Abstract
Objective
To determine the short-term outcome of neuropsychiatric (NP) events upon enrollment into an international, inception cohort of SLE patients.
Methods
The study was performed by the Systemic Lupus International Collaborating Clinics. Patients were enrolled within 15 months of diagnosis of SLE and NP events were characterized using the ACR case definitions. Decision rules were derived to identify NP events attributable to SLE. Physician outcome scores of NP events and patient derived mental (MCS) and physical (PCS) component summary scores of the SF-36 were recorded.
Results
There were 890 patients (88.7% female) with a mean (± SD) age of 33.8 ± 13.4 years and mean disease duration of 5.3 ± 4.2 months. Within the enrollment window 271/890 (33. 5%) patients had at least 1 NP event encompassing 15 NP syndromes. NP events attributed to SLE varied from 16.5% – 33.9% using alternate attribution models and occurred in 6.0% – 11.5% of patients. Outcome scores for NP events attributed to SLE were significantly better than for NP events due to non-SLE causes. Higher global disease activity was associated with worse outcomes. MCS scores were lower in patients with NP events, regardless of attribution, and were also lower in patients with diffuse and central NP events. There was a significant association between physician outcome scores and patient MCS scores only for NP events attributed to SLE.
Conclusion
In SLE patients the short-term outcome of NP events is determined by both the characteristics and attribution of the events.
Keywords: Systemic lupus erythematosus, Neuropsychiatric, Inception cohort, Attribution, Outcome
Nervous system disease is well recognized in systemic lupus erythematosus (SLE) and comprises both neurologic (N) and psychiatric (P) manifestations. Individual neuropsychiatric (NP) presentations include common disorders such as headaches, depression and cerebrovascular disease in addition to less common entities such as seizures, psychosis and demyelination [1–6]. In view of the non-specific nature of many of the NP syndromes, it is likely that some of the NP events in SLE patients are not a primary manifestation of the disease but rather occur due to complications of SLE, its therapy or a concurrent disease process [3, 4]. Regardless of attribution, the clinical significance of NP disease in SLE patients is reflected by the adverse impact on health-related quality of life [3, 4] and mortality [7–9].
There is relatively little information on the short or long-term outcome of NP events in SLE. Previous studies [10–16] have been limited by their small sample size and variable disease duration in individual patients, exclusion of NP events which were not attributable to SLE and restriction to individual NP manifestations rather than including the totality of possible NP events. We have assembled an international, multi-center, disease inception cohort of SLE patients and identified all NP events occurring at the time of diagnosis of SLE using standardized case definitions. In this study we report the short-term outcome of these NP events. In addition, several clinical variables, including the characteristics and attribution of events to SLE or non-SLE causes, patient demographics and global SLE disease activity were examined for association with improvement or deterioration in NP status.
Patients and Methods
Research study network
The study was conducted by the Systemic Lupus International Collaborating Clinics (SLICC) [17] which consists of 27 academic medical centres in eight countries with 30 investigators. SLICC centres were grouped into geographic locations (Canada, U.S.A./Mexico, Europe, and Asia). Data were collected prospectively on patients presenting with a new diagnosis of SLE. All information was submitted to the coordinating centre in Halifax, Nova Scotia, Canada. Additional information on the same patients was collected concurrently in a study of atherosclerosis in SLE and submitted to the coordinating centre at the University of Toronto, Ontario, Canada. Electronic data transfer occurred between the Toronto and Halifax sites and the merged dataset was available for analysis. The study protocol was approved by the Capital Health Research Ethics Board, Halifax, Nova Scotia, Canada and by each of the participating centre’s own institutional research ethics review boards in accordance with the Declaration of Helsinki’s guidelines for research in humans.
Patients
All patients fulfilled the ACR classification criteria for SLE [18] and provided written informed consent. The date of diagnosis was the time when at least four of the cumulative ACR criteria were first recognized. Enrollment in the study was encouraged as close as possible to the date of diagnosis but was permitted for up to 15 months following the diagnosis. Data included age, gender, ethnicity, education and medication history. Lupus-related variables included the ACR classification criteria for SLE [18], the SLE Disease Activity Index (SLEDAI) [19] and the SLICC/ACR damage index (SDI) [20]. Laboratory data included a complete blood count, serum creatinine, urinalysis and immunologic variables required for SLEDAI and SDI scores. Health related quality of life (HRQOL) was measured by the SF-36 [21].
Neuropsychiatric (NP) events
An enrollment window was defined within which all NP events, some of which are inherently evanescent, were captured. To ensure inclusion of NP events which may have been part of the presentation of lupus but which occurred prior to the accumulation of four ACR classification criteria, the enrollment window extended from 6 months prior to the date of diagnosis of SLE up to the enrollment date. As the latter could occur up to 15 months following the diagnosis of SLE, the maximum duration of the enrollment window was 21 months. The specific NP events identified were characterized using the ACR nomenclature and case definitions for 19 NP syndromes [22] described in SLE. Screening for NP syndromes was done primarily by clinical evaluation and subsequent investigations were performed only if clinically warranted. In order to further improve the consistency of data collection, a checklist of NP symptoms was distributed to each of the participating sites for use during patient encounters. In the majority of cases, the diagnosis of cognitive impairment was made on the basis of clinical assessment rather than formal neuropsychological testing.
All NP events within the enrollment window were identified and additional information was recorded depending upon the type of NP event and guided by the ACR glossary for NP syndromes [22]. This included potential etiologic factors other than SLE which were considered for exclusion, or recognized as an “association”, acknowledging that it is not always possible to be definitive about attribution. Collectively, these “exclusions” and “associations” were referred to as “non-SLE factors” and used in part to determine the attribution of NP events. Patients could have more than one type of NP event but repeated episodes of the same event occurring within the enrollment window were recorded only once. In the latter case the time of the first episode was taken as the date of onset of the NP event.
Attribution of NP events
Participating centres reported all NP events regardless of etiology. Decision rules were derived to determine the attribution of NP events which occurred within the enrollment window. Factors which were taken into account included: (i) onset of NP event(s) prior to study enrollment; (ii) presence of concurrent non-SLE factor(s) which were identified as part of the ACR definitions for each NP syndrome and considered to be a likely cause or significant contributor to the event and (iii) occurrence of “minor” NP events as defined by Ainiala et al who have previously reported a high frequency of such events in normal population controls [1]. These include all headaches, anxiety, mild depression (i.e. all mood disorders which fail to meet the criteria for “major depressive-like episodes”), mild cognitive impairment (deficits in less than 3 of the 8 specified cognitive domains) and polyneuropathy without electrophysiological confirmation.
The attribution of NP events to SLE was determined by two sets of decision rules (model A and B) of different stringency as described in detail elsewhere [4]. NP events which fulfilled the criteria for model A (the most stringent) or for model B (the least stringent) were attributed to SLE. Those NP events which did not fulfill these criteria were attributed to non-SLE causes:
Attribution Model A: NP events which had their onset within the enrollment window and had no “exclusions” or “associations” and were not one of the NP events identified by Ainiala [1] were attributed to SLE.
Attribution Model B: NP events which had their onset within 10 years of the diagnosis of SLE and had no “exclusions” and were not one of the NP events identified by Ainiala [1] were attributed to SLE.
Outcome of NP events
Two outcome measures of NP events were used to capture both the physician’s and patient’s assessment at study enrollment. These included (i) a physician generated 7-point Likert scale for individual NP events comparing the change in the NP status between the onset of the event and time of study enrollment (1 = patient demise, 2 = much worse, 3 = worse, 4 = no change, 5 = improved, 6 = much improved, 7 = resolved); (ii) patient generated mental (MCS) and physical (PCS) component summary scores of the SF-36 [21].
Statistical analysis
Individual NP manifestations were categorized by attribution to either SLE (model A or model B) or non-SLE causes. The distribution of patients in this hierarchy, and a no NP event class where relevant, was examined for associations with the outcome of the event as determined by physician assessment and patient generated SF-36 component summary scores. In addition, the NP manifestations were clustered into subgroups for additional analyses. Thus, the 19 NP syndromes were categorized into central and peripheral nervous system manifestations as previously described [22]. In addition, NP events were categorized into diffuse and focal manifestations; diffuse NP syndromes were aseptic meningitis, demyelinating syndrome, headache, acute confusional state, anxiety disorder, cognitive dysfunction, mood disorder and psychosis. Focal NP syndromes were cerebrovascular disease, Guillain Barré syndrome, movement disorder, myelopathy, seizure disorders, autonomic neuropathy, mononeuropathy, myasthenia gravis, cranial neuropathy, plexopathy and polyneuropathy. Descriptive statistics were used to summarize all variables with percentages, mean and standard deviation or median and range where appropriate. The relationships between the NP event outcome scores and geographical region, educational status, ethnicity, gender, age at diagnosis of SLE, disease duration, SLEDAI scores (with and without NP variables) and SDI scores (with and without NP variables) were examined by ordinal logistic regression, separately for events in the attribution hierarchy. The estimation was accomplished by generalized estimating equations (GEE) with an independence working correlation structure in order to adjust for multiple events within patients. In addition, we defined a time to case resolution variable for the NP events (i.e. NP event score = 7), and used Kaplan-Meier estimates and log rank tests to investigate the relationships between this event time variable and the time-invariant demographic variables for the attribution hierarchy. For SF-36 mental (MCS) and physical (PCS) component summary scores, separate analyses by linear regression were used for pre-defined groups: patients without NP events; patients with any attributable NP events in model A or in model B and patients with non-SLE NP events. The same attribution classification was used in the linear regression analyses that examined the association between physician generated NP outcome scores and patient generated SF-36 summary scores.
Results
Patients
A total of 890 patients were recruited in 24 centres between October 1999 and November 2006. The median (range) number of patients enrolled in each centre was 23 (2–104). The patients were predominantly women (88.7%), with a mean (± SD) age of 33.8 ± 13.4 years and a wide ethnic distribution although predominantly Caucasian (Table 1). At enrollment the mean disease duration was only 5.3 ± 4.2 months. The prevalence of individual ACR classification criteria reflected an unselected patient population. The mean SLEDAI and SDI scores revealed moderate global disease activity and minimal cumulative organ damage respectively. Therapy at the time of enrollment reflected the typical range of lupus medications. The mean duration of followup, representing the interval between the time of onset of NP events within the enrollment window and the date of assessment, was 3.7 ± 3.1 months.
Table 1.
Demographic and clinical manifestations of SLE patients
| Number of Patients | 890 |
| Gender | |
| Female | 746 (88.7%) |
| Male | 95 (11.3%) |
| Age (years) (mean ± SD) | 33.8 ± 13.4 |
| Ethnicity: | |
| Caucasian | 53.6% |
| Hispanic | 12.0% |
| Asian | 16.0% |
| Black | 14.4% |
| Other | 3.9% |
| Single/Married/Other | 45.8%/40.2%/14% |
| Post secondary education | 63.8% |
| Disease duration (months) (mean ± SD) | 5.3 ± 4.2 |
| Number of ACR criteria (mean ± SD) | 4.9 ± 1.0 |
| Cumulative ACR manifestations | |
| Malar rash | 36.9% |
| Discoid rash | 12.0% |
| Photosensitivity | 39.4% |
| Oral/nasopharyngeal ulcers | 37.8% |
| Serositis | 27.6% |
| Arthritis | 74.0% |
| Renal disorder | 28.1% |
| Neurological disorder | 5.8% |
| Hematologic disorder | 61.7% |
| Immunologic disorder | 76.6% |
| Antinuclear antibody | 96.6% |
| SLEDAI score (mean ± SD) | 5.5 ± 5.6 |
| SLICC/ACR damage index score (mean ± SD) | 0.37 ± 0.81 |
| Medications | |
| Corticosteroids | 68.5% |
| Antimalarials | 64.4% |
| Immunosuppressants | 37.5% |
| ASA | 14.25% |
| Antidepressants | 10.5% |
| Anticonvulsants | 4.7% |
| Warfarin | 3.0% |
| Antipsychotics | 0.6% |
Neuropsychiatric (NP) manifestations
Within the enrollment window 271/890 (33.5%) patients had at least 1 NP event and 90/890 (10.1%) had 2 or more events. There were a total of 407 NP events, encompassing 15 of the 19 NP syndromes (Table 2). The proportion of NP events attributed to SLE varied from 16.5% – 33.9% using alternate attribution models and occurred in 6.0 % [model A] – 11.5% [model B] of patients. Of the 407 NP events 379 (93%) affected the central nervous system and 28 (7%) involved the peripheral nervous system. The numbers of diffuse and focal events were 318 (78%) and 89 (22%), respectively.
Table 2.
Characteristics of neuropsychiatric syndromes in SLE patients as indicated by the number of NP events and their attribution using attribution models
| NP events (%) regardless of attribution | NP events due to SLE (Model A) | NP events due to SLE (Model B) | NP events due to non-SLE causes | |
|---|---|---|---|---|
| Headache | 171 (42.0) | 0 | 0 | 171 |
| Mood disorders | 67 (16.5) | 3 | 22 | 45 |
| Anxiety disorder | 26 (6.4) | 0 | 0 | 26 |
| Cerebrovascular disease | 23 (5.7) | 8 | 22 | 1 |
| Cognitive dysfunction | 26 (6.4) | 5 | 16 | 10 |
| Seizure disorder | 30 (7.4) | 16 | 26 | 4 |
| Acute confusional state | 16 (3.9) | 8 | 13 | 3 |
| Polyneuropathy | 10 (2.5) | 3 | 6 | 4 |
| Psychosis | 9 (2.2) | 3 | 8 | 1 |
| Mononeuropathy | 10 (2.5) | 7 | 10 | 0 |
| Cranial neuropathy | 7 (1.7) | 5 | 5 | 2 |
| Aseptic meningitis | 3 (0.7) | 2 | 2 | 1 |
| Myelopathy | 5 (1.2) | 4 | 5 | 0 |
| Movement disorder | 3 (0.7) | 2 | 2 | 1 |
| Autonomic disorder | 1 (0.3) | 1 | 1 | 0 |
| Guillain-Barre syndrome | 0 | 0 | 0 | 0 |
| Demyelinating syndrome | 0 | 0 | 0 | 0 |
| Myasthenia gravis | 0 | 0 | 0 | 0 |
| Plexopathy | 0 | 0 | 0 | 0 |
| Total | 407 | 67 | 138 | 269 |
- Attribution Model A: NP events which had their onset within the enrollment window and had no “exclusions” or “associations” and were not one of the NP events identified by Ainiala [1] were attributed to SLE.
- Attribution Model B: NP events which had their onset within 10 years of the diagnosis of SLE and had no “exclusions” and were not one of the NP events identified by Ainiala [1] were attributed to SLE.
Physician assessment of outcome of NP events
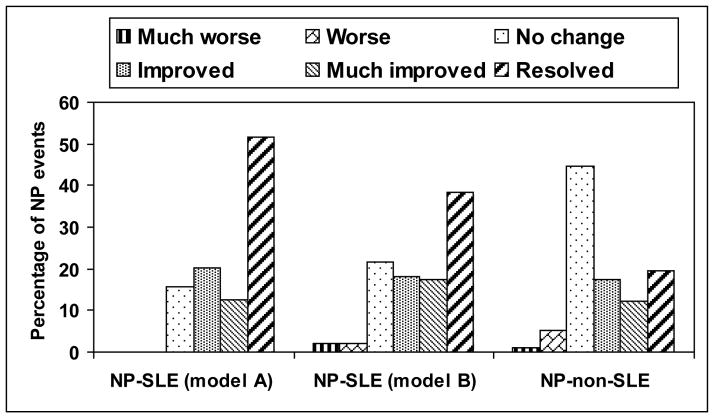
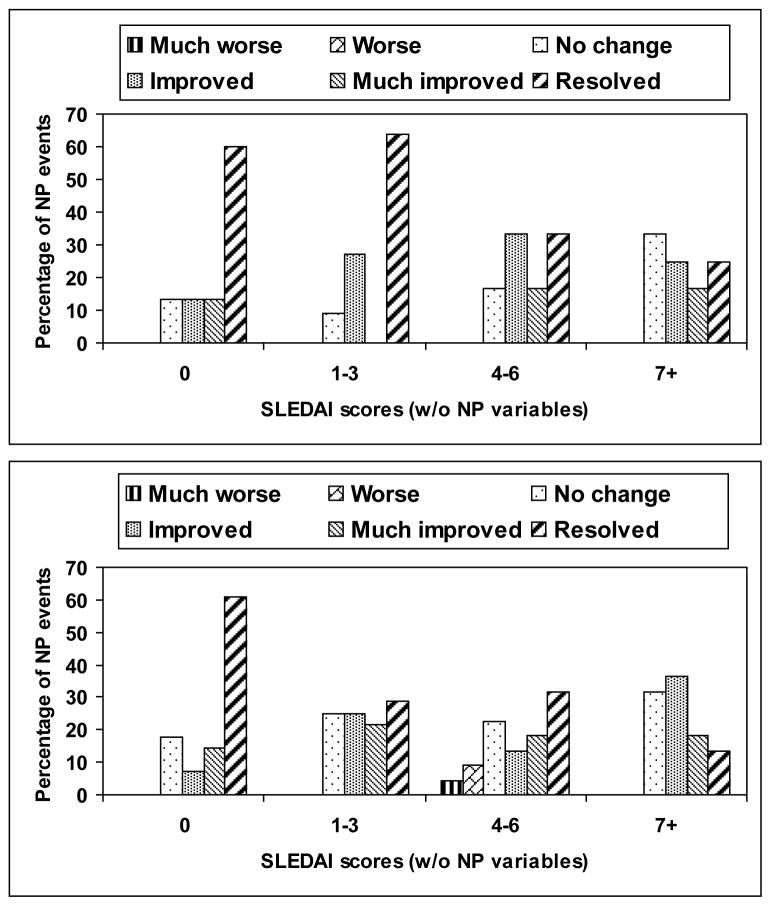
The outcome of individual NP events was examined following group assignment according to attribution (Figure 1), adjusting for multiple NP events in 90 (10.1%) patients. NP events attributed to SLE using either attribution model A or model B had a significantly better outcome compared to patients with NP events not attributed to lupus (p < 0.001). Outcome scores were significantly lower in patients with higher SLEDAI scores (including or excluding NP variables) (Figure 2) (attribution model A, p = 0.009, 0.017; attribution model B, p = 0.001, 0.002). There was no association between outcome scores and geographical region, educational status, ethnicity, gender or age of diagnosis demonstrated by ordinal logistic regression or the time to event analysis (data not shown). For NP events attributed to SLE using model A only, there was a significant association between worse outcome scores and shorter disease duration (p = 0.014) which likely indicates that insufficient time had elapsed for improvement to occur. For cumulative damage either including or excluding NP variables, we were not able to obtain reliable estimates in ordinal logistic regression because the data were too sparse to be informative, while time to event analysis indicated that there was no evidence of an association between time to case resolution and cumulative damage (including or excluding NP variables). Diffuse NP events had poorer outcome scores compared to focal NP events (p < 0.001). In subgroup analyses this was only significant for diffuse NP events not attributed to SLE (p = 0.019), but there was no evidence that the relationships differed across the attribution classes (model B, p = 0.143)
Figure 1.
Physician generated outcome scores at enrollment for NP events attributed to SLE using different attribution models A and B and for NP events attributed to non-SLE causes. Those NP events which were attributed to SLE using either attribution model A or model B had a significantly better outcome compared to patients with NP events not attributed to lupus (p < 0.001).
Figure 2.
Physician generated outcome scores at enrollment for NP events attributed to SLE using either attribution model A (upper panel) and model B (lower panel). Outcome scores were significantly lower in patients with higher SLEDAI scores (excluding NP variables). Attribution model A, p = 0.017; Attribution model B, p = 0.002.
Patient assessment of outcome of NP events
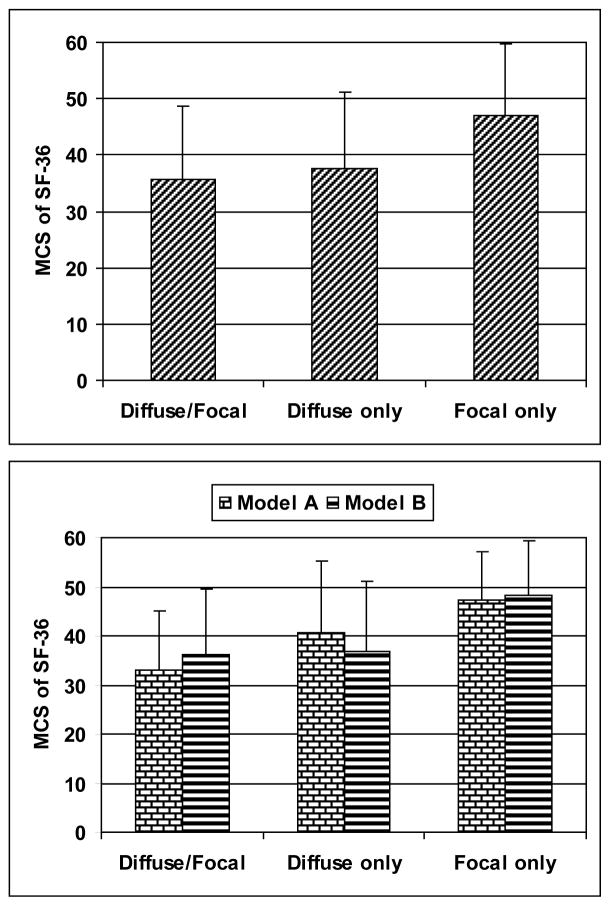
The SF-36 mental (MCS) and physical (PCS) component summary scores were used to assess the impact of NP events from the patients’ perspective. Patients with NP events, regardless of attribution, had significantly lower (worse) MCS scores compared to patients with no NP events (38.46 ± 13.63 vs. 43.04 ± 12.69; p < 0.001). There was no significant difference between MCS scores in patients with NP events attributed to SLE (attribution model A or B) and to non-SLE causes (attribution model B: 39.58 ± 14.05 vs. 37.79 ± 13.38; p = 0.363). There was a significant although less profound difference between PCS scores in patients with NP events from any cause compared to patients with no NP events (attribution model B: 35.29 ±11.83 vs. 37.47 ± 12.05; p = 0.035) although again there was no difference in PCS scores in patients with NP events attributed to SLE and to non-SLE causes (35.19 ± 12.28 vs. 35.35 ± 11.60; p = 0.927). These findings are comparable to our previous report of the first 572 patients enrolled in the cohort [4]. In addition, patients with NP events, regardless of attribution and clustered collectively into diffuse manifestations with or without concurrent focal NP events, had lower MCS scores compared to patients with focal NP events only (35.71 ± 12.85 vs 37.72 ± 13.52 vs 47.09 ± 12.58; p = 0.002) (Figure 3). The findings were similar when the analysis was restricted to patients with NP events attributed to SLE using either attribution model A (32.92 ± 12.19 vs 40.64 ± 14.49 vs 47.20 ± 9.83; p = 0.012) or model B (36.21 ± 13.18 vs 36.83 ± 14.43 vs 48.21 ± 11.14; p = 0.001) (Figure 3). Similarly, patients with central NP events with and without concurrent peripheral NP events, regardless of attribution, had lower MCS scores compared to patients with peripheral NP events only (35.28 ± 12.22 vs 38.16 ± 13.63 vs 49.51 ± 11.54; p = 0.020). In patients with NP events there were no significant associations between MCS scores and geographical region, educational status, ethnicity, gender, age of diagnosis, disease duration, SLEDAI or cumulative damage computed either including or excluding NP variables (data not shown).
Figure 3.
Mental component summary (MCS) scores (mean ± SD) at enrollment in patients with NP events assigned to groups with diffuse and focal NP disease, diffuse NP disease only and focal NP disease only. Regardless of attribution (upper panel), all patients with diffuse/focal and diffuse only events had lower MCS scores compared to patients with focal NP events only (p = 0.002). The findings were similar when the analysis was restricted to patients with NP events attributed to SLE (lower panel) using either attribution model A (p = 0.012) or model B (p = 0.001).
Agreement between physician assessment of outcome of NP events and patient SF-36 summary scores
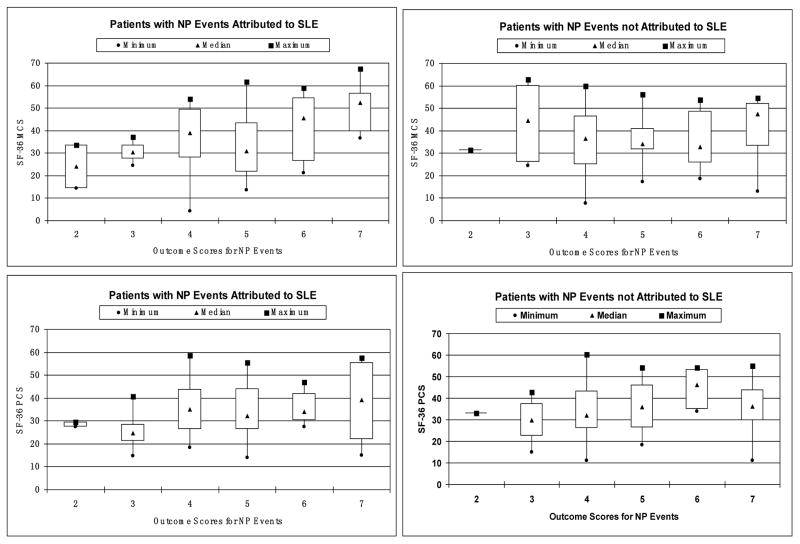
The 271 patients with NP events were stratified according to the physician generated outcome scores. In those patients with more than one outcome score due to the occurrence of multiple NP events, the lowest (worst) outcome score was used for group assignment. Patients were further segregated by attribution of NP events and differences in MCS scores and PCS scores were compared (Figure 4). In those patients with NP events attributed to SLE (attribution model A or B) there was a significant association between physician generated outcome scores and patient generated MCS scores (p < 0.001). Regression analysis provided an R-square value of 0.28 indicating that 28% of the variability in MSC scores in this group is explicable on the basis of NP event outcome scores. Similar associations were not seen for PCS scores in the patients with NP events attributed to SLE nor for MCS or PCS scores in patients with NP events not attributed to SLE (Figure 4).
Figure 4.
Boxplot of mental component summary (MCS) scores (median and range) and physical component summary (PCS) scores (median and range) at enrollment in patients with NP events segregated according to physician generated outcome scores. Patients were further divided by attribution of NP events and differences in MCS scores and PCS scores were compared. Only in patients with NP events attributed to SLE (attribution model A or B) was there a significant association between physician generated outcome scores and patient generated MCS scores (left upper panel; p < 0.001). Similar associations were not seen for PCS scores in the patients with NP events attributed to SLE nor for MCS scores or PCS scores in patients with NP events not attributed to SLE (p > 0.050).
Discussion
Neuropsychiatric events in SLE patients are frequent and varied [1–6], although the majority of events are not a primary feature of the disease [3, 4]. Despite the high prevalence of NP events, there have been relatively few attempts to determine their clinical outcome over the short or long-term [10–16]. In the present study of an international, multi-centre, inception cohort of SLE patients, all NP events were identified within a predefined window around the time of diagnosis of SLE. The short-term outcome of these events in the context of attribution, global disease activity and other clinical variables was examined. The results demonstrate that both physicians and patients identify significant group differences in the outcome of NP events when these are stratified by attribution either to SLE or non-SLE causes. For patients with NP events due to SLE, there was significant convergence in physician and patient assessments.
Open [13, 14, 23, 24] and controlled [12, 15, 25] clinical trials, retrospective [26, 27] and prospective [3, 28, 29] observational cohorts and case series [10] have provided information on the prognosis of NP events in SLE patients, particularly those events attributed to SLE. The conclusions have been inconsistent. For example, increased mortality, which is a crude but important outcome of NP disease, has been reported in some studies [7–9] but not in others [30–32]. Clinical trials of NP-SLE have been generally favourable [12–15, 23–25], although most have examined specific subsets of NP-SLE and lack controls or blinded assessment. Observational studies in patients with NP-SLE have also yielded mixed findings. Karassa et al [10] examined the prognosis of NP disease in 32 patients hospitalized for NP-SLE and followed for 2 years. The outcome was generally favorable with either substantial improvement (69%) or stabilization (19%). Likewise, in a five year prospective study of cognitive function in SLE [33], the majority of patients did not have a progressive decline in cognition, a finding supported by other studies [29, 34–36]. Conversely the adverse effect of cumulative NP events in SLE patients is evident from the significantly lower scores for HRQOL in SLE [3, 4], more fatigue [3] and more cumulative organ damage compared to patients without NP events [4]. Of interest these associations were independent of the attribution of the NP events to SLE or to an alternative etiology [3, 4], and did not occur in patients with a history of renal disease which was used as a comparator to assess the impact of another major organ system [3]. Jonsen et al [11] reported a higher frequency of disability in SLE patients with NP disease compared to patients without NP events and the general population. Thus, in contrast to the previous studies [12–15, 23–25, 29, 33, 34], these later data suggest that NP events in SLE patients, regardless of their etiology and attribution, have a negative impact on quality of life.
The current observational study has several novel features. A large inception cohort provides sufficient size and diversity to make the findings generalizable, guarantees universally short disease duration which limits the chronic psychological effects of the disease process and provides more certainty than a retrospective study for determining the correct attribution of NP events. A specific objective was to capture all NP events regardless of attribution, in order to measure the full impact of NP disease. Two attribution models of different stringency were employed based upon a composite of clinical decision rules. The physician generated measure demonstrated a significantly better outcome for NP events attributed to SLE compared to non-SLE causes which suggests that the former have greater potential for reversibility. An additional observation was the association between poor outcome scores and active generalized SLE disease activity outside of the nervous system indicating that NP disease which occurs in the context of a generalized lupus flare is less likely to improve, at least in the short term.
The MCS and PCS scores of the SF-36 were used to assess the patients’ perspective on NP events. As previously reported [4] the MCS scores were significantly lower in patients with NP events regardless of attribution. In the current study we also found that MCS scores were lower in patients with diffuse compared to focal NP disease and furthermore in central compared to peripheral NP disease. An additional new and important finding is a significant association between physician and patient assessments of outcome of NP events attributed to SLE as indicated by higher or lower MCS scores in patients assigned to groups with high or low physician outcome scores respectively. A similar association was not found for PCS scores in the same patient groups nor with either MCS or PCS scores in patients with NP events not attributed to SLE. This indicates enhanced sensitivity and specificity for changes in NP disease attributed to SLE.
There are a number of limitations to the present study. First, restriction of NP syndromes to the 19 identified in the ACR case definitions [22] could potentially have excluded other forms of nervous system disease. However, this did not emerge as an issue during the execution of the study. In fact, four of the 19 NP syndromes were never identified in this relatively large inception cohort. Second, formal neuropsychological assessments were not performed routinely on all patients, largely for logistical reasons. If included in the study protocol this would likely have increased the prevalence of cognitive impairment in our cohort, albeit subtle and sub-clinical in the majority of cases. Several cross-sectional and longitudinal studies have indicated that such deficits do not adversely affect HRQOL [33, 37, 38] or lead to long-term, clinically significant neurological sequelae [33–36]. Third, although the physician generated outcome was focused specifically on NP events, the patient generated outcome was based upon a generic instrument of HRQOL. However, the MCS score of the SF-36 performed well and despite the potential to be influenced by other factors it reflected a significant amount of the variability in outcome scores for patients with NP events attributed to SLE. Finally, this study evaluated only the short-term outcome of NP events occurring around the time of diagnosis of SLE. In spite of this limitation there were striking differences in the outcome and impact of NP events depending upon their characteristics and attribution. These findings inform our understanding of the evolution of NP events in SLE patients and how they can best be evaluated in trials of current and future therapies.
In summary, we have demonstrated that the short-term outcome of NP events in recently diagnosed SLE patients is different depending on the type and attribution of NP events. This is true for both physician and patient assessments. Longer followup of this cohort will determine the reproducibility of our findings and examine clinical and laboratory variables as potential prognostic indicators for the long-term outcome of NP events.
Acknowledgments
Financial support:
J.G. Hanly (Canadian Institutes of Health Research grant MOP-57752, Capital Health Research Fund), M.B. Urowitz (Canadian Institutes of Health Research grant MOP-49529, Lupus Foundation of Ontario, Ontario Lupus Association, Lupus UK, Lupus Foundation of America, Lupus Alliance Western New York, Conn Smythe Foundation, Tolfo Family (Toronto), S.C. Bae (Brain Korea 21 Program), C. Gordon (Lupus UK, arthritis research campaign, Wellcome Trust Clinical Research Facility in Birmingham, UK), G.S Alarcón (University of Alabama at Birmingham, grant P60AR48095), A. Clarke (Fonds de la recherche en sante de Quebec National Scholar, Singer Family Fund for Lupus Research), S. Bernatsky (Canadian Institutes of Health Research Junior Investigator Award; Fonds de la recherche en santé du Québéc Jeune Chercheure; Canadian Arthritis Network Scholar Award; McGill University Health Centre Research Institute), P.R. Fortin (The Arthritis Society/Institute of Musculoskeletal Health and Arthritis Investigator award, Arthritis Centre of Excellence), D. Gladman (Canadian Institutes of Health Research), M. Petri (Hopkins Lupus Cohort grant AR 43727, Johns Hopkins University General Clinical Research Center grant MO1 RR00052), O. Nived (Swedish Medical Research council grant 13489), G. Sturfelt (Swedish Medical Research council grant 13489), R. Ramsey-Goldman (National Institutes of Health research grants M01-RR00048; K24 AR02318; P60 AR 48098).
We are grateful for the generous donation of our patients’ time and the dedication of all the research coordinators and research assistants in the SLICC network to the completion of this work.
References
- 1.Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45(5):419–23. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31(11):2156–62. [PubMed] [Google Scholar]
- 4.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56(1):265–73. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 5.Sanna G, Bertolaccini ML, Cuadrado MJ, Laing H, Mathieu A, Hughes GR. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30(5):985–92. [PubMed] [Google Scholar]
- 6.Sibbitt WL, Jr, Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29(7):1536–42. [PubMed] [Google Scholar]
- 7.Mody GM, Parag KB, Nathoo BC, Pudifin DJ, Duursma J, Seedat YK. High mortality with systemic lupus erythematosus in hospitalized African blacks. Br J Rheumatol. 1994;33(12):1151–3. doi: 10.1093/rheumatology/33.12.1151. [DOI] [PubMed] [Google Scholar]
- 8.Samanta A, Feehally J, Roy S, Nichol FE, Sheldon PJ, Walls J. High prevalence of systemic disease and mortality in Asian subjects with systemic lupus erythematosus. Ann Rheum Dis. 1991;50(7):490–2. doi: 10.1136/ard.50.7.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonsson H, Nived O, Sturfelt G. Outcome in systemic lupus erythematosus: a prospective study of patients from a defined population. Medicine (Baltimore) 1989;68(3):141–50. [PubMed] [Google Scholar]
- 10.Karassa FB, Ioannidis JP, Boki KA, et al. Predictors of clinical outcome and radiologic progression in patients with neuropsychiatric manifestations of systemic lupus erythematosus [In Process Citation] Am J Med. 2000;109(8):628–34. doi: 10.1016/s0002-9343(00)00603-3. [DOI] [PubMed] [Google Scholar]
- 11.Jonsen A, Bengtsson AA, Nived O, Ryberg B, Sturfelt G. Outcome of neuropsychiatric systemic lupus erythematosus within a defined Swedish population: increased morbidity but low mortality. Rheumatology (Oxford) 2002;41(11):1308–12. doi: 10.1093/rheumatology/41.11.1308. [DOI] [PubMed] [Google Scholar]
- 12.Karlson EW, Liang MH, Eaton H, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(6):1832–41. doi: 10.1002/art.20279. [DOI] [PubMed] [Google Scholar]
- 13.Mok CC, Lau CS, Chan EY, Wong RW. Acute transverse myelopathy in systemic lupus erythematosus: clinical presentation, treatment, and outcome. J Rheumatol. 1998;25(3):467–73. [PubMed] [Google Scholar]
- 14.Mok CC, Lau CS, Wong RW. Treatment of lupus psychosis with oral cyclophosphamide followed by azathioprine maintenance: an open-label study. Am J Med. 2003;115(1):59–62. doi: 10.1016/s0002-9343(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 15.Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64(4):620–5. doi: 10.1136/ard.2004.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernatsky S, Clarke A, Gladman DD, et al. Mortality related to cerebrovascular disease in systemic lupus erythematosus. Lupus. 2006;15(12):835–9. doi: 10.1177/0961203306073133. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg D, Ramsey-Goldman R. Systemic Lupus International Collaborating Group--onwards and upwards? Lupus. 2006;15(9):606–7. doi: 10.1177/0961203306071868. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 19.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 20.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 21.Thumboo J, Fong KY, Ng TP, et al. Validation of the MOS SF-36 for quality of life assessment of patients with systemic lupus erythematosus in Singapore. J Rheumatol. 1999;26(1):97–102. [PubMed] [Google Scholar]
- 22.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.McCune WJ, Golbus J, Zeldes W, Bohlke P, Dunne R, Fox DA. Clinical and immunologic effects of monthly administration of intravenous cyclophosphamide in severe systemic lupus erythematosus. N Engl J Med. 1988;318(22):1423–31. doi: 10.1056/NEJM198806023182203. [DOI] [PubMed] [Google Scholar]
- 24.Tokunaga M, Saito K, Kawabata D, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66(4):470–5. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denburg SD, Carbotte RM, Denburg JA. Corticosteroids and neuropsychological functioning in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37(9):1311–20. doi: 10.1002/art.1780370907. [DOI] [PubMed] [Google Scholar]
- 26.Rosner S, Ginzler EM, Diamond HS, et al. A multicenter study of outcome in systemic lupus erythematosus. II. Causes of death. Arthritis Rheum. 1982;25(6):612–7. doi: 10.1002/art.1780250602. [DOI] [PubMed] [Google Scholar]
- 27.Mikdashi J, Handwerger B. Predictors of neuropsychiatric damage in systemic lupus erythematosus: data from the Maryland lupus cohort. Rheumatology (Oxford) 2004;43(12):1555–60. doi: 10.1093/rheumatology/keh384. [DOI] [PubMed] [Google Scholar]
- 28.Gladman DD, Urowitz MB, Slonim D, et al. Evaluation of predictive factors for neurocognitive dysfunction in patients with inactive systemic lupus erythematosus. J Rheumatol. 2000;27(10):2367–71. [PubMed] [Google Scholar]
- 29.McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005;64(2):297–303. doi: 10.1212/01.WNL.0000149640.78684.EA. [DOI] [PubMed] [Google Scholar]
- 30.Sibley JT, Olszynski WP, Decoteau WE, Sundaram MB. The incidence and prognosis of central nervous system disease in systemic lupus erythematosus. J Rheumatol. 1992;19(1):47–52. [PubMed] [Google Scholar]
- 31.Feinglass EJ, Arnett FC, Dorsch CA, Zizic TM, Stevens MB. Neuropsychiatric manifestations of systemic lupus erythematosus: diagnosis, clinical spectrum, and relationship to other features of the disease. Medicine (Baltimore) 1976;55(4):323–39. doi: 10.1097/00005792-197607000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs JA, Urowitz MB, Gladman DD. Dilemmas in neuropsychiatric lupus. Rheum Dis Clin North Am. 1993;19(4):795–814. [PubMed] [Google Scholar]
- 33.Hanly JG, Cassell K, Fisk JD. Cognitive function in systemic lupus erythematosus: results of a 5-year prospective study. Arthritis Rheum. 1997;40(8):1542–3. doi: 10.1002/art.1780400825. [DOI] [PubMed] [Google Scholar]
- 34.Waterloo K, Omdal R, Husby G, Mellgren SI. Neuropsychological function in systemic lupus erythematosus: a five-year longitudinal study. Rheumatology (Oxford) 2002;41(4):411–5. doi: 10.1093/rheumatology/41.4.411. [DOI] [PubMed] [Google Scholar]
- 35.Hay EM, Huddy A, Black D, et al. A prospective study of psychiatric disorder and cognitive function in systemic lupus erythematosus. Ann Rheum Dis. 1994;53(5):298–303. doi: 10.1136/ard.53.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlomagno S, Migliaresi S, Ambrosone L, Sannino M, Sanges G, Di Iorio G. Cognitive impairment in systemic lupus erythematosus: a follow-up study. J Neurol. 2000;247(4):273–9. doi: 10.1007/s004150050583. [DOI] [PubMed] [Google Scholar]
- 37.Hanly JG, Fisk JD, Sherwood G, Eastwood B. Clinical course of cognitive dysfunction in systemic lupus erythematosus. J Rheumatol. 1994;21(10):1825–31. [PubMed] [Google Scholar]
- 38.Ginsburg KS, Wright EA, Larson MG, et al. A controlled study of the prevalence of cognitive dysfunction in randomly selected patients with systemic lupus erythematosus. Arthritis Rheum. 1992;35(7):776–82. doi: 10.1002/art.1780350711. [DOI] [PubMed] [Google Scholar]