Abstract
Heart failure with preserved ejection fraction (HFpEF) is half of all HF, but standard HF therapies are ineffective. Diastolic dysfunction, often secondary to interstitial fibrosis, is common in HFpEF. Previously, we found that supra-physiologic levels of ω3-PUFAs produced by 12 weeks of ω3-dietary supplementation prevented fibrosis and contractile dysfunction following pressure overload [transverse aortic constriction (TAC)], a model that resembles aspects of remodeling in HFpEF. This raised several questions regarding ω3-concentration-dependent cardioprotection, the specific role of EPA and DHA, and the relationship between prevention of fibrosis and contractile dysfunction. To achieve more clinically relevant ω3-levels and test individual ω3-PUFAs, we shortened the ω3-diet regimen and used EPA- and DHA-specific diets to examine remodeling following TAC. The shorter diet regimen produced ω3-PUFA levels closer to Western clinics. Further, EPA, but not DHA, prevented fibrosis following TAC. However, neither ω3-PUFA prevented contractile dysfunction, perhaps due to reduced uptake of ω3-PUFA. Interestingly, EPA did not accumulate in cardiac fibroblasts. However, FFA receptor 4, a G protein-coupled receptor for ω3-PUFAs, was sufficient and required to block transforming growth factor β1-fibrotic signaling in cultured cardiac fibroblasts, suggesting a novel mechanism for EPA. In summary, EPA-mediated prevention of fibrosis could represent a novel therapy for HFpEF.
Keywords: eicosapentaenoic acid, docosahexaenoic acid, fibroblast, transverse aortic constriction, omega-3 polyunsaturated fatty acids
Heart failure (HF) is a complex and heterogeneous syndrome, and two broadly defined phenotypes have emerged in the last 10 years: HF with reduced ejection fraction (EF) and HF with preserved EF (HFpEF). Currently, over half of all HF diagnoses are HFpEF, and incidence is increasing rapidly as the population ages (1, 2). Generally, patients with HFpEF are older, female, and more likely to have hypertension, renal disease, atrial fibrillation, and/or pulmonary disease (2). More importantly, standard HF therapies show no efficacy in HFpEF (3, 4).
Numerous studies have established that ω3-PUFAs, EPA, and DHA are cardioprotective [reviews (5–7)]. Four large randomized clinical trials demonstrated that increased blood levels of ω3-PUFAs correlate with reduced mortality and hospitalizations in coronary heart disease (CHD) (8–11). This is supported by several meta-analyses indicating that ω3-PUFAs reduce the risk of CHD by preventing sudden death (12–16). Few studies have examined ω3-PUFAs in HF, but the most prominent is GISSI-HF (17). In GISSI-HF, ω3-PUFAs reduced total mortality 9% and cardiovascular mortality 8%, on top of standard care, whereas statins had no effect (17). Another small clinical trial examined the effects of ω3-PUFAs on left ventricular systolic function in patients with stable class II-IV NYHA HF secondary to nonischemic dilated cardiomyopathy (18). After 1 year, ω3-PUFAs significantly improved left ventricular EF, peak VO2, exercise duration, and mean NYHA functional class. While results from the GISSI-HF trial and this more recent study are promising, neither addressed patients with HFpEF (17, 18).
A limited number of studies have examined ω3-PUFAs mechanistically in cell or animal models of HF. Further, due to the phenotypic variation and complicated pathophysiology of HFpEF, no animal models perfectly replicate remodeling in HFpEF (19, 20). However, transverse aortic constriction (TAC), a surgical model of afterload-induced HF, approximates some aspects of remodeling in HFpEF (20). TAC induces hypertrophy, interstitial cardiac fibrosis, and systolic and diastolic dysfunction, which, excluding systolic dysfunction, are common features of HFpEF. Recently, we reported that dietary supplementation with a fish oil (FO) diet rich in ω3-PUFAs prevents interstitial fibrosis and cardiac systolic and diastolic dysfunction induced by TAC (21). More importantly, we identified a direct effect of ω3-PUFAs on cardiac fibroblasts to inhibit myofibroblast transformation and, thereby, prevent fibrosis. Prevention of fibrosis and diastolic dysfunction in the TAC model by ω3-PUFA supplementation might suggest that ω3-PUFA supplementation could be a novel therapy for HFpEF.
However, the ω3-index [erythrocyte ω3-PUFA levels defined by (DHA + EPA)/total FAs] and ω3-PUFA levels in heart tissue (15.3 and 36.5%, respectively) achieved in our previous study were well above the basal ω3-index observed in most human populations (∼4% in US; ∼9% in Japan), and significantly higher than the maximal cardioprotective effects in CHD associated with an ω3-index ≥8% (22). This raised several important questions including: 1) whether the cardioprotective effects we observed were due to the supra-physiologic ω3-levels; 2) which ω3-PUFA(s) mediated prevention of fibrosis; and 3) whether improvement in function was due solely to prevention of fibrosis, or whether ω3-PUFAs have a protective effect on function independent of prevention of fibrosis. To address these questions, we examined ventricular remodeling following TAC in mice fed diets supplemented with EPA or DHA, and control mice fed the standard ω3-diet (FO) or control diet [corn oil (CO)] from our previous study (21). To achieve more clinically relevant ω3-PUFA levels, we reduced the pre-TAC diet regimen to 2 weeks and continued the diet for 6 weeks post-TAC. Briefly, the shorter diet regimen produced ω3-PUFA levels closer to humans treated with ω3-PUFAs (∼10%). Further, we found that EPA prevented fibrosis, but did not accumulate in cardiac myocytes or nonmyocytes (fibroblasts), the traditional mechanism of action. Alternatively, we found that FFA receptor (FFAR)4, a G protein-coupled receptor (GPR) for long-chain FAs, including ω3-PUFAs, was both sufficient and required to prevent fibrotic signaling in cultured adult cardiac fibroblasts, suggesting a novel mechanism of action.
METHODS
Mice
In this study, 97 8-week-old C57BL/6J mice were randomly divided into four groups and started on diets supplemented with ω3-PUFAs (see Diets below). After 2 weeks, mice were subjected to TAC (see TAC below) and diets were continued for an additional 6 weeks. All procedures on animals conformed to the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee at Sanford Research.
Diets
Each diet contained 4% by weight test oil: 1) CO, 40 g CO per kilogram; 2) FO, 12 g menhaden oil and 28 g CO per kilogram; 3) EPA, 1.9 g EPA and 38.1 g CO per kilogram; and 4) DHA, 1.3 g DHA and 38.7 g CO per kilogram) (Dyets, Bethlehem, PA). The levels of EPA and DHA in the experimental diets were based on the relative amounts of EPA and DHA in the FO diet.
TAC
TAC surgery was performed as previously described (21, 23–25). TAC was validated by examining the relationship between aortic velocity and hypertrophy [heart weight-to-body weight ratio (HW/BW)], fibrosis, systolic dysfunction (EF), and diastolic dysfunction [early mitral valve filling velocity to early mitral annular tissue velocity (E/E’)], which all showed a direct (HW/BW, fibrosis, E/E’) or indirect correlation (EF) (supplementary Fig. 1). Additional details are included in the supplementary Methods.
Measurement of FAs
Blood was collected from the left ventricle for quantification of FA in erythrocytes. Five TAC mice from each diet group were used to quantitate FAs in cardiac myocytes and nonmyocytes (fibroblasts). Myocytes and nonmyocytes (fibroblasts) were isolated as previously described (26). FA levels were measured as previously described (21, 27). Additional details are included in the supplementary Methods.
Measurement of cardiac function
Echocardiography was performed before TAC and weekly after TAC using a Vevo 2100 system (VisualSonics, Toronto, Canada) with the MS250 (9–18 MHz) and MS400 (18–38 MHz) transducers. For all measurements, mice were anesthetized with isoflurane, gently restrained, and echocardiographic images were captured as mice were recovering from anesthesia to achieve a target heart rate of 500–550 bpm. Additional details on specific parameters measured are included in the supplementary Methods.
Measurement of fibrosis
Six weeks after TAC, hearts were arrested in diastole and perfusion-fixed for 20 min with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Hearts were paraffin-embedded, sectioned, and stained by the Sanford-Burnham Histopathology Core (La Jolla, CA). Paraffin-embedded transverse sections were stained with picrosirius red (collagen stain) to quantitate ventricular fibrosis. Ventricular fibrosis (as percent of total ventricular area) was quantified using Fiji software (National Institutes of Health). Ventricular fibrosis was quantified using images captured at 4× magnification and included both the right and left ventricle. The threshold settings were adjusted to highlight and calculate the total tissue area or the picrosirius red positively stained area.
Measurement and detection of FFAR gene expression
Total RNA was extracted from heart tissue or freshly isolated cardiac myocytes and nonmyocytes (fibroblasts) [cell isolations were as previously described (26)] using TRIzol reagent (Life Technologies) followed by RNeasy fibrous tissue mini kit (Qiagen). Total RNA was quantified using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific). Quantitative RT-PCR for FFAR1, FFAR2, FFAR3, and FFAR4 gene expression in whole hearts was measured by the Genomic-Microarray/qPCR Core at the Sanford/Burnham Institute (La Jolla, CA), as previously described (21, 28). Additional details are included in the supplementary Methods.
Culture of adult cardiac fibrosis and examination of FFAR4 signaling
Cardiac fibroblasts were isolated from hearts of a group of untreated C57BL/6J mice and cultured as previously described (21). Where indicated, fibroblasts were transfected with 5 nM siRNA directed against FFAR4 or control scrambled siRNA using Lipofectamine 2000 (Life Technologies). After 48 h, fibroblasts were treated with transforming growth factor β1 (TGFβ1) (1 ng/ml) and GW9508 (1–50 μM), an FFAR1/4 agonist. After 48 h, myofibroblast transformation was assessed by collagen I expression, fibroblast proliferation, and α-smooth muscle actin staining, as previously described (21). Additional details are included in the supplementary Methods.
Statistical analysis
Results are presented as mean ± SEM or with 95% confidence interval, as indicated in the figure legends. Data were analyzed by unpaired t-test with Welch’s correction, one-way ANOVA, or two-way ANOVA with a Tukey post-test, or linear/non-linear regression, as indicated in the figure legends. Data were tested for normal distribution with the D’Agostino and Pearson test. In cases where data were not normally distributed, data were log-transformed and anti-log values were presented. The unpaired t-test with Welch’s correction was used to adjust for small n values in the sham group. Where explanatory models were developed, Mallow’s Cp was used to identify the most parsimonious model with the least bias at the lowest parameter (p), where Cp < p. P < 0.05 was considered significant. Data were analyzed using Prism (GraphPad Software, version 6.0) or JMP Pro (SAS Institute, version 10.0.2).
RESULTS
Eight weeks of dietary supplementation with ω3-PUFAs produced an ω3-index similar to US patients treated with ω3-PUFAs
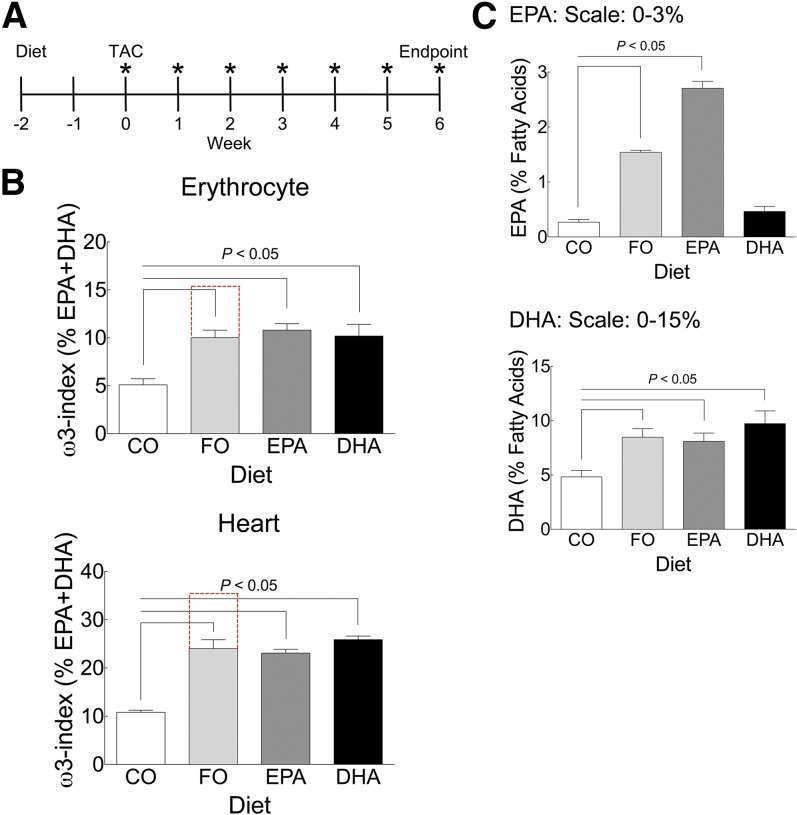
We previously demonstrated that 12 weeks of dietary supplementation with ω3-PUFAs produced high levels of ω3-PUFAs that prevented fibrosis and contractile dysfunction in mice subjected to pathologic pressure overload by TAC (21). However, ω3-PUFA levels achieved by 12 weeks of dietary supplementation were well above levels observed in most human populations. To achieve ω3-levels more relevant to human populations, we reduced the duration of dietary supplementation before TAC to 2 weeks, and continued for 6 weeks following surgery (timeline, Fig. 1A). To delineate the effects of specific ω3-PUFAs on preventing pathologic remodeling following TAC, mice were fed diets supplemented with specific ω3-PUFAs, including EPA or DHA, while control mice were fed diets supplemented with either CO or FO, as we previously described (21). The shorter diet regimen resulted in lower ω3-PUFA levels (EPA + DHA) in erythrocytes and heart tissue for mice fed the FO diet [FO ω3-PUFA levels (10.02 ± 0.74%, 24.00 ± 1.51%), CO ω3-PUFA levels (5.10 ± 0.60%, 10.79 ± 0.36%) for erythrocytes and heart, respectively (Fig. 1B; supplementary Tables 2, 4)], which was roughly half the increase achieved in our previous study (compare with red dashed lines) (21). With only 2 weeks of dietary supplementation before TAC versus 8 weeks in our previous study, ω3-PUFA levels were also likely significantly lower at the time of surgery in this study. Dietary supplementation with EPA or DHA alone also significantly increased ω3-PUFA levels (EPA + DHA) in erythrocytes and heart to a similar degree (Fig. 1B; and supplementary Tables 2, 4). Decreased levels of arachidonic acid (AA) (ω6) and docosapentaenoic acid (ω6) primarily offset the increased levels of ω3-PUFAs (supplementary Table 2). To validate the EPA- and DHA-specific diets, we also measured erythrocyte levels of each FA from each diet group. As expected, erythrocyte EPA or DHA levels were significantly increased in mice fed each respective diet (Fig. 1C, supplementary Table 2).
Fig. 1.
Eight weeks of dietary supplementation with ω3-PUFAs produced an ω3-index similar to US patients treated with ω3-PUFAs. A: Experimental design. Two weeks before TAC surgery (−2 weeks), mice were randomized to specific diets; CO, FO, EPA, or DHA. At time 0, TAC surgery was performed. Following surgery, cardiac function was assessed weekly by echocardiography (indicated by *), and 6 weeks after surgery, mice were euthanized and heart size, fibrosis, and blood ω3-PUFA levels were measured. B, C: The ω3-PUFA levels in erythrocytes and heart (B), and EPA and DHA (C) in erythrocytes. In (B) and (C), 8 weeks after initiation of dietary supplementation with ω3-PUFAs and at termination of the protocol, FA levels were measured (see Methods) and expressed as percent of total FAs. Total ω3-PUFA levels [percent EPA + percent DHA in erythrocytes or whole heart membranes (B)], and EPA or DHA levels in erythrocytes (C) are plotted as mean ± SEM. In (B), the red lines indicate ω3-PUFA levels from our previous study (21). Data were analyzed by one-way ANOVA with a Tukey post-test. The Tukey post-test compared all groups versus control and significance at P < 0.05 is indicated. CO, n = 19; FO, n = 19; EPA, n = 15; DHA, n = 15.
EPA prevented TAC-induced cardiac fibrosis; erythrocyte EPA abundance was inversely correlated with fibrosis
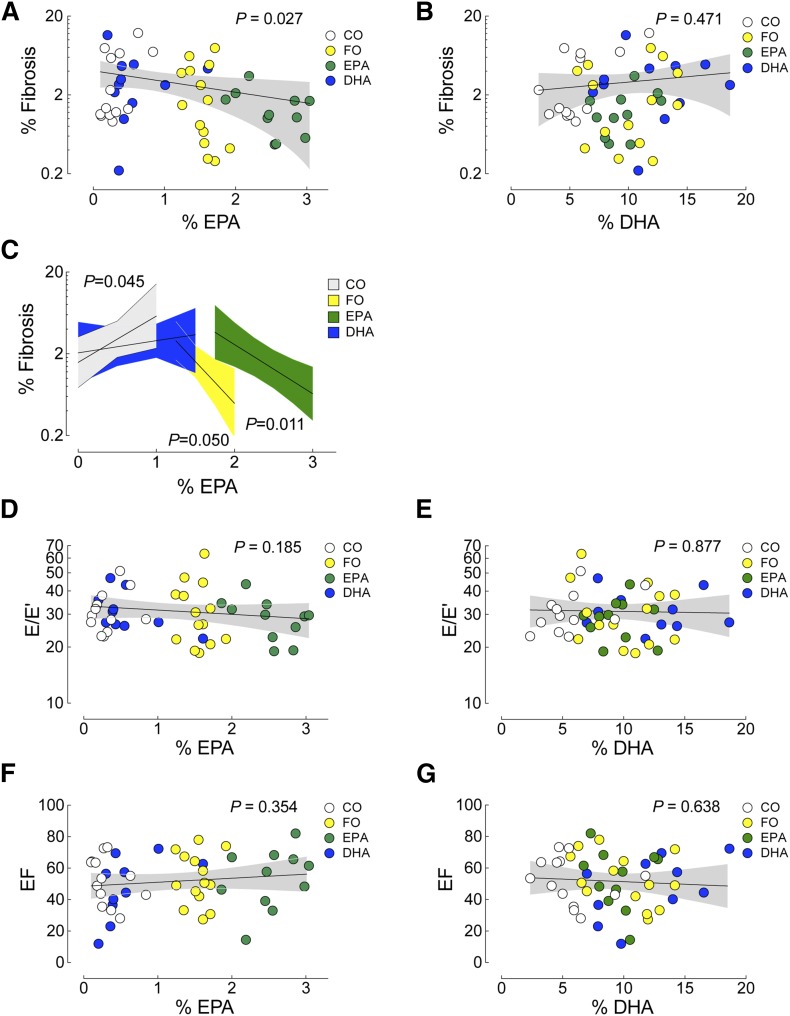
Pathologic remodeling induced by TAC is characterized by concentric remodeling/hypertrophy, interstitial fibrosis, and systolic and diastolic dysfunction. Six weeks after TAC, mice fed the control CO diet exhibited a significant increase in heart weight (HW) and heart weight-to-body weight ratio (HW/BW), a significant increase in ventricular fibrosis, a significant drop in EF, and a significant increase in E/E’ versus sham animals on the CO diet (Table 1), all indicative of pathologic remodeling. As with our prior study (21), dietary supplementation with ω3-PUFAs (FO, EPA, or DHA) had no effect on TAC-induced hypertrophy (HW or HW/BW). Interestingly, TAC-induced fibrosis (percent ventricular area in the TAC CO group) was not prevented by dietary supplementation with either the FO or DHA diets, but the degree of fibrosis in mice fed the EPA diet was similar to sham (sham CO: 1.46 ± 0.25%; EPA: 1.44 ± 0.29%), although this did not reach significance due to variability in the fibrotic response (one way ANOVA, comparing CO, FO, EPA, DHA; P = 0.22; Table 1). Alternatively, we examined the relationship between the abundance of erythrocyte ω3-PUFAs (defined as percent mass of total FAs) and the degree of fibrosis (Fig. 2A, B; EPA and DHA, respectively). Importantly, we found a significant inverse correlation between erythrocyte EPA levels and total fibrosis (n = 48, P = 0.027) that was not evident for DHA levels (n = 48, P = 0.471) (Fig. 2A, B). We next asked whether the inverse relationship between fibrosis and EPA abundance was different within each group, using model-fitting to test whether this simple relationship between percent EPA and percent fibrosis was sufficient or whether the dietary group provided a better explanation (Fig. 2C). Both diets supplemented with EPA (FO and EPA) were inversely correlated to myocardial fibrosis; however, the FO diet, providing both DHA and EPA, had low fibrosis at lower EPA levels than the EPA diet. Neither diet lacking EPA reduced fibrosis. Therefore, the current study indicated that EPA, but not DHA, prevents TAC-induced fibrosis, with a possible role for DHA in sensitizing the EPA response.
TABLE 1.
Summary of the effects of ω3-PUFA dietary supplementation on cardiac morphology and function following TAC
| CO Sham | CO TAC | FO TAC | EPA TAC | DHA TAC | |
| RBC FA% | |||||
| n | — | 18 | 20 | 15 | 16 |
| EPA | — | 0.27 ± 0.05 | 1.54 ± 0.04b | 2.71 ± 0.13b | 0.46 ± 0.09 |
| DHA | — | 4.83 ± 0.59 | 8.47 ± 0.79b | 8.09 ± 0.76b | 9.73 ± 1.17b |
| Pathology | |||||
| n | 5 | 18 | 20 | 16 | 19 |
| HW (mg) | 107.1 ± 4.2 | 176.8 ± 8.7a | 187.2 ± 8.6 | 183.4 ± 9.8 | 177.0 ± 8.6 |
| BW (g) | 24 ± 1 | 25 ± 0 | 25 ± 0 | 25 ± 0 | 24 ± 0 |
| HW/BW (mg/g) | 4.5 ± 0.2 | 7.1 ± 0.41a | 7.5 ± 0.3 | 7.5 ± 0.5 | 7.3 ± 0.4 |
| Echocardiography | |||||
| n | 5 | 19 | 19 | 15 | 19 |
| HR | 528 ± 6 | 517 ± 8 | 526 ± 4 | 533 ± 7 | 522 ± 5 |
| EF | 69.8 ± 4.3 | 52.4 ± 4.0a | 50.0 ± 3.9 | 48.1 ± 4.3 | 52.1 ± 4.2 |
| E/E’ | 19.4 ± 1.1 | 31.4 ± 1.9a | 34.2 ± 2.7 | 29.7 ± 2.1 | 28.7 ± 2.1 |
| AoV | 1001 ± 153 | 4736 ± 223a | 5000 ± 221 | 4459 ± 165 | 4676 ± 155 |
| Histology | |||||
| n | 5 | 14 | 14 | 10 | 14 |
| Fibrosis (%) | 1.46 ± 0.25 | 3.66 ± 0.97a | 2.55 ± 0.65 | 1.44 ± 0.29 | 3.54 ± 0.86 |
RBC FA%, erythrocyte FA percent; HR, heart rate; AoV, peak aortic velocity; Fibrosis, percentage of positive picrosirius red stain of ventricular tissue.
P < 0.05 versus CO Sham (t-test).
P < 0.05 versus CO TAC (one-way ANOVA, Tukey post-test).
Fig. 2.
EPA prevented TAC-induced cardiac fibrosis; erythrocyte EPA abundance was inversely correlated with fibrosis. A–C: Erythrocyte levels of EPA or DHA were correlated to ventricular fibrosis [(C) was a separate analysis examining the relationship between fibrosis and EPA abundance using model-fitting, as described in the Methods]. E/E’ (diastolic function) (D, E) or EF (systolic function) (F, G). Diet groups are identified in the legends (CO, white; FO, yellow; EPA, green; DHA, blue). Data were analyzed by linear regression, the P value for each analysis is indicated and the 95% confidence interval is shown in gray. All analyses, n = 48.
Previously we reported that dietary ω3-PUFA (FO)-mediated prevention of ventricular fibrosis induced by TAC was associated with prevention of systolic and diastolic dysfunction (21). However, TAC-induced systolic and diastolic dysfunction (EF and E/E’, respectively, in the TAC CO group) were not prevented by dietary supplementation in this study (FO, EPA, or DHA; P = NS for EF or E/E’ Table 1). Furthermore, we failed to detect a correlation between erythrocyte EPA or DHA levels and either EF or E/E’ (Fig. 2D, E and Fig. 2F, G, respectively).
In the current study, our intention was to achieve an ω3-index (erythrocyte EPA + DHA) closer to levels observed in patients treated with prescription ω3-PUFA supplementation in the US (9–10%) associated with the greatest protection from sudden death in CHD. In that regard, the lower overall levels of ω3-PUFA uptake, particularly prior to surgery (8 weeks prior to surgery previously, only 2 weeks in this study) might explain the inability to prevent contractile dysfunction in this study. In addition, a recent report examining pathology in human HFpEF indicated that while fibrosis was significantly increased in HFpEF patients, fibrosis alone could not account for systolic and diastolic dysfunction in HF, and other parameters contribute to contractile dysfunction (29).
Dietary supplementation with ω3-PUFAs failed to increase EPA levels in cardiac myocytes and nonmyocytes (fibroblasts)
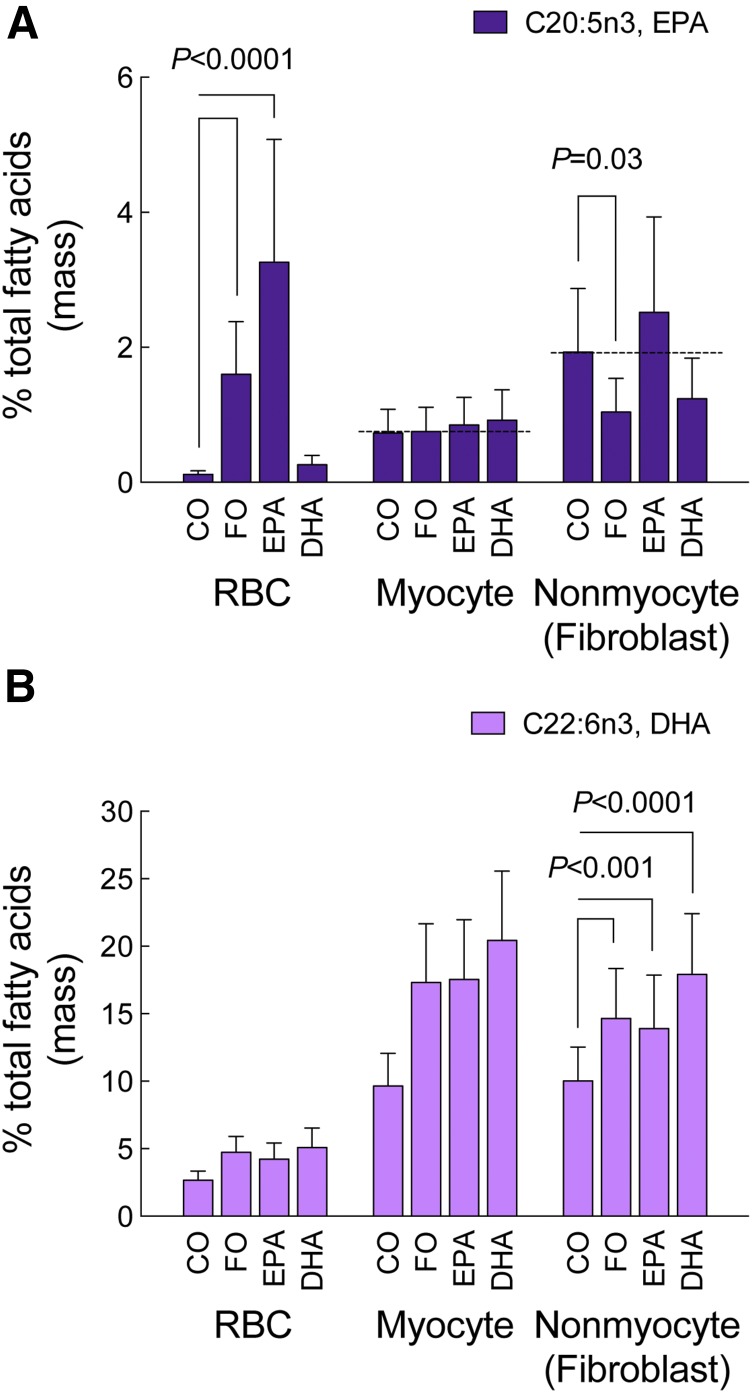
Our previous (21) and current data indicated that basal levels of ω3-PUFAs in the heart were relatively high and that dietary supplementation caused further significant increases in ω3-PUFA uptake in the heart. To further examine the distribution of ω3-PUFAs in the heart, we measured ω3-PUFA levels in isolated cardiac myocytes and nonmyocytes (based on the isolation procedure, we do not consider this population to be exclusively fibroblasts, but fibroblasts are likely the major component of this cell fraction) from mice in each diet group (supplementary Tables 4–6). Surprisingly, dietary supplementation with ω3-PUFAs (FO, EPA, or DHA) failed to increase EPA levels in either cardiac myocytes or nonmyocytes (Fig. 3A; supplementary Tables 5, 6), with EPA levels in nonmyocytes (fibroblasts) moderately reduced by the FO diet. In contrast, DHA levels were increased by FO, EPA, and DHA, and uniformly so, across all cell types (Fig. 3B; supplementary Tables 5, 6).
Fig. 3.
Dietary supplementation with ω3-PUFAs failed to increase EPA levels in cardiac myocytes and nonmyocytes (fibroblasts). Eight weeks after initiation of ω3-PUFA dietary supplementation and at the termination of the protocol, EPA (A) and DHA (B) levels were measured (see Methods) and expressed as percent of total FAs in erythrocytes (erythrocyte data reproduced from Fig. 1 solely for sake of comparison), cardiac myocytes, and nonmyocytes (enriched fibroblast population) isolated from a subset of mice. Data were analyzed by mixed model ANOVA and are presented as mean (95% confidence interval). Unadjusted contrasts were made to estimate post hoc differences. For all diet groups, n = 5 for both cardiac myocytes and nonmyocytes.
Diet-induced changes in cell-specific FA profiles
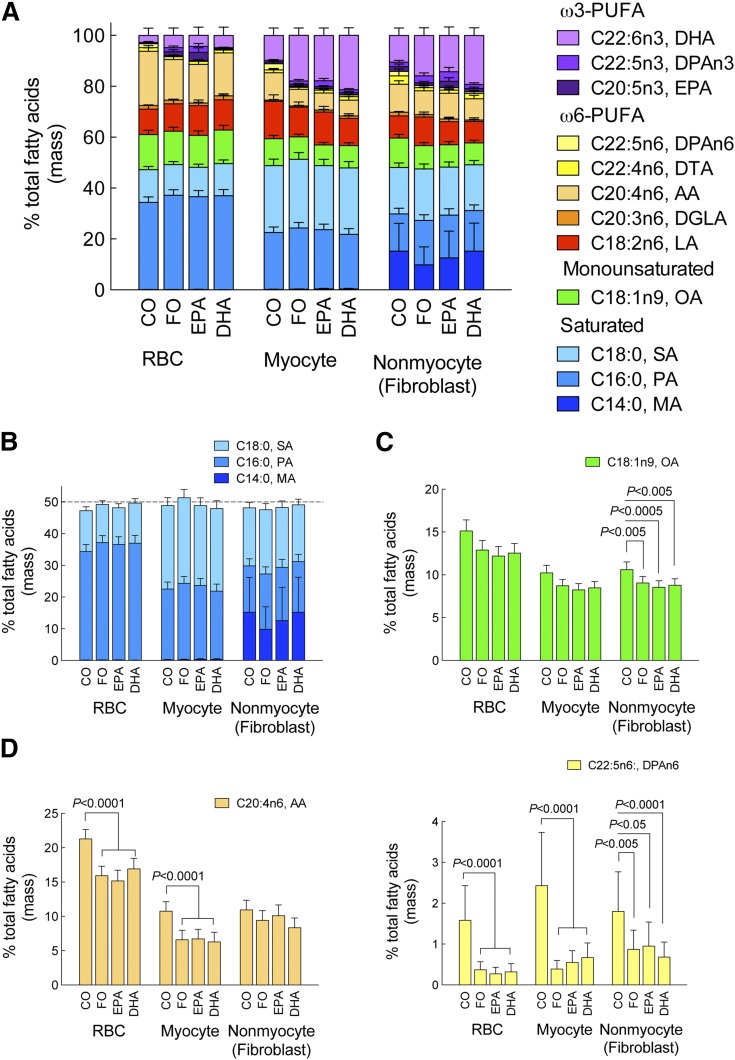
Typically, assessing the efficacy of an intervention on an outcome is straightforward. However, each dietary treatment had the potential to alter multiple FAs, including non-ω3 FAs. Here, we assessed changes in other FAs in order to best identify plausible molecular mechanisms to explain the outcome. Figure 4 illustrates the combined saturated, monounsaturated, and polyunsaturated FAs in erythrocytes, cardiac myocytes, and nonmyocytes (fibroblasts) (full list in the Fig. 4 legend). Due to limit of detection issues in the myocyte and nonmyocyte (fibroblast) fractions, we restricted the dataset to the 12 FAs whose levels were reliably detected (Fig. 4A) (Note: the full analysis of 24 FAs is included in supplementary Tables 2, 4–6). This approach reduced error, but resulted in a FA fractional abundance greater than usual. Approximately half of all FAs were saturated, and nonmyocytes (fibroblasts) were notably enriched in myristic acid; however, no effect of diet was detected (Fig. 4B). ω3-PUFAs appeared to replace other unsaturated FAs. The abundance of oleic acid was reduced by all diets compared with CO, regardless of cell type (Fig. 4C). The two major long-chain ω6 FAs, AA and docosapentaenoic acid n6, were reduced in erythrocytes and myocytes, but less so in nonmyocytes (fibroblasts) (Fig. 4D).
Fig. 4.
Diet-induced changes in cell-specific FA profiles. Eight weeks after initiation of ω3-PUFA dietary supplementation and at termination of the protocol, the levels of a reduced panel of 12 FAs (see below) were measured (see Methods) and expressed as percent of total FAs in erythrocytes (RBC), cardiac myocytes, and nonmyocytes (fibroblasts) isolated from a subset of mice. Data were analyzed by mixed model ANOVA and are presented as mean (95% confidence interval). Unadjusted contrasts were made to estimate post hoc differences. N = 5 for cardiac myocytes and nonmyocytes (fibroblasts) for all diet groups except EPA-fibroblasts, where one preparation was eliminated. Saturated FAs: myristic acid (C14:0, MA); palmitic acid (C16:0, PA); stearic acid (C18:0, SA). Monounsaturated FA: oleic acid (C18:1n9, OA). ω6-PUFAs: linoleic acid (C18:2n6 LA); dihomo gamma linoleic acid (C20:3n6, DGLA); AA (C20:4n6); docosatetraenoic acid (C22:4n6; DTA); docosapentaenoic acid n6 (C22:5n6, DPAn6). ω3-PUFAs: EPA (C20:5n3); docosapentaenoic acid n3 (C22:5n3, DPAn3); DHA (C22:6n3).
FFAR4 was expressed in cardiac myocytes and nonmyocytes (fibroblasts)
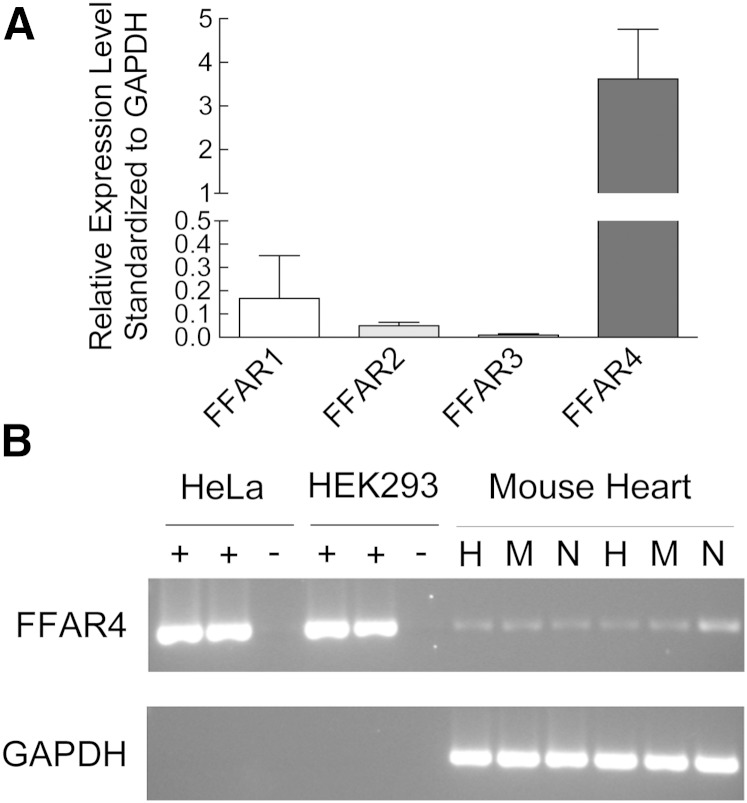
Although incorporation into cellular membranes is considered the traditional mechanism of action for ω3-PUFAs (30, 31), the failure of cardiac myocytes and nonmyocytes (fibroblasts) to accumulate EPA suggests an alternate mechanism for EPA-mediated prevention of fibrosis. In the last 10 years, a family of orphan GPRs was identified as receptors for FFAs. GPR41 and GPR43, now termed FFAR3 and FFAR2, respectively, were identified as receptors for short-chain FAs (<8 carbons) (32), and GPR40 and GPR120, now termed FFAR1 and FFAR4, respectively, were identified as receptors for long-chain FAs (33, 34). Furthermore, FFAR4 was identified as a specific receptor for ω3-PUFAs, both EPA and DHA (35). Therefore, we measured the expression of the FFAR family in heart tissue. FFAR4 was expressed at levels greater than 20-fold relative to FFAR1–3 in whole heart (Fig. 5A), and FFAR4 was expressed in both isolated cardiac myocytes and nonmyocytes (fibroblasts) (Fig. 5B). Previous studies indicated that cardiac expression of FFAR4 was low relative to other tissues, and we confirmed this by analyzing FFAR1 and FFAR4 expression in a variety of tissues (supplementary Fig. 2).
Fig. 5.
FFAR4 was expressed in cardiac myocytes and nonmyocytes (fibroblasts). A: FFAR expression in mouse heart. RNA was isolated from adult mouse heart and mRNA levels of FFAR1–4 were measured by semi-quantitative RT-PCR (n = 4). B: FFAR4 expression in whole heart (H), cardiac myocytes (M), and nonmyocytes (fibroblasts) (N). RNA was isolated from freshly isolated adult mouse cardiac myocytes and nonmyocytes (fibroblasts) and mRNA levels of FFAR4 were measured by qualitative PCR. PCR products for FFAR4 (520 bp) and GAPDH (326 bp) are indicated.
FFAR4 was sufficient to prevent TGFβ1-induced fibrotic signaling in cardiac fibroblasts
Previously, we found that both EPA and DHA inhibited fibrosis induced by TGFβ1, the main profibrotic cytokine in the heart that is induced by TAC, in primary cultures of cardiac fibroblasts (21). However, both EPA and DHA were also readily incorporated into membranes of primary cardiac fibroblasts and, therefore, we assumed membrane incorporation as a mechanism for ω3-PUFA prevention of fibrosis (21). However, our current data indicating that EPA is not incorporated into fibroblast membranes suggests that EPA might be actively excluded in vivo; whereas in cultured fibroblasts in vitro, EPA is readily accumulated in the membrane. To circumvent the ambiguity caused by EPA membrane incorporation in vitro, we employed a synthetic FFAR4 agonist, GW9508. As a small molecule with several benzyl ring structures, GW9508 is not likely acylated into lipids in fibroblast membranes, therefore allowing us to discriminate between membrane incorporation and receptor activation.
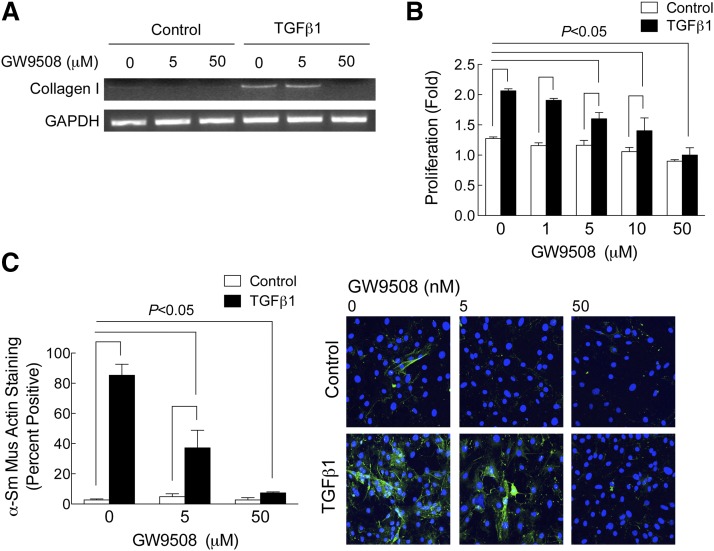
In primary cultures of adult mouse cardiac fibroblasts, TGFβ1, the main profibrotic cytokine in heart, increased collagen I expression, fibroblast proliferation, and α-smooth muscle actin expression, all hallmarks of myofibroblast transformation and a profibrotic response (Fig. 6A–C). GW9508, a small molecule agonist for FFAR1/4 (more potent at FFAR1, but EC50 at FFAR4, 3 μM), prevented TGFβ1 profibrotic signaling in a concentration-dependent manner in primary cardiac fibroblasts by inhibiting collagen I expression and proliferation, and α-smooth muscle actin expression (Fig. 6A–C). In short, our experiments with GW9508 suggest that activation of FFAR4 is sufficient to prevent TGFβ1-induced fibrosis without affecting membrane FA composition.
Fig. 6.
FFAR4 was sufficient to prevent TGFβ1-induced fibrotic signaling in cardiac fibroblasts. Cardiac fibroblasts isolated from adult mouse heart were cultured as described (see Methods). Fibroblasts were cultured for 48 h in serum-free medium, and then treated with TGFβ1 (1 ng/ml) in the presence of increasing concentrations of GW9508 (0–50 nM). After 48 h drug treatment, collagen expression (A), proliferation (B), and α-smooth muscle actin expression (C) were determined (see Methods). Collagen I expression was determined by RT-PCR, proliferation was determined by cell counts, and α-smooth muscle actin expression was determined by staining fibroblasts for α-smooth muscle actin and counting the percent of positive cells (Hoescht 33342 was used to stain nuclei). In (B) and (C), data are presented as mean ± SEM for n = 3 separate experiments. Data were analyzed by two-way ANOVA with a Sidak post-test. The post-test compared all groups and significance at P < 0.05 is indicated.
FFAR4 was required to prevent TGFβ1-induced fibrosis in primary cardiac fibroblasts
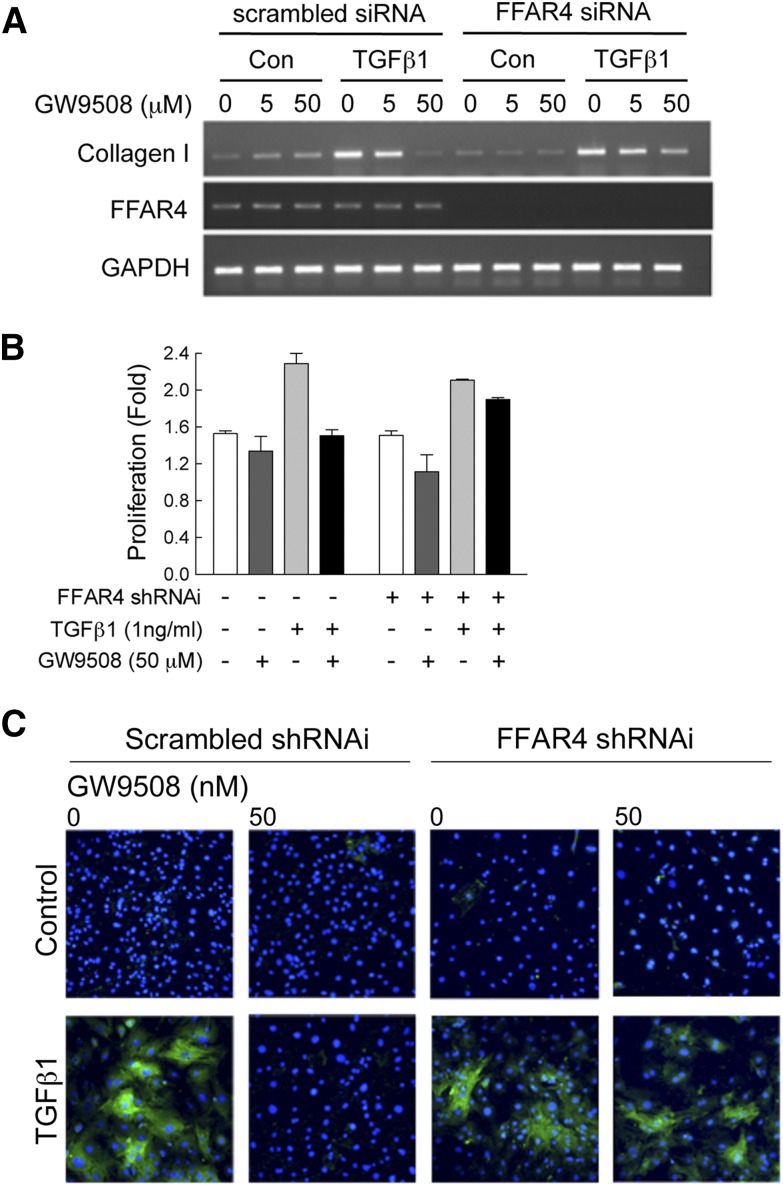
To address the role of FFAR4 more directly, we used siRNA to knockdown receptor expression and test the requirement for FFAR4 to prevent TGFβ1-induced fibrosis. We observed knockdown of FFAR4 mRNA within 48 h (Fig. 7A). More importantly, we found that in primary cardiac fibroblasts treated with TGFβ1, knockdown of FFAR4 blocked the anti-fibrotic effects of GW9508, indicating that FFAR4 is required for this effect (Fig. 7A–C).
Fig. 7.
FFAR4 was required to prevent TGFβ1-induced fibrosis in primary cardiac fibroblasts. Cardiac fibroblasts isolated from adult mouse heart were cultured as described (see Methods). Fibroblasts were cultured for 48 h in serum-free medium and transfected with 5 nM siRNA to FFAR4 (or 5 nM scrambled siRNA control), then treated with TGFβ1 (1 ng/ml) in the presence of increasing concentrations of GW9508 (0–50 nM) for another 48 h. After 48 h drug treatment, collagen expression (A), proliferation (B), and α-smooth muscle actin expression (C) were determined (see Methods). In (B) and (C), data are presented as mean ± SEM for n = 2 separate experiments.
DISCUSSION
Previously, we found that very high levels of ω3-PUFAs prevented fibrosis and contractile dysfunction following TAC (21), a mouse model of pressure overload-induced HF that resembles some aspects of HFpEF in humans (20). Here, we sought to determine how ω3-PUFA levels are correlated to these cardioprotective effects, which ω3-PUFA(s) mediate prevention of fibrosis and cardiac dysfunction in this HF model, and whether prevention of cardiac dysfunction was due solely to prevention of fibrosis. We tested ω3-PUFA-specific diets containing EPA or DHA and shortened the duration of dietary supplementation to 2 weeks before TAC and 6 weeks after TAC to reduce overall uptake of ω3-PUFAs. First, we achieved lower ω3-PUFA levels than our previous study (21), but closer to levels seen in the US population (22). Second, we found that dietary supplementation with EPA prevented fibrosis following TAC in a manner inversely proportional to erythrocyte levels of EPA (21), but did not prevent contractile dysfunction at the levels of EPA achieved here, unlike our previous study, which achieved higher levels of EPA. This suggests that either EPA-mediated prevention of fibrosis alone was not sufficient to prevent contractile dysfunction, that higher levels of EPA uptake are required, or that another ω3-PUFA is required to prevent contractile dysfunction. Third, we found that EPA was not accumulated in cardiac myocytes or nonmyocytes (fibroblasts), although EPA levels were increased in erythrocytes. Conventional mechanisms of action for ω3-PUFAs include incorporation into cell membranes and modulation of intracellular signaling. However, failure to accumulate EPA into nonmyocyte (fibroblast) membranes suggested an alternate mechanism of action. In fact, we detected expression of FFAR4, a GPR for long-chain FAs, in cardiac myocytes and fibroblasts, and found that FFAR4 was both sufficient and required to prevent TGFβ1-induced fibrotic signaling in cultured adult cardiac fibroblasts. Finally, our current data are consistent with our previous finding that ω3-PUFAs prevent cardiac fibrosis in a mouse model of pressure overload-induced HF that resembles some aspects of HFpEF in humans. Furthermore, our data suggest a dependency on EPA abundance (threshold) for EPA-mediated prevention of fibrosis, and suggest a novel mechanism of action through FFAR4.
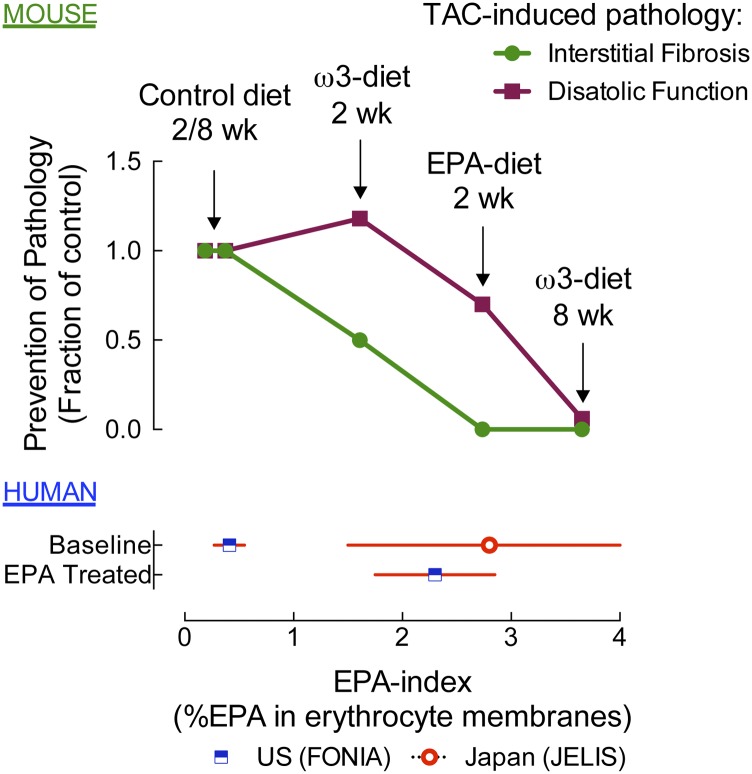
A recent meta-analysis demonstrated that patients given a fixed dose of ω3-PUFAs (EPA + DHA) had significant individual variation in ω3-blood levels, and that risk reduction in CHD correlated with higher ω3-PUFA levels (36). For example, in the JELIS trial, an EPA/AA ratio of 0.75 was associated with significantly lower risk of events in CHD patients (11). However, 28 days of EPA supplementation increased the EPA/AA ratio from 0.12 to 0.9, yet only 68% of the patients achieved a ratio of 0.78–1.02. More importantly, several trials that measured ω3-PUFA uptake never achieved an EPA/AA ratio of 0.75 (37–39). There are three practical implications of these findings: 1) there is a physiologic and genetic basis for the variability in erythrocyte levels of ω3-PUFAs in patients receiving a fixed dose; 2) erythrocyte levels of ω3-PUFAs are a better predictor of outcome than fixed dosing (all trials have given a fixed dose); and 3) patients that, for whatever reason, have lower erythrocyte levels of ω3-PUFAs might negatively affect trial results (36). A combined analysis of our previous and current data suggests EPA-mediated prevention of fibrosis and diastolic dysfunction is dependent on EPA abundance (Fig. 8). For this analysis, EPA levels were plotted against TAC-induced pathology (interstitial fibrosis and diastolic dysfunction). These rough calculations suggest a threshold concentration required for prevention of fibrosis of approximately 3% EPA, and slightly higher for prevention of diastolic dysfunction. In summary, our findings imply that there is a therapeutic index for ω3-PUFA function in general, and that best outcomes might be achieved by titrating the ω3-PUFA dose to reach optimal erythrocyte abundance.
Fig. 8.
Erythrocyte EPA abundance (EPA-index) and prevention of pathologic phenotypes following TAC in mice compared with EPA levels in humans. Upper section (mouse): EPA levels were plotted against TAC-induced pathology (interstitial fibrosis or diastolic dysfunction). EPA levels from this and our prior report (21) are plotted on the x axis (e.g., EPA-diet/2 week: mice were fed an EPA-supplemented diet for 2 weeks before TAC and the erythrocyte EPA levels at termination are plotted on the x axis). EPA-mediated prevention of fibrosis or diastolic dysfunction was assessed by calculating the percent inhibition [e.g., percent fibrosis in TAC EPA/(percent fibrosis TAC control diet − percent fibrosis sham control diet)] from this and our prior report (21), and is plotted on the y axis. Lower section (human): In FONIA, baseline erythrocyte EPA levels increased from 0.4 to 2.2% in a US patient (4 months, 3.4 g/day ω3-PUFAs; unpublished observations) versus untreated Japanese [2.8%, JELIS (52)].
The rightward shift in the IC50 for EPA prevention of diastolic dysfunction (Fig. 6) suggests that prevention of fibrosis alone might not necessarily account for the protective effects of EPA. Recently, microvascular rarefaction was detected in HFpEF patients at autopsy (along with myocardial fibrosis) (29). Microvascular rarefaction can result in impaired oxygen delivery, reduced systolic and diastolic reserve, and exacerbated exercise intolerance (40). The role of ω3-PUFAs in angiogenesis is poorly documented; however, one recent report indicates that ω3-PUFA dietary supplementation prevents microvascular rarefaction in a hind-limb ischemia model (41). Further, another report indicates that EPA induces VEGF-A expression through activation of FFAR4 in adipocytes (42). Therefore, prevention of microvascular rarefaction might, through EPA-mediated angiogenesis, represent another mechanism to improve outcomes in HFpEF.
This study provides unique insights into how FA metabolism differs among cell types. To our knowledge, no prior studies have demonstrated the uniqueness of basal FA composition by cell-type in the heart, or a cell-type specific response in the heart to dietary intervention. In order for a unique FA to emerge as having superior efficacy, we considered two characteristics: 1) superior association with lower fibrosis; and 2) a pattern of handling by fibroblasts that is unique in comparison to other possible mediators. The second is essential because EPA and DHA are highly correlated in human tissues (43), and an apparently superior association of one is often serendipitous. EPA fulfilled both characteristics, and it had the added benefit of being a target of primary intervention, which provides a simpler inference. Erythrocyte EPA is the best measure of global EPA abundance, and it has a significant inverse relationship to fibrosis. While the handling of EPA by cardiac myocytes and fibroblasts was unexpected, it was uniquely different from DHA or other FAs. No experimental diet changed EPA abundance from levels in the CO diet among cardiac myocytes; nonmyocytes (fibroblasts) also failed to accumulate EPA; however, diets containing DHA (FO and DHA) appeared to reduce EPA levels. The failure of cardiac cells to accumulate EPA was not a failure to absorb EPA, because erythrocyte EPA was enhanced 4.3-fold in the FO diet over CO. In contrast, DHA induced a 92% increase over CO regardless of cell type. Other PUFAs (4 total) were also cell dependent; however, the response to EPA was the most cell dependent and provided the simplest inference.
Although incorporation into cellular membranes is considered the traditional mechanism of action for ω3-PUFAs (30, 31), the failure of cardiac myocytes and nonmyocytes (fibroblasts) to accumulate EPA suggests an alternate mechanism. In the last 10 years, a family of orphan GPRs (FFAR1–4) was identified as a receptor for FFAs. Here, we found that a GPR for long-chain FAs (33), including EPA and DHA (35), was expressed in both cardiac myocytes and nonmyocytes (fibroblasts). The identification of FFAR4 in the heart and our data in cultured adult cardiac fibroblasts highlight (Figs. 6, 7) an entirely novel mechanism of action for FA signaling. To date, most studies of FFAR4 have focused on FFAR4’s role in inflammation and diabetes/obesity. Interestingly, FFAR4 knockout mice were used to show that ω3-PUFAs activate FFAR4-mediated anti-inflammatory signaling in macrophages (35). More recently, loss-of-function gene variants of FFAR4 were linked to insulin resistance in humans (44). However, the epidemiologic link between ω3-PUFAs and cardiovascular disease is stronger than the link between ω3-PUFAs and insulin sensitivity. In summary, our results indicate that EPA was clearly cardioprotective, consistent with previous findings in humans, but our data suggest a novel mechanism that requires FFAR4.
We cannot rule out a cardioprotective role for other FAs, specifically DHA, in response to pressure overload-induced HF. Four factors suggest that DHA might play a role. First, DHA is readily incorporated into cardiac myocytes and fibroblast membranes (Fig. 4), in line with more traditional mechanisms of action. Further, DHA appears to be unique compared with other ω3-PUFAs by virtue of its ability to alter membrane properties such as membrane fluidity, elasticity, phase behavior, ion permeability, fusion, and protein function (30, 31). DHA has known effects in the TAC model, including inhibition of mitochondrial transition pore opening (45, 46), but whether this improves survival is not clear (45). Our data suggest that EPA-mediated prevention of fibrosis does not completely reverse diastolic dysfunction (Fig. 8), although a standard ω3-diet (DHA + EPA) does, albeit at a higher overall level of ω3-uptake. Finally, when DHA was provided in diets alongside EPA, lower levels of EPA were associated with the same reduction in fibrosis, suggesting that DHA could sensitize the EPA-mediated suppression of fibrosis. Therefore, DHA might have an as yet undefined cardioprotective effect in response to pressure overload-induced HF.
Diastolic dysfunction is a defining feature of HFpEF. The hallmarks of diastolic dysfunction are impaired ventricular relaxation and filling caused by: 1) increased extracellular matrix (ECM) production (47); 2) structural changes that increase passive stiffness, such as alterations in titin isoform expression (48); or 3) altered calcium handling (49). Our results indicate that in the mouse TAC model, EPA prevents interstitial fibrosis, a prevalent comorbidity in HFpEF that contributes to diastolic stiffness, impaired ventricular filling, and exercise intolerance (3, 4, 47). Resident fibroblasts are thought to be the primary cells responsible for the production of ECM in response to injury. Upon transformation, activated myofibroblasts increase proliferation and ECM production, and alter the balance of matrix metalloproteinases and tissue inhibitors of metalloproteinases to favor fibrosis (50). Inhibition of myofibroblast transformation is a significant and novel therapeutic target for the treatment of cardiac fibrosis (51). Our previous results demonstrated that both EPA and DHA were sufficient to prevent myofibroblast transformation in primary cultures of adult mouse cardiac fibroblasts (21). Here, we found that EPA prevents fibrosis, potentially through activation of FFAR4. In summary, EPA-mediated prevention of fibrosis could represent a novel preventative therapy for HFpEF.
Finally, our primary finding is that EPA prevented fibrosis in a mouse model of pressure overload-induced HF (TAC) that resembles some aspects of remodeling in HFpEF, but uptake of EPA into cardiac nonmyocytes (fibroblasts) was not required. Our data suggest an alternate mechanism of action through activation of FFAR4. In short, this will potentially lay the groundwork for the development of a novel EPA-based therapeutic intervention for HFpEF.
Supplementary Material
Footnotes
Abbreviations:
- AA
- arachidonic acid
- BW
- body weight
- CHD
- coronary heart disease
- CO
- corn oil
- ECM
- extracellular matrix
- E/E’
- early mitral valve filling velocity to early mitral annular tissue velocity
- EF
- ejection fraction
- FFAR
- FFA receptor
- FO
- fish oil
- GPR
- G protein-coupled receptor
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- HW
- heart weight
- HW/BW
- heart weight-to-body weight ratio
- TAC
- transverse aortic constriction
- TGFβ1
- transforming growth factor β1
This work was supported by grants from the American Heart Association (Pre-doctoral Fellowship, 12PRE11780050; J.A.E.) and the National Institutes of Health (P20 RR017662; T.D.O., G.C.S., E.H.). The authors report no financial conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Owan T. E., Hodge D. O., Herges R. M., Jacobsen S. J., Roger V. L., and Redfield M. M.. 2006. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg B. A., Zhao X., Heidenreich P. A., Peterson E. D., Bhatt D. L., Cannon C. P., Hernandez A. F., and Fonarow G. C.; Get With the Guidelines Scientific Advisory Committee and Investigators. 2012. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 3.From A. M., and Borlaug B. A.. 2011. Heart failure with preserved ejection fraction: pathophysiology and emerging therapies. Cardiovasc. Ther. 29: e6–e21. [DOI] [PubMed] [Google Scholar]
- 4.Senni M., Paulus W. J., Gavazzi A., Fraser A. G., Diez J., Solomon S. D., Smiseth O. A., Guazzi M., Lam C. S., Maggioni A. P., et al. . 2014. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur. Heart J. 35: 2797–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris W. S., Poston W. C., and Haddock C. K.. 2007. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 193: 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Lavie C. J., Milani R. V., Mehra M. R., and Ventura H. O.. 2009. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 54: 585–594. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D., and Wu J. H.. 2011. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 58: 2047–2067. [DOI] [PubMed] [Google Scholar]
- 8.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. 1999. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 354: 447–455. [PubMed] [Google Scholar]
- 9.Burr M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., Elwood P. C., and Deadman N. M.. 1989. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 2: 757–761. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D., Lemaitre R. N., King I. B., Song X., Spiegelman D., Sacks F. M., Rimm E. B., and Siscovick D. S.. 2011. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann. Intern. Med. 155: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. . 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D., and Rimm E. B.. 2006. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 296: 1885–1899. [DOI] [PubMed] [Google Scholar]
- 13.Harris W. S., Mozaffarian D., Lefevre M., Toner C. D., Colombo J., Cunnane S. C., Holden J. M., Klurfeld D. M., Morris M. C., and Whelan J.. 2009. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J. Nutr. 139: 804S–819S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He K., Song Y., Daviglus M. L., Liu K., Van Horn L., Dyer A. R., and Greenland P.. 2004. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 109: 2705–2711. [DOI] [PubMed] [Google Scholar]
- 15.León H., Shibata M. C., Sivakumaran S., Dorgan M., Chatterley T., and Tsuyuki R. T.. 2008. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 337: a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marik P. E., and Varon J.. 2009. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin. Cardiol. 32: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavazzi L., Maggioni A. P., Marchioli R., Barlera S., Franzosi M. G., Latini R., Lucci D., Nicolosi G. L., Porcu M., and Tognoni G.. 2008. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 372: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 18.Nodari S., Triggiani M., Campia U., Manerba A., Milesi G., Cesana B. M., Gheorghiade M., and Dei Cas L.. 2011. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 57: 870–879. [DOI] [PubMed] [Google Scholar]
- 19.Butler J., Fonarow G. C., Zile M. R., Lam C. S., Roessig L., Schelbert E. B., Shah S. J., Ahmed A., Bonow R. O., Cleland J. G., et al. . 2014. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail. 2: 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horgan S., Watson C., Glezeva N., and Baugh J.. 2014. Murine models of diastolic dysfunction and heart failure with preserved ejection fraction. J. Card. Fail. 20: 984–995. [DOI] [PubMed] [Google Scholar]
- 21.Chen J., Shearer G. C., Chen Q., Healy C. L., Beyer A. J., Nareddy V. B., Gerdes A. M., Harris W. S., O’Connell T. D., and Wang D.. 2011. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 123: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris W. S. 2010. The omega-3 index: clinical utility for therapeutic intervention. Curr. Cardiol. Rep. 12: 503–508. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell T. D., Ishizaka S., Nakamura A., Swigart P. M., Rodrigo M. C., Simpson G. L., Cotecchia S., Rokosh D. G., Grossman W., Foster E., et al. . 2003. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J. Clin. Invest. 111: 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell T. D., Swigart P. M., Rodrigo M. C., Ishizaka S., Joho S., Turnbull L., Tecott L. H., Baker A. J., Foster E., Grossman W., et al. . 2006. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J. Clin. Invest. 116: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura A., Rokosh D. G., Paccanaro M., Yee R. R., Simpson P. C., Grossman W., and Foster E.. 2001. LV systolic performance improves with development of hypertrophy after transverse aortic constriction in mice. Am. J. Physiol. Heart Circ. Physiol. 281: H1104–H1112. [DOI] [PubMed] [Google Scholar]
- 26.O’Connell T. D., Rodrigo M. C., and Simpson P. C.. 2007. Isolation and culture of adult mouse cardiac myocytes. Methods Mol. Biol. 357: 271–296. [DOI] [PubMed] [Google Scholar]
- 27.Harris W. S., Sands S. A., Windsor S. L., Ali H. A., Stevens T. L., Magalski A., Porter C. B., and Borkon A. M.. 2004. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 110: 1645–1649. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q., Williams R., Healy C. L., Wright C. D., Wu S. C., and O’Connell T. D.. 2010. An association between gene expression and better survival in female mice following myocardial infarction. J. Mol. Cell. Cardiol. 49: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed S. F., Hussain S., Mirzoyev S. A., Edwards W. D., Maleszewski J. J., and Redfield M. M.. 2015. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 131: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaikh S. R. 2012. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J. Nutr. Biochem. 23: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassall S. R., and Stillwell W.. 2008. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem. Phys. Lipids. 153: 57–63. [DOI] [PubMed] [Google Scholar]
- 32.Tazoe H., Otomo Y., Kaji I., Tanaka R., Karaki S. I., and Kuwahara A.. 2008. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 59(Suppl 2): 251–262. [PubMed] [Google Scholar]
- 33.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., and Tsujimoto G.. 2005. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11: 90–94. [DOI] [PubMed] [Google Scholar]
- 34.Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., et al. . 2003. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 422: 173–176. [DOI] [PubMed] [Google Scholar]
- 35.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., and Olefsky J. M.. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Superko H. R., Superko S. M., Nasir K., Agatston A., and Garrett B. C.. 2013. Omega-3 fatty acid blood levels: clinical significance and controversy. Circulation. 128: 2154–2161. [DOI] [PubMed] [Google Scholar]
- 37.Donadio J. V., Bergstralh E. J., Bibus D. M., and Grande J. P.. 2006. Is body size a biomarker for optimizing dosing of omega-3 polyunsaturated fatty acids in the treatment of patients with IgA nephropathy? Clin. J. Am. Soc. Nephrol. 1: 933–939. [DOI] [PubMed] [Google Scholar]
- 38.Donadio J. V. Jr., Larson T. S., Bergstralh E. J., and Grande J. P.. 2001. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J. Am. Soc. Nephrol. 12: 791–799. [DOI] [PubMed] [Google Scholar]
- 39.Laidlaw M., and Holub B. J.. 2003. Effects of supplementation with fish oil-derived n-3 fatty acids and gamma-linolenic acid on circulating plasma lipids and fatty acid profiles in women. Am. J. Clin. Nutr. 77: 37–42. [DOI] [PubMed] [Google Scholar]
- 40.Hoenig M. R., Bianchi C., Rosenzweig A., and Sellke F. W.. 2008. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr. Vasc. Pharmacol. 6: 292–300. [DOI] [PubMed] [Google Scholar]
- 41.Turgeon J., Dussault S., Maingrette F., Groleau J., Haddad P., Perez G., and Rivard A.. 2013. Fish oil-enriched diet protects against ischemia by improving angiogenesis, endothelial progenitor cell function and postnatal neovascularization. Atherosclerosis. 229: 295–303. [DOI] [PubMed] [Google Scholar]
- 42.Hasan A. U., Ohmori K., Konishi K., Igarashi J., Hashimoto T., Kamitori K., Yamaguchi F., Tsukamoto I., Uyama T., Ishihara Y., et al. . 2015. Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPARgamma mediated pathways in 3T3–L1 adipocytes. Mol. Cell. Endocrinol. 406: 10–18. [DOI] [PubMed] [Google Scholar]
- 43.Shearer G. C., Pottala J. V., Spertus J. A., and Harris W. S.. 2009. Red blood cell fatty acid patterns and acute coronary syndrome. PLoS One. 4: e5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., et al. . 2012. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 483: 350–354. [DOI] [PubMed] [Google Scholar]
- 45.Galvao T. F., Khairallah R. J., Dabkowski E. R., Brown B. H., Hecker P. A., O’Connell K. A., O’Shea K. M., Sabbah H. N., Rastogi S., Daneault C., et al. . 2013. Marine n3 polyunsaturated fatty acids enhance resistance to mitochondrial permeability transition in heart failure but do not improve survival. Am. J. Physiol. Heart Circ. Physiol. 304: H12–H21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khairallah R. J., O’Shea K. M., Brown B. H., Khanna N., Des Rosiers C., and Stanley W. C.. 2010. Treatment with docosahexaenoic acid, but not eicosapentaenoic acid, delays Ca2+-induced mitochondria permeability transition in normal and hypertrophied myocardium. J. Pharmacol. Exp. Ther. 335: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berk B. C., Fujiwara K., and Lehoux S.. 2007. ECM remodeling in hypertensive heart disease. J. Clin. Invest. 117: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Heerebeek L., Borbely A., Niessen H. W., Bronzwaer J. G., van der Velden J., Stienen G. J., Linke W. A., Laarman G. J., and Paulus W. J.. 2006. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 113: 1966–1973. [DOI] [PubMed] [Google Scholar]
- 49.Zile M. R., and Brutsaert D. L.. 2002. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 105: 1503–1508. [DOI] [PubMed] [Google Scholar]
- 50.Hinz B., Phan S. H., Thannickal V. J., Prunotto M., Desmouliere A., Varga J., De Wever O., Mareel M., and Gabbiani G.. 2012. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 180: 1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wynn T. A. 2007. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oikawa S., Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Sasaki J., Hishida H., Itakura H., et al. ; JELIS Investigators, Japan. 2009. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: Sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 206: 535–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.