INTRODUCTION
Since its advent in 1982, implantable venous access systems have become increasingly utilized in health care as a convenient way to perform repeated blood draws and administer medications, particularly chemotherapy (1, 2). Between 1992 and 2011, placement of long-term central venous access devices in Medicare beneficiaries increased by 303% nationally, from 76,444 to 307,838 (3). Of the devices, chest ports play a critical role in patient care and minimize disruption to a patient’s life-style (4, 5).
While chest port placement was initially performed in an operating room (OR) by surgeons, there has been an increasing transition to placement by interventional radiologists (IR) in imaging suites (6). However, surgeons continue to be the dominant provider of chest port implantation at 62.4% in 2011, though radiologists increasingly provide these services, and have increased from placing 17.4% in 2004 to 27.0% in 2011 (3). Due to changes in health care reimbursements from fee for service to bundled care, healthcare providers must make cost-driven clinical decisions while maintaining quality of care (7, 8). Many previous studies have evaluated chest port complication and infection rates, but few have reviewed cost (9–13).
As there are similar training and certification requirements for interventional radiologists and surgeons, we hypothesize that there will be no difference in complication rates between services. However, we maintain that cost of implantation in IR will be less than in an OR setting, making IR placement more cost effective, which has been shown in prior studies (14). Therefore, the purpose of this study is to evaluate complication rates at a single institution as well as determine whether the overall cost of chest port implantation differs significantly depending on the specialty providing the service.
MATERIALS AND METHODS
The institutional review board (IRB) approved this retrospective study and waived informed consent. This study is HIPAA compliant. Consecutively placed chest ports in the departments of surgery and interventional radiology between 10/22/2010 and 2/26/2013 were queried to compare rates of complication. Because cost information was not available on all morbidity patients, cost data on a separate cohort of Medicare patients was collected from the hospital’s financial departments on IR placed ports from 8/9/2012–2/26/2013 and OR placed ports from 3/9/2012–2/15/2013.
Inclusion and Exclusion Criteria
Included patients underwent isolated port placements (no other simultaneous procedures), had documented encounters from initial chest port insertion to at least 30 days after chest port removal (to standardize follow up) or had chest ports in place or death. Patients lacking any of these types of post-procedural encounters were excluded.
Data Extraction
The medical records were queried for patients’ gender, age, number of catheter days (dwell time), inpatient/outpatient status, co-morbidities, indication, diagnosis, antibiotics, port type, image guidance, venous access site, and catheter tip position. Diagnosis was broadly categorized into solid tumors (e.g.: breast, pancreatic, colon, etc.) and hematologic malignancies (e.g.: lymphoma, etc.).
Procedural complications were documented: catheter tip malposition, arrhythmia, pneumothorax, and pleural effusion. Arrhythmias were attributed to chest port if they occurred during insertion or were documented as chest port related.
Post-procedural complications were documented and divided into early (within 30 days of chest port placement) and late (>30 days) in accordance with similar studies (11, 12). These included: catheter thrombosis/tip occlusion, venous thrombosis, non-functioning port, fibrin sheath, wound dehiscence, port leakage, intolerance to port, inflammation/necrosis/scarring of skin over implant site, flipped port, and port/catheter migration. Malposition was defined as chest ports whose catheter tip did not end in the cavoatrial junction, or lower 1/3 of the superior vena cava (SVC) and inability to aspirate requiring thrombolytic administration/removal. Catheter thrombosis/tip occlusion was defined as inability to aspirate requiring thrombolytic administration. Venous thrombosis was identified by ultrasound or venography. A non-functioning port was defined as those requiring replacement, as thrombolytic administration was ineffective or the port could not be accessed. A fibrin sheath was diagnosed by venography or if the patient required a stripping procedure.
We evaluated post-procedural infections including: catheter related blood stream infection, port pocket infection, and cellulitis. Catheter related blood stream infection was defined as a positive blood cultures and removal, while a port pocket infection was defined as positive blood cultures, positive tip cultures, or pus/cellulitis at the site of chest port insertion. Cellulitis was defined by erythema/tenderness at port site requiring the administration of antibiotics. Infections requiring chest port removal were considered a complication of the chest port; however, positive blood cultures where chest ports were not removed were not included in the chest port infection rate.
Port Placement Procedure
Chest ports in the IR department were placed either by an interventional radiologist, fellow, resident or advanced practicing nurse practitioners (APNP). Patients received conscious sedation (midazolam/fentanyl) administered by an IR nurse. Peri-procedural antibiotics were given, per protocol. Under direct ultrasound guidance, access to the vein was obtained. Fluoroscopy was used to determine optimal catheter tip position.
In contrast, surgically placed chest ports were placed in the operating room by a surgeon, resident, or physician assistant (PA). Patients received monitored anesthesia care (MAC) administered by a certified registered nurse anesthetist (CRNA) or an anesthesiologist. Ultrasound and/or fluoroscopy was used by some operators to access the vein. A chest radiograph was obtained in the immediately post-operative period.
All imaging during chest port insertion (IR) and/or immediate postoperative imaging (OR) was reviewed for catheter tip position.
Average room time was calculated based on time from patient entering the room to leaving for OR patients, compared to patient entering the room to technologist ending the encounter for IR patients (patient still in the room).
Cost
Hospital cost information (or cost incurred by the institution for the use of the facility) from the radiology and surgery financial departments for all isolated chest port placement procedures on consecutive Medicare outpatients were collected. Due to a change in the hospital’s electronic medical record system, only 6 months of information was accessible for IR patients. The costs were reviewed to remove procedure/pharmacy costs unrelated to chest port placement. Thus, cost and room time information was obtained for 100 IR placed ports and 49 OR placed ports. The cost information collected in this study excludes professional service fees (MD and CRNA) as well as charges billed to the payers and societal costs. The cost for each of the service lines was reviewed and extraneous costs were removed, in an attempt to standardize the methodology for collection of costs.
Costs to perform this procedure in the hospital outpatient setting were divided into variable cost (expenses that change with activity per service unit) and fixed costs (expenses that do not change with activity). The data was stratified into 4 components, and when applicable included appropriate pharmacy costs: total variable labor (TVL), total variable supply (TVS), total variable other (TVO), total fixed cost (TFC).
Room (IR suite or OR) TVL includes: operative staff salary (nurse, technologist, support staff) related to operative episode including anesthesia and recovery. Pharmacy TVL includes: staff salary (pharmacist, technician).
Room TVS: port, needles, syringes, surgical packs, gowns, sutures, sterilization equipment. Pharmacy TVS: medication related costs (anesthetic agents, sedatives, antibiotics).
Room TVO: lab testing, perfusion services, contracted labor (Pharmacy TVO was unavailable)
-
Room/Pharmacy TFC (variable between institutions): environmental control (temperature regulation), equipment maintenance, and salary of management and support staff.
(Note: radiography, fluoroscopy, and ultrasound costs were included across all non-pharmacy categories.)
Statistical Analysis
From each group, 239 eligible patients were included in this study, which was determined using 80% power to determine a difference of 15%. Data analysis was performed with SAS 9.3. (SAS institute, Cary, NC). Student’s t-test, X2 test, and Fisher’s exact test were performed on IR and OR data separately to identify if the following were related to an increased early or late complication/infection rate: demographics, catheter tip position, inpatient vs. outpatient status, indication, diagnosis.
Bivariate analysis was performed to identify if the following risk factors were related to an increased early complication/infection rate: age, gender, placement in IR vs. OR, tip position, diagnosis, inpatient status, use for chest port, comorbidities, and operator level of training. A binary variable was created for each comorbidity and the association between the previously listed risk factors was tested with the outcome of early complication/infection.
Statistical calculations for cost data was performed using STATA software Version 12.1 (StataCorp, Texas, USA). Continuous data was summarized as mean and standard deviation. Non-parametric tests for heterogeneity were performed using Kruskal-wallis method. Alpha was fixed at 0.05 for statistical significance.
RESULTS
Of 426 from IR and 402 OR patients, we included 239 consecutive patients from each cohort according to the inclusion criteria. All attempted port placements were successful. Six attending IRs and 14 attending surgeons staffed these procedures. One of 2 APNPs were primary operators in 66% of IR suite cases and 1 of 2 PAs were primary operators in 20% of OR cases. There was not a statistically significant difference in complication rates between APNP and PA vs. MD placement of chest ports. In 34% of IR cases and 57% of OR cases residents were involved.
Patient Characteristics
The patient demographics were similar with respect to age, total chest port dwell time, and inpatient vs. outpatient status (Table 1). X2 analysis demonstrated that more females had ports placed in the surgery department (p = 0.017). The IR service placed more chest ports for hematologic malignancies 30/239 (13%) versus 2/239 (1%) in the surgical group (p <.001).
Table 1.
Patient Demographics and Clinical Features for Chest Ports Placed in IR suite vs. OR
| Demographics | IR (%) | OR (%) |
|---|---|---|
| Patients | 239 | 239 |
| Female* | 142 (59) | 167 (70) |
| Male | 97 (41) | 72 (30) |
| Mean age | 57 | 58 |
| Age range | 21–83 | 22–85 |
| Mean total catheter days | 301.5 | 311 |
| Range catheter days | 7–570 | 3–796 |
| Total catheter days | 72,054 | 74,285 |
| Inpatient at time of insertion | 11 (5) | 12 (5) |
| Outpatient at time of insertion | 228 (95) | 227 (95) |
| Comorbidities | ||
| Cardiovascular disease | 121 (51) | 114 (48) |
| Pulmonary disease | 50 (21) | 32 (13) |
| Renal disease | 29 (12) | 8 (3) |
| Hepatic disease | 12 (5) | 7 (3) |
| Coagulopathy | 2 (1) | 5 (2) |
| HIV status | 3 (1) | 0 |
| Indication for port placement | ||
| Chemotherapy | 228 (95) | 234 (98) |
| Other† | 11 (5) | 5 (2) |
| Type of Malignancy | ||
| Solid Tumor | 199 (83) | 232 (97) |
| Breast | 38 (16) | 96 (40) |
| Pancreatic | 4 (2) | 85 (36) |
| Colon | 19 (8) | 22 (9) |
| Lung | 35 (15) | 1 (0.5) |
| Esophageal | 12 (5) | 5 (2) |
| Other‡ | 91 (38) | 23 (10) |
| Hematologic tumor | 30 (13) | 2 (1) |
| Lymphoma | 20 (8) | 2 (1) |
| Other§ | 10 (4) | 0 |
Statistically different between the cohorts (p = 0.017)
Other: indications for chest port placement include: sickle cell anemia, cystic fibrosis, Fabry disease, Crohn’s disease, total parenteral nutrition, hypomagnesia
Other: solid tumors include cancer of the: skin, brain, head/face/neck, lung, peritoneum, stomach, liver, appendix, adrenal glands, kidney, bladder, prostate, testicles, ovaries, endometrium, cervix, vulva, also: neuroendocrine carcinoma, leiomyosarcoma, angiosarcoma, synovial sarcoma, myxofibrosarcoma, spindle-cell sarcoma.
Other: hematologic tumors include: acute or chronic lymphocytic leukemia, acute promyelocytic leukemia, multiple myeloma.
Procedure Characteristics
Peri-procedural antibiotics were given to all patients in the radiology suite. For 2 of 239 (1%) surgical patients, retrospective chart review was unable to determine if antibiotics were administered. Of the remaining 237, 172 (73%) received antibiotics.
Interventional radiologists and surgeons placed 1 of 3 types of ports (Bard Peripheral Vascular Port: IR (n=125), OR (n=237), P.A.S. Port Power P.A.C.: IR (n=109), PortaCathII Deltec: OR (n=2). All IR chest ports were placed with both ultrasonography and fluoroscopy. Aside from 2 procedures, all surgeons used fluoroscopic guidance followed by a post-operative chest radiograph. Ultrasound was used in 79/239 (33%) of OR chest port placements. The site of venous access varied significantly between the two groups as 96% (229/239) of IR cases were internal jugular vein (IJ) and 90% (216/239) of OR cases were subclavian vein (SCV) (p < 0.001).
Complications
Early complications (excluding infection and including patients with multiple complications only once) occurred in 9.2% (22/239) of IR patients and 13.4% (32/239) of OR patients (p = .197), while 28.9% (69/239) and 27.6% (66/239) of IR and OR patients respectively had late port complications (p = .805).
Only patients with surgically placed chest ports experienced non-functioning ports, arrhythmias, and tip malposition. Port leakage, pneumothorax, pleural effusion, hemoptysis, and necrosis overlying the chest port diaphragm each occurred in 1 surgery patient. Only patients with radiologically placed chest ports experienced pain requiring port removal (n=1) or a flipped port (n=1).
A total of 9/239 (3.8%) radiologically placed ports and 18/239 (7.5%) surgically placed ports required early removal due to a non-infection related complication. IR ports required early removal for: fibrin sheath (2%, 4/239), catheter migration (1%, 2/239), pain (<.05%, 1/239), venous thrombus (<.05%, 1/239), and wound dehiscence (<.05%, 1/239). OR ports required early removal due to non-function (3%, 8/239), venous thrombus (3%, 6/239), malposition (1%, 2/239), inflammation/scarring (<.05%, 1/239) and dehiscence (<.05%, 1/239).
Early infections occurred in 4% (9/239) of patients with IR placed ports and 2% (4/239) of patients with OR placed ports. Of the 3 radiology patients with catheter related blood stream infections, all were receiving chemotherapy for solid tumors. One patient had a low ANC (1.47 cells/mm3) on day of insertion.
Late infections occurred in 4% (9/239) of patients in each population. Of the 3 IR patients with catheter related blood stream infections, only one patient had a hematologic malignancy. Of the 6 OR patients with catheter related blood stream infections, 5 had chest ports placed for solid tumors and 1 for total parenteral nutrition. For all patients with late infections, ANC levels were normal within 30 days of chest port placement. The total number of infections for patients with IR placed ports was 18/239 (8%) compared to 13/239 (5%) OR patients, which translates to .25 infections/1000 catheter days and .18 infections/1000 catheter days respectively.
Bivariate analysis of IR and OR patients’ early complication and infection rates demonstrated that females had a higher incidence of early complication/infection (p = 0.015) (Table 3). A pulmonary comorbidity also had a higher incidence of early complication (p = 0.032). Controlling for pulmonary disease, the odds of complication or infection for females is 2.1 fold higher than males (p = 0.022). Controlling for gender, the odds of complication or infection for patients with pulmonary disease is 1.87 fold higher than those without pulmonary disease (p = 0.049).
Table 3.
Bivariate Analysis of IR and OR Complication/Infection within 30 days
| Variables | Total | Yes | No | P Value |
|---|---|---|---|---|
| N=478 | N=64 (%) | N=414 (%) | ||
| Age | ||||
| Median (min–max) | 59 (21–89) | 60 (22–80) | 58 (21–89) | 0.672 |
| Mean ± SD | 57.8 ±12.6 | 58 ± 13.1 | 57.8 ± 12.5 | 0.893 |
| Gender | 0.015 | |||
| Male | 169 | 14 (8) | 155 (92) | |
| Female | 309 | 50 (16) | 259 (84) | |
| Group | 0.788 | |||
| IR | 239 | 31 (13) | 208 (87) | |
| OR | 239 | 33 (14) | 206 (86) | |
| Catheter tip position | 0.226 | |||
| RA/CAJ/low/SVC | 256 | 30 (12) | 226 (88) | |
| Other* | 219 | 34 (15.5) | 185 (84.5) | |
| Missing | 3 | 0 | 0 | |
| Diagnosis | 0.642 | |||
| Solid tumor | 431 | 56 (13) | 375 (87) | |
| Hematologic tumor | 32 | 6 (19) | 26 (81) | |
| Other† | 15 | 2 (13) | 13 (87) | |
| Inpt vs Outpt | 0.342 | |||
| Out | 455 | 59 (13.0) | 396 (87.0) | |
| In | 23 | 5 (21.7) | 18 (78.3) | |
| Chest port use | 1 | |||
| Chemotherapy | 462 | 62 (13) | 400 (87) | |
| Other | 16 | 2 (12.5) | 14 (87.5) | |
| Comorbidity | ||||
| Pulmonary | 0.032 | |||
| No | 396 | 47 (12) | 349 (88) | |
| Yes | 82 | 17 (21) | 65 (79) | |
| Cardiovascular | 0.082 | |||
| No | 243 | 39 (16) | 204 (84) | |
| Yes | 235 | 25 (11) | 210 (89) | |
| Renal | 1 | |||
| No | 441 | 59 (13) | 382 (87) | |
| Yes | 37 | 5 (13.5) | 32 (86.5) | |
| Hepatic | .762 | |||
| No | 459 | 62 (13.5) | 397 (86.5) | |
| Yes | 19 | 2 (10.5) | 17 (89.5) | |
| MD vs NP/PA | 0.668 | |||
| MD | 272 | 38 (14) | 234 (86) | |
| NP/PA | 206 | 26 (13) | 189 (87) |
Other: middle/upper SVC, R/L Brachiocephalic vein, jnc L BCV/SCV
Other: sickle cell anemia, cystic fibrosis, Fabry disease, Crohn’s disease, total parenteral nutrition, hypomagnesia
Bivariate analysis of IR patients alone demonstrates that inpatient vs. outpatient status was significantly related to complications (Table 4). There was also a significant correlation between pulmonary comorbidity and early complication in IR patients.
Table 4.
Bivariate Analysis: IR and OR Patients with Early Complications
| Variables | IR | P Value | OR | P Value |
|---|---|---|---|---|
| n=23 (%) | n=32 (%) | |||
| Age | 0.314 | 0.161 | ||
| Median (min–max) | 60 (22–78) | 60 (34–80) | ||
| Age | 0.199 | 0.21 | ||
| Mean ± SD | 53.9 ± 14.9 | 60.8 ± 11.4 | ||
| Gender | 0.297 | 0.020 | ||
| Male | 7 (7) | 4 (6) | ||
| Female | 16 (11) | 28 (17) | ||
| Tip | 1.000 | 0.069 | ||
| RA/CAJ/LowSVC | 19 (10) | 4 (7) | ||
| Other | 4 (10) | 28 (16) | ||
| Diagnosis | 0.803 | 0.264 | ||
| Solid | 18 (9) | 201 (87) | ||
| Hematological | 4 (13) | 1 (50) | ||
| Other* | 1 (10) | 5 (100) | ||
| Indication | 1.000 | 0.618 | ||
| Chemotherapy | 22 (10) | 202 (86) | ||
| Other† | 1 (9) | 5 (100) | ||
| In vs Out | 0.014 | 0.231 | ||
| Out | 19 (8) | 195 (86) | ||
| In | 4 (36) | 12 (100) | ||
| MD vs NP | 0.924 | 0.250 | ||
| MD | 8 (10) | 163 (85.3) | ||
| NP | 15 (9.5) | 44 (92) | ||
| Comorbidity | ||||
| Pulmonary | 0.032 | 0.160 | ||
| No | 14 (7) | 182 (88) | ||
| Yes | 9 (18) | 25 (78) | ||
| Cardiovascular | 0.110 | 0.215 | ||
| No | 15 (13) | 105 (84) | ||
| Yes | 8 (7) | 102 (89.5) | ||
| Renal | 0.749 | 0.602 | ||
| No | 21 (10) | 201 (87) | ||
| Yes | 2 (7) | 6 (75) | ||
| Hepatic | 1.000 | 0.598 | ||
| No | 22 (10) | 200 (86) | ||
| Yes | 1 (8) | 0 |
Other: sickle cell anemia, cystic fibrosis, Fabry disease, Crohn’s disease, total parenteral nutrition, hypomagnesia
Other: magnesium infusions, iron infusions, lab draws, poor venous access
Cost
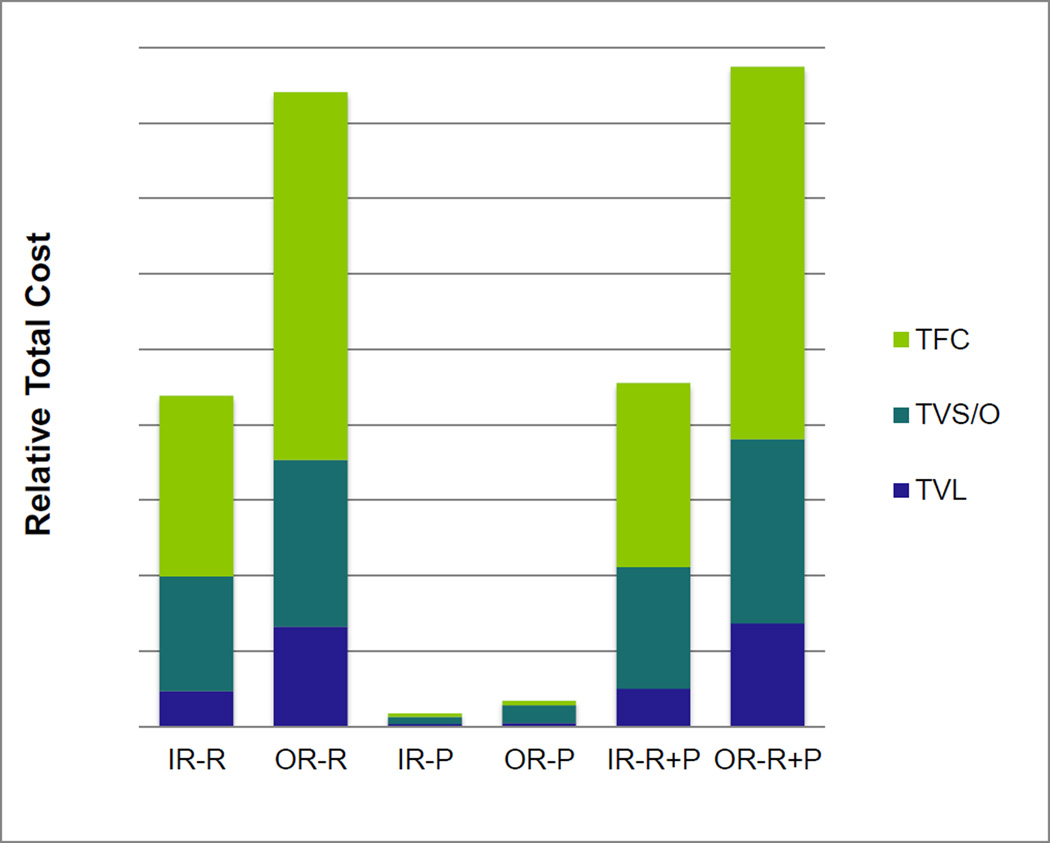
The relative total variable cost for labor, supply, other, and fixed costs for placing a port in the operating room and IR suite are reported in Figure 1. Room costs in all categories were higher in the operating room (p = 0.0001) as were the pharmacy costs (p = 0.0001).
Figure 1. Relative Cost of Chest Port Insertion in an IR Suite vs. OR.
The relative cost to the hospital to place a chest port in an IR suite is significantly less than compared to in the operating room for all of the variables. (p =.0001). The average total cost for an OR placed chest port is almost 2 times the cost in an IR suite due to higher TFC, TVL, and TVS/O costs (IR-R and OR-R). The IR and OR pharmacy costs (TVL contributes minimally to total pharmacy cost) shows the same trend (IR-P and OR-P). The total hospital cost for chest port placement is significantly lower for IR than for OR (p = 0.001) (IR-R+P and OR-R+P).
TFC: Total Fixed Cost, TVS/O: Total Variable Supply + Other Cost, TVL: Total Variable Labor Cost of the Room, IR-R: IR Room cost, OR-R: OR Room cost, IR-P: IR Pharmacy cost, OR-P: OR pharmacy cost, IR-R+P: IR Room + Pharmacy cost, OR-R+P: IR Room + Pharmacy cost
The variance of all components of room cost was greater for chest ports placed in the OR. This is also true of pharmacy costs with the exception of labor, which was estimated to be the same cost for each case giving a variance of 0 (based on combined 15 minutes of direct support time for OR pharmacist and pharmacy technician to ready, verify, and dispense orders for medications including anesthesia). Average room time for IR cases was found to be 37 minutes compared to 69 minutes for OR cases.
DISCUSSION
Given the current health care environment, at present there is a greater need for efficient use of resources, while maintaining a high standard of care (7). This study shows that patients at a single institution did not experience a significantly different rate of complication when interventional radiologists or surgeons placed chest ports; however, cost was significantly lower for chest ports placed in an IR suite.
Over the last several decades, the literature has reported varied rates of chest port complications. Most report either radiologic or surgical complications separately, while few compared them to one another. One such study concluded that “radiological placement is consistently more reliable than surgical placement” of central venous catheters as it found fewer complications and placement failures with IR than OR placed ports (9). A retrospective review of 368 ports by Sticca et al. demonstrated that early complications occurred in more frequently in IR (2.5%) than OR (1.1%) placed ports (15). Another study reporting over 100 port implantations found that overall complication rates ranged widely, from 4.3% to 46% (16). No similar studies found an increased complication rate in females or patients with pulmonary comorbidities. While articles that support chest port placement by one specialty over the other do exist, there is no consensus. As found in the current study, no difference in complication rates is seen between IR or OR chest port placement; therefore, cost becomes more significant.
There is a paucity of literature published on cost-effectiveness of IR vs. OR placed chest ports. A study of pediatric cancer patients found IR to be “slightly less costly than operative implantable venous access device (IVAD) insertion” when taking a societal costing perspective (including travels costs, productivity losses) (14). Another study comparing billed charges and reimbursement “revealed a financial advantage for surgically placed ports” (15).
The present study chose to evaluate cost from a hospital perspective, or cost incurred by the institution for the use of the facility but does not include depreciation, physician cost, or amortization. Charges billed to the patient were not included in this study as this information was not readily available and there is not a standard method of determining charges to the patient and/or their insurers. Professional service fees and Medicare reimbursement were not included as they would be the same between the two cohorts for the same procedure with the exception of anesthetist cost which would add only to the OR cohort. This study determined that the overall average cost to the hospital of placing a chest port in the OR was 193% greater than if placed in an IR suite. The lower IR room cost is likely influenced by the faster turnover time seen in the IR suite compared to the OR. The OR also requires a greater number of personnel (radiologic technologists, surgical technologists) not needed in the IR suites. OR pharmacy costs are driven up by the cost of MAC/local anesthesia used for the operative procedures (while only conscious sedation/local anesthesia are used in the IR suites). Additionally, since 100% of IR cases used antibiotics and only 73% of OR cases used antibiotics, this would mean costs would be higher in the surgical arm if perioperative antibiotic use was universal.
Professional service fees of radiologists, surgeons, anesthetists (MD and CRNA) were not included in this study as the intent was to review cost from a hospital perspective. Professional service fees between the two specialties should be the same as the Current Procedural Terminology code for this procedure is identical (17). Furthermore, because no significant difference in complications/infections was seen between the two services, costs associated with treatment and follow-up of complications were not included, as this was expected to be similar.
This study was limited by its retrospective design and small sample size, which precludes the ability to control for patient complexity, morbidity and risk, as statistical analysis could only demonstrate large effects. Financial data was not available for the same cohort of patients reviewed for complications. Due to difference in documentation between departments, IR room time was likely an underestimation of 10 minutes or less per case. Shorter time in the IR department was also likely influenced by the fact that APNPs were the primary operator in the majority of IR cases, while PAs were a minority of primary operators in the OR. Furthermore, trainees were involved in the majority of OR but not IR cases which may have led to increased time on the OR side. This in addition to the time added by the use of a CRNA or anesthesiologist may reflect the additional 32 minutes of OR room time.
Also, while the demographics were similar between the two groups, there was a difference in type of malignancy and gender, with IR placing more chest ports for hematologic malignancy. This likely reflects referral patterns, as surgical oncologists will place chest ports in their own patients, and women predominantly have their chest ports placed by their breast surgeons.
In conclusion, these results suggest that while surgeons and interventional radiologists have similar rates of complications, comparisons of hospital costs demonstrate that ports placed in an IR suite are more cost effective. A case could however be made for exclusively placing chest ports in a dedicated ambulatory setting by surgeons and/or interventionalists rather than in academic centers. In this way, both surgeons and interventional radiologists would be able to perform these procedures while minimizing overhead costs, thus decreasing cost to the patient. In academic hospitals, residents and fellows have the opportunity to learn how to perform these procedures and the cost of this education is intangible.
Table 2.
Total Numbers of Early and Late Chest Port Associated Complications
| Port Complications | EARLY (≤30 days after CPP*) |
LATE (>30 days after CPP*) |
||
|---|---|---|---|---|
| IR # (%) | OR # (%) | IR # (%) | OR # (%) | |
| Catheter thrombosis/tip occlusion | 21 (9) | 18 (8) | 60 (25) | 55 (23) |
| Catheter tip malposition† | 0 | 20 (8) | 0 | 0 |
| Venous thrombosis | 0 | 5 (2) | 2 (1) | 9 (4) |
| Infection | 9 (4) | 4 (2) | 9 (4) | 9 (4) |
| Other‡ | 1 (<1) | 9 (4) | 8 (3) | 9 (4) |
| TOTAL | 31 | 56 | 79 | 82 |
CPP: chest port placement
Catheter tip malposition: tips ending in upper 1/3 SVC (n=16), left BCV (n=2), junction of BCV (n=2) plus an additional port related complication.
Other: non-functioning port, fibrin sheath, arrhythmia, wound dehiscence, port leakage, pain, pneumothorax, pleural effusion, hemoptysis, inflammation/necrosis/scarring of skin, flipped port
ACKNOWEDGEMENTS
We would like to thank Qun Xiang, M.S., Aniko Szabo, PhD, and the Medical College of Wisconsin Division of Biostatistics supported, in part, by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055.
Sarah B. White, MD, MS – consultant for Guerbet LLC, IOrad, Cook, Grants: NIH 5 R25 CA 132822-03, RSNA Foundation Scholar Grant, SIR Foundation Grant
Stephanie Dybul, BSRT(R)(VI) CIRCC – consultant for SLD Consulting LLC
Kiran Turaga, MD, MPH – consultant for Ethicon, Caris Lifesciences
Parag J. Patel, MD, MS – consultant for Cook, Bard, Medtronic, Penumbra
Funding: RSNA 2013 Research Medical Student Grant
Footnotes
Conflict of Interest:
Jennifer LaRoy, BA – no conflicts of interest to disclose
Thejus Jayakrishnan, MD - no conflicts of interest to disclose
Dirk Ungerer, MBA - no conflicts of interest to disclose
REFERENCES
- 1.Niederhuber J, Ensminger W, Gyves J, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;(92):706–712. [PubMed] [Google Scholar]
- 2.Schutz JC, Patel AA, Clark TW, et al. Relationship between chest port catheter tip position and port malfunction after interventional radiologic placement. JVIR. 2004;15:581–587. doi: 10.1097/01.rvi.0000127890.47187.91. [DOI] [PubMed] [Google Scholar]
- 3.Duszak R, Bilal N, Picus D, Hughes DR, Xu BJ. Central Venous Access: Evolving roles of radiology and other specialties nationally over two decades. ACR. 2013;10:603–612. doi: 10.1016/j.jacr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Pandey N, Chittams JL, Trerotola SO. Outpatient placement of subcutaneous venous access ports reduces the rate of infection and dehiscence compared with inpatient placement. JVIR. 2013;24:849–854. doi: 10.1016/j.jvir.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58:323–346. doi: 10.3322/CA.2008.0015. [DOI] [PubMed] [Google Scholar]
- 6.Walser EM. Venous access ports: Indications, implantation technique, follow-up, and complications. Cardiovasc Intervent Radiol. 2012;35(4):751–764. doi: 10.1007/s00270-011-0271-2. [DOI] [PubMed] [Google Scholar]
- 7.Medicare Payment Advisory Commission. Report to the congress reforming the delivery system. Washington D.C.: 2008. [Accessed July 31, 2013]. p. 99. Available at: http://www.medpac.gov/documents/jun08_entirereport.pdf. [Google Scholar]
- 8.Ryder M. Peripheral access options. Surg Oncol Clin N Am. 1995;4(3):395–427. [PubMed] [Google Scholar]
- 9.McBride K, Fisher R, Warnock N, Winfield D, Reed M, Gaines P. A comparative analysis of radiological and surgical placement of central venous catheters. Cardiovasc Intervent Radiol. 1997;20:17–22. doi: 10.1007/s002709900103. [DOI] [PubMed] [Google Scholar]
- 10.Funaki B, Szymski G, Hackworth C, et al. Radiologic placement of subcutaneous infusion chest ports for long-term central venous access. AJR. 1997;169(5):1431–1437. doi: 10.2214/ajr.169.5.9353475. [DOI] [PubMed] [Google Scholar]
- 11.Nosher J, Bodner L, Ettinger L. Radiologic placement of a low profile implantable venous access port in a pediatric population. Cardiovasc Intervent Radiol. 2001;24:395–399. doi: 10.1007/s00270-001-0071-1. [DOI] [PubMed] [Google Scholar]
- 12.Seiler C, Frohlich B, Dorsam U, et al. Surgical technique for totally implantable access ports (TIAP) needs improvement: A multivariate analysis of 400 patients. JSO. 2005;92:24–29. doi: 10.1002/jso.20410. [DOI] [PubMed] [Google Scholar]
- 13.Koroglu M, Demir M, Koroglu B, et al. Percutaneous placement of central venous catheters: Comparing the anatomical landmark method with the radiologically guided technique for central venous catheterization through the internal jugular vein. Acta Radiol. 2006;47(1):43–47. doi: 10.1080/02841850500406845. [DOI] [PubMed] [Google Scholar]
- 14.Hancock-Howard R, Connolly BL, McMahon M, et al. Cost-effectiveness analysis of implantable venous access device insertion using interventional radiologic versus conventional operation room methods in pediatric patients with cancer. JVIR. 2010;21:677–684. doi: 10.1016/j.jvir.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Sticca RP, Dewing BD, Harris JD. Outcomes of surgical and radiologic placed implantable central venous access ports. Am J Surg. 2009;198:829–833. doi: 10.1016/j.amjsurg.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Teichgräber UK, Pfitzmann R, Hofmann HA. Central venous port systems as an integral part of chemotherapy. Dtsch Arztebl Int. 2011;108:147–153. doi: 10.3238/arztebl.2011.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services. Physician Fee Schedule Search. [Accessed August 11, 2014]; Available at http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx.