Abstract
Adolescence brings dramatic changes in behavior and neural organization. Unfortunately, for some 30% of individuals with autism, there is marked decline in adaptive functioning during adolescence. We propose a two-hit model of autism. First, early perturbations in neural development function as a “first hit” that sets up a neural system that is “built to fail” in the face of a second hit. Second, the confluence of pubertal hormones, neural reorganization, and increasing social demands during adolescence provides the “second hit” that interferes with the ability to transition into adult social roles and levels of adaptive functioning. In support of this model, we review evidence about adolescent-specific neural and behavioral development in autism. We conclude with predictions and recommendations for empirical investigation about several domains in which developmental trajectories for individuals with autism may be uniquely deterred in adolescence.
Keywords: adolescent peer relations, autism, brain, risk taking, social behavior
Adolescence is a time of dramatic physical, cognitive, emotional, behavioral, and social changes, which may have unique consequences for individuals with autism. This is the developmental period surrounding the transition from late childhood to early adulthood, which includes the awkward period between sexual maturation and the attainment of adult roles and responsibilities (Dahl & Spear, 2004). With adolescence comes pubertal maturation as well as the challenge of new developmental tasks (e.g., formation of high-quality friendships, acquiring autonomy from parents, forming romantic and sexual relationships). Among typically developing (TD) individuals, success in the ability to accomplish these adolescent-specific developmental tasks predicts levels of adaptive functioning in adulthood (e.g., work competence, romantic competence; Roisman, Masten, Coatsworth, & Tellegen, 2004). The ability to accomplish such tasks may require drastic reorganization in the functional organization of underlying neural networks to support the emergence of new behaviors (see Scherf & Scott, 2012; Scherf, Thomas, Doyle, & Behrmann, 2013).
In TD individuals, adolescence is a period during which we see the emergence of many social-emotional problems, including depression, anxiety disorders, and bipolar disorder, as well as a broad range of domains of problem behaviors that include risk taking, alcohol and substance use, aggression, and violence (Gardner & Steinberg, 2005; see Steinberg, 2008). Adolescents with autism spectrum disorders not only share these same risks with their non–autism spectrum disorder peers but also may face additional vulnerabilities during this developmental period. For example, although the transition from childhood through late adolescence is generally marked by improvements in core symptoms (Seltzer, Shattuck, Abbeduto, & Greenburg, 2004) and some social and cognitive skills (Anderson et al., 2007; Anderson, Oti, Lord, & Welch, 2009), an estimated 30% of children with autism experience deterioration in functioning for several years or more with the onset of puberty (J. Brown, 1969; Eisenberg, 1956; Gillberg & Schaumann, 1982; Rutter, 1970). This “pubertal deterioration” is associated with concomitant neurological complications (Gillberg & Steffenburg, 1987; Kanne, Gerber, Quirmbach, Sparrow, Cicchetti, & Saulnier, 2011), a substantial increase in social withdrawal (Anderson, Mayes, & Lord, 2011), and feelings of loneliness (Billstedt, Gillberg, & Gillberg, 2005).
These aspects of adolescent deterioration in autism are particularly concerning in light of findings that adolescent success in accomplishing developmental tasks is highly predictive of emerging adulthood outcomes among TD individuals (i.e., success in work, romantic relationships, and friendships; Seiffge-Krenke, Luyckx, & Salmela-Aro, 2014; Yu, Branje, Keijsers, & Meeus, 2014). Autism is fairly unique as a developmental disorder in terms of the marked failure of affected individuals to transition into autonomous adult social roles and levels of adaptive functioning. In contrast, individuals with other kinds of debilitating developmental disorders (i.e., Down’s syndrome, intellectual disabilities) do achieve fairly successful adult outcomes in that they get married, have children, live autonomously, and maintain employment (Esbensen, Bishop, Seltzer, Greenberg, & Taylor, 2010; Matson, Dempsey, & Fodstad, 2009; Whitehouse, Watt, Line, & Bishop, 2009). In this article, we propose that adolescence may be an especially vulnerable period of development in autism that makes the transition into adult social roles and adult levels of adaptive functioning overwhelmingly difficult. We frame this vulnerable developmental period in the context of a “two-hit” model (e.g., Knudson, 1971) that integrates genetic, environmental, and developmental factors to explain the onset and developmental course of autism through early adulthood.
The article is organized as follows. We begin by framing the notion of a two-hit model as it was first conceptualized and has been used to explain and make predictions about disorders, such as schizophrenia. Then we briefly review existing evidence for early perturbations in neural development as a potential “first hit” for individuals with autism that predisposes the neural system to a “second hit.” Next, we explain how the confluence of pubertal hormones, neural reorganization, and the increasing demands of adolescence may fundamentally interfere with the ability to transition into adult social roles and levels of adaptive functioning. In so doing, we review the scant evidence on adolescent-specific neural and behavioral development in autism and use our novel model to make predictions about how developmental trajectories for individuals with autism may be uniquely deterred in adolescence. We conclude with suggestions about several areas of future study we consider essential for understanding whether and how adolescence is a second hit in the developmental course of autism.
Developmentally Sensitive Two-Hit Model of Autism
Alfred Knudson (1971) originally formulated the two-hit hypothesis in an effort to explain a mechanism by which multiple genetic mutations can lead to cancerous cell development. In Knudson’s seminal model, an initial genetic mutation (i.e., a first hit) is inherited, which causes a predisposition for a second, later occurring mutation (i.e., a second hit) that greatly increases the likelihood of developing cancerous tumors. It is important to note that the second hit does not necessarily have to be a genetic mutation. It can present in the form of biological mechanisms, environmental insults, or some combination of harmful factors.
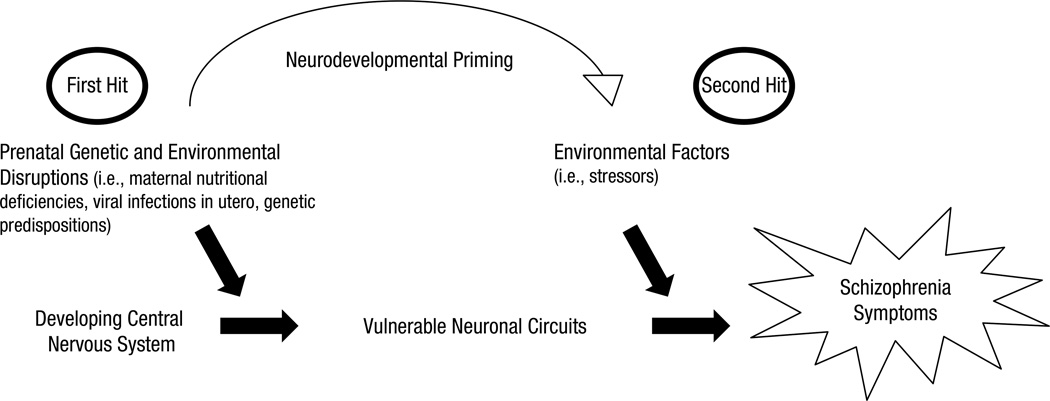
The two-hit hypothesis more recently has begun to garner attention in fields outside of genetics. For example, it has been used as a model for understanding the developmental course of schizophrenia. Maynard, Sikich, Lieberman, and LaMantia (2001) proposed that an early disruption in cell-to-cell signaling during neural development (e.g., neurogenesis, cell differentiation, and migration) is a first hit that makes the brain vulnerable to a second hit, which they argued occurs in adolescence and further compromises the already vulnerable brain. Maynard et al. suggested that the second hit is usually an environmental factor that triggers the onset of symptoms in the primed system, which is “built to fail” (see Fig. 1 for an adaptation of the Maynard et al. two-hit model of schizophrenia). This two-hit model has provided a valuable framework for integrating genetic, environmental, and developmental factors to explain the onset and developmental course of schizophrenia. Here, we have adapted this model to predict that individuals with autism experience two developmental insults that uniquely influence their ability to take on adult social roles and levels of adaptive functioning.
Fig. 1.
Schematic illustration of a two-hit model of schizophrenia. The model proposes that there is a first hit via prenatal genetic or environmental disruptions in the developmental central nervous system. As a result, the system is primed with vulnerable neuronal circuitry that is subsequently exacerbated by a second hit to the system via potential environmental factors that lead to the onset of schizophrenia systems, specifically during adolescence. Adapted from Maynard, Sikich, Lieberman, and LaMantia (2001).
We propose that a two-hit model will provide a framework for integrating the seemingly disparate literature on causal factors contributing to the developmental course of autism and will also direct research toward understanding how adolescence may be an especially vulnerable period for the developmental trajectory and outcomes of autism. We argue that for the model to have predictive power, it must be sensitive to findings that the behavioral and neural manifestations of autism differ throughout development. For example, although failing to respond to one’s own name is a hallmark feature of autism in infancy, it is not a diagnostic criterion in later childhood. Similarly, with respect to brain development, the intrinsic functional connectivity of neural networks appears to be enhanced in children with autism compared with TD children but reduced in adolescents and adults with autism compared with TD adults (Uddin, Supekar, & Menon, 2013).
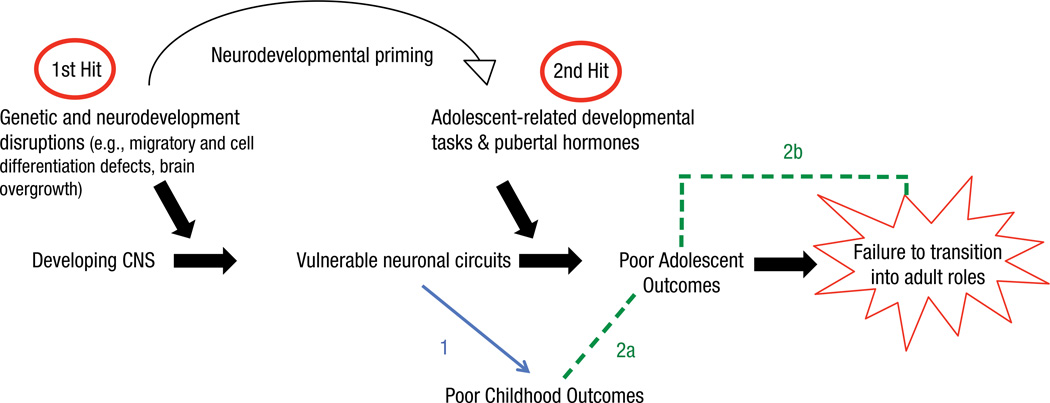
Our two-hit model of autism accommodates these developmental considerations and emphasizes the contributions of developmental influences that accompany the transitions into and out of adolescence, which are predictive of adult outcomes for TD individuals. Figure 2 provides a schematic representation of our model. We suggest that the first hit likely results from early disruptions to neural development (even as early as prenatal development) that fundamentally compromise developing neural circuits. In turn, these compromised neural circuits contribute to poor behavioral outcomes and atypical neural organization in infancy and childhood (Fig. 2, Prediction 1). This compromised neural circuitry lays the foundation for a subsequent hit, which we argue is the developmental period of adolescence (Fig. 2, Predictions 2a and 2b). More specifically, we contend that the concomitant pressures of adolescent-specific developmental tasks and pubertal maturation combine to create a secondary hit, thereby resulting in a failure to develop new behaviors that are essential for acquiring adult levels of adaptive functioning. In sum, this developmentally sensitive two-hit model strives to explain the mechanistic factors that prevent individuals with autism from transitioning into adult social roles and levels of adaptive functioning.
Fig. 2.
Schematic illustration of a developmentally sensitive two-hit model of autism. We propose that the first hit (i.e., genetic and neurodevelopmental disruptions) calibrates the system to be fundamentally vulnerable, which leads to poor childhood outcomes. A subsequent second hit (adolescent-specific developmental tasks and pubertal hormones) compounds previous neural vulnerabilities in addition to creating new and importantly different vulnerabilities, which lead to impediments in attaining adult levels of adaptive functioning. Colored lines within the diagram are numbered by area of research. The blue line predicts links between vulnerable neural circuits and poor childhood outcomes, which has been minimally characterized in the literature. The green lines are symbolic of two predictions that have yet to be addressed: how poor childhood outcomes cascade into poor adolescent outcomes and how poor adolescent outcomes (as a result of a second hit) lead to a failure to transition into adult social roles and levels of adaptive functioning.
It is important to note that we are not the first researchers to emphasize the role of early neural atypicalities in the developmental course of autism (e.g., Courchesne, Campbell, & Solso, 2011; Courchesne et al., 2007). However, with few exceptions, the vast majority of the work in this area has contributed to our understanding of (a) the relative stability (or lack thereof) of neural atypicalities (e.g., increased grey matter volume), without much regard for how these atypicalities affect levels of adaptive functioning or even behavior across development; or (b) atypical profiles of neural activation (e.g., atypical intrinsic functional connectivity) or structural organization (e.g., deficient macrostructural properties of particular fiber tracts) within a specific developmental period (e.g., childhood or adulthood) but not as a function of development. Existing research also has yet to consider the adolescent deterioration that is often reported in autism (e.g., Gillberg & Steffenburg, 1987).
The goal of our model is to provide a conceptual link between these early neural atypicalities, the reported adolescent deterioration, and the failure of individuals with autism to successfully transition into adult levels of adaptive functioning (e.g., low rates of independence from parents, high rates of unemployment, and social isolation; Henninger & Taylor, 2013; Howlin, Moss, Savage, & Rutter 2013). Whereas previous explanations of functional deterioration in autism have focused on the influence of increasing environmental demands that alter or magnify the presentation of symptoms (Levy & Perry, 2011), we highlight the unique biological (i.e., pubertal development and neural reorganization) and environmental (i.e., developmental tasks, increasing social demands and expectations) influences that uniquely combine during adolescence to alter developmental trajectories in autism. Also, unlike models of “regressive autism,” which attempt to explain the acquisition and subsequent loss of skills or behaviors (Hansen et al., 2008), our model is designed to explain why individuals with autism overwhelmingly fail to acquire adult levels of adaptive functioning.
Our hope is that this model will provide researchers with a conceptual framework to investigate potential adolescent-specific vulnerabilities that may contribute to deleterious outcomes for individuals with autism as they attempt to transition into adult social roles. In so doing, we build on the emerging findings of adolescent-specific neural and behavioral development in TD individuals, which have clearly identified adolescence as a crucial period that is predictive of adult levels of adaptive functioning.
What could be the first hit in autism?
We suggest that early (even prenatal) structural brain abnormalities, which occur before the onset of behavioral symptoms in autism (Courchesne, Campbell, & Solso, 2011; Stoner et al., 2014), are a likely first hit in the developmental course of autism. There is mounting evidence from genetic, prenatal, infant, and longitudinal neuroimaging studies that structural and functional connections in the brain are compromised in autism. For example, the genes that have been consistently implicated in autism etiology are involved in multiple aspects of neural circuitry development (i.e., neuronal migration, cell differentiation, signaling pathways, axon and synapse form/function, etc.; Abrahams & Geschwind, 2010; Benayed et al., 2005; Coe, Girirajan, & Eichler, 2012; Tabuchi et al., 2007). Abnormal expression of these genes during prenatal development leads to the disorganization of neurons across multiple cortical layers, particularly in the prefrontal and temporal lobes, which is likely related to neuronal migratory defects and gene-sequencing disruptions (Stoner et al., 2014).
Longitudinal brain imaging studies have revealed that early brain development follows an altered developmental course in autism, particularly in infancy and toddlerhood. Specifically, there are converging reports of early accelerated growth of both grey and white matter in the first years of life that is followed by a marked deceleration in overall brain growth in late childhood (8–9 years of age; Courchesne et al., 2007). There is also evidence of a plateau or decrease in neural growth rate specifically during the adolescent years, which is dramatically different from the typical trajectory of brain development (Courchesne, Webb, & Schumann, 2011; Hardan, Libove, Keshavan, Melhem, & Minshew, 2009). Critically, this decline in neural growth during adolescence is arguably not an extension of, but instead categorically different from, the early phase of brain overgrowth (Courchesne, Campbell, & Solso, 2011; Schumann & Amaral, 2006; van Kooten et al., 2008; Vargas, Nascimbene, Krishnan, Zimmerman, & Pardo, 2005). More specifically, the arrested and even decelerated patterns of brain development during adolescence may be a reactive consequence to the initial early disruption (Courchesne, Webb, & Schuman, 2011). These findings are compatible with our two-hit model in that the early developmental disruption in brain growth and organization may lead to early vulnerabilities (e.g., poor childhood outcomes), which may become compounded by later occurring developmental processes that lead to further deterioration, specifically during adolescence.
Similarly, studies of functional and structural neural connectivity in children and adults with autism are consistent with the notion that brain organization is atypical throughout the full developmental course of autism. Functional organization, that is, the coordinated neural activity between regions, is reportedly atypical during cognitive tasks (e.g., mental rotation, face processing, Tower of London, visuomotor) as well as during rest in all ages tested (i.e., school-age children through adulthood; Just, Cherkassky, Keller, Kana, & Minshew, 2007; Mizuno, Villalobos, Davies, Dahl, & Müller, 2006; Villalobos, Mizuno, Dahl, Kemmotsu, & Müller, 2005). In some cases, the extent of atypicality in these patterns of functional organization has been linked to symptom severity (Assaf et al., 2010; Monk et al., 2009; Supekar et al., 2013; Weng et al., 2010). One significant limitation of these studies is that they were not specifically designed to study age-related changes in functional brain organization to address how developmental trajectories in individuals with autism may differ from those in TD individuals. Uddin and colleagues (2013) have suggested that the nature of the atypicality in functional organization of neural circuits may be fundamentally different in children with autism compared with adolescents and adults with autism, which is altogether different from typical comparison groups. Similar to the findings of early gross structural brain development, this claim suggests that there may be a categorical shift in the functional organization of neural networks particularly during adolescence in autism, which lends support to our argument that adolescence may be a particularly vulnerable time (i.e., the second hit) in the developmental course of autism.
Finally, findings from structural imaging methodologies, such as diffusion tensor imaging, have suggested that there is reduced integrity in white matter tracts in both children and adults with autism, especially with respect to the corpus callosum, the largest white matter tract connecting the right and left hemispheres (Barnea-Goraly et al., 2004; Just et al., 2007; Kana, Keller, Cherkassky, Minshew, & Just, 2006; Lee et al., 2007; Schaer et al., 2013; Shukla, Keehn, & Müller, 2011). White matter tracts connecting many other brain regions are disrupted as well (e.g., Nair, Treiber, Shukla, Shih, & Müller, 2013; Schaer et al., 2013). Unfortunately, as with the functional connectivity studies, few studies have focused on investigating age-related changes in these abnormalities of structural connectivity.
The existing evidence overwhelmingly suggests that early structural brain abnormalities in individuals with autism introduce vulnerabilities in brain organization that have severe consequences for neural organization and are related to symptom severity. We argue that these early neural aberrations represent the first hit to individuals with autism, thereby setting up a neural system that is built to fail in the face of a second hit.
The second hit: Adolescence
In our model, we propose that the concomitant pressures of adolescent-specific developmental tasks together with the surge of pubertal hormones create a secondary hit for individuals with autism that their compromised neural circuitry cannot accommodate. The result is a failure to acquire the critical new behaviors that are essential for the transition to adult social roles and levels of adaptive functioning. In what follows, we provide examples of critical domains in which TD adolescents and adolescents with autism fundamentally diverge in their developmental trajectories in ways that are specific to adolescence. We contend that these diverging trajectories provide initial evidence for considering adolescence as a second hit to individuals with autism. These findings begin to reflect the extensive ways in which adolescence may uniquely and negatively affect individuals with autism, thereby setting them on an altered trajectory in their transition into adulthood.
There are particularly vulnerable neural regions in which reduced volume and increased neuron loss emerge in adolescence and extend into adulthood among individuals with autism (Courchesne, Campbell, & Solso, 2011; Schumann & Amaral, 2006; van Kooten et al., 2008). In particular, the amygdala exhibits early overgrowth in childhood followed by a distinct decline in volume during adolescence for individuals with autism (Mosconi et al., 2009; Nacewicz et al., 2006; Sparks et al., 2002). In contrast, the amygdala continues to grow in volume throughout adolescence in TD individuals (Hu, Pruessner, Coupé, & Collins, 2013; Uematsu et al., 2012). In addition, the caudate nucleus of the striatum evinces an atypical growth pattern; it shows an increase in volume during adolescent years for individuals with autism that is linked with more severe symptomology (Hollander et al., 2005; Rojas et al., 2006). Conversely, in TD individuals, the caudate decreases in volume during adolescent development (Giedd, 2006; Hu et al., 2013; Uematsu et al., 2012). These findings illustrate that growth patterns in some neural regions categorically diverge in adolescence for individuals with autism.
In the domain of visuoperceptual processing, TD individuals become increasingly attuned to the global properties of visual stimuli, particularly during adolescence, whereas individuals with autism do not (Scherf, Luna, Kimchi, Minshew, & Behrmann, 2008). Specifically, early in development, both TD children and those with autism are biased to detect more local features of objects. By early adolescence, TD individuals begin to become more biased for detecting the global properties of visual stimuli (Scherf, Behrmann, Kimchi, & Luna, 2009); however, this emerging sensitivity is not present among adolescents with autism. This puts individuals with autism at a disadvantage in processing the more global properties of objects, which are especially relevant for distinguishing perceptually homogenous visual objects, such as individual faces (Caron, Mottron, Berthiaume, & Dawson, 2006). In addition, the ability to recognize individual unfamiliar faces diverges specifically in adolescence between TD individuals and individuals with autism (O’Hearn, Schroer, Minshew, & Luna, 2010). Although TD individuals continue to improve in these skills, those with autism plateau in adolescence and remain relatively impaired as adults.
Executive functioning and working memory abilities also diverge during adolescence in individuals with autism. Specifically, individuals with autism worsen in their metacognitive abilities (i.e., working memory, planning and organization) in real-world executive functioning tasks during adolescence, whereas TD individuals improve (Rosenthal et al., 2013). Critically, these trajectories of metacognitive skills distinctly diverge during early adolescent development and continue to decline later in adolescence and into emerging adulthood (between 11– 13 and 14–18 years of age). Metacognitive abilities are critical for adaptive functioning in the social world; therefore, worsening of these important skills specifically during adolescence is especially problematic for individuals with autism.
Finally, adolescents with autism and TD adolescents begin to diverge even in more basic biological domains, such as sleep. For instance, there is evidence for worsening sleep problems that emerge disproportionately in individuals with autism compared with control individuals in the early stages of adolescent development (between ages 11 and 13; Sivertsen, Posserud, Gillberg, Lundervold, & Hysing, 2012). Given that disturbed sleep is related to emerging psychopathology specifically during adolescence for TD individuals (Dahl, 1996; Fredriksen, Rhodes, Reddy, & Way, 2004; Roberts & Duong, 2014), this finding might suggest that adolescents with autism are at even greater risk for developing adolescent-onset psychopathology.
In fact, individuals with autism may be at a heightened risk for developing comorbid depression or anxiety during adolescence (Brereton, Tonge, & Einfeld, 2006; Kuusikko et al., 2008; Mayes, Calhoun, Murray, & Zahid, 2011; McPheeters, Davis, Navarre, & Scott, 2011). Depression and anxiety are the most common disorders that co-occur with autism; as many as 65% of individuals with autism are affected with depression, anxiety, or both (Ghaziuddin, Ghaziuddin, & Greden, 2002; Green & Cox, 2000). In one large-scale study of 11- to 17-year-olds with autism, anxiety and depression symptoms were highly correlated with one another and were both linked with the severity of autism symptoms (Mayes, Calhoun, Murray, & Zahid, 2011). In contrast, other research has suggested that higher functioning children and adolescents with autism evince higher prevalence rates of anxiety (79%) and depression (54%) than do lower functioning individuals with autism (67% and 42%, respectively; Mayes, Calhoun, Murray, Ahuja, & Smith, 2011).
These findings converge on the notion that across many domains, developmental trajectories for individuals with autism plateau or worsen during adolescence, which means they fail to acquire skills or make the same transitions as do TD adolescents. This may in turn put them at great risk for developing psychopathology, worsening of basic cognitive abilities, and failing to accomplish the core developmental tasks of adolescence.
Critical Developmental Domains That May Be Especially Vulnerable for Adolescents With Autism
In what follows, we review findings that support our notion that the increasing social demands and challenges of adolescence cannot be accommodated by the atypical and vulnerable neural systems of individuals with autism. The resulting consequence is a severe limitation in the ability to transition into adult social roles and levels of adaptive functioning. We focus on findings from three primary domains: peer relationships, risk taking and sensation seeking, and cognitive control. Behavior in these domains directly contributes to meeting the challenges of adolescent-specific developmental tasks for TD adolescents and facilitates the transition to adulthood. For this reason, our model specifically predicts that the behavioral and neural foundation of these domains is likely to be particularly disrupted for individuals with autism. For each domain, we briefly discuss the findings from current research on typical adolescents, the evidence (if any) about how these domains develop in adolescents with autism, and hypotheses about how the domains may present specific challenges and increase vulnerabilities for individuals with autism during adolescence.
Peer relationships
During adolescence, peers begin to take on new salience and relevance for TD individuals, which amounts to a social reorientation from parents toward peers (B. Brown, 2004; Nelson, Leibenluft, McClure, & Pine, 2005).1 With this social reorientation emerges an unprecedented drive for acceptance from social peers and hypersensitivity to peer evaluation. Adolescent peer relationships are more elaborate than friendships at any earlier developmental period (for review, see B. Brown, 2004). Peers become a critical source of social support (B. Brown, Eicher, & Petrie, 1986) as well as the focus of new romantic and sexual interests (see Collins, Welsh, & Furman, 2009). Adolescents’ emotional responses to social stimuli are intensified and are modulated by social contexts involving peers. For example, in the presence of peers, TD adolescents are more prone to erratic and emotionally influenced behavior, even as they are achieving adultlike competence in many cognitive abilities (Dahl & Spear, 2004). All of these facets of social reorientation that are newly emerging in adolescence are critical for social competence with peers, which predicts work and romantic competence in young adulthood (Roisman et al., 2004).
This reorientation toward peers is also reflected in changes in the neural processing of peer-related information in TD adolescents. Specifically, a number of studies have yielded converging evidence that processing of peer-related information (e.g., peer presence, socially evaluative contexts, peer acceptance or rejection) at the neural level is categorically different in adolescence compared with childhood or adulthood, especially within regions of the “social brain” (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; Jankowski, Moore, Merchant, Kahn, & Pfeifer, 2014; Jones et al., 2014; Pfeifer et al., 2013). Specifically, regions supporting reward processing (e.g., ventral striatum) and social cognition (e.g., amygdala, temporoparietal junction, temporal pole) tend to show increased or differential activation patterns in adolescents in tasks that elicit processing of peer information (Jankowski et al., 2014; Jones et al., 2014; Peake, Dishion, Stormshak, Moore, & Pfeifer, 2013; Rodrigo, Padrón, de Vega, & Ferstl, 2014). These findings suggest that peers, compared with adults and children, are inherently more rewarding for TD adolescents, which may motivate the social reorientation toward peers during adolescent development. Critically, the same regions (caudate and amygdala) that are increasingly sensitive to peer-related information in TD adolescents show early and ongoing disrupted growth patterns in individuals with autism, which may severely limit alterations in the neural processing of peer-related information during adolescence.
Unfortunately, very little is known about whether adolescents with autism experience this kind of adolescent-specific peer reorientation or increasing neural salience of peer-related information. Adults with autism clearly struggle to manage the demands of adult relationships and social roles. Specifically, they struggle in forming romantic partnerships (Billstedt et al., 2005) and high-quality friendships (Orsmond, Krauss, & Seltzer, 2004), as well as in gaining autonomy from parents (Eaves & Ho, 2008). Here, we consider the possibility that individuals with autism fail to accomplish the developmental task of forming intimate peer relationships in part because of a failure to undergo peer reorientation during adolescence that may be related to atypical neural processing of information about peers.
Peer relationships differ in quantity, quality, and nature in autism
There is good evidence that the quantity, quality, and nature of friendships are different among adolescents with autism. One study reported that roughly 24% of adolescents with autism do not have reciprocal peer relationships (Mazurek & Kanne, 2010). Adolescents with autism, compared with TD adolescents, spend less time with peers and more quality time with parents and other adults (Kuo, Orsmond, Cohn, & Coster, 2013; Solish, Perry, & Minnes, 2010). The very notion of friendship appears to be qualitatively different in autism. Adolescents with autism describe a “friend” as someone with whom they share common interests (Carrington, Templeton, & Papinczak, 2003). This characterization is supported by reports that their friendships are less social in nature and more focused on shared circumscribed interests (e.g., playing video games; Bauminger & Schulman, 2003; Kuo et al., 2013). Adolescents with autism are less likely to rely on peers for social and romantic learning than are TD individuals (Stokes, Newton, & Kaur, 2007). This potentially deprives them of important contexts for learning about social relationships. For example, among TD adolescents, peer friendships foster vital social skills (e.g., conflict resolution, interpersonal competence, sharing, reciprocal communication; Hartup, 1996) that are transferable to other social relationships (e.g., romantic relationships; Bagwell, Newcomb, & Bukowski, 1998).
In addition, the nature of how peer friendships are initiated and evolve is different for adolescents with autism. In contrast with TD individuals, most friendships for adolescents with autism are prearranged and scaffolded by parents. One study has suggested that roughly 50% of friendships involving an adolescent with autism emerge in the context of a prearranged setting, and a minor 8% of friendships involve reciprocal activities outside of an organized or prearranged setting (Orsmond et al., 2004).
Finally, there is strong evidence that adolescents with autism often fail miserably in their attempts to form peer relationships, which is reflected in the excessively high rates of bullying (Little, 2002), an extreme form of peer rejection. Several studies have reported that adolescents with autism are often victims of bullying, with rates approaching 50% (Cappadocia, Weiss, & Pepler, 2012; Rowley et al., 2012; Sterzing, Shattuck, Narendorf, Wagner, & Cooper, 2012; van Roekel, Scholte, & Didden, 2010). Like TD adolescents, adolescents with autism report feelings of distress related to the experience of being socially excluded, even in laboratory simulations of peer rejection (Bolling et al., 2011; Masten et al., 2011). These findings reveal that adolescents with autism are cognizant of the fact that they are being rejected; however, it is unclear whether these feelings of distress are particularly meaningful to or motivating for them. Finally, given their difficulties forming and maintaining high-quality peer friendships, adolescents with autism are not likely to be able to draw on peer-based social support that is protective against the effects of peer rejection for TD adolescents (Hodges & Perry, 1999). Together, these findings suggest that individuals with autism are at a loss in terms of forming and maintaining intimate peer relationships in adolescence—a critical developmental task that foreshadows a successful transition into adult social roles and levels of adaptive functioning.
This difficulty initiating and maintaining peer relationships may contribute to the awkward attempts to cultivate romantic relationships seen among individuals with autism. According to some reports, 44% of adults with autism never date (Farley et al., 2009), which reflects the enormous challenge that sexual and romantic relationships pose for them (Henault, 2006). The adults with autism who do manage to engage in romantic relationships are often lacking in sexual knowledge (Byers, Nichols, Voyer, & Reilly, 2013). Although there is a paucity of research exploring the nature and frequency of romantic relationships among adolescents with autism, the work that does exist supports the notion that these individuals have immense difficulty initiating and engaging in these relationships. For example, adolescents with autism tend to focus their romantic attention and efforts toward strangers and engage in inappropriate courting behaviors (e.g., stalking behaviors; Stokes et al., 2007). They also do not appear to be able to learn about notions such as personal privacy and appropriate dating behavior even in the context of formal sexual educational training (Stokes & Kaur, 2005).
In sum, adolescents with autism struggle tremendously in their attempts to develop intimate peer relationships (friendship as well as romantic), which is a core developmental task of adolescence that predicts adult levels of adaptive social functioning. We propose that this difficulty likely stems from not only their existing impaired social skills, which are apparent even in childhood, and might be traced back to early aberrant development of neural regions in the social brain, but also a lack of increasing salience and relevance of peers during adolescence.
Neural processing of peer-related information is atypical in autism
Consistent with our notions that early atypical neural development lays the foundation for a subsequent second hit in the developmental course of autism, some evidence has suggested that neural processing of peer-related information is atypical in adolescents with autism, specifically within regions of the social brain that exhibit early aberrant growth patterns. Much of the work evaluating the neural processing of peer-related information in autism has employed experimental peer exclusion and reward-based learning paradigms. In two studies, researchers measured brain activation while participants played a game of Cyberball (Bolling et al., 2011; Masten et al., 2011). In this game, participants play catch with two other unfamiliar peer “players” who eventually exclude the participant and pass the ball only between themselves. During peer exclusion, adolescents with autism (aged 7–18 years) report similar levels of distress resulting from the exclusion as do TD adolescents; however, they evince weaker activation throughout a whole network of neural regions that have been previously implicated in processing social exclusion in adults, including right posterior insula, left ventral anterior cingulate cortex, left posterior cingulate cortex, hippocampus, and precuneus (Bolling et al., 2011). Moreover, individuals who exhibited more severe autism symptoms evinced the weakest activation in these regions. Similarly, adolescents with autism (mean age = 14 years) displayed less neural activation in the ventral anterior cingulate cortex, anterior insula, ventral striatum, and ventrolateral prefrontal cortex, which are regions that have been previously linked with the distressing responses of peer exclusion and with the process of regulating such responses during a game of Cyberball (Masten et al., 2011). These findings revealed that adolescents with autism report distress when being socially excluded by unfamiliar peers but that simulated social exclusion by peers may not be as stressful for them as it is for TD adolescents. On one hand, this relative insensitivity might be protective against the distress of peer rejection. On the other hand, a weakened drive for acceptance by peers and hyposensitivity to peer evaluation might also limit processing of important feedback signals about the relative success with which adolescents with autism form high-quality peer friendships.
In addition to becoming increasingly sensitive to the evaluation and acceptance of peers, the reward value of peer-related information increases dramatically in TD adolescents (Blakemore, 2008; Galvan, 2010), which is reflected in enhanced neural activation within the ventral striatum and orbitofrontal cortex (Chein et al., 2011). To our knowledge, no studies have specifically measured reward-related neural responses to peers among adolescents with autism. However, there is a related line of research that has focused on potential dysfunction of neural response to social versus monetary rewards in individuals with autism. Although this work does not use peer-related information as the social reward, it may reveal atypicalities in the basic neural processing of social reward that may be implicated in the failure among adolescents with autism to socially reorient toward peers.
This emerging literature converges on the notion that the neural basis of reward processing is disrupted in individuals with autism (Delmonte et al., 2012; Kohls et al., 2011; Kohls, et al., 2013; Lin, Rangel, & Adolphs, 2012); in comparison to responses observed in TD adolescents and adults, individuals with autism experience a disproportionate impairment in the processing of social rewards compared with monetary rewards. For example, compared with TD individuals, adolescents and adults (13–25 years old) with autism evinced reduced activity in the dorsal striatum during the receipt of social rewards (i.e., a smiling unfamiliar female face; Delmonte et al., 2012). Importantly, there were no such differences in the receipt of monetary rewards, thereby suggesting that these deficits are specific to social rewards. Also, decreased activation to the social rewards was related to increased repetitive behaviors in the autism group, which suggests that the core behavioral deficits of autism may fundamentally interfere with the ability to evaluate the salience and relevance of social stimuli. Recent findings of typical or even enhanced reward-related activation in response to nonsocial cues (e.g., food; Cascio et al., 2012) and personalized stimuli of interest (Cascio et al., 2014; Dichter et al., 2012) among adolescents with autism have demonstrated that the processing of social rewards may be disproportionately disrupted in autism.
Similarly, social rewards appear to be less motivating or useful in learning paradigms for individuals with autism. Specifically, in a reward-learning task with monetary (winning or losing money) and social (positive and negative faces) rewards, young adults with autism had a slower learning rate for the social-reward stimuli compared with the monetary-reward stimuli despite being able to accurately identify the valence of the social stimuli (Lin et al., 2012). In contrast, the age-matched TD participants showed the opposite pattern of behavioral results with better performance for the social-reward stimuli than for the monetary-reward stimuli. Similarly, in an implicit learning task executed in a functional MRI scanner with both social and monetary rewards, adolescents with autism exhibited significantly less activation in areas supporting reward learning (i.e., anterior cingulate cortex, ventral prefrontal cortex, and ventral striatum) during social-reward learning than did TD adolescents. There were no group differences during monetary-reward learning.
Hypotheses about peer relationships among adolescents with autism
On the basis of this literature, we hypothesize that there is a significantly weakened (and potentially nonexistent) social reorientation toward peers among adolescents with autism. Using our model, we predict that this limited social reorientation originates from early atypical neural organization, particularly within the mesocorticolimbic reward circuitry, that disproportionately disrupts processing of social rewards. The burden of this disruption becomes especially apparent during adolescence, when adolescents with autism are not driven to pursue high-quality peer friendships and navigate the increasingly complex nature of adolescent friendships. In turn, this severely limits the quality and nature of their peer relationships in adolescence, which ultimately restricts their ability to accomplish other core adolescent-specific developmental tasks, such as forming romantic and sexual relationships and becoming more autonomous from parents. In the Future Directions for Studying Adolescence as a Second Hit for Autism section, we discuss potential experimental paradigms and sets of findings that would help evaluate these hypotheses.
Risk taking and sensation seeking
During adolescence, TD individuals evince a marked increase in risk-taking behaviors, particularly in the context of peer interactions (Chein et al., 2011). Although the heightened risk-taking behavior that is common in typical adolescents can have severe consequences (i.e. delinquency, injury, death), these changes in behavior are normative. This interest in exploring new experiences (i.e., sensation seeking) is a hallmark feature of adolescence and is likely functionally important for motivating them to meet core developmental tasks of adolescence, including taking on more adult social roles and relationships and obtaining autonomy from parents (Spear, 2000; Steinberg, 2008).
Several models of brain development argue that the adolescent-specific increase in risk-taking behaviors is enabled by a temporal asynchrony in the structural and functional development of two primary systems, the motivational limbic systems (especially the amygdala and the striatum) and prefrontal control regions (e.g., Casey et al., 2010; Ernst, Pine, & Hardin, 2006; Steinberg, 2010). For example, according to the triadic model (Ernst et al., 2006), during early adolescence, there are impressive changes in the dopaminergic system that differentially influence the functional and structural development of the reward system (e.g., ventral striatum) when the harmavoidant (e.g., amygdala) and cognitive control (e.g., medial/ventral prefrontal cortex) systems are still relatively underdeveloped and weak. This imbalance arguably motivates the pursuit of rewards without much restraint but also allows adolescents to explore new social contexts, roles, and environments that may otherwise be frightening to them.
Much of the work on risk-taking behavior is conducted in the context of decision-making (i.e., gambling) tasks that use money as a reward. In the Iowa Gambling Task (IGT; Bechara, Damasio, Damasio, & Anderson, 1994), participants have to maximize monetary gain by choosing cards from multiple decks that vary with respect to their relative risk (e.g., high win/high loss, low win/low loss). A large literature has shown that reward-seeking behavior in this task increases from early to midadolescence, and risk aversion does not become a clear strategy until late adolescence and early adulthood (Cauffman et al., 2010; Steinberg, 2010). Also, neural activation in reward-related regions, including the ventral striatum and the ventral medial prefrontal cortex, follow the same age-related increase in activation (i.e., peaking in adolescence) during high-risk gambles compared with low-risk gambles (Van Leijenhorst et al., 2010). This work collectively suggests that adolescents evince uniquely strong reward-seeking behaviors and patterns of neural activation, particularly in high-risk situations.
There is a less clear understanding, across all developmental stages, of the profile of risk-taking behavior in individuals with autism. For example, one study of adolescents and adults with autism (mean age = 19.7 years) showed no differences in IGT performance between the adolescents and adults with autism and TD individuals with respect to choosing from the advantageous decks (South et al., 2008). Another study reported mild risk aversion for adults with autism in a slightly different version of a gambling task (De Martino, Harrison, Knafo, Bird, & Dolan, 2008). There are even fewer studies focused on risk-taking behavior specifically among adolescents with autism. In an IGT study by Johnson, Yechiam, Murphy, Queller, and Stout (2006), adolescents and adults with autism, compared with TD individuals, did not show consistently different risk-taking behavior. It is interesting that the TD individuals tended to follow a win-stay, loose-shift strategy, whereas the individuals with autism were more likely to consistently shift across decks regardless of the outcome. These findings may reflect differential sensitivity to rewards and losses or different learning strategies among the individuals with autism. They do not suggest that adolescents and adults with autism are more or less risk averse than are TD individuals.
There is only one study, to our knowledge, in which researchers have explicitly investigated risk-taking behaviors in adolescents with autism. South, Dana, White, and Crowley (2011) explored the relationship between age, anxiety symptoms, and risky decisions in typical children and adolescents (aged 8–18 years) and adolescents with autism in the context of the balloon analogue risk task. In this task, participants acquire money each time they pump a balloon without having it pop. It is important to note that the balloon can explode at any time, thereby causing participants to lose all their money. South and colleagues reported that the TD adolescents and those with autism exhibited similar levels of risk taking during adolescence. However, there were important moderators that differentially influenced their risk behavior. Age was a moderator of risky decisions but only for TD individuals. Thus, although the mean levels of risk were similar across the two groups, the rate of risk increased over time for the TD individuals. This suggests that compared with TD individuals, individuals with autism may exhibit higher levels of risk as children but potentially lower levels of risk taking as adolescents. Finally, as predicted, TD adolescents who had higher symptoms of anxiety were risk averse (i.e., made fewer risky decisions). However, adolescents with autism who had higher levels of anxiety made riskier choices. In contrast to the predictions, they were not risk averse. This complicated set of results is difficult to interpret but suggests that adolescents with autism exhibit comparable, and potentially lower, levels of risk-taking behavior to TD adolescents. Given this limited set of findings, it is critical to understand the status of the cognitive control system, which is largely mediated by the prefrontal cortex, in autism. If it is particularly compromised, individuals with autism may be especially challenged in adolescence when they have to manage even slight increases in the motivation to engage in risky behavior.
Compromised cognitive control systems in autism
Cognitive control is central to goal-directed decision making in that it keeps impulses (e.g., to engage in risky behavior) in check long enough to deliberate alternate choices (Chein et al., 2011). This aspect of cognitive control is empirically evaluated by asking participants to inhibit a prepotent behavioral response, such as in a go/no-go or antisaccade task. In TD individuals, there are marked improvements in cognitive control through adolescence (e.g., Somerville & Casey, 2010). A large literature has suggested that cognitive control is relatively weakened in children and adolescents with autism (Geurts, van den Bergh, & Ruzzano, 2014; for review, see O’Hearn, Asato, Ordaz, & Luna, 2008). For example, Minshew, Luna, and Sweeney (1999) found that children, adolescents, and adults with autism made significantly more errors than did age-matched TD control individuals in an antisaccade task, which requires participants to resist looking at a primed visual target and, instead, to look in the opposite direction. Among individuals with autism, this deficit exists in childhood and never improves as a function of age in adolescence and adulthood as it does among TD individuals. A recent meta-analysis of cognitive control in children and adolescents with autism concluded that the ability to inhibit proponent responses is significantly impaired in autism but actually reduces across the life span (Geurts et al., 2014).
Despite the large behavioral literature on cognitive control deficits in children and adolescents with autism, there are only a handful of neuroimaging studies on atypical neural activation during inhibition of prepotent responses in children and adolescents with autism. In one study, participants had to inhibit a regularized mapping between a visual stimulus and a button press (i.e., same side of screen). The adolescents with autism (aged 12–18 years) exhibited less activation within and functional connectivity (i.e., temporal coordination) between a network of neural regions implicated in cognitive control (Solomon et al., 2009). In a subsequent study using the same task, Solomon et al. (2013) specifically investigated age-related changes in the behavioral and neural basis of cognitive control from early (12–15 years) to later (16–18 years) adolescence in individuals with autism and age-matched TD control individuals. Although neither group improved in accuracy with age, both groups improved in the efficiency of their performance (i.e., faster reaction time to reach the same level of accuracy) from early to later adolescence. However, even though there were no behavioral differences between the two groups, the adolescents with autism differentially relied on a subset of regions in the cognitive control network (i.e., anterior cingulate cortex and ventrolateral prefrontal cortex) compared with the TD adolescents (i.e., dorsolateral prefrontal cortex and parietal cortex). Solomon and colleagues suggested that these findings might reflect strategic differences between the two groups with respect to how they manage cognitive control, particularly during cognitively demanding experiences.
Hypotheses about risk taking and cognitive control in adolescents with autism
The existing findings provide no evidence that individuals with autism show consistently higher levels of risk-taking behavior than do TD individuals. What remains to be seen is whether there are similar gradual increases in risk-taking behavior as a function of age that peak in adolescence (as in TD individuals) or whether adolescents with autism begin to show some evidence of risk aversion in adolescence. It is important to note that even modest increases in risk-taking behavior in adolescence may be especially problematic for individuals with autism (i.e., function as a second hit), given the consistent findings of compromised cognitive control abilities. We propose that the underlying neural and behavioral vulnerabilities in cognitive control may lead to an even more robust adolescent-specific imbalance between the approach/reward and regulatory systems for individuals with autism.
In addition, we predict that adolescents with autism may be less susceptible to the influence of peer contexts in the pursuit of risky behavior as a result of decreased sensitivity to social rewards that stems from early atypicality in the development of mesocorticolimbic neural circuitry. This potential insensitivity to the rewarding nature of peers may be protective, thereby making adolescents with autism less susceptible to the kinds of risk taking that lead to dire consequences for TD individuals (e.g., car accidents, drug use, binge drinking). Critically, future research is necessary to understand how findings about altered reward processing in autism relate to risk-taking behaviors in adolescents with autism. For example, if the peer context does not necessarily elicit stronger risk-taking behavior in adolescents with autism, it is important to understand whether other contexts do, such as those in which they can indulge restricted interests. For example, video games are a common focus of restricted interests for adolescent boys with autism (Mazurek & Engelhardt, 2013). It could be that sensation- and reward-seeking behaviors in these boys during adolescence might be excessively focused on video games at the expense of time spent pursuing peer social interactions.
Finally, we suggest that for adolescents with autism, any blunting of adolescent social risk taking likely impedes the development of social-emotional competencies that are necessary for pursuing autonomy and independence from parents, which compromises their ability to transition into adult levels of adaptive functioning.
Summary of vulnerable domains for adolescents with autism
We have identified several domains of development (i.e., peer reorientation, risk taking/sensation seeking, cognitive control) that are critical for accomplishing the developmental tasks of adolescence (e.g., forming intimate friendships and romantic relationships, gaining autonomy from parents) and will ultimately facilitate the transition from adolescence to adult levels of adaptive functioning. In addition, we have critically reviewed the existing (albeit scant) evidence that indicates that these domains of behavior are measurably disrupted as individuals with autism transition through adolescence. We summarize this evidence as follows:
Adolescents with autism struggle in their attempts to develop intimate peer relationships, which may be an indication that adolescent-specific social reorientation is significantly weaker in this population.
Peer rejection is disproportionately higher for individuals with autism in adolescence. This may lead to risk for developmental deterioration and increasing depression and anxiety during adolescence.
Compared with TD individuals, adolescents with autism appear to exhibit similar, or slightly reduced, levels of risk-taking behavior. However, nothing is known about differences in the context in which risk-taking behaviors occur for adolescents with autism (i.e., social contexts vs. contexts associated with repetitive and restricted interests).
The ability to inhibit a prepotent response is particularly difficult for individuals with autism and may be especially true in adolescence. There are also weaknesses in the contribution of the prefrontal cortex to cognitive control, which may contribute to a robust adolescent-specific imbalance between the approach/reward and regulatory systems for individuals with autism.
In sum, we argue that the limited evidence reviewed herein is consistent with our prediction that adolescence functions as a second hit to the already vulnerable neural system of individuals with autism; however, we also recognize that future research must be conducted to determine (a) the extent and nature of the difficulties individuals with autism exhibit in accomplishing the developmental tasks of adolescence, (b) a potential link between these difficulties and early alterations in the development of neural systems and their functional and structural organization, and (c) the relation between behavior in adolescence and the failure to take on adult social roles and reach adaptive levels of functioning during adulthood.
Future Directions for Studying Adolescence as a Second Hit for Autism
In this final section, we recommend three areas of future research that must proceed in order to understand whether and how adolescence functions as a second hit (i.e., a period of important vulnerability) to the developmental course of autism. First, we emphasize the need to investigate whether and to what extent there is peer reorientation among adolescents with autism. This includes an understanding of the contributing (or interfering) role of the mesocorticolimbic reward circuitry to this process. Second, we argue that the developmental trajectory of risk-taking behavior needs to be studied in autism with an emphasis on investigating the focus and contexts that drive approach and reward motivations (e.g., objects of restricted interests, peers). This method will need to be complemented by a parallel investigation of whether there is a disproportionate imbalance between approach and regulatory neural systems in autism, particularly during adolescence. Finally, we suggest that the role of pubertal development must be evaluated in the context of these other areas of research given the impressive pubertal deterioration that has been reported in the literature (Billstedt et al., 2005; Gillberg & Steffenburg, 1987) and the reports of the influence of pubertal maturation on many of these developing systems in TD adolescents (Forbes & Dahl, 2010; Moore et al., 2012; Pfeifer et al., 2013).
Investigating the role of peers in adolescent development
We propose that early neural atypicalities contribute to poor childhood social outcomes that cascade to interfere with peer reorientation in adolescents with autism. We also suggest that increasing levels of restricted interests that intensify during adolescence among individuals with autism may interfere with motivation to form peer relationships. The resulting consequence is that the ability to pursue friendships as well as romantic and sexual relationships is significantly compromised, which, in turn, leads to poor social competence in adulthood. These hypotheses can be evaluated by testing several empirical questions.
First, to what extent do adolescents with autism reorient toward peers? We suggest that future research addressing this question should focus on understanding whether there is increasing (a) complexity of peer relationships, (b) reliance on peers for social support, (c) quality of peer interactions, (d) emotional responses to social contexts involving peers, (e) drive for acceptance from peers, (f) sensitivity to peer evaluation, (g) neural reward-related responses to peers, and (h) motivation to engage in romantic or sexual relationships with peers. These are all hallmarks of peer reorientation among TD adolescents.
We also recommend that future studies on adolescent changes in the nature of peer interactions and complexity of friendships go beyond using parent and adolescent survey measures. Adolescents with autism and their parents appear to have fairly different notions of friendship (Orsmond et al., 2004), which may challenge the validity of such a measurement for use in autism. We suggest that researchers employ more ecologically valid observational methods of social interactions (e.g., social problem solving) between peer dyads (i.e., an adolescent with autism and a friend identified by the adolescent). It would also be useful to compare the nature of social interactions in peer dyads with those elicited in parent-adolescent dyads (i.e., an adolescent with autism and his or her parent).
In terms of evaluating the potential reward value of peer contexts, we recommend that this work proceed using very basic tasks. For example, one existing functional MRI study has reported that children and adolescents with autism exhibited levels of neural activation similar to TD individuals during passive viewing of familiar adult and same-aged unfamiliar peer faces (Pierce & Redcay, 2008). This particular study was focused on evaluating the neural signal in posterior visuoperceptual regions, such as the fusiform gyrus. However, a similar approach could be used to investigate the reward-related response to familiar and unfamiliar peer and adult faces in the reward circuitry. This would help evaluate whether peer faces are more rewarding to adolescents with autism than are adult faces or whether familiarity modulates such an effect. If adolescents with autism consistently show stronger reward-related activation to familiar faces, regardless of age, than to peer faces regardless of familiarity, the notion that peers may not take on the same reward value for adolescents with autism as for TD adolescents would be supported.
In addition, studies on more complex social processing of peer and adult faces, such as judging trustworthiness or attractiveness traits, could help determine the extent to which adolescents with autism are disproportionately socially motivated to process information about peers (e.g., higher ratings overall for trustworthiness and attractiveness for peer as compared with adult faces). A set of comparison studies that involve familiar and unfamiliar objects that are and are not the focus of each participant’s own restricted interest would help investigate the relative calibration of reward systems among adolescents with autism (e.g., restricted interest objects > common objects > familiar faces). It also is critical to investigate these same responses in children and adults with autism to examine whether there are adolescent-specific changes in these responses. This kind of approach would enable researchers to assess the extent to which developmental trajectories diverge between TD individuals and those with autism in terms of social-information processing about peers. Finally, it is important that future research assess whether individual differences in the magnitude of these reward-related responses to peer faces and objects of restricted interests are at all related to the abilities to form peer friendships during adolescence and to longer-term adult outcomes of social competence.
With regard to research on adolescent-specific developmental changes in the drive for acceptance from peers and sensitivity to peer evaluation, we suggest that investigators consider using the Chatroom task (Guyer, Choate, Pine, & Nelson, 2012), which was specifically designed to simulate ecologically valid adolescent social interactions (Bolling et al., 2011). In this task, participants first rank photographs of peers for social interest (i.e., how interested they are in interacting with the person) and are made to believe that they will also be ranked by each of the peers. In the return visit, participants are scanned while they view each photograph that they rated previously and are given “feedback” about whether the peer was mutually interested. Then participants have to judge how they feel after receiving the feedback. Thus, the participant experiences both acceptance and rejection by the virtual peers throughout the course of the experiment. Behavioral and neural responses collected during the Chatroom task could help determine the relative concern (or lack thereof) about peer acceptance and rejection among adolescents with autism. Furthermore, an assessment of individual differences in the response to peer rejection in early adolescence could be used to predict longitudinal changes in the magnitude of symptom severity and depression symptoms in later adolescence and early adulthood. This approach would provide foundational information about peer reorientation and sensitivity to peer rejection in autism and may also provide a clearer link between a failure to accomplish developmental tasks of adolescence and worsening outcomes for young adults with autism.
For TD individuals, the formation of romantic relationships during adolescence is an important antecedent to successful romantic relationships in adulthood (i.e., number of romantic partners, duration of romantic relationships; Rauer, Pettit, Lansford, Bates, & Dodge, 2013; Simpson, Collins, & Salvatore, 2011). Although there is an extremely sparse literature regarding romantic and sexual partnerships in adolescents with autism, findings in adults with autism suggest that outcomes are rather bleak. Recall that approximately 44% of adults with autism have never dated (Farley et al., 2009). This does not appear to be due to a lack of sexual well-being or interest; adults with autism do report sexual desire as well as arousability (Byers et al., 2013), and many express an interest in marriage and in having intimate and sexual relationships (Haracopos & Pedersen, 1992; Newport, Newport, & Bolick, 2002; Ousley & Mesibov, 1991). However, their knowledge of sexual behavior and romantic relationships is usually limited (Ousley & Mesibov, 1991). These findings suggest that individuals with autism appear to be interested in pursuing romantic and sexual relationships but do not have the social skills to be able to do so. This interpretation is confirmed by findings that have shown that adults and adolescents with autism engage in inappropriate courting behaviors (e.g., stalking behaviors; Stokes et al., 2007) and sexual behaviors (e.g., touching private body areas in public, removing clothing in public, masturbating in public, and touching members of the opposite sex inappropriately; Ruble & Dalrymple, 1993). Given that adolescence is the time when sexual behaviors are learned and practiced among TD individuals (Feldmann & Middleman, 2002), and that parents of adolescents with autism report that their child’s sexuality is a major concern, it is imperative that future work evaluate the emerging nature of romantic and sexual relationships among adolescents with autism.
First, it will be important to understand whether adolescents with autism are similarly motivated to pursue romantic and sexual relationships and whether they have the social skills to pursue such relationships. Part of this work needs to assess the frequency with which adolescents with autism are engaging in (or attempting to engage in) romantic relationships. Such work likely needs to be done using interviewing/survey methods, such as the Sexual Behavior Scale (Stokes & Kaur, 2005) and the Dating Questionnaire (Connolly, Craig, Goldberg, & Pepler, 2004), and may require parental input as well. It is also critical for future work to understand the developmental stages in the formation of romantic relationships in adolescents with autism. That is, to the extent that these relationships exist, do they follow a similar developmental progression as in TD adolescent romantic relationships (i.e., mixed-gender group affiliations, then group dates, and eventually formation of romantic partnerships; Connolly, Nguyen, Pepler, Craig, & Jiang, 2013)? This approach may reveal critical information about the specific aspects of forming romantic and sexual partnerships in adolescence that are particularly difficult for adolescents with autism. As a result, it could also lead to mechanistic explanations about why adolescents with autism overwhelmingly fail to meet this core developmental task that is so critical for later adult relationships.
Investigating risk taking among adolescents with autism
We suggest that compared with TD adolescents, adolescents with autism may have a disproportionate imbalance between their cognitive control and reward-processing systems. If this is the case, risk taking is likely to be a considerable vulnerability for individuals with autism, especially during adolescence. Establishing the developmental trajectory and contextual focus of risk-taking behavior in autism is essential to understanding whether and to what degree there is a disproportionate imbalance between the reward and regulatory systems in autism during adolescence.
On the basis of the existing literature, we suggest that there are four empirical questions that need to be addressed: (a) What is the developmental trajectory of risk-taking/sensation-seeking behavior in autism? (b) Is there emerging risk aversion? (c) If so, does this influence the ability to gain independence from parents and pursue more adult social roles? and (d) What is the relative influence of peers and other nonpeer contexts (e.g., involving restricted interests) on risk-taking behaviors?
To address the first two questions, researchers should use cross-sectional and longitudinal designs to investigate the trajectory of risk-taking behaviors from childhood through early adulthood using established parent and adolescent questionnaires and interviews, such as the Barratt Impulsiveness Scale (Patton, Stanford, & Barratt, 1995), Benthin Risk Perception Measure (Benthin, Slovic, & Severson, 1993), Youth Decision-Making Questionnaire (Ford, Wentzel, Wood, Stevens, & Siesfeld, 1989), and Zuckerman Sensation-Seeking Scale (Zuckerman, Eysenck, & Eysenck, 1978). These existing questionnaires may need to be modified to dissociate risk taking in and out of peer contexts for use with adolescents with autism. In addition, it is essential to evaluate the existence of risk-taking and risk-aversion behaviors depending on whether the context is social (i.e., with peers) or nonsocial (i.e., neutral, monetary, or focused on restricted interests). Future researchers should also aim to evaluate the potential link between alterations in reward-seeking behaviors among adolescents and subsequent autonomy in later adulthood. In other words, there may be individual differences in the degree to which individuals with autism engage in sensation-seeking and risk-taking behaviors that are systematically related to the likelihood that they accomplish some autonomy from parents (e.g., moving to a group home, going to college, obtaining a job).
To address the willingness of adolescents with autism to take risks to gain rewards, researchers should use new or modified experimental paradigms. For example, we recommend that researchers use simple decision-making tasks (such as the IGT) that manipulate peer presence (i.e., in the room or nearby when the adolescent with autism is participating in the task) or peer-related stimuli (i.e., pictures of peers or peer contexts) and stimuli of restricted individualized interests. These tasks (e.g., IGT) are traditionally nonsocial in nature and likely disproportionately motivate individuals who value monetary rewards. Thus, paradigms that target adolescents with autism may need to be tailored to the restricted interests of each participant (i.e., one individual may have cars as a restricted interest, whereas another individual may have video games as a restricted interest). Therefore, researchers may want to contact parents before administering the task to determine the stimuli that are most rewarding for the adolescents with autism. The decision-making task itself can be simple (e.g., guessing whether the value of a card is higher or lower than 5; Delgado, Nystrom, Fissell, Noll, & Fiez, 2000) and depend on rewards that are focused on the adolescents’ restricted interests. By evaluating the willingness of adolescents with autism to take risks with rewards focused on peers versus on restricted interests, researchers can better understand the risk-taking behavioral patterns and trajectories across contexts. This approach can dissociate whether, in adolescence, the reward-processing system in autism is calibrated differently but risk-taking behavior is comparable or whether both reward processing and risk taking are importantly altered.
With respect to evaluating the degree to which cognitive control is impaired using peer-based versus non-peer-based stimuli, a simple go/no-go task is an effective way to assess both contexts. For instance, one could test same-age peer faces versus adult faces, familiar peer faces versus unfamiliar peer faces, peer faces versus objects that are the focus of restricted interests, and neutral objects versus objects that are the focus of restricted interests. This approach can be used to target both the behavioral and the neural basis of the ability to inhibit a prepotent response despite differing contexts that may elicit more or less reward-related and risk- taking responses in adolescents with autism. Such research that directly focuses on potential differences in the reward-seeking and risk-taking behaviors of TD adolescents and adolescents with autism could inform meaningful forms of intervention (e.g., sexual-health education, other social adaptive functioning skills).
Pubertal development in adolescents with autism
An area of research that has yet to be explicitly explored in adolescents with autism is the role of pubertal development. Pubertal development is known to influence behavior (Feinberg & Campbell, 2010; Halpern, 2006; Marceau, Dorn, & Susman, 2012; Martin et al., 2002; Quevedo, Benning, Gunnar, & Dahl, 2009; Varlinskaya, Vetter-O’Hagen, & Spear, 2013; Wallen, 2001; Waylen & Wolke, 2004) and neural activation and organization (Blakemore, Burnett, & Dahl, 2010; Bramen et al., 2011; Forbes & Dahl, 2010; Forbes, Phillips, Silk, Ryan, & Dahl, 2011; Goddings, Burnett Heyes, Bird, Viner, & Blakemore, 2012; Herting, Maxwell, Irvine, & Nagel, 2012; Holm et al., 2009; Lenroot & Giedd, 2010; Romeo, 2003; Schulz, Molenda-Figueira, & Sisk, 2009; Sisk & Zehr, 2005; Spear, 2011) and is hypothesized to motivate and enable new behaviors in TD adolescents (Dahl & Spear 2004; Forbes & Dahl, 2010; Scherf et al., 2013; Scherf & Scott, 2012). There are no data that address whether or how puberty influences behavioral and brain development in autism. For example, given the reports of pubertal deterioration (Billstedt et al., 2005; Gillberg & Steffenburg, 1987), pubertal development may uniquely complicate development in these important adolescent domains. Even minor alterations to the timing or tempo of pubertal development may have significant consequences for emerging and fine-tuning all of the domains of adolescent development explored in this article.
We suggest that in future studies, researchers must begin to systematically investigate the influence of pubertal development on adolescent-specific deterioration in autism, including how it does or does not influence behavior in the domains we have outlined as well as the structural and functional organization in the developing brain. Recent evidence regarding human adolescence provides support for the mountain of evidence obtained from animal models (see Sisk & Zehr, 2005), which indicates that pubertal hormones sculpt both functional and structural organization of neural circuitry (Barnea-Goraly et al., 2004; Burnett & Blakemore, 2009; Gogtay et al., 2004; Ostby et al., 2009; Schmithorst & Yuan, 2010; Tamnes et al., 2010). With these findings in mind, and using our two-hit model, we predict that pubertal hormones contribute in important ways to disruptions in adolescent-specific neural organization and behavioral outcomes in autism. We suggest that the influx of pubertal hormones is forced to shape a pervasively disrupted neural system in individuals with autism. As a result, puberty-related influences on brain and behavioral development are expected to be markedly different in individuals with autism compared with TD individuals. Furthermore, these differences are likely to contribute to the failure to develop new behaviors that are essential for acquiring adult levels of adaptive functioning (e.g., independence from parents, intimate friendships, romantic and sexual partnerships). Future research on the relationship between pubertal development and pubertal hormones, neural functioning and organization, and behavior in adolescents with autism will provide critical evidence to evaluate this hypothesis.
Conclusions
We have proposed a novel theoretical framework for conceptualizing the full developmental trajectory of autism and have attempted to connect the most cutting-edge findings about atypicalities in early brain development, adolescent-specific deterioration in functioning, and poor levels of adaptive functioning in adulthood. It is important to note that our framework is particularly sensitive to the nonlinear atypicalities in the developmental course of autism. We focus on the role of adolescence as a developmental period of particular vulnerability that builds on the early first hit for individuals with autism and uniquely contributes to atypical trajectories toward adult outcomes. It is clear that adolescence is exceptionally difficult for individuals with autism, especially in comparison to individuals with other developmental disorders who fair reasonably well in taking on adult social roles and levels of adaptive functioning. For instance, compared with adults with autism, adults with Down’s syndrome achieve far more independence in their residential living status and social contact with friends (Esbensen, et al., 2010).
We provide evidence that suggests that the core developmental processes that support the acquisition of adult levels of social and adaptive functioning putatively fail to develop in autism, specifically during adolescence. We appreciate that there are important individual differences among individuals with autism in terms of adulthood outcomes and suggest that this variation can be used to evaluate some of the hypothesized trajectories. For instance, individuals with autism who evince more typical levels of social-reward processing may be capable of forming higher quality peer relationships in adolescence that lead to subsequent higher quality adult relationships. Also, we suggest that there may be individual differences with respect to how well this model characterizes the development of any one individual with autism and that there may be important moderating factors that mitigate or exacerbate the effects of the second hit of adolescence.
Specifically, individual differences in the manifestation of the first hit on atypical neural network organization will likely moderate the extent to which the second hit overwhelms the developing system in the face of new demands during adolescence. For example, individuals with autism who show disproportionately early (i.e., prenatal and infant development) and severe disruptions to the behaviors or neural networks that ultimately support cognitive control might exhibit more difficulty with indiscriminate risk taking (and lack of cognitive control) in adolescence, which could make them especially vulnerable to more harmful consequences of risk taking. Uncovering these potential outcomes of the first hit could reveal important targets for intervention that could moderate (or even prevent) subsequent consequences as individuals transition into adolescence.
Our goal is to provide a model that generally characterizes the full developmental trajectory of autism from early infancy to adulthood, that motivates basic research on this trajectory, and that will ultimately inform treatment and intervention approaches. For example, the go/ no-go paradigm that we outlined to evaluate cognitive control and approach tendencies may help identify adolescents with autism who exhibit enhanced approach behaviors for objects that are the focus of their restricted interests. This ultimately may be a tool for targeting such individuals for interventions designed to enhance the reward value of peers. The creation of interventions that involve hobbies or relate to restricted interests with same-age peers could encourage peer interaction and possibly enhance reward derived from peer interactions. We believe that this two-hit model of the developmental course of autism provides a framework that will guide both basic and translational research about the central factors that uniquely interfere with the ability to achieve adult levels of adaptive functioning in autism.
Acknowledgments
We would like to thank Elisabeth Whyte and Sara Barth for helpful comments on earlier versions of this manuscript.
Funding
This work was supported by Pennsylvania Department of Health SAP Grant 4100047862 to K. S. Scherf (co-principal investigator), the Social Science Research Institute, and the Department of Psychology at Pennsylvania State University, as well as a Graduate Research Fellowship from the National Science Foundation to G. Picci (DGE1255832). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
This is not to say that peers become the sole support system for adolescents; parents maintain a critical presence in adolescents’ lives (see Lam, McHale, & Crouter, 2012).
Author Contributions
G. Picci and K. S. Scherf jointly conceived and drafted the manuscript. Both authors approved the final version of the manuscript for submission.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Abrahams BS, Geschwind DH. Connecting genes to brain in the autism spectrum disorders. Archives of Neurology. 2010;67:395–399. doi: 10.1001/archneurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Lord C, Risi S, DiLavore P, Shulman C, Thurm A, Pickles A. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology. 2007;75:594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Anderson D, Mayes M, Lord C. Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. American Journal on Intellectual and Developmental Disabilities. 2011;116:381–397. doi: 10.1352/1944-7558-116.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Oti R, Lord C, Welch K. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2009;37:1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagwell C, Newcomb A, Bukowski W. Preadolescent friendship and peer rejection as predictors of adult adjustment. Child Development. 1998;69:140–153. [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Bauminger N, Shulman C. The development and maintenance of friendship in high-functioning children with autism. Autism: The International Journal of Research and Practice. 2003;7:81–97. doi: 10.1177/1362361303007001007. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio A, Damasio H, Anderson S. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Benayed R, Gharani N, Rossman I, Mancuso V, Lazar G, Kamdar S, Zimmerman RA. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. American Journal of Human Genetics. 2005;77:851–868. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benthin A, Slovic P, Severson H. A psychometric study of adolescent risk perception. Journal of Adolescence. 1993;16:153–168. doi: 10.1006/jado.1993.1014. [DOI] [PubMed] [Google Scholar]
- Billstedt E, Gillberg C, Gillberg C. Autism after adolescence: Population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. Journal of Autism and Developmental Disorders. 2005;35:351–360. doi: 10.1007/s10803-005-3302-5. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Kaiser MD, Pelphrey KA. Enhanced neural responses to rule violation in children with autism: A comparison to social exclusion. Developmental Cognitive Neuroscience. 2011;1:280–294. doi: 10.1016/j.dcn.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen J, Hranilovich J, Dahl R, Forbes E, Chen J, Toga A, Sowell E. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton AV, Tonge BJ, Einfeld SL. Psychopathology in children and adolescents with autism compared to young people with intellectual disability. Journal of Autism and Developmental Disorders. 2006;36:863–870. doi: 10.1007/s10803-006-0125-y. [DOI] [PubMed] [Google Scholar]
- Brown B. Adolescents’ relationships with peers. In: Lerner R, Steinberg L, editors. Handbook of adolescent psychology. Hoboken, NJ: John Wiley & Sons; 2004. pp. 363–394. [Google Scholar]
- Brown B, Eicher S, Petrie S. The importance of peer group (“crowd”) affiliation in adolescence. Journal of Adolescence. 1986;86:73–96. doi: 10.1016/s0140-1971(86)80029-x. [DOI] [PubMed] [Google Scholar]
- Brown J. Adolescent development of children with infantile psychosis. Seminars in Psychiatry. 1969;1:79–89. [Google Scholar]
- Burnett S, Blakemore SJ. Functional connectivity during a social emotion task in adolescents and in adults. European Journal of Neuroscience. 2009;29:1294–1301. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers ES, Nichols S, Voyer SD, Reilly G. Sexual well-being of a community sample of high-functioning adults on the autism spectrum who have been in a romantic relationship. Autism: The International Journal of Research and Practice. 2013;17:418–433. doi: 10.1177/1362361311431950. [DOI] [PubMed] [Google Scholar]
- Cappadocia MC, Weiss JA, Pepler D. Bullying experiences among children and youth with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42:266–277. doi: 10.1007/s10803-011-1241-x. [DOI] [PubMed] [Google Scholar]
- Caron MJ, Mottron L, Berthiaume C, Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129:1789–1802. doi: 10.1093/brain/awl072. [DOI] [PubMed] [Google Scholar]
- Carrington S, Templeton E, Papinczak T. Adolescents with Asperger syndrome and perceptions of friendship. Focus on Autism and Other Developmental Disabilities. 2003;18:211–218. [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM, Cao A. Response of neural reward regions to food cues in autism spectrum disorders. Journal of Neurodevelopmental Disorders. 2012;4:9–21. doi: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock J, Schauder KB, Loring WA, Rogers BP, Bolton S. Affective neural response to restricted interests in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2014;55:162–171. doi: 10.1111/jcpp.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Somerville LH. The storm and stress of adolescence: Insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46:193–207. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe BP, Girirajan S, Eichler EE. The genetic variability and commonality of neurodevelopmental disease. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2012;160C:118–129. doi: 10.1002/ajmg.c.31327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WA, Welsh DP, Furman W. Adolescent romantic relationships. Annual Review of Psychology. 2009;60:631–652. doi: 10.1146/annurev.psych.60.110707.163459. [DOI] [PubMed] [Google Scholar]
- Connolly J, Craig W, Goldberg A, Pepler D. Mixed-gender groups, dating, and romantic relationships in early adolescence. Journal of Research on Adolescence. 2004;14:185–207. [Google Scholar]
- Connolly J, Nguyen HN, Pepler D, Craig W, Jiang D. Developmental trajectories of romantic stages and associations with problem behaviours during adolescence. Journal of Adolescence. 2013;36:1013–1024. doi: 10.1016/j.adolescence.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann C, Redcay E, Buckwalter J, Kennedy D, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Webb SJ, Schumann CM. From toddlers to adults: The changing landscape of the brain in autism. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism spectrum disorders. New York, NY: Oxford University Press; 2011. pp. 611–631. [Google Scholar]
- Dahl R. The impact of inadequate sleep on children’s daytime cognitive function. Seminars in Pediatric Neurology. 1996;3:44–50. doi: 10.1016/s1071-9091(96)80028-3. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, Fagan AJ, Gallagher L. Social and monetary reward processing in autism spectrum disorders. Molecular Autism. 2012;3:7. doi: 10.1186/2040-2392-3-7. Retrieved from http://www.molecularautism.com/content/3/1/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Harrison NA, Knafo S, Bird G, Dolan RJ. Explaining enhanced logical consistency during decision making in autism. Journal of Neuroscience. 2008;28:10746–10750. doi: 10.1523/JNEUROSCI.2895-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LC, Ho HH. Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:739–747. doi: 10.1007/s10803-007-0441-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg L. The autistic child in adolescence. American Journal of Psychiatry. 1956;112:607–612. doi: 10.1176/ajp.112.8.607. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Bishop S, Seltzer MM, Greenberg JS, Taylor JL. Comparisons between individuals with autism spectrum disorders and individuals with Down syndrome in adulthood. American Journal on Intellectual and Developmental Disabilities. 2010;115:277–290. doi: 10.1352/1944-7558-115.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley MA, McMahon WM, Fombonne E, Jenson WR, Miller J, Gardner M, Coon H. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Research. 2009;2:109–118. doi: 10.1002/aur.69. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain and Cognition. 2010;72:56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Feldmann J, Middleman AB. Adolescent sexuality and sexual behavior. Current Opinion in Obstetrics and Gynecology. 2002;14:489–493. doi: 10.1097/00001703-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ME, Wentzel KR, Wood D, Stevens E, Siesfeld GA. Processes associated with integrative social competence: Emotional and contextual influences on adolescent social responsibility. Journal of Adolescent Research. 1989;4:405–425. [Google Scholar]
- Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: Tracking the effects of adolescent sleep loss during the middle school years. Child Development. 2004;75:84–95. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. Retrieved from http://journal.frontiersin.org/Journal/10.3389/neuro.09.006.2010/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Geurts HM, van den Bergh SF, Ruzzano L. Prepotent response inhibition and interference control in autism spectrum disorders: Two meta-analyses. Autism Research. 2014 doi: 10.1002/aur.1369. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Ghaziuddin N, Greden J. Depression in persons with autism: Implication for research and clinical care. Journal of Autism and Developmental Disorders. 2002;32:299–306. doi: 10.1023/a:1016330802348. [DOI] [PubMed] [Google Scholar]
- Giedd J, Clasen L, Lenroot R, Greenstein D, Wallace G, Ordaz S, Stayer C. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Schaumann H. Infantile autism and puberty. Journal of Autism and Developmental Disorders. 1982;11:365–371. doi: 10.1007/BF01531612. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Steffenburg S. Outcome and prognostic factors in infantile autism and similar conditions: A population-based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders. 1987;17:273–287. doi: 10.1007/BF01495061. [DOI] [PubMed] [Google Scholar]
- Goddings A-L, Burnett Heyes S, Bird G, Viner RM, Blakemore S-J. The relationship between puberty and social emotion processing. Developmental Science. 2012;15:108–111. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Cox A. Social and psychiatric function in adolescents with Asperger syndrome compared with conduct disorder. Journal of Autism and Developmental Disorders. 2000;30:279–293. doi: 10.1023/a:1005523232106. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern CT. Integrating hormones and other biological factors into a developmental systems model of adolescent female sexuality. New Directions for Child and Adolescent Development. 2006;112:9–22. doi: 10.1002/cd.159. [DOI] [PubMed] [Google Scholar]
- Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, Deprey LJ, Hertz-Picciotto I. Regression in autism: Prevalence and associated factors in the CHARGE Study. Ambulatory Pediatrics. 2008;8:25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Haracopos D, Pedersen L. Sexuality and autism: A nationwide survey in Denmark. Unpublished manuscript; 1992. [Google Scholar]
- Hardan AY, Libove RA, Keshavan MS, Melhem NM, Minshew NJ. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biological Psychiatry. 2009;66:320–326. doi: 10.1016/j.biopsych.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartup W. The company they keep: Friendships and their developmental significance. Child Development. 1996;67:1–13. [PubMed] [Google Scholar]
- Henault I. Asperger’s syndrome and sexuality from adolescence through adulthood. London, England: Jessica Kingsley; 2006. [Google Scholar]
- Henninger NA, Taylor JL. Outcomes in adults with autism spectrum disorders: A historical perspective. Autism: The International Journal of Research and Practice. 2013;17:103–116. doi: 10.1177/1362361312441266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cerebral Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges EV, Perry DG. Personal and interpersonal antecedents and consequences of victimization by peers. Journal of Personality and Social Psychology. 1999;76:677–685. doi: 10.1037//0022-3514.76.4.677. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. Journal of Adolescent Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, Rutter M. Social outcomes in mid- to later adulthood among individuals diagnosed with autism and average nonverbal IQ as children. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:572–581.e1. doi: 10.1016/j.jaac.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Hu S, Pruessner JC, Coupé P, Collins DL. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. NeuroImage. 2013;74:276–287. doi: 10.1016/j.neuroimage.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Jankowski KF, Moore WE, Merchant JS, Kahn LE, Pfeifer JH. But do you think I’m cool? Developmental differences in striatal recruitment during direct and reflected social self-evaluations. Developmental Cognitive Neuroscience. 2014;8:40–54. doi: 10.1016/j.dcn.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Yechiam E, Murphy RR, Queller S, Stout JC. Motivational processes and autonomic responsivity in Asperger’s disorder: Evidence from the Iowa Gambling Task. Journal of the International Neuropsychological Society. 2006;12:668–676. doi: 10.1017/S1355617706060802. [DOI] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, Casey BJ. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective, & Behavioral Neuroscience. 2014 doi: 10.3758/s13415-014-0257-z. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The role of adaptive behavior in autism spectrum disorders: Implications for functional outcome. Journal of Autism and Developmental Disorders. 2011;41:1007–1018. doi: 10.1007/s10803-010-1126-4. [DOI] [PubMed] [Google Scholar]
- Knudson AG. Mutation and cancer: Statistical study of retinoblastoma. Proceedings of the National Academy of Sciences, USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Rüther M, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Konrad K. Atypical brain responses to reward cues in autism as revealed by event-related potentials. Journal of Autism and Developmental Disorders. 2011;41:1523–1533. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte-Rüther M, Nehrkorn B, Müller K, Fink GR, Kamp-Becker I, Konrad K. Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2013;8:565–572. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MH, Orsmond GI, Cohn ES, Coster WJ. Friendship characteristics and activity patterns of adolescents with an autism spectrum disorder. Autism: The International Journal of Research and Practice. 2013;17:481–500. doi: 10.1177/1362361311416380. [DOI] [PubMed] [Google Scholar]
- Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila ML, Ebeling H, Moilanen I. Social anxiety in high-functioning children and adolescents with autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2008;38:1697–1709. doi: 10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- Lam CB, McHale SM, Crouter AC. Parent-child shared time from middle childhood to late adolescence: Developmental course and adjustment correlates. Child Development. 2012;83:2089–2103. doi: 10.1111/j.1467-8624.2012.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Lainhart JE. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Perry A. Outcomes in adolescents and adults with autism: A review of the literature. Research in Autism Spectrum Disorders. 2011;5:1271–1282. [Google Scholar]
- Lin A, Rangel A, Adolphs R. Impaired learning of social compared to monetary rewards in autism. Frontiers in Neuroscience. 2012;6:143. doi: 10.3389/fnins.2012.00143. Retrieved from http://journal.frontiersin.org/Journal/10.3389/fnins.2012.00143/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little L. Middle-class mothers’ perceptions of peer and sibling victimization among children with Asperger’s syndrome and nonverbal learning disorders. Issues in Comprehensive Pediatric Nursing. 2002;25:43–57. doi: 10.1080/014608602753504847. [DOI] [PubMed] [Google Scholar]
- Marceau K, Dorn LD, Susman EJ. Stress and puberty-related hormone reactivity, negative emotionality, and parent-adolescent relationships. Psychoneuroendocrinology. 2012;37:1286–1298. doi: 10.1016/j.psyneuen.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Masten CL, Colich NL, Rudie JD, Bookheimer SY, Eisenberger NI, Dapretto M. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Developmental Cognitive Neuroscience. 2011;1:260–270. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Dempsey T, Fodstad JC. The effect of autism spectrum disorders on adaptive independent living skills in adults with severe intellectual disability. Research in Developmental Disabilities. 2009;30:1203–1211. doi: 10.1016/j.ridd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, Zahid J. Variables associated with anxiety and depression in children with autism. Journal of Developmental and Physical Disabilities. 2011;23:325–337. [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, Ahuja M, Smith LA. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Research in Autism Spectrum Disorders. 2011;5:474–485. [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophrenia Bulletin. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Engelhardt CR. Video game use in boys with autism spectrum disorder, ADHD, or typical development. Pediatrics. 2013;132:260–266. doi: 10.1542/peds.2012-3956. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Kanne SM. Friendship and internalizing symptoms among children and adolescents with ASD. Journal of Autism and Developmental Disorders. 2010;40:1512–1520. doi: 10.1007/s10803-010-1014-y. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Davis A, Navarre JR, Scott TA. Family report of ASD concomitant with depression or anxiety among US children. Journal of Autism and Developmental Disorders. 2011;41:646–653. doi: 10.1007/s10803-010-1085-9. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Occulomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52:917–921. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Research. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WE, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: Associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Archives of General Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Davidson RJ. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Archives of General Psychiatry. 2006;63:1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. Impaired thalamocortical connectivity in autism spectrum disorder: A study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopa-thology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Newport J, Newport M, Bolick T. Autism-Asperger’s & sexuality: Puberty and beyond. Arlington, TX: Future Horizons; 2002. [Google Scholar]
- O’Hearn K, Asato M, Ordaz S, Luna B. Neurodevelopment and executive function in autism. Development and Psychopathology. 2008;20:1103–1132. doi: 10.1017/S0954579408000527. [DOI] [PubMed] [Google Scholar]
- O’Hearn K, Schroer E, Minshew N, Luna B. Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia. 2010;48:3955–3960. doi: 10.1016/j.neuropsychologia.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsmond GI, Krauss MW, Seltzer MM. Peer relationships and social and recreational activities among adolescents and adults with autism. Journal of Autism and Developmental Disorders. 2004;34:245–256. doi: 10.1023/b:jadd.0000029547.96610.df. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousley O, Mesibov G. Sexual attitudes and knowledge of high-functioning adolescents and adults with autism. Journal of Autism and Developmental Disorders. 1991;21:471–481. doi: 10.1007/BF02206871. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peake SJ, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. NeuroImage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, Dapretto M. Longitudinal change in the neural bases of adolescent social self-evaluations: Effects of age and pubertal development. Journal of Neuroscience. 2013;33:7415–7419. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who.”. Biological Psychiatry. 2008;64:552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: Effects on the psychophysiology of defensive and appetitive motivation. Development and Psychopathology. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauer AJ, Pettit GS, Lansford JE, Bates JE, Dodge KA. Romantic relationship patterns in young adulthood and their developmental antecedents. Developmental Psychology. 2013;49:2159–2171. doi: 10.1037/a0031845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37:239–244. doi: 10.5665/sleep.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo MJ, Padrón I, de Vega M, Ferstl EC. Adolescents’ risky decision-making activates neural networks related to social cognition and cognitive control processes. Frontiers in Human Neuroscience. 2014;8:60. doi: 10.3389/fnhum.2014.00060. Retrieved from http://journal.frontiersin.org/Journal/10.3389/fnhum.2014.00060/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman G, Masten A, Coatsworth J, Tellegen A. Salient and emerging developmental tasks in the transition to adulthood. Child Development. 2004;75:123–133. doi: 10.1111/j.1467-8624.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Rojas D, Peterson E, Winterrowd E, Reite M, Rogers S, Tregellas J. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. Retrieved from http://www.biomedcentral.com/1471-244X/6/56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R. Puberty: A period of both organizational and activational effects of steroid hormones on neurobehavioural development. Journal of Neuroendocrinology. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, Kenworthy L. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology. 2013;27:13–18. doi: 10.1037/a0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley E, Chandler S, Baird G, Simonoff E, Pickles A, Loucas T, Charman T. The experience of friendship, victimization and bullying in children with an autism spectrum disorder: Associations with child characteristics and school placement. Research in Autism Spectrum Disorders. 2012;6:1126–1134. [Google Scholar]
- Ruble L, Dalrymple N. Social/sexual awareness of persons with autism: A parental perspective. Archives of Sexual Behavior. 1993;22:229–240. doi: 10.1007/BF01541768. [DOI] [PubMed] [Google Scholar]
- Rutter M. Autistic children: Infancy to adulthood. Seminars in Psychiatry. 1970;2:435–450. [PubMed] [Google Scholar]
- Schaer M, Ottet MC, Scariati E, Dukes D, Franchini M, Eliez S, Glaser B. Decreased frontal gyrification correlates with altered connectivity in children with autism. Frontiers in Human Neuroscience. 2013;7:750. doi: 10.3389/fnhum.2013.00750. Retrieved from http://journal.frontiersin.org/Journal/10.3389/fnhum.2013.00750/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K, Behrmann M, Kimchi R, Luna B. Emergence of global shape processing continues through adolescence. Child Development. 2009;80:162–177. doi: 10.1111/j.1467-8624.2008.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Luna B, Kimchi R, Minshew N, Behrmann M. Missing the big picture: Impaired development of global shape processing in autism. Autism Research. 2008;1:114–129. doi: 10.1002/aur.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Scott LS. Connecting developmental trajectories: Biases in face processing from infancy to adulthood. Developmental Psychobiology. 2012;54:643–663. doi: 10.1002/dev.21013. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Thomas C, Doyle J, Behrmann M. Emerging structure-function relations in the developing face processing system. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht152. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain and Cognition. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and Behavior. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. Journal of Neuroscience. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffge-Krenke I, Luyckx K, Salmela-Aro K. Work and love during emerging adulthood: Introduction to the special issue. Emerging Adulthood. 2014;2:3–5. [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:234–247. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52:286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, Collins WA, Salvatore JE. The impact of early interpersonal experience on adult romantic relationship functioning: Recent findings from the Minnesota Longitudinal Study of Risk and Adaptation. Current Directions in Psychological Science. 2011;20:355–359. doi: 10.1177/0963721411418468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Posserud MB, Gillberg C, Lundervold AJ, Hysing M. Sleep problems in children with autism spectrum problems: A longitudinal population-based study. Autism: The International Journal of Research and Practice. 2012;16:139–150. doi: 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- Solish A, Perry A, Minnes P. Participation of children with and without disabilities in social, recreational and leisure activities. Journal of Applied Research in Intellectual Disabilities. 2010;23:226–236. [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Yoon JH, Ragland JD, Niendam TA, Lesh TA, Fairbrother W, Carter CS. The development of the neural substrates of cognitive control in adolescents with autism spectrum disorders. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.036. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Dana J, White SE, Crowley MJ. Failure is not an option: Risk-taking is moderated by anxiety and also by cognitive ability in children and adolescents diagnosed with an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41:55–65. doi: 10.1007/s10803-010-1021-z. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, Suchy Y, Kesner RP, McMahon WM, Lainhart JE, Passingham R. Intact emotion facilitation for nonsocial stimuli in autism: Is amygdala impairment in autism specific for social information? Journal of the International Neuropsychological Society. 2008;14:42–54. doi: 10.1017/S1355617708080107. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Sterzing PR, Shattuck PT, Narendorf SC, Wagner M, Cooper BP. Bullying involvement and autism spectrum disorders: Prevalence and correlates of bullying involvement among adolescents with an autism spectrum disorder. Archives of Pediatrics and Adolescent Medicine. 2012;166:1058–1064. doi: 10.1001/archpediatrics.2012.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MA, Kaur A. High-functioning autism and sexuality: A parental perspective. Autism: The International Journal of Research and Practice. 2005;9:266–289. doi: 10.1177/1362361305053258. [DOI] [PubMed] [Google Scholar]
- Stokes M, Newton N, Kaur A. Stalking, and social and romantic functioning among adolescents and adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2007;37:1969–1986. doi: 10.1007/s10803-006-0344-2. [DOI] [PubMed] [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Courchesne E. Patches of disorganization in the neocortex of children with autism. New England Journal of Medicine. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, Menon V. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Reports. 2013;5:738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Südhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in Human Neuroscience. 2013;7:458. doi: 10.3389/fnhum.2013.00458. Retrieved from http://journal.frontiersin.org/Journal/10.3389/fnhum.2013.00458/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. Retrieved from http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten I, Palmen S, Von Cappeln P, Steinbusch H, Korr H, Heinsen H, Schmitz C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131:987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- van Roekel E, Scholte RH, Didden R. Bullying among adolescents with autism spectrum disorders: Prevalence and perception. Journal of Autism and Developmental Disorders. 2010;40:63–73. doi: 10.1007/s10803-009-0832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroin-flammation in the brain of patients with autism. Annals of Neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Vetter-O’Hagen CS, Spear LP. Puberty and gonadal hormones: Role in adolescent-typical behavioral alterations. Hormones and Behavior. 2013;64:343–349. doi: 10.1016/j.yhbeh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. NeuroImage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Sex and context: Hormones and primate sexual motivation. Hormones and Behavior. 2001;40:339–357. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Waylen A, Wolke D. Sex ‘n’ drugs ‘n’ rock ‘n’ roll: The meaning and social consequences of pubertal timing. European Journal of Endocrinology. 2004;151:U151–U159. doi: 10.1530/eje.0.151u151. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Carrasco M, Swartz JR, Wiggins JL, Kurapati N, Liberzon I, Monk CS. Neural activation to emotional faces in adolescents with autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2011;52:296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Watt HJ, Line EA, Bishop DV. Adult psychosocial outcomes of children with specific language impairment, pragmatic language impairment and autism. International Journal of Language & Communication Disorders. 2009;44:511–528. doi: 10.1080/13682820802708098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Branje S, Keijsers L, Meeus W. Brief report: How adolescent personality moderates the effect of love history on the young adulthood romantic relationship quality. Journal of Adolescence. 2014 doi: 10.1016/j.adolescence.2014.02.006. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck SB, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. Journal of Consulting and Clinical Psychology. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]