Abstract
Proper grading of the cribriform prostate cancer pattern has not previously been supported by outcome-based evidence. Among 153 men who underwent radical prostatectomy, 76 with prostate-specific antigen (PSA) failure (≥0.2 ng/mL [0.2 µg/L]) were matched to 77 without failure. Frequencies of high-grade patterns included fused small acini, 83.7%; papillary, 52.3%; large cribriform, 37.9%; small (≤12 lumens) cribriform, 17.0%; and individual cells, 22.9%. A cribriform pattern was present in 61% (46/76) of failures but 16% (12/77) of nonfailures (P < .0001). Multivariate analysis showed the cribriform pattern had the highest odds ratio for PSA failure, 5.89 (95% confidence interval, 2.53–13.70; P < .0001). The presence of both large and small cribriform patterns was significantly linked to failure. The cumulative odds ratio of failure per added square millimeter of cribriform pattern was 1.173 (P = .008), higher than for any other pattern. All 8 men with a cribriform area sum of 25 mm2 or more had failure (range, 33–930). Regrading cribriform cancer as Gleason 5 improved the grade association with failure, although half of all cases with individual cells also had a cribriform pattern, precluding a precise determination of the independent importance of the latter. The cribriform pattern has particularly adverse implications for outcome.
Keywords: Cribriform, Papillary, Pattern, Prostate cancer, Gleason, Grading, Digital
In 1966, a 5-tier prostate cancer grading system that relies entirely on architectural features was devised by Gleason and colleagues, who correlated the histologic patterns in 270 Veterans Administration patients with patient outcome.1 The Gleason system is now the most widely used system for the grading of prostate cancer, and it predicts pathologic stage and guides treatment choice. The grading system has since undergone a few refinements, some implemented by Gleason himself.2,3 The lowest grades of 1 and 2 are now deemed clinically irrelevant,4,5 and grade 5 is not common; thus, most cancers are grade 3, grade 4, or a combination of the 2, creating 3 diagnostic bins. The presence of any grade 4 pattern as a secondary grade6 or even a tertiary grade7 is now sufficient to consider a cancer as high-grade overall. Also, the proportion of grade 4 independently predicts outcome.8,9 Grade 4 was broadened by several changes implemented by the International Society of Urological Pathology (ISUP) in 2005.10 In Gleason’s original study, it was narrowly defined as ragged or fused glands whose cells frequently had clear to pale “hypernephroid” cytoplasm.1 Only 10 of 270 cases had a primary grade 4, and 4 was a secondary grade in 20 of 270 cases.11 Since then, its scope has been expanded considerably.
The complex continuum of patterns encompassed by grades 3 and 4 includes small acini (separate or fused, with or without blue mucin) and larger acini with flat, undulated, papillary, or cribriform contours. Whether the heterogeneous patterns “lumped” together under these grades have disparate biologic potentials is uncertain. In Gleason’s original 1966 work, cribriform to papillary cancer was used as the illustration of grade 3.1 Gleason later designated a large acinar, undulating pattern of separate acini as pattern 3A and a cribriform/papillary carcinoma as pattern 3C.3 Cribriform cancer in 2000 was still deemed grade 3.12 Particularly for the large acinar patterns, outcome-based evidence for classification is lacking. Gleason was reportedly uncertain whether cribriform cancer could be considered a higher grade than 3,13 and today the grading of cribriform cancer remains a point of controversy.3,10,13–15
In 1966, grading was based mainly on transurethral resections and was calibrated to systemic recurrence and survival outcomes. In approximately 60,000 US men annually, prostate-specific antigen (PSA)-only progression develops within 10 years of treatment, and in one third of these patients, the disease progresses to clinical manifestations of metastases.16 Hence, pattern association with PSA failure now provides a logical basis for grading. In the 1974 study by Gleason et al,2 86% of men had extraprostatic cancer.
Cancers are now much smaller and more often grade 3 at diagnosis, raising the urgent issue of whether radical prostatectomy constitutes overtreatment for many men. The current work isolates 5 high-grade morphologic types in contemporary prostatectomy slide sets to determine the association of each with biochemical recurrence or death of prostate cancer after several years’ follow-up, in an effort to stratify more finely their implications for grading.
Materials and Methods
Because a consecutive series of unselected cases would have furnished relatively too few failures, a paired case-control study was devised. Each patient with failure was matched to a patient without failure. Exclusion criteria were a history of receiving cryotherapy, radiotherapy, or androgen deprivation before failure. All had postoperative drops in PSA levels to undetectable. Men chosen for the study came from 3 medical centers: the University of Colorado Denver Hospital, Aurora (n = 44); University of Wisconsin Health System, Madison (n = 60); and Methodist Hospital, Houston, TX (n = 49). Most had Gleason score 7 cancer (∼95% in the 6–8 range) because it is in this range that outcome varies the most. Biochemical failure was considered as a rise to 0.2 ng/mL (0.2 µg/L) or more, without evidence of later, lower measurements that would invalidate the 0.2 level. All men except 2 with nonfailure had at least 2 years’ follow-up; 2 had between 1 and 2 years’ follow-up. The follow-up duration was the primary matching criterion. Secondary criteria for matching were the potential confounders of stage, grade, margin status, and patient age. Prostates were completely sampled at 4- to 6-mm intervals at all contributing sites. Gland volume was calculated by the ellipsoidal method.17
Histologic Pattern Annotation
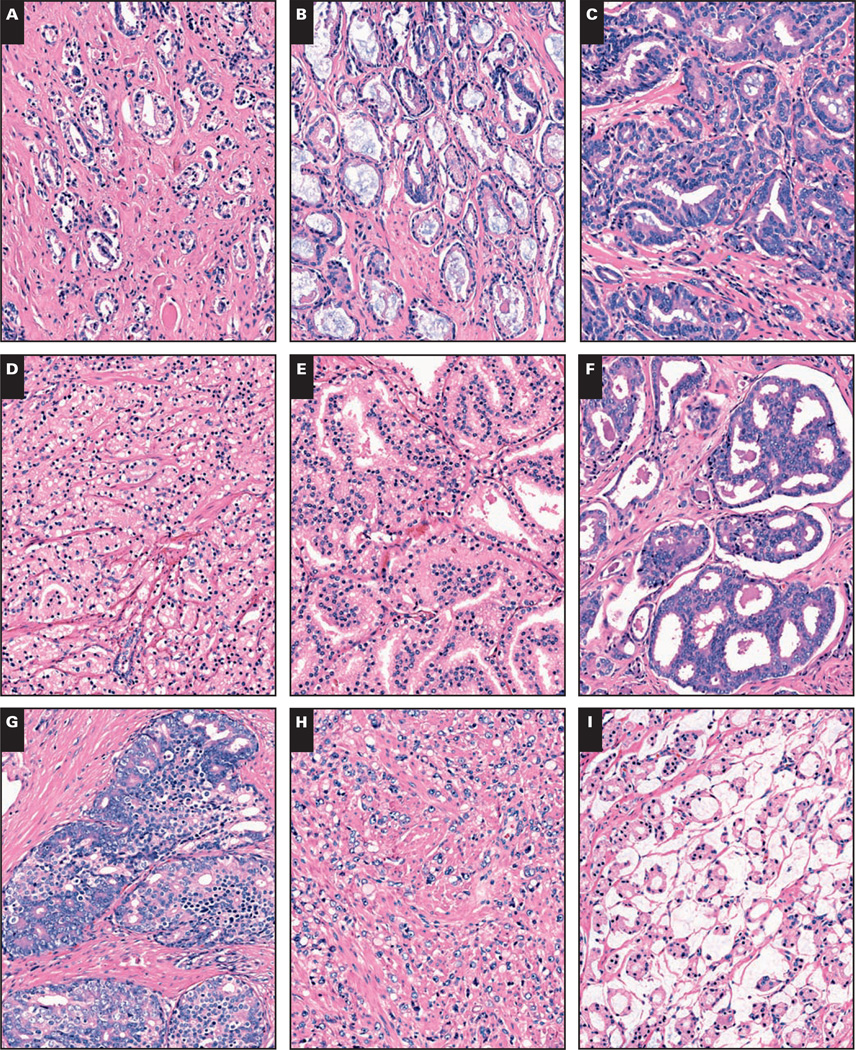
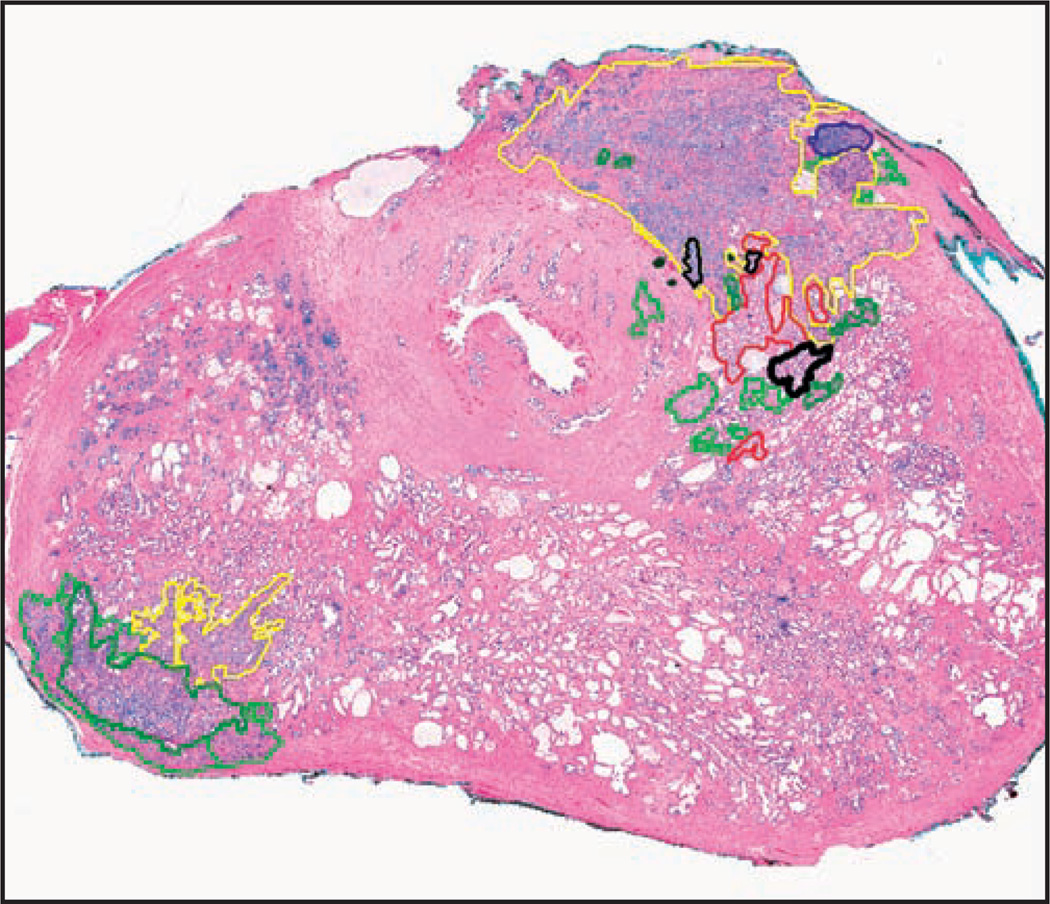
Patients’ entire prostatectomy slide sets were rereviewed. The slides containing cancer (average ± SD per case, 8.0 ± 4.3) were digitally scanned as virtual slides using a ScanScope XT (Aperio Technologies, Vista, CA). By using ImageScope software (Aperio), we manually annotated all foci of 9 histologic patterns in a nonoverlapping manner, using a different color for each pattern, denoted as follows: (1) S, single, separate small acini, like 3B pattern3 without blue mucin Image 1A; (2) B, single, separate small acini with blue mucin Image 1B; (3) U, undulated, stellate, or branching medium acini, like 3A pattern3 lined by a single cell layer Image 1C; (4) F, fused, ragged small acini, including those with mucin Image 1D; (5) P, (micro)papillary consisting of medium to large spaces with stromal cores or strands of cells with 1 or more cell layers bridging across the acinus, with intervening slit-like spaces Image 1E; (6) SC, small cribriform, mediumsized acinar spaces with rounded contour, no solid foci, and 12 or fewer lumen spaces (inclusive of the glomeruloid pattern18) Image 1F; (7) LC, large cribriform, with expansive cribriform to focally solid large acini with more than 12 lumen spaces Image 1G; (8) I, individual infiltrating or sheet-like cells lacking lumen formation Image 1H; and (9) M, mucinous (colloid) carcinoma in which nonfused acini (excluding single cells) floated in mucin pools Image 1I. Note that although the lumen contours of the U and P patterns were somewhat similar, strict criteria (as stated) were adhered to for P, (micro)papillary. Based on the assertion that small, rounded cribriform cancer might be graded as Gleason grade 3,10,13,15 specimens with any cribriform pattern were further subdivided by annotating the SC and LC areas. Image 2 shows an example of an annotated slide.
Image 1.
Nine histologic prostate cancer patterns were annotated in the study (H&E, ×100). A, The S pattern, single, small separate acini. B, The B pattern, luminal blue mucin–containing single, separate acini. C, The U pattern, undulating, branched, or angulated larger acini that are not truly papillary—no bridging or stromal cores. D, The F pattern, fused small acini. E, The P pattern, true papillary with stromal cores or bridging across acinar spaces. F, The SC pattern, small cribriform, defined as rounded acinar spaces with ≤12 lumens and no solid area. G, The LC pattern, large cribriform, with more sprawling, cribriform to focally solid formations. H, The I pattern, individual cells. I, The M pattern, mucinous/colloid carcinoma without fusion or individual cells.
Image 2.
A sample virtual slide is shown after annotation of histologic cancer patterns (H&E, ×3).
The rare cancer subtypes of ductal19,20 (excluding some papillary patterns that were encompassed in a broader definition of ductal21), and adenoid cystic/basal cell carcinoma22 were denoted, if present, according to established criteria. To verify all annotations considered to be LC or SC or to have ductal cancer, consensus conferences were held by 4 of us (K.A.I., G.R.K., F.G.L., and M.S.L.), at which annotated slide images were projected on a screen. For each specimen, taking together all pertaining slides, the area sum of each cancer pattern was determined by summation of all digitally annotated foci belonging to that pattern. In this manner, each distinct pattern could be considered individually from a statistical viewpoint. The amount of each pattern per specimen was also expressed as percentage of the total cancer area. However, area sums rather than pattern percentages were used as the main method of analysis to approximate the cancer contribution of each pattern.
To determine the relative tendency of each pattern toward extraprostatic extension or seminal vesicle invasion, the cancer patterns responsible for these findings were tabulated. As a separate analysis, to explore the effect of having a sizable amount of large acinar (LA, or P plus cribriform [C] patterns) cancer, a cut point of P + C patterns forming more than one third of the total cancer area per patient was chosen. The one-third cut point was chosen for its ease of perception at diagnosis and because it produced a category that included 17 cases, allowing meaningful statistical comparison with the other 136.
Statistical Analysis
For each patient, biochemical failure status was recorded. To determine whether failure was related to the presence or area sum per patient for each of the patterns, as well as to the preoperative serum PSA level, pathologic stage, grade, and margin status (but not Gleason score, owing to collinearity with the measured data), univariate and multivariate analyses were used. Only the latter results are shown.
Depending on the distribution of the underlying data, parametric or nonparametric tests were used to determine the associations of biochemical failure with clinical and pathologic parameters. Logistic regression analysis was used to test associations with failure while adjusting for potential confounding variables. All tests were 2-sided, and significance levels were set at a P value of less than .05. SAS, version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
Results
To control for potential confounding, we attempted to match our cases and controls on length of follow-up, age at surgery, stage, and grade. Table 1 shows the clinicopathologic data for the cases, from which 1,100 slides were digitized and annotated, proving we were successful in our match on age and length of follow-up. With our limited number of possible controls, however, we were not successful matching on stage and grade. To account for any mismatching, we adjusted our multivariate models for these potential confounders. Because we did match, or attempted to match on age, stage, grade, and length of follow-up, we were not able to study the independent relationship of these variables to PSA failure because the distribution of these variables in controls does not represent a random sample.
Table 1.
Clinicopathologic Features for 153 Men in the Study*
| PSA Failure |
P | ||
|---|---|---|---|
| Yes (n = 76) | No (n = 77) | ||
| Mean (SD) age at surgery (y) | 59.2 (7.2) | 58.5 (7.0) | .547† |
| Median (range) follow-up (d) | 2,156 (35–4,612) | 2,017 (398–4,198) | .817‡ |
| Gleason score ≥7 | 59 (78) | 46 (60) | .017§ |
| American Joint Committee on Cancer stage ≥pT3 | 46 (61) | 21 (27) | <.0001§ |
| Positive margins | 34 (45) | 17 (22) | .003§ |
| Median (range) preoperative PSA (ng/mL) | 7.9 (2.0–77.6) | 6.3 (1.0–737.0) | .026‡ |
| Median (range) gland volume (mL) | 37.7 (11.5–103.9) | 35.3 (9.4–102.1) | .835‡ |
| Mean (range) measured cancer area per patient (mm2) | 444.1 (1.1–3,900.7) | 192.7 (0.7–2,191.7) | <.0001‡ |
PSA, prostate-specific antigen.
Data are given as number (percentage) unless otherwise indicated.
t test.
Wilcoxon rank sum test.
χ2.
By our definition of PSA failure, the 153 total patients comprised 76 (49.7%) with PSA failure and 77 (50.3%) without failure. In 5 patients, there were known metastases, and 6 had prostatic fossa recurrence. Of the men in the study, 7 died, 5 of prostate cancer (3.3%) and 2 of other causes. As expected, patient age, follow-up days, and prostate volume were similar with respect to PSA failure status. Of the 153 specimens, 105 (68.6%) were Gleason grade 7 or more, 67 (43.8%) were stage T3, and 51 (33.3%) had positive margins. Significant differences included associations of failure with cancer stage (odds ratio [OR], 4.236; 95% confidence interval [CI], 2.160, 8.306; P < .0001), margin status (P = .003), cancer area sum per patient (P < .0001), preoperative PSA level (P = .026), and Gleason score (P = .017).
Significant differences between contributing sites included that the Colorado group had a lower cancer area sum (P = .023), and the median patient age was more than 2 years younger (P = .04), and that the Baylor group had a larger prostate size (P = .001). There was no difference in Gleason score among sites (P = .49).
Table 2 depicts the frequency of the presence of each pattern and ORs for association with PSA failure status in our selected population by multivariate analysis. The most common of all patterns was S, in 98.7% of men, but such acini with blue mucin (B pattern) occurred in 51.0% of men. The second and third most common patterns were F (83.7%) and U (79.9%). For analysis, the S, B, U, and M patterns were combined because all are traditionally grade 3; all (except M) were very common; and when the presence of each was analyzed separately, the associated OR for failure was less than unity (data not shown). Together, their 0.314 OR for failure points to a favorable outcome relative to the higher grade patterns. With cancer with a Gleason score of 7 or more constituting about 67% of the study cases, cribriform (C) prostate cancer (large or small) was present in a sizable minority, namely, 58 patients (37.9%). No specimen had any necrosis within the C component. Less common than the C pattern was the I/Gleason 5 pattern, seen in 35 patients (22.9%).
Table 2.
Presence of Nine Histologic Prostate Cancer Patterns and Their Association With PSA Failure in 153 Cases*
| Pattern | Present | PSA Failure (n = 76) |
Non-PSA Failure (n = 77) |
P(χ2) | OR for PSA Failure |
95% CI | P for OR |
|---|---|---|---|---|---|---|---|
| Low-grade (S, B, U, and M) | All, 151 (98.7) | 75 (99) | 76 (99) | .754† | 0.314 | 0.018–5.464 | .427 |
| S, 151 (98.7) | |||||||
| B, 78 (51.0) | |||||||
| U, 122 (79.7) | |||||||
| M, 9 (5.9) | |||||||
| Fused small | 128 (83.7) | 68 (89) | 60 (78) | .053 | 1.403 | 0.499–3.945 | .521 |
| Papillary | 80 (52.3) | 50 (66) | 30 (39) | .0009 | 2.155 | 0.999–4.645 | .050 |
| Individual | 35 (22.9) | 25 (33) | 10 (13) | .003 | 2.654 | 1.069–6.589 | .035 |
| All cribriform | 58 (37.9) | 46 (61) | 12 (16) | <.0001 | 5.891 | 2.534–13.698 | <.0001 |
| Any large | 58 (37.9) | 46 (61) | 12 (16) | <.0001 | 5.583 | 2.416–12.901 | <.0001 |
| Any small | 26 (17.0) | 21 (28) | 5 (6) | .0005 | 6.062 | 1.931–19.037 | .002 |
| Large acinar‡ | 17 (11.1) | 15 (20) | 2 (3) | .0007 | 10.806 | 2.152–54.256 | .004 |
B, luminal, blue mucin–containing, single, separate acini; CI, confidence interval; M, mucinous/colloid carcinoma without fusion or individual cells; OR, odds ratio; PSA, prostate-specific antigen; S, single, small separate acini; U, undulating, branched, or angulated larger acini that are not truly papillary—no bridging or stromal cores.
Data are given as number (percentage) unless otherwise indicated. ORs were determined by multivariate analysis adjusting for the effects of stage, age, margin status, total cancer area, and prostate volume. The F pattern is fused small acini; papillary, true papillary with stromal cores or bridging across acinar spaces; individual, individual cells; small cribriform, rounded acinar spaces with ≤12 lumens and no solid area; and large cribriform, with more sprawling, cribriform to focally solid formations.
This value derived from the Fisher exact test.
More than one third of cancer volume is composed of large acinar (papillary + cribriform) patterns.
On multivariate analysis, the histologic patterns whose presence was significantly linked to PSA failure were C (LC plus SC, P < .0001), P (P = .0009), and I (P = .003). The C pattern (SC or LC) was present in 61% of men with PSA failure (46/76) but only 16% of matched men without failure (12/77), resulting in an OR for PSA failure of 5.89, higher than for any other pattern. The association of the presence of fused small acini (F pattern) with PSA failure was minimal (P = .053, for an OR of 1.403 with P = .521), probably because of being the second-most frequent pattern in this population.
Of the 58 men, 32 (55%) had an exclusive LC pattern; 26 had coexistent LC and SC (45%), and no man had exclusive SC. Any LC and SC present was associated with failure (OR, 5.583 with P < .0001 and OR, 6.062 with P = .0005, respectively). Moreover, a subset of 17 specimens was defined as LA-preponderant based on having more than one third of the cancer volume composed of P plus C patterns. For the LA group, the OR for PSA failure rose to 10.81 (P = .0007).
Table 3 shows the analysis of the associations of area sums of a pattern with failure, based on only the specimens that had it present; that is, absence of a pattern did not count as a zero. All were not normally distributed. The area sums of the 4 low-grade S, B, U, and M patterns taken together did not correlate with failure (P = .122). Among the 4 high-grade patterns, the F (P < .0001) and P (P = .006) patterns correlated with failure. The C and I patterns also lacked significance for failure (P = .09 and P = .64 respectively). However, logistic regression analysis (adjusted for stage, age, margin, ellipsoid gland volume, and total cancer area) revealed that of the 35 cases with the I pattern, 18 had a coexistent C pattern (OR, 14.741; CI, 2.876–75.56; P = .001), while 17 did not (OR, 1.608; CI, 0.502–5.146; P = .424). Thus, the frequent coexistence of the C pattern with the I pattern was multiplicative in regard to the OR for failure and probably minimized the independent associative value for the fairly rare I pattern. No other pattern’s area sum correlated significantly with another. Eight men had a cribriform area sum exceeding 25 mm2 (range, 33–930 mm2), and all had PSA failure. Various clinicopathologic features were excluded as confounding factors because area sums of patterns did not correlate with any of them: age at surgery, preoperative PSA level, prostate volume, and total cancer volume.
Table 3.
Area Sum per Specimen of Nine Histologic Prostate Cancer Patterns and Their Association With PSA Failure, Only When Pattern Is Present*
| Pattern | Median Area | Median Area (Range) in PSA Failures (n = 76) |
Median Area (Range) in Non-PSA Failures (n = 77) |
P† |
|---|---|---|---|---|
| Low-grade (S, B, U, and M) | 82.4 | 103.1 (0.04–886.1) | 72.0 (0.35–2,091.0) | .122 |
| Fused small | 28.0 | 54.8 (0.20–775.7) | 11.6 (0.04–445.3) | <.0001 |
| Papillary | 7.2 | 12.2 (0.09–762.3) | 5.0 (0.06–222.7) | .006 |
| Individual | 17.8 | 18.2 (0.004–3,749.1) | 10.7 (0.3–310.4) | .637 |
| Cribriform, all | 4.2 | 5.4 (0.06–930.3) | 1.6 (0.1–22.5) | .094 |
| Large | 4.0 | 5.1 (0.06–929.4) | 1.3 (0.1–21.2) | .109 |
| Small | 0.1 | 0.1 (0.01–2.4) | 0.1 (0.02–1.3) | .896 |
B, luminal, blue mucin–containing, single, separate acini; M, mucinous/colloid carcinoma without fusion or individual cells; PSA, prostate-specific antigen; S, single, small separate acini; U, undulating, branched, or angulated larger acini that are not truly papillary—no bridging or stromal cores.
The F pattern is fused small acini; papillary, true papillary with stromal cores or bridging across acinar spaces; individual, individual cells; small cribriform, rounded acinar spaces with ≤12 lumens and no solid area; and large cribriform, with more sprawling, cribriform to focally solid formations. Areas are given in mm2.
Wilcoxon rank sum. Based on multivariate analysis adjusting for the effects of American Joint Committee on Cancer stage, age, margin status, total cancer area, and prostate volume.
Large acinar (C + P) pattern associations with failure were also analyzed based on their percentages. Notably, the percentage of the C pattern never exceeded 11.8% in the nonfailures, but ranged to highs of 57%, 63%, and 99% in some failures.
Next, an analysis was done in which the absence of a pattern in a specimen did count as a zero Table 4. The cumulative effect of the presence and amount of each pattern was expressed as the OR of failure per added square millimeter of a given pattern. An increasing area sum of low-grade S, B, U, and M patterns conferred an OR less than 1 and significantly less likelihood of progression, perhaps by precluding the formation of the higher grade patterns. The OR was 1.173 for the C pattern (P = .008), whereas it was only 1.00 to 1.02 for the F, P, and I patterns. The OR was still significant for the F and P patterns but not the I pattern. Thus, among only the 58 specimens with the C pattern, its area sum fell short of significance (Table 3), but among all 153 specimens, counting the absence of a C pattern as a zero, the effect of its area sum became significant (Table 4).
Table 4.
Cumulative Effect of Area Sum of Nine Histologic Prostate Cancer Patterns on PSA Failure, With Absence of a Pattern Counted as Zero
| Pattern* | Odds Ratio† of PSA Failure per Additional mm2 |
95% Confidence Interval |
P |
|---|---|---|---|
| Low-grade (S, B, U, and M) | 0.993 | 0.989–0.997 | .0007 |
| Fused small | 1.006 | 1.001–1.012 | .024 |
| Papillary | 1.018 | 1.003–1.034 | .017 |
| Individual | 1.002 | 0.995–1.009 | .552 |
| Cribriform, all | 1.173 | 1.042–1.321 | .008 |
| Large | 1.173 | 1.040–1.324 | .009 |
| Small | 4.131 | 0.483–35.362 | .195 |
B, luminal, blue mucin–containing, single, separate acini; M, mucinous/colloid carcinoma without fusion or individual cells; PSA, prostate-specific antigen; S, single, small separate acini; U, undulating, branched, or angulated larger acini that are not truly papillary—no bridging or stromal cores.
The F pattern is fused small acini; papillary, true papillary with stromal cores or bridging across acinar spaces; individual, individual cells; small cribriform, rounded acinar spaces with ≤12 lumens and no solid area; and large cribriform, with more sprawling, cribriform to focally solid formations.
By logistic regression analysis adjusting for the effects of American Joint Committee on Cancer stage, age, margin status, total cancer area, and prostate volume.
The stage was pT3 in 67 men. In 66 men (43.1% of total), there was extraprostatic extension (EPE). One man had seminal vesicle invasion alone, and 11 (7.2% of total) had EPE and seminal vesicle invasion. To determine the likelihood of EPE based on pattern, the patterns responsible for EPE were tabulated. EPE by the small acinar S, B, and F patterns accounted for 69 foci, compared with 9 foci of LA P and C patterns. This difference was not significant relative to the mean percentages of these patterns. With seminal vesicle invasion, however, 11 foci of small acinar pattern occurred vs 8 foci of LA pattern, such that LA patterns invaded more frequently than expected (P < .001; χ2) based on mean percentages.
In 17 men with LA-preponderant (> one third C + P pattern) cancer, the occurrence of EPE was 41% (n = 7) vs 32% (n = 44) in the remaining 136 men (P = .47; χ2). The failure rate in men with EPE was 75% (6/8) vs 78% (7/9) for men without EPE (P = .91; χ2). However, seminal vesicle invasion was relatively more frequent, with 4 in the LA-preponderant group but 7 in the larger, non-LA group (P = .0047).
It was postulated that because the C pattern carried the highest elevation of OR for failure per square millimeter of the pattern (Table 4), it could be hypothetically regraded as Gleason 5. A regrading exercise was undertaken Table 5 and Table 6 in which new grades and scores were assigned to all specimens based on proportional areas of patterns, in square millimeters. The percentage of grade 3 area was considered as the sum of the percentages of areas of S, B, U, and M patterns; grade 4 was the sum of F and P patterns; and grade 5 included the sum of C pattern along with the usual I pattern. Secondary patterns were required to constitute 5% or more of the total cancer area, as recommended for prostatectomy.10 The McNemar test was used to compare the proportion of patients with PSA failure after rescoring.
Table 5.
Reallocated Prostate Cancer Gleason Scores: Distribution of New Scores*
| Original Score | Reallocated Score |
||||
|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | |
| 5 | — | 2 | — | — | — |
| 6 | 26 | 17 | 3 | — | — |
| 7 | 14 | 63 | 7 | 9 | — |
| 8 | — | 2 | 1 | — | 2 |
| 9 | — | 2 | — | 4 | 1 |
After allocating the cribriform pattern along with the individual cell pattern as grade 5, the papillary and fused patterns as grade 4, and all others (S, B, U, and M) as grade 3. For the original scores, multivariate odds ratio (OR) for failure of score ≥7 vs score ≤6 is 1.474 (95% confidence interval [CI], 0.653–3.327; P = .35). For the reallocated scores, the OR is 5.667 (95% CI, 2.046–15.693; P = .0008). B, luminal, blue mucin–containing, single, separate acini; M, mucinous/colloid carcinoma without fusion or individual cells; S, single, small separate acini; U, undulating, branched, or angulated larger acini that are not truly papillary—no bridging or stromal cores. Bold type indicates how many scores remained the same.
Table 6.
Reallocated Prostate Cancer Gleason Scores: Dose-Response Relationship of Original and Reallocated Scores to PSA Failure*
| Score 6 |
Score 7 |
Score ≥8 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | OR | No. | OR | 95% CI | P | No. | OR | 95% CI | P | |
| Original score | 48 | 1.0 | 93 | 1.26 | 0.54–2.96 | .588 | 12 | 3.47 | 0.81–14.9 | .094 |
| Reallocated score | 40 | 1.0 | 86 | 4.64 | 1.62–13.28 | .004 | 27 | 10.04 | 2.66–37.87 | .0007 |
CI, confidence interval; OR, odds ratio; PSA, prostate-specific antigen.
Adjusted dose response with increasing Gleason score (age, preoperative PSA, American Joint Committee on Cancer stage, margin, gland volume, and cancer area).
The score rose for 41 men, fell for 18, and remained the same in 94 (Table 5). Adjusted for age, preoperative PSA level, stage, margin, gland volume, and cancer area, the new score groupings provided a stronger association with failure than did the original groupings. The dose-response relationship was significant for new scores of 7 compared with 6 (OR, 4.64; P = .004) and 8 or more compared with 6 (OR, 10.04; P < .0007) (Table 6), while these relationships were not significant under the original scores. The proportion of new scores of 7 or more among failures was higher, with 89% (68/76) having a score of 7 or more vs 58% of nonfailures (P < .0001; χ2).
We determined the degree to which the area sums of patterns per patient correlated with each other. Weak but significant correlations were obtained for the area sums of the F and I patterns (34 occurrences; ρ = 0.42979; P = .0112) and the area sums of the P and C pattern (49 occurrences; ρ = 0.37582; P = .0078). The P pattern also correlated with the LC pattern (49 occurrences; ρ = 0.36133; P = .0107) but not significantly with the SC pattern. For the S, B, U, and M patterns, correlations with all other patterns were insignificant, with ρ values of 0.2 or less.
According to the criteria for ductal morphology,20,22,23 9 men (5.9%) had a ductal component. Ductal cancer always occurred within P pattern, being present in 9 (11%) of the 80 men with the P pattern. The only case in the entire study with any necrosis had necrosis within the ductal component of the P pattern. All 9 of these men had PSA failure, precluding logistic regression analysis; but their 100% failure rate exceeded the 54% (38/71) of men with the P pattern without ductal cancer who had failure (P = .003; Fisher exact test). The rare glomeruloid pattern,18 consisting of a dilated gland with an intraluminal tuft, had been proposed as a form of pattern 4.24 It was noted in only 4 of our cases, too few to draw conclusions. Adenoid cystic/basal cell carcinoma is a rare cancer subtype that can assume a cribriform pattern. This cancer was considered indolent until 2003, when 21% of patients were reported to have developed metastases.25 This diagnosis requires a component of basaloid cells, and no specimens in the study fit the criteria for adenoid cystic/basal cell carcinoma.
Discussion
This study addressed the uncertain biologic potential of LA prostate cancer by comparing it with other architectural patterns. The C pattern of any diameter (dichotomized into large or small) emerged as a uniquely adverse finding. Its presence had a stronger OR (5.89) for PSA failure than did any other pattern. The C pattern was present in 61% of men with PSA failure but only 16% of matched men without failure. The area sum of the C pattern was not significant among only the 58 cribriform-containing cases (Table 3) but became a significant outcome predictor when the fact of its presence was also considered—that is, among the entire 153 cases (Table 4). In the prostate cancer population studied, the frequency of any cribriform cancer was 37.9% of specimens, a higher frequency than individual cells (I pattern), the prototypical Gleason grade 5. Yet, PSA failure was more strongly associated with the presence of the C pattern than with the presence of the I pattern. This could be attributable to the I pattern being the rarest pattern and to its frequent association (18 of 35 cases) with the C pattern, reducing its independent associative value (as explained for Table 3). The P pattern, the other LA pattern, had a somewhat lower but significant 2.155 OR for PSA failure. Strikingly, among 17 cases with a preponderance of LA (C and P) patterns amounting to one third of the cancer area, the OR for failure reached 10.80. The knowledge that the C pattern is a particularly adverse prognostic finding could help stratify prognosis, especially considering the wide variability in the histologic appearances of grade 4 cancer.
It has been suggested that one rationale for grading all cribriform foci as grade 4 regardless of size would be to improve interobserver reproducibility.26 But none of Gleason’s studies addressed the prognostic differences between small, rounded cribriform clusters and irregular, sprawling ones. Table 2 shows that the presence of the C pattern was strongly linked to PSA failure regardless of whether it was small or large. Only 1 man in the study had an exclusive SC pattern, and this patient eventually had PSA failure. Failure was also associated with the cumulative area sum of cribriform cancer, and while 1.173 per mm2 is a small OR, cumulatively, for the 27 (47%) of 58 men whose cribriform cancer was more than 5 mm2, the OR would have gone much higher. The cumulative OR was significant for large cribriform cancer but not small cribriform cancer (Table 4), but this probably reflects the greater number of men with the former. Thus, it has been demonstrated, albeit in a population of cancers enriched for PSA failure, that neither the small size nor small amount of cribriform cancer can justify a low grade, such as grade 3.
Recent evidence for an elevated biologic potential of cribriform cancer came from Kronz et al,14 who, in their biopsy study of “atypical cribriform lesions,” found that 55% of patients had cancer on repeated biopsy. Also, of 10 patients with subsequent carcinoma, 6 had a component of Gleason pattern 4.14 The only major molecular alteration, to our knowledge, that was correlated with a C pattern was TMPRSS2-ERG fusion, which is associated with a worse outcome in some studies, although there is recent evidence to the contrary.27 The C pattern was present in 24% of TMPRSS2-ERG fusion-positive cases vs 7% of fusion-negative cases (OR, 9.4; P = .002).28 Moreover, 75% of intraductal prostate cancer that was cribriform was demonstrated to have this rearrangement, compared with 0% for cribriform high-grade prostatic intraepithelial neoplasia.29 However, Fine et al30 found no difference, calling the preceding data into question.
In a 1992 review of grading, “cribriform epithelium in smooth rounded cylinders” was grade 3, but in the accompanying schematic diagram, cribriform formations appeared to straddle the barrier with grade 4, and if there is necrosis, grade 5.3 A consensus statement of the College of American Pathologists in 2000 placed cribriform and papillary cancer under grade 3.12 Kronz et al14 in 2001 considered that the C pattern 3 “has cribriform glands that are smaller and more regular than…cribriform pattern 4.” Pathologists’ view of cribriform cancer has evolved in the past decade, so that it is now usually assigned grade 4. The ISUP grading consensus conference of 2005, while shifting most cribriform acini to grade 4, ruled that such proliferations that were rounded and of comparable size to benign acini could be graded as grade 3.10
Since then, no outcome-based data have been produced to support grading of cribriform cancer as 3, 4, or even 5. Among genitourinary pathologists surveyed, 58 (88%) of 66 remained willing to assign cribriform cancer to grade 3.13 In a 2008 interobserver consensus study, 10 experts in prostate pathology were shown 36 images of cribriform cancer that would qualify as grade 3 under the ISUP consensus. Consensus was reached on the grading of 24 of these images, and in 23 of the 24, the consensus grade was 4.15
Thus, our findings largely validate a practice that is becoming habitual, at least for urologic pathologists, and support the new ISUP 2005 rules. Two recent studies showed trends toward upgrading of cases after 2005 compared with those before 2004 by applying the new ISUP criteria. In biopsy specimens, for example, the percentage of cases with Gleason scores of 6 or less fell from 68% to 55%, while cases with Gleason score 7 rose from 30% to 43%.31 In radical prostatectomy specimens, Gleason 7 cases rose from 48% to 60%.30 Another study stratifying biopsy cases into prognostic groups (2–4, 5–6, 7, and 8–10) determined that the ISUP criteria shifted 25% into a higher prognostic group.32 Application of our findings will maintain this overall tendency toward higher grades.
What are the implications of these findings about cribriform cancer for clinical practice? At a minimum, cribriform cancer of any diameter should be grade 4. The main criterion to consider prostate cancer high grade is the emergence of grade 4 patterns as a primary, secondary,6 or even tertiary7 grade. In multivariate analysis with age, serum PSA level, grade, stage, and margin status, the combined percentage of Gleason patterns 4 + 5 independently predicts survival after radical prostatectomy.8 Even with cribriform cancer graded as Gleason 4, our findings may merit a comment or extra line in biopsy and prostatectomy reports when cribriform cancer is present. Moreover, PSA failure should be recognized as almost certain when the cribriform area sum exceeds about 25 mm2 (eg, 5 × 5 mm total), as was true for all 8 men in the present study. A more radical approach, equating cribriform cancer of any size or amount to grade 5, the individual/cell sheet pattern (Table 6), is speculative. Before implementing this grading, validation would be required in a population that is free of the selection biases inherent in this study, by enriching for the number of PSA failures.
Our study is preliminary, with several limitations; validation studies using unselected cases will be needed before results are applied to the population of patients with prostate cancer diagnosed by biopsy. First, PSA failures were enriched. Because failure is a rarer outcome than nonfailure, it was necessary to overrepresent failures in the matching process to achieve statistical significance. Matching the nonfailures for stage, grade, and margins also skewed the nonfailure population to cases with more adverse features.
Second, the use of prostatectomy specimens selected out men who chose surgery and who probably had more aggressive cancer than average. This too was necessary, to quantify the entire cancer in the gland without the sampling error inherent in biopsies. A prospective study on biopsy material would be needed to represent the entire spectrum of men with cancer, some of whom elect watchful waiting, hormone ablation, radiation, or cryotherapy. Hence, this study’s findings apply best to men who are surgical candidates.
Third, the cancer area sum and patient age were significantly lower at 1 institution, and the gland volume was significantly larger at 1 institution. These discrepancies probably reflect the differential application of “gatekeeper” selection criteria for undergoing surgery at different institutions. These discrepancies are probably unavoidable, but their net effect should be null as long as patients were matched within contributing institutions.
Fourth, using PSA failure is a much softer end point than death due to cancer, particularly because failure is very dependent on a long follow-up; however, it would have been prohibitively hard to find enough such men for meaningful analysis, and failure is a clinically relevant end point.
Ductal carcinoma comprises papillary/cribriform structures with uniquely tall stratified columnar cells, occurs alone in 1% of prostate cancer cases, and occurs admixed with acinar carcinoma in about 3% of cases.20 Its status as a distinct entity was questioned by Bock and Bostwick.19 Among the current subjects, ductal carcinoma occurred in 9 (5.9%), all admixed with acinar cancer. All 9 of these men experienced PSA failure, whereas 4 of 6 with follow-up in a study by Lee et al21 did not have failure. Lee et al21 used a broader definition than we did; we would have relegated their “micropapillary ductal” and “cystically dilated ductal” variants21 to the spectrum of papillary carcinoma. Associated with adverse outcome in most studies,22 ductal carcinoma is recommended to be graded as grade 4,10,22 although it was suggested that high-grade prostatic intraepithelial neoplasia–like forms be considered grade 3.23
The data on area sum associations revealed that papillary and cribriform acini had a weak tendency to occur together, as did individual cells with fused small acini. These findings are logical, considering that P and C are high-grade, large acinar patterns and F and I are high-grade patterns with acini breaking down from poorly formed to nonexistent. Whether these associations signify divergent pathways of cancer development cannot be deduced because the ρ values suggest they are weak, and their spatial relationships were not studied. In fact, the decades-old question of whether grade 3 cancers evolve over time into patterns designated 4 or 53 could be answered only by spatial analysis.
Also inconclusive were the data regarding 2 other prostate cancer patterns. The B pattern, separate acini with luminal blue mucin, present in 50% of cases, was not previously studied as a prognostic feature, only as a diagnostic feature favoring cancer in biopsy specimens.20,33 Blue mucin emerged as one of the morphologic features significantly associated with TMPRSS2-ERG fusion status, being present in 50% of fusion-positive cases but 15% of fusion-negative cases, for an OR of 11.6 (P < .0001).27 Because of frequent admixture of high-grade patterns in the current study, the independent effect of the B pattern could not be determined. We cannot draw any conclusion about the M pattern, mucinous (colloid) cancer—glands floating in mucinous pools—because it formed a component of cancer in only 9 subjects in the study. It has been suggested to be pattern 3.10 In 2 recent studies, mucinous carcinoma did not confer a poorer prognosis than that of nonmucinous.34,35
The LA patterns—C and, to a lesser extent, P—are shown, by a number of measures, to have biologic potential at least equal to that of the small acinar high-grade patterns and possibly similar to individual cells/grade 5. Future studies may use an unselected population to validate these findings or could examine the effect on outcome of lumen size, shape, and spacing within each annotated pattern.
Upon completion of this activity you will be able to:
describe the variety of prostate cancer patterns that comprise the frequently used Gleason grades 3 and 4 and estimate their relative frequencies.
discuss the reasons that certain cribriform structures might have been graded as Gleason 3 based on customary practice, and the findings in outcome-based evidence in the literature.
estimate the odds ratio for prostate-specific antigen failure and seminal vesicle failure in prostatic carcinomas with large acinar patterns, particularly with the cribriform pattern.
Footnotes
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
References
- 1.Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50:125–128. [PubMed] [Google Scholar]
- 2.Gleason DF, Mellinger GT and the VA Cooperative Urological Research Group. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 3.Gleason DF. Histologic grading of prostate cancer: a perspective. Hum Pathol. 1992;23:273–279. doi: 10.1016/0046-8177(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 4.Berney DM. Low Gleason score prostatic adenocarcinomas are no longer viable entities. Histopathology. 2007;50:683–690. doi: 10.1111/j.1365-2559.2007.02596.x. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI. Gleason score 2–4 adenocarcinoma of the prostate on needle biopsy: a diagnosis that should not be made [editorial] Am J Surg Pathol. 2000;24:477–478. doi: 10.1097/00000478-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Billis A, Rogerio F, Oliveira RV, et al. The border between low- and high-grade Gleason score for prostate carcinoma: 6 or 7a (3+4) [abstract]? Mod Pathol. 2008;21:149A. [Google Scholar]
- 7.Pan CC, Potter SR, Partin AW, et al. The prognostic significance of tertiary Gleason patterns of higher grade in radical prostatectomy specimens: a proposal to modify the Gleason grading system. Am J Surg Pathol. 2000;24:563–569. doi: 10.1097/00000478-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Davidson DD, Lin H, et al. Percentage of Gleason pattern 4 and 5 predicts survival after radical prostatectomy. Cancer. 2007;110:1967–1972. doi: 10.1002/cncr.23004. [DOI] [PubMed] [Google Scholar]
- 9.Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459–3464. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Allsbrook WC, Amin MB, et al. The 2005 International Society of Urologic Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–1246. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 11.Bailar JC, Mellinger GT, Gleason DF. Survival rates of patients with prostatic cancer, tumor stage, and differentiation: preliminary report. Cancer Chemother Rep. 1966;50:129–136. [PubMed] [Google Scholar]
- 12.Srigley JR, Amin MB, Bostwick DG, et al. for the Members of the Cancer Committee, College of American Pathologists. Updated protocol for the examination of specimens from patients with carcinomas of the prostate gland: a basis for checklists. Arch Pathol Lab Med. 2000;124:1034–1039. doi: 10.5858/2000-124-1034-UPFTEO. [DOI] [PubMed] [Google Scholar]
- 13.Egevad L, Allsbrook WC, Jr, Epstein JI. Current practice of Gleason grading among genitourinary pathologists. Hum Pathol. 2005;36:5–9. doi: 10.1016/j.humpath.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Kronz JD, Shaikh AA, Epstein JI. Atypical cribriform lesions on prostate biopsy. Am J Surg Pathol. 2001;25:147–155. doi: 10.1097/00000478-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Latour M, Amin MB, Billis A, et al. Grading of invasive cribriform carcinoma on prostate needle biopsy: an interobserver study among experts in genitourinary pathology. Am J Surg Pathol. 2008;32:1532–1539. doi: 10.1097/PAS.0b013e318169e8fd. [DOI] [PubMed] [Google Scholar]
- 16.Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Clin Pract Urol. 2005;2:174–182. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- 17.Werahera PN, Miller JG, Torkko K, et al. Biomorphometric analysis of human prostatic carcinoma by using three-dimensional computer models. Hum Pathol. 2004;35:798–807. doi: 10.1016/j.humpath.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Pacelli A, Lopez-Beltran A, Egan AJ, et al. Prostatic adenocarcinoma with glomeruloid features. Hum Pathol. 1998;29:543–546. doi: 10.1016/s0046-8177(98)90073-9. [DOI] [PubMed] [Google Scholar]
- 19.Bock BJ, Bostwick DG. Does prostatic ductal adenocarcinoma exist? Am J Surg Pathol. 1999;23:781–785. doi: 10.1097/00000478-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Grignon DJ. Unusual subtypes of prostate cancer. Mod Pathol. 2004;17:316–327. doi: 10.1038/modpathol.3800052. [DOI] [PubMed] [Google Scholar]
- 21.Lee TK, Miller JS, Epstein JI. Rare histological patterns of prostatic ductal adenocarcinoma. Pathology. 2010;42:319–324. doi: 10.3109/00313021003767314. [DOI] [PubMed] [Google Scholar]
- 22.Brinker DA, Potter SR, Epstein JI. Ductal adenocarcinoma of the prostate diagnosed on needle biopsy: correlation with clinical and radical prostatectomy findings and progression. Am J Surg Pathol. 1999;23:1471–1479. doi: 10.1097/00000478-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Tavora F, Epstein JI. High-grade prostatic intraepithelial neoplasialike ductal adenocarcinoma of the prostate: a clinicopathologic study of 28 cases. Am J Surg Pathol. 2008;32:1060–1067. doi: 10.1097/PAS.0b013e318160edaf. [DOI] [PubMed] [Google Scholar]
- 24.Lotan TL, Epstein JI. Gleason grading of prostatic adenocarcinoma with glomeruloid features on needle biopsy. Hum Pathol. 2009;40:471–477. doi: 10.1016/j.humpath.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iczkowski KA, Ferguson KL, Grier DD, et al. Adenoid cystic carcinoma of the prostate: clinicopathologic findings in 19 cases. Am J Surg Pathol. 2003;27:1523–1529. doi: 10.1097/00000478-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Lotan TL, Epstein JI. Clinical implications of changing definitions within the Gleason grading system. Nat Rev Urol. 2010;7:136–142. doi: 10.1038/nrurol.2010.9. [DOI] [PubMed] [Google Scholar]
- 27.Gopalan A, Leversha MA, Satagopan JM, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosquera JM, Mehra R, Regan MM, et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res. 2009;15:4706–4711. doi: 10.1158/1078-0432.CCR-08-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han B, Suleman K, Wang L, et al. ETS gene aberrations in atypical cribriform lesions of the prostate: implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia. Am J Surg Pathol. 2010;34:478–485. doi: 10.1097/PAS.0b013e3181d6827b. [DOI] [PubMed] [Google Scholar]
- 30.Fine SW, Gopalan A, Leversha MA, et al. TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high-grade morphological features. Mod Pathol. 2010;23:1325–1333. doi: 10.1038/modpathol.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zareba P, Zhang J, Yilmaz A, et al. The impact of the 2005 International Society of Urological Pathology (ISUP) consensus on Gleason grading in contemporary practice. Histopathology. 2009;55:384–391. doi: 10.1111/j.1365-2559.2009.03405.x. [DOI] [PubMed] [Google Scholar]
- 32.Guimaraes MS, Billis A, Quintal MM, et al. The impact of the 2005 International Society of Urological Pathology (ISUP) consensus conference on standard Gleason grading of prostatic carcinoma [abstract] Mod Pathol. 2006;19:139A. [Google Scholar]
- 33.Iczkowski KA, Bostwick DG. Criteria for biopsy diagnosis of minimal volume prostatic adenocarcinoma: analytic comparison with nondiagnostic but suspicious atypical small acinar proliferation. Arch Pathol Lab Med. 2000;124:98–107. doi: 10.5858/2000-124-0098-CFBDOM. [DOI] [PubMed] [Google Scholar]
- 34.Lane BR, Magi-Galluzzi C, Reuther AM, et al. Mucinous adenocarcinoma of the prostate does not confer poor prognosis. Urology. 2006;68:825–830. doi: 10.1016/j.urology.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Osunkoya AO, Nielsen ME, Epstein JI. Prognosis of mucinous adenocarcinoma of the prostate treated by radical prostatectomy: a study of 47 cases. Am J Surg Pathol. 2008;32:468–472. doi: 10.1097/PAS.0b013e3181589f72. [DOI] [PubMed] [Google Scholar]