Abstract
Background
Cardiac resynchronization therapy (CRT) reduces morbidity and mortality among individuals with dyssynchronous systolic heart failure (HF). However, patient outcomes vary with some at higher risk than others for HF progression and death.
Objective
To develop a risk prediction score incorporating variables associated with mortality, left ventricular assist device (LVAD) implant or heart transplant in recipients of primary prevention cardiac resynchronization therapy-defibrillators (CRT-D).
Methods
We followed 305 CRT-D patients from the Prospective Observational Study of Implantable Cardioverter-Defibrillators for the composite outcome of all-cause mortality, LVAD implant or heart transplant soon after device implantation. Serum biomarkers, electrocardiographic and clinical variables were collected at implant. Multivariable analysis using Cox-proportional hazards model with stepwise selection method was used to fit the final model.
Results
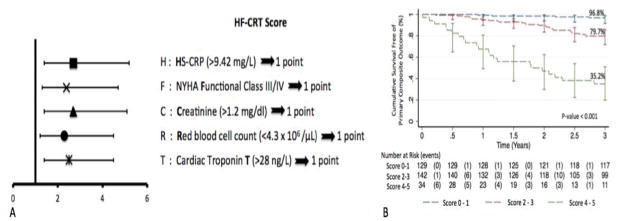
Among 305 patients, 53 experienced the composite endpoint. In multivariable analysis, 5 independent predictors (HF-CRT) were identified: HS-CRP >9.42ng/L (HR=2.5 [1.4, 4.5]), NYHA Functional Class III/IV (HR=2.3 [1.2, 4.5]), Creatinine >1.2mg/dL (HR=2.7 [1.4, 5.1]), Red blood cell count <4.3×106/μL (HR=2.4 [1.3, 4.7]) and cTnT >28ng/L (HR=2.7 [1.4, 5.2]). One point was attributed to each predictor and three score categories were identified. Patients with scores 0–1, 2–3 and 4–5 had a three year cumulative event-free survival of 96.8%, 79.7% and 35.2%, respectively (log-rank, p<0.001).
Conclusion
A simple score combining clinical and readily available biomarker data can risk stratify CRT patients for HF progression and death. These findings may help identify patients with limited benefit from CRT who are in need of closer monitoring or early application of more aggressive circulatory support.
Keywords: cardiac resynchronization therapy, heart failure, mortality, left ventricular assist device, heart transplant
INTRODUCTION
Cardiac resynchronization therapy (CRT) reduces heart failure (HF) hospitalization, mortality, and improves quality of life among patients with dyssynchronous systolic heart failure (1,2). However, patients within this group are prognostically heterogeneous and vary in their progression to more advanced HF states (3). Our current ability to identify potential CRT candidates with a higher risk for death or early need of advanced circulatory support is limited. Hence, a pressing need exists for a comprehensive prognostic tool to address this knowledge gap in order to better guide application of these therapies (e.g, CRT, left ventricular assist device (LVAD) implantation, heart transplantation) in this cohort.
Multiple clinical and biomarker metrics have been used (4) with varying degrees of validation (5–7) to predict outcomes in HF patients. These prior risk modeling studies have been limited by highly selected patient groups and the absence of serum biomarkers now known to play an integral role in HF progression (8,9). We sought to identify predictors of mortality and early need for advanced circulatory support interventions among the cohort of primary prevention CRT-defibrillator (CRT-D) recipients in the Prospective Observational Study of Implantable Cardioverter-Defibrillators (PROSE-ICD).
METHODS
Study Design, Data Collection and Patient Follow-up
PROSE-ICD is a prospective, multi-center study that enrolled 1,189 patients with systolic HF undergoing primary prevention implantable cardioverter defibrillator (ICD) or CRT-D implantation from December 2003 till January 2013. The PROSE-ICD design and study protocol have been previously described (10). Briefly, all patients underwent a comprehensive history and cardiovascular physical examination, electrocardiographic (ECG) evaluation, cardiac imaging to assess ejection fraction and a blood draw. Patients were followed every 6 months and after any patient perceived ICD therapy. During follow-up visits, a cardiac focused history and physical exam, signal averaged ECG, device interrogation and blood collection were performed. We analyzed the 305 participants who received a CRT-D. The Institutional Review Board of the participating institutions approved the study and all participants provided signed informed consent.
Device Implantation
All patients met contemporary clinical practice guidelines for CRT-D implantation (11). Device programming was left to the discretion of the implanting physician. Right ventricular leads were targeted to the RV apex and left ventricular pacing leads were targeted to a lateral or posterolateral branch of the coronary sinus with care to avoid apical placement (12). Atrioventricular and ventriculoventricular delays were not systemically optimized but programmed to allow for a fully biventricular paced QRS complex with a right bundle branch block conduction morphology in lead V1 (13).
Biomarker Analysis
Serum biomarker analysis was carried out on baseline fasting samples as previously described (10). Criteria for biomarker selection relied on pathophysiologic relevance and supporting evidence in the literature for a prognostic role in HF (14). Four inflammatory biomarkers were tested using high sensitivity ELISA including high sensitivity C-reactive protein (HS-CRP), interleukin 6 (IL-6), IL-10, and soluble tumor necrosis factor alpha receptor IIa (TNF-αRIIa). Serum levels of N-terminal pro-brain natriuretic peptide (NT pro-BNP) and markers of myocardial injury (eg, cardiac troponin T (cTnT), troponin I (cTnI), creatine kinase MB (CK-MB)) were also measured (see supplemental section for more details).
Patient Outcomes, Event Adjudication and Endpoints
The primary endpoint was a composite of all-cause mortality, heart transplant or LVAD implant. Long-term survival data were obtained from our study follow-up and verified with queries of the US Social Security Death Index. Two cardiologists adjudicated the cause of death using data from hospital records, death certificates and interviews with next-of-kin. Input from a third adjudicator was used in case of disagreement. Deaths were categorized as cardiac, non-cardiac, or unknown. Cardiac deaths were further classified as sudden or due to progressive HF. Patients who withdrew consent, had their devices explanted or turned off were censored. Time-to-event was calculated as time from device implant to first occurrence of any of the endpoint events contributing to the composite outcome. Follow-up outcomes were assessed at one and three years. Our pre-specified cut-off of three years for assessment of CRT outcomes in this analysis was chosen based on recent extended CRT trial follow-up as well as observational studies reporting durability of CRT benefit beyond three years (15). Thus, CRT-D recipients who experience poor outcomes within this time frame from CRT-D implant, represent a special group with limited benefit from CRT who may benefit from more aggressive medical therapy and alternate advanced circulatory support therapies (LVAD, heart transplant). Identifying this high-risk group is imperative to improve therapy selection and outcomes in advanced systolic heart failure patients.
Statistical Analysis
Descriptive statistics for categorical variables are summarized as frequency (%) and compared among cases and non-cases using Pearson χ2 or Fischer-exact test as appropriate. Continuous variables are presented as mean±SD or as median (interquartile range, 25th–75th percentile) and compared using the unpaired student t-test or the non-parametric Wilcoxon Rank Sum test, respectively. Continuous variables were transformed to binary variables using pre-specified cut-offs to facilitate the formulation of an easily implemented score (Supplement Table 1). To avoid bias, pre-specified thresholds for cut-offs were either derived from clinical and laboratory recognized normal reference limits or from cut-offs validated in the literature. With the exception of cardiac enzymes where normal reference cut-offs were used, cut-offs for biomarker data were assigned according to the 75th percentile. Cox-proportional hazards models with pre-defined censorship criteria were implemented to account for our time-to-event data. Clinical, echocardiographic and ECG variables in addition to basic laboratory and biomarker data were entered in to univariable Cox-proportional hazards models to assess for associations with the primary endpoint at three years of follow-up. All variables with a p<0.05 in univariable analysis were considered for inclusion in the multivariable model. A p<0.05 was required for entry into the model and a p≥0.1 was required for removal. We employed both backward and forward stepwise selection in the derivation of the final multivariable model and arrived at the same final model using both methods. After stepwise selection, significant variables remaining in the model were entered into a bootstrapped Cox regression with 1,000 samples. Variables with a bootstrapped p<0.05 were assigned a weighted point score based on their associated hazard ratio and a simple score was calculated by summing all the points. Kaplan-Meier estimates methodology with the log-rank significance test was used to assess survival across the score categories. Consecutive score categories with no significant survival difference were combined into one category. This analysis was repeated to identify predictors of the primary composite outcome at one year. The validity of the proportional hazards assumption was verified by using the log-negative-log(Survival(t)) plot. The Breslow method was used to handle event ties. The final model was bootstrap validated with 1000 samples (16). The Harrell’s c-statistic was used to assess discriminative power and the Cox-Snell residuals were calculated to determine goodness of fit. A two-sided p<0.05 was considered statistically significant. All analyses were done using STATA 12 (StataCorp LP; College Station, TX).
RESULTS
Three-year Patient Outcomes
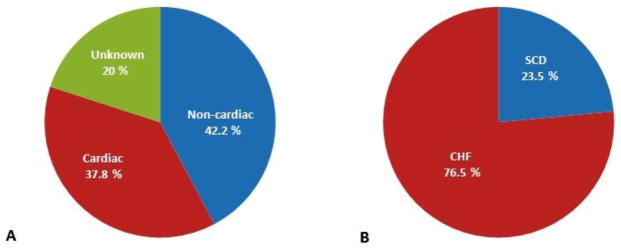
53 patients (45 deaths, 4 LVAD, 4 heart transplantation) experienced the primary endpoint. Mode of death was attributed to a cardiac cause in 17/45 (37.8%), non-cardiac cause in 19/45 (42.2%) and was unable to be determined in 9 (Figure 1A). Sudden cardiac death (SCD) constituted 4/17 and progressive HF 13/17 of the cardiac cases (Figure 1B). Participants who met the primary endpoint were older, were more likely to have a history of atrial fibrillation, ischemic cardiomyopathy, renal dysfunction, more advanced NYHA functional class and be treated with loop diuretics (Table 1). In addition, anemia, low serum chloride and calcium, elevated red blood cell distribution width, elevated inflammatory markers (eg, HS-CRP, IL-6, IL-10 and TNF-αRIIa), elevated NT-ProBNP and cTnT were also associated with the primary endpoint (Tables 2 and 3). In multivariable analysis, 5 independent predictors were associated with the primary composite outcome at three-years: HS-CRP >9.42mg/L (hazard ratio (HR)=2.5 [1.4, 4.5]; p=0.002), NYHA Functional Class III/IV (HR=2.3 [1.2, 4.5]; p=0.01), serum Creatinine >1.2mg/dL (HR=2.7 [1.4, 5.1]; p=0.003), Red blood cell count <4.3 × 106/μL (HR=2.4 [1.3, 4.7]; p=0.007) and cTnT >28ng/L (HR=2.7 [1.4, 5.2]; p=0.002) (Supplement Table 2). The c-statistic of the multivariable model was 0.81 [0.76, 0.86]. The HR point estimates for the different variables were similar with overlapping confidence intervals (Figure 2A). Hence, each variable was assigned 1 point for the scoring construct. A simple acronym HF-CRT was derived reflecting the different risk factor components of the score. After combining contiguous score categories with no significant survival difference, three distinct score categories remained (category 1: score 0–1 (n=129), category 2: score 2–3 (n=142) and category 3: score 4–5 (n=34)). Three-year cumulative survival in the first, second and third categories was 96.8%, 79.7% and 35.2%, respectively (log-rank, p<0.001) (Figure 2B). In the third category, approximately half of the events occurred within one year of CRT-D implant. Given this and the current recommendations against implantation in those with less than 1 year survival, we examined predictors of outcomes at one year.
Figure 1.
A. Mode of death at three years follow-up classified as cardiac versus non-cardiac cause and B. Mode of cardiac death at three years follow-up defined as congestive heart failure (CHF) related or sudden cardiac death (SCD)
Table 1.
Baseline characteristics grouped by primary composite outcome status at three years follow-up
| Baseline Characteristics | Primary Composite Outcome † | P-Value | |
|---|---|---|---|
| Yes (N = 53) | No (N = 252) | ||
| Age (years) ‡ | 67.3 ± 12.8 | 61.8 ± 12.3 | 0.003 |
| Male (%) | 39 (73.6) | 154 (61.1) | 0.09 |
| Race (%) | 0.86 | ||
| African American | 17 (32.1) | 63 (25) | |
| White Caucasian | 35 (66) | 177 (70.2) | |
| Other | 1 (1.9) | 12 (4.8) | |
| Body Mass Index (kg/m2) | 28.8 ± 7.0 | 29.6 ± 6.1 | 0.40 |
| Systolic blood pressure (mmHg) | 120.3 ± 19.1 | 124.5 ± 21.9 | 0.19 |
| Diastolic blood pressure (mmHg) | 70.1 ± 11.8 | 73.3 ± 11.4 | 0.06 |
| Heart rate (beats/min) | 78.7 ± 19.0 | 75.8 ± 15.8 | 0.24 |
| QRS (ms) | 149.8 ± 29.5 | 147.2 ± 25.8 | 0.53 |
| QTc (ms) | 490.2 ± 41.3 | 476.9 ± 47.9 | 0.06 |
| LBBB (%) | 27 (50.9) | 132 (52.4) | 0.85 |
| NYHA Class (%) ‡ | 0.02 | ||
| I | 4 (7.6) | 28 (11.1) | |
| II | 12 (22.6) | 104 (41.3) | |
| III | 37 (69.8) | 116 (46) | |
| IV | 0 (0) | 4 (1.6) | |
| Ejection fraction (%) | 19.6 ± 7.9 | 20.9 ± 7.2 | 0.25 |
| Ischemic Cardiomyopathy (%) ‡ | 26 (49.1) | 85 (33.7) | 0.04 |
| History of Myocardial Infarction (%)‡ | 20 (37.7) | 61 (24.2) | 0.04 |
| History of CABG (%) ‡ | 18 (34.0) | 42 (16.7) | 0.004 |
| History of PCI (%) | 15 (28.3) | 44 (17.5) | 0.07 |
| Diabetes (%) | 23 (43.4) | 88 (34.9) | 0.24 |
| Hypertension (%) | 35 (66.0) | 147 (58.3) | 0.30 |
| Dyslipidemia (%) | 26 (49.1) | 109 (43.3) | 0.44 |
| Chronic kidney disease (%) ‡ § | 28 (52.8) | 76 (30.2) | 0.002 |
| Atrial fibrillation (%) ‡ | 24 (45.3) | 65 (25.8) | 0.01 |
| Smoking (%) | 41 (77.4) | 164 (65.1) | 0.08 |
| Family History of Sudden Cardiac Death (%) | 15 (28.3) | 72 (28.6) | 0.97 |
| Family History of Myocardial Infarction (%) | 24 (45.3) | 112 (44.4) | 0.91 |
|
| |||
| Medications | |||
|
| |||
| Beta Blocker (%) | 45 (84.9) | 227 (90.1) | 0.27 |
| ACE-I (%) | 32 (60.4) | 177 (70.2) | 0.16 |
| ARB (%) | 13 (24.5) | 56 (22.2) | 0.72 |
| Aldosterone Antagonist (%) | 20 (37.7) | 69 (27.4) | 0.13 |
| Statin (%) | 36 (67.9) | 156 (61.9) | 0.41 |
| Loop Diuretic (%) ‡ | 46 (86.8) | 177 (70.2) | 0.01 |
| Thiazide (%) | 8 (15.1) | 26 (10.3) | 0.32 |
| Calcium Channel Blocker (%) | 9 (17.0) | 25 (9.9) | 0.14 |
| Digoxin (%) | 19 (35.9) | 70 (27.8) | 0.24 |
| Aspirin (%) | 35 (66.0) | 152 (60.3) | 0.44 |
| Clopidogrel (%) | 8 (15.1) | 34 (13.5) | 0.76 |
Composite Outcome: All-cause mortality, LVAD implant or Heart Transplant
P-Value < 0.05
Chronic kidney disease is defined as estimated glomerular filtration rate < 60 ml/min/1.73m2
ACE-I: Angiotensin Converting Enzyme Inhibitor; ARB: Angiotensin II Receptor Blocker; CABG: Coronary Artery Bypass Graft; LBBB: Left Bundle Branch Block; NYHA Class: New York Heart Association Class; PCI: Percutaneous Coronary Intervention.
Table 2.
Basic laboratory data grouped by primary composite outcome status at three years follow-up
| Laboratory Data | Primary Composite Outcome † | P-Value | |
|---|---|---|---|
| Yes (N = 53) | No (N = 252) | ||
| Sodium (meq/L) | 139.1 ± 3.7 | 139.1 ± 3.0 | 0.97 |
| Potassium (meq/L) | 4.2 ± 0.5 | 4.2 ± 0.4 | 0.60 |
| Chloride (meq/L) ‡ | 101.8 ± 4.1 | 103.0 ± 4.1 | 0.046 |
| Blood Urea Nitrogen (mg/dL) ‡ | 30.5 ± 16.1 | 22.4 ± 9.4 | < 0.001 |
| Creatinine (mg/dL) ‡ | 1.6 ± 0.8 | 1.1 ± 0.4 | < 0.001 |
| Estimated glomerular filtration rate (ml/min/1.73m2) ‡ § | 56.0 ± 25.0 | 73.2 ± 22.7 | < 0.001 |
| Calcium (mg/dL) ‡ | 9.1 ± 0.6 | 9.3 ± 0.6 | 0.03 |
| Magnesium (mg/dL) | 1.7 ± 0.2 | 1.7 ± 0.3 | 0.80 |
| White Blood cell count (/μL) | 7702.1 ± 2728.7 | 7373.5 ± 2618.3 | 0.41 |
| Red blood cell count (x106/μL) ‡ | 4.1 ± 0.6 | 4.4 ± 0.6 | < 0.001 |
| Hemoglobin (g/dL) ‡ | 12.3 ± 1.7 | 13.3 ± 1.8 | < 0.001 |
| Hematocrit (%) ‡ | 37.4 ± 4.9 | 39.7 ± 4.7 | 0.002 |
| Mean corpuscular volume (fL) | 91.0 ± 6.0 | 89.9 ± 5.4 | 0.18 |
| Red cell distribution width (%) ‡ | 15.2 ± 1.5 | 14.3 ± 1.7 | < 0.001 |
| Platelets (x103/μL) | 217.3 ± 72.6 | 210.3 ± 57.9 | 0.51 |
Composite Outcome: All-cause mortality, LVAD or Heart Transplant.
P-Value < 0.05.
Estimated glomerular filtration rate was calculated using the CKD-EPI equation.
Table 3.
Biomarker data grouped by primary composite outcome status at three years follow-up
| Biomarker Data | Primary Composite Outcome † | P-Value | |
|---|---|---|---|
| Yes (N = 53) | No (N = 252) | ||
| HS-CRP (mg/L) ‡ | 10.4 (4.3 – 17.8) | 3.0 (1.3 – 6.9) | <0.001 |
| HS-IL-6 (pg/ml) ‡ | 4.1 (2.1 – 7.7) | 1.7 (1.0 – 3.1) | <0.001 |
| TNF-αRIIa (ng/ml) ‡ | 4.8 (3.1 – 6.3) | 3.1 (2.3 – 4.4) | <0.001 |
| IL-10 (pg/ml) ‡ | 1.9 (1.3 – 3.9) | 1.3 (0.9 – 2.5) | 0.003 |
| NT-pro-BNP (ng/L) ‡ | 4500 (2900 – 8800) | 2600 (1700 – 3800) | <0.001 |
| Cardiac Troponin T (ng/L) ‡ | 32(5 – 88) | 4 (0 – 29) | <0.001 |
| Cardiac Troponin I (ng/L) | 23 (0 – 82) | 11 (0 – 51) | 0.12 |
| CK-MB (ng/ml) | 2.8 (1.7 – 4.3) | 2.6 (1.6 – 4.2) | 0.37 |
Composite Outcome: All-cause mortality or LVAD or Heart Transplant
P-Value < 0.05
CK-MB: creatine kinase MB; HS-CRP: high sensitivity C-reactive protein; HS-IL-6: high sensitivity interleukin 6; IL-10: interleukin 10; NT-proBNP: N-terminal pro-brain natriuretic peptide and TNF-αRIIa: soluble tumor necrosis factor alpha receptor II
Figure 2.
A. Forest plot showing multivariable HRs associated with predictors included in HF-CRT score B. Plot of Kaplan Meier estimates of three-year survival free of primary composite endpoint according to HF-CRT score categories
One Year Patient Outcomes
Within the first year of CRT-D implantation, 19 patients (17 deaths and 2 heart transplantation) experienced the primary endpoint. Mode of death was attributed to a cardiac cause in 7/17 (41.2%), non-cardiac cause in 8/17 (47%) and was unable to be determined in 2 (Supplement Figure 1A). Sudden cardiac death (SCD) constituted 1/7 and progressive HF 6/7 of cardiac mortality cases (Supplement Figure 1B). Predictors of the primary endpoint at one year included atrial fibrillation (HR=4.1 [1.5, 10.9]; p=0.006), HS-CRP >9.42 mg/L (HR=3.8 [1.5, 9.7]; p=0.005), serum creatinine >1.2mg/dL (HR=6.3 [2.2, 18.4]; p=0.001) and red blood cell count <4.3 × 106/μL (HR=3.7 [1.2,11.3]; p=0.02) (Supplement Table 3). The c-statistic of the multivariable model was 0.88 [0.82,0.94]. Patients with all four risk factors had a significantly elevated risk of mortality or progression to LVAD/heart transplant (66.7 %) within one year of CRT-D implant.
DISCUSSION
Among primary prevention CRT-D recipients, a simple score combining baseline clinical and serum biomarker data can identify patients at high (score 4–5), intermediate (score 2–3) and low risk (score 0–1) of death or need for advanced circulatory support within 3 years of CRT-D implantation. Notably, patients with four or more HF-CRT variables had a 64.8% probability of mortality or need for advanced circulatory support. Interestingly, half of the outcomes in this high risk subgroup occurred within one year of CRT implant despite the fact that all patients enrolled were deemed to have a meaningful 1 year life expectancy. In fact, individuals with atrial fibrillation, elevated hS-CRP (>9.42mg/L), elevated creatinine (>1.2 mg/dL) and anemia (RBC count <4.3×106/μL) were at very high risk of HF progression or death at one year with a specificity of 98.9% and characterize a subgroup of patients where CRT-D may have limited ability in modulating overall patient outcomes.
This analysis was focused on describing patient outcomes after implantation rather than CRT response, a distinction worth noting. A given individual may have an ephemeral response to CRT (as defined by various soft or hard endpoints) soon after device implant, but their outcome over time may still be death or need for more advanced circulatory support. Hence, focusing on what the patient outcome is after implantation may have more meaningful impact on overall patient management. The HF-CRT score is focused on outcome-based risk-stratification in a way that may better inform clinicians on the optimal management of these patients.
HF-CRT: Clinical Variables Associated with Risk of Death or Heart Failure Exacerbation
The variables identified in our study are aligned with several earlier studies. Advanced HF status (9), CKD (17,18) and anemia(19) have been previously reported to impart an increased risk for death and HF progression and it is not surprising that these variables significantly contributed to our risk score. CKD, in particular, is present in approximately 40% of CRT candidates (17). Despite consistent reductions in morbidity and mortality among CKD and non-CKD patients from CRT, patients with CKD do experience a higher rate of adverse outcomes. In addition, anemia has been shown to be an independent predictor of poor outcomes in HF patients likely through mechanisms related to myocardial ischemia, increased hemodynamic load and neurohormonal activation. While many of the variables identified in our analysis predicted both one year and three-year outcomes, a history of atrial fibrillation appeared as a significant predictor in the one-year model only. Recently, there has been increasing appreciation of the impact of atrial fibrillation burden on CRT outcomes (20,21). CRT patients with persistent atrial fibrillation have been shown to experience worse outcomes when compared to patients in sinus rhythm (21). On the other hand, patients with paroxysmal atrial fibrillation have been shown to derive equal benefit and experience similar survival when compared to CRT patients in sinus rhythm (22). Our data lack the granularity for the type of atrial fibrillation that was present as well as the degree of biventricular pacing following CRT implant. It may be that patients with higher burden of atrial fibrillation experienced early adverse outcomes and thus atrial fibrillation was a predictor at 1-year. Longer follow up analyses necessarily selected “healthier” patients, thus leaving patients with lower burden of atrial fibrillation for the 3 year analysis.
HF-CRT: Serum Biomarkers Associated with Risk of Death or Heart Failure Exacerbation
Inflammation and subclinical myocardial injury contribute to the pathogenesis and progression of HF. In a study of 65 CRT patients, elevated pre-implant HS-CRP has been shown to predict echocardiographic non-response and cardiac mortality (23). Other inflammatory mediators including TNF-alpha, IL-10 and IL-6 have also been associated with CRT response (24). Our findings extend observations made in these early studies. While elevations in inflammatory markers are associated with poorer outcomes, we found HS-CRP to be the strongest predictor. Similarly, elevated cTnT levels, a common finding in chronic HF patients, have been shown to be an independent predictor of HF progression and mortality in CRT recipients (25).
Previous CRT Prediction Scores
Prior studies have generated risk scores to predict CRT patient outcomes. A recent paper identified NYHA functional class IV, eGFR <60 ml/min/1.73m2, age ≥70 years, atrial fibrillation and ejection fraction <22% to be associated with mortality over a mean follow-up of 36.2 ± 29.2 months (8). Another study looking at predictors of long-term mortality in CRT found larger left ventricular end-systolic volume, less distance covered in the 6 min walking test, poor renal function, more severe heart failure, male gender, and presence of atrial fibrillation, no posterolateral left ventricular (LV) lead, and no LV dyssynchrony to be associated with poor prognosis after CRT (9). While the variables incorporated in our scoring construct are similar to prior reports, it is our hope that a simplified score combining clinical and biomarker data such as the HF-CRT is easier to remember and apply. More importantly, we focused our endpoint to include not just mortality but other events that could guide decision making when there is clinical equipoise between CRT-D implantation and application of more advanced circulatory support solutions such as LVAD implantation or heart transplant.
Given the difference in focus from a scoring system centered on CRT response, it is not surprising that our scoring construct did not include commonly known variables such as baseline QRS duration and morphology. In fact, prior studies aimed at patient survival after CRT implant also did not identify QRS duration or morphology as being statistically significant. Gasparini et al. found no difference in survival after CRT implantation among patietns with baseline QRS durations between 120–149 ms and 150–199 ms (26). In addition, Dupont et al. found that while baseline QRS duration and morphology modulate CRT response, these variables had minimal effect in predicting a clinical composite outcome that included LVAD implant, heart transplant or death (27).
While the clinical variables identified in our study findings are aligned with prior reports, our study is unique in its incorporation of serum-based biomarkers for risk prediction. Identifying these variables as being important in predicting death or need for early advanced circulatory support underscores the importance of inflammation and subclinical ischemia in the mechanisms of HF progression. Our data suggest that elevated levels of inflammatory and ischemic markers may identify individuals at higher risk for early death or need for advanced circulatory support despite CRT implantation.
Limitations
This study has a few limitations. First, the proportion of CRT-D recipients from PROSE is small and its composition (e.g., 36.4% ischemic) may limit its applicability to all CRT patients. Furthermore, the size of this cohort limited our ability to assess predictors of the different modes of death. Nevertheless, this multi-center cohort is one of the most extensively phenotyped primary prevention cohorts described and represents a more racially diverse group than prior reports. Second, we attempted to use variables that are commonly collected in usual standard practice. Hence, information typically obtained from advanced imaging (e.g., cardiac magnetic resonance imaging for myocardial scar, detailed echocardiographic measurements) was not used in the derivation of our score. Third, data on final left ventricular lead location and its relation to scar tissue was not collected. Fourth, certain serum variables including albumin, uric acid and lipid levels were not accounted for in our score derivation because of missing data. In addition, our score was not validated in a separate population but was internally validated using bootstrapping methodologies. Further validation of our findings in larger cohorts is needed. Finally, our cohort lacked follow-up data on functional status, therefore, we were unable to include measures of quality life in our composite endpoint. Nevertheless, we focused on hard endpoints in the derivation of our score as functional status improvement may be ephemeral and not necessarily correlate with ultimate patient outcomes.
Conclusion
The HF-CRT score is derived from a racially diverse and extensively phenotyped primary prevention CRT cohort that better identifies individuals with limited benefit from CRT implant who are at higher risk for poor patient outcomes. While further validation in larger cohorts will be needed, this information can potentially guide decision-making in individuals with advanced dyssynchronous HF when there is clinical equipoise between CRT-D implantation and use of more advanced circulatory support interventions.
Supplementary Material
CLINICAL PERSPECTIVES.
Comprehensive risk prediction scores incorporating multiple pathophysiologic determinants in advanced heart failure (HF) patients eligible for CRT is critically needed to guide clinical decision-making especially where there is clinical equipoise between CRT-D implantation and application of more advanced circulatory support interventions. Leveraging pre-implant clinical, ECG and serum biomarker data from a primary prevention CRT recipient cohort, we developed a five-component risk score (HF-CRT: HS-CRP >9.42mg/L, NYHA Functional Class III/IV, Creatinine >1.2mg/dL, Red blood cell count <4.3 × 106/μL and cTnT >28ng/L) for the composite outcome of LVAD implant, heart transplant or death at three-years following CRT implant. Patients with scores 0–1, 2–3 and 4–5 had a three year cumulative event-free survival of 96.7%, 78.6% and 33.3%, respectively (log-rank, p<0.001). Application of this scoring construct in patients with dyssynchronous systolic HF may better guide clinical management and potentially lead to improvement in patient outcomes.
Acknowledgments
Funding was provided by the Donald W. Reynolds Foundation and NIH R01 grants HL091062 (GT) and NIH R01 HL103946 (AC). VN was supported by the Johns Hopkins Murex Research Award and partly supported on Fogarty training grant NIH-FIC-D43TW009118 awarded to the Scholars in Health Research Program.
Abbreviations
- CKD
Chronic Kidney Disease
- CRT-D
Cardiac Resynchronization Therapy-Defibrillators
- HF
Heart Failure
- ICD
Implantable Cardioverter Defibrillator
- LVAD
Left Ventricular Assist Device
- PROSE-ICD
Prospective Observational Study of Implantable Cardioverter-Defibrillators
- SCD
Sudden Cardiac Death
Footnotes
Relationships with Industry: AC received honoraria from Boston Scientific, Medtronic and St. Jude Medical for participation in fellows’ educational programs and advisory committee participation. KAE received honoraria from Medtronic, Boston Scientific, Biotronik, served as a consultant for Medtronic, Boston Scientific, St. Jude Medical and received fellowship support from Medtronic and Boston Scientific. JR has received honoraria from St. Jude Medical for participation in educational programs. All other authors have no relevant disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 2.McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, Page RL, Hlatky MA, Rowe BH. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–2514. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 3.Birnie DH, Tang AS. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol. 2006;21:20–26. doi: 10.1097/01.hco.0000198983.93755.99. [DOI] [PubMed] [Google Scholar]
- 4.Kandala J, Altman RK, Park MY, Singh JP. Clinical, laboratory, and pacing predictors of CRT response. J Cardiovasc Transl Res. 2012;5:196–212. doi: 10.1007/s12265-012-9352-0. [DOI] [PubMed] [Google Scholar]
- 5.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 6.Regoli F, Scopigni F, Leyva F, Landolina M, Ghio S, Tritto M, Calo L, Klersy C, Auricchio A collaborative study group. Validation of Seattle Heart Failure Model for mortality risk prediction in patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2013;15:211–220. doi: 10.1093/eurjhf/hfs162. [DOI] [PubMed] [Google Scholar]
- 7.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, Laskar S, Puskas J, Dunbar S, Vega D, Levy WC, Butler J. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol. 2009;53:334–342. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Khatib M, Tolosana JM, Trucco E, Borras R, Castel A, Berruezo A, Doltra A, Sitges M, Arbelo E, Matas M, Brugada J, Mont L. EAARN score, a predictive score for mortality in patients receiving cardiac resynchronization therapy based on pre-implantation risk factors. Eur J Heart Fail. 2014;16:802–809. doi: 10.1002/ejhf.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Bommel RJ, Borleffs CJ, Ypenburg C, Marsan NA, Delgado V, Bertini M, van der Wall EE, Schalij MJ, Bax JJ. Morbidity and mortality in heart failure patients treated with cardiac resynchronization therapy: influence of pre-implantation characteristics on long-term outcome. Eur Heart J. 2010;31:2783–2790. doi: 10.1093/eurheartj/ehq252. [DOI] [PubMed] [Google Scholar]
- 10.Cheng A, Dalal D, Butcher B, Norgard S, Zhang Y, Dickfeld T, Eldadah ZA, Ellenbogen KA, Guallar E, Tomaselli GF. Prospective observational study of implantable cardioverter-defibrillators in primary prevention of sudden cardiac death: study design and cohort description. J Am Heart Assoc. 2013;2:e000083. doi: 10.1161/JAHA.112.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Singh JP, Klein HU, Huang DT, et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011;123:1159–1166. doi: 10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 13.Ellenbogen KA, Gold MR, Meyer TE, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 15.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase. Eur Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 16.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 17.Lin G, Gersh BJ, Greene EL, Redfield MM, Hayes DL, Brady PA. Renal function and mortality following cardiac resynchronization therapy. Eur Heart J. 2011;32:184–190. doi: 10.1093/eurheartj/ehq403. [DOI] [PubMed] [Google Scholar]
- 18.Garg N, Thomas G, Jackson G, Rickard J, Nally JV, Jr, Tang WH, Navaneethan SD. Cardiac resynchronization therapy in CKD: a systematic review. Clin J Am Soc Nephrol. 2013;8:1293–1303. doi: 10.2215/CJN.00750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar S, Koehler J, Crossley GH, Tang WH, Abraham WT, Warman EN, Whellan DJ. Burden of atrial fibrillation and poor rate control detected by continuous monitoring and the risk for heart failure hospitalization. Am Heart J. 2012;164:616–624. doi: 10.1016/j.ahj.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Ousdigian KT, Borek PP, Koehler JL, Heywood JT, Ziegler PD, Wilkoff BL. The epidemic of inadequate biventricular pacing in patients with persistent or permanent atrial fibrillation and its association with mortality. Circ Arrhythm Electrophysiol. 2014;7:370–376. doi: 10.1161/CIRCEP.113.001212. [DOI] [PubMed] [Google Scholar]
- 22.Ruwald AC, Pietrasik G, Goldenberg I, Kutyifa V, Daubert JP, Ruwald MH, Jons C, McNitt S, Wang P, Zareba W, Moss AJ. The effect of intermittent atrial tachyarrhythmia on heart failure or death in cardiac resynchronization therapy with defibrillator versus implantable cardioverter-defibrillator patients: a MADIT-CRT substudy (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2014;63:1190–1197. doi: 10.1016/j.jacc.2013.10.074. [DOI] [PubMed] [Google Scholar]
- 23.Kamioka M, Suzuki H, Yamada S, Kamiyama Y, Saitoh S, Takeishi Y. High sensitivity C-reactive protein predicts nonresponders and cardiac deaths in severe heart failure patients after CRT implantation. Int Heart J. 2012;53:306–312. doi: 10.1536/ihj.53.306. [DOI] [PubMed] [Google Scholar]
- 24.Osmancik P, Herman D, Stros P, Linkova H, Vondrak K, Paskova E. Changes and prognostic impact of apoptotic and inflammatory cytokines in patients treated with cardiac resynchronization therapy. Cardiology. 2013;124:190–198. doi: 10.1159/000346621. [DOI] [PubMed] [Google Scholar]
- 25.Aarones M, Gullestad L, Aakhus S, Ueland T, Skaardal R, Aass H, Wergeland R, Smith HJ, Aukrust P, Kongsgaard E. Prognostic value of cardiac troponin T in patients with moderate to severe heart failure scheduled for cardiac resynchronization therapy. Am Heart J. 2011;161:1031–1037. doi: 10.1016/j.ahj.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Gasparini M, Leclercq C, Yu CM, Auricchio A, Steinberg JS, Lamp B, Klersy C, Leyva F. Absolute survival after cardiac resynchronization therapy according to baseline QRS duration: a multinational 10-year experience: data from the Multicenter International CRT Study. Am Heart J. 2014;167:203–209. e1. doi: 10.1016/j.ahj.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Dupont M, Rickard J, Baranowski B, Varma N, Dresing T, Gabi A, Finucan M, Mullens W, Wilkoff BL, Tang WH. Differential response to cardiac resynchronization therapy and clinical outcomes according to QRS morphology and QRS duration. J Am Coll Cardiol. 2012;60:592–598. doi: 10.1016/j.jacc.2012.03.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.