Abstract
Fatty acid binding protein 4 (FABP4), also known as adipocyte FABP or aP2, is secreted from adipocytes in association with lipolysis as a novel adipokine, and elevated serum FABP4 level is associated with obesity, insulin resistance, and atherosclerosis. However, little is known about the modulation of serum FABP4 level by therapeutic drugs. Sitagliptin (50 mg/day), a dipeptidyl peptidase 4 (DPP-4) inhibitor that increases glucagon-like peptide 1 (GLP-1), was administered to patients with type 2 diabetes (n = 24) for 12 weeks. Treatment with sitagliptin decreased serum FABP4 concentration by 19.7% (17.8 ± 1.8 vs. 14.3 ± 1.5 ng/ml, P < 0.001) and hemoglobin A1c without significant changes in adiposity or lipid variables. In 3T3-L1 adipocytes, sitagliptin or exendin-4, a GLP-1 receptor agonist, had no effect on short-term (2 h) secretion of FABP4. However, gene expression and long-term (24 h) secretion of FABP4 were significantly reduced by sitagliptin, which was not mimicked by exendin-4. Treatment with recombinant DPP-4 increased gene expression and long-term secretion of FABP4, and the effects were cancelled by sitagliptin. Furthermore, knockdown of DPP-4 in 3T3-L1 adipocytes decreased gene expression and long-term secretion of FABP4. In conclusion, sitagliptin decreases serum FABP4 level, at least in part, via reduction in the expression and consecutive secretion of FABP4 in adipocytes by direct inhibition of DPP-4.
Keywords: fatty acid binding protein 4, adipokine, adipocyte, glucagon-like peptide 1
Fatty acid binding proteins (FABPs) are about 14–15 kDa predominantly cytosolic proteins that can reversibly bind hydrophobic ligands, such as saturated and unsaturated long-chain fatty acids, with high affinity (1–3). FABPs have been proposed to facilitate the transport of lipids to specific compartments in the cell (1). Among FABPs, fatty acid binding protein 4 (FABP4), also known as adipocyte FABP or aP2, is mainly expressed in both adipocytes and macrophages and plays an important role in the development of insulin resistance and atherosclerosis (4–6). Furthermore, it has been demonstrated in experimental models that the use of a small-molecule FABP4 inhibitor might be a therapeutic strategy against insulin resistance, type 2 diabetes mellitus, and atherosclerosis (7).
Recent studies have demonstrated that FABP4 is secreted from adipocytes in association with lipolysis regulated by adenylyl cyclase/protein kinase A signaling and guanylyl cyclase/protein kinase G signaling via a nonclassical secretion pathway (8–11), though there are no typical secretory signal peptides in the sequence of FABP4 (1). It has also been shown that FABP4 acts as an adipokine for the development of hepatic insulin resistance (9), suppression of cardiomyocyte contraction (12), and inhibition of endothelial nitric oxide synthase in endothelial cells (13). Furthermore, elevated serum concentration of FABP4 has been shown to be associated with obesity, insulin resistance, type 2 diabetes mellitus, hypertension, cardiac dysfunction, renal dysfunction, dyslipidemia, atherosclerosis, and cardiovascular events (8, 14–24). However, little is known about the modulation of serum FABP4 level by therapeutic drugs including antidiabetic agents except for thiazolidinedione (25).
For treatment of type 2 diabetes mellitus, incretin-based therapies using glucagon-like peptide 1 (GLP-1) receptor agonists or dipeptidyl peptidase 4 (DPP-4) inhibitors have become available (26, 27). GLP-1 receptor agonists directly activate the GLP-1 receptor, whereas DPP-4 inhibitors slow GLP-1 degradation, thereby increasing the concentration of endogenous GLP-1, which activates the GLP-1 receptor (28–30). The potential glycemic benefits of these treatments include glucose-dependent stimulation of insulin production and secretion; suppression of inappropriate glucagon secretion; slowing of gastric emptying, which reduces the rate of glucose appearance in the circulation; and satiety, which may reduce food intake (29). In the present study, we investigated the impact of DPP-4 inhibitor therapy on serum FABP4 level in patients with type 2 diabetes mellitus and its mechanisms.
SUBJECTS AND METHODS
The present study consisted of a human study (study 1) and an in vitro study (study 2). Study 1 conformed to the principles outlined in the Declaration of Helsinki and was performed with the approval of the Ethical Committee of Fujita Health University. Written informed consent was received from all of the study subjects. Study 2 was approved by the Committee for Animal Research, Sapporo Medical University, and was conducted in accordance with the Guidelines of Sapporo Medical University for Animal Use in Research.
Study 1: effects of sitagliptin on FABP4 level in patients with type 2 diabetes mellitus
A total of 24 type 2 diabetic patients treated with only diet and exercise therapy [n = 14; male/female (M/F): 7/7] or glimepiride (0.5–2 mg/day), a sulfonylurea, for more than 8 weeks (n = 10, M/F: 8/2) were enrolled from outpatient clinics affiliated with Fujita Health University. Entry criteria were no treatment with antidiabetic agents except for sulfonylurea before the enrollment and no evidence of complications such as hepatic, cerebrovascular, cardiovascular, or renal disease. Before and after treatment with sitagliptin (50 mg/day), a DPP-4 inhibitor, for 12 weeks, blood samples were collected, and plasma and serum samples were analyzed immediately or stored at −80°C until biochemical analyses.
Serum concentration of FABP4 was measured using a commercially available enzyme-linked immunosorbent assay kit for FABP4 (Biovendor R and D, Modrice, Czech Republic). The accuracy, precision, and reproducibility of the kit have been described previously (8). The intra- and interassay coefficient variances in the kits were <5%. Plasma glucose was determined by the glucose oxidase method. Fasting plasma insulin was measured by a radioimmunoassay method. Hemoglobin A1c (HbA1c) was determined by a latex coagulation method and was expressed in NGSP scale. Creatinine (Cr), 1,5-anhydroglucitol (1,5-AG) and lipid profiles, including total cholesterol, HDL cholesterol, and triglycerides, were determined by enzymatic methods. LDL cholesterol level was calculated by the Friedewald equation. High-sensitivity C-reactive protein (hsCRP) was measured by a nephelometry method. HOMA-R, an index of insulin resistance, was calculated by the previously reported formula: insulin (μU/ml) × glucose (mg/dl)/405. As an index of renal function, estimated glomerular filtration rate (eGFR) was calculated by an equation for Japanese individuals (31): eGFR (ml/min/1.73 m2) = 194 × Cr(−1.094) × age(−0.287) × 0.739 (if female). BMI was calculated as body weight (in kilograms) divided by the square of body height (in meters).
Study 2: effects of sitagliptin and exendin-4 on FABP4 expression and secretion in 3T3-L1 adipocytes
All biochemical reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Preadipocyte 3T3-L1 cells were obtained from Health Science Research Resources Bank (Osaka, Japan). Differentiation of 3T3-L1 cells was induced as previously described (10). After an overnight serum depletion by 0.5% BSA in DMEM (Invitrogen, Carlsbad, CA), differentiated 3T3-L1 adipocytes were stimulated with 0–50 µM sitagliptin, 0–50 nM exendin-4 (a GLP-1 receptor agonist), and 0–500 ng/ml recombinant DPP-4 (Prospec, East Brunswick, NJ) in the absence or presence of 10 µM isoproterenol in DMEM supplemented with 0.5% BSA for 2 or 24 h. The doses of reagents and incubation periods were varied according to the experimental protocol. Each experiment was done in at least triplicate. Cytotoxicity was assessed by lactate dehydrogenase (LDH) assay kit (Promega, Madison, WI).
Small interfering RNA knockdown.
Small interfering RNA (siRNA) analysis was performed by using Stealth RNAi (Invitrogen) targeting mouse DPP-4 mRNA (cat. #MSS203657) and negative control (cat. #12935-300). Using a method of density-based separation followed by replating of enriched adipocytes in a monolayer (32), differentiated 3T3-L1 adipocytes were transfected with specific or control Stealth RNAi by lipofectamine 2000 (Invitrogen) and cultured for 48 h.
Quantitative real-time PCR
Total RNA was isolated using Trizol reagent (Invitrogen). One microgram of total RNA was reverse transcribed by using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR analysis was performed using SYBR Green in a real-time PCR system (Applied Biosystems). The thermal cycling program was 10 min at 95°C for enzyme activation and 40 cycles of denaturation for 15 s at 95°C, 30 s annealing at 58°C, and 35 s extension at 72°C. Two pairs of specific primers used were 5′- AAG GTG AAG AGC ATC ATA ACC CT -3′ and 5′- TCA CGC CTT TCA TAA CAC ATT CC -3′ for FABP4, 5′- TTG TGG ATA GCA AGC GAG TTG -3′ and 5′- CAC AGC TAT TCC GCA CTT GAA -3′ for DPP-4, 5′- TCG CTG ATG CAC TGC CTA TG -3′ and 5′- GAG AGG TCC ACA GAG CTG ATT -3′ for PPARγ2, 5′- CAA GAA CAG CAA CGA GTA CCG -3′ and 5′- GTC ACT GGT CAA CTC CAG CAC -3′ for CCAAT/enhancer binding protein α (C/EBPα), and 5′- AGT CCC TGC CCT TTG TAC ACA -3′ and 5′- CGA TCC GAG GGC CTC ACT A -3′ for 18S rRNA as an internal control gene.
Western blot analysis
The conditioned medium (CM) used for adipocytes was filtered to obtain 10-50 kDa fractions of proteins using Amicon Ultra 10K and 50K devices (Millipore, Billerica, MA) for detection of FABP4. Total protein content of the cell lysate (CL) in a cell lysis buffer, containing 50 mM Tris-HCl (pH 7.0), 2 mM EGTA, 5 mM EDTA, 30 mM NaF, 10 mM Na3VO4, 10 mM Na4P2O7, 40 mM β-glycerophosphate, 0.5% NP-40, and 1% protease inhibitor cocktail, was assessed by a microplate protein assay based on the Lowry method (Bio-Rad, Hercules, CA). Western blot analysis using primary antibodies for FABP4 (Abcam, Tokyo, Japan), DPP-4 (R&D Systems, Minneapolis, MN), adiponectin (Cell Signaling, Danvers, MA), and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) and densitometry analysis using ImageJ software were performed as previously described (10). FABP4 secretion was relatively expressed as densitometry of FABP4 in the CM divided by those of FABP4 in the CL and GAPDH in the CL as previously described (33).
Statistical analysis
Numeric variables are expressed as means ± SEM. The distribution of each parameter was tested for its normality using the Shapiro-Wilk W test, and nonnormally distributed parameters were logarithmically transformed for regression analyses. The correlation between two variables was evaluated using Pearson’s correlation coefficient. Comparison between two groups was done with Wilcoxon signed-rank test for paired samples and Mann-Whitney’s U test for unpaired samples. One-way ANOVA and the Tukey-Kramer post hoc test were used for detecting significant differences in data between more than two groups. A P value of <0.05 was considered statistically significant. All data were analyzed by using JMP 9 for Macintosh (SAS Institute, Cary, NC).
RESULTS
Study 1
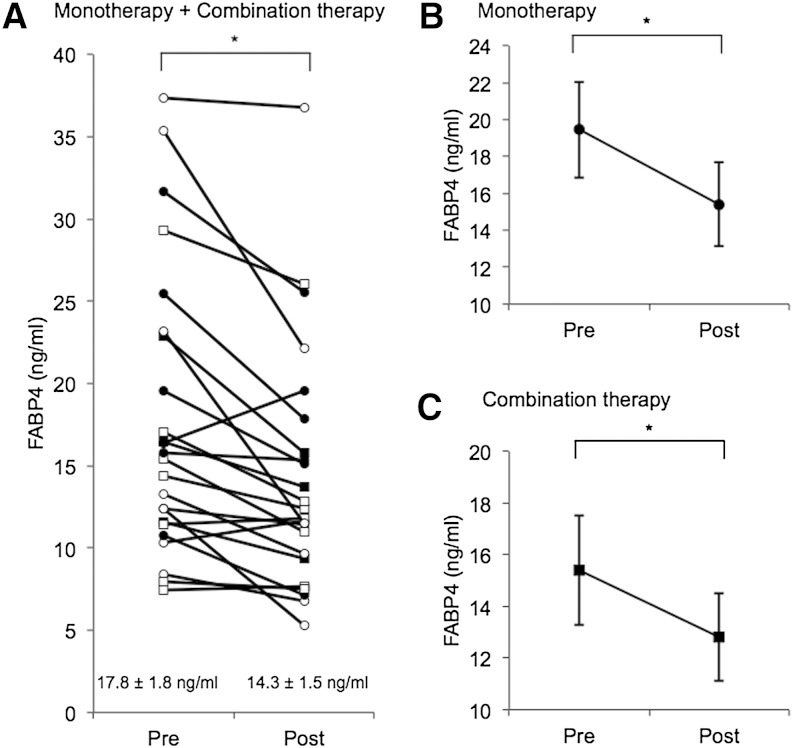
Characteristics of the patients in study 1 are shown in Table 1. Mean age, BMI, and waist circumference of the recruited patients were 70.2 ± 2.2 years old, 25.1 ± 1.0 kg/m2, and 88.1 ± 2.3 cm, respectively. Approximately 90% of the patients had hypertension and dyslipidemia, and most of the patients had received antihypertensive agents and statins. In all of the patients, treatment with sitagliptin for 12 weeks significantly decreased levels of glucose (167.5 ± 8.9 vs. 149.3 ± 6.9 mg/dl, P = 0.048), HOMA-R (4.2 ± 1.0 vs. 2.5 ± 0.3, P = 0.048), and HbA1c (7.7 ± 0.2 vs. 6.8 ± 0.2%, P < 0.001) and increased 1,5-AG (5.8 ± 1.4 vs. 9.4 ± 1.6 µg/ml, P < 0.001), an indicator of reduction in postprandial hyperglycemia (Table 1). Sitagliptin tended to decrease insulin level (9.3 ± 1.8 vs. 6.8 ± 0.6 µU/ml, P = 0.111), but no significant difference between pre- and posttreatment levels was found in BMI, waist circumference, blood pressure, pulse rate, or levels of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides. Treatment with sitagliptin significantly decreased serum FABP4 level by 19.7% (17.8 ± 1.8 vs. 14.3 ± 1.5 ng/ml, P < 0.001) (Fig. 1A). Change (Post − Pre) in FABP4 level was not significantly correlated with change in the level of glucose (r = −0.37, P = 0.081), HOMA-R (r = 0.38, P = 0.095), 1,5-AG (r = 0.37, P = 0.096), HbA1c (r = −0.37, P = 0.083), or other variables.
TABLE 1.
Characteristics of the patients treated with sitagliptin for 12 weeks
| Whole | Monotherapy | Combination Therapy | ||||
| Pre | Post | Pre | Post | Pre | Post | |
| n (M/F) | 24 (15/9) | 14 (7/7) | 10 (8/2) | |||
| Age (years) | 70.2 ± 2.2 | 72.1 ± 3.1 | 67.5 ± 3.1 | |||
| BMI (kg/m2) | 25.1 ± 1.0 | 25.2 ± 1.0 | 24.7 ± 1.4 | 24.6 ± 1.4 | 25.8 ± 1.6 | 25.9 ± 1.6 |
| Waist circumference (cm) | 88.1 ± 2.3 | 87.0 ± 2.2 | 87.6 ± 3.4 | 85.7 ± 3.2 | 89.0 ± 2.6 | 89.0 ± 2.4 |
| Systolic blood pressure (mmHg) | 140.7 ± 4.0 | 138.6 ± 2.7 | 142.1 ± 5.8 | 137.5 ± 3.6 | 138.8 ± 5.3 | 140.6 ± 3.8 |
| Diastolic blood pressure (mmHg) | 78.7 ± 1.7 | 79.4 ± 2.0 | 79.1 ± 2.7 | 77.3 ± 2.8 | 78.1 ± 1.8 | 83.1 ± 1.9 |
| Pulse rate (beats/min) | 71.1 ± 1.7 | 70.1 ± 1.0 | 72.7 ± 2.5 | 70.9 ± 1.1 | 68.7 ± 1.3 | 67.5 ± 2.1 |
| Biochemical data | ||||||
| Glucose (mg/dl) | 167.5 ± 8.9 | 149.3 ± 6.9a | 170.5 ± 10.4 | 144.1 ± 8.1a | 162.9 ± 16.7 | 156.7 ± 12.2 |
| Insulin (µU/ml) | 9.3 ± 1.8 | 6.8 ± 0.6 | 10.9 ± 2.9 | 6.4 ± 0.9 | 7.1 ± 1.6 | 7.4 ± 0.9 |
| HOMA-R | 4.2 ± 1.0 | 2.5 ± 0.3a | 4.9 ± 1.4 | 2.2 ± 0.4a | 3.1 ± 1.3 | 2.9 ± 0.5 |
| 1,5-AG (µg/ml) | 5.8 ± 1.4 | 9.4 ± 1.6a | 7.3 ± 2.3 | 11.9 ± 2.5a | 3.7 ± 0.7 | 6.2 ± 1.0a |
| HbA1c (%) | 7.7 ± 0.2 | 6.8 ± 0.2a | 7.4 ± 0.2 | 6.6 ± 0.2a | 8.0 ± 0.3 | 7.2 ± 0.3a |
| Total cholesterol (mg/dl) | 193.8 ± 7.2 | 180.0 ± 11.2 | 195.6 ± 11.6 | 185.7 ± 11.2 | 191.6 ± 8.1 | 172.9 ± 21.4 |
| HDL cholesterol (mg/dl) | 46.2 ± 2.2 | 46.3 ± 1.8 | 48.6 ± 3.3 | 47.0 ± 2.0 | 42.8 ± 2.6 | 45.2 ± 3.3 |
| LDL cholesterol (mg/dl) | 115.2 ± 5.7 | 112.8 ± 5.4 | 112.4 ± 8.7 | 106.6 ± 8.2 | 119.7 ± 5.9 | 120.7 ± 6.2 |
| Triglycerides (mg/dl) | 169.6 ± 15.7 | 154.7 ± 13.6 | 172.1 ± 19.6 | 157.6 ± 16.3 | 166.0 ± 27.0 | 150.7 ± 24.2 |
| Cr (mg/dl) | 0.75 ± 0.04 | 0.78 ± 0.03 | 0.76 ± 0.05 | 0.79 ± 0.05 | 0.74 ± 0.05 | 0.76 ± 0.04 |
| eGFR (ml/min/1.73 m2) | 73.6 ± 3.0 | 71.3 ± 14.1 | 70.5 ± 3.7 | 68.0 ± 4.0 | 79.0 ± 4.9 | 76.5 ± 3.8 |
| hsCRP (mg/dl) | 0.28 ± 0.14 | 0.22 ± 0.08 | 0.09 ± 0.03 | 0.07 ± 0.02 | 0.52 ± 0.31 | 0.41 ± 0.18 |
| Diagnosis | ||||||
| Hypertension | 22 (91.7) | 13 (92.9) | 9 (90.0) | |||
| Dyslipidemia | 21 (87.5) | 12 (85.7) | 9 (90.0) | |||
| Medication | ||||||
| Sulfonylurea (glimepiride) | 10 (41.7) | 0 (0) | 10 (100) | |||
| Angiotensin II receptor blocker | 11 (45.8) | 7 (50.0) | 4 (40.0) | |||
| ACE inhibitor | 4 (16.7) | 2 (14.3) | 2 (20.0) | |||
| Direct renin inhibitor | 7 (29.2) | 4 (28.6) | 3 (30.0) | |||
| Calcium channel blocker | 13 (54.2) | 8 (57.1) | 5 (50.0) | |||
| α Blocker | 1 (4.2) | 1 (7.1) | 0 (0) | |||
| β Blocker | 5 (20.8) | 4 (28.6) | 1 (10) | |||
| Diuretics | 1 (4.2) | 1 (7.1) | 0 (0) | |||
| Statin | 10 (41.7) | 7 (50.0) | 3 (30.0) | |||
Variables are expressed as n (%) or means ± SEM.
P < 0.05 versus Pre.
Fig. 1.
Effect of sitagliptin on serum FABP4 level. A: Treatment with sitagliptin (50 mg/day; n = 24; M/F: 15/9) as a monotherapy (n = 14; M/F: 7/7) or a combination therapy with glimepiride, a sulfonylurea (n = 10; M/F: 8/2), for 12 weeks significantly decreased FABP4 levels in patients with type 2 diabetes mellitus. Open circle, male in the monotherapy group; closed circle, female in the monotherapy group; open square, male in the combination therapy group; closed square, female in the combination therapy group. B, C: Both monotherapy with sitagliptin (B) and combination therapy with sitagliptin and sulfonylurea (C) for 12 weeks significantly decreased FABP4 levels in patients with type 2 diabetes mellitus. * P < 0.01.
When the study subjects were divided into a sitagliptin-monotherapy group (n = 14; M/F: 7/7) and combination therapy (sitagliptin and glimepiride) group (n = 10; M/F: 8/2), similar results were obtained for changes in biochemical data before and after treatment with sitagliptin, though changes in levels of glucose and HOMA-R did not reach statistical significance in the combination therapy group (Table 1). Treatment with sitagliptin significantly decreased serum FABP4 concentration by 20.6% (19.4 ± 2.6 vs. 15.4 ± 2.3 ng/ml, P = 0.007) in the monotherapy group (Fig. 1B) and by 16.9% (15.4 ± 6.7 vs. 12.8 ± 1.7 ng/ml, P = 0.007) in the combination therapy group (Fig. 1C).
Study 2
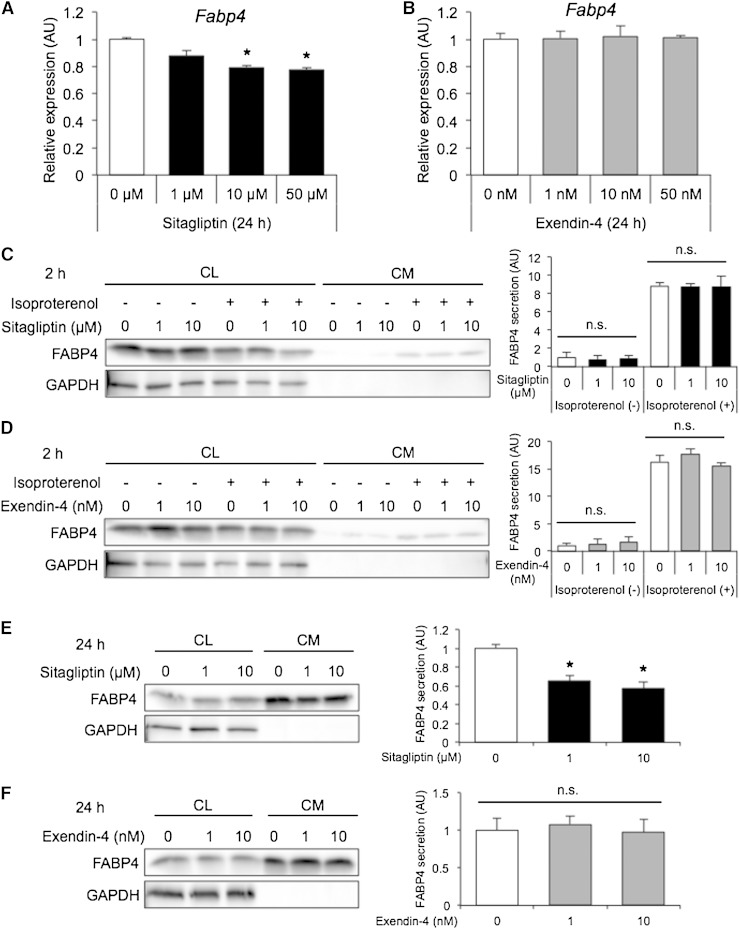
Treatment of differentiated 3T3-L1 adipocytes with sitagliptin, but not exendin-4, for 24 h decreased gene expression of FABP4 in a dose-dependent manner (Fig. 2A, B). Sitagliptin significantly decreased gene expression of other adipogenic markers, including PPARγ2 and C/EBPα, in a dose-dependent manner (supplementary Fig. 1A, B). For assessing secretion of FABP4, 3T3-L1 adipocytes were treated with sitagliptin or exendin-4 for 2 or 24 h in the absence and presence of 10 µM isoproterenol, a pan-β-adrenergic agonist, known as an inducer of FABP4 secretion (9, 10). Western blot analysis showed that FABP4 was present in both the CL and CM of 3T3-L1 adipocytes and that GAPDH, a nonsecretory protein, was not present in the CM (Fig. 2C–F), indicating that FABP4 is secreted from adipocytes but not leaked by injury of cell membranes. No cytotoxic effect, measured as LDH leakage, was observed in any of the experiments performed (data not shown). Treatment with sitagliptin or exendin-4 did not affect short-term (2 h) secretion of FABP4 from adipocytes in the absence or presence of isoproterenol (Fig. 2C, D). However, treatment with sitagliptin for 24 h significantly decreased FABP4 secretion from adipocytes, though the effect of sitagliptin was not mimicked by exendin-4 (Fig. 2E, F).
Fig. 2.
Expression and secretion of FABP4 in 3T3-L1 adipocytes treated with sitagliptin or exendin-4. A, B: Gene expression of FABP4 determined by quantitative real-time PCR in differentiated 3T3-L1 adipocytes treated with 0–50 µM sitagliptin (A) or 0–50 nM exendin-4 (B) for 24 h (n = 3 in each group). * P < 0.05 versus 0 µM. AU, arbitrary units. C, D: Western blot analysis of FABP4 and GAPDH using the CL and filtered CM of 3T3-L1 adipocytes treated with 0–10 µM sitagliptin (C) or 0–10 nM exendin-4 (D) for 2 h in the absence and presence of 10 µM isoproterenol (n = 4 in each group). E, F: Western blot analysis of FABP4 and GAPDH using the CL and filtered CM of 3T3-L1 adipocytes treated with 0–10 µM sitagliptin (E) or 0–10 nM exendin-4 (F) for 24 h (n = 3 in each group). FABP4 secretion was relatively expressed as densitometry of FABP4 in the CM divided by those of FABP4 in the CL and GAPDH in the CL. n.s., not significant. * P < 0.05 versus 0 µM.
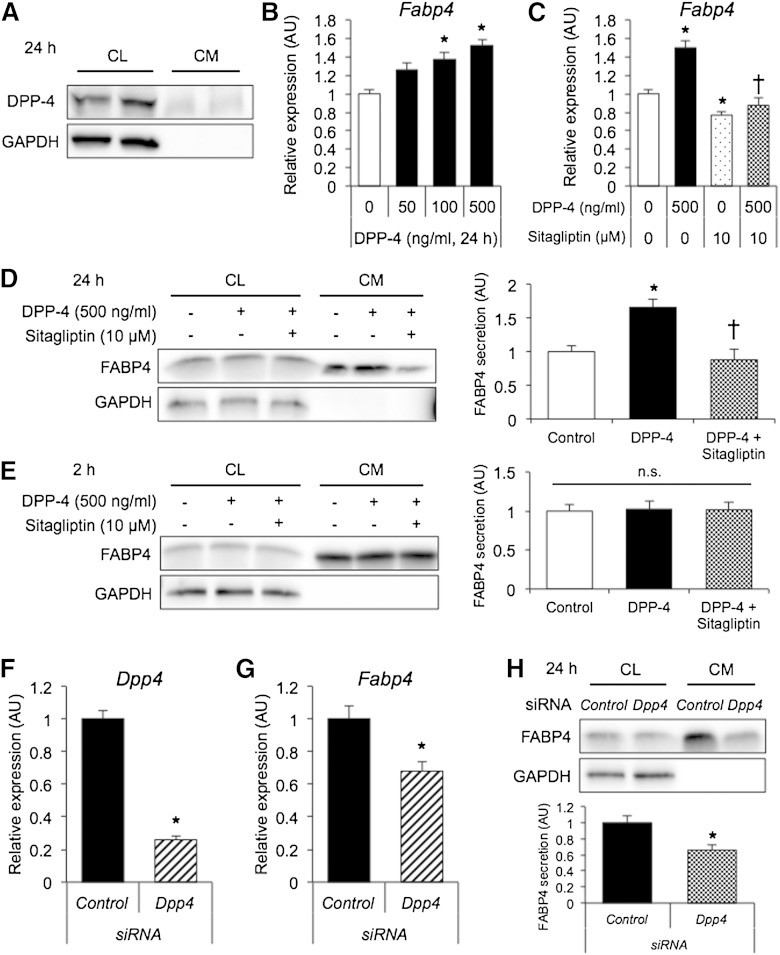
DPP-4 was present in both the CL and CM of 3T3-L1 adipocytes incubated for 24 h (Fig. 3A), indicating that DPP-4 is released from adipocytes as previously reported (34). Treatment of differentiated 3T3-L1 adipocytes with recombinant DPP-4 for 24 h increased gene expression of FABP4 in a dose-dependent manner (Fig. 3B), and the effect of DPP-4 was inhibited by sitagliptin (Fig. 3C). Similar results were obtained for gene expression of PPARγ2 and C/EBPα (supplementary Fig. 2A–D). Western blot analysis showed that treatment with recombinant DPP-4 significantly increased long-term (24 h) secretion of FABP4 from adipocytes, which was inhibited by sitagliptin (Fig. 3D). However, sitagliptin and/or DPP-4 did not affect short-term (2 h) secretion of FABP4 (Fig. 3E) or adiponectin (supplementary Fig. 3).
Fig. 3.
Expression and secretion of FABP4 in 3T3-L1 adipocytes treated with DPP-4 and sitagliptin. A: Western blot analysis of DPP-4 and GAPDH using the CL and nonfiltered CM of 3T3-L1 adipocytes incubated for 24 h. B, C: Gene expression of FABP4 determined by quantitative real-time PCR in differentiated 3T3-L1 adipocytes treated with 0–500 ng/ml DPP-4 (B) or with 500 ng/ml DPP-4 and 10 µM sitagliptin (C) for 24 h (n = 3 in each group). * P < 0.05 versus DPP-4 (0 ng/ml). † P < 0.05 versus DPP-4 (500 ng/ml). AU, arbitrary units. D, E: Western blot analysis of FABP4 and GAPDH using the CL and filtered CM of 3T3-L1 adipocytes treated with 500 ng/ml DPP-4 and 10 µM sitagliptin for 24 h (D) or 2 h (E) (n = 3 in each group). FABP4 secretion was relatively expressed as densitometry of FABP4 in the CM divided by those of FABP4 in the CL and GAPDH in the CL. * P < 0.05 versus control. † P < 0.05 versus DPP-4. n.s., not significant. F, G: Gene expression of DPP-4 (F) and FABP4 (G) determined by quantitative real-time PCR in DPP-4-knockdown 3T3-L1 adipocytes (n = 3 in each group). * P < 0.05 versus control. H: Western blot analysis of FABP4 and GAPDH using the CL and filtered CM of DPP-4-knockdown 3T3-L1 incubated for 24 h (n = 3 in each group). * P < 0.05 versus control.
In differentiated 3T3-L1 adipocytes, siRNA-mediated DPP-4-knockdown resulted in efficient suppression of gene expression of DPP-4 by 74.1% (Fig. 3F). Knockdown of DPP-4 in 3T3-L1 adipocytes significantly decreased gene expression and long-term (24 h) secretion of FABP4 (Fig. 3G, H). There was no further reduction of gene expression of FABP4 by treatment with 10 µM sitagliptin for 24 h in DPP-4-knockdown 3T3-L1 adipocytes (supplementary Fig. 4).
DISCUSSION
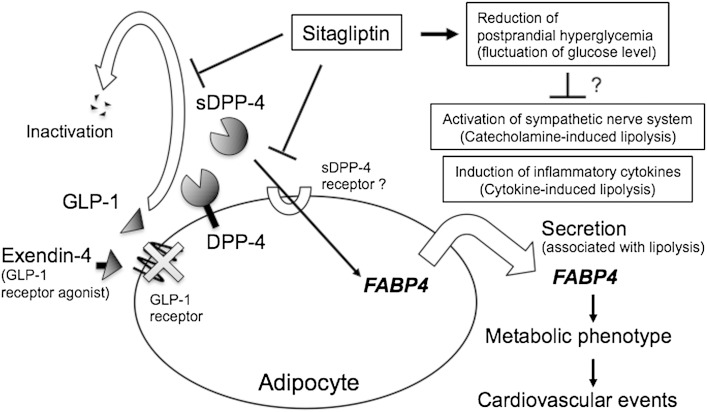
The present study showed for the first time that sitagliptin, a DPP-4 inhibitor, decreased serum FABP4 concentration in patients with type 2 diabetes mellitus. Treatment of 3T3-L1 adipocytes with sitagliptin for 24 h significantly decreased gene expression and consecutive secretion of FABP4. We also confirmed that DPP-4 is released from 3T3-L1 adipocytes as previously reported (34). Treatment with recombinant DPP-4 for 24 h increased gene expression and consecutive secretion of FABP4 in 3T3-L1 adipocytes, the effect of which was cancelled by sitagliptin. Furthermore, knockdown of DPP-4 in 3T3-L1 adipocytes decreased gene expression and consecutive secretion of FABP4. It has recently been reported that DPP-4 is one of the adipocyte-derived bioactive molecules known as adipokines, though the receptor for soluble DPP-4 remains obscure (35). Exogenous DPP-4 decreased insulin signaling in adipocytes, skeletal muscle cells, and smooth muscle cells, which was rescued by a DPP-4 inhibitor (35). In addition, exogenous DPP-4 has been reported to promote adipogenesis through increased expression of PPARγ, which is upstream of FABP4 regulation (36). Taken together, the present findings indicate that pharmacological inhibition of the activity of soluble DPP-4 reduces serum FABP4 level by decreasing the expression of FABP4 in adipocytes (Fig. 4).
Fig. 4.
Possible mechanisms of reduced FABP4 concentration caused by sitagliptin. FABP4 is secreted from adipocytes in association with lipolysis and may act as an adipokine for the development of insulin resistance and atherosclerosis, leading to cardiovascular events (3, 9). Sitagliptin, a DPP-4 inhibitor, slows GLP-1 degradation, thereby increasing endogenous GLP-1 concentration. The GLP-1 receptor has been reported to be expressed in the stromal vascular fraction of adipose tissue but not in adipocytes (39). Furthermore, exendin-4, a GLP-1 receptor agonist, did not affect expression and secretion of FABP4, suggesting that reduction in serum FABP4 level by sitagliptin is independent of a canonical GLP-1 receptor signaling in adipocytes. DPP-4 is released from cell surface in adipocytes (34), and soluble DPP-4 (sDPP-4) has been suggested to act as an adipokine (35), though the receptor for sDPP-4 remains obscure. Direct inhibition of sDPP-4 as an adipokine by sitagliptin may contribute to a decrease in the expression of FABP4 in adipocytes, consequently leading to a reduction in serum FABP4 level. Another possible explanation for the reduction in serum FABP4 levels by sitagliptin is a decrease in lipolysis via suppression of sympathetic nerve activation and/or inflammatory cytokines as pleiotropic effects of DPP-4 inhibitors.
Inhibition of DPP-4 leads to an increase in incretins such as GLP-1 and gastric inhibitory polypeptide (GIP) and thereby an increase in insulin secretion by pancreatic β cells (29, 30). GLP-1 has been reported to increase cyclic AMP levels downstream of GLP-1 receptor signaling and to subsequently increase insulin secretion by pancreatic β cells (29, 30, 37). Notably, FABP4 is secreted from adipocytes in association with lipolysis via the β-adrenergic receptor-adenyl cyclase-cyclic AMP pathway (10). However, expression of the GLP-1 receptor in tissues has been obscure because of a lack of reliable antibodies (38), and a recent study demonstrated that the GLP-1 receptor is expressed in the stromal vascular fraction but not in the adipocyte fraction of adipose tissue (39). Furthermore, a significant difference has been found between pancreatic GLP-1 receptor-expressing cells and 3T3-L1 adipocytes in signal transduction activated by exendin-4, a GLP-1 receptor agonist (40). In the present study, exendin-4 did not modulate gene expression and secretion of FABP4 in 3T3-L1 adipocytes (Fig. 2B, D, F), though doses of exendin-4 were well above the reported dissociation constant (Kd) value of the GLP-1 receptor (41, 42). Hence, the present results suggest that reduction in serum FABP4 level is independent of a canonical GLP-1 receptor signaling in adipocytes (Fig. 4).
In addition to its effect on pancreatic β cells, activation of the GIP receptor has been reported to increase lipogenesis, enhance secretion of adipokines, and promote expansion of fat depots in adipocytes (30, 43). The extra-pancreatic effects of GIP possibly increase circulating FABP4 level because there is a close correlation between serum FABP4 level and adiposity mass (8, 16). Hence, it is unlikely that augmentation of GIP by DPP-4 inhibition, if any, contributes to the reduction in FABP4 concentration by sitagliptin.
Change in the FABP4 level caused by sitagliptin was not significantly correlated with change in the level of glucose, HbA1c, or other variables in the present study. However, 1,5-AG level was significantly increased by treatment with sitagliptin, indicating an improvement of postprandial hyperglycemia and fluctuation of glucose level in both the monotherapy and combination therapy groups. It has been suggested that augmented fluctuation of glucose level causes sympathetic nerve activation (44), and sympathetic tone has been reported to be reduced by sitagliptin and vildagliptin, another DPP-4 inhibitor, in an obese rat model (45). DPP-4 inhibitors have also been shown to decrease several inflammatory cytokines, including tumor necrosis factor α and interleukin 6 (46, 47), which are known to increase lipolysis in adipocytes (48). Secretion of FABP4 is associated with lipolysis (3, 9, 10), and two of the regulatory factors of lipolysis, sympathetic tone and inflammatory cytokines, are potentially modulated by pharmacological inhibition of DPP-4. Hence, suppression of sympathetic nerve activation and/or inflammatory cytokines may have been an additional mechanism by which sitagliptin reduced serum FABP4 levels as its pleiotropic effects (Fig. 4).
Serum FABP4 level has been reported to predict long-term cardiovascular events (22–24). Together with accumulating evidence indicating a significant role of FABP4 in insulin resistance and atherosclerosis (4–6), the decrease in FABP4 level caused by DPP-4 inhibitors might have a preventive effect of cardiovascular events, though this issue has not yet been critically proved by randomized controlled trial studies using DPP-4 inhibitors.
The present study has several limitations. First, the number of subjects enrolled in study 1 was small, and the possibility of type 1 error in statistical tests cannot be excluded. Second, most of the study subjects had been treated with several drugs at baseline, including angiotensin II receptor blockers (33, 49) and a statin (50), which have been reported to affect circulating FABP4 concentration. Therefore, such drugs might have modulated the change in FABP4 level. Third, the present study lacked a placebo control group and a GLP-1 receptor agonist group in study 1. Hence, a prospective and placebo-controlled study with larger numbers of subjects is necessary for confirming the impact of DPP-4 inhibitor treatment on circulating FABP4 level and for determining the clinical benefit of a DPP-4 inhibitor. Fourth, results obtained from in vitro experiments using mouse 3T3-L1 adipocytes, a well-used cell line of adipocytes, might not be directly extrapolated to human adipocytes. Finally, we cannot exclude the possibility that the effect of sitagliptin on serum FABP4 level was partly mediated by changes in expression and secretion of FABP4 in cells other than adipocytes. There has been accumulating evidence indicating FABP4 is expressed in several types of cells, in addition to adipocytes and macrophages, under both physiological and pathological conditions (3, 51–55), though the predominant contributors of circulating FABP4 level are adipocytes rather than macrophages or other types of cells (3, 9).
In conclusion, treatment with sitagliptin decreases serum FABP4 concentration in patients with type 2 diabetes mellitus, at least in part, via reduction of FABP4 expression and its secretion from adipocytes by direct inhibition of DPP-4. Reduction of serum FABP4 level as a pleiotropic effect of DPP-4 inhibitors might be beneficial for patients with metabolic and cardiovascular diseases. A further understanding of the mechanisms of FABP4 expression and secretion in adipocytes and their pharmacological modulation may enable development of new therapeutic strategies for cardiovascular and metabolic diseases.
Supplementary Material
Acknowledgments
The authors thank Ms. Shiho Ishikawa for technical help.
Footnotes
Abbreviations:
- 1
- 5-AG, 1,5-anhydroglucitol
- C/EBPα
- CCAAT/enhancer binding protein α
- CL
- cell lysate
- CM
- conditioned medium
- Cr
- creatinine
- DPP-4
- dipeptidyl peptidase 4
- eGFR
- estimated glomerular filtration rate
- FABP
- fatty acid binding protein
- FABP4
- fatty acid binding protein 4
- GIP
- gastric inhibitory polypeptide
- GLP-1
- glucagon-like peptide 1
- M/F
- male/female
M.F. has been supported by grants from the Japan Society for the Promotion of Science, Ministry of Education, Culture, Sports, Science and Technology (MEXT) Translational Research Network Program, Uehara Memorial Foundation, Senshin Medical Research Foundation, Japan Diabetes Foundation, Takeda Medical Research Foundation, Ono Medical Research Foundation, Takeda Science Foundation, Akiyama Life Science Foundation, Yamaguchi Endocrine Research Foundation, Naito Foundation Natural Science Scholarship, Suhara Memorial Foundation, and Kondou Kinen Medical Foundation. The authors declare no conflict of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Furuhashi M., and Hotamisligil G. S.. 2008. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 7: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuhashi M., Ishimura S., Ota H., and Miura T.. 2011. Lipid chaperones and metabolic inflammation. Int. J. Inflam. 2011: 642612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuhashi M., Saitoh S., Shimamoto K., and Miura T.. 2014. Fatty acid-binding protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin. Med. Insights Cardiol. 8: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil G. S., Johnson R. S., Distel R. J., Ellis R., Papaioannou V. E., and Spiegelman B. M.. 1996. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 274: 1377–1379. [DOI] [PubMed] [Google Scholar]
- 5.Makowski L., Boord J. B., Maeda K., Babaev V. R., Uysal K. T., Morgan M. A., Parker R. A., Suttles J., Fazio S., Hotamisligil G. S., et al. . 2001. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 7: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuhashi M., Fucho R., Gorgun C. Z., Tuncman G., Cao H., and Hotamisligil G. S.. 2008. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Invest. 118: 2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuhashi M., Tuncman G., Gorgun C. Z., Makowski L., Atsumi G., Vaillancourt E., Kono K., Babaev V. R., Fazio S., Linton M. F., et al. . 2007. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 447: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu A., Wang Y., Xu J. Y., Stejskal D., Tam S., Zhang J., Wat N. M., Wong W. K., and Lam K. S.. 2006. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 52: 405–413. [DOI] [PubMed] [Google Scholar]
- 9.Cao H., Sekiya M., Ertunc M. E., Burak M. F., Mayers J. R., White A., Inouye K., Rickey L. M., Ercal B. C., Furuhashi M., et al. . 2013. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mita T., Furuhashi M., Hiramitsu S., Ishii J., Hoshina K., Ishimura S., Fuseya T., Watanabe Y., Tanaka M., Ohno K., et al. . 2015. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring). 23: 359–367. [DOI] [PubMed] [Google Scholar]
- 11.Ertunc M. E., Sikkeland J., Fenaroli F., Griffiths G., Daniels M. P., Cao H., Saatcioglu F., and Hotamisligil G. S.. 2015. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J. Lipid Res. 56: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamounier-Zepter V., Look C., Alvarez J., Christ T., Ravens U., Schunck W. H., Ehrhart-Bornstein M., Bornstein S. R., and Morano I.. 2009. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ. Res. 105: 326–334. [DOI] [PubMed] [Google Scholar]
- 13.Aragonès G., Saavedra P., Heras M., Cabré A., Girona J., and Masana L.. 2012. Fatty acid-binding protein 4 impairs the insulin-dependent nitric oxide pathway in vascular endothelial cells. Cardiovasc. Diabetol. 11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu A., Tso A. W., Cheung B. M., Wang Y., Wat N. M., Fong C. H., Yeung D. C., Janus E. D., Sham P. C., and Lam K. S.. 2007. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 115: 1537–1543. [DOI] [PubMed] [Google Scholar]
- 15.Tso A. W., Xu A., Sham P. C., Wat N. M., Wang Y., Fong C. H., Cheung B. M., Janus E. D., and Lam K. S.. 2007. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 30: 2667–2672. [DOI] [PubMed] [Google Scholar]
- 16.Ishimura S., Furuhashi M., Watanabe Y., Hoshina K., Fuseya T., Mita T., Okazaki Y., Koyama M., Tanaka M., Akasaka H., et al. . 2013. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One. 8: e81318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ota H., Furuhashi M., Ishimura S., Koyama M., Okazaki Y., Mita T., Fuseya T., Yamashita T., Tanaka M., Yoshida H., et al. . 2012. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am. J. Hypertens. 25: 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuseya T., Furuhashi M., Yuda S., Muranaka A., Kawamukai M., Mita T., Ishimura S., Watanabe Y., Hoshina K., Tanaka M., et al. . 2014. Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc. Diabetol. 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabré A., Lázaro I., Girona J., Manzanares J. M., Marimón F., Plana N., Heras M., and Masana L.. 2008. Plasma fatty acid-binding protein 4 increases with renal dysfunction in type 2 diabetic patients without microalbuminuria. Clin. Chem. 54: 181–187. [DOI] [PubMed] [Google Scholar]
- 20.Cabré A., Lázaro I., Girona J., Manzanares J. M., Marimón F., Plana N., Heras M., and Masana L.. 2008. Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J. Lipid Res. 49: 1746–1751. [DOI] [PubMed] [Google Scholar]
- 21.Yeung D. C., Xu A., Cheung C. W., Wat N. M., Yau M. H., Fong C. H., Chau M. T., and Lam K. S.. 2007. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27: 1796–1802. [DOI] [PubMed] [Google Scholar]
- 22.Furuhashi M., Ishimura S., Ota H., Hayashi M., Nishitani T., Tanaka M., Yoshida H., Shimamoto K., Hotamisligil G. S., and Miura T.. 2011. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One. 6: e27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Eynatten M., Breitling L. P., Roos M., Baumann M., Rothenbacher D., and Brenner H.. 2012. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler. Thromb. Vasc. Biol. 32: 2327–2335. [DOI] [PubMed] [Google Scholar]
- 24.Chow W. S., Tso A. W., Xu A., Yuen M. M., Fong C. H., Lam T. H., Lo S. V., Tse H. F., Woo Y. C., Yeung C. Y., et al. . 2013. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J. Am. Heart Assoc. 2: e004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabré A., Lázaro I., Girona J., Manzanares J. M., Marimón F., Plana N., Heras M., and Masana L.. 2007. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis. 195: e150–e158. [DOI] [PubMed] [Google Scholar]
- 26.Amori R. E., Lau J., and Pittas A. G.. 2007. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. J. Am. Med. Assoc. 298: 194–206. [DOI] [PubMed] [Google Scholar]
- 27.Aroda V. R., Henry R. R., Han J., Huang W., DeYoung M. B., Darsow T., and Hoogwerf B. J.. 2012. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin. Ther. 34: 1247–1258.e22. [DOI] [PubMed] [Google Scholar]
- 28.Mentlein R. 1999. Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul. Pept. 85: 9–24. [DOI] [PubMed] [Google Scholar]
- 29.Drucker D. J., and Nauck M. A.. 2006. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 30.Campbell J. E., and Drucker D. J.. 2013. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., and Hishida A.. 2009. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 32.Kajimoto K., Takayanagi S., Sasaki S., Akita H., and Harashima H.. 2012. RNA interference-based silencing reveals the regulatory role of fatty acid-binding protein 4 in the production of IL-6 and vascular endothelial growth factor in 3T3–L1 adipocytes. Endocrinology. 153: 5629–5636. [DOI] [PubMed] [Google Scholar]
- 33.Furuhashi M., Mita T., Moniwa N., Hoshina K., Ishimura S., Fuseya T., Watanabe Y., Yoshida H., Shimamoto K., and Miura T.. 2015. Angiotensin II receptor blockers decrease serum concentration of fatty acid-binding protein 4 in patients with hypertension. Hypertens. Res. 38: 252–259. [DOI] [PubMed] [Google Scholar]
- 34.Das S. S., Hayashi H., Sato T., Yamada R., Hiratsuka M., and Hirasawa N.. 2014. Regulation of dipeptidyl peptidase 4 production in adipocytes by glucose. Diabetes Metab. Syndr. Obes. 7: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamers D., Famulla S., Wronkowitz N., Hartwig S., Lehr S., Ouwens D. M., Eckardt K., Kaufman J. M., Ryden M., Muller S., et al. . 2011. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 60: 1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosmaninho-Salgado J., Marques A. P., Estrada M., Santana M., Cortez V., Grouzmann E., and Cavadas C.. 2012. Dipeptidyl-peptidase-IV by cleaving neuropeptide Y induces lipid accumulation and PPAR-gamma expression. Peptides. 37: 49–54. [DOI] [PubMed] [Google Scholar]
- 37.Drucker D. J., Philippe J., Mojsov S., Chick W. L., and Habener J. F.. 1987. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA. 84: 3434–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyke C., Heller R. S., Kirk R. K., Orskov C., Reedtz-Runge S., Kaastrup P., Hvelplund A., Bardram L., Calatayud D., and Knudsen L. B.. 2014. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 155: 1280–1290. [DOI] [PubMed] [Google Scholar]
- 39.Panjwani N., Mulvihill E. E., Longuet C., Yusta B., Campbell J. E., Brown T. J., Streutker C., Holland D., Cao X., Baggio L. L., et al. . 2013. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology. 154: 127–139. [DOI] [PubMed] [Google Scholar]
- 40.Montrose-Rafizadeh C., Yang H., Wang Y., Roth J., Montrose M. H., and Adams L. G.. 1997. Novel signal transduction and peptide specificity of glucagon-like peptide receptor in 3T3–L1 adipocytes. J. Cell. Physiol. 172: 275–283. [DOI] [PubMed] [Google Scholar]
- 41.Göke R., Fehmann H. C., Linn T., Schmidt H., Krause M., Eng J., and Göke B.. 1993. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J. Biol. Chem. 268: 19650–19655. [PubMed] [Google Scholar]
- 42.Gao W., and Jusko W. J.. 2012. Target-mediated pharmacokinetic and pharmacodynamic model of exendin-4 in rats, monkeys, and humans. Drug Metab. Dispos. 40: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamont B. J., and Drucker D. J.. 2008. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes. 57: 190–198. [DOI] [PubMed] [Google Scholar]
- 44.Di Flaviani A., Picconi F., Di Stefano P., Giordani I., Malandrucco I., Maggio P., Palazzo P., Sgreccia F., Peraldo C., Farina F., et al. . 2011. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care. 34: 1605–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apaijai N., Pintana H., Chattipakorn S. C., and Chattipakorn N.. 2013. Effects of vildagliptin versus sitagliptin, on cardiac function, heart rate variability and mitochondrial function in obese insulin-resistant rats. Br. J. Pharmacol. 169: 1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satoh-Asahara N., Sasaki Y., Wada H., Tochiya M., Iguchi A., Nakagawachi R., Odori S., Kono S., Hasegawa K., and Shimatsu A.. 2013. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. 62: 347–351. [DOI] [PubMed] [Google Scholar]
- 47.Makdissi A., Ghanim H., Vora M., Green K., Abuaysheh S., Chaudhuri A., Dhindsa S., and Dandona P.. 2012. Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 97: 3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masoodi M., Kuda O., Rossmeisl M., Flachs P., and Kopecky J.. 2015. Lipid signaling in adipose tissue: connecting inflammation & metabolism. Biochim. Biophys. Acta. 1851: 503–518. [DOI] [PubMed] [Google Scholar]
- 49.Miyoshi T., Doi M., Hirohata S., Kamikawa S., Usui S., Ogawa H., Sakane K., Izumi R., Ninomiya Y., and Kusachi S.. 2011. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart Vessels. 26: 408–413. [DOI] [PubMed] [Google Scholar]
- 50.Karpisek M., Stejskal D., Kotolova H., Kollar P., Janoutova G., Ochmanova R., Cizek L., Horakova D., Yahia R. B., Lichnovska R., et al. . 2007. Treatment with atorvastatin reduces serum adipocyte-fatty acid binding protein value in patients with hyperlipidaemia. Eur. J. Clin. Invest. 37: 637–642. [DOI] [PubMed] [Google Scholar]
- 51.Lee M. Y., Tse H. F., Siu C. W., Zhu S. G., Man R. Y., and Vanhoutte P. M.. 2007. Genomic changes in regenerated porcine coronary arterial endothelial cells. Arterioscler. Thromb. Vasc. Biol. 27: 2443–2449. [DOI] [PubMed] [Google Scholar]
- 52.Elmasri H., Karaaslan C., Teper Y., Ghelfi E., Weng M., Ince T. A., Kozakewich H., Bischoff J., and Cataltepe S.. 2009. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 23: 3865–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iso T., Maeda K., Hanaoka H., Suga T., Goto K., Syamsunarno M. R., Hishiki T., Nagahata Y., Matsui H., Arai M., et al. . 2013. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler. Thromb. Vasc. Biol. 33: 2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka M., Furuhashi M., Okazaki Y., Mita T., Fuseya T., Ohno K., Ishimura S., Yoshida H., and Miura T.. 2014. Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron Clin. Pract. 128: 345–351. [DOI] [PubMed] [Google Scholar]
- 55.Okazaki Y., Furuhashi M., Tanaka M., Mita T., Fuseya T., Ishimura S., Watanabe Y., Hoshina K., Akasaka H., Ohnishi H., et al. . 2014. Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS One. 9: e115429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.