Abstract
Objectives
To review the literature and provide an updates on the mechanisms of action and therapeutic uses of oral colchicine in arthritis and inflammatory conditions.
Methods
We performed PubMed database searches through June 2014 for relevant studies in the English literature published since the last update of colchicine in 2008. Searches encompassed colchicine mechanisms of action and clinical applications in medical conditions. A total of 381 articles were reviewed.
Results
The primary mechanism of action of colchicine is tubulin disruption. This leads to subsequent down regulation of multiple inflammatory pathways and modulation of innate immunity. Newly described mechanisms include various inhibitory effects on macrophages including the inhibition of the NACHT-LRRPYD-containing protein 3 (NALP3) inflammasome, inhibition of pore formation activated by purinergic receptors P2X7 and P2X2, and stimulation of dendritic cell maturation and antigen presentation. Colchicine also has anti-fibrotic activities and various effects on endothelial function. The therapeutic use of colchicine has extended beyond gouty arthritis and Familial Mediterranean Fever, to osteoarthritis, pericarditis and atherosclerosis.
Conclusion
Further understanding of the mechanisms of action underlying the therapeutic efficacy of colchicine will lead to its potential use in a variety of conditions.
Keywords: colchicine, mechanism of action, inflammation, pharmacokinetics, therapeutic use
1. INTRODUCTION
Colchicine is an alkaloid extracted from plants of the genus Colchicum (autumn crocus). The therapeutic use of colchicine has been well documented in gout and familial Mediterranean fever (FMF); it has also been used in other diseases including Behcet’s disease (BD), pericarditis, coronary artery disease and other inflammatory and fibrotic conditions. The pharmacotherapeutic mechanism of action of colchicine in diverse disorders is not fully understood. The aim of this article is to review the literature and provide an update on the mechanisms of action and therapeutic uses of colchicine in various inflammatory conditions.
2. METHODS
We performed a PubMed database search for relevant studies published using the search terms “mechanism of action” OR “inflammation” OR “pharmacokinetics” OR “toxicity” OR “therapeutic use” AND “colchicine” between 1st Jan 2008 to 15th June 2014 and restricted to the English language. The start date was chosen to cover literature published since the topic was last comprehensively reviewed in 2008 [1][2] We focused on original articles that provided new information on the mechanisms of action of colchicine in various conditions and clinical applications of colchicine in medical conditions. Additionally, references noted in relevant articles were also reviewed. A total of 1,705 articles published in English were identified from the PubMed search and 1,354 of these articles were deemed to be outside the scope of this review. A total of 351 relevant articles from the search and 30 articles from relevant references were retrieved for full text review. The final review included 116 articles on mechanisms of action, 23 articles on pharmacokinetics and toxicities, 39 articles on therapeutic uses, 80 review articles, 49 observational studies, and 74 case reports.
3. RESULTS
3.1 Mechanism of action
3.1.1 Tubulin Disruption and anti-mitotic effect of colchicine
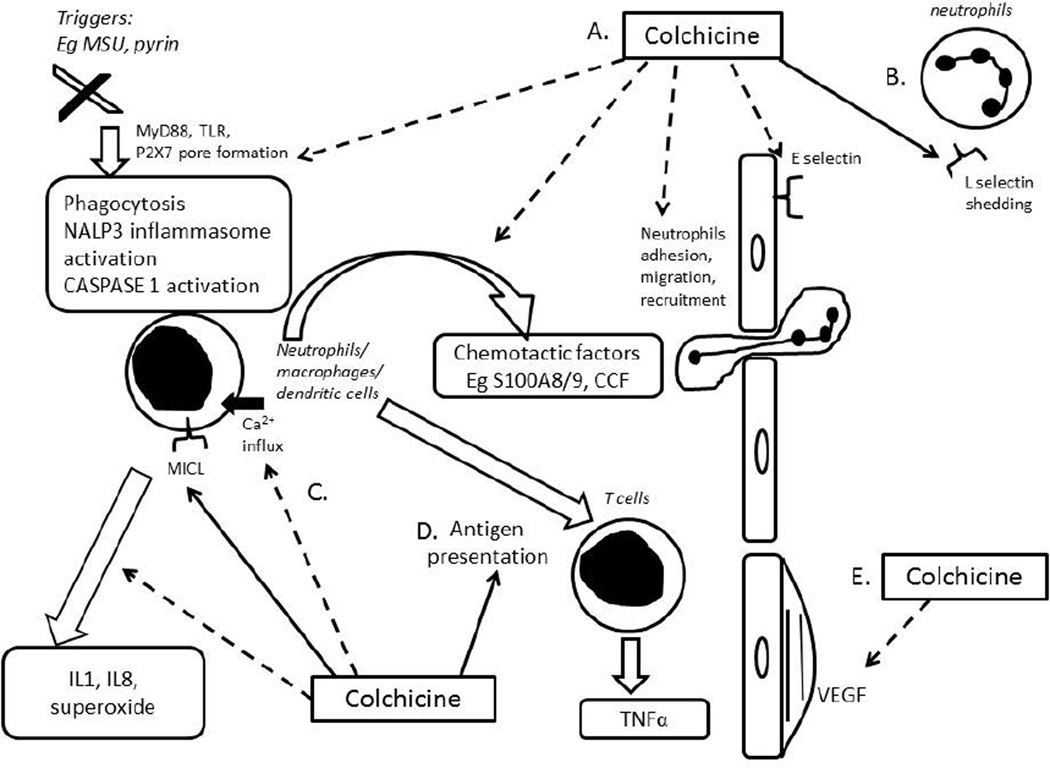
The most studied therapeutic mechanism of action of colchicine is its capacity to bind to tubulins, thereby blocking the assembly and polymerization of microtubules. Microtubules, key components of the cytoskeleton, are made up of αβ-tubulin heterodimers. Microtubules are involved in various cellular processes including maintenance of cell shape, intracellular trafficking, cytokine and chemokine secretion, cell migration, and regulation of ion channels and cell division. Colchicine is a classical anti-mitotic drug which blocks mitotic cells in metaphase. It binds to soluble tubulin to form tubulin-colchicine complexes in a poorly reversible manner, which then binds to the ends of microtubules to prevent the elongation of the microtubule polymer. At low concentrations, colchicine arrests microtubule growth and, at higher concentrations, colchicine promotes microtubule depolymerisation. It causes severe toxicity to normal tissues at high dose, which limits its use in cancer therapies [3]. Although none has yet developed to the point of clinical application in anticancer therapy, numerous compounds with the capability of interacting with the colchicine binding site have been described over the last decade [4]. Other effects of colchicine on malignancy include inhibition of cancer cell migration and metastatic potential [5], cell blebbing via a Rho/ Rho effector kinase (ROCK)/myosin light chain kinase (MLCK) pathway [6], inhibition of angiogenesis [7], limitation of adenosine triphosphate (ATP) influx into mitochondria [8] and release of cysteine-dependent aspartate-directed proteases (caspases) and cytochrome-c, leading to apoptotic cell death [3]. Colchicine also has anti-inflammatory effects, mainly related to disruption of microtubules and downstream cellular functions of leucocytes. Figure 1 summarizes the main anti-inflammatory mechanisms of action of colchicine.
Figure 1. Summary of the main anti-inflammatory mechanisms of action of colchicines.
Dashed lines refer to inhibitory activities and continuous lines to stimulatory activities of colchicine. The main mechanisms of action include the following: A. Colchicine inhibits activation of innate immunity, NALP3 inflammasome activation, CASPASE-1 activation; inhibits release of chemotactic factor release from neutrophils and then neutrophil recruitment; B. At low concentrations, colchicine inhibits expression of E-selectin on endothelial cells and prevents neutrophil adhesion. At high concentrations, colchicine promotes shedding of L-selectin from neutrophils and prevents further recruitment; C. Colchicine inhibits neutrophil activation and release of IL1, IL8, and superoxide; D. Colchicine promotes maturation of dendritic cells to act as antigen presenting cells; E. Colchicine inhibits vascular endothelial growth factor (VEGF) and endothelial proliferation. CASPASE= cysteine-dependent aspartate-directed proteases, CCF= crystal-derived chemotactic factor, MSU=monosodium urate (gout) crystals, MICL= myeloid inhibitory C-type lectin-like receptor, NALP3=NACHT-LRRPYD-containing protein 3, TLR=toll-like receptors, MyD88= myeloid differentiation primary response gene 88, VEGF= vascular endothelial growth factor.
3.1.2 Mechanisms of action of colchicine on the immune system
Recent advances in the understanding of the pathophysiology of crystal-induced inflammation, summarized by Nuki et al [1], have provided new insights into the mechanisms of action of colchicine in crystal arthropathies. The main mechanisms are inhibition of neutrophil chemotaxis, adhesion and mobilization, and superoxide production in addition to inhibition of NACHT-LRRPYD-containing protein 3 (NALP3) inflammasomes and interleukin (IL)1β processing and release.
3.1.3 Neutrophils
3.1.3a Inhibition of neutrophil chemotaxis
Colchicine concentrates intensively in leukocytes. In vitro, colchicine at concentrations as low as 0.1nM inhibits neutrophil chemotaxis and the release of a glycopeptide crystal-derived chemotactic factor (CCF) from neutrophil lysosomes after phagocytosis of monosodium urate crystals (MSU) [9]. A MSU-induced chemotactic factor S100A8/9 (thought to be the same molecule as CCF [10]) released from neutrophils was shown to substantially amplify neutrophil recruitment [11]. Recently, colchicine was shown to dampen inflammation via regulation of the myeloid inhibitory C-type lectin-like receptor (MICL), which is expressed by macrophages, monocytes, neutrophils, myeloid and plasmacytoid dendritic cells. Gagné et al demonstrated that colchicine inhibits the MSU-induced loss of cell surface MICL in neutrophils and subsequent IL8 production [12].
3.1.3b Neutrophil adhesion, mobilization and recruitment
Through microtubule depolymerisation, colchicine interferes with neutrophil adhesion and recruitment to inflamed tissue [13][14]. At nanoconcentrations (50% inhibitory concentration, IC50 of 3 nM) or typical doses used as prophylaxis, colchicine alters the distribution of E-selectin on endothelial cell surfaces and eliminates the adhesiveness for neutrophils. At higher microconcentrations (IC50 = 300 nM), colchicine induces shedding of neutrophil adhesion molecules (L-selectin) and prevents further neutrophil recruitment [14]. Colchicine was shown to inhibit neutrophil adhesion and mobility in crystal-induced neutrophil activation by selective inhibition of tyrosine phosphorylation [15]. A recent study has demonstrated that colchicine reduces crystal-induced tyrosine phosphorylation of Tec, which is likely the principle kinase in crystal induced neutrophil activation [16]. Paschke et al found that colchicine inhibits the deformability and motility of human neutrophils in confined spaces, which is crucial for neutrophil extravasation during inflammation [17].
3.1.3c Inhibition of superoxide production from neutrophil
Colchicine selectively suppresses MSU-induced superoxide production by neutrophils in vitro; this effect is mediated by inhibition of microtubules [15]. Recently, Chia et al demonstrated that colchicine inhibits MSU-induced superoxide production by murine peritoneal macrophages in vivo at doses 100 times lower than that required to inhibit neutrophil infiltration [18]. This suggests that superoxide anion production is more sensitive to suppression by colchicine than microtubule formation involved in cell migration. Colchicine has been shown to reduce oxidative stress by reducing calcium (Ca2+) influx into neutrophils [19].
3.1.4 Inhibition of NALP3 inflammasome and innate immune responses
MSU and calcium pyrophosphate dihydrate crystals (CPPD) were shown to specifically activate the NALP3 inflammasome, also known as the cryopyrin inflammasome. At high concentrations (5µM), colchicine suppresses MSU-induced NALP3 inflammasomes, responsible for caspase-1 activation and subsequent IL1β and IL18 processing and release [20]. The mechanism by which colchicine inhibits this NALP3 inflammasome activation is still unknown. It could possibly be related to the disruption of microtubule dependent transport of mitochondria to the endoplasmic reticulum. The assembly of NALP3 in the endoplasmic reticulum with its adaptor, Apoptosis associated Speck like protein containing Caspase recruitment domain (ASC), is required for the activation of inflammasomes [21][22][23]. Suppression of MSU-induced NALP3 inflammasome activity occurs at doses much higher than those used therapeutically and thus this may not be the primary therapeutic action of colchicine responsible for aborting flares of acute crystal arthropathies [24][1]. However, the concentration of colchicine in neutrophils may be more than 16 times the peak concentration in plasma [25]. It may be possible for a continuous low prophylactic dose of colchicine to achieve a high enough intracellular concentration in macrophages to inhibit NALP3 inflammasome activation. Colchicine may increase the threshold for initiation of full-blown NALP3 inflammasome activation in part by diminishing (without eliminating) subclinical inflammation [26]. Prophylactic therapy with colchicine also regulates the innate inflammatory response by blocking intracellular signalling pathways by targeting nuclear factor kB (NF-kB) or caspase-1 [27].
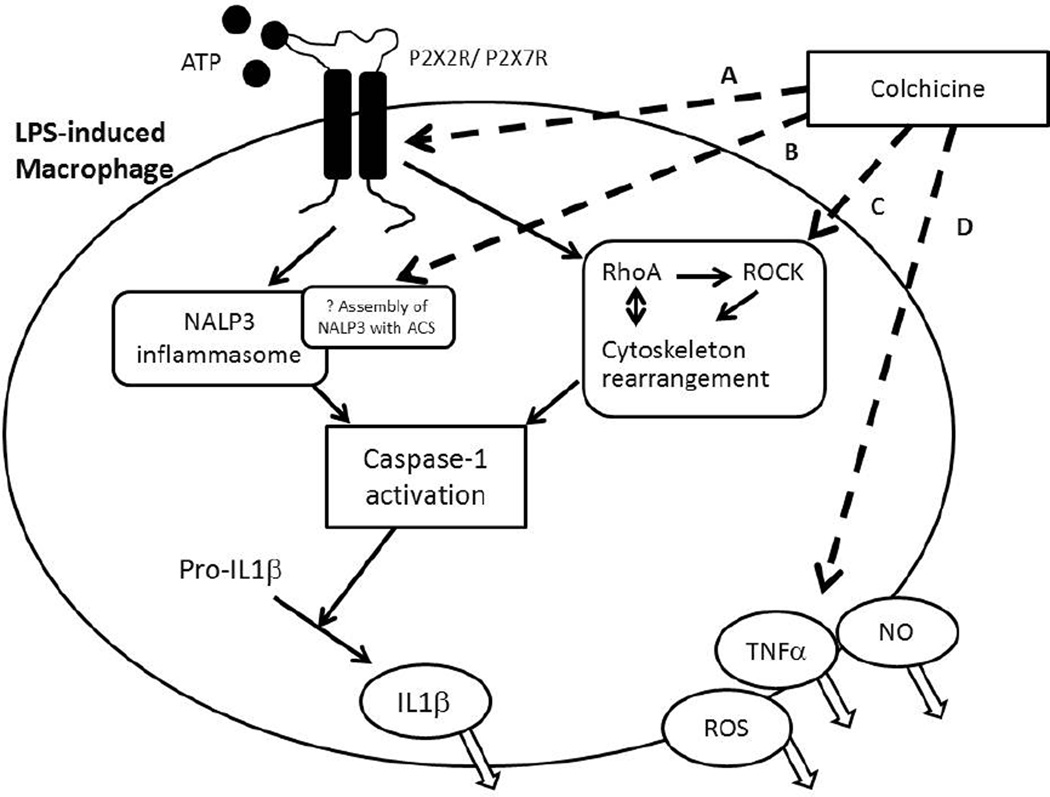
The effects of colchicine on macrophages have received much attention (Figure 2). Colchicine was shown to modulate lipopolysaccharide-induced secretion of tumor necrosis factor (TNF)a by liver macrophages in a rat model [28]. In a mouse brain macrophage cell line, colchicine inhibited ATP-induced release of IL1β via preventing microtubule reorganization and inhibiting activation of the Ras homolog gene family, member A (RhoA)/Rho-associated, coiled-coil containing protein kinase (ROCK) pathway [29]. Marques-da-Silva et al. recently demonstrated that colchicine is a potent inhibitor of pore formation induced by activation of both purinergic receptors P2X7 and P2X2 both in vitro and in vivo [30]. In the presence of colchicine, peritoneal mouse macrophages showed less ATP-induced permeability to ethidium bromide, and less reactive oxygen species (ROS) formation, nitric oxide (NO) and IL1β release. After colchicine treatment, mice inoculated with lipopolysaccharide and ATP also had diminished ROS, IL1β, interferon-γ and NO production. The formation of P2X7 pores is a necessary step in the innate immune response for triggering ATP-induced NALP3 inflammasome activation [31]. This event is upstream to microtubule depolymerisation and may represent a new therapeutic target for treatment of chronic inflammation [32].
Figure 2. Summary of the effects of colchicine on macrophages.
Dashed lines refer to inhibitory activities and continuous lines to stimulatory activities of colchicine. A. Colchicine inhibits activation of P2X2 and P2X7 receptors and blocks cationic dye uptake (via recruitment of the pannexin-1 membrane pore) and further pro-inflammatory cascades without affecting cell death. B. Colchicine inhibits the NACHT-LRRPYD-containing protein 3 (NALP3) inflammasome, possibly via inhibition of assembly of NALP3 with Apoptosis associated Speck like protein containing Caspase recruitment domain (ACS); C. Colchicine inhibits the RhoA/ Rho effector kinase (ROCK) pathway via cytoskeleton rearrangement and thus the activation of caspase- 1 and downstream maturation and release of IL1β; D. Colchicine inhibits release of various substances including reactive oxygen (ROS), nitrite oxide (NO) and tumor necrosis factor (TNF)α. Caspase-1= cysteine-dependent aspartate-directed proteases-1, IL= interleukin, LPS= lipopolysaccharide, P2X2 and P2X7= purinergic receptors.
3.1.5 Stimulation of antigen presentation
At relatively low concentrations (3µg/ml), mice colchicine promotes maturation of dendritic cells, generation of cytokines and the presentation of antigen to allogenic naive CD4+ lymphocytes [33]. Highlighting the importance of microtubules in cells processing of antigens, this stimulatory effect of colchicine on dendritic cells (as antigen presenting cells) was further demonstrated in human dendritic cells [34][35][36].
3.1.6 Anti-fibrotic and cardiovascular protective effects
Colchicine has anti-fibrotic effects. In a rat model of cyclosporine nephrotoxicity, colchicine inhibited tubulointerstitial fibrosis by stimulating B-cell lymphoma 2 (Bcl-2) expression and suppression of caspase-3 and thereby suppressed renal cell apoptosis [37]. In a rat model of hypertensive chronic kidney disease, colchicine inhibited renal fibrosis via inhibition of RhoA signalling and infiltration of inflammatory cells [38]. In a rat model, colchicine inhibited liver fibrosis by inhibiting the activation of hepatic stellate cells and induced stellate cell apoptosis [39]. In an encapsulating peritoneal sclerosis model, colchicine inhibited anti-transforming growth factor (TGF)-β1 activity [40]. In an in vitro study using human lung fibroblasts, colchicine inhibited myofibroblast differentiation via Rho/ serum response factor (SRF) dependent, but Smad independent signalling [41]. The cardiovascular applications of colchicine have been evolving in the last decade. FMF patients treated with colchicine were observed to have lower markers for endothelial dysfunction and cardiovascular risk such as β-thromboglobulin and mean platelet volume [42] [43]. Colchicine inhibited intimal hyperplasia and leukocyte vascular endothelial growth factor (VEGF) expression in an angioplasty model in dogs [44]. In a rat pulmonary arterial hypertension model, colchicine suppressed smooth muscle cell proliferation, increased cell apoptosis and reduced protein expression of inflammation (TNF-a and NF-κB) [45]. Colchicine was shown to have synergistic protective effects with atorvastatin on endothelial function, reduced C-reactive protein (CRP) and lipoprotein associated phospholipase A2 (Lp-PLA2), and enhanced NO production in rats [46]. The clinical applications of colchicine in pericarditis and atherosclerosis have been under intense investigation.
3.2 Colchicine metabolism and toxicities
Colchicine has a narrow therapeutic window. When prescribed daily and chronically for FMF, the most common adverse reactions (up to 20%) are abdominal pain, diarrhoea, nausea, and vomiting. These effects are usually mild, transient, and reversible upon lowering the dose. Prescribed acutely for gout flares with doses up to 1.8 mg within 2 hours, the most common adverse reaction is diarrhoea (23%) and pharyngolaryngeal pain (3%) [47]. At therapeutic doses of colchicine, blood dyscrasias have been reported including myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, and aplastic anemia [48]. Colchicine is a substrate for intestinal and hepatic cytochrome P450 3A4 (CYP3A4), and also a substrate for P-glycoprotein 1 (P-gp) reflux transporter. Colchicine is primarily eliminated by hepatobiliary excretion. Renal excretion accounts for 10–20% of colchicine elimination in patients with normal renal function. Consistent with the current understanding of colchicine metabolism, certain drugs increase the potential for colchicine toxicity via modulation of P-gp and CYP3A4 activity. Cases of myopathy and/or rhabdomyolysis in patients receiving colchicine have been reported with concomitant use of statins, fenofibrate/gemfibrozil, cyclosporine, or digoxin. Toxicity has also been reported in a patient who regularly (daily) consumed grapefruit juice while being treated chronically with colchicine. Once colchicine is stopped, the symptoms generally resolve within 1 week to several months. Life-threatening and fatal drug interactions have been reported in patients treated with colchicine with concomitant P-gp inhibitors (eg cyclosporine, ranolazine) and strong CYP3A4 inhibitors (eg. clarithromycin, telithromycin, itraconazole, ketoconazole, nefazodone, and some protease inhibitors). In the majority of cases, the doses of colchicine were within the therapeutic range. An algorithm for dosage adjustments of colchicine during concomitant treatment with multiple CYP3A4/P-gp inhibitors to improve safety have been proposed based on an in vivo single dose pharmacokinetic study [49]. P-gp is encoded by the multidrug resistance gene-1 (MDR1, adenosine triphosphate-binding cassette transporter: ABCB1, P-glycoprotein) and is an ATP-dependent efflux pump that regulates the tissue distribution of colchicine. The P-gp/MDR1 gene is highly polymorphic with more than 70 single nucleotide polymorphisms (SNPs) defined to date [50]. Polymorphisms of the MDR1 gene have been shown to change both the expression level and function of P-gp, and might be associated with colchicine resistance in some small sample studies in FMF [51][52][53] and BD [54]; but not in all studies [55][56]. In general, further studies with a larger sample size are needed to better understand the role of MDR1 gene polymorphisms in the colchicine response of patients with different conditions.
Apart from avoidance of the use of colchicine with adversely interacting drugs, colchicine dose reductions should be considered in patients with renal or hepatic impairment, and the elderly. Some recommendations suggest reducing the colchicine dose by 50% in patients with creatinine clearance below 50 ml/minute [2]. The American College of Rheumatology guidelines recommended exercising caution and instituting colchicine dose adjustment in renal impairment at the discretion of the treating clinician [57].
3.3 Therapeutic uses of colchicine
The use of colchicine has been better established in gout and FMF. Beyond these, the possible therapeutic roles of colchicine in rheumatic osteoarthritis (OA), BD or non-rheumatic conditions, including pericarditis, artherosclerosis and liver cirrhosis are still ongoing and as described below, appear promising for some of these conditions.
3.3.1 Rheumatic diseases
3.3.1a Gout
Colchicine has been used ubiquitously in abortion of acute gouty attacks, yet there remain few large randomized controlled trials evaluating the optimal dosing frequency and dosage (Table 1). There has only been one recent randomized, double blinded, placebo controlled trial in 2010, the AGREE trial (Acute Gout Flare Receiving Colchicine Analysis) that has looked at high versus low doses of colchicine, i.e. 8 versus 3 tablets (0.6 mg) in 24 hours in early acute gout flares [47]. Superiority of colchicine was demonstrated over placebo in 185 patients randomized to high dose or low dose of colchicine or placebo with responder rates 32.7%, 37.6% and 15.5% respectively; there was no significant difference between the high and low dose groups. The high dose treatment arm had more associated gastrointestinal side effects. The general consensus for the treatment of acute gout is to use low doses of colchicine. In view of its potential side effects, including renal, hepatic and gastrointestinal side effects, dosage adjustments also need to be considered. The European League against Rheumatism EULAR 2011consensus guidelines recommended low dose colchicine, up to 3 doses of 0.5mg in the first 24 hours for the treatment of gout [58]. The ACR guidelines also recommended colchicine as an appropriate primary treatment option in acute gout attacks, with a loading dose of 1.2 mg followed by 0.6mg [57].
Table 1.
Summary of the uses of colchicine in arthritis.
| Author Country Year |
Disease group |
Study Desig n |
subject | n | Interventio n |
FU | Outcome |
|---|---|---|---|---|---|---|---|
| Terkeltaub, et al USA, 2010 [47] | Gouty arthritis | RCT, DB | Acute gouty arthritis | 184 | low-dose colchicine −(1.8 mg total over 1h) vs. high-dose colchicine −(4.8 mg total over 6h) vs. placebo |
24h |
Positive
|
| Das, et al India 2002 [67] | Knee OA | RCT, DB | Knee OA with inflammation
|
39 | Colchicine 0.5mg bid vs. Placebo |
20 w |
Positive Higher proportion in colchicine group achieved 30% improvement
|
| Das, et al India 2002 [68] | Knee OA | RCT, DB | Primary knee OA Addition of NSAID |
36 | Colchicine 0.5mg bid vs. placebo |
20 w |
Positive Higher proportion in colchicine group achieved 30% |
improvement
|
|||||||
| Aran, et al Iran 2011 [69] | Knee OA | RCT, DB | primary knee OA Women, Postmenopausal |
61 | Colchicine 0.5mg bid vs. placebo |
16 w |
Positive In colchicine group:
|
| Leung, et al Singapore NCT02176460 [70] |
Knee OA | RCT, DB | Primary knee OA | 120 | Colchicine 0.5mg bid vs. placebo |
16 w | In recruitment phase |
n=sample size; OA= osteoarthritis; RCT = randomized controlled trial; DB = double blinded; SC = single center; NSAIDs = non-steroidal anti-inflammatory drugs; IA = intra-articular; vs. = versus; bid = twice daily; h= hour; d = day; w = week; VAS = visual analogue scale; KGMC = total King George’s Medical College (KGMC) scale; WOMAC= total Western Ontario and McMaster University Osteoarthritis scores.
The effectiveness of colchicine in prophylaxis of gout flares after the initiation of urate lowering therapy has been demonstrated [59][60]. The EULAR guidelines recommended prophylaxis for acute gout attacks in the first 6 to 12 months of therapy with urate-lowering agents [58].
3.3.1b Osteoarthritis
The association of uric acid and OA has long been observed [61][62], and a pathological link between the two conditions has been proposed [63]. In a recent large study of human cadaveric ankles, MSU were strongly associated with cartilage lesions as well as immunohistochemical changes of cartilage degeneration and repair [64]. A study of human chondrocytes and cartilage explants also demonstrated the profound inhibitory effect of MSU on chondrocyte viability and function [65]. In a study among OA subjects without clinical gout, a strong positive association was identified between synovial fluid uric level and IL18, IL1β and OA severity as measured by imaging [66]. This suggested that monosodium uric acid may contribute both to the initiation and/or propagation of cartilage degradation in OA although it may do so in forms other than crystalline.
The pain and symptom relieving effects of colchicine for knee OA have already been demonstrated in three small RCTs (Table 1) [67][68][69]; In the first study of 39 knee OA subjects with clinical signs of inflammation, despite background piroxicam therapy, colchicine in addition to intraarticular steroid treatment produced significantly greater symptomatic improvement and reduction of signs of knee inflammation than intraarticular steroid and placebo [67]. The second study showed that despite background therapy with nimesulide, 36 subjects with knee OA had greater symptomatic improvement defined by acheiving a 30% reduction in Western Ontario and McMaster Universities Arthritis Index at 20 weeks with colchicine than placebo (57.9% versus 23.5%) [68]. Another study of 61 knee OA subjects demonstrated that colchicine added to usual treatment (analgesics, non steroidal anti-inflammatory drugs (NSAIDs) and physiotherapy) led to greater improvement in patient and physician global assessment at the end of 3 months versus placebo [69]. A registered single center RCT (NCT02176460) with larger sample size has started, aiming to compare colchicine versus placebo in reducing pain and function, in addition to usual treatment in subjects with knee OA. It includes evaluation of serum and synovial fluid biomarkers in an attempt to delineate colchicine's underlying mechanisms of action and impact on joint tissue metabolism [70].
3.3.1c Behcet’s disease
In Behcet’s disease, colchicine is postulated to be useful for mucocutaneous involvement. A small open-label study of 14 subjects in Japan showed equivocal response in the frequency of ocular attacks between treatment with monotherapy with infliximab compared with combination therapy with colchicine (p<0.05) [71]. A multicentered randomized controlled trial in Iran involving 169 patients demonstrated significant improvement based on the Iran Behcet’s Disease Dynamic Activity Measure (IBDDAM) in the colchicine treatment arm compared with placebo (mean difference colchicine versus placebo, −0.80 versus 0.46, p = 0.00016) [72]. An open-label study in subjects with mucocutaneous BD revealed significant reduction in oral ulcers and serum inflammatory cytokines IL-6, IL-8 and TNFα with the combination of colchicine and levamisole [73].
3.2.1d Familial Mediterranean fever (FMF)
Familial Mediterranean fever (FMF) is a rare inherited autosomal recessive autoinflammatory disorder caused by mutations of the FMF gene (MEFV, Mediterranean Fever) on chromosome 16p13.3, which encodes pyrin. It is characterized by recurrent and self-limited episodes of fever and painful serositis, related to neutrophil activation at serosal surfaces. It affects predominantly ethnic groups around the Mediterranean basin [74]. Colchicine represents the standard first line treatment for FMF and has to be continued for life. A good, partial response to colchicine was seen in 87% of the patients in reducing severity, duration and frequency of attacks [75][76][77]. Although colchicine does not totally prevent febrile attacks, its long term use can arrest the progression of AA amyloidosis. Consensus guidelines have been developed for the chronic use of colchicine in children with FMF. Compliance should be carefully monitored [77].
3.3.2 Non rheumatic diseases
3.3.2a Pericarditis
Exciting new developments have been made in recent years on the application of colchicine for treating acute, recurrent pericarditis, as well as post-pericardiotomy syndrome. This is especially relevant as there are no definitive therapies for the treatment of pericarditis. In an open-label randomized controlled trials, COlchicine for REcurrent pericarditis (CORE) [78] and COlchicine for acute PEricarditis (COPE) [79], colchicine given as an adjunct to conventional treatment, has demonstrated significant reduction in recurrence of pericarditis at 18 months. Subsequent larger multicenter RCTs, Colchicine for Recurrent Pericarditis (CORP) [80] and the CORP-2 trial [81], colchicine added to conventional anti-inflammatory treatment significantly reduced the rate of subsequent recurrences of pericarditis in subjects with multiple recurrences. In a multicenter RCT, colchicine was shown to reduce the risk of the first recurrent attack of pericarditis (relative risk reduction in the colchicine group, 0.56; 95% confidence interval, 0.27 to 0.73; number needed to treat 3) [82]. The efficacy and safety of colchicine for the primary prevention of the postpericardiotomy syndrome (PPS), postoperative effusions, and postoperative atrial fibrillation (POAF) has been shown in the COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS) trial [83]. A larger multicenter COPPS2 trial evaluating the use of colchicine for the primary prevention of PPS, postoperative effusions, and POAF is currently in a recruitment phase (NCT00128453); potentially it may provide evidence to support the use of preoperative colchicine to prevent several postoperative complications [84]. Several meta-analyses support the use of colchicine in the treatment of pericarditis [85][86][87][88][89](Table 2). Colchicine compared to standard treatment with aspirin or NSAIDs was associated with comparable adverse effects and drug discontinuation rates. Gastrointestinal intolerance was the most frequent side effect (mean incidence 8%). Based on early observational trials, the European Society of Cardiology has recommended colchicine as a treatment option for pericarditis and post-pericardiotomy syndrome (level of evidence B, class IIa), either as an adjunct to NSAIDs or as monotherapy [90]. The evidence regarding the clinical utility of colchicine in pericarditis is still evolving, in general it has a high potential for future clinical use.
Table 2.
Summary of studies on the adjunctive use of colchicine in pericarditis.
| Author | Year | design | setting | n | FU | Trial Name |
Subject character |
Treatment | Primary Outcome |
Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Imazio et al [78] | 2005 | RCT, OL | SC | 84 | 6 m | CORE | Recurrent pericarditis | colchicine (1.0–2.0 mg D1, maintenance 0.5–1.0 mg/d | Recurrent pericarditis at 18m; symptom persistence at 72 h |
Positive Recurrent rates at 18 months −(24.0% colchicine vs 50.6% placebo; p = .02; NNT = 4.0) Symptom persistence at 72h −(10% colchicine vs 31% placebo; p = 0.03). |
| Imazio et al [80] | 2011 | RCT, DB | MC | 120 | 6 m | CORP | Recurrent pericarditis | Colchicine 1–2mg D1, maintenance 0.5–1mg/d | 2nd recurrence of pericarditis |
Positive 24% colchicine vs. 55% placebo; p <0.05 |
| Imazio et al [81] | 2014 | RCT, DB | MC | 240 | 6 m | CORP 2 | Recurrent pericarditis | Colchicine 1–2mg D1, maintenance 0.5 mg/d | Subsequent recurrence (>2nd) |
Positive 21.6% colchicine vs 51% placebo, p=0.009 |
| Imazio et al [79] | 2005 | RCT, OL | SC | 120 | 3 m | COPE | Acute pericarditis | colchicine (1.0–2.0 mg D1, maintenance 0.5–1.0 mg/d | Recurrent pericarditis at 18m; symptom persistence at 72 h |
Positive Recurrent rates at 18 months −(10.7% colchicine vs. 32.3% placebo; p=0.004; NNT=5) Symptom persistence at 72h −(11.7% colchicine vs. 36.7% placebo; p=0.003). |
| Imazio et al [82] | 2013 | RCT, DB | MC | 240 | 3 m | ICAP | Acute pericarditis | Colchicine 0.5 mg bid (for BW >70 kg) Colchicine 0.5 mg/d (for BW ≤70 kg) |
1st recurrence of acute pericarditis |
Positive 16.7% colchicine vs 37.5% placebo, p<0.001 RRR 0.56; CI 0.30 to 0.72; NNT 4.0; p<0.001 |
| Imazio et al [83] | 2010 | RCT, DB | MC | 360 | 1 m | COPP S | PPS | POD3: 1mg bd, maintenance 0.5mg bd | Incidence of PPS |
Positive 8.9% colchicin e vs 21.1% placebo, p<0.002 |
| Imazio et al [84] | 2013 | RCT, DB | MC | 360 | 1 m | COPP S2 | PPS | POD3: 1mg bd, maintenance 0.5mg bd | Incidence of PPS, Postopera tive effusions, POAF | in recruitment phase |
| Meta Analyses | ||||||||||
| Author | Yea r |
Number of Trials |
Setting | Results | ||||||
| Kuo et al [85] | 2009 | 3 | Primary and secondary prophylaxis of pericarditis | Positive | AR 21.6% NNT 5 | |||||
| Imazio et al [86] | 2012 | 5 | Pericarditis prevention | Positive | RR 0.40 CI 0.30–0.54 | |||||
| Lotrionte et al [88] | 2013 | 7 | Acute or recurrent pericarditis | Positive | Reduced risk of treatment failure (OR 0.23 CI 0.11–0.49) recurrent pericarditis (OR 0.39 CI 0.20–0.77) | |||||
| Imazio et al [87] | 2011 | 4 | Primary prevention of PPs | Positive | OR 0.38 CI 0.22–0.6 | |||||
| Alam et al [89] | 2012 | 5 | Recurrent pericarditis or primary Prevention of PPs | Positive | Overall (RRR 20.3%, NNT 4.9) Recurrent Pericarditis (RRR 33.3% and NNT 3.0). Primary prevention of PPS (RRR 12.1, NNT 8.3) |
|||||
n = sample size; FU= Follow Up duration; RCT: randomized control trial, SC = single centre; MC = multi centre; OL = open-label; DB = double blinded; y = year; m = month; d=day; POD =: Post-operation day, PPS = post pericardiotomy syndrome; POAF Post-operative Atrial Fibrillation;, P = Placebo; vs. = versus; OR = odd ratio; RR = relative risk; AR = absolute risk ratio; RRR = relative risk reduction; CI = 95% confident interval; NNT = number needed to treat; CORE = Colchicine for Recurrent Pericarditis; CORP =Colchicine for Acute Pericarditis; ICAP= Investigation on Colchicine for Acute Pericarditis; COPPS= Colchicine for Prevention of the PPS and POAF;
3.3.2b Use of colchicine in atherosclerosis and cardiovascular diseases
A recent large randomized, double blinded study in Canada consisting of 532 subjects who had coronary artery disease and who took who took colchicine 0.5mg daily for a median of three years, demonstrated a reduction in the composite incidence of acute cardiovascular events, or non-hospital cardiac arrests or non-cardioembolic ischaemic strokes in the colchicine treatment arm (5.3% vs 16% p<0.001) [91]. Deftereos et al also demonstrated a significant reduction in bare metal stent stenosis of 196 patients post percutaneous coronary intervention who were randomized to either colchicine 0.5mg twice a day or placebo (16% versus 33%, p<0.007) [92]. In a RCT of 80 subjects with acute coronary or cerebrovascular event, low dose colchicine given at 0.5mg twice daily compared with placebo, did not show a significant difference in high sensitivity CRP levels or platelet function at 30 days [93]. In another RCT in 267 subjects with chronic heart failure, colchicine compared with placebo did not reduce the proportion of subjects achieving improvement in the New York Heart Association class or the composite acute coronary syndrome or stroke end points, although CRP and IL6 were significantly reduced in the colchicine group [94].
3.3.2c Hepatic Diseases
Colchicine has anti-fibrotic effect and possibly a modulatory role in bile composition. However, the results of clinical trials have been inconsistent. In a placebo-controlled multicenter study in 549 patients with advanced alcoholic cirrhosis [95], colchicine failed to reproduce the previously reported favorable effect on mortality [96]. A meta-analysis combining the results of 15 RCTs encompassing 1,714 subjects with alcoholic or non-alcoholic liver cirrhosis, demonstrated no significant effects of colchicine on mortality, liver related mortality, complications and other outcomes [97]. In a recent double blind, randomized controlled trial in 74 subjects with chronic liver cirrhosis and who could not be treated with alpha-interferon, Muntoni et al demonstrated that colchicine 1mg daily significantly increased survival (94.6% vs 78.4% p=0.001), and decreased serum N-terminal peptide of type III procollagen (a biomarker of liver fibrosis) over a follow up period of 4.4 years [98].This suggests there may be a beneficial effect of colchicine for selected subjects with liver cirrhosis. In an open-label study of 91 subjects with primary biliary cirrhosis who had an incomplete response to ursodeoxycholic acid, a regimen of 6 months of add-on colchicine followed by add-on methotrexate, demonstrated a significant decrease in liver enzymes and histological fibrosis and inflammation scores [99].
3.3.2d Other indications
Colchicine has been tested in a variety of dermatological conditions and has shown a certain degree of efficacy in recurrent aphthous stomatitis [100][101] and chronic urticaria [102][103], but not in hidradenitis suppurativa [104] or peyronie’s disease [105]. The benefits of colchicine were demonstrated in a small three-arm study comparing prednisolone alone versus prednisolone and cyclophosphamide versus prednisolone and colchicine in pulmonary fibrosis. There were significant improvements in dyspnoea in the prednisolone and colchicine group compared with the two other groups [106].
4. CONCLUSION
Colchicine has been the first line therapy for the treatment of acute gouty arthritis and FMF. Due to the anti-inflammatory and anti-fibrotic activities, the therapeutic use of colchicine has extended beyond arthritis. The exact mechanisms of action underlying its efficacy are not completely understood and remain under active investigation. Current results suggest that colchicine downregulates multiple inflammatory pathways and modulates innate immunity. As demonstrated by this review, there are many potential therapeutic uses for colchicine or its analogues.
Acknowledgments
Funding source
This review is funded in part by the Singapore National Medical Research Council, transitional award (NMRC-TA-0007-2012), the National Institutes of Health USA (NIH P30-AG-028716) and a grant from the Duke-NUS Governing Board for funding support to VBK and Y-YL.
List of abbreviations
- ABCB1
adenosine triphosphate-binding cassette transporter 1
- ASC
Apoptosis associated Speck like protein containing Caspase recruitment domain
- ATP
adenosine triphosphate
- Bcl-2
B-cell lymphoma 2
- BD
Behcet’s disease
- Ca2+
calcium
- Caspase
cysteine-dependent aspartate-directed proteases
- CCF
crystal-derived chemotactic factor
- CPPD
calcium pyrophosphate dihydrate crystals
- CRP
C-reactive protein
- CYP3A4
cytochrome P450 3A4
- FMF
familial Mediterranean fever
- IC50
50% inhibitory concentration
- IL
interleukin
- MDR1
multidrug resistance gene-1
- MLCK
myosin light chain kinase
- MICL
myeloid inhibitory C-type lectin-like receptor
- MSU
monosodium urate crystals
- MyD88
myeloid differentiation primary response gene 88
- NALP3
NACHT-LRRPYD-containing protein 3
- NF-kB
nuclear factor kB
- NO
nitric oxide
- NSAIDs
non steroidal anti-inflammatory drugs
- P-gp
P-glycoprotein 1
- P2X2
P2X7, purinergic receptors P2X2, P2X7
- ROS
reactive oxygen species
- RhoA
Ras homolog gene family, member A
- ROCK
Rho-associated, coiled-coil containing protein kinase
- SNPs
single nucleotide polymorphisms
- SRF
serum response factor
- TLR
toll-like receptors
- TGF-β1
transforming growth factor beta-1
- TNFα
tumor necrosis factor α
- VGEF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
All authors contributed equally and approved the final version of the manuscript.
Conflict of interest
There is no conflict of interest in regard to the content of the manuscript.
References
- 1.Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10(3):218–227. doi: 10.1007/s11926-008-0036-3. [DOI] [PubMed] [Google Scholar]
- 2.Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum. 2009;38(6):411–419. doi: 10.1016/j.semarthrit.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya B, Panda D, Gupta S, Banerjee M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev. 2008;28(1):155–183. doi: 10.1002/med.20097. [DOI] [PubMed] [Google Scholar]
- 4.Massarotti A, Coluccia A, Silvestri R, Sorba G, Brancale A. The tubulin colchicine domain: a molecular modeling perspective. ChemMedChem. 2012;7(1):33–42. doi: 10.1002/cmdc.201100361. [DOI] [PubMed] [Google Scholar]
- 5.Charpentier MS, Whipple RA, Vitolo MI, Boggs AE, Slovic J, Thompson KN, et al. Curcumin targets breast cancer stem-like cells with microtentacles that persist in mammospheres and promote reattachment. Cancer Res. 2014;74(4):1250–1260. doi: 10.1158/0008-5472.CAN-13-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meshki J, Douglas SD, Hu M, Leeman SE, Tuluc F. Substance P induces rapid and transient membrane blebbing in U373MG cells in a p21-activated kinase-dependent manner. PloS one. 2011;6(9):e25332. doi: 10.1371/journal.pone.0025332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganguly A, Yang H, Zhang H, Cabral F, Patel KD. Microtubule dynamics control tail retraction in migrating vascular endothelial cells. Mol Cancer Ther. 2013;12(12):2837–2846. doi: 10.1158/1535-7163.MCT-13-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado EN, Patnaik J, Mullins MR, Lemasters JJ. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 2010;70(24):10192–10201. doi: 10.1158/0008-5472.CAN-10-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phelps P. Polymorphonuclear leukocyte motility in vitro: IV. Colchicine inhibition of chemotactic activity formation after phagocytosis of urate crystals. Arthritis Rheum. 2008;58(2 Suppl):S25–S33. doi: 10.1002/art.23357. [DOI] [PubMed] [Google Scholar]
- 10.McCarty DJ. Urate crystals, inflammation, and colchicine. Arthritis Rheum. 2008;58(2 Suppl):S20–S24. doi: 10.1002/art.23069. [DOI] [PubMed] [Google Scholar]
- 11.Ryckman C, McColl SR, Vandal K, de Médicis R, Lussier A, Poubelle PE, et al. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003;48(8):2310–2320. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- 12.Gagné V, Marois L, Levesque JM, Galarneau H, Lahoud MH, Caminschi I, et al. Modulation of monosodium urate crystal-induced responses in neutrophils by the myeloid inhibitory C-type lectin-like receptor: potential therapeutic implications. Arthritis Res Ther. 2013;15(4):R73. doi: 10.1186/ar4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asako H, Kubes P, Baethge BA, Wolf RE, Granger DN. Colchicine and methotrexate reduce leukocyte adherence and emigration in rat mesenteric venules. Inflammation. 1992;16(1):45–56. doi: 10.1007/BF00917514. [DOI] [PubMed] [Google Scholar]
- 14.Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G, et al. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96(2):994–1002. doi: 10.1172/JCI118147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberge CJ, Gaudry M, de Médicis R, Lussier A, Poubelle PE, Naccache PH, et al. Crystal-induced neutrophil activation IV. Specific inhibition of tyrosine phosphorylation by colchicine. J Clin Invest. 1993;92(4):1722–1729. doi: 10.1172/JCI116759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popa-Nita O, Marois L, Paré G, Naccache PH. Crystal-induced neutrophil activation: X. Proinflammatory role of the tyrosine kinase Tec. Arthritis Rheum. 2008;58(6):1866–1876. doi: 10.1002/art.23801. [DOI] [PubMed] [Google Scholar]
- 17.Paschke S, Weidner AF, Paust T, Marti O, Beil M, Ben-Chetrit E, et al. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J Leukoc Biol. 2013;94(5):1091–1096. doi: 10.1189/jlb.1012510. [DOI] [PubMed] [Google Scholar]
- 18.Chia EW, Grainger R, Harper JL. Colchicine suppresses neutrophil superoxide production in a murine model of gouty arthritis: a rationale for use of low-dose colchicine. Br J Pharmacol. 2008;153(6):1288–1295. doi: 10.1038/bjp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korkmaz S, Erturan I, Nazıroğlu M, Uğuz AC, Ciğ B, Övey IS, et al. Colchicine modulates oxidative stress in serum and neutrophil of patients with Behçet disease through regulation of Ca2+ release and antioxidant system. J Membr Biol. 2011;244(3):113–120. doi: 10.1007/s00232-011-9404-4. [DOI] [PubMed] [Google Scholar]
- 20.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 21.Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14(5):454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 22.Taskiran EZ, Cetinkaya A, Balci-Peynircioglu B, Akkaya YZ, Yilmaz E. The effect of colchicine on pyrin and pyrin interacting proteins. J Cell Biochem. 2012;113(11):3536–3546. doi: 10.1002/jcb.24231. [DOI] [PubMed] [Google Scholar]
- 23.Yu JW, Farias A, Hwang I, Fernandes-Alnemri T, Alnemri ES. Ribotoxic stress through p38 mitogen-activated protein kinase activates in vitro the human pyrin inflammasome. J Biol Chem. 2013;288(16):11378–11383. doi: 10.1074/jbc.M112.448795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cronstein BN, Sunkureddi P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. J Clin Rheumatol. 2013;19(1):19–29. doi: 10.1097/RHU.0b013e31827d8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fordham JN, Kirwan J, Cason J, Currey HL. Prolonged reduction in polymorphonucler adhesion following oral colchicine. Ann Rheum Dis. 1981;40(6):605–608. doi: 10.1136/ard.40.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual E, Castellano JA. Treatment with colchicine decreases white cell counts in synovial fluid of asymptomatic knees that contain monosodium urate crystals. J Rheumatol. 1992;19:600–603. [PubMed] [Google Scholar]
- 27.Chae JJ, Wood G, Richard K, Jaffe H, Colburn NT, Masters SL, et al. The familial Mediterranean fever protein, pyrin, is cleaved by caspase-1 and activates NF-kappaB through its N-terminal fragment. Blood. 2008;112(5):1794–1803. doi: 10.1182/blood-2008-01-134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viktorov AV, Yurkiv VA. Albendazole and colchicine modulate LPS-induced secretion of inflammatory mediators by liver macrophages. Bull Exp Biol Med. 2011;151(6):683–685. doi: 10.1007/s10517-011-1415-8. [DOI] [PubMed] [Google Scholar]
- 29.Takenouchi T, Iwamaru Y, Sugama S, Sato M, Hashimoto M, Kitani H, et al. Lysophospholipids and ATP mutually suppress maturation and release of IL-1 beta in mouse microglial cells using a Rho-dependent pathway. J Immunol. 2008;180(12):7827–7839. doi: 10.4049/jimmunol.180.12.7827. [DOI] [PubMed] [Google Scholar]
- 30.Marques-da-Silva C, Chaves MM, Castro NG, Coutinho-Silva R, Guimaraes MZP. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: implications for its therapeutic action. Br J Pharmacol. 2011;163(5):912–926. doi: 10.1111/j.1476-5381.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 32.Pelegrín P. Many ways to dilate the P2X7 receptor pore. Br J Pharmacol. 2011;163(5):908–911. doi: 10.1111/j.1476-5381.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizumoto N, Gao J, Matsushima H, Ogawa Y, Tanaka H, Takashima A, et al. Discovery of novel immunostimulants by dendritic-cell-based functional screening. Blood. 2005;106(9):3082–3089. doi: 10.1182/blood-2005-03-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizumoto N, Tanaka H, Matsushima H, Vishwanath M, Takashima A. Colchicine promotes antigen cross-presentation by murine dendritic cells. J Invest Dermatol. 2007;127(6):1543–1546. doi: 10.1038/sj.jid.5700699. [DOI] [PubMed] [Google Scholar]
- 35.Marin-Esteban V, Charron D, Gelin C, Mooney N. Chemotherapeutic agents targeting the tubulin cytoskeleton modify LPS-induced cytokine secretion by dendritic cells and increase antigen presentation. J Immunother (1997) 2010;33(4):364–370. doi: 10.1097/CJI.0b013e3181cd1094. [DOI] [PubMed] [Google Scholar]
- 36.Peachman KK, Rao M, Palmer DR, Zidanic M, Sun W, Alving CR, et al. Functional microtubules are required for antigen processing by macrophages and dendritic cells. Immunol Lett. 2004;95(1):13–24. doi: 10.1016/j.imlet.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Yang CW, Ahn HJ, Kim WY, Park CW, Park JH, et al. Colchicine decreases apoptotic cell death in chronic cyclosporine nephrotoxicity. J Lab Clin Med. 2002;139(6):364–371. doi: 10.1067/mlc.2002.124397. [DOI] [PubMed] [Google Scholar]
- 38.Guan T, Gao B, Chen G, Chen X, Janssen M, Uttarwar L, et al. Colchicine attenuates renal injury in a model of hypertensive chronic kidney disease. Am J Physiol Renal Physiol. 2013;305(10):F1466–F1476. doi: 10.1152/ajprenal.00057.2013. [DOI] [PubMed] [Google Scholar]
- 39.Shu JC, He YJ, Lv X, Ye GR, Wang LX. Curcumin prevents liver fibrosis by inducing apoptosis and suppressing activation of hepatic stellate cells. Journal of natural medicines. 2009;63(4):415–420. doi: 10.1007/s11418-009-0347-3. [DOI] [PubMed] [Google Scholar]
- 40.Bozkurt D, Bicak S, Sipahi S, Taskin H, Hur E, Ertilav M, et al. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(Suppl 5):S53–S57. [PubMed] [Google Scholar]
- 41.Sandbo N, Ngam C, Torr E, Kregel S, Kach J, Dulin N, et al. Control of myofibroblast differentiation by microtubule dynamics through a regulated localization of mDia2. J Biol Chem. 2013;288(22):15466–15473. doi: 10.1074/jbc.M113.464461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abanonu GB, Daskin A, Akdogan MF, Uyar S, Demirtunc R. Mean platelet volume and β-thromboglobulin levels in familial Mediterranean fever: effect of colchicine use? European journal of internal medicine. 2012;23(7):661–664. doi: 10.1016/j.ejim.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Terekeci HM, Oktenli C, Ozgurtas T, Nalbant S, Top C, Celik S, et al. Increased asymmetric dimethylarginine levels in young men with familial Mediterranean fever (FMF): is it early evidence of interaction between inflammation and endothelial dysfunction in FMF? J Rheumatol. 2008;35(10):2024–2029. [PubMed] [Google Scholar]
- 44.Atta HM, El-Rehany MA, Abdel Raheim SR, Fouad R, Galal AMF. Colchicine inhibits intimal hyperplasia and leukocyte VEGF expression in dogs. J Surg Res. 2008;146(2):184–189. doi: 10.1016/j.jss.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Lee FY, Lu HI, Zhen YY, Leu S, Chen YL, Tsai TH, et al. Benefit of combined therapy with nicorandil and colchicine in preventing monocrotaline-induced rat pulmonary arterial hypertension. Eur J Pharm Sci. 2013;50(3–4):372–384. doi: 10.1016/j.ejps.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Huang C, Cen C, Wang C, Zhan H, Ding X. Synergistic effects of colchicine combined with atorvastatin in rats with hyperlipidemia. Lipids Health Dis. 2014;13:67. doi: 10.1186/1476-511X-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW, et al. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060–1068. doi: 10.1002/art.27327. [DOI] [PubMed] [Google Scholar]
- 48.U.S. Food and Drug Administration, online. [Accessed 20 Dec. 2013]; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm174315.htm.
- 49.Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63(8):2226–2237. doi: 10.1002/art.30389. [DOI] [PubMed] [Google Scholar]
- 50.National Center for Biotechnology Information. [Accessed 20 Dec 2014]; Online. Available at: http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?chooseRs=coding&locusId=5243&mrna.
- 51.Ozen F, Silan C, Uludag A, Candan F, Silan F, Ozdemir S, et al. Association between ABCB1 (MDR1) gene 3435 C>T polymorphism and colchicine unresponsiveness of FMF patients. Ren Fail. 2011;33(9):899–903. doi: 10.3109/0886022X.2011.605980. [DOI] [PubMed] [Google Scholar]
- 52.Tufan A, Babaoglu MO, Akdogan A, Yasar U, Calguneri M, Kalyoncu U, et al. Association of drug transporter gene ABCB1 (MDR1) 3435C to T polymorphism with colchicine response in familial Mediterranean fever. J Rheumatol. 2007;34(7):1540–1544. [PubMed] [Google Scholar]
- 53.Uludag A, Silan C, Atik S, Akurut C, Uludag A, Silan F, et al. Relationship between response to colchicine treatment and MDR1 polymorphism in familial Mediterranean fever patients. Genetic testing and molecular biomarkers. 2014;18(2):73–76. doi: 10.1089/gtmb.2013.0293. [DOI] [PubMed] [Google Scholar]
- 54.Rustemoglu A, Gül Ü, Gümüş-Akay G, Gönül M, Yiğit S, Bozkurt N, et al. MDR1 gene polymorphisms may be associated with Behçet's disease and its colchicum treatment response. Gene. 2012;505(2):333–339. doi: 10.1016/j.gene.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 55.Dogruer D, Tug E, Bes C, Soy M. Lack of an effect of CYP3A4 and MDR1 gene polymorphisms on colchicine pharmacogenetics in the treatment of Familial Mediterranean fever. Genet Mol Res. 2013;12(3):3521–3528. doi: 10.4238/2013.January.24.2. [DOI] [PubMed] [Google Scholar]
- 56.Saricaoglu H, Yilmaz M, Karkucak M, Ozturk HZY, Yakut T, Gulten T, et al. Investigation of ABCB1 gene polymorphism with colchicine response in Behçet's disease. Genet Mol Res. 2011;10(1):1–6. doi: 10.4238/vol10-1gmr824. [DOI] [PubMed] [Google Scholar]
- 57.Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis care & research. 2012;64(10):1447–1461. doi: 10.1002/acr.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamburger M, Baraf HSB, Adamson TC, Basile J, Bass L, Cole B, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123(6 Suppl 1):3–36. doi: 10.3810/pgm.2011.11.2511. [DOI] [PubMed] [Google Scholar]
- 59.Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386–2397. doi: 10.1016/j.clinthera.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31(12):2429–2432. [PubMed] [Google Scholar]
- 61.Acheson RM, Collart AB. New Haven survey of joint diseases XVII. Relationship between some systemic characteristics and osteoarthrosis in a general population. Ann Rheum Dis. 1975;34(5):379–387. doi: 10.1136/ard.34.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Y, Brenner H, Sauerland S, Günther KP, Puhl W, Stürmer T, et al. Serum uric acid and patterns of radiographic osteoarthritis--the Ulm Osteoarthritis Study. Scand J Rheumatol. 2000;29(6):380–386. doi: 10.1080/030097400447589. [DOI] [PubMed] [Google Scholar]
- 63.Roddy E, Zhang W, Doherty M. Are joints affected by gout also affected by osteoarthritis? Ann Rheum Dis. 2007;66(10):1374–1377. doi: 10.1136/ard.2006.063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muehleman C, Li J, Aigner T, Rappoport L, Mattson E, Hirschmugl C, et al. Association between crystals and cartilage degeneration in the ankle. J Rheumatol. 2008;35(6):1108–1117. [PMC free article] [PubMed] [Google Scholar]
- 65.Chhana A, Callon KE, Pool B, Naot D, Gamble GD, Dray M, et al. The effects of monosodium urate monohydrate crystals on chondrocyte viability and function: implications for development of cartilage damage in gout. J Rheumatol. 2013;40(12):2067–2074. doi: 10.3899/jrheum.130708. [DOI] [PubMed] [Google Scholar]
- 66.Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A. 2011;108(5):2088–2093. doi: 10.1073/pnas.1012743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das SK, Mishra K, Ramakrishnan S, Srivastava R, Agarwal GG, Singh R, et al. A randomized controlled trial to evaluate the slow-acting symptom modifying effects of a regimen containing colchicine in a subset of patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2002;10(4):247–252. doi: 10.1053/joca.2002.0516. [DOI] [PubMed] [Google Scholar]
- 68.Das SK, Ramakrishnan S, Mishra K, Srivastava R, Agarwal GG, Singh R, et al. A randomized controlled trial to evaluate the slow-acting symptom-modifying effects of colchicine in osteoarthritis of the knee: a preliminary report. Arthritis Rheum. 2002;47(3):280–284. doi: 10.1002/art.10455. [DOI] [PubMed] [Google Scholar]
- 69.Aran S, Malekzadeh S, Seifirad S. A double-blind randomized controlled trial appraising the symptom-modifying effects of colchicine on osteoarthritis of the knee. Clin Exp Rheumatol. 2011;29(3):513–518. [PubMed] [Google Scholar]
- 70.Leung YY, Thumboo J, Wong BS, Haaland B, Chowbay B, Chakraborty B, et al. Colchicine effectiveness in symptom and inflammation modification in knee osteoarthritis (COLKOA): study protocol for a randomized controlled trial. Trials. 2015;16(1):200. doi: 10.1186/s13063-015-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeuchi M, Asukata Y, Kawagoe T, Ito N, Nishide T, Mizuki N, et al. Infliximab monotherapy versus infliximab and colchicine combination therapy in patients with Behçet's disease. Ocul Immunol Inflamm. 2012;20(3):193–197. doi: 10.3109/09273948.2012.665124. [DOI] [PubMed] [Google Scholar]
- 72.Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, Shahram F, Nadji A, Shams H, et al. Colchicine versus placebo in Behçet’s disease: randomized, double-blind, controlled crossover trial. Mod Rheumatol. 2009;19(5):542–549. doi: 10.1007/s10165-009-0200-2. [DOI] [PubMed] [Google Scholar]
- 73.Sun A, Wang YP, Chia JS, Liu BY, Chiang CP. Treatment with levamisole and colchicine can result in a significant reduction of IL-6, IL-8 or TNF-alpha level in patients with mucocutaneous type of Behcet's disease. J Oral Pathol Med. 2009;38(5):401–405. doi: 10.1111/j.1600-0714.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 74.Portincasa P, Scaccianoce G, Palasciano G. Familial mediterranean fever: a fascinating model of inherited autoinflammatory disorder. Eur J Clin Invest. 2013;43(12):1314–1327. doi: 10.1111/eci.12170. [DOI] [PubMed] [Google Scholar]
- 75.Dinarello CA, Wolff SM, Goldfinger SE, Dale DC, Alling DW. Colchicine therapy for familial mediterranean fever. A double-blind trial. N Engl J Med. 1974;291(18):934–937. doi: 10.1056/NEJM197410312911804. [DOI] [PubMed] [Google Scholar]
- 76.Ben-Chetrit E. Familial Mediterranean fever (FMF) and renal AA amyloidosis--phenotype-genotype correlation, treatment and prognosis. J Nephrol. 2003;16(3):431–434. [PubMed] [Google Scholar]
- 77.Kallinich T, Haffner D, Niehues T, Huss K, Lainka E, Neudorf U, et al. Colchicine use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics. 2007;119(2):e474–e483. doi: 10.1542/peds.2006-1434. [DOI] [PubMed] [Google Scholar]
- 78.Imazio M, Bobbio M, Cecchi E, Demarie D, Pomari F, Moratti M, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med. 2005;165(17):1987–1991. doi: 10.1001/archinte.165.17.1987. [DOI] [PubMed] [Google Scholar]
- 79.Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112(13):2012–2016. doi: 10.1161/CIRCULATIONAHA.105.542738. [DOI] [PubMed] [Google Scholar]
- 80.Imazio M, Brucato A, Cemin R, Ferrua S, Belli R, Maestroni S, et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med. 2011;155(7):409–414. doi: 10.7326/0003-4819-155-7-201110040-00359. [DOI] [PubMed] [Google Scholar]
- 81.Imazio M, Belli R, Brucato A, Cemin R, Ferrua S, Beqaraj F, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicenter, double-blind, placebo-controlled, randomized trial. Lancet. 2014;383(9936):2232–2237. doi: 10.1016/S0140-6736(13)62709-9. [DOI] [PubMed] [Google Scholar]
- 82.Imazio M, Brucato A, Cemin R, Ferrua S, Maggiolini S, Beqaraj F, et al. A Randomized Trial of Colchicine for Acute Pericarditis. N Engl J Med. 2013;369(16):1522–1528. doi: 10.1056/NEJMoa1208536. [DOI] [PubMed] [Google Scholar]
- 83.Imazio M, Belli R, Brucato A, Ferrazzi P, Patrini D, Martinelli L, et al. COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS): a multicenter, randomized, double-blind, placebo-controlled trial. Eur Heart J. 2010;31:2049–2054. doi: 10.1093/eurheartj/ehq319. [DOI] [PubMed] [Google Scholar]
- 84.Imazio M, Belli R, Brucato A, Ferrazzi P, Patrini D, Martinelli L, et al. Rationale and design of the COlchicine for Prevention of the Post-pericardiotomy Syndrome and Post-operative Atrial Fibrillation (COPPS-2 trial): A randomized, placebo-controlled, multicenter study on the use of colchicine for the primary prevention of the postpericardiotomy syndrome, postoperative effusions, and postoperative atrial fibrillation. Am Heart J. 2013;166:13–19. doi: 10.1016/j.ahj.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 85.Kuo IF, Pearson GJ, Koshman SL. Colchicine for the Primary and Secondary Prevention of Pericarditis: An Update. Ann Pharmacother. 2009;43:2075–2081. doi: 10.1345/aph.1M234. [DOI] [PubMed] [Google Scholar]
- 86.Imazio M, Brucato A, Forno D, Ferro S, Belli R, Trinchero R, et al. Efficacy, safety of colchicine for pericarditis prevention. Systematic review and meta-analysis. Heart. 2012;98:1078–1082. doi: 10.1136/heartjnl-2011-301306. [DOI] [PubMed] [Google Scholar]
- 87.Imazio M, Brucato A, Markel G, Cemin R, Trinchero R, Spodick DH, et al. Meta- Analysis of Randomized Trials Focusing on Prevention of the Postpericardiotomy Syndrome. Am J Cardio. 2011;108:575–579. doi: 10.1016/j.amjcard.2011.03.087. [DOI] [PubMed] [Google Scholar]
- 88.Lotrionte M, Biondi-Zoccai G, Imazio M, Castagno D, Moretti C, Abbate A, et al. International collaborative systematic review of controlled clinical trials on pharmacological treatments for acute pericarditis and its recurrences. Acute Ischemic Heart Disease J. 2010;160:662–670. doi: 10.1016/j.ahj.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Alam M, Kayani WT, Bandeali SJ, Shahzad SA, Huang HD, Virani SS, et al. Impact of Colchicine on pericardial inflammatory syndromes - An analysis of randomized controlled trials. Int J Cardiol. 2012;161(1):59–62. doi: 10.1016/j.ijcard.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 90.Maisch B, Seferović PM, Ristić AD, Erbel R, Rienmüller R, Adler Y, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 91.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 92.Deftereos S, Giannopoulos G, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, et al. Colchicine Treatment for the Prevention of Bare Metal Stent Restenosis in Diabetic Patients. J Am Coll Cardiol. 2013;61:1679–1685. doi: 10.1016/j.jacc.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 93.Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW, et al. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: a pilot randomized controlled trial. J Thromb Thrombolysis. 2012;33:88–94. doi: 10.1007/s11239-011-0637-y. [DOI] [PubMed] [Google Scholar]
- 94.Deftereos S, Giannopoulos G, Panagopoulou V, Bouras G, Raisakis K, Kossyvakis C, et al. Anti-Inflammatory Treatment With Colchicine in Stable Chronic Heart Failure, A Prospective, Randomized Study. JACC Heart Fail. 2014;2(2):131–137. doi: 10.1016/j.jchf.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Morgan TR, Weiss DG, Nemchausky B, Schiff ER, Anand B, Simon F, et al. Colchicine treatment of alcoholic cirrhosis: a randomized, placebo-controlled clinical trial of patient survival. Gastroenterology. 2005;128(4):882–890. doi: 10.1053/j.gastro.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 96.Kershenobich D, Vargas F, Garcia-Tsao G, Perez Tamayo R, Gent M, Rojkind M, et al. Colchicine in the treatment of cirrhosis of the liver. N Engl J Med. 1988;318(26):1709–1713. doi: 10.1056/NEJM198806303182602. [DOI] [PubMed] [Google Scholar]
- 97.Rambaldi A, Gluud C. Colchicine for alcoholic and non-alcoholic liver fibrosis and cirrhosis. Cochrane Database Syst Rev. 2005;18(2):CD002148. doi: 10.1002/14651858.CD002148.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muntoni S, Rojkind M, Muntoni S. Colchicine reduces procollagen III and increases pseudocholinesterase in chronic liver disease. World J Gastroenterol. 2010;16(23):2889–2894. doi: 10.3748/wjg.v16.i23.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaplan MM, Bonder A, Ruthazer R, Bonis PAL. Methotrexate in Patients with Primary Biliary Cirrhosis Who Respond Incompletely to Treatment with Ursodeoxycholic Acid. Dig Dis Sci. 2010;55:3207–3217. doi: 10.1007/s10620-010-1291-5. [DOI] [PubMed] [Google Scholar]
- 100.Fontes V, Machet L, Huttenberger B, Lorette G, Vaillant L. [Recurrent aphthous stomatitis: treatment with colchicine. An open trial of 54 cases] Ann Dermatol Venereol. 2002;129(12):1365–1369. [PubMed] [Google Scholar]
- 101.Pakfetrat A, Mansourian A, Momen-Heravi F, Delavarian Z, Momen-Beitollahi J, Khalilzadeh O, et al. Comparison of colchicine versus prednisolone in recurrent aphthous stomatitis: A double-blind randomized clinical trial. Clin Invest Med. 2010;33(3):E189–E195. doi: 10.25011/cim.v33i3.13725. [DOI] [PubMed] [Google Scholar]
- 102.Criado RFJ, Criado PR, Martins JEC, Valente NYS, Michalany NS, Vasconcellos C, et al. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19(2):92–96. doi: 10.1080/09546630701499309. [DOI] [PubMed] [Google Scholar]
- 103.Lawlor F, Black AK, Ward AM, Morris R, Greaves MW. Delayed pressure urticaria, objective evaluation of a variable disease using a dermographometer and assessment of treatment using colchicine. Br J Dermatol. 1989;120(3):403–408. doi: 10.1111/j.1365-2133.1989.tb04167.x. [DOI] [PubMed] [Google Scholar]
- 104.van der Zee HH, Prens EP. The anti-inflammatory drug colchicine lacks efficacy in hidradenitis suppurativa. Dermatology. 2011;223(2):169–173. doi: 10.1159/000332846. [DOI] [PubMed] [Google Scholar]
- 105.Safarinejad MR. Therapeutic effects of colchicine in the management of Peyronie's disease: a randomized double-blind, placebo-controlled study. Int J Impot Res. 2004;16(3):238–243. doi: 10.1038/sj.ijir.3901185. [DOI] [PubMed] [Google Scholar]
- 106.Fiorucci E, Lucantoni G, Paone G, Zotti M, Li BE, Serpilli M, et al. Colchicine, cyclophosphamide and prednisone in the treatment of mild-moderate idiopathic pulmonary fibrosis: comparison of three currently available therapeutic regimens. Eur Rev Med Pharmacol Sci. 2008;12(2):105–111. [PubMed] [Google Scholar]