Abstract
DLG mediates the clustering of synaptic molecules. Here we demonstrate that synaptic localization of DLG itself is regulated by CaMKII. We show that DLG and CaMKII colocalize at synapses, and exist in the same protein complex. Constitutively activated CaMKII phenocopied structural abnormalities of dlg mutant synapses, and dramatically increased extrajunctional DLG. Decreased CaMKII activity caused opposite alterations. In vitro, CaMKII phosphorylated a DLG fragment with a stoichiometry close to one. Moreover, expression of site-directed dlg mutants that blocked or mimicked phosphorylation had effects similar to those observed upon inhibiting or constitutively activating CaMKII. We propose that CaMKII-dependent DLG phosphorylation regulates the association of DLG with the synaptic complex during development and plasticity, thus providing a link between synaptic activity and structure.
INTRODUCTION
The mechanisms by which the complex organization of synaptic junctions develops have been the object of intense inquiry (Sanes and Lichtman, 1999). Such mechanisms must involve not only the formation of multimolecular complexes, which bring together functionally related proteins, but also regulatory processes which allow dynamic assembly and disassembly of these complexes. This dynamic regulation is thought to be essential for developmental, structural, and functional plasticity.
In recent years membrane associated guanylate kinases (MAGUKs), such as the mammalian PSD-95/SAP90, SAP97/hdlg, SAP102/NE-dlg, and Chapsyn-110/PSD93, and the insect DLG, have emerged as synapse organizing elements (Woods and Bryant, 1991; Lahey et al., 1994; Garner and Kindler, 1996; Sheng, 1996; Kennedy, 1997; Gramates and Budnik, 1999). MAGUKs are composed of three PDZ repeats, an SH3 domain, and a guanylate kinase-like (GUK) domain. Cell biological and in vitro studies in mammals, as well as genetic studies in Drosophila, show that MAGUKs function as clustering and synapse-targeting components. Synaptic MAGUK binding partners include Shaker K+-channels, NMDA-type glutamate receptors, SynGAP and neuroligins (Kim et al., 1995; Kornau et al., 1995; Müller et al., 1996; Kim et al., 1998; Irie et al., 1997; Chen et al., 1998). These proteins interact with specific PDZ domains via a carboxy-terminal T/SXV-motif. Binding partners for other MAGUK domains are also beginning to be identified, including kainate receptor subunits for the SH3 domain, and GKAP/SAPAPs for the GUK domain (Kim et al., 1997; Takeuchi et al., 1997; Garcia et al., 1998). Studies at the Drosophila larval NMJ demonstrated that DLG is essential for the development of proper synapse structure and function (Lahey et al., 1994; Guan et al. 1996, Budnik et al., 1996) as well as for the localization and clustering of Shaker K+-channels and the cell adhesion molecule Fasciclin II (FasII) (Tejedor et al., 1997; Thomas et al., 1997b; Zito et al., 1997). FasII is required for the maintenance and plasticity of NMJs (Schuster et al., 1996a,b; Davis and Goodman, 1998). Therefore, the regulated synaptic targeting and localization of its anchoring molecule, DLG, can also be assumed to affect plasticity. However, the requirements for synaptic localization of DLG are mostly unknown.
Recent in vitro studies point to palmitoylation as one mechanism by which the association of PSD-95 with the plasma membrane is controlled (Topinka and Bredt, 1998). The cysteine residues that become palmitoylated in PSD-95 have also been implicated in the head to head multimerization of this MAGUK (Hsueh and Sheng, 1999). These cysteine residues are absent in SAP97 and the fly MAGUK DLG, suggesting that additional mechanisms for the synaptic localization of MAGUKs exist.
Several studies implicate phosphorylation as one mechanism by which the association of proteins with cell junctions is regulated. A classical example is the case of cadherins (Balsamo et al., 1996). Cadherins are Ca++-dependent cell adhesion molecules whose function depends on their association with the actin cytoskeleton. This association is mediated by α-, β-, and γ-catenins (Yap et al., 1997). Tyrosine phosphorylation of β-catenin has been correlated with loss of cadherin mediated adhesion (Balsamo et al., 1996). These studies demonstrate that only non-phosphorylated β-catenin associates with N-cadherin in chick retina. In contrast, the phosphorylation of a tyrosine phosphatase (PTP1B) results in its association with N-cadherin. Either the dephosphorylation of the phosphatase or the tyrosine phosphorylation of β-catenin prevents their association with N-cadherin, and prevents N-cadherin mediated cell adhesion. Thus in this system phosphorylation and dephosphorylation maintain a dynamic equilibrium that regulates the association of β-catenin and the phosphatase with N-cadherin at cell junctions.
Calcium/calmodulin-dependent protein kinase II (CaMKII) is highly concentrated at vertebrate central synapses (Erondu and Kennedy, 1985). The kinase is regulated by intracellular calcium levels and autophosphorylation. At resting conditions (low intracellular calcium), CaMKII is inactive due to the interaction of an autoinhibitory domain with the catalytic part of the enzyme. Peptides corresponding to this autoinhibitory domain can operate as exogenous inhibitors of CaMKII (Soderling, 1993; Griffith et al., 1993). When calcium is elevated, Ca++/calmodulin disrupts the interaction of the autoinhibitory and catalytic domains and allows the kinase to phosphorylate itself and exogenous substrates. In this state, the kinase autophosphorylates a highly conserved threonine residue within the regulatory domain (T286 in mammals and T287 in the fly). This weakens the ability of the autoinhibitory domain to suppress activity and the kinase becomes Ca++-independent with up to 80% of maximal activity (Hanson and Schulman, 1992). This transformation of a transient calcium signal into a longer lasting change in enzyme activity has led to models in which CaMKII is centrally involved in synaptic plasticity (Miller and Kennedy, 1986; Lisman, 1994).
In flies, CaMKII is encoded by a single gene located at chromosome 4 (Cho et al., 1991). Transgenic flies expressing a peptide inhibitor of CaMKII (Griffith et al., 1993; Wang et al., 1994) and flies expressing mutant forms of CaMKII with altered Ca++-dependence (Jin et al, 1998) have been generated and used to implicate CaMKII in behavioral and synaptic plasticity. In this paper, we use these transgenic flies to examine the consequences of altering CaMKII activity on synapse structure.
We find that constitutive CaMKII activation at motorneurons and postsynaptic muscles leads to dramatic changes in synaptic structure, similar to those encountered in dlg mutants. This activation also leads to extrasynaptic DLG localization and consequently to reduced levels of synaptic FasII. Biochemical and anatomical analysis of flies carrying mutations in a conserved CaMKII phosphorylation consensus sequence in a dlg transgene suggest that the changes in synaptic structure are mediated by direct phosphorylation of DLG. We propose a model by which CaMKII-dependent phosphorylation of DLG is responsible for regulating the association of DLG with the synaptic complex, and thereby its ability to cluster and target synaptic components. This model provides a mechanism for the dynamic regulation of synapse assembly by neuronal activity.
RESULTS
DLG and CaMKII are associated in a synaptic protein complex
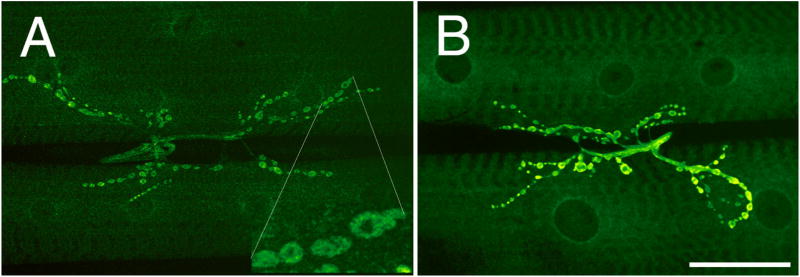
We used antibodies against Drosophila CaMKII to determine its distribution at the larval NMJ. CaMKII was localized at wild type NMJs around type I synaptic boutons in a pattern similar to DLG (Fig. 1A). Double labeling with anti-DLG revealed that DLG and CaMKII were colocalized at bouton borders (Fig. 1A, A1, A2). In addition, transgenic CaMKII was targeted to synaptic boutons, as visualized by increased immunoreactivity levels at NMJs upon expression of a CaMKII transgene in both motorneurons and muscles by using Gal4 activators (Brand and Perrimon, 1993) BG487 (muscle specific) and C380 (motorneuron-specific) (Fig. 1B; Budnik et al., 1996)
Figure 1.
CaMKII immunoreactivity at the NMJ. (A) Confocal image of NMJs at muscles 6 and 7 of a wild type third instar NMJ stained with anti-CaMKII. Inset A1 is a high magnification view of a subset of type I boutons. The same boutons are shown in A2, but superimposed to include anti-DLG signal (red). Note that DLG and CaMKII colocalize at the bouton border. (B) Confocal image of CaMKII immunoreactive boutons at NMJs of larvae overexpressing a wild type CaMKII transgene at both muscles and presynaptic motorneurons using the Gal4 lines BG487 and C380. (C) Coimmunoprecipitation of DLG from body wall muscle extracts of wild type (lane 1), wild type expressing CaMKII (R3 isoform; lane 2), dlgX1-2 (lane3), wild type expressing CaMKII-T287D (lane 4), and dlgX1-2 expressing transgenic DLG (lane 5) using anti-CaMKII. In lane 2, anti-CaMKII antibody has been substituted by preimmune serum (NIS). Molecular weights are in Kd. Bar is 45 μm in A, B, and 15 μm in A1 and A2.
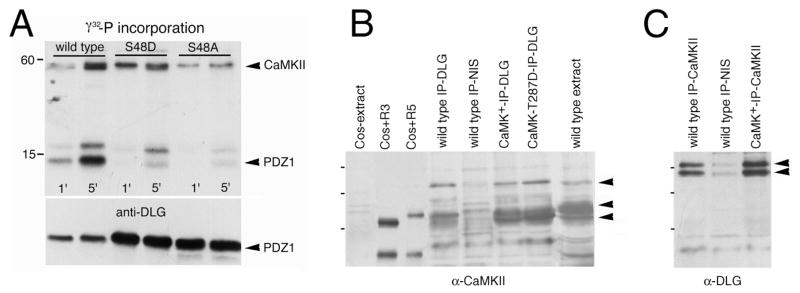
CaMKII and DLG colocalization was further supported by immunoprecipitation experiments, which demonstrated that both proteins exist in the same complex in body wall muscles (Fig. 1C). When anti-CaMKII was used for immunoprecipitations of body wall muscle extracts, DLG coimmunoprecipitated from wild type, CaMKII+, or CaMKII-T287D overexpressing extracts (Fig. 1C; lanes 1, 3,4), but not from extracts immunoprecipitated with preimmune serum (lane 2). Moreover, transgenic DLG expressed in dlgX1-2 mutants, was also coimmunoprecipitated by CaMKII (lane 5). Thus, DLG and CaMKII are closely associated at type I NMJs.
Constitutive activation of CaMKII alters synaptic bouton structure
To determine the role of CaMKII in the development of NMJ structure, we modified CaMKII activity in subsets of motorneurons and muscles by Gal4-targeted expression of mutant forms of CaMKII (Jin et al., 1998; Wang et al., 1998). In one of these variants, substitution of a threonine at position 287 by aspartate (CaMKII-T287D) mimics autophosphorylation rendering the enzyme constitutively active even in the absence of Ca++. In another variant, substitution of the same threonine residue by alanine (CaMKII-T287A) blocks autophosphorylation making the kinase exclusively Ca++-dependent. We also partially inhibited endogenous CaMKII activity by expression of an autoinhibitory peptide (ala; Griffith et al., 1993).
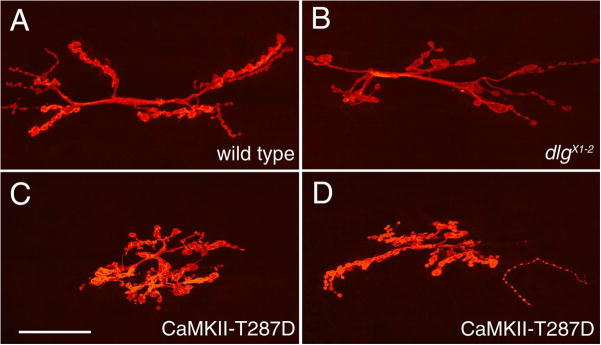
Synaptic structure was dramatically altered in flies expressing CaMKII-T287D at both pre- and postsynaptic sites (Fig. 2). Unlike wild type NMJs (Fig. 2A), NMJs from CaMKII-T287D larvae had enlarged synaptic boutons, and the distribution pattern of these boutons over the muscle cells was abnormal (Fig. 2C, D). In wild type, type I synaptic boutons appear as beads on a string; each bouton is connected to the next by a short neuritic process (Fig. 2A). In CaMKII-T287D larvae, synaptic boutons were conglomerated in a smaller area, and the beaded appearance of the junction was less evident (Fig. 2C, D). In contrast, expression of wild type CaMKII, CaMKII-T287A, or ala had no significant effect (not shown).
Figure 2.
The morphology of NMJs and their distribution are altered in larvae expressing constitutively active CaMKII. (A–D) Confocal images of NMJs at muscles 6 and 7 stained with anti-HRP in (A) wild type, (B) a dlgX1-2 mutant, and (C, D) larvae expressing a constitutively active (CaMKII-T287D) transgene. Note that the size and shape of synaptic boutons, as well as the overall pattern of NMJ branching is altered in CaMKII-T287D NMJs. (E–H) Confocal images of NMJs stained with anti-FasII antibodies in (E) wild type, (F) a dlgX1-2 mutant, (G) a larva expressing constitutively active (CaMKII-T287D) transgene, and (H) a larva expressing a wild type CaMKII transgene. Note that both in CaMKII-T287D and dlgX1-2 mutants, FasII immunoreactivity is low and delocalized. Bar is 40 μm in A–D, and 10 μm in E–H.
The cell adhesion molecule Fas II is highly concentrated at type I boutons (Fig. 2 E; Schuster et al., 1996a; Thomas et al., 1997b). The distribution of FasII was also altered in CaMKII-T287D larvae. Similar to the situation at dlgXI-2 mutant NMJs (Thomas et al., 1997b), FasII signal was low and dispersed in an abnormally wide area around boutons (Fig. 2G). This phenotype was not observed when wild type CaMKII was overexpressed at these NMJs (Fig. 2H).
Constitutive activation of CaMKII alters synaptic bouton structure in a manner similar to mutations in dlg
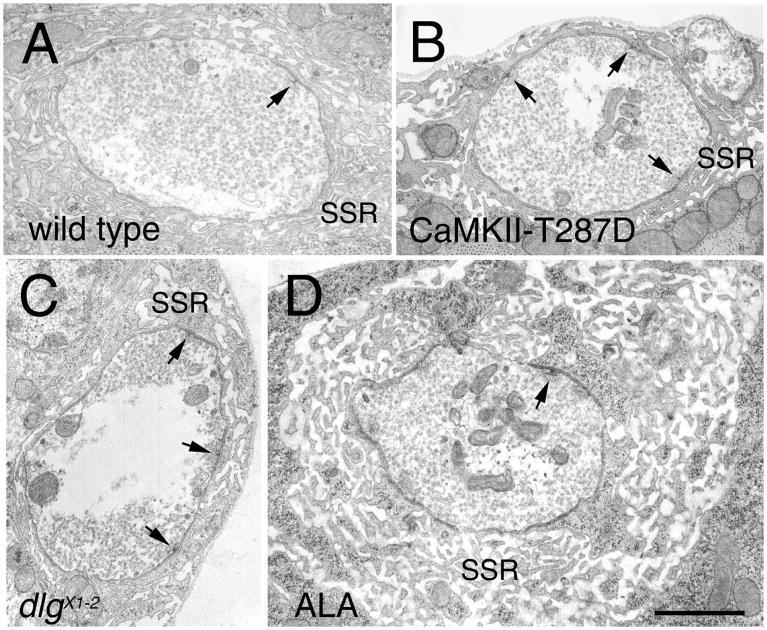
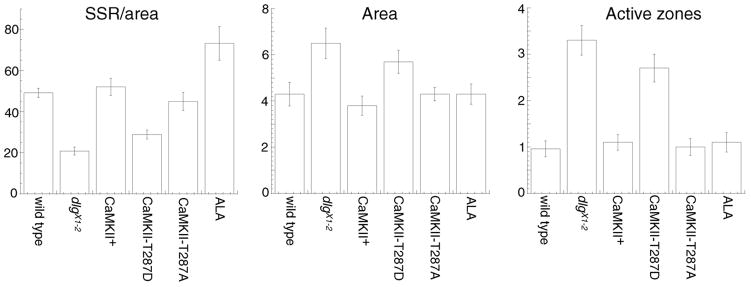
Intriguingly, the structural defects observed at NMJs expressing constitutively active CaMKII were reminiscent to those observed in severe dlg hypomorphic mutants such as dlgX1-2. These dlg mutants also show enlarged boutons, abnormal NMJ arbors, and defects in the clustering of FasII (Fig. 2B, F; Thomas et al., 1997a,b). To determine the extent of the similarity between the phenotypes of CaMKII-T287D and dlg mutants, we examined the boutons at the EM level (Fig. 3). Previous studies demonstrated that dlgX1-2 mutants have an abnormal organization of synaptic boutons, with the junctional membrane (subsynaptic reticulum —SSR) markedly reduced, a 3–4 fold increase in the number of active zones, and an increase in the size of synaptic boutons (Fig. 3B; Lahey et al., 1994; Budnik et al., 1996; Thomas et al., 1997b). All these phenotypes were clearly present in CaMKII-T287D larvae (Fig. 3C). This was confirmed by morphometric analysis of synaptic boutons (Fig. 4). In CaMKII-T287D the normalized cross-sectional SSR length (cross-sectional SSR length/cross-sectional bouton area) was 28.9±2.2 μm−1— 40% smaller than in wild type (49.2±2.0 μm−1; p<0.0001) or larvae expressing wild type CaMKII control (52.1±4.2 μm−1; p<0.0001). This reduction is similar to that in dlgX1-2 mutants (20.8±1.9 μm−1; Fig. 4). In addition, the number of active zones was increased by 3 fold and the area of the boutons was significantly larger (38%) in CaMKII-T287D type I boutons (Fig 4).
Figure 3.
Ultrastructure of type I synaptic boutons in strains with altered CaMKII activity. The micrographs are cross-sections through bouton midlines examined by TEM in (A) wild type, (B) in a dlgX1-2 mutant, (C) in a strain expressing constitutively active CaMKII (CaMKII-T287D), and (D) in larvae expressing the CaMKII inhibitory peptide ala. Note that as in dlg mutants, CaMKII-T287D larvae have a reduced SSR and an increased number of active zones (arrows). In contrast, synaptic boutons from larvae expressing ala show an overdeveloped SSR. Bar is 0.7 μm.
Figure 4.
Morphometric analysis of type I synaptic boutons in larvae with altered CaMKII activity. (A) Normalized cross-sectional SSR length at the bouton midline (cross-sectional SSR length/cross-sectional bouton area). (B) Cross-sectional bouton midline area. (C) Number of active zones at the bouton midline. Number of boutons analyzed and method for quantification are specified in the Methods section.
The EM analysis also revealed that larvae expressing the ala peptide, in which endogenous CaMKII activity is reduced, had a significant alteration in bouton ultrastructure (Fig. 3D), even though gross morphological differences were not observed at the light microscopic level (but see Table 1). In these boutons the SSR appeared more extensive than wild type, measuring 73.2±8.2 μm−1— 49% longer than wild type (Fig. 4; p<0.001). However, the number of active zones in ala type I boutons was similar to wild type. A similar increase in SSR size has been observed when DLG is overexpressed in muscles (Budnik et al., 1996). Thus, constitutive activation of CaMKII mimics many aspects of the structural defects found at severe dlg mutant synaptic boutons. Moreover, reduced levels of CaMKII activity lead to an effect that is similar to that of overexpressing DLG at the NMJ — an overdeveloped SSR (Budnik et al., 1996).
Table 1.
Intensity of DLG immunoreactivity at type I boutons
| genotype parameter | wild type | CaMKII+ | ala | CaMKII-T287A | CaMKII-T287D |
|---|---|---|---|---|---|
| %Imax | 85.8±1.3 | 86.2±6.7 | 102.0±1.4 | 99.7±2.6 | 66.4±1.0 |
| N | 119 | 97 | 93 | 57 | 99 |
%Imax: % of maximum intensity on a relative, linear scale of 0–255
N: number of boutons scored
Constitutive CaMKII activation leads to abnormal localization of DLG at the synaptic membrane
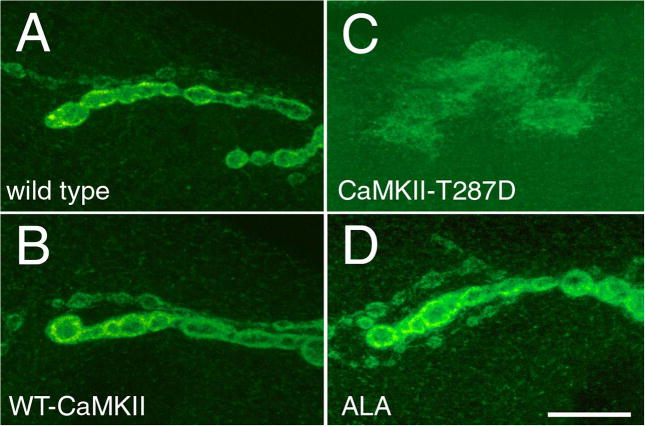
The above observations, together with the demonstration that CaMKII and DLG exist in the same complex suggested that CaMKII might be involved in the DLG-dependent regulation of synapse structure. This possibility was further explored by examining the distribution of DLG at NMJs of larvae with altered CaMKII. Figure 5 shows confocal images of NMJs in larvae expressing wild type and mutant CaMKII forms, obtained using identical confocal settings. In wild type and in larvae expressing wild type CaMKII, DLG is distributed at the border of synaptic boutons (Fig. 5A, B). Previous immuno-EM examination shows that this pattern corresponds to both pre- and postsynaptic expression (Lahey et al., 1994). In contrast, in transgenic larvae expressing constitutively active CaMKII at both pre- and postsynaptic sites, DLG signal around synaptic boutons was reduced and there was a substantial increase in immunoreactivity at extrasynaptic regions (Fig. 5D,E). Extrasynaptic DLG was most intense at the perisynaptic region, but was evident throughout the muscle membrane and cytoplasm. Thus constitutive activation of CaMKII results in the dissociation of DLG from the synaptic complex.
Figure 5.
The synaptic distribution of DLG is altered in NMJs with altered CaMKII activity. (A–E) Confocal images of NMJs stained with anti-DLG in (A) wild type, (B) NMJs expressing a wild type CaMKII transgene, (C) NMJs expressing the ala peptide, and (D, E) NMJs expressing the CaMKII-T287D transgene at low (D) and high (E) magnification. (F) Anti-HRP staining of the same preparation in (E) to show the presynaptic boutons. Note that DLG signal appears diffuse in CaMKII-T287D larvae and with an increase of signal in extrasynaptic regions. Also note that the intensity of the signal is increased in ala larvae. Bar is 15 μm in A–C and E–F, and 45 μm in D.
An opposite phenotype was observed when CaMKII activity was inhibited by expressing the ala peptide (Fig. 5C). At these NMJs DLG immunoreactivity was consistently increased at synaptic boutons with respect to wild type and larvae expressing wild type CaMKII controls. In addition, no increase in extrajunctional DLG was observed (Table 1). Taken together, the delocalization of DLG by constitutive activation of CaMKII, and the increased localization of DLG by inhibiting CaMKII activity, may explain the phenotypic similarity between CaMKII-T287D and dlg mutant NMJs, and between ala and NMJs overexpressing DLG.
Unlike the case of DLG, extrasynaptic FasII was low. This may be due to the much lower levels of FasII compared to DLG, which may prevent its visualization when it is not concentrated in a small area.
DLG is phosphorylated by CaMKII in vitro and mutations in dlg that block or mimic phosphorylation affect DLG distribution in vivo
The changes in synaptic bouton structure, together with the changes in DLG distribution in larvae with altered CaMKII activity, are consistent with the hypothesis that CaMKII phosphorylation regulates the association of DLG with the synapse, and therefore its clustering function. This regulation may be the result of direct phosphorylation of DLG by CaMKII. To test this hypothesis, we performed in vitro phosphorylation assays. In addition, we examined the in vivo distribution of transgenic DLG variants in which a putative CaMKII phosphorylation site is missing, or whose phosphorylation is mimicked.
Several CaMKII phosphorylation consensus sequences are distributed throughout the DLG protein. However, a perfectly conserved site in all MAGUKs is a RGNS motif present at the beginning of PDZ1. We therefore generated purified DLG fragments spanning PDZ1 (amino acids 31 to 102) or PDZ1-2 (amino acids 39 to 251), as well as a fragment spanning the GUK domain (amino acids 764 to 960; Woods and Bryant, 1991), which contains a non-conserved CaMKII site, for in vitro phosphorylation assays using recombinant fly CaMKII (R3 isoform; Griffith and Greenspan, 1993). Moreover, we used site directed mutagenesis to substitute the conserved target serine (S48) to alanine (S48A) or aspartate (S48D) to block phosphorylation. We found that PDZ1 and PDZ1-2 fragments were indeed phosphorylated by fly CaMKII (Fig. 6) with a stoichiometry of 0.6 and 0.95 mol phosphate/mol DLG fragment respectively. Moreover, substitution of S48 by alanine or aspartate virtually eliminated phosphorylation. In contrast, the GUK domain fragment was not phosphorylated by CaMKII (not shown).
Figure 6.
CaMKII phosphorylates DLG in vitro. Phosphate incorporation into PDZ1 fragments after 1 and 5 minutes of CaMKII phosphorylation reaction (TOP). Note that wild type PDZ1 is phosphorylated by CaMKII, and that phosphorylation is virtually eliminated in PDZ1 mutant fragments in which S48 has been substituted by A or D. The band above PDZ1 is a contaminant as demonstrated by immunoblotting the same gel with anti-DLG antibody (BOTTOM).
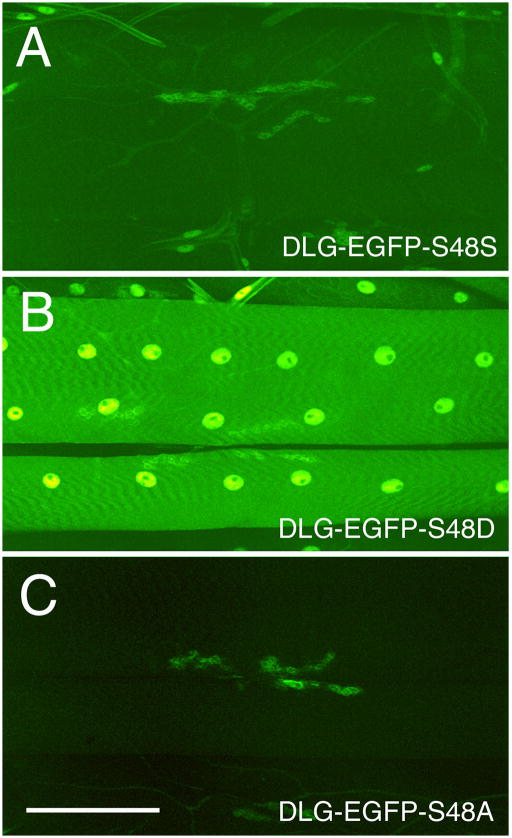
The above results show that CaMKII is capable of directly phosphorylating DLG. To further test the hypothesis that the changes in DLG distribution observed in larvae with altered CaMKII activity are due to direct phosphorylation of DLG, we generated transgenic flies carrying dlg transgenes tagged with enhanced green fluorescent protein (eGFP). Three different variants of the eGFP-dlg transgene were used, wild type (eGFP-dlg-S48S), a mutant form in which S48 was substituted by aspartate to mimic phosphorylation (eGFP-dlg-S48D), and a mutant form in which S48 was substituted by alanine to block phosphorylation (eGFP-dlg-S48A). These dlg transgenes were expressed in dlgX1-2 mutant body wall muscles using the BG487 or the daughterless (da) Gal4 drivers (Thomas et al., 1997a). The localization of DLG at the body wall muscles was directly visualized as GFP fluorescence in fixed preparations simultaneously processed for eGFP imaging. Figure 7 shows images of eGFP fluorescence at NMJs which were obtained by confocal microscopy using the same settings for each genotype. We found that the eGFP-DLG-S48S signal was strongly localized at synaptic boutons (Fig. 7A). In addition, very weak, but detectable fluorescence was observed along the muscle membrane and cytoplasm (Fig. 7A, B). In striking contrast, eGFP-DLG-S48D fluorescence was comparatively weak and diffusely localized at synaptic boutons but strong in extrasynaptic regions of the muscle surface and cytoplasm (Fig. 7C). The localization of eGFP-DLG-S48A also differed somewhat from wild type eGFP-DLG. In eGFP-DLG-S48A, fluorescence was strong at synaptic boutons but virtually undetectable over the muscle surface (Fig. 7D). Similar results were observed with two or more independent transformant strains for each of the eGFP-dlg transgenes. The difference in DLG distribution in the eGFP-DLG variants was not likely to result from large differences in the level of expression of the transgenes. Western blot analysis of body wall muscle extracts probed with anti-GFP or anti-DLG antibodies revealed that the strains had comparable levels of the eGFP-DLG protein (Fig. 7G). Thus, mimicking DLG phosphorylation changes the distribution of DLG from primarily synaptic to extrasynaptic, and blocking phosphorylation strongly restricts DLG localization to the synaptic region.
Figure 7.
Distribution of DLG at NMJs expressing wild type or mutant eGFP-dlg transgenes containing mutations in S48. (A–F) eGFP fluorescence at NMJs expressing (A, B) eGFP-DLG-S48S, (C) eGFP-DLG-S48D, (D) eGFP-DLG-S48A, (E) eGFP-DLG-S48S and CaMKII-T287D, and (F) eGFP-DLG-S48D and CaMII-T287D. Note that in wild type fluorescence is concentrated at NMJs, while in eGFP-DLG-S48D flies, fluorescence is strong in muscle but is still detected, although diffuse at NMJs. In eGFP-DLG-S48A all of the eGFP signal is observed at the NMJ, with virtually no detectable signal at muscle. Compare to (B) (G) Western blot analysis of eGFP-DLG protein expressed in wild type body wall muscle extracts, and probed with anti-GFP (top) or anti-DLG (bottom) antibodies. Transgenic eGFP-DLG bands are indicated by the green arrowheads, and can be distinguished from endogenous DLG (black arrowhead) because of the increased molecular weight due to the eGFP fusion. Note that the three transgenic stains (eGFP-DLG-S48S, eGFP-DLG-S48A, and eGFP-DLG-S48D) show comparable amounts of transgenic protein, although eGFP-DLG-S48D shows slightly higher levels. Bar is 12 μm in A, B, and 140 μm in C, D. Molecular weights are in Kd. (H) Model of the regulation of synaptic DLG distribution by CaMKII-dependent phosphorylation. Dephosphorylated DLG associates with the synaptic complex and clusters binding partners such as FasII and Shaker. However, upon CaMKII-dependent phosphorylation, DLG dissociates from the synaptic complex and becomes unable to cluster synaptic proteins to the synapse.
A prediction of this model is that constitutive CaMKII activation should not change the synaptic distribution of eGFP-DLG-S48A. Moreover, eGFP-DLG-S48A should rescue the synaptic abnormalities observed at both dlg mutants and at larvae expressing constitutively active CaMKII. We therefore co-expressed CaMKII-T287D together with eGFP-DLG-S48A or eGFP-DLG-S48S in dlgX1-2 mutant larvae. As predicted, eGFP-DLG-S48A was found in tight association with the synaptic membrane even when CaMKII-T287D was simultaneously expressed (Fig. 7F). In contrast, an intermediate phenotype was observed when CaMKII-T287D and eGFP-DLG-S48S were co-expressed (Fig. 7e). Although some of the eGFP signal was found to be associated with synapses, a considerable portion was also found dispersed around the boutons and on the muscles. These observations provide compelling evidence for specific CaMKII-dependent regulation of DLG localization at the NMJ.
EM analysis of dlgX1-2 synapses expressing eGFP-DLG-S48A demonstrated that this dlg transgene completely rescued the ultrastructural defects (Fig. 4). Consistent with its unaffected localization, eGFP-DLG-S48A retained its rescue activity when CaMKII-T287D was simultaneously expressed at postsynaptic muscles of dlgX1-2 (Fig. 4).
DISCUSSION
An important property of synapses is their dynamic regulation during development and plasticity. Therefore, a deep understanding of how synapses are organized must involve the identification of regulatory mechanisms that allow assembly and disassembly of synaptic components. CaMKII has been implicated in activity dependent regulation of synaptic plasticity (Lisman, 1994). Particularly relevant is its ability to transform a transient change in Ca++ levels into a long-lasting change in kinase activity. Substrates of CaMKII include proteins that function in synaptic plasticity, such as transmitter receptors, synaptic vesicle proteins, and signal transduction components (e.g. Bahler and Greengard, 1987; Greengard et al., 1993; Roche et al., 1996; Barria et al., 1997; Chen et al., 1998). In this paper we have established a close functional link between changes in CaMKII activity and the involvement of the Drosophila MAGUK DLG in the process of synapse assembly. MAGUKs of the PSD-95 and DLG family have emerged in the past few years as major players in targeting and clustering of synaptic proteins (Garner and Kindler, 1996; Sheng, 1996; Gramates and Budnik, 1999). In this paper we used a mutational and transgenic expression approach to identify a mechanism for the dynamic association of DLG with the synaptic complex. We showed that CaMKII is highly enriched at NMJs, and that changes in its activation state lead to modification of synaptic structure and composition. Most significantly, we demonstrated that constitutive activation or partial inhibition of CaMKII results in opposite alterations in DLG distribution, and consequently in changes in synapse structure. Several lines of evidence that are consistent with a role of CaMKII in DLG phosphorylation further substantiated this finding. Together these results demonstrate that the association of DLG with the synaptic complex is regulated by CaMKII-dependent phosphorylation, and provide a novel mechanism for the regulation of synapse structure during development and plasticity.
Synaptic DLG localization is regulated by CaMKII-dependent phosphorylation
Constitutive activation or partial inhibition of CaMKII elicited opposite changes in synaptic structure. This was most clearly demonstrated by examining the size of the postsynaptic junctional membrane — the SSR. Constitutive CaMKII activation resulted in poorly developed SSR, while CaMKII inhibition resulted in an overdeveloped SSR. These alterations phenocopied previously described effects of changing DLG levels at the synaptic membrane. Our previous studies revealed that mutations in dlg resulted in a poorly developed SSR, while overexpressing wild type DLG resulted in an overdeveloped SSR (Lahey et al., 1994; Budnik et al., 1996). The similarity between the phenotypes elicited by constitutive activation of CaMKII and those observed in dlg mutants was not solely restricted to the morphology of the SSR. We also observed an increase in the number of active zones, an enlargement of the boutons, and an abnormal FasII clustering around these boutons (Thomas et al., 1997b). Thus, these results strongly suggest that the changes in synaptic structure and composition elicited by changing CaMKII activity were the result of changes in DLG distribution.
In this paper, two approaches were used to demonstrate that CaMKII activity changes the synaptic localization of DLG. Constitutive activation of CaMKII, or a point mutation that mimicked CaMKII phosphorylation of DLG, both resulted in a significant increase in extrasynaptic DLG. The similar localization of DLG and CaMKII at synaptic boutons, the in vitro phosphorylation assays, the coimmunoprecipitation of CaMKII by DLG, and the in vivo analysis of DLG distribution in larvae carrying transgenic DLG in which the conserved CaMKII phosphorylation site is either blocked or mimicked, all support the idea that DLG is directly phosphorylated by CaMKII in vivo. However, the participation of an intermediate kinase which is directly or indirectly activated by CaMKII cannot be completely ruled out.
Phosphorylation of mammalian MAGUKs by CaMKII has not been demonstrated. However, the S48 CaMKII consensus site in DLG is conserved in all MAGUKs described so far, supporting the notion that this may be a functionally essential site. This site is at the beginning of PDZ1, raising the possibility that its phosphorylation may also affect the binding of PDZ-associated partners. Interestingly, we have found that simultaneous expression of CaMKII-T287D and eGFP-SAP 97 to larval NMJs also induces delocalization of SAP97 (Y.-H. Koh, U. Thomas, and V. Budnik, unpublished).
In mammals, expression of constitutively active CaMKII and loss of PSD-95 function appear to yield divergent effects on long-term plasticity. Constitutive CaMKII activation in mouse forebrain resulted in a loss of hippocampal LTP, and defects in spatial memory and fear conditioning (Silva et al 1992; Mayford et al., 1996). In contrast, a mutation in PSD-95 that gives rise to a truncated protein resulted in a decreased threshold for LTP (Migaud, et al., 1998). Intriguingly, a similar mutation in dlg (dlgm52) leads to an increase in neurotransmitter release, and other dlg mutations (dlgX1-2) increase the number of active zones (Budnik et al., 1996; Thomas et al., 1997b).
DLG may link CaMKII-activity to adhesion-dependent structural plasticity at the NMJs
What is the significance of our finding with regard to development and plasticity of glutamatergic fly synapses? Drosophila larval NMJs are continuously expanding during the larval stages due to an exponential increase in muscle fiber size (Gorczyca et al., 1993; Keshishian et al., 1993; Guan et al., 1996). The expansion is affected both by levels of activity as well as by cell adhesion (Budnik et al., 1990; Schuster et al., 1996a, b). Mutations that increase the level of electrical activity result in NMJs that are larger, contain an elevated number of synaptic boutons, and exhibit more elaborate NMJ branching (Budnik et al., 1990). These activity-dependent changes in NMJ expansion are tightly regulated by FasII, a cell adhesion molecule that requires DLG for proper synaptic localization (Thomas et al., 1997b; Zito et al., 1997). Mutations that dramatically reduce FasII levels lead to poorly elaborated NMJs which contain fewer boutons, presumably due to the inability of many synapses to be maintained (Schuster et al., 1996a). In contrast, moderate reduction in FasII level lead to larger than normal NMJ expansion. These observations have led to a model in which the degree of NMJ expansion is dependent on FasII-mediated adhesion. According to this model, enhanced cell adhesion maintains stable synapses that are restricted in their ability to grow. Nonetheless, when FasII levels are decreased, this restrictive influence is at least partially lifted, and the synaptic arbor can expand.
The studies of Schuster et al. (1996b) show that increased levels of activity result in a decrease of FasII at the synaptic membrane. This decrease in FasII could explain the more elaborated NMJs observed in mutants with increased electrical activity (Budnik et al., 1990; Schuster et al., 1996b). Similarly in Aplysia, long-term sensitization of the gill and siphon withdrawal reflex results in endocytosis of apCAM, a FasII homolog expressed on sensory neurons (Mayford et al., 1992). This internalization of apCAM is correlated with an increase in the size of the sensory arbors, the number of synaptic contacts, and the strength of the synaptic connection (Bailey and Kandel, 1993; Abel et al., 1998).
How is FasII-dependent regulation of synaptic plasticity at the NMJ accomplished? Previous studies demonstrated that the synaptic localization of FasII depends on DLG on one hand, and on electrical activity on the other. In this paper we provide a mechanism by which synaptic activity can impinge upon changes in FasII localization through CaMKII-dependent alterations of DLG.
We propose that the anchoring of DLG at the synapse is optimal when DLG is in the dephosphorylated state. Upon phosphorylation DLG is less restricted to the synaptic complex (Fig. 7H). Because DLG is essential for proper synaptic localization of FasII and Shaker (Tejedor et al., 1997; Thomas et al., 1997b), these binding partners are similarly free to move away from the synaptic complex upon CaMKII-dependent phosphorylation. An alternative, though not mutually exclusive possibility is that CaMKII dependent phosphorylation of DLG affects its targeting to the synapse during development. However, in our studies we find that CaMKII and DLG colocalize at synapses. Therefore it is likely that CaMKII-dependent phosphorylation of DLG occurs at the synapse.
Schuster et al.(1996b) have shown that synaptic FasII is down-regulated by increased cAMP levels, suggesting that PKA is also involved in coupling synaptic activity to structural plasticity at NMJs. Both PKA and CaMKII can be activated by various synaptic stimuli. Whether both signal transduction pathways act together or in parallel to regulate DLG-dependent localization of FasII remains to be determined.
METHODS
Flies
The following fly strains were used in these studies. (1) UAS-CAMKII strains (UAS-CaMKII+, UAS-CaMKII-T287D, UAS-CaMKII-T287A, UAS-ala; described in Griffith et al., 1993, Jin et al., 1998, and below). (2) UAS-eGFP-DLG strains (UAS-eGFP-DLG-S48S, UAS-eGFP-DLG-S48D, UAS-eGFP-DLG-S48A; described below). (3) Gal4 driver strains (BG487, C380, and C57 (Budnik et al., 1996), da-Gal4 (Thomas et al., 1997a)). (4) Mutations in dlg (dlgX1-2; dlgm52, described in Tejedor et al., 1997, Thomas et al., 1997a).
Immunocytochemistry and transmission electron microscopy (TEM)
The immunocytochemical procedure is described in Thomas et al. (1997b). The following antibodies were used. (1) Rabbit or rat anti-DLG-PDZ (see below; 1:40,000 and 1:1,000 respectively). (2) Anti-CaMKII (1:4,000). (3) anti-FasII polyclonal (see below; 1:4,000). (4) Anti-HRP (Sigma; 1:400). (5) Secondary antibodies (Jackson labs; 1:200). To visualize eGFP signal, body wall muscles were fixed for 45–60 min with 4% paraformaldehyde in 0.1 M Phosphate buffer, pH 7.2. Samples were then washed for 10 min in PBT (0.1M phosphate buffer, pH 7.2, 0.2% Triton X-100), and mounted using Vectashield (Vector Labs). The intensity of anti-DLG signal at synapses was determined as in Thomas et al. (1997b) using the NIH Image program (version 1.67). Larvae used for intensity analysis were processed simultaneously for immunocytochemistry and confocal images were acquired under identical conditions. Briefly, a line (L) beginning from the center of a synaptic bouton and extending through the outer limit occupied by DLG immunoreactivity was traced. Then, the maximum intensity along L (on a linear relative scale of 0 to 250) was determined using the plot profile function of NIH Image. Four measurements were taken for each bouton, averaged, and expressed as a percentage of maximum relative intensity.
The procedures for transmission electron microscopy and morphometric analysis of synaptic boutons are extensively described in Budnik et al. (1996) and Thomas et al. (1997b). For this analysis synaptic boutons from muscles 6 and 7 (segments A2) were serially sectioned, and the section of largest area (defined as the bouton midline) used for quantitative analysis. To determine SSR length, electron micrographs were printed at 30,000X, the SSR traced, scanned, and analyzed using NIH Image. The function “Analyze particles” of NIH Image was used to determine the length of SSR segments, which were then added to obtain the total cross-sectional SSR length. To determine the cross-sectional area of the boutons, the presynaptic terminal (at the bouton midline) was traced as above, and the area determined by using NIH Image software. The number of active zones was determined by counting the number of electron dense “presynaptic T-bars” at the bouton midline. At least 4 different preparations for the morphometric analysis of each genotype were used. The number of synaptic boutons analyzed in each case are 12 boutons in wild type (Canton-S), 19 boutons in dlgX1-2, 17 boutons in CaMKII+ larvae, 23 boutons in CaMKII-T287D larvae, 22 boutons in CaMKII-T287A larvae, 18 boutons in ala larvae, 18 boutons in dlgX1-2 eGFP-dlg-S48A, and 11 boutons in dlgX1-2 eGFP-dlg-S48A CaMKIIT287D.
Site directed mutagenesis, generation of transgenes, and germline transformation
PDZ1 and PDZ1-2 DLG fragments were generated by PCR using a dlg-A cDNA as a template, and subcloned into the pET expression vector (Novagen). To generate mutations in the putative CaMKII phosphorylation site the Quickchange site-directed mutagenesis Kit (Stratagene) was used according to manufacturer instructions. Upon IPTG-induced expression of plasmid constructs in E. coli BL21, purified recombinant proteins were obtained by affinity chromatography on a Ni2+ resin column (Novagen). The UAS-CAMKII+ strain was generated by cloning the gene for the wild type R3 isoform of CaMKII into the polylinker of the pUAST vector (Brand and Perrimon, 1993).
To generate eGFP-dlg constructs, PCR fragments comprising bp −7 to 894 of the dlg coding region were generated using dlg-A cDNA (Woods and Bryant, 1991) or its point-mutated derivatives (see above) as a template. The upstream primer was designed to allow for in-frame HindIII-linkage of the dlg PCR fragment to the 3′-end of the eGFP coding region within the vector pEGFP-C1 (Clontech). All PCR products were verified by sequencing. An internal SalI-site at bp 883 was used to ligate the PCR-derived fragment of dlg -A to the remaining downstream region of the cDNA. The eGFP-dlg fusion genes were then cloned as NheI-XbaI fragments into the polylinker of pUAST. Transgenic flies were generated by P-element mediated germline transformation of w1118 flies (Spradling, 1986) using the Δ2–3 “turbo” helper plasmid as a source for transposase.
Immunoprecipitations
Coimmunoprecipitations were performed essentially as described in Thomas et al. (1997b). Briefly, 10 dissected body wall muscle preparations (consisting of body wall muscles and CNS) were homogenized in 100 μl RIPA buffer containing protease inhibitors at 4°C. After centrifugation at 3,000 g for 5 min, the supernatant was precleared with preimmune serum and protein A+G beads for 1 hr. The cleared homogenate was then incubated with rabbit anti-CaMKII antibody (5μl crude serum) at 4°C for one hour. Immunoprecipitates were collected with protein A-sepharose, separated in a 7.5% SDS PAGE gel and immunoblotted with rat anti-DLG antibodies (1:2000). Bands were visualized with peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents (Amersham).
Phosphorylation assays
Purified recombinant fly CaMKII (R3 isoform) was purified as described in GuptaRoy and Griffith (1996). About 100 ng of purified PDZ1 or PDZ1-2 fragments were phosphorylated in 20 μl reaction mixture (50 mM PIPES buffer, pH 7, 1 mM CaCl2, 15 mM MgCl2, 10 μg/ml bovine calmodulin, 50 μM γ32-P-ATP (1 Ci/mmol) and 15 ng of purified CaMKII. The reaction was started by adding radioactively labeled ATP, and was allowed to proceed for 1–5 min at 30°C and stopped by boiling in sample buffer. The amount of phosphate incorporated was measured with a Phosphorimager after running the reaction on a 15% SDS-PAGE gel. The stoichiometry of the reaction was determined by allowing the reaction to proceed for 1 hr at 30°C in the presence of excess kinase to saturate the reaction. The number of moles of phosphate transferred per mole of DLG was calculated based on a γ32-P-ATP standard curve.
Generation of polyclonal antibodies
To generate anti-DLG and anti-FasII polyclonal antibodies, either PDZ1-2 of DLG (amino acids #39–251), or the last 90 amino acids of the transmembrane FasII splice form (Thomas et al., 1997b) was obtained by PCR using a dlg-A cDNA or a FasII cDNA as a template. Fragments were subcloned into the pET expression vector, and recombinant protein purified as above used for immunization of rabbits or rats. The specificity of the sera was determined by Western blot analysis of recombinant protein and body wall muscle extracts. Anti-CaMKII serum was generated by using a GST fusion protein containing the catalytic domain of Drosophila CaMKII. Rabbits were boosted with a histidine tagged R3 catalytic domain.
Acknowledgments
We thank Xiaomeng Long for generating the CaMKII antibody, Michael Gorczyca and Mary Packard for critical reading of the manuscript and helpful discussions, and Mary Packard for help with EM. We also thank the EM Facility and the BCRC at the University of Massachusetts. Supported by NIH grants RO1 NS30072 and KO4 NS01786 to V.B., and R01 GM54408 and K02 NS01897 to L.C.G. U.T. was supported by the Max Kade Foundation.
References
- Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes; inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Balsamo J, Leung T, Ernst H, Zanin MK, Hoffman S, Lilien J. Regulated binding of PTP1B-like phosphatase to N-cadherin: control of cadherin-mediated adhesion by dephosphorylation of beta-catenin. J Cell Biol. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptor by CaMKII during long term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axon terminals in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene, dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Cho KO, Wall JB, Pugh PC, Ito M, Mueller SA, Kennedy MB. The alpha subunit of type II Ca2+/calmodulin-dependent protein kinase is highly conserved in Drosophila. Neuron. 1991;7:439–450. doi: 10.1016/0896-6273(91)90296-c. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity:homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol. 1998;8:149–156. doi: 10.1016/s0959-4388(98)80018-4. [DOI] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Garner C, Kindler S. Synaptic proteins and the assembly of synaptic junctions. Trends Cell Biol. 1996;6:429–433. doi: 10.1016/s0962-8924(96)10036-2. [DOI] [PubMed] [Google Scholar]
- Gorczyca MG, Augart C, Budnik V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J Neurosci. 1993;13:3692–3704. doi: 10.1523/JNEUROSCI.13-09-03692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates LS, Budnik V. Assembly and maturation of the Drosophila larval neuromuscular junction. In: Gramates LS, Budnik V, editors. Neuromuscular Junctions in Drosophila. San Diego, California: Academic Press; 1999. in press. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoprotein and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, Greenspan RJ. Inhibition of Calcium/Calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- Griffith LC, Greenspan RJ. The diversity of calcium/calmodulin-dependent protein kinase II isoforms in Drosophila is generated by alternative splicing of a single gene. J Neurochem. 1993;61:1534–1537. doi: 10.1111/j.1471-4159.1993.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Guan B, Hartmann B, Koh YH, Gorczyca M, Budnik V. The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Current Biology. 1996;6:695–706. doi: 10.1016/s0960-9822(09)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GuptaRoy B, Griffith LC. Functional heterogeneity of alternatively spliced isoforms of Drosophila calcium/calmodulin-dependent protein kinase II. J Neurochem. 1996;66:1282–1288. doi: 10.1046/j.1471-4159.1996.66031282.x. [DOI] [PubMed] [Google Scholar]
- Hannan F, Zhong Y. Second messenger systems underlying plasticity at the neuromuscular junction. In: Budnik V, Gramates LS, editors. Neuromuscular Junctions in Drosophila : International Review of Neurobiology. Vol. 43. San Diego: Academic Press; 1999. pp. 119–138. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Ann Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Sheng M. Requirement of N-terminal cysteines of PSD-95 for PSD-95 multimerization and ternary complex formation, but not for binding to potassium channel Kv1.4. J Biol Chem. 1999;274:532–536. doi: 10.1074/jbc.274.1.532. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jia X, Gorczyca M, Budnik V. Ultrastructure of Neuromuscular Junctions in Drosophila: Comparison of Wild Type and Mutants with Increased Excitability. J Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Griffith LC, Murphey RK. Presynaptic Ca2+/calmodulin-dependent protein kinase II regulates habituation of a simple reflex in adult Drosophila. J Neurosci. 1998;18:8955–8964. doi: 10.1523/JNEUROSCI.18-21-08955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:103–113. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Keshishian H, Chiba A, Chang TN, Halfon MS, Harkins EW, Jarecki J, Wang L, Anderson M, Cash S, Halpern ME, et al. Cellular mechanisms governing synaptic development in Drosophila melanogaster. J Neurobiol. 1993;24:757–87. doi: 10.1002/neu.480240606. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kim E, Cho KO, Rothschild AR, Sheng M. Heteromultimerization and NMDA receptor clustering activity of chapsyn-110, a member of the PSD-95 family of synaptic proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia X, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994;17:406. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Mayford M, Barzilai A, Keller F, Schachers S, Kandel ER. Modulation of NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256:638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of CaMKII transgenes. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RGM, Morrison JH, O’Dell TJ, Grant SGN. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: A Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Müller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, Lau LF, Veh RW, Huganir RL, Gundelfinger ED, Garner CC. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–265. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Ann Rev Neurosci. 1999;22:389–342. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Sheng M. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 1996;17:575–578. doi: 10.1016/s0896-6273(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I Fasciclin II controls synaptic stabilization and growth. Neuron. 1996a;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman GS. Genetic dissection of structural and functional components of synaptic plasticity. II Fasciclin II controls presynaptic structural plasticity. Neuron. 1996;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Deficient hippocampal long-term potentiation in α-calcium calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Soderling TR. Protein kinases and phosphatases: regulation by autoinhibitory domains. Biotechnol Appl Biochem. 1993;18:185–200. [PubMed] [Google Scholar]
- Spradling AC. P-element-mediated transformation. In: Roberts DB, editor. Drosophila – A practical approach. Oxford: IRL Press; 1986. [Google Scholar]
- Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- Tejedor FJ, Bokhari A, Rogero O, Zhang J, Gorczyca M, Kim E, Sheng M, Budnik V. Essential Role for dlg in Synaptic Clustering of Shaker K+ Channels in vivo. J Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U, Phannavong B, Müller B, Garner CC, Gundelfinger ED. Functional expression of rat synapse-associated proteins SAP97 and SAP102 in Drosophila dlg-1 mutants: effects on tumor suppression and synaptic bouton structure. Mech Dev. 1997a;62:161–174. doi: 10.1016/s0925-4773(97)00658-8. [DOI] [PubMed] [Google Scholar]
- Thomas U, Kim E, Kuhlendahl S, Koh YH, Gundelfinger E, Sheng M, Garner C, Budnik V. Synaptic Clustering of the Cell Adhesion Molecule Fasciclin II by Discs Large and its Role in the Regulation of Presynaptic Structure. Neuron. 1997b;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topinka JR, Bredt DS. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron. 1998;20:125–134. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Ann Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Wang J, Renger J, Griffith LC, Greenspan RJ, Wu CF. Concomitant alterations of physiological and developmental plasticity at CaM kinase II-inhibited synapses in Drosophila. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Palmer G, Griffith LC. Regulation of Drosophila Ca2+/calmodulin-dependent protein kinase II by autophosphorylation analyzed by site-directed mutagenesis. J Neurochem. 1998;71:328–387. doi: 10.1046/j.1471-4159.1998.71010378.x. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The disc-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Zito K, Fetter RD, Goodman CS, Isacoff EY. Synaptic clustering of Fasciclin II and Shaker: essential targeting sequences and role of Dlg. Neuron. 1997;19:1007–1016. doi: 10.1016/s0896-6273(00)80393-1. [DOI] [PubMed] [Google Scholar]