Abstract
Background
Temporomandibular disorders (TMD) have a relatively high prevalence and in many patients pain and masticatory dysfunction persist despite a range of treatments. Non-invasive brain neuromodulatory methods, namely transcranial direct current stimulation (tDCS), can provide relatively long-lasting pain relief in chronic pain patients.
Objective
To define the neuromodulatory effect of five daily 2×2 motor cortex high-definition tDCS (HD-tDCS) sessions on clinical pain and motor measures in chronic TMD patients. It is predicted that M1 HD-tDCS will selectively modulate clinical measures, by showing greater analgesic after-effects compared to placebo, and active treatment will increase pain free jaw movement more than placebo.
Methods
Twenty-four females with chronic myofascial TMD pain underwent five daily, 20-minute sessions of active or sham 2 milliamps (mA) HD-tDCS. Measurable outcomes included pain-free mouth opening, visual analog scale (VAS), sectional sensory-discriminative pain measures tracked by a mobile application, short form of the McGill Pain Questionnaire, and the Positive and Negative Affect Schedule. Follow-up occurred at one-week and four-weeks post treatment.
Results
There were significant improvements for clinical pain and motor measurements in the active HD-tDCS group compared to the placebo group for: responders with pain relief above 50% in the VAS at four-week follow-up (p=0.04); pain-free mouth opening at one-week follow-up (p<0.01); and sectional pain area, intensity and their sum measures contralateral to putative M1 stimulation during the treatment week (p<0.01). No changes in emotional values were shown between groups.
Conclusion
Putative M1 stimulation by HD-tDCS selectively improved meaningful clinical sensory-discriminative pain and motor measures during stimulation, and up to four weeks post-treatment in chronic myofascial TMD pain patients.
Keywords: transcranial direct current stimulation, temporomandibular disorder, pain, PainTrek, clinical trial
Introduction
Temporomandibular disorders (TMD) have a relatively high prevalence [1] and in many patients pain and masticatory dysfunction persist despite a range of treatments [2]. Chronic pain can be caused by an untreated peripheral insult, by sensitization of the central nervous system, or both [3]. As such, resolving pain in a patient with longstanding symptoms might involve addressing both the peripheral source of the pain and the central nervous system changes that facilitate or augment nociceptive signals along the affected pathway. Several studies with motor cortex stimulation (MCS) have shown that epidural electrodes in the primary motor cortex (M1) are effective in providing analgesia in patients with refractory central pain [4-6]. Evidently, the invasive nature of such a procedure limits its indication to highly severe chronic pain disorders. However, among the methods of central neurostimulation, two of them, repetitive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), are appealing as they can change brain activity in a non-invasive and safe way. There is a growing body of scientific evidence that both methods can provide relatively lasting pain relief in chronic trigeminal pain patients [7-9], and even modulate and activate the μ-opioid system [10, 11]. The advantages of tDCS are small portable size and relatively low cost; nevertheless, the electric fields generated by conventional tDCS analgesic montages are widely distributed across the brain, lacking specificity on the pain-related structures directly targeted. Recently, a novel high-definition tDCS (HD-tDCS™) approach was able to more precisely target the cortical areas of interest [12]. Our group further optimized the HD-tDCS montage for non-invasive putative M1 modulation following neurological (e.g., homunculus) and technical (e.g., direction of the current) MCS principles for effectual analgesia.
Given the persistent sensory and motor clinical dysfunction reported in chronic TMD patients, and the opportunity for non-surgical modulation of cortical function, this study aims to define the effect of five daily putative M1 HD-tDCS sessions on chronic pain measures in myofascial TMD, specifically general and sectional sensory-discriminative pain measurements (e.g., pain area and intensity), and evaluate its impact on pain-free jaw mobility. It is predicted that M1 HD-tDCS will selectively modulate clinical measures, by showing greater analgesic after-effects compared to placebo, and active treatment will increase pain free jaw movement more than placebo.
Materials and Methods
This was a randomized, placebo-controlled, single-blind, parallel-group study conducted in a research-only outpatient hospital setting. Participants were enrolled September-November 2013 and data was collected September 2013-January 2014.
Participants
Subjects with chronic myofascial TMD pain were recruited using flyers, the patient-clinical study matching service www.umclinicalstudies.org, and by referral from the TMD and Orofacial Pain Clinic at the University of Michigan. This was followed by a TMD physical exam performed by an orthodontic resident, trained and supervised by an orofacial pain specialist, according to the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD). We used the following inclusion criteria: A) between the ages of 18 to 65; B) daily chronic TMD pain and dysfunction for at least one year matching RDC/TMD Axis I Group I: Myofascial pain diagnosis [13] not adequately controlled by previous conventional therapies (TMJ open-surgery naïve) for more than 1 year; C) with self-reported pain score of at least 3 on a 0-10 scale in spite of existing treatment in the two weeks preceding the onset of the study; D) subjects that were taking stable doses of medications for at least 4 weeks, and willing to limit the introduction of new medications for chronic TMD symptoms during the study. Exclusion criteria: A) if pain was NOT primarily due to masticatory myofascial TMD; B) history or current evidence of neurological disorder (e.g. epilepsy, major depression, stroke, neuropathy, neuropathic pain); C) pregnant or expecting to become pregnant during the study.
The University of Michigan Institutional Review Board approved the study (HUM00070766), and written informed consent was obtained from all participants.
Temporomandibular Disorder Diagnosis and Pain Assessment
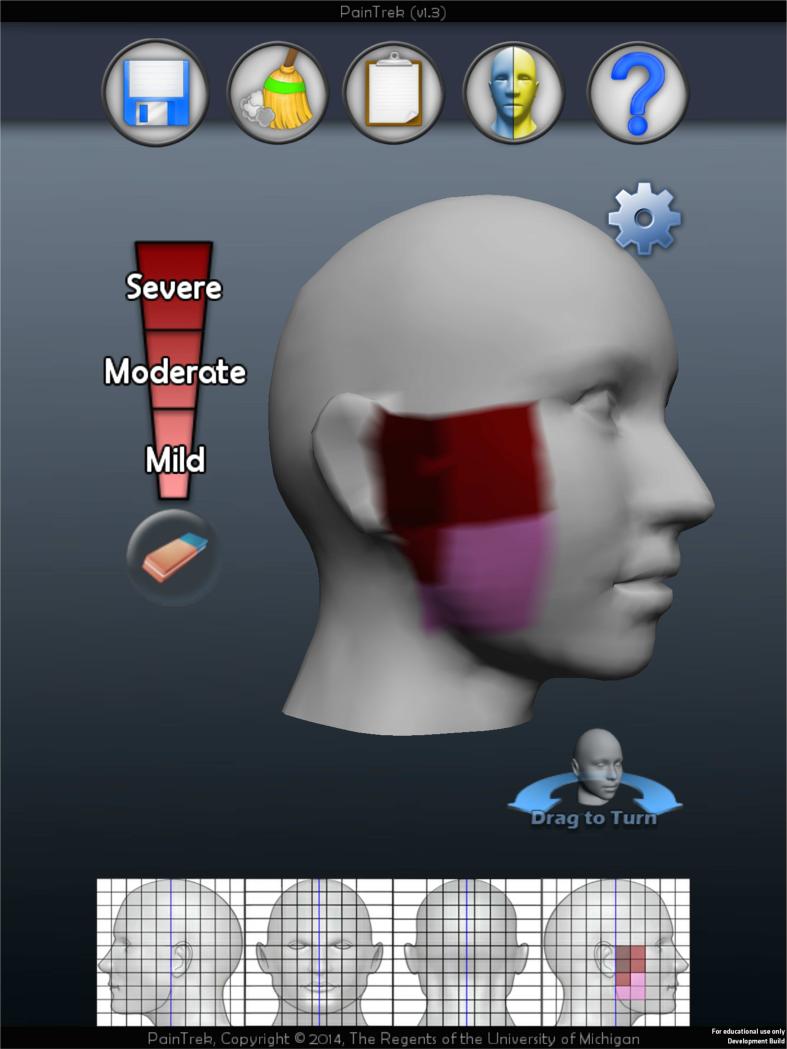
TMD pain was measured using the visual analog scale (VAS), short form of the McGill Pain Questionnaire (SF-MPQ), and PainTrek. The VAS allows the patient to self-rate his or her pain on a 0-10 horizontal grading scale and is widely used in studies measuring pain outcomes. It has been shown to be both a valid and reproducible pain measure [14]. Any patient with a VAS decrease of 50% or greater from week one to week six was considered a responder, as defined by previous tDCS pain studies [15-17]. The SF-MPQ is a valid pain measure that allows subjects to describe their sensory and affective pain experiences using 15 adjectives [18]. PainTrek is an in-house and free mobile application developed by the Headache and Orofacial Pain Effort (H.O.P.E.) at the University of Michigan. PainTrek provides a 3-D head and facial map based on a squared grid system with vertical and horizontal coordinates using anatomical landmarks. Each quadrangle, measuring approximately 1.6 cm × 1.6 cm, frames well-detailed craniofacial and cervical areas for the patient to express his or her exact global and sectional pain location and intensity, as well as associated signs and symptoms.
Using the application on an iPad® (Apple Inc., Cupertino), participants drew their pain in one of three shades of red on the touch sensitive screen: pink shade (mild pain), red shade (moderate pain), and dark red shade (severe pain), see Figure 1. The pain drawing was quantified by scoring each of the 220 cells on a 0-3 point scale: 0- no pain, 1- mild pain, 2- moderate pain, and 3- severe pain. From these values, three pain measures were generated from the PainTrek drawing. Average pain was the average score of all cells that are marked as painful, with a scale of 1-3. Pain area was the percent of the area of the head and neck that was experiencing pain, with a scale of 0-100% of all cells. At last, Pain Area and Intensity Number Summation (P.A.I.N.S.) was the cumulative score for the 220 cells, with a scale of 0-660 (equal to 220 × 3 for maximum severe pain). For the three pain measures, the analysis was performed for the entire head and neck area, or unilaterally, to understand how sensory-discriminative pain measures changed ipsilateral or contralateral to the putative M1 stimulation.
Figure 1.
Example of a pain drawing in PainTrek. In this example, the pain is depicted as severe immediately anterior to the patient's right ear, moderate along the right zygoma and ramus of the mandible, and mild in the area of mandibular angle. All grey areas have no pain.
The Positive and Negative Affect Schedule (PANAS), a reliable and valid affect measure, was used to assess if mood was a co-factor in pain experience [19].
General Study Design
Participants were initially screened by phone. After clinical exam and diagnosis of RDC/TMD Axis I Group: myofascial pain, participants were randomized to the treatment or placebo group using the Taves covariate adaptive randomization method. Using this method, a new participant was sequentially assigned to a group based upon the previous assignments of other participants and certain covariates [20]. The first eight participants were randomized by coin flip and the remaining participants were randomized by age and gender, following the Taves covariate adaptive randomization protocol.
Once assigned to the active or sham HD-tDCS group, participants presented during week one for a baseline visit. At this visit VAS, PANAS, SF-MPQ and PainTrek data were collected and a TMD exam was performed
HD-tDCS: Neuromodulation Protocol
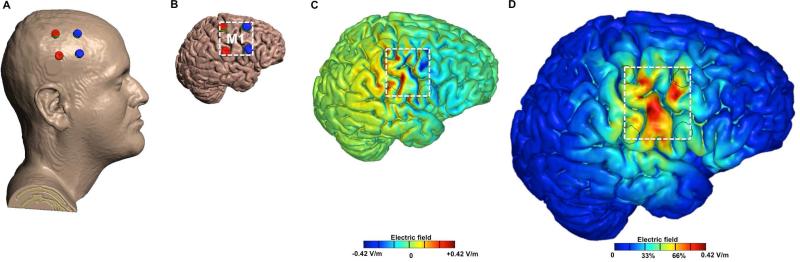
During week two, subjects participated in five-daily sessions of either active or sham HD-tDCS. A modified 2×2 HD-tDCS montage was developed and used, with four electrodes arranged at the corners of a 4 cm × 4 cm square centered over the caudal portion of the putative M1 (Figure 2), where the homuncular head and facial region is represented.
Figure 2.
Figure caption: Brain modulation using 2×2 HD-tDCS Montage. A. 3D rendered head built from the MRI derived segmentation masks used in the study. Anode electrodes (red) were placed over C3 and C5 and Cathode electrodes were placed over FC3 and FC5. B. Skin,skull, and CSF masks are suppressed to reveal the underlying gray matter mask. C. Induced cortical surface electric-field directional plot for 2 mA stimulation. Red denotes inward (anodal) stimulation while blue denotes outward (cathodal) stimulation. D. Induced cortical surface electric-field magnitude plot for 2 mA stimulation.
The putative M1 stimulation area (Brodmann area 4) was estimated using the International Electroencephalography (EEG) 10-20 system, with the upper anode placed on C3 and lower anode on C5, and the upper and lower cathodes placed anteriorly on FC3 and FC5, respectively. The computational model shown in Figure 2 corroborated that our HD-tDCS montage produced maximum (postero-anterior) current flow on the putative M1 head and facial in the precentral gyrus region, immediately anterior to the central sulcus, and at the level of the inferior frontal sulcus [21]. An individualized finite element (FE) head model [22] was from a 1 mm3 resolution MRI scan of a neurologically normal adult male. Using a combination of tools (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK; Simpleware Ltd, Exeter, UK), the HD-tDCS electrodes were modeled as computer-aided design (CAD) files and incorporated into the model. The FE mesh produced from the segmented tissue compartments and the electrodes was imported into COMSOL Multiphysics 4.3 (Burlington, MA) for computation of electric fields [23]. The standard Laplace equation with purely conductive properties was solved [24] and the following isotropic electrical conductivities were assigned (S/m): skin: 0.465, skull: 0.01, CSF: 1.65, grey matter: 0.276, white matter: 0.126, air: 10−15, conductive gel: 1.4, electrode: 5.99 ×107. Current density corresponding to 1 mA was applied to both anodes and ground applied to the two cathodes. The entire model workflow preserved precision beginning from the 1 mm3 resolution MRI to the induced cortical electric field maps in our 2×2 HD-tDCS montage [25].
During the HD-tDCS sessions, the patient's vertex was found by measuring the midpoint between nasion and inion and the midpoint between the pre-auricular areas. The midpoint from the vertex to the pre-auricular point on the side contralateral to the patient's worst pain was marked so that stimulation could be applied to the same spot each day. A perforated cloth cap with a chinstrap was then placed on the head to secure plastic casings in the desired position for each of the four electrodes, as described in the details above. Approximately 3 mL of Lectron II Conductivity Gel was injected into the electrode casings. Ag/AgCl sintered ring electrodes with the rough surface directed towards the skin were placed into the gel and held in place with plastic caps. For active HD-tDCS subjects, 2 mA of transcranial direct current stimulation was applied for 20 minutes. For sham-controlled HD-tDCS sessions, the same montage was used; however, the current was only applied for 30 seconds at the beginning and end of the session, as sensations arising from HD-tDCS treatment most frequently occur at the beginning and end of application [26]. Before and after each HD-tDCS session VAS, SF-MPQ, PANAS, and PainTrek data were collected.
Follow-up appointments were scheduled one-week and one-month after the HD-tDCS sessions, consistent with previous HD-tDCS pain studies [15, 27-32]. At these appointments, the RDC/TMD Axis I exam, VAS, SF-MPQ, PANAS, and PainTrek were completed.
Statistical Methods
The effect of treatment group on pain was analyzed using linear mixed regression models for two separate timeframes of analysis. The session effect of treatments was analyzed using pre- and post- treatment pain assessments during the 5 days of protocol treatment. The cumulative effect of treatment was explored using a separate model of pain scores from baseline, post treatment, one week follow-up and one month follow-up pain assessments. Models for each timeframe - treatment and study - were performed for all pain measures resulting in 8 total models for analysis. In addition, PainTrek was analyzed in 4 supplementary models treating ipsilateral and contralateral pain as distinct outcomes. P-values are reported from a two-sided t-test performed within the linear mixed regression model framework. Each pain measure was treated as a continuous independent variable for modeling. In the linear regression models, individuals were assigned a compound symmetry covariance structure for within subject correlation across repeated measures of the same pain index and a random effect for individual differences at baseline was employed. The distribution of each pain measure was explored graphically but no transformations were performed prior to modeling. VAS and PainTrek response rates, defined as >50% response, were also tested across treatment using one-sided Fisher Exact tests. All statistical analysis was performed using the SAS® system Version 9.2 (SAS Institute Inc., Cary, NC).
Results
Seventy-eight patients were screened for this study, and of those, 24 (30.8%) met the inclusion criteria, were enrolled, and completed the study (Supplementary Figure 1). The patients were randomized, with 12 per group; all were female, though males were eligible to participate and were screened. Demographic information, TMD diagnoses, and baseline pain values are presented in Supplementary Supplementary Table. All patients had an RDC/TMD Axis I Group I diagnosis of myofascial pain, and 10 in the active and 8 in the sham group had concurrent limited opening. Demographics and baseline values for VAS and pain free opening were comparable between groups. PainTrek pain area, intensity, and their sum values varied more at baseline, with p-values approaching significance.
TMD Outcomes
General Visual Analog Scale and McGill Pain Measures
General TMD pain relief was measured using VAS at baseline, before and after each HD-tDCS session, and at both follow-ups. Throughout the study more participants in the active HD-tDCS group compared to controls (X2=4.20; p=0.04) experienced pain relief, as defined by VAS decrease of 50% or greater from week one (baseline) to week 6, with nine high responders in the active group compared to four in the sham group (Table 1). The results of a one-sided Fisher's exact test yielded a similar p-value (p=0.0498). When “response” was defined as a moderate VAS decrease of 30% or more, we saw evidence of a trend for a better response rate in the active group after week six (X2=3.56; p=0.059), and a Fisher's Exact test yielded a similar result (p=0.08). There was no significant difference between groups in number of responders above 30% or 50% before week six.
Table 1.
VAS responders, defined by a decrease in VAS by 50% or greater from baseline to the one-month follow-up.
| VAS 50% Responders from Week 1 to Week 6 | |||
|---|---|---|---|
| Group | Active | Sham | Total |
| <50% VAS decrease | 3 | 8 | 11 |
| ≥50% VAS decrease | 9 | 4 | 13 |
| Total | 12 | 12 | 24 |
| Chi-Square | X2=4.1958 | p=0.04 | |
We also examined total descriptor values from the SF-MPQ across time points in the study, in addition to pre vs post treatment within-day effect, but neither one was significantly affected by treatment group. Total descriptor values decreased for both groups from week one to week two (p<0.0001) and thereafter remained lower than baseline scores. No significant differences were found between treatment groups for this effect (p=0.16).
Sectional Sensory-Discriminative Pain Measures Across the Head and Face
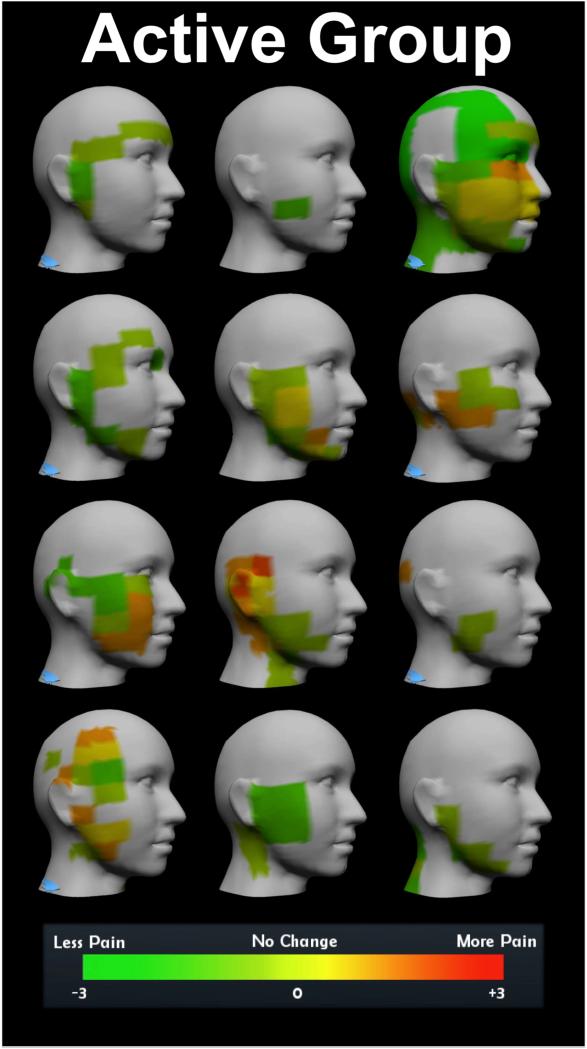
Three sectional clinical pain measures were generated from the PainTrek tool: pain area, average pain intensity, and their number sum. These dependent variables were analyzed bilaterally, taking into account the pain experience on both sides across the head and face, as well as unilaterally, analyzing separately the pain measures contralateral or ipsilateral to the side of HD-tDCS application. Patients received stimulation on the side of the head contralateral to their worst TMD pain. These pain measures were analyzed for a time effect, a group effect, and time*group interaction throughout the study, and for a time effect, group effect, pre-post HD-tDCS effect, and prepost*group interaction during the HD-tDCS sessions (Table 2). In the short term, there was evidence that during week two active HD-tDCS, compared to sham, was effective in immediately reducing pain area (p=0.008), intensity (p=0.0012), and their number sum (P.A.I.N.S.: p=0.01) for pre-post PainTrek measurements of the contralateral side to the stimulation (Figure 3). Over the long-term, contralateral change from baseline to week six was not significant across treatment groups (p=0.3852). As expected, no significant analgesic effects were noticed in pain measures ipsilateral to the stimulation at any time-point (Table 2).
Table 2.
Analysis of PainTrek pain measures: pain sum, average pain, and pain area. Separate analyses were performed accounting for pain bilaterally on the head and neck and unilaterally on the side contralateral or ipsilateral to tDCS stimulation. The time frame “Study” refers to pain measures at weeks 1, 3, and 6, while “Treatment” refers to pain measures before and after (PrePost) the tDCS sessions during week 2.
| Location | Time Frame | Effect | Pain Sum | Ave Pain | Pain Area |
|---|---|---|---|---|---|
| Bilateral | Study | Week | 0.0042 | <0.0001 | 0.0027 |
| Group | 0.0851 | 0.9845 | 0.1057 | ||
| Week*Group | 0.3450 | 0.6252 | 0.3403 | ||
| Treatment | Day | 0.0071 | <0.0001 | 0.0052 | |
| Group | 0.1186 | 0.4078 | 0.0977 | ||
| PrePost | 0.2945 | 0.0084 | 0.2237 | ||
| PrePost*Group | 0.6300 | 0.1176 | 0.3567 | ||
| Ipsilateral | Study | Week | 0.0132 | <0.0001 | 0.0095 |
| Group | 0.1170 | 0.6522 | 0.1623 | ||
| Week*Group | 0.3136 | 0.8908 | 0.2470 | ||
| Treatment | Day | 0.1713 | 0.0013 | 0.0139 | |
| Group | 0.2058 | 0.6991 | 0.1932 | ||
| PrePost | 0.2871 | 0.7001 | 0.4564 | ||
| PrePost*Group | 0.2107 | 0.9903 | 0.3297 | ||
| Contralateral | Study | Week | 0.0083 | <0.0001 | 0.0045 |
| Group | 0.0735 | 0.9820 | 0.0758 | ||
| Week*Group | 0.3852 | 0.0924 | 0.4903 | ||
| Treatment | Day | 0.0005 | <0.0001 | 0.0057 | |
| Group | 0.0747 | 0.4471 | 0.0553 | ||
| PrePost | 0.0007 | <0.0001 | 0.0035 | ||
| PrePost*Group | 0.0118 | 0.0012 | 0.0088 | ||
*p-value is for Type 3 test of fixed effect from linear mixed model for particular time frame (over study or over treatment) of particular dependent variable.
Figure 3.
PainTrek summary. Colors represent change in pain from baseline to one-month follow-up on the side of head contralateral to stimulation for each patient.
RDC/TMD Pain Free Mouth Opening
During the RDC/TMD exam, pain free mouth opening was measured (Table 3). Pain free opening significantly increased in the active group from week one to week three and then plateaued at healthy, functional opening values. Sham group patients did not experience such a drastic initial increase, indicating active group patients achieved greater pain free opening faster than the sham group patients. The results of a linear mixed model support this graphical observation. There was a significant difference in the change in pain-free opening from weeks one to three between groups (p<0.01), but not at week six.
Table 3.
RDC/TMD pain free mouth opening, measured during weeks 1, 3, and 6.
| RDC/TMD Pain Free Opening (SD), mm | ||||
|---|---|---|---|---|
| Group | Active | Sham | Group Effect, p | |
| Week | 1 | 28.7 (9.6) | 30.0 (12.3) | 0.77 |
| 3 | 40.1 (8.8) | 32.5 (10.5) | <0.01 | |
| 6 | 39.8 (8.9) | 36.8 (9.3) | 0.24 | |
| Week Effect, p | <0.01 | <0.01 | Week × Group, p=0.02 | |
Positive And Negative Affect Scale (PANAS)
Mood, as measured by PANAS, changed during the course of the study, but there were no significant differences between treatment groups. In most cases, Positive Affect Score decreased for patients in both groups from week one to week two and remained below baseline level thereafter. Negative Affect scores tended to stay around baseline levels throughout the study in both groups.
HD-TDCS Side Effects
There was a low rate of adverse events during the trial, and those that did occur were mild. Following each HD-tDCS session patients completed a side effects questionnaire rating each of 10 parameters on a 0-3 scale, representing side-effect absent, mild, moderate, or severe. They varied from headache to scalp burn (sensation). No skin lesions were observed under or neighboring the areas of stimulation by the investigators. The rate of each side effect occurring at least once per patient is presented in Table 4. During any given appointment, patients in the active group experienced 3.33 side effects per session while patients in the sham group experienced 3.32 side effects per session (out of 10 possible).
Table 4.
Rate of side effect occurring at least once per patient during the study.
| Rate of Side Effect Occurring At Least Once Per Patient | |||
|---|---|---|---|
| Group | Active | Sham | |
| Side Effect | Headache | 50.0% | 43.3% |
| Neck Pain | 35.0% | 31.7% | |
| Scalp Pain | 45.0% | 45.0% | |
| Scalp Burn (Sensation) | 43.3% | 30.0% | |
| Tingling | 56.7% | 60.0% | |
| Skin Redness | 16.7% | 3.3% | |
| Sleepiness | 60.0% | 61.7% | |
| Trouble concentrating | 18.3% | 31.7% | |
| Mood Change | 6.7% | 18.3% | |
| Other | 1.7% | 6.7% | |
Discussion
This study assessed the neuromodulatory effect of repetitive 2×2 M1 HD-tDCS on pain and motor dysfunction in patients with chronic myofascial TMD pain diagnosis. We have noticed very selective improvements for clinical measurements in the active HD-tDCS group, with more TMD patient “responders” to treatment above 50% pain relief at one-month follow-up, pain-free mouth opening at one-week follow-up, and pain area and intensity pain measures contralateral to the cortical stimulation during the treatment week. No changes in emotional values were noted with PANAS following treatment at any time point.
Central Mechanisms Associated with Somatotopic Analgesia
Direct M1 stimulation elicits contralateral somatotopic analgesia by inhibiting thalamic sensory neurons and disinhibiting the neurons in the PAG, an area rich in μ-opioid receptors [33]. In fact, a recent PET study from our group using C11 carfentanil, a selective μ-opioid radiotracer, showed that conventional M1-SO tDCS induces immediate μ-opioid activation in the PAG in humans [11]. Reciprocal connections from M1 to the primary somatosensory cortex (S1) could also contribute to topographical analgesic descending mechanisms for the precise modulation of facial sensory-discriminative and motor clinical measures in our active HD-tDCS group. However, as demonstrated in the computational model of current distribution, our montage mostly targeted the putative M1 head and facial homuncular region, not S1, with peak of intensity in the lower precentral gyrus at the level of the inferior frontal sulcus. Furthermore, the centered location of M1 between the anodes and cathodes facilitated the postero-anterior stimulation of the precentral gyrus’ superficial fibers, tangential to the surface. These parameters of current distribution in M1 are sought by other non-invasive and invasive neuromodulatory techniques to produce optimal pain relief, including transcranial magnetic stimulation (TMS) and MCS, respectively [34].
Sensory-Discriminative Pain Modulation with M1 HD-tDCS
The specific clinical analgesia noticed during the first week of HD-tDCS stimulation was highly selective and sectional. This suggests that measuring pain area, intensity, and their sum with PainTrek provided information that VAS alone does not, and can be a useful tool to more objectively assess suffering based on specific sensory-discriminative neuromechanisms. Nonetheless, general analgesic effects measured by VAS built up with time, reaching significance at the one-month follow-up, when responders to treatment had higher than 50% pain relief. As reported by other studies, clinical tDCS effects are cumulative and develop slowly, possibly due to resilient neuroplastic changes related to the chronic ascending pain inputs to the brain. The long-term general pain relief in our study probably incorporated other therapeutic perceptions by the TMD patients in the active group indirectly associated with cortical functions beyond the unilateral M1 modulation. For instance, M1 stimulation can induce either excitatory or inhibitory effects on M1 in the opposite cortical side, and several functional connectivity studies show that it can remotely influence other (sub)cortical regions with varied functions, such as cognitive-emotional (e.g., DLPFC). However, those indirect therapeutic effects seem to be subtle as they failed to show significance separately for the active HD-tDCS group in our study, at least in the sectional ipsilateral pain relief (PainTrek), McGill pain questionnaire values, and mood changes as measured by PANAS.
Placebo Effect with HD-tDCS
As expected, sham HD-tDCS had a positive time-effect in several clinical measurements. Pain-free opening significantly improved to healthy functional values in the active group from week one to week three compared to sham group, but not in week six when the sham group also ameliorated to similar levels. Recent studies show that placebo can activate part of the μ-opioid and dopaminergic mechanisms in the brain induced by M1 stimulation [35]. As it relates to TMD, placebo groups consistently perform in line with active treatment groups, and outperform no-treatment groups in TMD pain studies. A review of the placebo effect in TMD studies found that one-third of patients in blinded placebo groups had considerable or complete pain relief, and in studies where the placebo was augmented by writing prescriptions, patient education by the doctor, or elaborate treatment procedures the analgesic effect was seen in nearly two-thirds of placebo patients [36]. The sham group in this TMD HD-tDCS study was blinded, and the placebo was augmented by eight hours of appointments in a six-week time frame and an elaborate treatment procedure. Of the patients in the sham group, by week six of the study six of the 12 had no RDC-TMD Group I diagnosis, four had a VAS less than one, and five had a PainTrek pain sum score of two or less. As such, approximately one-third to one-half the placebo patients in this study experienced some kind of pain relief, which is in line with the published rate of placebo effect [36].
Side Effects
The overall rate of side effects in the active group was 33.3%, or 200 reported adverse events of a possible 600. In a systematic review, the most common side effects of tDCS, reported here with corresponding rates of occurrence in active groups, were itching (39.3%), tingling (22.2%), headache (14.8%), discomfort (10.4%), and burning sensation (8.7%) [37]. The rates of occurrence for similar side effects measured in the active group of our current study were tingling (56.7%), headache (50.0%), scalp pain (45.0%), and a burning sensation (43.3%). This study had a 0.8% occurrence rate of severe side effects in the active group on a 0-3 rating scale, compared to a 2.5% rate of severe side effects on a 1-5 rating scale in the largest published tDCS side effects survey [38]. Therefore, while the overall rate of side effects for this study was slightly higher than reported by the systematic review, the rate of severe side effects was lower than previously published.
2 × 2 M1 HD-tDCS Montage: Caveats and Lessons for Protocol Improvement
A recent Cochrane Review concluded that tDCS was not effective for treating chronic pain[39], however of the 14 studies included in the review only one used the high-definition montage. The 2 × 2 M1 HD-tDCS montage used in this study was a novel 2 × 2 electrode design. Two anode electrodes were placed posterior to the motor cortex and two cathode electrodes were placed anterior to the motor cortex, at the corners of a 4 × 4 cm square. The objective was to focally target M1, on a postero-anterior direction, however, with decreasing area of stimulation the margin for error for electrode placement becomes smaller. In fact, due to variations in skull and brain anatomy, localizing the M1 using the EEG 10-20 system is only accurate to within 21 mm [40]. Thus, a small error in landmark identification or any shift in electrode placement by the operator or patient could cause the area of peak current flow to miss the motor cortex. It has been shown using implants and tDCS that stimulation of the motor cortex can effectively treat chronic pain, but if stimulation is directed to an area adjacent to the motor cortex the effect on pain is less certain. In the context of this study, it is possible that between participants and between days a combination of ideal, marginal, and absent current flow to the motor cortex was delivered based upon accuracy of electrode placement and variations in individual anatomy. For future studies, the stimulation target could be confirmed by using TMS and/or image-guided navigation to help non-invasively identify the location of the motor cortex.
Conclusion
Stimulation using the 2 × 2 M1 HD-tDCS montage was effective for improving short-term, highly selective sensory-discriminative and motor clinical TMD measures compared to sham group. It also induced meaningful long-term general pain relief, defined as VAS decrease of 50% or more from week one to week six. While not definitive, non-invasive stimulation of the motor cortex using the novel 2 × 2 HD-tDCS montage demonstrated to be a reliable research tool to somatotopically modulate clinical pain and motor dysfunction related to TMD, and could potentially be used in the future as a less empirical therapeutic option for chronic pain conditions in the head and facial region, such as migraine. Our M1 montage could also be adapted for other pain conditions associated with neighboring homuncular regions. Nevertheless, additional studies should be performed to better understand the longer-term clinical effects and neuromechanisms associated with M1 HD-tDCS analgesia.
Supplementary Material
Acknowledgements
This project was funded by grants from the American Academy of Orofacial Pain and the University of Michigan Rackham Graduate School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8(3):291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 2.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993 Oct;124(10):115–21. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007 Aug 2;55(3):353–64. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008 Jun 10;70(24):2329–37. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 5.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–9. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 6.Velasco F, Arguelles C, Carrillo-Ruiz JD, Castro G, Velasco AL, Jimenez F, et al. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomized double-blind trial. J Neurosurg. 2008 Apr;108(4):698–706. doi: 10.3171/JNS/2008/108/4/0698. [DOI] [PubMed] [Google Scholar]
- 7.DaSilva AF, Datta A, Mendonca ME, Zaghi S, Lopes M, DosSantos MF, et al. Chronic Migraine Alleviation by tDCS Is Predicted To Be Associated with Current Flow through Pain-Related (Sub) Cortical Regions. Headache. 2011 Jun;51:48–9. [Google Scholar]
- 8.DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp. 2011;(51) doi: 10.3791/2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014 Nov;125(11):2150–206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 10.DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer M, Schambra HM, et al. Building up Analgesia in Humans via the Endogenous μ-Opioid System by Combining Placebo and Active tDCS: A Preliminary Report. PLoS One. 2014 Jul; doi: 10.1371/journal.pone.0102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DosSantos MF, Love TM, Martikainen IK, Nascimento TD, Fregni F, Cummiford C, et al. Immediate effects of tDCS on the mu-opioid system of a chronic pain patient. Front Psychiatry. 2012;3:93. doi: 10.3389/fpsyt.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villamar MF, Volz MS, Bikson M, Datta A, Dasilva AF, Fregni F. Technique and considerations in the use of 4×1 ring high-definition transcranial direct current stimulation (HD-tDCS). J Vis Exp. 2013;(77):e50309. doi: 10.3791/50309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffman EL, Ohrbach R, Truelove EL, Tai F, Anderson GC, Pan W, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J Orofac Pain. 2010 Winter;24(1):63–78. [PMC free article] [PubMed] [Google Scholar]
- 14.Bolton JE, Wilkinson RC. Responsiveness of pain scales: a comparison of three pain intensity measures in chiropractic patients. J Manipulative Physiol Ther. 1998 Jan;21(1):1–7. [PubMed] [Google Scholar]
- 15.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006 May;122(1-2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI. Transcranial Direct Current Stimulation to Lessen Neuropathic Pain After Spinal Cord Injury: A Mechanistic PET Study. Neurorehabil Neural Repair. 2014 Mar;28(3):250–9. doi: 10.1177/1545968313507632. [DOI] [PubMed] [Google Scholar]
- 17.Goncalves GS, Borges IC, Goes BT, de Mendonca ME, Goncalves RG, Garcia LB, et al. Effects of tDCS Induced Motor Cortex Modulation on Pain in HTLV-1: A Blind Randomized Clinical Trial. Clin J Pain. 2013 Nov 28; doi: 10.1097/AJP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 18.Wright KD, Asmundson GJ, McCreary DR. Factorial validity of the short-form McGill pain questionnaire (SF-MPQ). Eur J Pain. 2001;5(3):279–84. doi: 10.1053/eujp.2001.0243. [DOI] [PubMed] [Google Scholar]
- 19.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988 Jun;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 20.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008 Apr-Jun;43(2):215–21. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier A, Fontaine D, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999 Sep;82(3):245–51. doi: 10.1016/S0304-3959(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Dmochowski JP, Su Y, Datta A, Rorden C, Parra LC. Automated MRI segmentation for individualized modeling of current flow in the human head. J Neural Eng. 2013 Dec;10(6):066004. doi: 10.1088/1741-2560/10/6/066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009 Oct;2(4):201–7. 7 e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007 Apr 15;35(3):1113–24. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Bikson M, Datta A. Guidelines for precise and accurate computational models of tDCS. Brain Stimul. 2012 Jul;5(3):430–1. doi: 10.1016/j.brs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006 Apr;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Wrigley PJ, Gustin SM, McIndoe LN, Chakiath RJ, Henderson LA, Siddall PJ. Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain. 2013 Oct;154(10):2178–84. doi: 10.1016/j.pain.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006 Dec;54(12):3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 29.Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2(3):353–61. [PMC free article] [PubMed] [Google Scholar]
- 30.Antal A, Terney D, Kuhnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage. 2010 May;39(5):890–903. doi: 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Mori F, Codeca C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010 May;11(5):436–42. doi: 10.1016/j.jpain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012 Sep;52(8):1283–95. doi: 10.1111/j.1526-4610.2012.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franca NR, Toniolo EF, Franciosi AC, Alves AS, de Andrade DC, Fonoff ET, et al. Antinociception induced by motor cortex stimulation: somatotopy of behavioral response and profile of neuronal activation. Behav Brain Res. 2013 Aug 1;250:211–21. doi: 10.1016/j.bbr.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Zaghi S, Thiele B, Pimentel D, Pimentel T, Fregni F. Assessment and treatment of pain with non-invasive cortical stimulation. Restor Neurol Neurosci. 2011;29(6):439–51. doi: 10.3233/RNN-2011-0615. [DOI] [PubMed] [Google Scholar]
- 35.DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer MD, Schambra HM, et al. Building up analgesia in humans via the endogenous mu-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014;9(7):e102350. doi: 10.1371/journal.pone.0102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greene CS, Goddard G, Macaluso GM, Mauro G. Topical review: placebo responses and therapeutic responses. How are they related? J Orofac Pain. 2009;23(2):93–107. Spring. [PubMed] [Google Scholar]
- 37.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011 Sep;14(8):1133–45. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 38.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012 Apr;5(2):155–62. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;4:CD008208. doi: 10.1002/14651858.CD008208.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Sparing R, Buelte D, Meister IG, Paus T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008 Jan;29(1):82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.