Abstract
Objective
To describe the frequency, attribution, outcome and predictors of seizures in SLE
Methods
The Systemic Lupus International Collaborating Clinics (SLICC) performed a prospective inception cohort study. Demographic variables, global SLE disease activity (SLEDAI-2K), cumulative organ damage (SLICC/ACR Damage Index (SDI)) and neuropsychiatric events were recorded at enrollment and annually. Lupus anticoagulant, anticardiolipin, anti-β2 glycoprotein-I, anti-ribosomal P and anti-NR2 glutamate receptor antibodies were measured at enrollment. Physician outcomes of seizures were recorded. Patient outcomes were derived from the SF-36 mental (MCS) and physical (PCS) component summary scores. Statistical analyses included Cox and linear regressions.
Results
The cohort was 89.4% female with a mean follow up of 3.5±2.9 years. 75/1631 (4.6%) had ≥1 seizure, the majority around the time of SLE diagnosis. Multivariate analysis indicated a higher risk of seizures with African race/ethnicity (HR(CI):1.97 (1.07–3.63); p=0.03) and lower education status (1.97 (1.21–3.19); p<0.01). Higher damage scores (without NP variables) were associated with an increased risk of subsequent seizures (SDI=1:3.93 (1.46–10.55)); SDI=2 or 3:1.57 (0.32–7.65); SDI≥4:7.86 (0.89–69.06); p=0.03). There was an association with disease activity but not with autoantibodies. Seizures attributed to SLE frequently resolved (59/78(76%)) in the absence of anti-seizure drugs. There was no significant impact on the MCS or PCS scores. Anti-malarial drugs in absence of immunosuppressive agents were associated with reduced seizure risk (0.07(0.01–0.66); p=0.03).
Conclusion
Seizures occurred close to SLE diagnosis, in patients with African race/ethnicity, lower educational status and cumulative organ damage. Most seizures resolved without a negative impact on health-related quality of life. Anti-malarial drugs were associated with a protective effect.
Keywords: Systemic lupus erythematosus, Neuropsychiatric, Seizures, Inception cohort
Nervous system disease in systemic lupus erythematosus (SLE) includes neurological and psychiatric events (1–5) of which 19–38% are attributable to lupus.(6) Previous studies clustered individual neuropsychiatric (NP) events into groups to study outcomes and impact on health-related quality of life (HQol). Although meritorious, assessment of individual NP manifestations is preferable but requires a large cohort of patients studied over a prolonged period.
Seizures are a manifestation of NPSLE and occur in 6%–51% (1–5) of adult and pediatric patients. Most studies have been cross-sectional and have not assessed patients from the time of diagnosis of SLE, an important issue in view of reports that seizures are most frequent early in the disease course. Here, we report our findings from a large, prospective, international, inception cohort of SLE patients. Our objectives were to describe the frequency, attribution and outcome of seizure disorders in SLE and to identify clinical and laboratory predictors of their occurrence.
Patients and Methods
Research study network
The study was conducted by the Systemic Lupus International Collaborating Clinics (SLICC) (7) consisting of 37 investigators in 30 international academic medical centres in 11 countries. Data were collected per protocol at enrollment and annually thereafter, submitted to the coordinating centre in Halifax, Nova Scotia, Canada and entered into a centralized Access database. Appropriate procedures ensured data quality, management and security. Capital Health Research Ethics Board, Halifax, and each of the participating centre’s institutional research ethics review boards approved the study.
Patients
Patients fulfilled the ACR SLE classification criteria SLE,(8) taken as the date of diagnosis, and provided written informed consent. Enrollment was permitted up to 15 months following the diagnosis. Demographic variables included age, gender, ethnicity, education and medication history. Lupus-related variables included the SLE Disease Activity Index (SLEDAI-2K) (9) and SLICC/ACR damage index (SDI).(10) Laboratory testing included hematology, chemistry and immunology required for SLEDAI-2K and SDI scores.
Neuropsychiatric (NP) events
An enrollment window extended from 6 months prior to the diagnosis of SLE up to the enrollment date. NP events were characterized within this window using the ACR case definitions for NP syndromes (11), diagnosed by clinical evaluation and supported by investigations if clinically warranted as per the guidelines. Patients were reviewed annually with a 6-month window around the anticipated assessment date. New NP events since the previous study visit were determined at each assessment.
Seizures were classified into either primary generalized seizures with 4 subtypes (tonic clonic, atonic, absence and myoclonic) or focal seizures with 2 subtypes (simple and complex).(11) The diagnosis was confirmed by independent description from a reliable witness and a normal EEG did not exclude the diagnosis.
Other potential causes (“exclusions”) or contributing factors (“associations”) for each NP event (11) were sought. These “non-SLE factors” in part determined the attribution of events. For patients with seizures several “exclusions” (vasovagal and cardiac syncope, hysteria, hyperventilation, tics, narcolepsy and cataplexy, labyrinthitis, alcohol and drug withdrawal, medications quinolones and imipenem, subarachnoid hemorrhage, hypoglycemia, panic attacks, conversion disorders, malingering) and “associations” (thrombotic thrombocytopenic purpura/microangiopathy, stroke, transient ischemic attack, migraine, hypoglycemia (mild), hypoxemia, uremia, intra-cranial tumour and infection) were considered.
Recurrent episodes of the same NP event within the enrollment window or follow-up assessment period were recorded once. The date of the first episode was taken as the onset of the event.
Attribution of NP events
Decision rules were used to determine the attribution of all NP events including seizures. To optimize consistency this was performed at the coordinating centre utilizing data provided in the case record form by individual SLICC sites. Factors considered in the decision rules included: (i) onset of NP event(s) prior to the diagnosis of SLE; (ii) concurrent non-SLE factor(s) identified from the ACR glossary for each NP syndrome and (iii); “common” NP events which are frequent in normal population controls as described by Ainiala et al.(12) These include all headaches, anxiety, mild depression (mood disorders failing to meet criteria for “major depressive-like episodes”), mild cognitive impairment (deficits in less than 3 of the 8 specified cognitive domains) and polyneuropathy without electrophysiological confirmation.
Decision rules of different stringency (models A and B) determined attribution of NP events, described in detail elsewhere.(6, 13) NP events that fulfilled criteria for model A (most stringent) or for model B (least stringent) were attributed to SLE. By definition, all NP events attributed to SLE using model A were included in the group of NP events using model B. Events not fulfilling these criteria were attributed to non-SLE causes.
Outcome of seizures
Physician generated 7-point Likert scale compared the change in seizure activity between onset and study assessment (1=patient demise, 2=much worse, 3=worse, 4=no change, 5=improved, 6=much improved, 7=resolved).(14) Patient generated SF-36 questionnaire provided mental (MCS) and physical (PCS) component summary scores.(14, 15) These were not available to the physicians at the time of assessment.
Autoantibodies
Lupus anticoagulant, IgG anticardiolipin, anti-β2 glycoprotein-I, anti-ribosomal P and anti-NR2 glutamate receptor antibodies were measured at the enrollment visit at the Oklahoma Medical Research Foundation, USA using previously described methodology.(16–19)
Statistical analysis
The associations of explanatory variables with the time to the first occurrence of all seizures and those attributed to SLE (model B) were examined using Cox proportional hazards regression. SF-36 analyses used linear regression and generalized estimating equations with a first-order autoregressive correlation structure to allow for correlation between multiple SF-36 measurements for the same patient. (See online supplementary text S1).
Results
Patients
1631 patients were recruited between October 1999 and January 2011. The median (range) number of patients enrolled in SLICC centres was 36 (8–202). Patients were most frequently women (89.4%), with a mean (±SD) age of 35.0±13.4 years and a wide ethnic distribution although predominantly Caucasian (Table 1).
Table 1.
Demographic and clinical manifestations of SLE patients at enrollment
| N* | ||
|---|---|---|
| Number of Patients | 1631 | 1631 |
| Gender (%) | ||
| Female | 1458 (89.4) | 1631 |
| Male | 173 (10.6) | |
| Age (years) (mean ± SD) | 35.0 ± 13.4 | 1629 |
| Race/Ethnicity (%): | ||
| Caucasian | 780 (47.9) | 1629 |
| Asian | 264 (16.2) | |
| African | 264 (16.2) | |
| Hispanic | 254 (15.6) | |
| Other | 67 (4.1) | |
| Single/Married/Other (%) | 743 (45.7)/674 (41.4)/210(12.9) | 1627 |
| Post secondary education (%) | 953 (62.0) (range 32.4–100) | 1536 |
| Disease duration (months) (mean ± SD) | 5.6 ± 4.8 | 1631 |
| Number of ACR criteria (mean ± SD) | 4.9 ± 1.1 | 1631 |
| Cumulative ACR manifestations (%) | 1631 | |
| Malar rash | 589 (36.1) | |
| Discoid rash | 208 (12.8) | |
| Photosensitivity | 585 (35.9) | |
| Oral/nasopharyngeal ulcers | 611 (37.5) | |
| Serositis | 441 (27.0) | |
| Arthritis | 1212 (74.3) | |
| Renal disorder | 441 (27.0) | |
| Neurological disorder | 84 (5.2) | |
| Hematologic disorder | 1008 (61.8) | |
| Immunologic disorder | 1238 (75.9) | |
| Antinuclear antibody | 1546 (94.8) | |
| SLEDAI-2K score (mean ± SD) | 5.3 ± 5.4 | 1616 |
| SLICC/ACR damage index score (mean ± SD) | 0.29 ± 0.73 | 620 |
| Medications (%) | 1631 | |
| Corticosteroids | 1143 (70.1) | |
| Anti-malarials | 1075 (65.9) | |
| Immunosuppressants | 645 (39.6) | |
| ASA | 232 (14.2) | |
| Antidepressants | 164 (10.1) | |
| Warfarin | 87 (5.3) | |
| Anticonvulsants | 75 (4.6) | |
| Antipsychotics | 11 (0.7) | |
| Autoantibodies [# positive (%)] | ||
| Lupus anticoagulant | 237 (21.2) | 1116 |
| Anticardiolipin | 134 (12.5) | 1074 |
| Anti-β2 glycoprotein-I | 155 (14.4) | 1074 |
| Anti-ribosomal P | 102 (9.6) | 1068 |
| Anti-NR2 glutamate receptor | 126 (12.7) | 996 |
N is the total number of patients with valid data.
At enrollment the mean disease duration was 5.6±4.8 months and 937 (57.5%) patients had disease duration <6 months. The prevalence of ACR classification criteria reflected an unselected patient population, receiving a typical range of lupus medications. The mean SLEDAI-2K and SDI scores indicated moderate global disease activity and minimal cumulative organ damage respectively. The number of annual assessments varied from 1 to 12 with a mean followup of 3.5±2.9 years.
Neuropsychiatric (NP) manifestations
NP (≥1) occurred in 747/1631(45.8%) patients and 336/1631(20.6%) had ≥2 events over the study period. The events and their attribution are summarized in Table 2.
Table 2.
Characteristics of cumulative neuropsychiatric syndromes incorporating attribution models A and B over the study period in SLE patients.
| NP events (%) regardless of attribution | NP events due to SLE (model A) | NP events due to SLE (Model B) | NP events due to non-SLE causes | ||
|---|---|---|---|---|---|
| Headache | 657 | (48.4) | 0 | 0 | 657 |
| Mood disorders | 213 | (15.7) | 33 | 73 | 140 |
| Seizure disorder | 91 | (6.7) | 59 | 78 | 13 |
| Anxiety disorder | 70 | (5.2) | 0 | 0 | 70 |
| Cerebrovascular disease | 70 | (5.2) | 28 | 68 | 2 |
| Cognitive dysfunction | 61 | (4.5) | 12 | 35 | 26 |
| Polyneuropathy | 44 | (3.2) | 16 | 20 | 24 |
| Acute confusional state | 33 | (2.4) | 16 | 26 | 7 |
| Mononeuropathy | 27 | (2.0) | 14 | 27 | 0 |
| Psychosis | 23 | (1.7) | 10 | 22 | 1 |
| Cranial neuropathy | 24 | (1.8) | 20 | 20 | 4 |
| Movement disorder | 11 | (0.8) | 4 | 8 | 3 |
| Myelopathy | 13 | (1.0) | 7 | 12 | 1 |
| Aseptic meningitis | 8 | (0.6) | 5 | 5 | 3 |
| Demyelinating syndrome | 6 | (0.4) | 1 | 6 | 0 |
| Autonomic disorder | 2 | (0.2) | 2 | 2 | 0 |
| Plexopathy | 1 | (0.1) | 0 | 0 | 1 |
| Guillain-Barre syndrome | 2 | (0.2) | 2 | 2 | 0 |
| Myasthenia gravis | 2 | (0.2) | 0 | 2 | 0 |
|
| |||||
| Total | 1358 | 229 | 406 | 952 | |
| % among 1358 NP events | 16.9 | 29.9 | 70.1 | ||
The attribution of neuropsychiatric (NP) events to SLE was determined using two attribution models:
Attribution Model A: NP events which had their onset within the enrollment window and had no “exclusions” or “associations” and were not one of the NP events identified by Ainiala (12) were attributed to SLE.
Attribution Model B: NP events which had their onset within 10 years of the diagnosis of SLE and were still present within the enrollment window and had no “exclusions” and were not one of the NP events identified by Ainiala (12) were attributed to SLE.
There were 1358 NP events, involving all 19 NP syndromes (11). The proportion of NP events attributed to SLE varied from 16.9% (model A) to 29.9% (model B) and occurred in 10.3% (model A) – 17.4% (model B) of patients. Of the 1358 NP events, 1256 (92.5%) involved the central nervous system and 102 (7.5%) the peripheral nervous system. The classification of events into diffuse and focal was 1071 (78.9%) and 287 (21.1 %), respectively.
Seizures
Over the study 75/1631 (4.6%) patients had a seizure. In 59/75 (78.7%), these occurred during one observation period and in 16/75 (21.3%) patients, seizures occurred during two observation periods. There were a total of 91 seizures (66% generalized; 34% focal) in the 75 patients and 9 patients had generalized and focal seizures (Table 3).
Table 3.
Characteristics of cumulative seizures incorporating attribution models A and B over the study period in SLE patients.
| Seizures regardless of attribution | Seizures due to SLE (model A) | Seizures due to SLE (Model B) | Seizures due to non-SLE causes | |
|---|---|---|---|---|
| Primary generalized | 60 | 39 | 50 | 10 |
| Tonic clonic | 57 | 38 | 47 | 10 |
| Atonic | 1 | 1 | 1 | 0 |
| Absence | 2 | 0 | 2 | 0 |
| Myoclonic | 0 | 0 | 0 | 0 |
| Focal seizures | 31 | 20 | 28 | 3 |
| Simple | 16 | 8 | 15 | 1 |
| Complex | 15 | 12 | 13 | 2 |
Of the 91 seizures 78 (86%) were attributed to SLE. The majority of seizures presented early in the disease course with a median (range) interval from the time of SLE diagnosis to onset of first seizure of 0.14 (−0.50–7.57) years.
Seizures and other NP events
Of the 91 seizures 36 (39%) occurred in the absence of other NP events within the same period of observation; 28 (31%) presented concurrently with other NP events attributed to SLE (model B) and 27 (30%) with other NP events not attributed to SLE (model B). Seizures attributed to SLE (model B) were more frequent in the absence of other NP events compared to those not attributed to SLE (34/78 (44%) vs 2/13 (15%)) although statistical significance was not achieved (p=0.07, Fisher’s exact test).
Predictors of seizures
Single-factor analyses revealed that female gender, race/ethnicity, lack of post-secondary education, younger age at SLE diagnosis, SDI (total), SDI (without NP) and SDI (without seizures) score were individually associated with a higher risk of first occurrence of seizures (Table 4).
Table 4.
Single-factor ANOVA of clinical and laboratory variables at enrollment and occurrence of seizures regardless of attribution (95% CI)).
| Predictor | N* | Hazard Ratio | Lower 95% CI | Higher 95% CI | P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Female gender | 75 | 0.48 | 0.27 | 0.84 | 0.010 | |
|
| ||||||
| Race/Ethnicity | Caucasian | 75 | 0.036 | |||
| Asian | 0.47 | 0.18 | 1.21 | |||
| African | 1.70 | 0.95 | 3.03 | |||
| Hispanic | 1.79 | 0.99 | 3.23 | |||
| Other | 1.18 | 0.36 | 3.85 | |||
|
| ||||||
| Geography | Canada | 74 | 0.307 | |||
| USA | 1.25 | 0.63 | 2.47 | |||
| Europe | 1.34 | 0.68 | 2.64 | |||
| Asia | 0.81 | 0.29 | 2.24 | |||
| Mexico | 2.01 | 0.96 | 4.23 | |||
|
| ||||||
| Post-secondary education | No | 70 | 2.15 | 1.34 | 3.44 | 0.002 |
|
| ||||||
| Age at diagnosis† | Linear | 75 | 0.83 | 0.64 | 1.07 | 0.050 |
| Quadratic | 1.22 | 1.04 | 1.43 | |||
|
| ||||||
| SLEDAI-2K score‡ | Linear | 26 | 1.43 | 0.88 | 2.32 | 0.315 |
| Quadratic | 0.94 | 0.81 | 1.10 | |||
|
| ||||||
| SLEDAI-2K score (w/o NP variables) | Linear | 25 | 1.18 | 0.72 | 1.93 | 0.792 |
| Quadratic | 0.97 | 0.84 | 1.14 | |||
|
| ||||||
| SLEDAI-2K score (w/o seizure) | Linear | 25 | 1.32 | 0.80 | 2.20 | 0.553 |
| Quadratic | 0.94 | 0.79 | 1.12 | |||
|
| ||||||
| SDI score | 21 | 1.41 | 1.05 | 1.90 | 0.024 | |
|
| ||||||
| SDI score (w/o NP variables) | 20 | 1.44 | 1.05 | 1.99 | 0.025 | |
|
| ||||||
| SDI score (w/o seizure) | 20 | 1.40 | 1.03 | 1.89 | 0.033 | |
|
| ||||||
| Medications | Corticosteroids | 26 | 6.29 | 1.41 | 28.02 | 0.015 |
| Antidepressants | 0.86 | 0.26 | 2.88 | |||
| ASA, Warfarin anticoagulant | 1.69 | 0.75 | 3.78 | |||
| Anti-malarials | 0.443 | 0.20 | 0.97 | |||
| Immuno-suppressants | 1.23 | 0.52 | 2.91 | |||
|
| ||||||
| Lupus anticoagulant | 51 | 1.58 | 0.87 | 2.89 | 0.135 | |
|
| ||||||
| Anticardiolipin | 50 | 1.31 | 0.61 | 2.78 | 0.488 | |
|
| ||||||
| Anti-β2-GPI | 50 | 1.50 | 0.75 | 3.01 | 0.248 | |
|
| ||||||
| Anti-ribosomal P | 50 | 0.58 | 0.18 | 1.87 | 0.364 | |
|
| ||||||
| Anti-NR2 | 44 | 0.47 | 0.15 | 1.52 | 0.207 | |
N is the total number of available events (first occurrence of seizures) for analysis.
Age at diagnosis was standardized by taking (Age at diagnosis-35)/14.
SLEDAI-2K and SLEDAI-2K (w/o NP variables) were standardized by taking (SLEDAI-2K-4)/4.
Concurrent medications were also jointly associated with the risk of first seizure occurrence. In particular the current use of corticosteroids and anti-malarial drugs were associated with increased and reduced risks, respectively. There was no association with autoantibodies individually (Table 4) or after combining lupus anticoagulant, IgG anticardiolipin or anti-β2 glycoprotein-I into a single antiphospholipid (aPL) panel either for all seizures (HR (CI) 1.42 (0.82–2.47) or for seizures attributed to SLE (model B) (1.37 (0.78–2.47). Among 1070 patients with available data for all 3 antibodies, 101 (9.4%) of them had >1 aPL, 279 (26.1%) had only 1 aPL. Likewise there was no association with individual SLEDAI-2K scores. Using the adjusted mean SLEDAI-2K (20) (AMS), 9/25 events which occurred prior to the first follow-up assessment, were excluded as computing AMS scores requires a minimum of 2 SLEDAI-2K scores. Nevertheless, there was evidence of a relationship with seizure occurrence based on these analyses (p=0.07 for AMS (w/o NP variables) and p=0.04 for AMS (w/o seizures)). Similar results were obtained for seizures attributed to SLE (model B) with the exception that the association with SDI scores (without seizures) and current use of anti-malarial drugs did not reach statistical significance at the 0.05 level.
Multivariate analysis (Table 5, analysis I) indicated a significant relationship with race/ethnicity (global test p-value = 0.03) with a higher risk of seizures in African race/ethnicity (HR (CI): 1.97 (1.07–3.63)). Higher risk was also associated with lack of post-secondary education (1.96 (1.21–3.19); p=0.006) after adjustment for gender, race/ethnicity and age at diagnosis, and there was no interaction between race/ethnicity and education (p=0.964). Inclusion of SDI scores in the analysis (Table 5, analysis II) reduced the available events for analysis from 70 to 20 as SDI scores cannot be computed in patients with <6 months disisease duration. Nevertheless, when SDI scores were included, post-secondary education remained an important predictor and the association with race/ethnicity was reduced. Adjusting for gender, race/ethnicity, age at diagnosis and post-secondary education, patients with higher SDI scores (without NP) were associated with an increased risk of subsequent seizures, reaching statistical significance when SDI scores were treated both as a continuous variable (1.50 (1.04–2.17); p=0.03) and as a categorical variable (SDI = 1:3.93 (1.46–10.55); SDI = 2 or 3:1.57 (0.32–7.65); SDI≥4:7.86 (0.89–69.06); p=0.03). After adjustment for prior medications (Table 5, analysis III), this risk was less significant (p=0.07) demonstrating confounding between disease severity and medications. An overall association with the four specified medication variables was found (global test p-value=0.04). Corticosteroids were associated with an increased risk of seizures but with a wide confidence interval. Prior use of anti-malarial drugs in the absence of immunosuppressive agents was the most notable treatment effect on seizures, conferring a reduced risk (0.07 (0.01–0.66)). A formal test for an interaction between anti-malarial drugs and immunosuppressive agents was significant (p=0.03).
Table 5.
Multivariate analysis of clinical and laboratory variables at enrollment and occurrence of seizures regardless of attribution. The total numbers of first seizures for Analysis I–IV are 70, 20, 20 and 25, respectively*.
| Predictor | Analysis I | Analysis II | Analysis III | Analysis IV | ||||
|---|---|---|---|---|---|---|---|---|
| HR** (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
|
| ||||||||
| Female gender | 0.61 (0.32,1.15) | 0.13 | 0.88 (0.25,3.04) | 0.84 | 1.10 (0.32,3.82) | 0.88 | 1.35 (0.39,4.62) | 0.64 |
| Race/Ethnicity | 0.03 | 0.24 | 0.57 | 0.43 | ||||
| Caucasian | ||||||||
| Asian | 0.47 (0.18,1.23) | Not estimable | Not estimable | Not estimable | ||||
| African | 1.97 (1.07, 3.63) | 3.05 (0.99,9.45) | 2.12 (0.67,6.68) | 1.86 (0.67,5.14) | ||||
| Hispanic | 1.56 (0.80, 3.04) | 1.82 (0.48,6.95) | 1.32 (0.33,5.34) | 1.11 (0.34,3.66) | ||||
| Other | 1.34 (0.41,4.42) | 1.13 (0.28,4.61) | 0.98 (0.23,4.09) | 0.68 (0.20,2.34) | ||||
| Lack of post- secondary education | 1.96 (1.21, 3.19) | <0.01 | 4.55 (1.68,12.33) | <0.01 | 4.07 (1.50,11.07) | <0.01 | 3.05 (1.31,7.10) | <0.01 |
| Age at diagnosis* | ||||||||
| Linear | 0.83 (0.63, 1.09) | 0.09 | 0.91 (0.53,1.57) | 0.92 | 0.93 (0.53,1.63) | 0.96 | 0.93 (0.57,1.52) | 0.95 |
| Quadratic | 1.21 (1.02,1.44) | 0.99 (0.67,1.46) | 1.00 (0.68,1.49) | 0.99 (0.67,1.45) | ||||
| SDI score (w/o NP variables) | 0.03 | 0.07 | ||||||
| 0 | ||||||||
| 1 | 3.93 (1.46,10.55) | 3.36 (1.26,9.02) | ||||||
| 2 or 3 | 1.57 (0.32,7.65) | 1.22 (0.25,5.95) | ||||||
| ≥4 | 7.86 (0.89,69.06) | 5.22 (0.58,46.70) | ||||||
| Medications | 0.04 | 0.01 | ||||||
| Corticosteroids | 9.01 (1.14,71.44) | 5.87 (1.29,26.70) | ||||||
| Antimalarials | 0.07 (0.01,0.66) | 0.10 (0.02,0.50) | ||||||
| Immunosuppressants | 0.39 (0.11,1.43) | 0.46 (0.15,1.39) | ||||||
| Interaction (Antimalarials, Immunosuppressants) | 13.85 (1.21,158.05) | 8.56 (1.33,55.16) | ||||||
The number of patients included in Analysis I was reduced to 70 due to missing data on 5 patients for post-secondary education status. In Analysis II and III the number was reduced to 20 because of the small number of SDI scores available due either to the inability to compute scores at the enrollment visit in patients with disease duration < 6 months or the occurrence of seizures prior to the enrollment visit.
Hazard ratio
Age at diagnosis was standardized by taking (Age at diagnosis-35)/14.
Without adjusting for SDI scores (see Table 5, analysis IV), the global medication test generated a p-value of 0.01 with again a significant interaction (p=0.02). This result derives primarily from a comparison of 7 events in 715 observation intervals for patients not taking immunosuppresants or anti-malarials versus 2 events in 2210 observation intervals for patients taking only anti-malarials. Because of missing values, adjustment for AMS (w/o NP variables) alters this finding because 5 events and 225 intervals in the first group and 1 event and 655 intervals in the second group are excluded so the interaction, and the global medication effect, is not detected. If the missing AMS values are replaced by the current SLEDAI-2K values, the significant findings are again seen. Thus, the paucity of data makes a definitive statement on the possible confounding of medication and disease activity effects difficult. Similar results were obtained for seizures attributed to SLE (model B).
Comparable results were also obtained in a multivariate analysis of medication effects based on the inclusion of propensity scores for the use of steroids and anti-malarials alone. Factors included in the propensity scores included all those in Table 5, analysis III, as well as SLEDAI-2K. More severe disease was linked to steroid use while less severe disease was linked to anti-malarials alone. In this analysis, steroid use had an estimated HR of 9.9 with a confidence interval of (1.26, 78.57) while anti-malarials alone had an estimated HR of 0.08 (0.01, 0.70).
Outcome of seizures
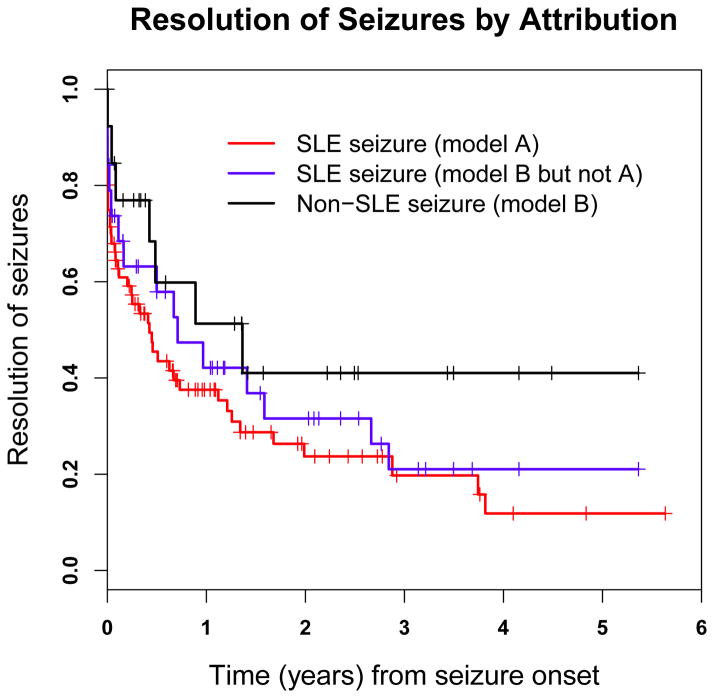
Using physician assessment (Figure 1), seizures attributed to SLE were more likely to resolve (59/78, (76%)) compared to seizures attributed to non-SLE causes (7/13, (54%)). While more patients with seizures attributed to SLE stopped taking anti-seizure drugs at the first followup assessment compared to patients with seizures attributed to non-SLE causes (19/66=29% vs. 2/12=17%) and similarly by the second followup assessment (16/50=32% vs. 1/9=11%), the small number of seizures (especially non-SLE seizures) during the follow-up period precluded formal statistical testing. Using patient self-report the mean (± SD) MCS score in patients with seizures and no other NP events was comparable to patients without NP events (45±13.1 vs. 48.5 ± 10.9; p=0.21). A similar outcome was seen in the mean (± SD) PCS scores (40.0 ± 10.8 vs. 42.8 ± 11.2; p=0.34).
Figure 1.
Kaplan-Meier curves for time to resolution of seizures incorporating attribution models A and B over the study period in SLE patients.
Discussion
Seizures are a manifestation of NPSLE but may follow a different course to idiopathic seizures. This has implications for diagnosis and treatment. Previous studies have been limited by cross-sectional design, small sample size and short duration of follow-up. We studied a large, prospective, international, multi-ethnic, disease inception cohort of adult SLE patients. The risk of seizures was higher in patients of African race/ethnicity, lower educational status and in patients with organ damage outside of the nervous system. Most seizures were attributable to lupus and occurred in close proximity to the diagnosis of SLE. Most seizures resolved, did not require long-term anti-seizure medication and were not associated with a negative impact on mental or physical health-related quality of life. The association with lupus related therapies was complex but anti-malarial drugs were associated with a protective effect.
Prevalence estimates of seizures in SLE have varied between 6% (3) and 51%.(5) The SLICC cohort is well positioned to determine the frequency of seizures due to the size of the cohort, wide global geographic distribution, enrollment of patients close to diagnosis, standardized data collection and attribution rules. The cumulative frequency of 4.6% over a mean of 3.5 years in our study is comparable to other large studies. For example, 600 patients in the LUMINA cohort (21) had a frequency of 6.7%, usually early in the disease course. Gonzalez-Duarte et al (22) studied 1200 SLE patients of variable disease duration and reported long-term outcomes for a subset of the cohort over a mean of 5 years. Seizures occurred in 12.5% of patients, 54% of them within one year of SLE diagnosis, and recurred in 53%.
Although single-factor analyses identified several variables at enrollment with subsequent seizures, African race/ethnicity and lack of post-secondary education remained significant in multivariate analysis. The same association with race/ethnicity was reported in the LUMINA cohort.(21) Lack of education is a surrogate for socioeconomic status (23) and a predictor of functional status (24) and mortality (25) in SLE. In the present study the association with education may be due to impaired access or adherence to anti-seizure or lupus related therapies. The association with cumulative organ damage has been reported in the LUMINA cohort (21) and also in the current study albeit with some confounding with concurrent medication use. Overall, these data indicate that lupus patients with seizures have more severe disease both within and without the nervous system.
Previous studies have noted that seizures occur in patients with higher global SLE disease activity.(21, 26, 27) We did not find an association with higher SLEDAI-2K scores, even in patients whose seizures were attributed to SLE. As SLEDAI-2K scores were computed at the enrollment and follow-up visits, rather than at the time of seizure occurrence, they do not reflect global disease activity at the precise time of the NP event. However, the association with AMS and with corticosteroids supports the likelihood that SLE patients with seizures likely had more active SLE.
The autoantibodies selected for study reflected those most frequently associated with NPSLE. Antiphospholipid antibodies have previously been reported in association with seizures in some (26, 27) but not all studies (22) and no such association was found in the current study. This discrepancy may be due to differences in patient selection and study design. Alternatively, autoantibody status at the enrollment visit only may be insufficient to determine the true risk of these autoantibodies. Rather, an assessment of autoantibodies over time may be required to identify a potential clinical-serological linkage.
Of considerable interest is the inverse association between seizures and anti-malarials which suggests a possible protective effect, an observation also reported in the LUMINA cohort.(21) The potential for confounding by indication was addressed in the current study by propensity scores and regression modeling in the analysis. Both approaches produced similar results in keeping with the findings of Shah et al.(28) Although the precise mechanism remains to be determined, our findings further supports the short-term (29) and long-term (30–33) benefit associated with anti-malarial use in SLE. This may also explain the ability to discontinue anticonvulsant therapy which was more frequent in patients with seizures attributed to SLE. Overall, seizure disorders in SLE patients have a favourable outcome as indicated by a lower recurrence rate, more frequent discontinuation of anti-seizure medications, and no detectable impact on patient self-report HRQoL.
There are limitations to the current study. First, a measure of global SLE disease activity was not universally available at close proximity to the time of seizure occurrence which limited the ability to examine the association with generalized disease activity. Second, the association with persistent autoantibodies needs to be examined in subsequent studies. Third, the number of patients with seizures attributed to non-SLE causes was relatively small which limited the power of some statistical analyses. Finally, although the duration of follow-up extended to 12 years, the mean follow-up was 3.5 years which is insufficient to capture the life-time experience of SLE patients with seizures. Despite these limitations, the size and characteristics of the SLICC NPSLE inception cohort and the standardized approach to data collection and analyses has provided information which will help to guide management of seizure disorders in SLE patients worldwide. Our findings support the recent EULAR recommendations on seizures in SLE patients.(34)
Supplementary Material
Acknowledgments
Financial support:
J.G. Hanly (Canadian Institutes of Health Research grant MOP-57752, Capital Health Research Fund)
Li Su (MRC(UK) U105261167), V. Farewell (MRC(UK) U105261167).
Dr. Sang-Cheol Bae’s work was supported by the Korea Healthcare technology R & D project, Ministry for Health and Welfare, Republic of Korea (A080588).
The Montreal General Hospital Lupus Clinic is partially supported by the Singer Family Fund for Lupus Research. Dr. Clarke is a National Scholar of the Fonds de la recherché en santé de Quebec.
Dr. Paul R. Fortin is a Distinguished Senior Investigator of The Arthritis Society with additional support from the Arthritis Centre of Excellence, University of Toronto.
Dr. Ramsey-Goldman’s work was supported by the NIH (grants UL-1RR-025741, K24-AR-02318, and P60-AR-48098).
Dr. Ruiz-Irastorza is supported by the Department of Education, Universities and Research of the Basque Government.
References
- 1.Ainiala H, Loukkola J, Peltola J, et al. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57:496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58:1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, McCurdy G, Fougere L, et al. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31:2156–62. [PubMed] [Google Scholar]
- 4.Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30:985–92. [PubMed] [Google Scholar]
- 5.Sibbitt WL, Jr, Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29:1536–42. [PubMed] [Google Scholar]
- 6.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56:265–73. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 7.Isenberg D, Ramsey-Goldman R. Systemic Lupus International Collaborating Group--onwards and upwards? Lupus. 2006;15:606–7. doi: 10.1177/0961203306071868. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 9.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 10.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 11.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45:419–23. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Hanly JG, Urowitz MB, Su L, et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum. 2008;59:721–9. doi: 10.1002/art.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanly JG, Urowitz MB, Jackson D, et al. SF-36 summary and subscale scores are reliable outcomes of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:961–967. doi: 10.1136/ard.2010.138792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thumboo J, Fong KY, Ng TP, et al. Validation of the MOS SF-36 for quality of life assessment of patients with systemic lupus erythematosus in Singapore. J Rheumatol. 1999;26:97–102. [PubMed] [Google Scholar]
- 16.Merrill JT, Zhang HW, Shen C, et al. Enhancement of protein S anticoagulant function by beta2-glycoprotein I, a major target antigen of antiphospholipid antibodies: beta2-glycoprotein I interferes with binding of protein S to its plasma inhibitor, C4b-binding protein. Thromb Haemost. 1999;81:748–57. [PubMed] [Google Scholar]
- 17.Merrill JT, Shen C, Gugnani M, et al. High prevalence of antiphospholipid antibodies in patients taking procainamide. J Rheumatol. 1997;24:1083–8. [PubMed] [Google Scholar]
- 18.Erkan D, Zhang HW, Shriky RC, et al. Dual antibody reactivity to beta2- glycoprotein I and protein S: increased association with thrombotic events in the antiphospholipid syndrome. Lupus. 2002;11:215–20. doi: 10.1191/0961203302lu178oa. [DOI] [PubMed] [Google Scholar]
- 19.Hanly JG, Urowitz MB, Siannis F, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58:843–53. doi: 10.1002/art.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibanez D, Gladman DD, Urowitz MB. Adjusted mean Systemic Lupus Erythematosus Disease Activity Index-2K is a predictor of outcome in SLE. J Rheumatol. 2005;32:824–7. [PubMed] [Google Scholar]
- 21.Andrade RM, Alarcon GS, Gonzalez LA, et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV) Ann Rheum Dis. 2008;67:829–34. doi: 10.1136/ard.2007.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Duarte A, Cantu-Brito CG, Ruano-Calderon L, et al. Clinical description of seizures in patients with systemic lupus erythematosus. Eur Neurol. 2008;59:320–3. doi: 10.1159/000121423. [DOI] [PubMed] [Google Scholar]
- 23.Jolly M, Mikolaitis RA, Shakoor N, et al. Education, zip code-based annualized household income, and health outcomes in patients with systemic lupus erythematosus. J Rheumatol. 2008;37:1150–7. doi: 10.3899/jrheum.090862. [DOI] [PubMed] [Google Scholar]
- 24.Callahan LF, Pincus T. Associations between clinical status questionnaire scores and formal education level in persons with systemic lupus erythematosus. Arthritis Rheum. 1990;33:407–11. doi: 10.1002/art.1780330315. [DOI] [PubMed] [Google Scholar]
- 25.Ward MM. Education level and mortality in systemic lupus erythematosus (SLE): evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheum. 2004;51:616–24. doi: 10.1002/art.20526. [DOI] [PubMed] [Google Scholar]
- 26.Appenzeller S, Cendes F, Costallat LT. Epileptic seizures in systemic lupus erythematosus. Neurology. 2004;63:1808–12. doi: 10.1212/01.wnl.0000144178.32208.4f. [DOI] [PubMed] [Google Scholar]
- 27.Mikdashi J, Krumholz A, Handwerger B. Factors at diagnosis predict subsequent occurrence of seizures in systemic lupus erythematosus. Neurology. 2005;64:2102–7. doi: 10.1212/01.WNL.0000165959.98370.D5. [DOI] [PubMed] [Google Scholar]
- 28.Shah BR, Laupacis A, Hux JE, et al. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol. 2005;58:550–9. doi: 10.1016/j.jclinepi.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 29.A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. N Engl J Med. 1991;324:150–4. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 30.Fessler BJ, Alarcon GS, McGwin G, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52:1473–80. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15:577–83. doi: 10.1177/0961203306071872. [DOI] [PubMed] [Google Scholar]
- 32.Alarcon GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann Rheum Dis. 2007;66:1168–72. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinjo SK, Bonfa E, Wojdyla D, et al. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum. 2010;62:855–62. doi: 10.1002/art.27300. [DOI] [PubMed] [Google Scholar]
- 34.Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69:2074–82. doi: 10.1136/ard.2010.130476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.