure, including four in the efavirenz group and three in the ritonavir-boosted lopinavir group are shown in Table 3. Two of the four children in the efavirenz group were returned to ritonavir-boosted lopinavir and later re-suppressed. Both children had NNRTI (K103N) and NRTI resistance (M184V/I). Viral elevation in one child occurred following treatment interruption due to elevated ALT. After resolution of the hepatitis and resumption of the efavirenz-based regimen, the child re-suppressed. This child had no resistance detected. The fourth child in the efavirenz group had viral failure in association with severe household disruption. Both K103N and M184V were detected. The child was transitioned to lamivudine monotherapy as a holding therapy until adherence could be attained. The child did not reach a point where a suppressive regimen could be re-introduced and did not complete follow-up. All children in the ritonavir-boosted lopinavir group with viral failure re-suppressed without regimen change despite two of three having M184V and the other, E138A (Table 3).
Table 3.
Details on seven children with viral failure, defined as confirmed (i.e. two or more) HIV RNA measurements >1000 copies/ml by 48 weeks after randomization
| Sex | Age at treatment start (months) |
Other two antiretrovirals |
HIV RNA at failure (copies/ml) |
Time after randomization of first failure (months) |
Age at first failure (years) |
Change in regimen? | Re- suppress? |
Protease resistance mutations at failure |
Reverse transcriptase resistance mutations at failure |
HIV-1 Subtype |
|---|---|---|---|---|---|---|---|---|---|---|
| Ritonavir-boosted Lopinavir group | ||||||||||
| Girl | 9.9 | Abacavir lamivudine |
1925 | 5.5 | 5.2 | No | Yes | None | M184V | C |
| Boy | 6.2 | Abacavir lamivudine |
1370 | 11.5 | 4.1 | No | Yes | None | M184V | C |
| Boy | 22.8 | Abacavir lamivudine |
744682 | 11.5 | 4.2 | No | Yes | None | E138A | C |
| Efavirenz group | ||||||||||
| Boy | 5.7 | Abacavir lamivudine |
12785 | 3.5 | 5.5 | Changed to ritonavir- boosted lopinavir |
Yes | None | M184I, K103N, E138A, N348I |
C |
| Girl | 11.7 | Stavudine lamivudine |
88390 | 11.3 | 4.5 | Treatment interrupted due to toxicity, restarted efavirenz |
Yes | None | None | C |
| Girl | 4.7 | Stavudine lamivudine |
16120 | 3.8 | 4.0 | Changed to lamivudine Monotherapy |
No | None | M184V, K103N | C |
| Boy | 2.5 | Stavudine lamivudine |
1025 | 3.4 | 4.0 | Changed to ritonavir- boosted lopinavir |
Yes | None | M184V, K103N | C |
There were no differences in viral outcomes in those receiving stavudine vs abacavir. Effects of efavirenz vs ritonavir-boosted lopinavir were also unchanged if stratified by stavudine vs abacavir.
Secondary outcomes
The lipid profile of children in the efavirenz group was better than that of the ritonavir-boosted lopinavir group 40 weeks after randomization. Children in the efavirenz group had lower total cholesterol, LDL, and triglycerides than the ritonavir-boosted lopinavir group (Table 4).
Table 4.
Lipids at baseline and 40 weeks post randomization among HIV-infected children remaining on a ritonavir-boosted lopinavir-based regimen or switched to an efavirenz-based regimen
| Baseline | Week 40 | |||||
|---|---|---|---|---|---|---|
| Measurement | Ritonavir-boosted Lopinavir (N=148) |
Efavirenz (N=150) |
P-Value | Ritonavir-boosted Lopinavir (N=148) |
Efavirenz (N=144) |
P-Value |
| Total Cholesterol, N (%) Acceptable: <170 mg/dL Borderline: 170-199 mg/dL Elevated: ≥200 md/dL Missing |
66 (44.6) 36 (24.3) 46 (31.1) 0 |
59 (39.3) 53 (35.3) 38 (25.3) 0 |
0.11 |
59 (40.7) 50 (34.5) 36 (24.8) 3 |
84 (58.7) 40 (28.0) 19 (13.3) 1 |
0.005 |
| LDL, N (%) Acceptable: <110 mg/dL Borderline: 110-129 mg/dL Elevated: ≥130 mg/dL Missing |
92 (62.6) 20 (13.6) 35 (23.8) 1 |
92 (61.3) 32 (21.3) 26 (17.3) 0 |
0.13 |
92 (63.5) 26(17.9) 27(18.6) 3 |
113 (79.0) 16 (11.2) 14 (9.8) 1 |
0.01 |
| HDL, N (%) Normal: ≥35 mg/dL Abnormal: <35 mg/dL Missing |
141 (95.3) 7 (4.7) 0 |
147 (98.0) 3 (2.0) 0 |
0.22 |
138 (95.2) 7 (4.8) 3 |
137 (95.8) 6 (4.2) 1 |
0.80 |
| Triglycerides, N (%) Normal: ≤150 mg/dL Abnormal: >150 mg/dL Missing |
112 (75.7) 36 (24.3) 0 |
126 (84.0) 24 (16.0) 0 |
0.07 |
112 (77.2) 33 (22.8) 3 |
128 (89.5) 15 (10.5) 1 |
0.005 |
P-values calculated from chi-square tests. Subjects with missing data were excluded from the analysis.
Both groups remained within the normal range for CD4 percentages and CD4 cell counts. At baseline and at 24 weeks there were no differences in CD4 percentages or CD4 cell counts between the groups. By 48 weeks, CD4 percentage was 2.88 (95% CI: 1.26, 4.49) units higher in the efavirenz than in the ritonavir-boosted lopinavir group. There were no significant differences in weight- or height-for-age z-scores between groups at visits after randomization. Neither anemia nor neutropenia were more common in the efavirenz group. ALT elevations were more common in the efavirenz group, but were primarily of grade 1 or 2. Skin manifestations did not differ across the groups. There were no deaths in either group and hospital admissions were rare. Two children in the efavirenz group were initiated on tuberculosis medication after randomization (eTable 1).
Four weeks after randomization, 26% of children in the efavirenz group reported trouble sleeping or having nightmares compared to no children in the ritonavir-boosted lopinavir group (p<.001). However, this difference between groups was no longer present after 8 weeks through the end of the study. There were no significant differences between the groups in behavioral problems on the SDQ although a high proportion of children in both groups had an abnormal SDQ total difficulties score (30.8 vs 34.0%, p=0.30). Nausea was reported slightly more frequently in the efavirenz group (28.0 vs 17.6%, p=0.03). Other symptoms were similar across the groups (eTable 1).
Two children experienced seizure disorders suspected to be related to efavirenz. One child was diagnosed with absence seizures, thought related to delayed efavirenz clearance due to CYP 2B6-516 T/T homozygosity which resolved after discontinuing the drug (previously reported15). The other child was diagnosed with generalized tonic-clonic seizures attributed to abnormally high efavirenz blood levels of 20 mg/L (reference range 1-4 mg/L)16 resulting from two genetic mutations – CYP2B6 516 G/T and CYP2B6 785 A/G. The seizures resolved after efavirenz was stopped and ritonavir-boosted lopinavir re-started.
Over all follow-up visits, 73.2% of children in the ritonavir-boosted lopinavir group returned the medication containers for adherence calculations and 86.1% of children in the efavirenz group did so. Non-adherence defined as returning >10% of the expected volume of either ritonavir-boosted lopinavir or efavirenz was similar in the two groups: 12.9% and 15.8% respectively (p=0.23). Non-adherence with the other two drugs in the regimen was also similar in the two groups (eTable 1).
Discussion
Switching to efavirenz-based therapy compared with continuing ritonavir-boosted lopinavir-based therapy did not result in significantly higher rates of viral rebound (i.e., HIV RNA >50 copies/ml) or viral failure (i.e., confirmed HIV RNA >1000 copies/ml) in this cohort of nevirapine-exposed children initially virally-suppressed on ritonavir-boosted lopinavir.
There are several potential advantages of switching to efavirenz, including preserving ritonavir-boosted lopinavir for second-line treatment, harmonizing pediatric and adult treatment guidelines, and reducing the cost of national programs. Ritonavir-boosted lopinavir syrup is a medication of poor palatability that has to be dosed twice-per-day and requires a cold storage to maintain long-term stability.6 New formulations will address some, but not all, of these limitations.17 Efavirenz offers a more adherence-friendly formulation, including being dosed once-daily. Since abacavir and lamivudine can also be used once-daily in children already virally-suppressed18 a once-daily pediatric fixed-dose combination could be formulated. In adults, simplifications in formulations have had significant adherence benefits.19
Tuberculosis is a common co-infection among children with HIV in South Africa.20,21 Rifampicin is a potent inducer of the cytochrome P450 enzyme class. The use of ritonavir-boosted lopinavir as part of pediatric ART is problematic in that lopinavir is metabolized by these enzymes which may in turn lead to diminished virologic efficacy. WHO guidelines for co-treatment of tuberculosis and HIV in children ≥ 3 years recommend addressing this issue by substituting efavirenz or a third NRTI for ritonavir-boosted lopinavir. Alternatively, the guidelines also suggest the option of using ritonavir-boosted lopinavir combined with additional ritonavir to maintain adequate lopinavir concentrations; the South African pediatric treatment guidelines recommend this latter approach.3,22 The poor palatability of ritonavir-boosted lopinavir is made considerably worse by the introduction of additional ritonavir syrup thus complicating adherence and possibly affecting virological suppression. Reduced viral suppression in young children on tuberculosis co-treatment when on a ritonavir-boosted lopinavir-based regimen has been previously reported.23-26 Although efavirenz is also metabolized by the same hepatic enzyme system, there does not appear to be a requirement to increase its dose, nor is there the need for any additional drug to boost its levels, thereby making this regimen option the more suitable alternative.
Long-term use of ritonavir-boosted lopinavir also raises concerns about metabolic toxicities, and our trial demonstrated a more favorable lipid profile with efavirenz, with lower levels of total cholesterol, LDL, and triglycerides. Our data suggest that further benefits of efavirenz include less frequent low level viremia and more robust CD4 cell response. We previously noted both of these benefits with nevirapine relative to ritonavir-boosted lopinavir-based therapy.10, 11 Although this study was designed as a non-inferiority study, we observed higher rates of low level viral rebound in the ritonavir-boosted lopinavir group. One study has suggested that low level viremia may portend a higher risk of future virologic failure.27 The dose of ritonavir-boosted lopinavir used in this study was on the lower end of what is now recommended, which may have played a role in low level viremia. No statistically significant differences in adherence were observed between the groups.
Neuropsychiatric adverse effects of efavirenz are well known. Efavirenz adverse events are generally associated with high drug concentrations, and specific genetic mutations predispose to inadequate drug clearance.28 In our study two children developed seizure disorders while receiving efavirenz; both were likely to be due to these issues and resolved with change in treatment. Almost a quarter of children reported having had nightmares or trouble sleeping 4 weeks after switching to efavirenz, but these adverse events subsequently resolved. Efavirenz is associated with psychiatric adverse events in adults, but its manifestations in children are less well described.29 Reassuringly, on a standardized instrument assessing emotional/behavioral problems there was no additional evidence of increased risk. In summary, although for the majority of children the drug was safe and well-tolerated, clinical vigilance is necessary.
We believe it is unlikely that shifts away from short duration nevirapine for PMTCT to wider use of efavirenz-based ART for mothers combined with longer durations of nevirapine prophylaxis for infants in the current era will affect the generalizability of our results. This is because a single base-pair mutation is sufficient to confer resistance to efavirenz. There is now an extensive body of literature that almost all infected infants exposed to even one dose of nevirapine will have at least one of these mutations.30, 31 Hence, there is a ceiling effect that longer durations of nevirapine cannot plausibly exceed. One would need to speculate that resistance selected in the current era is qualitatively different either in persistence or in mutation mix but this has not been observed in recent surveillance data.9 Nevertheless, ongoing monitoring of the resistance profile of children acquiring infection in the current era is warranted. Our strategy requires viral load monitoring to identify children who may benefit from switching. Post-switch virological monitoring is also advisable. We are conducting a follow-up study of the children in the trial reported herein. Our strategy fits well with efforts to expand access to viral load testing within treatment programs in low resource settings.34
Three of four children in the efavirenz group with viral failure had K103N. They also had M184V, as did two of three children in the ritonavir-boosted lopinavir group with viral failure. All the children in the ritonavir-boosted lopinavir group re-suppressed on the same regimen (including lamivudine) with adherence counseling. Both children in the efavirenz group switched back to ritonavir-boosted lopinavir re-suppressed. It is interesting that K103N predominated in viral failure in these children as all studies of PMTCT-exposed infants prior to ART initiation have observed a predominance of Y181C. This contrasts to patterns observed among their mothers and other adults.30, 31 Y181C confers high-level resistance to nevirapine, but only intermediate resistance to efavirenz.32 This may explain why we observed excellent efficacy of efavirenz in this trial, but more viral failure in our prior trial attempting the reintroduction of nevirapine following induction of viral suppression with ritonavir-boosted lopinavir,10, 11 both trials among nevirapine-exposed children.
Several limitations should be considered in interpreting this study. All children in our trial were over 3 years of age and although the eligibility criteria only required a year of treatment, the mean duration of treatment was 3.5 years. Thus we are not able to determine whether such a long initial period of treatment with ritonavir-boosted lopinavir is necessary. A further limitation is that our results cannot be generalized to children under three years of age. We designed our trial before efavirenz dosing for children under three years of age was available and dosing below this age remains controversial, given volatility in drug metabolism related to enzyme system maturation.33 Thirdly, the treatment strategy was designed for a population suppressed on their ritonavir-boosted lopinavir-based regimen and we cannot address the generalizability of this approach to children failing ritonavir-boosted lopinavir.
There is little guidance available as to what clinicians ought to do when confronted with a child above the age of three years who began treatment with ritonavir-boosted lopinavir. As a result, it has been left to individual interpretation and there are anecdotal reports of clinicians switching to efavirenz anyway in the absence of data to support such a practice. This study provides evidence to support the safety and efficacy of switching to efavirenz, the recommended drug for children older than 3 years, among virally suppressed children.
Conclusions
Among HIV-infected children exposed to nevirapine for PMTCT and initially virally-suppressed on ritonavir-boosted lopinavir-based therapy, switching to efavirenz-based therapy compared with continuing ritonavir-boosted lopinavir-based therapy did not result in significantly higher rates of viral rebound or viral failure.
Supplementary Material
Acknowledgments
Funding/Support: The study was supported by grants (HD061255) from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We would like to thank Lynne Mofenson, M.D. (at the time at the National Institutes for Child Health and Human Development) for assistance with the study as well as the members of the Data Safety and Monitoring Board: Mark Cotton, M.D. (Stellenbosch University), Brian Eley, M.D. (University of Cape Town), Mary-Glenn Fowler, M.D. (Johns Hopkins University), Carl Lombard, Ph.D. (South African Medical Research Council), Paul E. Palumbo, M.D. (Geisel School of Medicine at Dartmouth), and Avy Violari, M.D (Perinatal HIV Research Unit). The named individuals were not compensated for their participation. We also thank all members of the study team in South Africa and New York. We appreciate the dedication and commitment to the study of the participants and their caregivers.
Footnotes
Trial Registration: Clinical Trials.gov NCT01146873
Author Contributions: Drs. Coovadia and Kuhn have had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures:
Previous Presentation: Coovadia A, Abrams EJ, Strehlau R, Shiau S, Pinillos F, Martens L, Hunt G, Tsai WY, Kuhn L, NEVEREST 3 Study Team. Virologic Efficacy of Efavirenz Maintenance Therapy in Nevirapine Prophylaxis-Exposed Children (Oral Abstract 73) Conference on Retroviruses and Opportunistic Infections (CROI). Boston, MA; 3-6 March 2014.
References
- 1.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 2.Abrams EJ, Simonds RJ, Modi S, Rivadeneira E, Vaz P, Kankasa C, et al. PEPFAR scale-up of pediatric HIV services: innovations, achievements, and challenges. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S105–12. doi: 10.1097/QAI.0b013e31825cf4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Jun, 2013. ISBN 978 92 4 150572 7 (NLM classification: WC 503.2) 2013. [PubMed]
- 4.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363(16):1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366(25):2380–9. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohn AH, Nuttall JJ, Zhang F. Sequencing of antiretroviral therapy in children in low- and middle-income countries. Curr Opin HIV AIDS. 2010;5(1):54–60. doi: 10.1097/COH.0b013e3283339bd8. [DOI] [PubMed] [Google Scholar]
- 7.Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58(9):1297–307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommendations for a Public Health approach 2010;whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf?ua=1:(accessed December 14, 2014) [PubMed]
- 9.Kuhn L, Hunt G, Technau KG, Coovadia A, Ledwaba J, Pickerill S, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS. 2014;28(11):1673–8. doi: 10.1097/QAD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coovadia A, Abrams EJ, Stehlau R, Meyers T, Martens L, Sherman G, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304(10):1082–90. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn L, Coovadia A, Strehlau R, Martens L, Hu CC, Meyers T, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis. 2012;12(7):521–30. doi: 10.1016/S1473-3099(12)70051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorthy A, Kuhn L, Coovadia A, Meyers T, Strehlau R, Sherman G, et al. Induction therapy with protease-inhibitors modifies the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children. Clin Infect Dis. 2011;52(4):514–21. doi: 10.1093/cid/ciq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 14.Pillay V, Ledwaba J, Hunt G, Rakgotho M, Singh B, Makubalo L, et al. Antiretroviral drug resistance surveillance among drug-naive HIV-1-infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antivir Ther. 2008;13(Suppl 2):101–7. [PubMed] [Google Scholar]
- 15.Strehlau R, Martens L, Coovadia A, Dandara C, Norman J, Maisel J, et al. Absence seizures associated with efavirenz initiation. Pediatr Infect Dis J. 2011;30(11):1001–3. doi: 10.1097/INF.0b013e318223b680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirt D, Urien S, Olivier M, Peyriere H, Nacro B, Diagbouga S, et al. Is the recommended dose of efavirenz optimal in young West African human immunodeficiency virus-infected children? Antimicrob Agents Chemother. 2009;53(10):4407–13. doi: 10.1128/AAC.01594-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musiime V, Fillekes Q, Kekitiinwa A, Kendall L, Keishanyu R, Namuddu R, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66(2):148–54. doi: 10.1097/QAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 18.Musiime V, Kendall L, Bakeera-Kitaka S, Snowden WB, Odongo F, Thomason M, et al. Pharmacokinetics and acceptability of once- versus twice-daily lamivudine and abacavir in HIV type-1-infected Ugandan children in the ARROW Trial. Antivir Ther. 2010;15(8):1115–24. doi: 10.3851/IMP1695. [DOI] [PubMed] [Google Scholar]
- 19.Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, et al. Systematic review and meta-analysis: Patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health. 2014;19(5):501–13. doi: 10.1111/tmi.12297. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Global Tuberculosis Report. WHO/HTM/TB/2014.08 2014.
- 21.Martinson NA, Moultrie H, van Niekerk R, Barry G, Coovadia A, Cotton M, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis. 2009;13(7):862–7. [PMC free article] [PubMed] [Google Scholar]
- 22.National Department of Health South Africa Guidelines for the management of HIV in children. 2nd 2010.
- 23.Frohoff C, Moodley M, Fairlie L, Coovadia A, Moultrie H, Kuhn L, et al. Antiretroviral therapy outcomes in HIV-infected children after adjusting protease inhibitor dosing during tuberculosis treatment. PLoS One. 2011;6(2):e17273. doi: 10.1371/journal.pone.0017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reitz C, Coovadia A, Ko S, Meyers T, Strehlau R, Sherman G, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis. 2010;201(8):1121–31. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Impact of tuberculosis cotreatment on viral suppression rates among HIV-positive children initiating HAART. AIDS. 2011;25(1):49–55. doi: 10.1097/QAD.0b013e32833f9e04. [DOI] [PubMed] [Google Scholar]
- 26.Soeters HM, Napravnik S, Patel MR, Eron JJ, Jr., Van Rie A. The effect of tuberculosis treatment on virologic and CD4+ cell count response to combination antiretroviral therapy: a systematic review. AIDS. 2014;28(2):245–55. doi: 10.1097/01.aids.0000434936.57880.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grennan JT, Loutfy MR, Su D, Harrigan PR, Cooper C, Klein M, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205(8):1230–8. doi: 10.1093/infdis/jis104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahri K, Ensom MH. Efavirenz and nevirapine in HIV-1 infection : is there a role for clinical pharmacokinetic monitoring? Clin Pharmacokinet. 2007;46(2):109–32. doi: 10.2165/00003088-200746020-00002. [DOI] [PubMed] [Google Scholar]
- 29.Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf. 2013;12(6):841–6. doi: 10.1517/14740338.2013.823396. [DOI] [PubMed] [Google Scholar]
- 30.Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L. Impact of antiretroviral drugs in pregnant women and their children in Africa: HIV resistance and treatment outcomes. J Infect Dis. 2013;207(Suppl 2):S93–100. doi: 10.1093/infdis/jit110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ton Q, Frenkel L. HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission. Curr HIV Res. 2013;11(2):126–36. doi: 10.2174/1570162x11311020005. [DOI] [PubMed] [Google Scholar]
- 32.Basson AE, Rhee SY, Parry CM, El-Khatib Z, Charalambous S, De Oliveira T, et al. Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2015;59(2):960–71. doi: 10.1128/AAC.04215-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukonzo JK. The challenge of paediatric efavirenz dosing: implications and way forward for the sub-Saharan Africa. AIDS. 2014;28(13):1855–7. doi: 10.1097/QAD.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 34.Roberts T, Bygrave H, Fajardo E, Ford N. Challenges and opportunities for the implementation of virological testing in resource-limited settings. J Int AIDS Soc. 2012;15(2):17324. doi: 10.7448/IAS.15.2.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.