Abstract
In this retrospective analysis of electroencephalograms were to identify a surrogate biomarker for the Dopamine D2 receptors in the brain by comparing patients diagnosed with Schizophrenia taking Atypical Antipsychotics to Depressive patients medicated with Selective Serotonin Reuptake Inhibitors. To achieve this, thirty-seconds of resting EEG were spectrally transformed in sLORETA. Three-dimensional statistical non-paramentric maps (SnPM) for the sLORETA Global Field Power within each band were then computed. Our results illustrated that the Right Superior Frontal Gyrus (t=2.049, p=0.007), along the dopamine mesolimbic pathway, had higher neuronal oscillations in the delta frequency band in the 100 Schizophrenia patients as compared to the 32-depressive female patients. The comparisons with both the 48 depressive patient cohort or the sixteen male depressive patient cohort did not yield any statistically significant findings. We conclude that the Superior Frontal Gyrus should be investigated as a possible surrogate biomarker for preclinical and clinical drug discovery in neuropharmacology.
Keywords: Dopamine Receptors, Schizophrenia, sLORETA, Mesolimbic
1.1. Introduction
Clinical Sciences often compare disease states to healthy controls, in hopes of identifying the difference between the healthy versus the sick patients. However, in these days where there are increasing numbers of drug repurposing efforts and searches for orphan drug receptors comparing differences between diagnostic patient groups will become more prevalent, especially in neuropharmacology. Current trends have used Positron Emission Tomography (PET) scans to evaluate receptor binding to construct pharmacokinetic-receptor occupancy models, however, often times, participants enrolled in the study rarely reach 50 in each cohort. Electroencephalography (EEG) neuroimaging techniques are refined and the cost is very attractive for major drug companies, such as Merck, Pfizer, and AstraZeneca, are looking to use EEG as a surrogate biomarker in both preclinical and clinical programs(Leiser et al., 2011; Wilson et al., 2014).
Electroencephalographic recordings, with patients at rest, have been sited to very sensitive as a diagnostic tool, for neurodevelopmental disability, behavioral pathologies, and for genetic associations (Pievani et al., 2011). Moreover, there have been consistent reports, throughout the years, that patients suffering from schizophrenia have an increased slow wave, “delta and theta frequency band,” neuronal activity on recorded electroencephalograms, while there is a decrease in neuronal activity of the higher frequencies, alpha and beta (Sponheim et al., 1994, 1997; Williamson and Mamelak, 1987).
In this retrospective electroencephalography neuroimaging analysis, we will investigate the pharmacodynamics neuroimaging differences of patients with Schizophrenia medicated with Atypical Antipsychotics (ATAPs) and compare them to patients with Major Depressive Disorder medicated with Selective Serotonin Reuptake Inhibitors (SSRIs). The ATAPs act mainly via the Dopamine-D2 receptors and Serotonin 5HT2 receptors while the SSRIs increase serotonin in the brain. Our hypothesize is that conducting the whole brain, voxel-by-voxel, t-tests in these diagnostic groups, we expect to isolate the Dopamine D2-receptor pathway, due to there being no statistical differences in the Serotonin 5HT2 neurotransmitter effects in the ATAPs and SSRIs.
1.2. Methods
Patients
This study was a retrospective chart review of the electroencephalogram database at the Neurophysiology Unit at the Medical University of Lublin’s Department of Psychiatry. All patients were diagnosed by a board-certified Psychiatrist using the diagnostic criteria from the International Statistical Classification of Diseases and Related Health Problems (ICD-10) section on Mental, Behavioral, and Neurodevelopmental Disorders.
During the patient’s electroencephalogram selection in our experimental electrophysiological neuroimaging analysis, we decided to compare EEG Neuroimaging results of 100 Schizophrenia patients to 48 Depressive patients, for our first comparison. Following that, we kept the 100 Schizophrenia patients consistent and compared gender differences in the depressive patients by evaluating 16 depressive male patients in the second analysis, and in the third and final comparison, we compared the 100 Schizophrenia patients to 32 depressive female patients. For the 100 Schizophrenia (ICD-10 Code F20) patients, the group wide and gender-specific average ages and standard deviation of the ages are as follows: 100 Schizophrenia Patients (average age = 32 ± 11.8) as one comparative cohort with 65 Schizophrenia Males (average age = 31 ± 10.6) and 35 Schizophrenia Females (average age = 35 ± 13.7). Next, for the 48 patients diagnosed with Major Depression (ICD-10 Code F32) or Depressive Episode (ICD-10 Code F33), the group wide and gender-specific average ages and standard deviation of the ages are as follows: 48 Major Depressive Disorder and Depressive Episode Patients (mean age =49 ± 12.9), 16 Major Depressive Disorder or Depressive Episode Males (average age =45 ± 15.1), 32 Major Depressive Disorder or Depressive Episode Females (average age =50 ± 11.7).
All patients diagnosed with Schizophrenia were medicated with atypical antipsychotics, Olanzapine (dose 5mg/day to 20mg/day) or Risperidone (dose 1mg/day to 4mg/day) blocking both Dopamine D2 and Serotonin 5-HT2 receptors. Whereas, all of the patients diagnosed with Major Depression or Depressive Episode were medicated with Sertraline (50mg/day to 200mg/day maximum dose) or Citalopram (20mg/day to 40mg/day maximum dose). This research protocol was approved by the Bioethical Commission of the Medical University of Lublin.
EEG Recording
All patients were comfortably seated at a semi-recumbent position in a sound and lighted attenuated room, while 20-minutes or more of routine eye-open and eyes-closed resting EEG data were recorded using the 19-channel EEG Analysis Station (ELMIKO Medical, Poland) and Ag/AgCl electrodes. Patients EEG recordings were in accordance to the international 10/20 system with electrodes placed at Fp2, F8, T8/T4, P8/T6, O2, F4, C4, P4, Fp1, F7, T7/T3, P7/T5, O1, F3, C3, P3, Fz, Cz, and Pz. Electrodes were referenced to linked earlobes and impedances were kept below 5 kΩ. The data sampling rate was 250 Hz, and the acquired signals were filtered with a band-pass filter of 0.15–70 Hz after sampling. Prior to data analysis, artifact detection was performed, visually, to exclude eye-movements, head-movements, muscle-movements, and segments of decreased alertness. EEG recordings were then exported using ELMIKO’s EEG DigiTrak Analysis Software to the ASCII format for later processing.
Neuroimaging – LORETA
Following the export of the ASCII formatted data from the ELMIKO EEG acquisition system, 30-seconds of eyes-open EEG signals were recomputed to the average reference. Subsequently, spectral analysis was performed for the same 30-seconds of artifact-free data of each ICD-10 diagnostic group. The cross-spectra were averaged across the 50% overlapping windows, which yielded 7 EEG frequency bands: delta (1.5–6 Hz), theta (6.5–8 Hz), alpha-1 (8.5–10 Hz), alpha-2 (10.5–12 Hz), beta-1 (12.5–18 Hz), beta-2 (18.5–21 Hz) and beta-3 (21.5–30 Hz) (Kubicki et al., 1979). Lastly, LORETA was used to estimate the 3-dimensional intracerebral current density distribution (Pascual-Marqui, 2002; Pascual-Marqui et al., 1994).
The three-dimensional intracerebral neuronal source activity, illustrating statistical non-parametric maps of neuronal activity, are derived from the 19-channel electrodes were assessed using Low Resolution Brain Electrotomography (LORETA) (Pascual-Marqui et al., 1994). When considering the available methodologies of detecting intracerebral activity, it is important to note that of all available published three-dimensional, discrete, distributed, linear EEG/Magnetoecephalography (MEG) tomography methods for solving the classic EEG inverse problem, LORETA has been identified to report the lowest localization error (to within 1 voxel resolution on average) (Pascual-Marqui et al., 1994). The source localization results from LORETA, even without utilizing an individual patient’s MRI anatomical scans, has demonstrated that with as many as 16-electrodes, and using the approximate three-shell head model registered to the Talairach human brain (Talairach and Tournoux, 1988), localization accuracy of EEG is 10 mm, for worst cases (Cohen et al., 1990). By adding the expected localization error, as a result of the head model, the average error is not expected to exceed 2 to 3 cm in the final LORETA source localization results. LORETA inverse solutions are a model of the 3D distribution of electric neuronal activity, represented by adjacent voxels, has maximum synchrony relative to the orientation and strength between neighboring neuronal populations (Pascual-Marqui et al., 1994). LORETA inverse solutions are restricted to 2394 voxels (spatial resolution=7 mm) within cortical gray matter and hippocampi, as determined by the digitized Talairach and probability atlases of the Brain Imaging Centre, Montreal Neurological Institute (MNI305).
EEG electrode coordinates are derived from cross-registrations between spherical and head geometry (Towle et al., 1993). Concerning the validity of the neuronal activation results, currently, LORETA has received robust theoretical and cross-modal validation from localization studies combining this method with both structural and functional Magnetic Resonance Imaging, Positron Emission Tomography (PET), visual and auditory event-related potentials, and well as intracranial recordings (Pascual-Marqui et al., 1994).
In our investigation of the gender-based diagnostic (ICD-10) group differences, Standardized LORETA (sLORETA) (Pascual-Marqui et al., 1994) was used. The sLORETA inverse solutions are constrained to the MNI152 (Mazziotta et al., 2001) template composed of 6239 cortical gray matter voxels at 5mm spatial resolution.
The sLORETA software package was used as a new improvement relative to LORETA. With sLORETA, realistic scalp electrode coordinates are adapted to a 10/5 electrode system (Oostenveld and Praamstra, 2001) and are registered to the Montreal Neurological Institute’s MNI152 (Mazziotta et al., 2001) scalp, with a 12-parameter transformation followed by a spline solution that projects the electrodes onto the scalp with minimal distortion (Jurcak et al., 2007). By transforming the electrode system, sLORETA provides a much more realistic head-surface based coordinate system. Moreover, the sLORETA transformation matrix for the inverse solution uses the electric potential lead field computed within the boundary element method applied to the MNI152 digitized structural MRI template (Fuchs et al., 2002). Futher, the identified sLORETA inverse solutions are reported on MNI152 template which is composed of 6239 cortical gray matter voxels at 5 mm with anatomical labels as Brodmann areas are also reported using MNI space, with correction to Talairach space (Brett et al., 2002).
Statistical Analysis
The demographic differences between the patient groups were graphically represented using Microsoft Excel. The localization of the differences in activity between the two clinical diagnostic groups were assessed by voxel-by-voxel unpaired t-tests of the LORETA images, based on the log-transformed power of the estimated electric current density, which resulted in t statistic 3-dimensional images. In these images, cortical voxels of statistically significant differences were displayed as statistical parametric maps (SPMs) using a randomization strategy that determined the critical probability threshold values for the actually observed statistic with corrections for multiple testing (Holmes et al., 1996).
To visualize the global distributions of the t-test differences, for each band we computed the location of the mean center of gravity of all voxels with positive and negative t-values. To correct for multiple comparisons, a nonparametric single-threshold test was applied on the basis of the theory of randomization and on permutation tests (Holmes et al., 1996). The omnibus null hypothesis of no activation anywhere in the brain was rejected if at least one t-value (i.e. voxel, t-max) was above the critical threshold for p = 0.05, determined by 5,000 randomizations (Horacek et al., 2007). Lastly, to visualize the age distribution of the Schizophrenia patients, Epi Info™ 6 (http://www.cdc.gov/epiinfo), public domain statistical software for epidemiology developed by Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia, United States of America was used.
Results
The results of the t-tests yielded non-statistically significant results when comparing the one-hundred schizophrenia patients to the entire cohort of forty-eight depressive patient cohort with the one-tailed test identifying the Left Middle Frontal Gyrus (t=1.851, p=0.12300, BA 10, MNI X= −35, Y=25, Z=50). Similarly, the where non-statistically significant results for the one-tailed t-tests comparing the one-hundred schizophrenia patients to the sixteen male depressive patients; the Right Superior Temporal Gyrus (t=2.285, p=0.35840, BA 41, MNI X= 40, Y= −35, Z= 5) was identified.
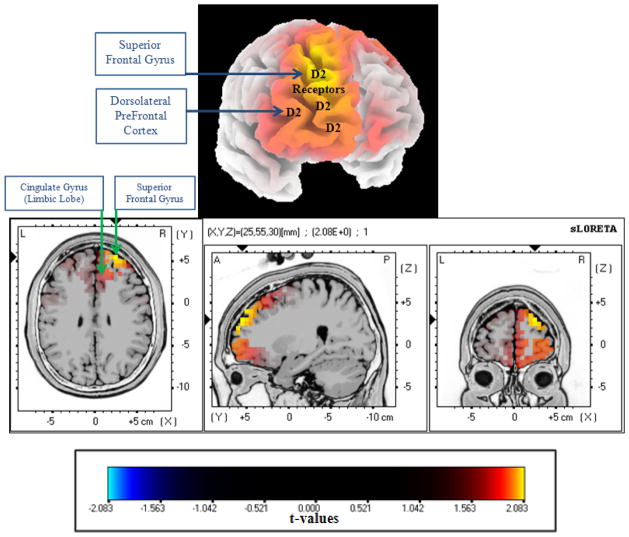
Contrastingly, the results of the one-hundred males and females diagnosed with Schizophrenia compared to the thirty-two females diagnosed with Major Depressive Disorder or Depressive Episodes. The one-hundred male and female Schizophrenia patients had an average age of 32-years-old and a Standard Deviation of 11.8-years. Whereas, the Depressive Episode and Major Depressive Disorder females had an average age of 50-years old and a Standard Deviation of 11.7-years. The statistically significant neuroimaging results identified the Delta (1.5–6 Hz) frequency band at the neuroanatomical location of the region of the Superior Frontal Gyrus (p=0.007; t=2.08, BA 10, X=25, Y=55, Z=30) with greater neuronal oscillations and synchrony in the one-hundred males and females diagnosed with Schizophrenia than the thirty-two females diagnosed with Depressive Episodes and Major Depressive Disorder.
1.3. Discussion
Superior Frontal Gyrus Volumes Size
In a Genome Wide Association Study (GWAS) of 281 patients with schizophrenia (52.0% males, 146 males and 135 females; mean age 36.0±12.4 years) and 413 healthy controls (49.6% males, 205 males and 208 females; mean age 36.4±12.8 years) investigating schizophrenia-related phenotypes, they found single-nucleotide polymorphisms (SNPs) associated with Superior Frontal Gyrus volumes in schizophrenia-related social functions (Hashimoto et al., 2014). In this same study, the team found genotype effects of rs4654899 on gray matter volume of right SFG were found in patients with schizophrenia as well controls.
A study at Harvard Medical School with seventeen schizophrenia patients, gender and parental socio-economic status-matched subjects, with all seventeen affective (mainly manic) psychosis, and seventeen normal comparison subjects, age-matched within a narrow age range (18–29 years), identified gray matter volume reduction Superior Frontal Gyrus (SFG) in the schizophrenia patients (Hirayasu et al., 2001). This study found significant left prefrontal gray matter volume reduction and trend level reduction on the right (Hirayasu et al., 2001).
Another study identified that patient with schizophrenia had selectively activity in the SFG that extended medially to the anterior cingulate region and caudally to the paracingulate region, as with the findings in our study (Goldberg et al., 2006). Similarly, another study at Harvard University with twenty-six individuals with schizophrenia and twenty-nine controls demonstrated that schizophrenia participants had thinner cortex in a region of the superior frontal gyrus (BA10) (Tully et al., 2014). With all of these findings finding an associative decreased cortical volume in patients with schizophrenia will naturally result in neurophysiological abnormalities and may serve as a biomarker both, morphologically, and in our case, neurophysiologically with patients with schizophrenia.
Potential Treatment Options
Based on these findings, what is practical way for a patient to improve their brain volumes? A study set out to examine exercise effects on cerebral volume and cortical thickness in schizophrenia patients and resulted that cardio-respiratory fitness improvement attenuated brain volume changes in schizophrenia patients and increased thickness in large areas of the cortex in both schizophrenia patients and healthy controls. Similarly, another study found an improvement of brain volume, specifically the hippocampus, with exercise in patients suffering from schizophrenia (Pajonk et al., 2010). Exercise should be recommended for patients with schizophrenia.
In a double blind, randomized, placebo-controlled clinical trial of 92 patients with schizophrenia treated with risperidone where subjects were randomly assigned to receive 200mg per day of minocycline found that rates of treatment response (43.6%) in the minocycline group were significantly higher than those in the placebo group (10.0%) after 16weeks of treatment (Liu et al., 2014).
Associations of Toxoplasmosis and Psychosis
A meta-analysis of the association of psychosis and Anti-Toxoplasma Gondii IgM Antibodies, at the Department of Psychiatry and Health Behavior at the Georgia Regents University, found an increased seroprevalence of Toxoplasma Gondii IgM in patients with acute psychosis suggesting that infections may be relevant to the etiopathophysiology of relapse in some patients with schizophrenia (Monroe et al., 2014).
Moreover, a study in Prague identified that Toxoplasmosis triggers schizophrenia in predisposed subjects (Flegr et al., 2014). It may very well be that the Toxoplasmosis parasite increasing dopamine secretions in the frontal lobes may very well lead to decreased cerebral volumes. Cat feces, sexual contact, and eating raw or undercooked pork are well known transmitters of the Toxoplasmosis parasite and may be advisable that patients take precautions of the possibility of these triggering symptoms of psychosis with these possible routes of infection. Another adjunct to Atypical Antipsychotics may be to treat the potential cerebral Toxoplasmosis infection with Cotrimoxazole or Pyrimethamine/Clindamycin (Patil et al., 2011).
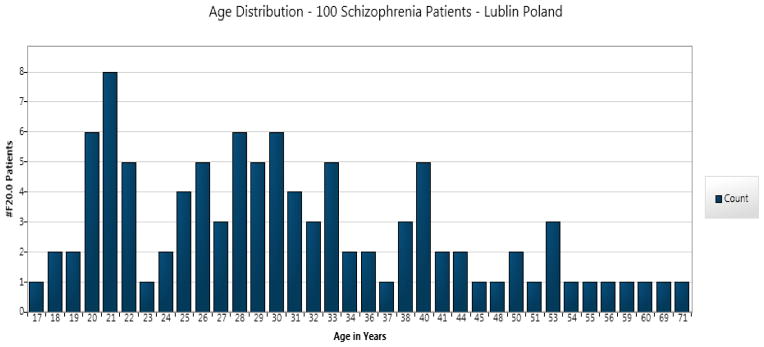
The figure below is a histogram distribution of the ages of one hundred Schizophrenia patients in this study. There appears to be a cyclical peak every ten-years cycles at 20, 30, 40, and 50-year-old patients. This may suggest a recent finding that CD8 T cells, which play a pivotal role in mediating long-term immunity to Toxoplasma, are down-regulated in schizophrenia patients (Bhadra et al., 2013). Based on the aforementioned statements, a vaccine for Toxoplasmosis may be difficult to achieve, but the treatment and screening possibilities in patients with psychosis are available today.
1.4. Conclusion
From our analysis, the findings indicate that in comparing patient groups of schizophrenia versus depression, there is increased neuronal oscillatory activity in the Superior Frontal Gyrus may be indicative of a translatable biomarker for targeting new drug receptor binding in neuropharmacology. Moreover, the pharmacodynamic effects of Atypical Antipsychotics, Olanzapine or Risperidone in our Schizophrenia patients resulted in resting EEG frequencies in the delta band and may be improved with adjunct compounds. From a treatment perspective, there may be room aiding in management of patients with schizophrenia with anti-inflammatory compounds, exercise regimens, minocycline, cotrimoxazole, or Pyrimethamine/Clindamycin in facilitating the healing process of schizophrenia and psychosis.
Figure 1.
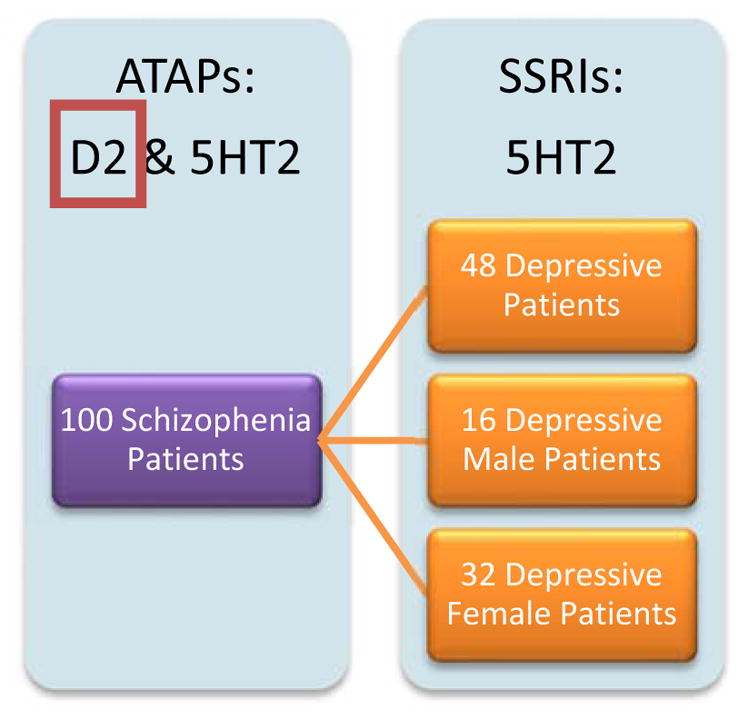
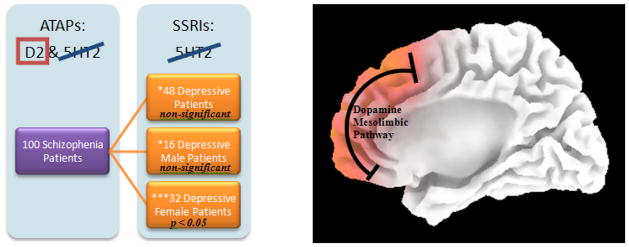
Group comparisons designed for isolating the mesolimbic Dopamine D2-receptor pathway using statistical non-Paramentric Mapping unpaired t-test. The expectation is to have the Serotonin 5HT2 Receptor Pathway cancel out and result in the Dopaminergic pathway. ATAP: Atypical Antipsychotics
Figure 2.
Resting State neuroimaging findings illustrating the action of atypical antipsychotics on post-synaptic dopamine D2-receptors following whole brain voxel-by-voxel unpaired t-tests that are based on statistical non-parametric map (SnPM) of sLORETA images in 100 Schizophrenia patients. The Yellow/Orange shades indicate increased neuronal activity in the delta frequency band in the right Superior Frontal Gyrus (Brodmann Area=10; MNI: X=25, Y=55, Z=30) along the dopamine mesolimbic pathway. Structural anatomy is shown in grey scale (A – anterior; S – superior; P – posterior; L – left; R – right).
Figure 3.
Resulting comparisons isolating the Dopamine D2 Mesolimbic Pathway using statistical non-Paramentric Mapping (SnPM) indicating statically significant (p<0.05), via unpaired t-tests, results of 100 Schizophrenia patients medicated with Atypical Antipsychotics (ATAPs) compared with 32 Depressive patients medicated with Selective Serotonin Reuptake Inhibitors (SSRIs). *Indicates the non-statistically significant findings. ***Indicates the group SnPM pathway with statistically significant p<0.05 findings.
Figure 4.
Histogram age distributions of the 100 Schizophrenia patients illustrating clusters of patients at 20, 30, 40, and 50 years old.
Table 1.
Patient group results of sLORETA analysis between the 100 Schizophrenia Patients vs. 32 Depressive Females.
| sLORETA Brain Mapping Results | |
|---|---|
| Patient Group with the Statistically Significant Neuronal Synchronization | 100 Schizophrenia Patients |
| Whole Brain Voxel-wise t-test Result | t=2.08 |
| p-Value | p=0.007 |
| Frequency Band | Delta Frequency Band (1.5Hz–6Hz) |
| Neuroanatomical Locations(s) | Right Superior Frontal Gyrus |
| Lobe | Frontal Lobe |
| Brodmann Area | 10 |
| Montreal Neurological Institute Coordinates | X=25, Y=55, Z=30 |
Acknowledgments
We would like to thank Ms. Katarzyna Ziniuk for consistently recording the electroencephalograms in the neurophysiology laboratory.
Funding: Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008685.
Footnotes
1.6. Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Andy R. Eugene, Email: eugene.andy@mayo.edu.
Jolanta Masiak, Email: jolantamasiak@wp.pl.
1.7. References
- Bhadra R, Cobb DA, Weiss LM, Khan IA. Psychiatric disorders in toxoplasma seropositive patients--the CD8 connection [Internet] Schizophr Bull. 2013;39:485–9. doi: 10.1093/schbul/sbt006. [cited 2015 Jan 25] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3627775&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, et al. MEG versus EEG localization test using implanted sources in the human brain. Ann Neurol. 1990;28:811–817. doi: 10.1002/ana.410280613. [DOI] [PubMed] [Google Scholar]
- Flegr J, Klapilová K, Kaňková S. Toxoplasmosis can be a sexually transmitted infection with serious clinical consequences. Not all routes of infection are created equal. Med Hypotheses. 2014 doi: 10.1016/j.mehy.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS. A standardized boundary element method volume conductor model. Clin Neurophysiol. 2002;113:702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. When the Brain Loses Its Self: Prefrontal Inactivation during Sensorimotor Processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Ikeda M, Yamashita F, Ohi K, Yamamori H, Yasuda Y, et al. Common variants at 1p36 are associated with superior frontal gyrus volume [Internet] Transl Psychiatry. 2014;4:e472. doi: 10.1038/tp.2014.110. [cited 2015 Jan 24] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25335168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, et al. Prefrontal gray matter volume reduction in first episode schizophrenia [Internet] Cereb Cortex. 2001;11:374–81. doi: 10.1093/cercor/11.4.374. [cited 2015 Jan 24] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11278200. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Horacek J, Brunovsky M, Novak T, Skrdlantova L, Klirova M, Bubenikova-Valesova V, et al. Effect of low-frequency rTMS on electromagnetic tomography (LORETA) and regional brain metabolism (PET) in schizophrenia patients with auditory hallucinations. Neuropsychobiology. 2007;55:132–142. doi: 10.1159/000106055. [DOI] [PubMed] [Google Scholar]
- Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems [Internet] Neuroimage. 2007;34:1600–11. doi: 10.1016/j.neuroimage.2006.09.024. [cited 2014 Jul 10] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17207640. [DOI] [PubMed] [Google Scholar]
- Kubicki S, Herrmann WM, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatr Neuropsychopharmakol. 1979;12:237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Dunlop J, Bowlby MR, Devilbiss DM. Aligning strategies for using EEG as a surrogate biomarker: A review of preclinical and clinical research. Biochem Pharmacol. 2011;81:1408–1421. doi: 10.1016/j.bcp.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo X, Wu R, Ou J, Zheng Y, Zhang B, et al. Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: A double blind, randomized, controlled trial. Schizophr Res. 2014;153:169–176. doi: 10.1016/j.schres.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe JM, Buckley PF, Miller BJ. Meta-Analysis of Anti-Toxoplasma gondii IgM Antibodies in Acute Psychosis. [Internet] Schizophr Bull. 2014 doi: 10.1093/schbul/sbu159. [cited 2015 Jan 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25385789. [DOI] [PMC free article] [PubMed]
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 2001;112:713–719. doi: 10.1016/s1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- Pajonk F-G, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain [Internet] Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7876038. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Patil HV, Patil VC, Rajmane V, Raje V. Successful treatment of cerebral toxoplasmosis with cotrimoxazole [Internet] Indian J Sex Transm Dis. 2011;32:44–46. doi: 10.4103/2589-0557.81255. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L361929610\nhttp://dx.doi.org/10.4103/0253-7184.81255\nhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=02537184&id=doi:10.4103/0253-7184.81255&atitle=Successful+treatment+of+cerebra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011;10:829–843. doi: 10.1016/S1474-4422(11)70158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Iacono WG, Clementz BA, Beiser M. Season of birth and electroencephalogram power abnormalities in schizophrenia [Internet] Biol Psychiatry. 1997;41:1020–7. doi: 10.1016/S0006-3223(96)00184-9. [cited 2014 Dec 18] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9129782. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. 1988 [Google Scholar]
- Towle VL, Bolaños J, Suarez D, Tan K, Grzeszczuk R, Levin DN, et al. The spatial location of EEG electrodes: locating the best-fitting sphere relative to cortical anatomy. Electroencephalogr Clin Neurophysiol. 1993;86:1–6. doi: 10.1016/0013-4694(93)90061-y. [DOI] [PubMed] [Google Scholar]
- Tully LM, Lincoln SH, Liyanage-Don N, Hooker CI. Impaired cognitive control mediates the relationship between cortical thickness of the superior frontal gyrus and role functioning in schizophrenia [Internet] Schizophr Res. 2014;152:358–64. doi: 10.1016/j.schres.2013.12.005. [cited 2015 Jan 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24388000. [DOI] [PubMed] [Google Scholar]
- Williamson P, Mamelak M. Frontal spectral EEG findings in acutely ill schizophrenics [Internet] Biol Psychiatry. 1987;22:1021–4. doi: 10.1016/0006-3223(87)90011-4. [cited 2014 Dec 18] Available from: http://www.ncbi.nlm.nih.gov/pubmed/3607126. [DOI] [PubMed] [Google Scholar]
- Wilson FJ, Leiser SC, Ivarsson M, Christensen SR, Bastlund JF. Can pharmaco-electroencephalography help improve survival of central nervous system drugs in early clinical development? Drug Discov Today. 2014;19:282–288. doi: 10.1016/j.drudis.2013.08.001. [DOI] [PubMed] [Google Scholar]