Abstract
Background
Cardiac resynchronization therapy (CRT) reduces mortality and morbidity in selected heart failure (HF) patients. However, not all patients respond to CRT.
Objective
We hypothesized that a novel measure of electrical dyssynchrony, sum absolute QRST integral (SAI), predicts CRT response independent of QRS duration and morphology.
Methods
We retrospectively analyzed baseline 12-lead electrocardiograms (ECGs) of SMART-AV study participants [N=234, mean age 67 y, 70% male, 60% ischemic cardiomyopathy (ICM), mean left ventricular ejection fraction (LVEF) 25%, mean QRS duration 152ms, 77% had left bundle branch block (LBBB)]. Baseline pre-implant ECGs were digitized, transformed into orthogonal XYZ, and analyzed automatically by customized Matlab software. SAI was measured as an averaged arithmetic sum of absolute areas under the QRST curve. Patients were followed prospectively 6 months after CRT-D implantation. Patients with a decrease in left ventricular end-systolic volume ≥ 15mls after 6 months of CRT were considered responders. Logistic regression model was adjusted for age, gender, BBB morphology, LVEF, type of cardiomyopathy and QRS duration.
Results
Patients with the high mean SAI (3rd tertile) had 2.5 times greater odds of response than those with low mean SAI (1st tertile; OR 2.5, 95% CI 1.3–5.0, p=0.010), and 1.9 times greater than the lower two tertiles combined (OR 1.9, 95%CI 1.1–3.5; P=0.03). Adjustment for renal function (OR 2.33 (95%CI 1.32, 4.11); P=0.003) and LV lead position in RAO/LAO (OR 1.7 (95%CI 0.9, 3.2); P=0.087) did not attenuate association of SAI with outcome.
Conclusion
High SAI QRST independently predicts CRT response in the SMART-AV study.
Keywords: Cardiac resynchronization therapy, heart failure, remodeling, SAI QRST
Introduction
Cardiac resynchronization therapy (CRT) is a powerful electrical treatment for patients with systolic HF and electrical dyssynchrony. However, about a third of CRT recipients do not improve with pacing optimally. Despite the initial promise, assessment of mechanical dyssynchrony did not improve patient selection1. It is now accepted that electrical, rather than mechanical, dyssynchrony needs to be present for CRT to be beneficial. Electrical dyssynchrony is traditionally characterized by QRS duration2–4 and morphology3, 5. However, QRS duration is an imperfect predictor of CRT response. A positive correlation between QRS duration and CRT benefit was shown in many, but not all, studies6. Marked QRS widening (≥178 ms) in patients with LBBB was associated with non-responsiveness to CRT7. Moreover, similar CRT response was shown in LBBB patients with QRS duration 120–150 ms and above 150 ms8. Likewise, there is a discrepancy regarding the definition of a CRT-responding conduction abnormality.
SAI QRST9, 10 is a simple ECG marker of electrical remodeling and arrhythmia vulnerability, which in post-MI patients showed a correlation with the time passed since MI11. Moreover, SAI QRST predicted sustained ventricular arrhythmias with appropriate ICD therapies9–11 and cardiac death11 in patients with and without conduction abnormalities. SAI QRST was developed as a simplified measure of action potential heterogeneity in the heart.. In healthy heart, electrical activation behaves as a dipole, with non-dipolar components of activation12 < 20%, whereas in heart failure multipolarity of activation is increasing13. Theoretically multipolarity of electrical activation means electrical dyssynchrony. SAI QRST reflects multipolarity of electrical activation, consistent with electrical dyssynchrony, and global electrical heterogeneity in the heart14. We hypothesized that SAI QRST is independently associated with CRT response.
Methods
Study population
The study population was drawn from the SMART AV randomized clinical trial15. The SMART AV trial was conducted with the goal to compare three different CRT optimization strategies: AV delay optimization with echocardiography, intracardiac electrogram – based SmartDelay™ algorithm, and a fixed nominal AV delay. The trial was conducted in accordance with the Helsinki Declaration and the ethics or regulatory committee of each participating institution. All study participants gave written informed consent before entering the study.
The SMART AV trial randomized 980 HF patients with NYHA class III–IV despite optimal medical therapy, with LVEF ≤ 35% and QRS duration ≥ 120 ms. Complete heart block was an exclusion criterion in the SMART AV trial. Baseline resting 12-lead ECGs were collected before CRT device implantation as an image of paper-printed routine clinical ECG. All SMART AV participants with available: (1) paired baseline and 6 month echocardiographic studies, and (2) baseline 12-lead ECG were considered for inclusion in this study. For the present analysis, patients were excluded if the ECG image quality was insufficient (resolution < 200 DPI and a grayscale color depth < 8 bit), to permit ECG digitization.
Remodeling response to CRT
LV volumetric changes were measured as the mechanical response to CRT. LV systolic function and LV volumes were assessed by 2-dimensional echocardiography before and 6 months after CRT15. LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LVEF were measured. Consistent with the pre-defined SMART AV study design, patients with a decrease in LVESV ≥ 15mls after 6 months of CRT were prospectively defined as primary endpoint responders. In addition, relative changes in LVESV were assessed retrospectively, as a secondary outcome. Patients with a decrease in LVESV ≥ 15% following 6 months of CRT were defined as secondary outcome responders.
ECG digitization
Routine clinical 12-lead ECGs were collected before CRT device implantation as images of ECG paper printouts. ECG printout images were digitized using the ECGScan 2.0 (AMPS LLC, Italy) if image resolution was ≥ 200 DPI and a grayscale color depth was ≥ 8 bit. ECGScan produced high resolution (500 Hz) digital ECG output in XML standard file as required by the Food and Drug Administration.
ECG analysis: sum absolute QRST integral
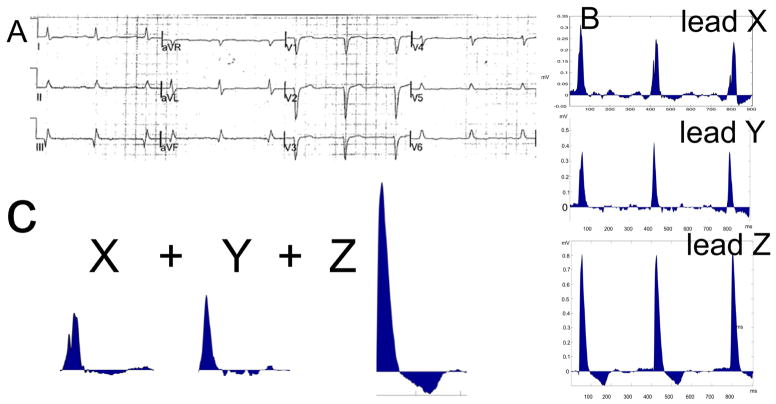
ECG analysis was performed by investigators (JP and LGT) blinded to the study outcome and patients clinical characteristics. Customized Matlab (MathWorks, Inc, Natick, MA) software was used. The digitized 12-lead ECG signal was transformed into orthogonal XYZ ECG by using inverse Dower transformation matrix. The absolute value of the area under the entire QRST waveform was calculated for each orthogonal lead (X, Y, and Z), and averaged per beat. Absolute QRST integral values on X, Y, and Z leads were then added together to obtain averaged SAI QRST9–11, 16 (Figure 1).
Figure 1.
Schematic presentation of the method. A. 12-lead ECG. B. Digtized transformed XYZ ECG. C. Averaged area under QRST curve on X, Y, and Z leads is summed to obtain SAI.
Statistical analysis
SAS 9.3 (SAS Institute, Inc, Cary, NC) was used for data analysis. Continuous variables were reported as mean ±standard deviation (SD), unless otherwise specified. SAI QRST variable was categorized into tertiles. One-way ANOVA was used to compare continuous variables among tertiles of SAI QRST. The Fisher’s exact test was used to compare categorical variables. Multivariate logistic regression analysis was performed to determine association of SAI QRST with CRT response, after adjustment for covariates: age, gender, type of cardiomyopathy (ischemic vs. non-ischemic), LVEF, QRS duration and BBB morphology (LBBB vs. non-LBBB). Renal function and sodium value at baseline, as well as LV lead position in right anterior oblique (RAO) and left anterior oblique (LAO) views were also considered for inclusion in the multivariate model, but ultimately excluded to improve model fit. Another reason to exclude LV lead position in the final model was its different nature: LV lead position represents a procedural characteristic, while all other variables in the final model represent baseline patient characteristics. The remodeling response to CRT served as an outcome in this analysis: absolute reduction in LVESV ≥ 15mls served as a primary outcome; relative reduction in LVESV ≥ 15% served as a secondary outcome. Relationship of SAI QRST to CRT response was tested in clinically important subgroups: patients with LBBB vs. non-LBBB; elderly (≥65y) vs. younger (<65 y) participants; males vs. females; QRS duration ≥ 150 ms vs. < 150ms; patients with ischemic vs. non-ischemic cardiomyopathy, apical / basal vs. mid RAO LV lead position, and anterior / anterolateral / anteroseptal vs. posterior/posterolateral LAO LV lead position. We also tested interaction of SAI QRST tertiles with covariates expressed as the binary variables described above. A P-value of <0.05 was considered significant.
Results
Study population characteristics and SAI QRST
This study population was composed of 234 SMART AV study participants with successfully digitized ECGs and available paired echocardiography data at baseline and 6 months CRT. Mean age was 67±10 years, N=163 (70%) were male. More than half (N=140, 60%) of the cohort have had ischemic cardiomyopathy with mean LVEF 25±7%, and N=179 (77%) had LBBB. Mean QRS duration was 152±20 ms. Clinical characteristics of study participants are shown in Table 1.
Table 1.
Demographic and clinical charactristics of SMART-AV participants with SAI QRST tertiles.
| Baseline Characteristic | All Patients N=234 |
Tertile 1 (6.8–24.3) N=79 |
Tertile 2 (24.3–67.8) N=77 |
Tertile 3 (67.9–986.3) N=78 |
ANOVA P-value |
|---|---|---|---|---|---|
| Age(years), Mean±SD | 67 ± 10 | 67 ± 10 | 66 ± 10 | 66 ± 11 | 0.83 |
| Female, N(%) | 71 (30.3%) | 27 (34.2%) | 23 (29.9%) | 21 (26.9%) | 0.62 |
| White, N(%) | 181 (79.7%) | 65 (82.3%) | 61 (81.3%) | 55 (75.3%) | 0.55 |
| BMI (kg/m2), Mean±SD | 30.2 ± 6.3 | 29.9 ± 6.5 | 30.3 ± 6.5 | 30.3 ± 5.9 | 0.92 |
| NYHA Class I, N(%) | 1 (0.4%) | 1 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0.055 |
| NYHA Class II, N(%) | 9 (3.9%) | 7 (9.0%) | 1 (1.3%) | 1 (1.3%) | |
| NYHA Class III, N(%) | 215 (92.3%) | 69 (88.5%) | 72 (93.5%) | 74 (94.9%) | |
| NYHA Class IV, N(%) | 8 (3.4%) | 1 (1.3%) | 4 (5.2%) | 3 (3.8%) | |
| LBBB, N(%) | 179 (76.5%) | 63 (79.7%) | 55 (71.4%) | 61 (78.2%) | 0.43 |
| Ischemic cardiomyopathy, N(%) | 140 (59.8%) | 47 (59.5%) | 51 (66.2%) | 42 (53.8%) | 0.30 |
| LVEF(%), Mean±SD | 24.7 ± 6.9 | 25.8 ± 6.5 | 24.9 ± 6.9 | 23.5 ± 7.2 | 0.12 |
| LVESV(ml), Mean±SD | 126 ± 66 | 109 ± 51 | 115 ± 55 | 154 ± 80 | < 0.001 |
| QRS(ms), Mean±SD | 152 ± 20 | 147 ± 21 | 150 ± 20 | 160 ± 15 | < 0.001 |
| Diabetes, N(%) | 95 (40.6%) | 36 (45.6%) | 28 (36.4%) | 31 (39.7%) | 0.50 |
| Lead Position (LAO) | |||||
| Anterior/ Anterolateral/ Anteroseptal, N (%) | 66 (29.1%) | 26 (33.3%) | 16 (21.3%) | 24 (32.4%) | 0.19 |
| Posterior/ Posterolateral, N (%) | 161 (70.9%) | 52 (66.7%) | 59 (78.7%) | 50 (67.6%) | |
| Lead Position (RAO) | |||||
| Apical, N (%) | 25 (11.0%) | 5 (6.4%) | 8 (10.7%) | 12 (16.2%) | 0.061 |
| Basal, N (%) | 9 (4.0%) | 2 (2.6%) | 1 (1.3%) | 6 (8.1%) | |
| Mid, N (%) | 193 (85.0%) | 71 (91.0%) | 66 (88.0%) | 56 (75.7%) | |
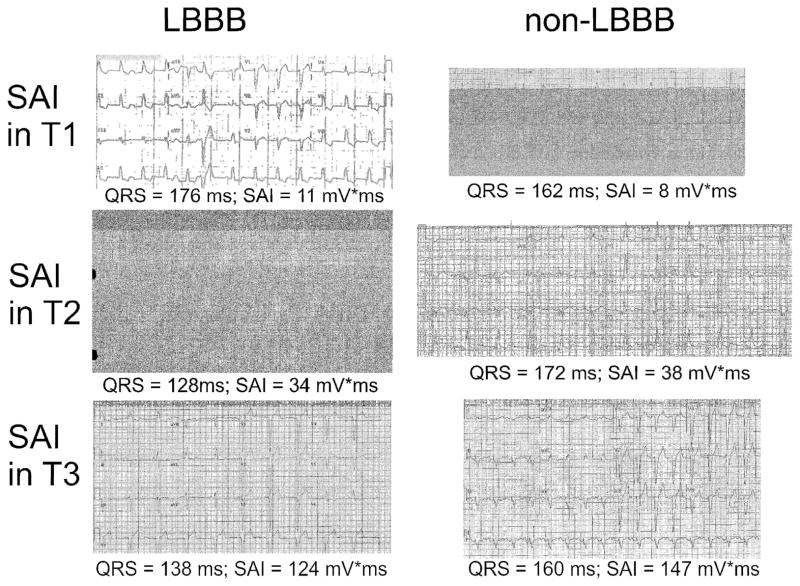
On average, 2.7±0.9 beats were included in analysis. Distribution of SAI QRST was skewed. Mean SAI was 64.5±79.4 mV*ms. Median SAI was 48.0 (interquartile range 18.1–81.5) mV*ms. Representative examples of 12-lead ECGs in study participants with SAI in the 1st, 2nd, and the 3rd tertile are shown in Figure 2.
Figure 2.
Representative examples of 12-lead ECGs in participants with LBBB and non-LBBB QRS morphology and SAI in the 1st, 2nd, and the 3rd tertile (T1-T2-T3).
In univariate analysis (Table 1) demographic characteristics (age, sex, race), body composition (body mass index), type of cardiomyopathy, type of conduction abnormality, and major comorbidities (diabetes) did not affect SAI. However, severity of HF was clearly associated with SAI. Baseline LVESV gradually and significantly increased from the lowest to the highest SAI QRST tertile, whereas LVEF trended towards steady decreasing. There were significantly more patients with advanced NYHA class III–IV amongst participants with SAI QRST in the two upper tertiles. QRS duration progressively widened with increasing SAI QRST from the lowest to middle, and from middle to the highest tertile (Table 1).
Association of SAI QRST with primary outcome mechanical response on CRT
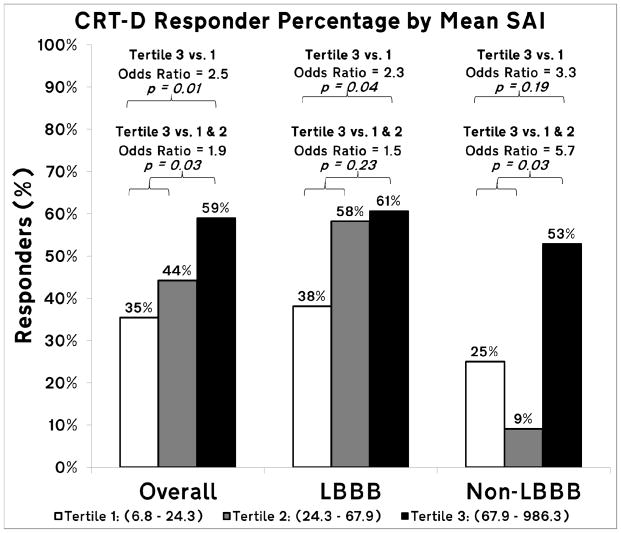
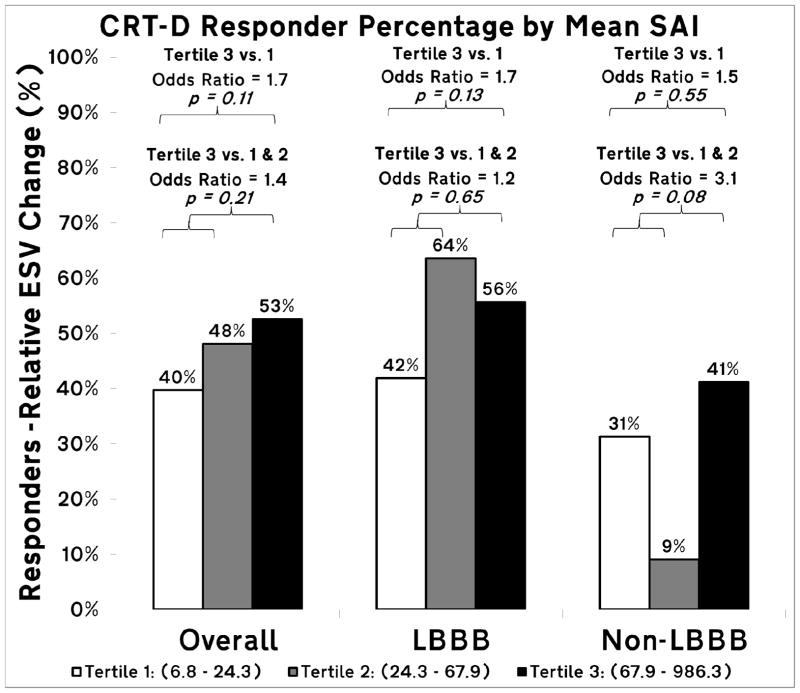
Among the 234 patients included in the analysis, 108 (46%) were observed to be primary CRT responders. Patients with the high mean SAI (3rd tertile) had 2.5 times greater odds of response to CRT than those with low mean SAI (1st tertile), and 2.0 times greater than the lower two tertiles combined (Figure 3; CRT response of 35%, 44%, and 59% for 1st, 2nd and 3rd tertiles, respectively), in spite of more frequent unfavorable apical LV lead placement (Table 1). A trend towards the interaction between SAI and BBB morphology was observed (P=0.17). The effect size was larger in the non-LBBB patients (N=54). Non-LBBB patients with the SAI in the highest 3rd tertile had nearly 6 times greater odds of response than those with SAI in the lower two tertiles combined.
Figure 3.
Bar graph of primary CRT responder percentage by mean SAI QRST tertiles for all study participants, and for bundle branch block (BBB) morphology subgroups (LBBB and non-LBBB). Reported odds ratios are adjusted by age, gender, type of cardiomyopathy, LVEF, QRS duration and BBB morphology.
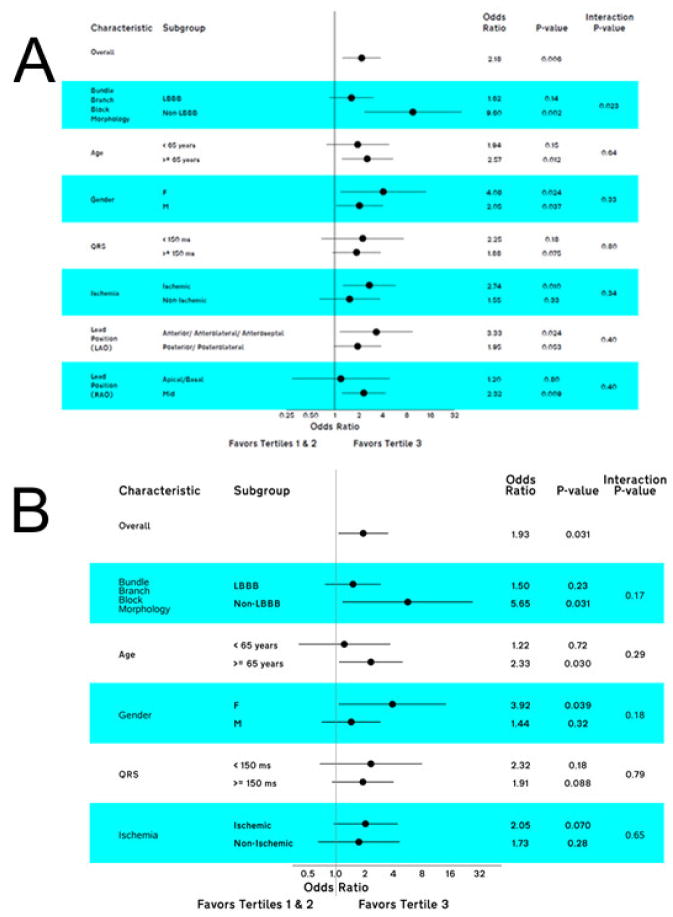
In adjusted logistic regression analyses no statistically significant interactions of SAI with clinically important baseline characteristics were observed (Figure 4). Importantly, there was a significant association of SAI QRST with CRT response in the following subgroups: patients with non-LBBB, females and patients at least 65 years old. Additionally, there was a trend towards stronger association of SAI with CRT response in patients with ischemic cardiomyopathy and QRS duration < 150 ms.
Figure 4.
A. Unadjusted and B. adjusted primary CRT response, associated with SAI QRST tertiles in clinically relevant subgroups.
Further adjustment of minimally (by demographics) adjusted logistic regression model (Table 2) by cardiomyopathy type, LVEF, renal function, sodium level, did not affect strength of association between SAI and primary outcome. Addition in the model QRS duration and BBB morphology only slightly attenuated odds ratio. IIn the final adjusted model (Table 3) SAI remained significant predictor of primary outcome after adjustment by major known predictors of CRT response: gender, BBB morphology, QRS duration, LVEF, age and cardiomyopathy type.
Table 2.
Association of SAI QRST with CRT response.
| Predictor=SAI QRST tertiles | Odds Ratio (95%CI) (Tertile 3 vs. Tertiles 1 & 2) | P | AIC* |
|---|---|---|---|
| Model 1 (unadjusted) | 2.18 (1.25, 3.79) | 0.011 | 319.26 |
| Model 2 (adjusted by age, sex) | 2.33 (1.32, 4.11) | 0.005 | 315.02 |
| Model 3 (model 2 + by cardiomyopathy type) | 2.21 (1.25, 3.93) | 0.007 | 311.17 |
| Model 4 (model 2 + LVEF) | 2.23 (1.26, 3.95) | 0.006 | 314.57 |
| Model 5 (model 2 + BBB morphology, QRS) | 2.00 (1.10, 3.62) | 0.023 | 307.23 |
| Model 6 (model 2 + renal function) | 2.33 (1.32, 4.11) | 0.003 | 316.92 |
| Model 7 (model 2 + sodium) | 2.26 (1.28, 4.00) | 0.005 | 312.98 |
| Model 8 (model 2+ LVEF, CM type, QRS, BBB morph, renal function, Na) | 1.86 (1.02, 3.41) | 0.044 | 307.01 |
| Model 9 (model 2+ LVEF, CM type, QRS, BBB morph, LV lead position (RAO and LAO)) | 1.71 (0.92, 3.17) | 0.087 | 306.44 |
| FINAL MODEL, (model 2+ LVEF, CM type, QRS, BBB morph) | 1.87 (1.03, 3.42) | 0.041 | 306.06 |
Smaller AIC indicates better model fit
Table 3.
Full final logistic regression model predicting primary CRT response.
| Predictor | Comparison | Odds ratio (95%CI) | P |
|---|---|---|---|
| SAI QRST tertiles | T3 vs. T1/T2 | 1.87 (1.03, 3.42) | 0.041 |
| Gender | M vs. F | 0.52 (0.27, 0.99) | 0.048 |
| Ischemic cardiomyopathy | Ischemic vs. Non-Ischemic | 0.60 (0.32, 1.10) | 0.100 |
| Age | Per 5 years | 1.02 (0.89, 1.18) | 0.74 |
| BBB morphology | LBBB vs Non-LBBB | 2.29 (1.11, 4.71) | 0.025 |
| QRS duration | Per 10 ms | 1.16 (1.00, 1.34) | 0.049 |
| LVEF | Per 1% | 0.97 (0.93, 1.01) | 0.16 |
Association of SAI QRST with secondary outcome mechanical response on CRT
A total of 109 of the 234 patients (46%) were observed to be secondary responders, in which response was defined by a relative reduction in LVESV by at least 15%. Agreement between the primary and secondary definitions of response was high (Kappa coefficient = 0.89). Despite the high agreement between the two outcomes of response, the effect size of association of SAI QRST with the secondary outcome was smaller, as compared to the association of SAI with primary outcome (Figure 5). Accordingly, statistical power of the study was insufficient to demonstrate statistical significance of the association. Importantly, no differences in direction and trends in the association of SAI with primary and secondary outcomes were observed.
Figure 5.
Bar graph of secondary CRT responder percentage by mean SAI QRST tertiles for all study participants, and for bundle branch block (BBB) morphology subgroups (LBBB and non-LBBB). Unadjusted odds ratios are reported.
Discussion
The main finding of the study is that simple ECG metric SAI QRST, easily obtainable from automated analysis of 12-lead ECG, is independently (beyond QRS duration, BBB morphology, LVEF, cardiomyopathy type, gender, age) associated with CRT response in the SMART-AV randomized clinical trial. The observed association of baseline high SAI QRST with CRT response suggests that SAI QRST estimates electrical dyssynchrony. After validation of our finding in another prospective study, measurement of SAI QRST could become an important component of the “electrical dyssynchrony score” for selection of CRT candidates, especially in the “gray area” subgroups: HF patients with non-LBBB conduction defect, QRS duration 120–149 ms, and ischemic cardiomyopathy.
After more than 10 years of CRT application, the recent ACCF/AHA/HRS device guideline update17 narrowed the recommendations for CRT based on electrical dyssynchrony measures: QRS duration and morphology. These appropriately implemented guideline changes were based on a strong evidence of CRT benefit in LBBB5, 18 patients with QRS ≥150ms3, 6, 19: more than 8,000 patients in NYHA class I-IV HF were evaluated in landmark randomized controlled trials18, 20, 21. It was shown that electrical, but not mechanical22, dyssynchrony should guide electrical CRT therapy. However, currently used measures of electrical dyssynchrony (QRS duration and morphology) have limitations.
QRS duration is an imperfect measure of electrical dyssynchrony8, 23. QRS duration characterizes only duration of depolarization, but does not describe the electrical substrate, amenable for cardiac resynchronization. On one hand, a non-negligible percentage of patients with QRS ≥150 ms do not respond23. A marked QRS widening (≥178 ms) as a sign of HF severity was related to less CRT benefit7, raising the question that QRS duration is not capable of distinguishing electrical dyssynchrony from severe mechanical dyssynchrony due to LV and frequently accompanied RV dilatation24, 25, hypertrophy, and scar, which is not responsive to resynchronization. On the other hand, a high percentage of patients with QRS 120–149 ms does respond, and could even demonstrate super-response,3, 8 on CRT.
QRS morphology is an imperfect measure of electrical dyssynchrony26, as well. In addition to LBBB patients5, non-LBBB patients with ≥15% decrease in LVESV index significantly improved long-term survival with CRT26. Detailed anatomy of the His-Purkinje system is extremely complex and highly individualized. At the same time, QRS morphology does carry important information. Josephson and Wellens25 recently highlighted known ECG features that could help to separate electrical dyssynchrony due to LBBB from the non-amenable to CRT mechanical dyssynchrony due to RV dilatation27. These features include a marked discrepancy in QRS voltages in the extremity and precordial leads28. SAI QRST, by calculating summed area under QRST curve, takes into account amplitudes of orthogonal XYZ leads. Reynolds et al24 showed that ECG signs of RV dilation in LBBB may help identify HF patients unlikely to benefit from CRT. SAI QRST, by calculating summed area under QRST curve, incorporates all QRST morphology features, which are not taken into account by classical definition of ventricular conduction abnormalities.
ECGi evaluates 3-dimensional activation of LV and RV, and therefore, could potentially characterize specific details of electrical dyssynchrony in individual patients. A small pilot ECGi study predicted clinical CRT response better than QRS duration or morphology29. However, ECGi is an expensive and time-consuming technology. In contrast, SAI QRST could be easily calculated from routinely available 12-lead ECG, while providing 3-dimensional data regarding activation and recovery.
SAI QRST is a composite ECG marker that measures summed area under the QRST curve in 3-dimensions (X, Y, and Z), and therefore, accounts for both QRS duration and QRS morphology. SAI QRST is better suited to capture complexity of electrical dyssynchrony in HF than either QRS duration, or QRS morphology alone. In this study, SAI QRST was strongly associated with baseline LVESV and NYHA HF class. Even after adjustment for QRS duration, BBB morphology, LVEF, cardiomyopathy type, gender, and age, SAI QRST predicted primary CRT response. No test for interaction reached statistical significance in this study, likely due to insufficient statistical power for the subgroups analysis. However, observed trends suggest that SAI QRST could become an important asset for selection of CRT candidates amongst HF patients with non-LBBB conduction defect, QRS duration 120–149 ms, and ischemic cardiomyopathy. Future prospective studies are needed to test this hypothesis.
Recently van Deursen et al30 in a smaller study showed that, similar to SAI QRST, ECG parameter QRSAREA, which is a sum of the areas under the QRS on three orthogonal XYZ ECG leads (i.e. SAI QRS), was significantly larger in CRT responders, as compared to non-responders. In univariable analysis, QRSAREA predicted CRT response better than QRS duration and conventionally defined LBBB morphology and was comparable with the proposed refined definition of LBBB31. The findings of van Deursen et al30 are consistent with results of our study. Notably, unlike QRSAREA, SAI QRST considers the entire cardiac cycle, including the repolarization phase. Including the repolarization potentially allows detection of cardiac memory due to old BBB, and provides insight in into “new or intermittent” vs. “old or chronic” ventricular conduction abnormality32. Furthermore, calculation of SAI QRST (rather than SAI QRS) simplifies the ECG metric and makes it more robust and potentially more reproducible.
Consistently with the results of this study, in MADIT II11 and PROSE-ICD9, 10 SAI QRST was associated with HF severity (LVEF9, 11, LV diastolic diameter9) and HF outcomes11. Risk of HF hospitalizations and death was higher in MADIT II participants with SAI QRST in the highest quartile. Notably, SAI QRST correlated with the time after MI11 and many clinical characteristics, which suggested that SAI QRST is a measure of electrical remodeling. Consistently with clinical findings, Kozmann et al14 in silico showed that SAI QRST is a measure of non-dipolarity of activation and heterogeneity of action potential morphology distribution. Combining together SAI QRST, QRS duration, and QRS morphology into an “electrical dyssynchrony score” could conceivably improve selection of CRT candidates. More electrical dyssynchrony predicts more remodeling and more remodeling is associated with fewer hospitalizations and less ventricular arrhythmia. Future prospective studies are needed to test this hypothesis.
Limitations
Several limitations of this study must be considered. This study is a post-hoc analysis of a large multicenter randomized controlled trial. Moreover, only a subgroup of study participants, but not the entire SMART AV study population was analyzed. Therefore, before implementation in clinical practice all results should be validated in a prospective study. Definition of a primary outcome CRT response was based on absolute LVESV value reduction, whereas a standard measure of CRT response (relative reduction in LVESV ≥ 15%) served as a secondary outcome. Additionally, we analyzed digitized ECGs, rather than digitally recorded ECGs, as in all our previous studies of SAI QRST9–11, 16, and therefore SAI thresholds are directly not comparable. Future studies are needed to compare SAI QRST measured on digitally recorded ECG vs. digitized ECG. It is clearly preferable to use digitally recorded ECGs for any automated analysis. However, this study showed that automated analysis of digitized ECG can provide meaningful results, which opens an important avenue for post-hoc analyses of numerous previously conducted studies that collected ECG printouts, but did not save digital ECG. Retrospective post-hoc analyses of conducted clinical studies are encouraged by NIH as a wise use of resources. Finally, limited statistical power did not allow conclusive analyses of subgroups. Future studies are needed to compare predictive value of SAI QRST in patients with QRS duration 120–149 ms, non-LBBB patients, and ischemic cardiomyopathy patients.
Clinical Perspectives.
In this study we showed that ECG measure of electrical dyssynchrony, SAI QRST, is independently (after adjustment for QRS duration, BBB morphology, LVEF, cardiomyopathy type, gender, age) associated with CRT response in the SMART-AV randomized clinical trial. This finding suggests that development of the “electrical dyssynchrony score” (which would combine several markers of electrical dyssynchrony: QRS duration, QRS morphology, and SAI QRST) is feasible, and may improve selection of approriate CRT candidates in the future, especially in the “gray area” subgroups: HF patients with non-LBBB conduction defect, QRS duration 120–149 ms, and ischemic cardiomyopathy. However, validation of our findings in another prospective study needs to be done first, before implementation in clinical practice. The observed trend towards stronger association of SAI with both non-LBBB conduction defect, and anterior/anteroseptal/anterolateral LV lead position, suggests that anterior /anteroseptal /anterolateral LV lead placement may be recommended for patients with non-LBBB conduction defect (e.g. anterior fascicular block) and SAI in the highest 3rd tertile. This hypothesis should be tested in the future randomized clinical trial.
Acknowledgments
Authors thank Fabio Badilini, PhD, and his team, for providing AMPS ECGScan software for ECG digitizing. We also thank undergraduate students of the Johns Hopkins University (Albert Feeny, Kutay Muslu, Kavya Singampalli, Patrick Jongeneel, Valeriya Aranovich, Akudo Ogubunka, Malvi Hemani) for help with ECG digitizing.
Funding Sources: The SMART AV study was sponsored by Boston Scientific. This research was supported in part by the National Institute of Health #1R01HL118277 (LGT).
Abbreviations
- CRT
Cardiac resynchronization therapy
- HF
heart failure
- LBBB
left bundle branch block
- MI
myocardial infarction
- SAI QRST
Sum absolute QRST integral
- ICD
implantable cardioverter defibrillator
- AV
atrioventricular
- NYHA
New York Heart Association
- LVEF
left ventricular ejection fraction
- RV
right ventricular
- ECGi
Electrocardiographic imaging
Footnotes
Conflict of interest: None
Clinical Trial Registration—http://www.clinicaltrials.gov. Unique identifier: NCT00677014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 2.Ploux S, Whinnett Z, Lumens J, Denis A, Zemmoura A, De Guillebon M, Ramoul K, Ritter P, Jais P, Clementy J, Haissaguerre M, Bordachar P. Acute hemodynamic response to biventricular pacing in heart failure patients with narrow, moderately, and severely prolonged QRS duration. Heart Rhythm. 2012;9:1247–1250. doi: 10.1016/j.hrthm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Gold MR, Thebault C, Linde C, Abraham WT, Gerritse B, Ghio S, St John Sutton M, Daubert JC. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. 2012;126:822–829. doi: 10.1161/CIRCULATIONAHA.112.097709. [DOI] [PubMed] [Google Scholar]
- 4.Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med. 2011;171:1454–1462. doi: 10.1001/archinternmed.2011.247. [DOI] [PubMed] [Google Scholar]
- 5.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang AS. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–3556. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sassone B, Gambetti S, Bertini M, Beltrami M, Mascioli G, Bressan S, Fucà G, Pacchioni F, Pedaci M, Michelotti F, Bacchi Reggiani ML, Padeletti L. Relation of QRS Duration to Response to Cardiac Resynchronization Therapy. The American Journal of Cardiology. 2015;115:214–219. doi: 10.1016/j.amjcard.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Dupont M, Rickard J, Baranowski B, Varma N, Dresing T, Gabi A, Finucan M, Mullens W, Wilkoff BL, Tang WH. Differential response to cardiac resynchronization therapy and clinical outcomes according to QRS morphology and QRS duration. J Am Coll Cardiol. 2012;60:592–598. doi: 10.1016/j.jacc.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 9.Tereshchenko LG, Cheng A, Fetics BJ, Butcher B, Marine JE, Spragg DD, Sinha S, Dalal D, Calkins H, Tomaselli GF, Berger RD. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol. 2011;44:208–216. doi: 10.1016/j.jelectrocard.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tereshchenko LG, Cheng A, Fetics BJ, Marine JE, Spragg DD, Sinha S, Calkins H, Tomaselli GF, Berger RD. Ventricular arrhythmia is predicted by sum absolute QRST integralbut not by QRS width. J Electrocardiol. 2010;43:548–552. doi: 10.1016/j.jelectrocard.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tereshchenko LG, McNitt S, Han L, Berger RD, Zareba W. ECG marker of adverse electrical remodeling post-myocardial infarction predicts outcomes in MADIT II study. PLoS One. 2012;7:e51812. doi: 10.1371/journal.pone.0051812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lux RL, Evans AK, Burgess MJ, Wyatt RF, Abildskov JA. Redundancy reduction for improved display and analysis of body surface potential maps. I. Spatial compression. Circ Res. 1981;49:186–196. doi: 10.1161/01.res.49.1.186. [DOI] [PubMed] [Google Scholar]
- 13.Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res. 2010;106:981–991. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozmann G, Tuboly G, Szathmáry V, Švehlíková J, Tyšler M. Computer modelling of beat-to-beat repolarization heterogeneity in human cardiac ventricles. Biomedical signal processing and control. 2014;14:285–290. [Google Scholar]
- 15.Ellenbogen KA, Gold MR, Meyer TE, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 16.Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One. 2013;8:e57175. doi: 10.1371/journal.pone.0057175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. NEnglJMed. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 19.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. NEnglJ Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 20.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. NEnglJ Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 21.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De MT, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. NEnglJ Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 22.Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 23.Bax JJ, Gorcsan J., 3rd Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933–1943. doi: 10.1016/j.jacc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds MR, Joventino LP, Josephson ME. Relationship of baseline electrocardiographic characteristics with the response to cardiac resynchronization therapy for heart failure. Pacing Clin Electrophysiol. 2004;27:1513–1518. doi: 10.1111/j.1540-8159.2004.00669.x. [DOI] [PubMed] [Google Scholar]
- 25.Josephson ME, Wellens HJ. The ECG in left bundle branch block and heart failure. Heart Rhythm. 2015;12:250–251. doi: 10.1016/j.hrthm.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Gold MR, Daubert C, Abraham WT, Ghio S, St John Sutton M, Hudnall JH, Cerkvenik J, Linde C. The effect of reverse remodeling on long-term survival in mildly symptomatic patients with heart failure receiving cardiac resynchronization therapy: results of the REVERSE study. Heart Rhythm. 2015;12:524–530. doi: 10.1016/j.hrthm.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Bommel RJ, Marsan NA, Delgado V, van Rijnsoever EPM, Schalij MJ, Bax JJ, Wellens HJ. Value of the Surface Electrocardiogram in Detecting Right Ventricular Dilatation in the Presence of Left Bundle Branch Block. The American Journal of Cardiology. 2011;107:736–740. doi: 10.1016/j.amjcard.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 28.Goldberger AL, Dresselhaus T, Bhargava V. Dilated cardiomyopathy: Utility of the transverse: Frontal plane QRS voltage ratio. Journal of Electrocardiology. 1985;18:35–40. doi: 10.1016/s0022-0736(85)80032-7. [DOI] [PubMed] [Google Scholar]
- 29.Ploux S, Lumens J, Whinnett Z, et al. Noninvasive Electrocardiographic Mapping to Improve Patient Selection for Cardiac Resynchronization Therapy: Beyond QRS Duration and Left Bundle Branch Block Morphology. Journal of the American College of Cardiology. 2013;61:2435–2443. doi: 10.1016/j.jacc.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 30.van Deursen CJ, Vernooy K, Dudink E, Bergfeldt L, Crijns HJ, Prinzen FW, Wecke L. Vectorcardiographic QRS area as a novel predictor of response to cardiac resynchronization therapy. J Electrocardiol. 2015;48:45–52. doi: 10.1016/j.jelectrocard.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–934. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Shvilkin A, Bojovic B, Vajdic B, Gussak I, Ho KK, Zimetbaum P, Josephson ME. Vectorcardiographic and electrocardiographic criteria to distinguish new and old left bundle branch block. Heart Rhythm. 2010;7:1085–1092. doi: 10.1016/j.hrthm.2010.05.024. [DOI] [PubMed] [Google Scholar]