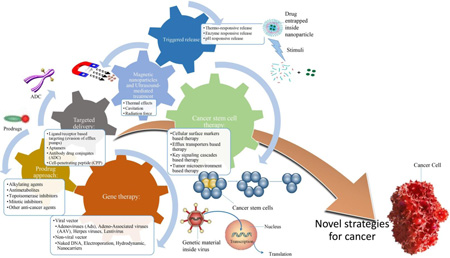
Abstract
Currently, a majority of cancer treatment strategies are based on the removal of tumor mass mainly by surgery. Chemical and physical treatments such as chemo- and radiotherapies have also made a major contribution in inhibiting rapid growth of malignant cells. Furthermore, these approaches are often combined to enhance therapeutic indices. It is widely known that surgery, chemo- and radiotherapy also inhibit normal cells growth. In addition, these treatment modalities are associated with severe side effects and high toxicity which in turn lead to low quality of life. This review encompasses novel strategies for more effective chemotherapeutic delivery aiming to generate better prognosis. Currently, cancer treatment is a highly dynamic field and significant advances are being made in the development of novel cancer treatment strategies. In contrast to conventional cancer therapeutics, novel approaches such as ligand or receptor based targeting, triggered release, intracellular drug targeting, gene delivery, cancer stem cell therapy, magnetic drug targeting and ultrasound-mediated drug delivery, have added new modalities for cancer treatment. These approaches have led to selective detection of malignant cells leading to their eradication with minimal side effects. Lowering, multi-drug resistance and involving influx transportation in targeted drug delivery to cancer cells can also contribute significantly in the therapeutic interventions in cancer.
Keywords: Chemotherapeutics, Tumor, Cancer, Targeted Drug Delivery, Stem cells, Cell Penetrating Peptides, Efflux Pumps, Gene therapy, Immunotherapy
Graphical Abstract
1. INTRODUCTION
Cancer is a group of diseases involving uncontrolled growth and spread of abnormal cells. Such cells undergo transformations to obtain inexhaustible replication and thus traverse to other organs leading to malignancy. Failure to regulate or prevent such a spread of cancerous cells often leads to death of the patient. Cancer represents the most common cause of death in the US, and accounts for nearly 1 of every 4 deaths [3]. Approximately, 14.5 million Americans with a history of cancer were surviving on January 1, 2014. Survival statistics vary with cancer type and diagnosis stage. In U.S., 1,658,370 new cancer cases are expected to be diagnosed in 2015 and about 589,430 Americans are expected to die of cancer. Within the years 2004–2010, the relative survival rate for all cancers diagnosed was 68%, compared to 49% in 1975–1977 [3]. Several internal and external factors are responsible for this deadly disease [4][5]. External factors include infectious organisms, unhealthy diet, pesticides, environmental toxins and tobacco while internal factors includes inherited genetic mutations, immune conditions and hormones. These factors may act together or in series to develop cancer. There are several stages in cancer progression which is generally established with tumor size, extent of primary tumor and spreading capability to nearby lymph nodes or other organs. Diagnosis and staging are essential elements to initiate therapy. The conventional cancer treatment methods include surgery, radiation and chemotherapy.
Several differences in normal and cancer biology render cancer therapy as a multidisciplinary task. Targeted therapy based on distinct tumor type aiming to maximize efficacy and minimize toxicity has remained extremely challenging. Targeted therapy has not been particularly effective in treating certain tumors [4][6].The cancer genome atlas (TCGA) was founded to explore opportunities that may provide a holistic approach for classification. TCGA researchers hypothesized a multiplatform analysis of 12 cancer types, unravelling similarity of tumors based on genetics and molecular biology. This analysis generated a reclassification of approximately 10% of tumors based on their cell origin instead of tissue site. Interestingly, TCGA’s integrative analysis unified classification of 12 cancer types into 11 major subtypes. Eleven major cancer types include lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), breast adenocarcinoma (BRCA), uterine corpus endometrial carcinoma (UCEC), glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSC), colon and rectal carcinoma (COAD, READ), bladder urothelial carcinoma (BLCA), kidney renal clear cell carcinoma (KIRC), ovarian serous carcinoma (OV) and acute myeloid leukemia (AML) which were standardized according to their mutational data from 3281 tumors. Analysis revealed that some tumors are molecularly heterogeneous while others are homogenous [6]. As mentioned above, histological classification includes 100 different types of cancer that are classified into 6 major categories: i.e. carcinoma, sarcoma, myeloma, leukemia, lymphoma and mixed types (Table 1).
Table 1.
Summary of six major categories of cancer [7]
| Category | Types | Examples |
|---|---|---|
| Carcinoma | Adenocarcinoma Squamous Cell Carcinoma |
Breast, Lung, Colon, Prostate, Bladder |
| Sarcoma | Osteosarcoma | Bone |
| Chrondosarcoma | Cartilage | |
| Leiomyosarcoma | Smooth muscle | |
| Rhabdomyosarcoma | Skeletal muscle | |
| Mesothelial sarcoma | Membranous linings | |
| Fibrosarcoma | Fibrous tissue | |
| Angiosarcoma | Blood vessels | |
| Liposarcoma | Adipose tissue | |
| Glioma or astrocytoma | Neurogenic tissue in brain | |
| Myxosarcoma | Embryonic connective tissue | |
| Mesenchymous | Mixed connective tissue | |
| Myeloma | -- | Plasma cells of bone marrow |
| Leukemia | Myelogenous or granulocytic | Myeloid and granulocytes |
| Lymphatic, Lymphoblastic | Lymphoid and lymphocytic | |
| Polycythemia vera or erythemia | Red cells and blood cells | |
| Lymphoma | Hodgkin Lymphoma, Non Hodgkin Lymphoma |
Stomach, breast or brain |
| Mixed types | Adenosquamous carcinoma Mixed mesodermal tumor Carcinosarcoma |
Breast, Lung, Colon, Prostate etc. |
2. Novel strategies for cancer targeted delivery
For effective cancer therapy, it is necessary to improve and develop novel strategies for effective delivery of chemotherapeutics to cancer cells. Conventional chemotherapeutic agents accumulate both in normal and tumor cells due to non-specificity. The ultimate goal of cancer therapy is to reduce the systemic toxicity and improve the quality of life. The landscape of cancer treatment has improved significantly over the past four decades.
Direct drug administration may be associated with embolism, non-specificity and drug induced toxicity. Additionally, orally administered drug regimen is required to overcome biological barriers, protein binding and first pass metabolism to reach therapeutic concentrations in cancer cells. Direct drug administration into the tumor environment may be effective when the cancer is benign. However, the prognosis is completely different when tumors metastasize and invade surrounding normal tissues. Under such conditions (metastasis) tumor cells invade other organs by altering phenotype. Moreover, such cells over express efflux pumps (P-glycoprotein, MRP and BCRP) and metabolizing enzymes relative to normal cells [8]. Such overexpression aids tumor cell survival and imparts resistance to xenobiotics (anticancer agents). For example, glioblastoma multiforme (GBM) is one of the metastatic diseases which is very challenging to treat till now.
Delivery of anticancer agents to metastatic cancer cell at therapeutic levels is extremely challenging. Targeted drug delivery may counteract metastatic tumor and minimize off target toxicity. Targeted drug delivery may be achieved by exploiting overexpression of transporters and receptors on cancer cell plasma membrane. Also ion channels such as potassium (K+), sodium (Na+), calcium (Ca+2), chloride (Cl-) and AQP4 channels may be targeted to regulate tumor metastases. Cancer cells, particularly GBM utilize these channels for migration [8].
2.1. Ligand/receptor based targeting
Ligand/receptor targeting has proven to be an effective method for drug delivery [9]. In order to improve efficacy chemotherapeutic agents need to be delivered into tumor cell cytoplasm or sub-cellular organelles such as nucleus and mitochondria. Such targeting may be achieved by proper selection, tailoring and designing ligands. Specificity of antibody targeting an antigen overexpressed in cancer cells needs to be well delineated. In addition, if a linker is involved such as cell penetrating peptide (CPP) drug conjugates, a stable covalent bond formation is essential between the ligand/receptor and the drug. However, mechanism of drug release at tumor site is crucial. Premature drug release may result in systemic toxicity.
Tumor targeting ligands such as antibody, aptamer, siRNA, and peptides are being explored to target metastatic cells and block migration and invasion. Targeted drug delivery may be broadly classified as active and passive. Active targeting includes ligand mediated drug delivery. These ligands may be covalently conjugated to active agent or on to the surface of a carrier system such as nanoparticles, liposomes or nanomicelles [16]. Passive targeting exploits systemic and lymphatic systems in tumor architecture. Several targeting strategies have been developed for anticancer drug delivery, of which a few are discussed in this review article.
2.1.1. Antibody-drug conjugates (ADC)
Development of monoclonal antibodies targeting various antigens highly expressed on malignant cells is a novel approach. ADC represents a broad class of effective biopharmaceutical agents designed for targeted therapy including cancer. Antibodies may target malignant cells that overexpress a specific antigen. A few antibody drug conjugates have been marketed for oncology therapeutics such as brentuximab vedotin (Adcetris®) and trastuzumab emtansine (Kadcyla®). Currently, a majority of antibody drug conjugates are in different stages of clinical trials and some biologics are still in early stages of development. Robertson et al. studied the application of a recombinant human interleukin 18 (rhIL-18), a cytokine with antitumor activity conjugated to Rituximab. This CD20 monoclonal antibody against B cell lymphoma is highly effective for the treatment of Non-Hodgkin Lymphoma. Phase I studies ( 19 patients) with intravenous rituximab (375 mg/m2/wk) and rhIL-18(2 hours IV infusion/wk) demonstrated elevated plasma levels for pro-inflammatory cytokines, transient lymphopenia, and baseline lymphocyte count [8]. In another study, DM1, a cytotoxic agent that binds and destabilizes microtubule activity was studied by Lopus et al. The researchers established the application of antibody-DM1 conjugates in cancer therapy. Such antibody-drug conjugates were formulated as Trastuzumab emtansine (T-DM1) consisting of monoclonal antibody trastuzumab (Herceptin) conjugated to DM1 [10, 11]. Trastuzumab emtansine has been recently approved by FDA for breast cancer treatment. T-DM1 targets epidermal growth factor receptor 2 (HER2) overexpressed in aggressive breast cancer. Trastuzumab emtansine destroys cancer cells due to interaction of microtubules and DM1 [12]. Brentuximab Vedotin is another example for antibody-drug conjugate approved for treating Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL). Brentuximab Vedotin targets cell membrane protein (CD30) and an antimitotic agent, monomethyl auristatin E (MMAE) [13]. A phase II study on relapsed/ refractory sALCL indicated that 86% patients reached the objective response (57% complete remission and 17 % partial remission) [14]. However, phase II study on relapsed/ refractory diffuse large B cell lymphoma (DLBCL) demonstrated 44 % objective rate achievement [15]. Based on the presence of CD33 on acute myeloid leukemia (AML) blasts and some leukemic stem cells, gemtuzumab ozogamicin (GO), an anti-CD33 antibody carrying the cytotoxic calicheamicin has been developed to treat AML [231]. Several other markers including CD44 [232], IL-3RA or CD123 [233], the immunoglobulin mucin TIM-3 [234] and folate receptor beta (FRβ) [235], have been extensively explored to specifically target leukemic stem cells in human AML.
2.1.2. Aptamers
These macromolecules are single stranded DNA or RNA that binds to proteins and peptides with higher specificity and affinity. Aptamers recognize various targets ranging from small molecules to macromolecules [16, 17]. Therefore, aptamers are utilized in therapeutic and diagnostic applications [18]. Several studies demonstrated that aptamer drug conjugates minimized systemic toxicity and enhanced drug delivery at the tumor site [19, 20]. For example, modified TLS11a-GC aptamer conjugated to doxorubicin (DOX) demonstrated high binding affinity (7.16 ±0.59 nM) towards hepatocellular carcinoma cells (LH86). However, negligible binding was observed towards normal human liver cell line (Hu1082) [21, 22]. Similarly, Cao et al. studied cy-apt 20 aptamer as a biomarker and selected it as a targeting ligand for gastric cancer [23]. Results indicate a significant rise in binding of cy-apt 20 to gastric carcinoma cells (AGS) with prolonged incubation time. In addition, concentration dependent increase in binding affinity was demonstrated. Cell uptake studies with FITC labelled cy-apt 20 AGS on Hepatoma cells (HepG2) and colon carcinoma cells (SW620) confirmed significant staining of AGS cells. Negligible staining was observed in HepG2 and SW620 [23]. Similar results were obtained with cy-apt 20, a diagnostic agent for gastric cancer. This study found cy-apt 20 aptamer to possess high and specific binding for AGS cells. Similar results were obtained with aptamer in human prostate cancer. Elevation in prostatic acid phosphatase (PAP) is associated with prostate cancer [24]. Kong and Byun reported that 6N 2’-FY RNA aptamer with 50 nucleotide sequence contains two hairpins that facilitate binding with PAP. Moreover, 6N 2’-FY RNA aptamer demonstrated higher binding (118 nM) for positive PAP mammalian cells (LNCaP and PC-3) relative to normal human lung fibroblast (IMR-90) [24]. These results indicate potential application of 6N 2’-FY RNA aptamer as a targeting ligand.
2.1.3. siRNA
Surface of calcium carbonate nanoparticles decorated with siRNA demonstrated active targeting to vascular endothelial growth factor C (VEGF-C) in gastric tumors. Similarly, surface modified nanoparticles generated higher transfection efficiency in human gastric cell line (SGC-7901) relative to unconjugated blank nanoparticles. In vivo studies suggest that siRNA decorated calcium carbonate nanoparticles can inhibit angiogenesis and growth of cancer cells [25]. Wang et al. demonstrated targeted delivery of anti CD47 siRNA conjugated to liposomal protamine hyaluronic nanoparticles (LPH-NPs) for lung cancer cells. The study demonstrated significant cancer metastasis inhibition (~27%) suggesting that active targeted siRNA delivery is highly effective [26]. Targeted drug delivery can prolong residence time at tumor site. In vivo studies demonstrated that GBM therapy with DOX PLGA nanoparticles surface decorated with siRNA and angiopep-2 (ANG) caused significantly higher DOX accumulation in glioma region [27]. Main advantages of such targeting include low dose requirement, reduced frequency of drug administration and minimal toxicity. None or minimal hematopoietic toxicity was observed with anti-CD47 siRNA encapsulated in a lipostamine-hyaluronic acid (LPH) formulation targeting CD47 receptors. This study indicated that LPH formulation was safe and well-tolerated [26].
2.1.4. Peptides
Chlorotoxin, a 36 amino acid peptide derived from scorpion venom [28] and AaCtx (70% homology with chlorotoxin peptide) are examples of such peptides which potentiate the anti-angiogenic effect of bevacizumab and may be targeted as anti-angiogenic agents for tumors [29]. Phase display peptide library series were screened at large to identify peptides with high affinity towards cancer cells. Recently, McGuire, et al. isolated 11 peptides with high affinity towards non-small lung cancer cells and low affinity for normal cells [30]. Ligands are designed to generate high binding affinity to plasma membrane of cancer cells [31–33]. For example, conjugation of paclitaxel to a peptide ligand demonstrated significant tumor size reduction relative to paclitaxel alone. Pallechia et al. conjugated paclitaxel (PTX) to dYNH peptide [ySAYPDSVP(L-norleucine)(L-homoserine)S] and YNH peptide [YSAYPDSVP(L-norleucine)(L-homoserine)S] to target EphA2 receptor overexpressed on various neoplastic cells including prostate cancer cells. dYNH-PTX- conjugate appeared to be more stable relative to YNH-PTX conjugate. Additionally, tumor reduction was more noticed with dYNH-PTX relative to mice treated with YNH-PTX [34]. A comparison of two studies indicate that dYNH peptide displays higher affinity and more anti-tumor activity than YNH peptide. Enhanced permeation and retention (EPR) effect due to hyper vascularization, poorly differentiated vasculature and ineffective lymphatic drainage are mostly responsible for development of weak, fragile and leaky vasculature [33, 35]. Such passive targeting exploits systemic and lymphatic systems in tumor architecture. Certain aggressive tumors may develop a 100 to 800 nm pore due to neovascularization [36]. Drug carriers with nanometer size range may take advantage of such pores and accumulate in the tumor site due to EPR effect. There are reports that small particle size (20 nm-100 nm) with surface pegylation may prolong circulation. Such carrier properties may aid in higher particulate accumulation at tumor site and enhance diffusion within tumor tissues [37, 38].
2.1.5. Cell-penetrating peptide (CPP)
CPP can serve as an effective ligand for targeting conventional as well as oligonucleotide based cancer therapeutics. Cell-penetrating peptides are generally composed of 5–30 amino acids, basic or amphiphilic [39]. CPP efficiently translocates plasma membrane and may aid in drug translocation across cell membrane. Hence, CPPs may be a potential targeting ligands for cancer chemotherapeutics or delivery systems. Charge specific CPP conjugated to DOX was studied by Lelle et al. [40]. Positively charged octa-arginine and negatively charged proline rich aliphatic CPP conjugated to DOX were studied. In vitro cytotoxicity of DOX conjugated to octa-arginine peptide on MCF-7 and HT-29 demonstrated an IC50 of 11.4µM and 19.0 µM, respectively. However, DOX conjugated to proline rich peptide displayed a higher IC50 of 27.0 µM on MCF-7 and 24.7 µM on HT-29 relative to DOX alone on MCF-7 (0.4 µM) and on HT-29 (1.2 µM) [40]. Confocal laser scanning microscopy revealed that oligo-arginine-DOX had significantly higher cellular uptake relative to proline peptide DOX indicating octa-arginine peptide as an efficient targeting ligand. Furthermore, a 16 base pair CPP (KKLFKKILKKL─ NH2) (B16) was conjugated to chlorambucil (CLB) to form CLB-BP16 demonstrated an IC50 value of 8.7 µM. On the other hand, an arginine analogue with 308 base pair (BP308) conjugated to CLB (CLB-BP308) had an IC50 of 25.5µM against CAPAN-1, MCF-7, PC-3, 1BR3G and SKMEL-28 cell lines compared to CLB alone which had an IC50 of 73.7µM. A significant improvement in activity of CLB was evidenced when conjugated to either at the N or C-terminal of B16 [41]. A number of small molecular weight peptides have also been applied to deliver targeted MNP (see section: Magnetic nanoparticles)
3. Intracellular targeted drug delivery
The most effective techniques to target tumor cells is to direct DNA inhibiting drug molecules to nuclei of cancer cells. Nuclear targeting not only causes primarily tumor cell death but simultaneously minimizes damage to surrounding normal cells. The major problem of such targeted drug delivery is to avoid translocation of active agents into endosomal or lysosomal vesicles. Drug delivery mechanism requires active molecules to escape from subcellular cytoplasmic vesicles and translocate into nuclei [42]. The cancer cells develop intracellular resistance mechanisms such as overexpression of drug efflux pumps, metabolism and sequestration into acidic compartments and deactivation [43]. Sui et al. described two nuclear drug delivery strategies. One strategy involves indirect nuclear targeting in which drug molecules are carried into cytosol in large quantities subsequently allowing complete sequestration of nuclear DNA. The other strategy provides direct nuclear targeting in which nanocarriers carry molecules to cancer cells across the cell membrane into cytosol and finally localize in the nuclei where the active molecules may be released [44].
3.1. Barriers for nuclear drug delivery
(a) Plasma membrane: The foremost barrier to nuclear drug delivery to mammalian cells is the plasma membrane that restrict the passage of charged and large hydrophilic molecules [45]. Large nanocarriers are taken by different endocytotic mechanisms into the cell [46]. Physical properties of nanocarriers such as geometry may have an effect on phagosome formation. Pinocytosis may be subdivided into clathrin mediated endocytosis (CME), caveolin-mediated endocytosis, clathrin and caveolin- independent endocytosis, and macropinocytosis. Trafficking of nanocarriers through different types of pinocytotic mechanisms is an important factor to develop targeted therapies. For instance, polarized MDCK epithelial cells that lack caveolae on apical surface cationic as well as anionic nanoparticles are trafficked by clathrin mediated endocytosis (CME) [47]. However, anionic particles are trafficked through CME and caveolae mediated endocytosis whereas cationic particles are restricted to CME and micropinocytosis in non-polarized HeLa cells [48]. In MDCK cells anionic particles access lysosomal compartments whereas cationic particles are directed to transcytosis [49]. These studies highlight the significance of shape and charge of the particulate delivery system which must be considered to overcome the foremost barrier of nuclear drug delivery.
(b) Nuclear membrane and nuclear transport: Multicellular nature of eukaryotes allows for compartmentalized architecture to regulate cell differentiation. The nuclear envelope surrounds the nucleus separating the nucleoplasm and genetic material from cytoplasm. The nuclear envelope comprises nuclear pore complex (NPC) through which exchange of molecules occurs [50]. NPC consists of a central channel, a nuclear and cytoplasmic ring which are made up of 50 different proteins called nucleoporins [51]. Each individual NPC translocates approximately 1000 proteins per second in a bidirectional way [52]. Molecules ≥45KDa or 9nm in diameter are transported though targeting signals to enter or exit the nucleus. On the other hand, small molecules pass through the NPC by passive diffusion [53]. Interestingly, a few studies reported the entry of particles over 100 nm [54]. Therefore, there is no consensus about exact mechanisms of transport across the NPC [55].
3.2. Strategies for targeted nuclear delivery
Four major nuclear targeted delivery systems have been studied extensively, i.e., 1) NLS mediated delivery system 2) TAT conjugated nuclear targeting 3) cationic polymer based delivery system and 4) pH triggered charge reversal approach. In vitro/In vivo studies demonstrated effective nuclear localization of NLS functionalized nanocarriers which are presented in Table 2. Positive charge residues in NLS and CPP sequences render them non-specific for in vivo applications. Even though brain penetration of CPP was documented, CPP and NLS exhibit rapid clearance mechanisms [56, 57]. Block copolymer micelles (BCM) surface-grafted with Trastuzumab-Fab-NLS generated 5-fold higher tumor uptake in HER2-overexpressing mice model. Moreover, the clearance of NLS conjugated Tm-Fab-NLS conjugates is attributed to mononuclear phagocyte system (MPS) without lowering tumor accumulation [58]. TAT functionalized liposomes conjugated to DOX increased cancer cell apoptosis by 37.1%. PEG modified liposomes are activated extracellularly by cysteine to achieve higher in vivo tumor uptake [59]. Similarly, DOX conjugated dextran and phenylboronic acid cholesterol (chol-PBA) nanomicelles demonstrated significant lysosomal-acidity dependent nuclear uptake of DOX nanomicelles. DOX-loaded Dex/Chol-PBA nano assembly demonstrated 100% survival rate and reduced tumor volume in mice model [60]. Cytotoxicity of DOX is largely dependent on its intercalation between two base pairs of DNA forming DNA adduct thereby inducing separation of DNA strands and DNA helicase [61]. Increased DNA cleavage activity and nuclear accumulation are dual functions that may enhance the efficacy of an anticancer drug. On the other hand, Wang et al. developed Graphene Quantum Dots conjugated DOX (DOX/GQD) and reported significant nuclear DOX accumulation and enhanced DNA cleavage. Further, at cellular level DOX/GQD conjugates evaded efflux transporters overexpressed in MCF-7/ADR cell lines [62].
Table 2.
Drug delivery vehicles and their effect on nuclear translocation
| Drug | Formulation | In vitro | In vivo | Reference |
|---|---|---|---|---|
| Doxorubicin | Dox-NLS loaded PLGA NP |
six times higher uptake than native DOX in MCF-7 cell line |
N/A | [63] |
| Carboplatin | PEGylated NLS- carboplatin conjugate |
Rapid internalization and accumulation in nucleus in M109FR cell lines that have overexpressed folic acid receptors |
N/A | [64] |
| Doxorubicin | TAT modified micelles consisting 2 block co polymers |
Suppressed tumor growth in Xenograft mice models |
[65] | |
|
Camptotheticin |
PLL & PAMAM dendrimer |
Facilitates nuclear accumulation by endosomal and lysosomal uptake in SKOV3 ovarian cancer cells |
N/A |
[66] |
| Doxorubicin | Targeted charge reversal nanoparticles [TCRN] |
Increased antitumor activity in SKOV3 ovarian cancer cells |
N/A | [67] |
3.3. Gene Therapy
Apparently, more than 2100 gene therapy clinical trials have been conducted as well as approved early till to date. Several gene therapy approaches have been introduced which include suicide gene therapy, immunomodulatory gene therapy, genetic modulation, pro-apoptotic and corrective gene therapy, antiangiogenic gene therapy, and siRNA therapy [68, 69]. In comparison to conventional chemotherapy which can cause high toxicity due to the lack of specificity, gene therapy provides a unique and powerful approach to combat cancer. Gene therapy works at molecular level, in which genetic materials or functioning genes are inserted into patients’ cells to either repair or replace the defective genes. The cancer cells carry mutated genes such as p53, bax and other oncogenes. Therefore, gene therapy can play crucial role in treating cancer.
3.3.1. Mechanism of gene therapy in cancer treatment
Gene therapy involves the delivery of genetic materials into cancer cells. A vector is employed as a carrier to ensure the delivery of genetic materials. After therapeutic genes are transported into cells, these sequences exert their action through various mechanisms such as silencing, up or down-regulation, repair or modification of the particular target genes. Suicide genes may cause cell death and/or tumor necrosis. Gene silencing inhibits cell growth and tumor regression. While gene modification may lead to higher response from other combination therapies (e.g. chemotherapy, immunotherapy, or radiation).
3.3.2. Gene delivery
Several delivery methods have been developed for gene therapy, which generally can be divided into two categories, viral and non-viral delivery system.
3.3.2.1. Viral vector
Viruses are microscopic infectious agents that can replicate in living cells [70]. Researchers have used viruses to deliver therapeutic genes into cell nuclei because of high transfection efficiency, ability to penetrate, express and replicate in host cells [71]. In order to utilize virus as vector, it is necessary to remove the pathogenic part of viral genes and replace with the therapeutic genes [71]. The remaining non-pathogenic segments in virus carries therapeutic gene constituting the viral vector.
(a) Adenovirus (Ads) are non-enveloped viruses that possess linear ds (double stranded) DNA [72]. Ads can cause transduction safely along with high transgene expression, which makes it a very powerful vector to treat glioblastoma multiforme (GBM) [72]. However, the application of Ads in gene therapy is limited by the low therapeutic efficacy after systemic administration and severe toxicity [73]. Many attempts have been made to minimize toxicity and improve therapeutic efficacy. Yao et al. demonstrated higher tumor-selective transgene expression with PEGylated adenovirus vector relative to Ads [74, 75]. The breakthrough in Ads gene therapy was the approval of Gendicine (Ad-p53) in 2003 in US and Oncorine/ONYX-015 (E1B-defective Ad) in 2005 in China. Both drugs are approved for treating head and neck squamous cell carcinoma [76].
(b) Adeno-Associated viruses (AAV) are small single-stranded (ss) DNA viruses which offer several advantages such as wide host range, low immune response (due to lack of pathogenicity) and long-term expression [77]. Recently several AAV-mediated genes have been developed to treat cancers such as prostate cancer, glioblastoma, cervical and breast cancer, nasopharyngeal carcinoma and lung carcinoma [78–81]. The safety of cytotoxic T lymphocytes (CTLs) with recombinant AAV has been evaluated in cancer patients [82]. AAV-mediated bevacizumab has been reported to inhibit ovarian tumor growth [83]. In another study, down-regulations of p16 (INK4), p27 (KIP1), p21 (WAF1), and p53 tumor suppressors using AAV type 2 was noted [84]. Another breakthrough of AAV associated cancer therapy was the approval of Glybera (alipogene tiparvovec) by European Union in 2013. It is an adeno-associated viral vector to treat lipoprotein lipase deficiency [85].
(c) Herpes viruses are large enveloped dsDNA viruses that can carry large transgene. A combination of oncolytic herpes simplex virus and vinblastine to deliver interleukin (IL)-12 to enhance antitumor effect in prostate cancer model has been evaluated [86]. Zeng et al. demonstrated synergistic effects of a combination therapy with paclitaxel and oncolytic herpes simplex virus G47Δ [87] in breast cancer. Similarly, Goshima et al. also showed promising effect of a combination of HF-10, herpes simplex virus (HSV) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in murine ovarian cancer [88].
(d) Lentiviruses are ssRNA genome retroviruses. They have emerged as a promising vector in cancer gene therapy. Lentiviruses possesses many advantages over other types of viral systems i.e. in low immunogenicity and the capability of transducing a variety of cells [89]. Various attempts have been made with lentivirus as vector in cancer gene therapy. Lentivirus mediated RNA interference was utilized to target protein phosphatase magnesium/manganese-dependent 1D (PPM1D) [90] or high mobility group box 1(HMGB1) [91]. These constructs suppress growth of bladder cancer. Similarly, other research groups have applied lentivirus-mediated short hairpin RNA to knockdown PPM1D in lung [92] and colorectal carcinoma [93].
3.3.2.2. Non-viral
Many non-viral systems have been investigated for gene delivery, including the injection of naked DNA or physical methods such as electroporation, gene gun, hydrodynamic distribution, sonoporation, and nanocarriers (nanoparticles and neutral or cationic liposomes) [94]. Non-viral methods are superior for large-scale production, low immunogenicity and they provide high level of transfection efficiencies compared to viral methods [95].
(a) Naked DNA: This is the simplest way to deliver therapeutic genes with direct injection of free DNA into particular tissues resulting in gene expression. In cancer gene therapy, DNA can be directly injected either inside the tumor or into tumor-surrounding tissues to express tumor antigens that might work as cancer vaccine. This method is less immunogenic and comparatively economical in terms of production cost. However, the low overall expression level restricts its use. Despite limitations, some success has been reported regarding clinical trials of naked DNA plasmid delivery [96], [97], [98].
(b) Electroporation (or electro-permeabilization) is a technique in which electrical field is applied to enhance the penetrability of DNA into cells [99] [100]. Electroporation offers several advantages such as accurate delivery of therapeutic genes, localized gene expression and less adverse effects. Studies have been conducted to ensure the safety and tolerability of this method in cancer and infectious diseases. The first clinical trial started in 2004 [101], [102]. Daud et al. have demonstrated the effectiveness of interlieukin-12 plasmid electroporation in metastatic melanoma patients in a phase I trial [103], [104]. Another study reported an intravaginal therapeutic DNA vaccine by electroporation. This involves coding calreticulin linked to E7 (CRT/E7) against cervicovaginal cancer [105].
(c) Hydrodynamic is another simple but effective non-viral gene delivery method. It works by employing a physical force that increases intravascular pressure [106]. This process is widely applied for gene delivery in animals. Hydrodynamic gene delivery is capable of delivering transgenes into mammalian cells in efficient and safe manner [107]. Yazawa et al. have examined hydrodynamic injection of plasmid encoding a soluble form of fetal liver kinase-1 (Flk-1) gene for angiogenesis inhibition in mouse model [108].
(d) Nanocarriers is another widely studied area in cancer gene therapy involving nanoparticle-based delivery of genetic material. These artificially synthesized non-bioactive nonviral vectors provide an efficient way to deliver genetic material to cells. Low immunogenic, less toxic and flexible for chemical modifications are unique advantages associated with this approach. However relatively low transfection efficiency is the main drawback with this method [109]. The nano-vectors i.e. nanoparticles or nanocapsules are usually prepared with biodegradable materials. These 10 to 100 nm particles form a nano complex by encapsulation or adsorption of genetic materials. The flexibility to chemical modification of nanomaterials provides excellent capability to adsorb, concentrate and protect genetic materials. Such nano-vectors can be divided into two categories: polymeric nano-vectors made of dendrimers, lipids, PLGA, and chitosan; and inorganic nano-vectors made of silica, iron oxide, and gold nanoparticles [110] [111] [112, 113]. Endocytosis is considered as the main delivery mechanism. In addition, coupling of specific molecules (antibodies/monoclonal antibodies, and peptides) to the surface of nanoparticles may be utilized to target tumor complex through binding to specific receptors on cell surface. The targeted genes can thus be safely and effectively transfected. The nanocarriers used for gene therapy in cancer are summarized in Table 3.
Table 3.
Nanocarriers for gene therapy in cancer
| Nanoparticle types |
Gene/Drugs | Target Cancer | Reference |
|---|---|---|---|
|
Cationic Liposomes |
ZEB1 siRNA and doxorubicin | lung cancer | [114] |
| Anti- Prostate-specific membrane antigen (PSMA) liposome complex |
prostate cancer | [115] | |
|
Neutral/zwitteri onic Liposomes |
Chlorotoxin-coupled nanoparticles for antisense oligonucleotides or siRNAs |
glioblastoma | [116, 117] |
|
Magnetic nanoparticles |
Vascular endothelial growth factor (VEGF)- siRNA | ovarian cancer | [118] |
| siRNA with multimodal mesoporous silica-based nanocarrier. |
lung cancer | [119] | |
| Notch-1 shRNA | breast cancer | [120] | |
|
Gold nanoparticles |
MicroRNA | prostate and breast cancer |
[121] |
| siRNA delivery with polyion complexes and gold nanoparticles. |
cervical cancer | [122] | |
| siRNA by novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold NPs |
retinoblastoma | [123] | |
| Chitosan-based | systemic delivery of survivin (SVN) siRNA | prostate cancer | [124] |
| siRNA | ovarian cancer | [125] | |
|
Hyaluronic acid based |
siRNA | lung cancer | [126] |
3.4. Cancer stem cell therapy
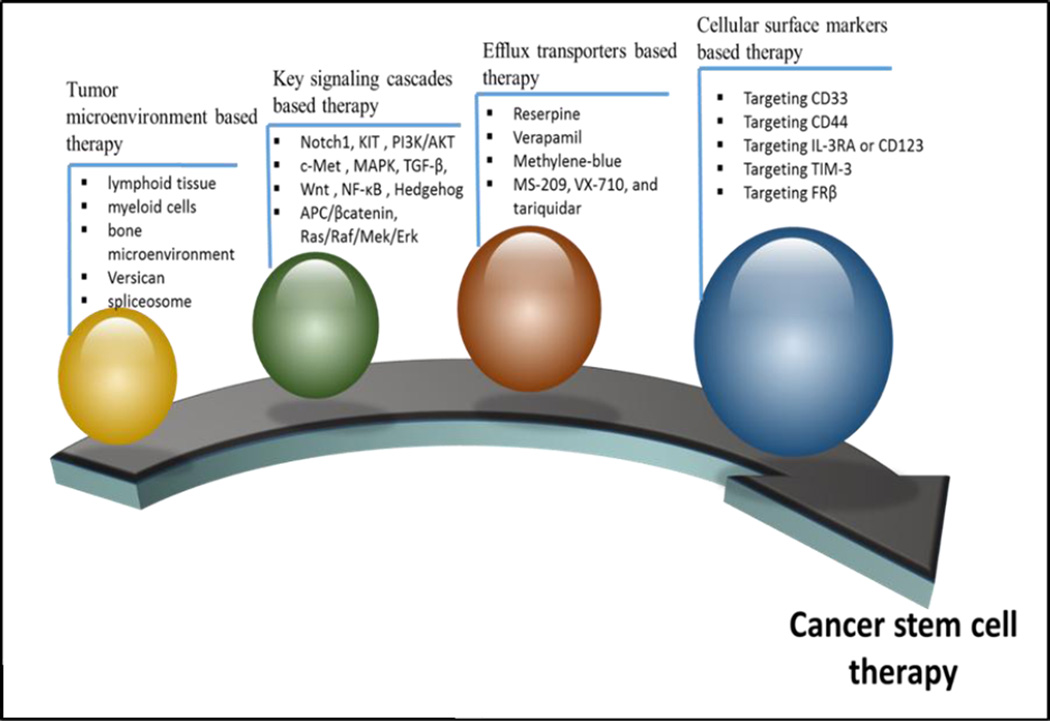
Self-renewal and proliferation of cancer stem cell (CSC) cause tumor initiation, development, metastasis and recurrence. So far, CSC has been discovered in a wide variety of solid tumors, including lung, colon, prostate, ovarian, and brain cancer, as well as melanoma. The primary reasons for treatment failure in multiple malignancies include resistance and lack of selectivity for chemotherapeutics. Furthermore, CSC populations are more resistant to convention chemotherapy relative to non-CSC population. Therefore, elimination of CSC is crucial in cancer treatment. Recently, multiple novel therapeutic systems have been explored for elimination of CSC and alteration of microenvironment which supports the growth of such cells. Chen et al. has reported various targets of current cancer treatment strategies. (Fig. 1). Surface markers and signaling pathways are the two potential targets. Multiple potential CSC therapeutic targets, including the ABC superfamily, anti-apoptotic factors, detoxifying enzymes, DNA repair enzymes and distinct oncogenic cascades have been identified. Currently, a few therapeutic strategies have been developed that can destroy CSC successfully while others are still under preclinical and clinical stages [127].
Fig 1.
Cancer Stem Cell Therapy
3.4.1. Key signaling cascades based therapy
Understanding the anti-apoptotic pathway and inactive pro apoptotic pathway/ mechanisms are emerging areas for the development of cancer chemotherapeutics. Notch1 signaling plays a major role in the development of tumors. Xanthohumol (XN) that inhibits Notch1 signaling pathway leading to accelerated apoptosis of tumor cells [128]. Merkelbach and co-workers described apoptosis modulation of KIT. Several mRNAs can induce apoptosis such as the PI3K/AKT signaling pathways in gastrointestinal stromal tumors [129]. Several other signaling cascades including c-Met [130], MAPK [131], TGF-β, Wnt [132], NF-κB [133], Hedgehog [134], APC/βcatenin, Ras/Raf/Mek/Erk and others play an important role in the recurrence and maintenance of CSC.
3.4.2. Tumor microenvironment based therapy
Tumor microenvironment also plays a vital role in creating a niche for nursing and protecting CSC from drug induced apoptosis. Caro et al. reported an important role of tertiary lymphoid tissue in T cell recruitment and local activation of immune cells in the tumor microenvironment. Several approaches involving targeting myeloid cells [135], bone microenvironment [136], Versican, (a 'bridge' connecting inflammation with tumor progression) [137], spliceosome [138] and NADPH oxidase-derived reactive oxygen species [139] have been explored.
4. Cancer Immunotherapy
Cancer immunotherapy is an alternative treatment intended to activate the immune system to induce disease stabilization [140]. In contrast to the other delivery approaches, immunotherapy primarily aims to prevent the metastatic spread of the disease. Cancer immunotherapy can utilize specificity of the immune system for the treatment of melanoma. Although the immune system is capable of recognizing and eliminating cancer cells but the tumor interferes with immune responses [141]. During tumor growth, cancer cells gain the ability to conquer the surrounding healthy tissue, thereby spreading the disease. Owing to genetic instability, cancer cells usually express abnormal proteins. Tumor-associated antigens (TAAs), produce none or limited expression on non-cancer cells. Such TAAs expose new, potentially immunogenic epitopes, which can be recognized by the host immune system. Therefore, tumor cells must be determined at early stages by immune system causing destruction of these abnormal cells [142]. Currently, immune check point inhibitors have been utilized to treat cancer. The immune system check points include PD-1 (programmed cell death protein 1) and CLTA-4 (Cytotoxic T-lymphocyte-associated antigen 4) which can protect normal cells. Targeting of these check points may be a new tool for the treatment of cancer [143]. Approaches to cancer immunotherapy include, active and passive immunotherapy. Cytokines regulate both the cells of the innate immune system and the adaptive immune system. Cytokines exert the effects upon binding to their respective receptors on the target cells. US Food and Drug Administration (FDA) approved IL-2 and IFN-α2b for treatment of a variety of cancers. IL-2 is indicated in the treatment of renal cell carcinoma, leukemia and lymphoma. IFN-α2b has been approved for the treatment of Kaposi’s sarcoma and various types of leukemia. Active immunotherapy includes cancer vaccines in which tumor antigen(s) are co-administered with an adjuvant to raise a specific T cell or B cell response. Passive immunotherapy includes monoclonal antibodies that block immune checkpoints such as CTLA-4 and PD-1 [142], [140]
5. Strategies for controlled drug delivery
5.1. Triggered Release
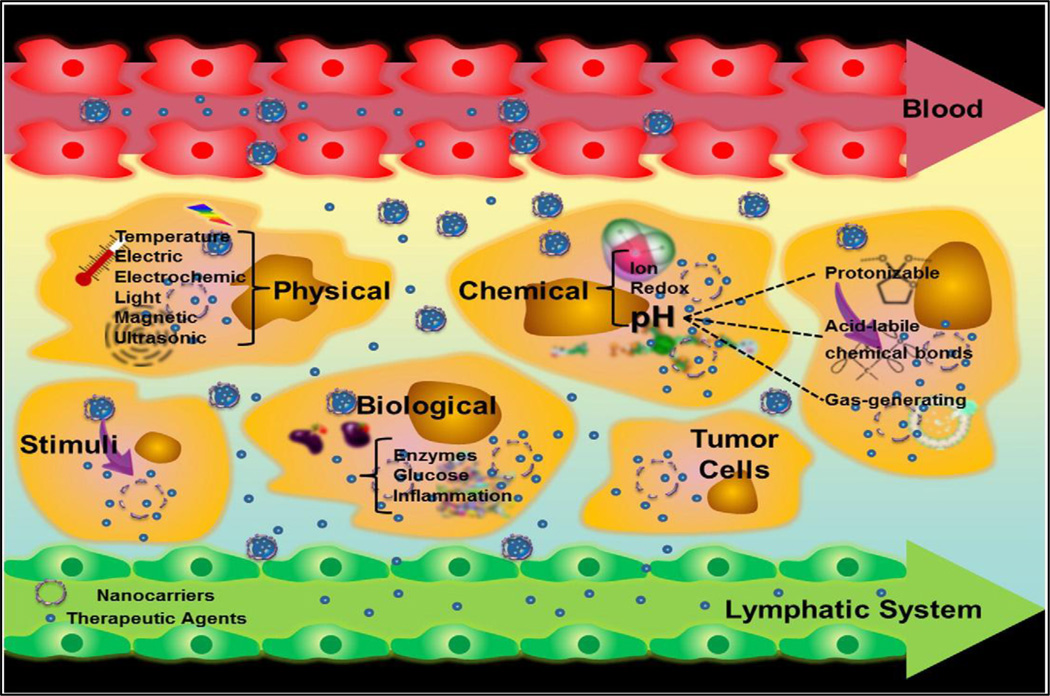
Noninvasive stimuli-responsive controlled drug delivery is attractive since such systems allow remote, repeatable, and reliable switching on or off drug release based on need. A complete noninvasive remote-controlled drug delivery system comprises of drug, an external/internal stimulus, stimulus-sensitive materials, and stimulus-responsive carriers. The external stimulus can be a magnetic field, light, ultrasound or radio-frequency. The internal stimulus can be pH, temperature or cellular enzymes. With the help of these response-triggered delivery systems, not only one can achieve targeted delivery of therapeutic agents, but can control the duration and extent of drug release also into tumor cell. A schematic has been providing to explain the different types of triggered release systems (Fig. 2).
Fig. 2.
Nanocarriers enhance the permeability and retention of drugs in tumors. The figure depicts various stimuli that can be used to trigger drug-release from appropriately responsive materials in tumor cells. Reproduced with the permission from Liang et al. [1]
5.2. Thermo-responsive release
Temperature-responsive or thermoresponsive polymers exhibit a sharp and discontinuous change of their physical properties with temperature [144]. These polymers can be functionalized with groups that bind to specific biomolecules. The polymer-biomolecules-conjugate can be precipitated from solution by a small change in temperature. Several examples of thermoresponsive delivery systems have been discussed. Gnaim et al. synthesized and characterized a novel γ-cyclodextrin (γ-CD) based carrier for molecular encapsulation of cancer chemotherapeutic agent DOX. The γ-CD derivative, with a β-naphthyl alanine residue attached in its primary surface, exhibited potent binding with DOX. The encapsulation efficiency was assessed under various temperatures and pH. The carrier-DOX inclusion complex is highly stable under a wide range of acidic conditions (pH 1.0–7.0). However, the encapsulated drug is slowly released under hyperthermic conditions (up to 50°C). Cell culture studies showed that the complexation of DOX with the carrier inhibited cellular uptake thereby significantly lowering toxicity. Thermo-triggered DOX release was validated and rise in cellular uptake was observed [145]. Magnetic-based core-shell particles (MBCSPs) were developed to target skin cancer cells while delivering chemotherapeutic drugs in a controlled fashion. MBCSPs consist of a thermoresponsive shell of poly (N-isopropylacrylamide–acrylamide–allylamine) and a core of poly (lactic-co-glycolic acid) (PLGA) embedded in magnetite nanoparticles. To target melanoma cancer cells, MBCSPs were conjugated with Gly–Arg–Gly–Asp–Ser (GRGDS) peptides that specifically bind to the a5b3 receptors of melanoma cells. MBCSPs consist of unique multifunctional controlled drug delivery properties. Specially, these particles can provide dual drug release mechanisms (a sustained release through degradation of PLGA core and a controlled release in response to changes in temperature via thermo-responsive polymer shell), as well as dual targeting mechanisms (magnetic localization and receptor-mediated targeting). Results from in vitro studies demonstrated that GRGDS-conjugated MBCSPs were less than 300nm in diameter. No cytotoxicity was observed in human dermal fibroblasts. These particles sustained the release of curcumin. A temperature-dependent release of doxorubicin from the shell of MBCSPs was observed. The particles also produced a dark contrast signal in magnetic resonance imaging. Finally, the particles accumulated at the tumor site in a B16F10 melanoma orthotopic mouse model, especially in the presence of a magnet [146]. To improve the efficacy of gemcitabine (GEM) in the treatment of advanced pancreatic cancer a multi-functional nanoplatform permitting both in vivo heating and drug delivery was developed by Kim et al. [147]. The researchers utilized a chemo-hypothermia approach to achieve high intra-tumoral GEM concentrations simultaneously inducing hyperthermia for enhanced tumor cell death as well as growth inhibition. MRI visible hydroxypropyl cellulose (HPC) grafted porous magnetic drug carrier may permit in vivo visualization of bio distribution. The magnetic drug carriers produced strong T2 weighted image contrast and permitted efficient heating with low magnetic field intensities. The thermo-mechanical response of HPC triggered GEM release was confirmed by in vitro drug release studies. In vitro studies confirmed that, pancreatic cancer cell growth was significantly inhibited (~82% reduction) with chemohyperthermia compared to chemotherapy or hyperthermia alone. Chemohyperthermia with intra-tumoral injections of GEM-magnetic carriers (followed by heating) resulted in significant rise in apoptotic cells compared to tumors treated with GEM-magnetic carrier injections. Chemohyperthermia with GEM-magnetic carrier offers the potential to significantly improve the therapeutic efficacy of gemcitabine in the treatment of pancreatic cancer [147].
5.3. Enzyme responsive release
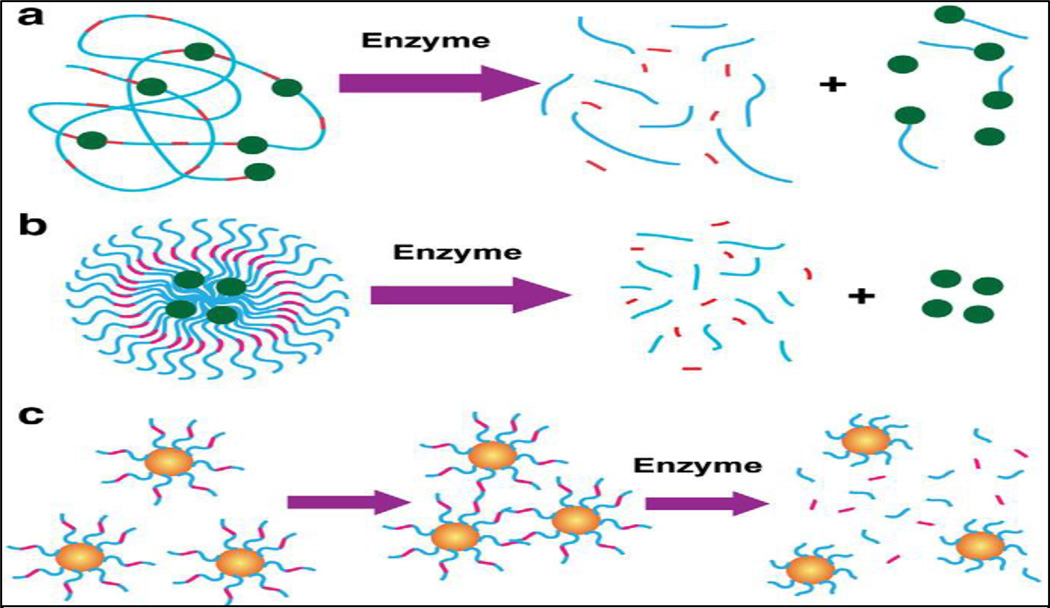
Enzymes are key components of the bio-nanotechnology toolbox that provides exceptional bio-recognition capabilities and outstanding catalytic properties. Combined with unique physical properties of nanomaterials, the resulting enzyme-responsive nanoparticles can be designed to perform efficiently with specificity for the triggering stimulus (Fig.3). This powerful concept has been successfully applied in the fabrication of drug delivery systems where the tissue of interest is targeted via release of cargo triggered by the biocatalytic action of an enzyme [2] [148]. When the enzymatic activity associated to a particular tissue is expressed at higher concentrations at the target site, the nanomaterial can be programmed to deliver drugs via enzymatic conversion of the carrier [149]. Excitingly, the detection of enzyme activity can be an extremely useful tool in diagnostics, up and down regulation of enzyme expressions that may be associated with many disorders. Also, the exceptional efficiency of enzymes in catalyzing a chemical reaction can be harnessed to amplify the signal generated by the recognition of a certain analyte, i.e. immunoreaction in enzyme-linked immunoassays. In some cases, the nanoparticles are prepared with a material that is sensitive to enzymatic transformation stemming from recognition of chemical structure by a biocatalyst and/or transformation of an enzymatic reaction by the product. Polymeric nanoparticles incorporating biological motifs are cleaved via such enzymatic digestion. Under these conditions, it is possible to program the nanomaterials to release cargo (e.g. anticancer agent) by triggering the degradation of the polymeric shell [150]. Nazli et al. developed magnetic iron oxide nanoparticles (MIONPs) coated with matrix-metalloproteinase (MMP)-sensitive PEG-hydrogel. High concentrations of proteolytic enzymes such as MMP are secreted by tumor tissues. These enzymes can degrade the basement membrane and natural extracellular matrix opening up more space for tumor growth. MMP-2 and MMP-9 are highly expressed in some malignant tissues such as breast, colon and brain tumors [151]. Elevated concentrations of MMP in tumor tissues can lead to proteolytic degradation of peptide linkages conjugated to drugs and/or delivery systems. Multifunctional MIONP was designed to serve as contrast agent for MRI as well as carry chemotherapeutic doxorubicin to target overexpressed receptors on cancer cell membrane. Nanoparticles consist of proteolytically degradable PEG hydrogel coatings with integrin-binding RGDS domains. In presence of MMP, such nanoparticles may degrade due to cleavage of the MMP sensitive domains causing anticancer drug release. Studies demonstrated that such nanoparticles may penetrate cancer cells eleven times more efficiently than blank nanoparticles. Confocal laser scanning microscopy further showed that the targeted nanoparticles released doxorubicin into the nuclei of HeLa cells within 2 hrs. This strategy may become a promising and highly efficient alternative to existing methods of cancer treatment [151].
Fig. 3.
Enzyme-responsive nanomaterials for drug delivery and diagnostics. a) polymer based nanoparticles covalently modified with drugs through an enzyme cleavable linker with enzyme triggered drug delivery in malignant tissue; b) polymer stabilized liposomes loaded with drugs, where programmed degradation triggered by an enzyme; c) Inorganic nanoparticles used for diagnostics where the activity of the target hydrolase the assembly or disassembly of the nanoparticles. Reproduced with the permission from Stevens et al. [2]
5.4. pH responsive release
Among the different types of stimuli, pH sensitive system has been the most widely employed nano-systems in cancer therapy [1]. It is well known that pH varies significantly in different tissues or organs, such as stomach and liver, and in disease states, such as ischemia, infection, inflammation, and tumorigenesis. Due to high rate of glycolysis in cancer cells, aerobic and anaerobic conditions and lower pH in tumors can be exploited to target chemotherapeutics to these cells. Tumors have been demonstrated to exhibit acidic pH values ranging from 5.7 to 7.8, while the pH of normal tissue is 7.4 [152][153]. Several approaches have been developed to design pH-responsive drug release. One of the most commonly used approach is to introduce an “ionizable” chemical group such as amines, phosphoric and carboxylic acids among others, into polymeric structures. These groups, with different chemical structures and pKa values, can accept or donate protons and undergo pH-dependent changes in physical or chemical properties such as swelling ratio and/or solubility, resulting in drug release [154]. Another approach is to introduce acid-labile chemical bonds either by covalently conjugating drug molecules directly to the surface of existing nanocarrier or to construct a new nanocarrier. These acid-labile linkages are stable at neutral pH but easily degraded or hydrolyzed under acidic environment. The acid-labile linkers most commonly employed are acetal, orthoester, hydrazone, imine, and cis-aconyl bonds. A novel approach to prepare pH-responsive delivery systems is to incorporate carbon dioxide-generating precursors that generate CO2 in an acidic environment, leading to disintegration of the carrier and release of active molecules [155]. For effective delivery of anticancer drugs, pH-sensitive nanosystems may encapsulate and stabilize the anticancer agent at physiological pH but rapidly release the cargo at tumor pH.
6. Prodrug approach: analog / chemical conjugation for cancer chemotherapeutics
Prodrug approach in the field of anticancer drug delivery has gained tremendous interest. This strategy can improve the efficacy and reduce systemic or unwanted tissue/organ toxicity of the parent drugs. The structures of parent anticancer drugs and their prodrugs developed in last two years have been summarized in the following sections:
6.1. Prodrugs of alkylating agents
Selective DNA cross linking triggered by oxidation, reduction, fluoride induction and photo-irradiation have been developed. Han et al. recently described a series of binitroimidazole prodrugs that generated DNA inter-strand cross–links (ICL) and direct strand breaks (DSB) upon UV irradiation. Such DNA cross linking may lead to highly selective inhibition of DNA replication and gene expression, thereby leading to cell death [156]. Paci and co-workers designed preactivated ifosfamide (IFO), an oxazaphosphorine (alkylating agent). This prodrug requires bioactivation by cytochrome P450 enzymes to release the active entity. This approach led to the inhibition of toxic metabolites which is the major drawback of IFO delivery [157]. A hypoxia-selective anti-tumor agent, demonstrated preparation of mono-N-oxide analogue by removal of 4-oxide from tirapazamine (parent di-N-dioxide). It bears a nitrogen mustard unit, which exhibits significant alkylating properties [158]. Peng and group developed H2O2 activated aromatic nitrogen mustard based prodrugs where the DNA alkylating agent is connected to a H2O2 responsive trigger by an electron withdrawing group. Such an approach of ROS-activation provided better specificity and lower toxicity [120, 159–161]. A melphalan derived prodrug, melflufen (J1) which releases the parent drug by hydrolytic cleavage of the peptide bond by aminopeptidase N (APN) thereby eliciting anti-anigiogenic effects [162]. Wietrzyk and co-workers developed ester prodrugs of isophosphoramide mustard (iPAM), an active metabolite of IFO which can be easily hydrolyzed by esterases to release the parent drug [163]. Boger et al. reported reductively cleaved novel phenolic prodrugs of duocarmycin that alkylates DNA in a selective sequential manner [164]. Oxidative quenching of quinone methide precursors prevented quinone methide regeneration leading to formation of transient derivatives that can cause reversible alkylation in DNA duplex [165].
Platinum drugs (cisplatin, carboplatin, and oxaliplatin) are not grouped under alkylating agents. However, these compounds cause tumor cell death following same mechanism as alkylating agents. Recently, Lippard and co-workers developed a series of Pt (IV) prodrugs of cisplatin and carboplatin bearing axial halides by oxidative halogenation [166, 167]. Similarly derivatives of oxaliplatin with axial valproato ligands were developed [168]. Cisplatin analogs conjugated with cyclooxygenase (COX) inhibitors were also designed to overcome cisplatin resistance [169, 170]. Cisplatin-based aliphatic bis(carboxylato) Pt(IV) prodrugs with varying carbon chain length are reported by Osella and group [171].
Recently, several nanoformulations of anticancer drugs have gained attention. Bilgicer and co-workers developed photosensitive Pt (IV)-azide prodrug-loaded nanoparticles that selectively induce active Pt (II) at the tumor site by UVA irradiation [172]. The platin-A prodrug is developed to overcome the nephrotoxicity and ototoxicity of cisplatin [173]. Xing and group developed near-infrared (NIR) light-activated nanoplatform for a platinum (IV) prodrug [174]. Many other prodrugs with nano formulations of anti-tumor agents are currently under development [175–182]
6.2. Prodrugs of antimetabolites
Sakamoto and co-workers developed prodrug which releases 5-fluorouracil (5-FU) anticancer drug from oligonucleotide strands following photo irradiation [183]. Prodrugs of gemcitabine containing bioorthogonal Pd(0)-cleavable groups that generate biologically inert precursors of gemcitabine [184]. Cui et al. discussed a tripeptide prodrug, 13F-1 of 5-FU that provides activity against tumor cells by targeting an enzyme aminopeptidase N [185]. N-Acyl-phosphoramidates derivatives of gemcitabine can overcome acquired resistance to gemcitabine caused by deoxycytidine kinase deficiency [186]. Similarly, Mcguigan et al. have disclosed NUC-1031, a gemcitabine phosphoramidate prodrug that has shown significant reduction in viability of tumor cells [187]. Zhao et al. demonstrated the efficacy of cell penetrable dendrimer as a potential antitumor drug carrier by 5-FU and lysine dendrimer conjugates [188]. Several nanocarriers like PEGylated lipids for squalenoyl amphiphilic prodrugs of gemcitabine and dideoxycytidine [189] and polymeric micelles for stearoyl-gemcitabine [190] are being developed to improve the efficacy and sustainability of the anticancer properties. A glycol chitosan (GC) grafted with 2,3-dimethylmaleic acid (DMA) and fullerene (C60) nanogels display both photo and low pH responsive properties for cancer therapy [191]. A few other recent advancements in the field of prodrugs of cancer therapy have been listed; prodrugs activated by histone deacetylases and a tumour-associated protease [192]; squalene-based prodrug [193]; fluorouracil/zidovudine glyceryl prodrug [194]; 1,2- and 1,3-diacylglycerophosphates of clofarabine [195].
6.3. Prodrugs of anthracyclines/ anti-tumor antibiotics
A non-hormonal therapeutic approach is a growing area that provides an alternative to various treatment modalities for hormone refractory cancers. Antibody-directed enzyme prodrug therapy (ADEPT) and gene-directed enzyme prodrug therapy (GDEPT) are being explored recently to overcome nondiscriminatory drug exposure to normal tissues by chemotherapeutics. Kim and co-workers recently developed an induced phenotype targeted therapy (IPTT) where DEVD-S-DOX prodrug containing DOX linked to a peptide moiety (DEVD) is cleaved by an enzyme caspase-3. Caspase three expression is elevated in tumor region by radiation induced apoptosis [196]. Several enzyme cleavable peptide prodrug conjugates and formulations of DOX have been recently developed to increase the specificity and tolerability of chemotherapeutic agents [197–205]. Selective DOX prodrug activation using photoirradiation [206], pH active linkages [176, 207–209], photothermal linkages [210], and reduction [211] have paved the way for the development of new selective chemotherapeutic agents.
6.4. Prodrugs of topoisomerase inhibitors
Kim and co-workers developed a prodrug of SN-38, having a biotin subunit for localization and a piperazine-rhodol moiety for fluorescent based monitoring. These two segments are connected by a self-immolative linker through disulfide bonds. Such a strategy can offer better targeting and drug release. It can also aid in cancer diagnosis and treatment [212]. Ashley and group developed a macromolecular prodrug of SN-38 by a linker between the macromolecular carrier and the drug molecule. The linker undergoes self-cleavage by a non-enzymatic B-elimination which is highly predictable. This process in turn prolongs the circulation time and lower glucoronidation of the parent drug [213]. Several hyaluronan [214], methylenthiol group [215], folate targeted [216] based prodrugs of campothecin and malic acid based ester prodrugs of etoposide [208] have been reported. Wang et al. developed a prodrug of SN-38, which releases the parent drug upon hydrolysis in the presence of glutathione. It leads to reactive oxygen species which can elicit high anticancer therapeutic activity [217]. Nano-formulations such as glutathione responsive nanoparticles [218], phosphorylcholine micelles responsive to reducing agent [219] and PEG based nanomicelle [220] have been prepared recently. Chen and co-workers came up with a very innovative approach of co-delivering camptothecin and small interfering RNA in the form of a prodrug (CPTssR5H5) to overcome multi-drug resistance [221].
6.5. Prodrugs of mitotic inhibitors
Recently the development of mitotic chemotherapeutic agents has made rapid progress. The representative drugs for this class include PTX. Poor aqueous solubility and systemic toxicity led to derivatization of PTX prodrugs. Conjugation of PTX with a hydrophilic macromolecule through a bio-cleavable linker is one of the strategies widely studied [222–226]. Silicate ester derivatives of PTX and docetaxel (Dtx) also demonstrated improved physiochemical properties. Targeted therapy based on distinct tumor types faces a major challenge. As classification has been primarily based on histology of tumor, targeted therapy has not been effective in treating tumors with similar histology [6, 227]. Similarly, several Dtx derivatives with better targetibility and stability were developed such as CD44 targeted-hyaluronic acid based derivatives [228]. Sun and co-workers reported a prodrug with fluorinated Dtx conjugated to rhodamine B (imaging reporter/targeting domain) through a biodegradable ester linkage. This prodrug demonstrated high plasma/blood stability and specificity to mitochondria. [229]. Other derivatives of colchicine [230], estradiol [231], combretastatin [232, 233] and phenstatin [234] have been synthesized and have shown better efficacy relative to parent drug.
6.6. Steroid prodrugs as anticancer agents
Corticosteroids are also known to exhibit anticancer activity. However, several serious side effects are associated with their use. Gilmer and co-workers prepared nitrophenyl based prodrugs of prednisolone, budesonide and celecoxib where the parent drugs generated by the nitro reductase action of the colonic microflora thus improving the targetibilty and lowering the toxicity of parent drugs [235].
7. Magnetic nanoparticles (MNP)
Widder and colleagues were the first to utilize magnetic microsphere to direct anticancer agents to tumor tissue with the aid of external magnetic field [236]. Magnetism-assisted therapy has undergone a significant evolution to become a sophisticated theranostic (combination of therapeutics and diagnostics) approach. Magnetic nanoparticles (MNP) are flexible systems offering a variety of modification for therapeutic and diagnostic applications. Owing to super paramagnetic property, MNP have proven to be excellent magnetic resonance imaging (MRI) contrast agents. These agents are biocompatible and well tolerated. As a result, a number of MNP’s are in clinical trials or approved as contrast agent for MRI to detect a variety of cancers. Iron oxide NP may produce reactive oxygen species in vivo (Fenton reaction) leading to DNA damage and toxicity [237]. However, this can be easily avoided by coating MNP’s with polymers, surfactant, inorganic metals or oxides. Some of the commonly used materials for preparation of MNP’s include magnetite (Fe3O4), maghemite (γ-Fe2O3), iron-based metal oxides (CoFe2O4, NiFe2O4, MnFe2O4), iron alloys (FePt and FeAu), rare earth metal alloys and transition metals. Cobalt, nickel and chromium are less preferred as biomedical agents, because these metals are highly toxic and require impervious coating. Iron oxide and iron alloy based materials are generally safer to use.
7.1. Therapeutic applications of MNP
MNP’s are typically 10 to 100 nm in size with very narrow size distribution, which allow them to exploit enhanced permeation and retention effect (passive targeting). This particle size range aids in evasion of both renal clearance and phagocytosis of circulating particles. Chemotherapeutic agents can be incorporated in the polymeric film of MNP or chemically conjugated to polymer chains via suitable linker. MNP can also be directed to the tumor site by using an external magnetic field to provide a localized delivery of anticancer agents thereby limiting off-target toxicity. In a clinical trial, MNP’s were employed to deliver DOX hydrochloride to hepatocellular carcinoma assisted by external magnetic field. Particle localization was monitored by MRI. In a similar study Wilson et al. treated inoperable hepatocellular carcinoma with MNP targeted DOX hydrochloride. Using an external magnet DOX hydrochloride coated MNP’s were directed to tumor. The fraction of tumor volume (ranged from 0.64 to 0.91) was treated with minimal off target effect [238].
Surface coating also provides numerous advantages such as prevention of agglomeration of sub-nano particles. It also limits nonspecific interactions, improves pharmacokinetics and allows ligand mediated active targeting. Coating also enables development of multifunctional nanocarriers facilitating delivery of a multiple class of therapeutic agents simultaneously. A schematic representation of multifunctional nanoparticles is shown in Fig.4. MNP can be actively targeted to cancer cells via receptor-ligand mediated specific interactions. A number of cancer cell specific surface biomarkers have been targeted for this purpose. Ligand-targeted MNP have been utilized for diagnosis as well as theranostics application for MR imaging. Location of tumor lesions as small as ~2–3 mm in diameter can be identified following cellular uptake [239]. Chlorotoxin-conjugated MNP was utilized to target glioma cells. Active targeting resulted in higher accumulation in cancer cells with significantly improved contrast MR imaging [240]. Targeting ligands conjugated to MNP was exploited for theranostics purposes ranging from small molecule nutrient transporters such folate [241–243] to macromolecules like Trastuzumab [244–246], single-chain anti-epidermal growth factor antibody [247], anti-epidermal growth factor receptor antibody [248], anti-vascular endothelial growth factor antibody (VEGF) [249, 250] and aptamers [251], [252]. A number of small molecular weight peptide have also been investigated including arginylglycylaspartic acid (RGD) [243, 253], bombesin [254, 255], and luteinizing hormone releasing hormone [256, 257]; highly cationic peptides like CPP [258], myristoylated poly-arginine peptide (MPAP) [259, 260] and HIV-TAT [261]. Multifunctional MNP have also been developed where nanocomposites are surface modified with fluorescent probes enabling both fluorescence and MR imaging for enhanced in vivo tracking. Lin et al. developed methotrexate loaded folate receptor targeted dual probe MNP where cyanin dye (Cy5.5) was used as fluorescent probe [262]. Such theranostic nanocomposite can be delivered to treat tumors and monitored in real time via MRI or fluorescence imaging.
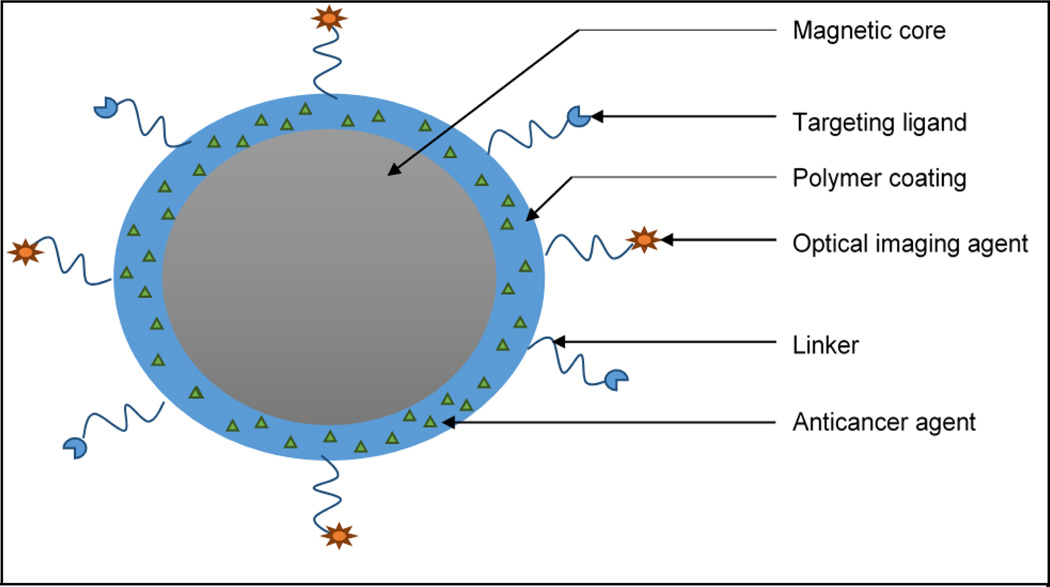
Fig.4.
Schematic representation of core shell type magnetic nanoparticles. The external polymer coating entrapping anticancer agent can be decorated with imaging agent for enhanced imaging and/or targeting ligand for active targeting via a suitable linker.
Hyperthermia is another application of superparamagnetic iron oxide nanoparticles (SPIONs) [263]. MNP can raise local temperature to ~ 41–46 °C under alternating magnetic field which has been shown to destroy cancer cells [264]. Exposure to moderate temperature 41–46 °C leads to activation of myriad of intra and extracellular degradation mechanisms such as protein denaturation, protein folding, aggregation and DNA cross linking. Temperatures above 46 °C (up to 56 °C) causes direct tissue necrosis, coagulation or carbonization leading to cell death. MNP in the 14–16 nm range have been reportedly most effective in producing hyperthermia [265]. However, this technique requires precise special controls to avoid damage to normal cells. Hyperthermia has also been employed as a mechanism to trigger chemotherapeutic release within tumor microenvironment, potentially minimizing off-target effect due to premature drug release. Hyperthermia-mediated drug release is achieved via either bond cleavage, where drug is chemically conjugated to MNP via thermolabile bond or enhanced permeability where chemotherapeutics are encapsulated in polymer film coated MNP. Thermoresponsive poly (N-isopropylacrylamide) (PNiPAM) microgel coated MNP containing anticancer agents were encapsulated inside microgel. Release is achieved via enhanced permeation mechanism [266]. Similarly, PNiPAM based hydrogel was encapsulated with SPIONs. Model agents (vitamin B12 and methylene blue) showed drug release in the presence of oscillating magnetic field via enhanced permeation mechanism [267]. Derfus et al. have reported multifunctional MNP for remote controlled delivery of fluorescein-labeled DNA via oscillating magnetic field [268].
8. Current non-invasive strategies for cancer treatment
8.1. Ultrasound-mediated treatment
Ultrasound as a traditional diagnostic tool has been indicated in non-invasive therapy and anticancer drug delivery. The potential mechanisms of ultrasound for drug delivery involves three strategies including thermal effects, cavitation and radiation forces. Ultrasound has been utilized to facilitate intracellular delivery of a specific drug as well as to enhance the overall efficiency of the cytotoxic effects from carriers such as microbubbles and nanobubbles [269–274]. Ultrasound as a component of drug delivery system has the potential to be coupled with various drug carriers for the treatment of cancer [275–277].
8.1.1. Thermal effects
Localized tissue heating depends on the absorption of energy, intensity and frequency of the ultrasound, and the rates of thermal diffusion and conversion. Even a moderate temperature increase may significantly increase permeability of blood capillaries and/or cause cell membrane fluidization [278, 279]. Thermal effect of ultrasound has been utilized with temperature sensitive liposomes which is the most commonly investigated ultrasound-responsive drug delivery vehicle. In combination with localized hyperthermia under ultrasound, thermosensitive liposomes improved the delivery of various anticancer drugs to tumors [280–282]. Liposomes undergo a gel-to-fluid phase transition in the phospholipid membrane and become more permeable. This process allows rapid drug release in the target region within non-destructive hyperthermia range (39–41 °C) [279, 280, 283, 284]. An increase in local drug delivery causes significant inhibition of tumor growth [285].
8.1.2. Cavitation
Acoustic cavitation causes oscillation, and collapse of small stabilized gas bubbles under an ultrasonic field in a fluid medium. It is considered to be the most important of all non-thermal ultrasound mechanisms. This mechanism has the potential to produce cavitation in biological tissues, especially for enhancing drug delivery. Cavitation can be considerably improved by combining gas-filled microbubbles [271]. There are two distinct types of acoustic cavitation activity such as non-inertial (or stable) cavitation and inertial (or transient) cavitation [286]. The non-inertial cavitation bubbles may stably oscillate and persist for a many acoustic cycles. Non-inertial cavitation of systemically injected microbubbles induces alternating invagination and distention of vascular walls. In turn it causes damage to the endothelial lining and temporarily raises blood vessel permeability for enhanced extravasation and can improve delivery to whole tissue [287–289]. In ultrasound, inertial (transient) cavitation can also alter the permeability of individual cells for improved delivery of genes and drugs. Inertial cavitation bubbles grow and expand two to three times of their resonant size, and finally collapse. Inertial cavitation is generally considered as the primary mechanism for structurally altering intact cells including irreversible damage and non-destructive membrane permeability [290, 291]. Inertial cavitation of microbubbles causes microjets and shock waves which create holes in blood vessels and cell membranes causing higher permeability of drugs and the carriers. The process of ultrasound-induced creation of pores in cell membranes is known as sonoporation [292–295]. Acoustic cavitation approach involves the path way of ultrasound for deploying drugs from the carrier. However, the exact mechanism by which the interactions between the bubbles and the carriers create these effects is still unclear. Ultrasound-induced cavitation has been incorporated for opening liposomal membranes and ultrasound-responsive stable liposomes demonstrated prolonged circulation time and effective tumor targeting [296–299]. Drug-loaded microbubbles are attractive and ultrasound-responsive drug carriers may be very beneficial for drug targeting to intravascular targets [300–306]. Targeted and ultrasound-triggered liposomes co-modified with single stranded DNA aptamers can target platelet-derived growth factor receptors (PDGFRs) expressed in breast cancer cells. Poly (NIPMAM-co-NIPAM) as the thermosensitive polymer can sensitize these liposomes at high temperature [307]. Ideal ultrasound-mediated tumor-targeted drug carrier requires good drug stability in circulation, long drug retention until activated, small size allowing extravasation through defective tumor vasculature and high ultrasound responsiveness [308]. However, currently used contrast agents as tumor-targeted drug carriers have many inherent difficulties. The commercially available microbubbles failed to effectively extravagate into tumor tissue for effective drug targeting due to their very short circulation time and relatively large size (2–10 microns). After release from microbubbles into circulation, a majority of drug fraction may circulate within the systemic circulation and eventually reach off-target sites. As a result only a small fraction reaches the tumor site.
8.1.3. Radiation force
Radiation has been defined as a unidirectional force that is generated with a transfer of momentum from the ultrasound wave to the medium under relatively high amplitudes of ultrasound exposures. Radiation forces are proportional to the rate of energy being applied and the absorption coefficient of the medium while it is inversely proportional to the speed of the ultrasound wave in the medium. [309]. Acoustic streaming can reduce heating through the process of increasing the mass transport of nanoparticles for improved transdermal delivery [310, 311]. Acoustic streaming and radiation force can also facilitate nanoparticle transfer across blood capillaries, consequently enhancing extravasation of drug carriers and/or macromolecular drugs [312–317]. Additionally, ultrasound radiation force may assist in modulating ligand exposure onto the surface of nanoparticles. The ligand in the droplet of primary nanoparticle can be exposed to the cell receptor with ultrasound [318]. Ultrasound effect on drug carriers and biological tissues is to enhance perfusion, extravasation of drugs and/or carriers, and drug diffusion through tumor tissue. Overall effect is drug penetration through various biological barriers. Enhanced intracellular uptake of nanoparticles, genes, and drugs extensively improve therapeutic efficacy of these agents [312, 319–325]. Furthermore, ultrasound treatment has also been associated with an induced immune response to tumors [326–328].
9. Chemotherapy and Drug Resistance
Chemotherapy is one of the foremost therapeutic interventions in cancer. Despite advances in drug discovery and treatment protocols, patients acquire multidrug resistance (MDR). Consequently response to chemotherapy remains far below expectations. MDR is a phenomenon where tumor cells develop resistance to functionally and structurally unrelated anticancer drugs [329]. While cancer cells initially respond to chemotherapy but relapse is common. Clinically drug resistance ensues prior to or in response to chemotherapy. It is either acquired or inherent and can be caused via multiple pathways. The mechanisms of drug resistance adapted by cancer cells include modifications in drug metabolism and transport, gene mutation, amplification of drug targets and genetic rewiring leading to gene repair and impaired apoptosis. Tumor heterogeneity (heterogeneous population of cells with distinct genetic fingerprints) is another aspect of drug resistance where a small subpopulation remain dormant and unresponsive to chemotherapy. But these cells later emerge as virulent type which become difficult to treat [330]. Onset of drug resistance is a complex process. It involves pharmacokinetic and pharmacodynamic mechanisms. Extensive reviews are available on the mechanisms of drug resistance [331–334]. Pharmacokinetic mechanisms involving sub-therapeutic concentration, elevated efflux, due to overexpression of MDR genes and enhanced biotransformation by cytochrome P450 (CYP) metabolizing enzymes are implicated. All these mechanisms indicate up-regulation of efflux transporters and metabolizing enzymes that constitute a major resistance phenotype. A concerted effects of efflux transporters and metabolizing enzymes leading to MDR may possibly occur due to overlapping substrate specificity and coordinated regulation of their expression. The expressions of MDR genes and CYP are mainly governed by nuclear receptors, particularly pregnane X receptor (PXR). These mechanisms may function independently or synergistically, leading to treatment failure. Therefore, ways to overcome such integrated role of efflux transporters [P-glycoprotein (P-gp), multidrug resistant proteins (MRP) and breast cancer resistance protein (BCRP)] along with CYP activity have been discussed in the following sections:
9.1. Role of Transporters in Drug Delivery
Transporters are integral part of cell membrane proteins. These proteins are intricately associated with selective absorption of endogenous substances (substances such as anions and cations, vitamins, sugars, nucleosides, amino acids, and peptides) and exclusion of toxic elements. Indeed, influx transporters allow absorption of essential nutrients and ions whereas efflux transporter eliminate cellular metabolites and xenobiotics. These proteins play a vital role in drug absorption, distribution, elimination, as well as drug-drug interactions. Although the occurrence of multidrug resistance in bacteria was identified more than fifty years ago, role of P-gp as a major factor for efflux of xenobiotics has been established recently. It is clear now that, these efflux proteins are highly expressed on different tumor cells which leads to drug resistance in chemotherapy.
In fact, these transporters play an important role in the process of drug absorption, distribution, metabolism and elimination (ADME). Almost 2000 genes have been identified for expression of transporters or transporter-related proteins [335]. Approximately 400 membrane transporters, in two super families, have been identified and characterized for specific tissue localization and a number of these transporters have been cloned [335]. From pharmacological perspective two major super families, ABC (ATP binding cassette) and SLC (solute carrier) transporters, are most important. Importance of drug transporters in the process of ADME and drug-drug interactions is now well documented. Recently US Food and Drug Administration (FDA) and International Transporter Consortium (ITC) together have considered a few transporters i.e., organic anion transporter (OAT), organic anion transporting polypeptide (OATP), organic cation transporter (OCT), peptide transporter (PEPT), P-gp, multidrug resistance protein (MRP) and breast cancer resistance protein (BCRP) which are crucial for xenobiotics absorption, and drug-drug interactions. From pharmacokinetic view point, localization and function of these transporters in the intestine, liver and kidney are receiving foremost consideration.
(a) ABC Transporters: The ABC transporter genes stand for the largest family of transmembrane proteins. So far 49 human genes have been recognized. Based on sequence homology and domain structure, these proteins are categorized into seven different classes (ABCA-ABCG). So far, thirteen different transporters have been identified from classes A, B, C and G and these transporters play a significant role in the development of multidrug resistance (MDR) [334, 336]. Biedler and Riehm [337] reported that Chinese hamster ovary (CHO) cells identified for resistance to actinomycin D, also impart cross-resistance to many other drugs (duramycin, democoline, mithramycin, mytomycin C, puromycin, vinblastin and vincristine). These efflux pumps also impart resistance to HIV-protease inhibitors. Mammalian cells can acquire cross resistance to a variety of therapeutic agents with diverse chemical structures and functions. This phenomenon is referred to as multidrug resistance. Juliano and Ling revealed that drug resistance in CHO cells is due to the presence of P-glycoprotein. These ABC transporters function in a unidirectional manner as opposed to several others transporters which are bidirectional [338]. All ABC proteins show in general a minimal requirement of two transmembrane domains (TMDs) and two nucleotide binding domains (NBDs) except BCRP. The hydrolysis of ATP is required as a standard power for translocation by these transporters [339]. The structural requirements of the ABC transporter for drug binding have been discussed previously [340, 341].
(b) Efflux transporters: Three important ABC transporters: P-gp, MRP and BCRP are expressed at physiological barriers (e.g. intestinal absorption barrier, BBB, placental barrier, corneal and retinal barriers), in the liver, cancer cells, and other tissues and are responsible for clearance and excretion of xenobiotics. These transporters significantly influence ADME of a number of drugs and their metabolites and cause drug resistance for many therapeutic agents. Such proteins along with primary metabolizing enzyme cytochrome P450 (CYP) together constitute a highly efficient barrier for oral drug absorption. P-gp is the most extensively studied efflux transporter which functions as a biological barrier by extruding toxins and xenobiotics into extracellular fluid. BCRP and MRP also belong to the same ABC super family. Substrate specificity and tissue localization of P-gp and BCRP differ from MRP. As a consequence of efflux, drug absorption is reduced and bioavailability of xenobiotics diminishes at the target tumor tissue. In recent reviews we have elaborately discussed the role of these efflux transporters in drug resistance [342]. A list of anticancer agents that are substrate for various efflux transporters is presented in Table 4.
Table 4.
A list of chemotherapeutic agents which are substrates for various efflux transporters
| Efflux transporters |
Chemotherapeutic substrates |
|---|---|
| MDR1 | Bisantrene, daunorubicin, docetaxel, DOX, epirubicin, etoposide, idarubicin, methotrexate, mitoxantrone, paclitaxel, teniposide, vinblastine, vincristine |
| MRP1 | Daunorubicin, DOX, epirubicin, etoposide, imatinib, melphalan, methotrexate, mitoxantrone, paclitaxel, vinblastine, vincristine |
| MRP2 | Cisplatin, DOX, etoposide, irinotecan, methotrexate, SN-38, vinblastine, vincristine |
| MRP3 | Carboplatin, cisplatin, etoposide, methotrexate, teniposide |
| MRP4 | Methotrexate, topotecan |
| MRP5 | 5-Fluorouracil, methotrexate |
| MRP6 | Cisplatin, etoposide, teniposide |
| MRP7 | Docetaxel, paclitaxel, vinblastine, vincristine |
| MRP8 | 5-Fluorouracil |
| BCRP | Bisantrene, daunorubicin, DOX, epirubicin, etoposide, gefitinib, imatinib, irinotecan, methotrexate, mitoxantrone, SN-38, teniposide, topotecan |
9.2. Strategies to overcome efflux
A major impediment in the effective delivery of anticancer agents is efflux. Hence developing strategies to circumvent efflux proteins are indispensable.
9.2.1. Pharmacological inhibition of efflux proteins
Co-administration of appropriate chemical agents that can reverse the action of efflux proteins either by competitive or non-competitive binding may be a smart strategy. The efflux modulators can efficiently prevent the activity of efflux proteins and consequently augment the permeability of desired drug molecules in the targeted tumor. A schematic of this mechanism is depicted in Fig. 5.
Fig. 5.
Mechanism of action of combination therapy: efflux pump modulator upon co-administration with anticancer agents- substrates of MDR efflux transporters
(a) First generation MDR modulators: Clinically approved, Ca2+ channel blocker verapamil can enhance cellular accumulation of vincristine in P-gp overexpressing cell lines [343, 344]. Though these first generation inhibitors are potent for MDR reversal in vitro, these compounds require very high doses to inhibit drug efflux pumps in human causing severe toxicity [345].
(b) Second generation MDR modulators: In order to circumvent the complications and toxicities associated with the first generation modulators, MDR modulators with lower toxicities were developed. Structural analogues of verapamil and cyclosporine A, such as dexverapamil and PSC-833 (valspodar) respectively are generated as second-generation of MDR modulators [346, 347]. These compounds alter the pharmacokinetic properties of co-administered anticancer drugs, such as paclitaxel, and DOX [348, 349] along with nonspecific interactions with CYP enzymes and other resistance proteins such as P-gp [350]. Often a consequence of these changes is associated with dose adjustment of co-administered anticancer drugs, which may otherwise adversely affect the outcome of therapy.
(c) Third generation MDR modulators: These agents are designed to overcome the limitations of the earlier modulators. Zosuquidar [351], tariquidar [352] and elacridar [353] are the MDR inhibitors that have shown potential in pre-clinical mouse models and in the clinic. These agents do not cause pharmacokinetic interactions with other therapeutic agents and drug metabolizing (CYP-450) enzymes. Preclinical studies indicate MDR-bearing human tumour reduction in mice and prolonged survival rate [354]. No alterations in pharmacokinetic parameters of anticancer drugs such as DOX, etoposide, daunorubicin, vincristine and paclitaxel, have been reported upon co-administration. Several Phase I and phase II studies were conducted with co-administration of zosuquidar with docetaxel and duanorubicin and DOX for the treatment of breast cancer, leukaemia and non-Hodgkin’s lymphoma [355–358]. The results indicate clinical improvements. Several tyrosine kinase inhibitors (TKI) and other kinase inhibitors are being developed as MDR modulators. Quantitative structure-activity relationship to overcome the problems associated with first and second generation modulators are ongoing. TKI modulators (imatinib, nilotinib, ponatinib, icotinib, laptinib, erlotinib, canertinib, telatib, sunitinib, and vandetanib) are very effective at nanomolar concentrations and clinically relevant for cancer therapy [359].
9.3. Metabolizing enzymes
Apart from MDR, metabolizing enzymes such as cytochrome P450 (CYP450) are also induced by anticancer drugs causing rapid biotransformation resulting in treatment failure. Several chemotherapeutic agents require enzymatic activation to exert cytotoxic effects. For example, a nucleoside drug cytarabine (indicated for leukemia) requires phosphorylation by deoxycytidine kinase to active cytarabine triphosphate. To reduce the drug effect, cancer cells acquire resistance by lowering drug activation. Such resistance develops via down regulation/mutation of involved metabolic enzyme [360, 361].
Two main approaches have been explored so far to overcome efflux pumps and metabolizing enzymes. These include either co-administering an anticancer agent with an efflux modulator or employing anticancer agents which are not substrates of these efflux transporters. Unfortunately there are no modulators, so far identified, which can act at molecular level to prevent activation of MDR gene and metabolizing enzymes simultaneously. A novel common modulator which can inhibit the resistance mechanisms (activation of efflux transporters and metabolizing enzymes) needs to be explored for successful chemotherapy. Table 5 depicts representative chemotherapeutic agents which are metabolized by CYP450.
Table 5.
Chemotherapeutic agents metabolized by different isoforms of CYP450
| CYP450 isoforms | Chemotherapeutic substrates |
|---|---|
| 1A1 | Dtx, erlotinib, tamoxifen |
| 1A2 | Erlotinib, etoposide, flutamide, imatinib, tamoxifen |
| 2B6 | Cyclophosphamide, ifosfamide, tamoxifen |
| 2C9 | Cyclophosphamide, ifosfamide, imatinib, tamoxifen |
| 2C19 | Cyclophosphamide, ifosfamide, imatinib, tamoxifen, teniposide |
| 2D6 | Imatinib, tamoxifen, vinorelbine |
| 2E1 | Cisplatin, etoposide, tamoxifen, vinorelbine |
| 3A4/5 | Cisplatin, cyclophosphamide, cytarabine, dexamethasone, Dtx, DOX, erlotinib, etoposide, exemestane, flutamide, fulvestrant, gefitinib, ifosfamide, imatinib, irinotecan, letrozole, medroxyprogresterone acetate, mitoxantrone, paclitaxel, tamoxifen, targretin, teniposide, topotecan, vinblastine, vincristine |
9.4. Ritonavir and reversal of chemo-resistance
Our laboratory has examined the strategy of combining ritonavir-a retroviral protease inhibitors with anticancer drugs to control drug efflux, and metabolism thereby allowing sufficient drug entry into tumor cells [342]. Vinblastine mediated induction of efflux transporters (MDR1, MRP2), metabolizing enzyme (CYP3A4) and nuclear receptor (PXR) is reversed in the presence of ritonavir in human colon adenocarcinoma cells (LS-180). Vinblastine induced overexpression of these genes completely deactivated co-treated cells with ritonavir. Uptake of [3H] Lopinavir and VIVID™ assay further confirmed the functional activity of transcribed genes upon co-treatment. When any one of the anticancer agents (DOX, paclitaxel, tamoxifen and vinblstine) was combined with ritonavir, a significantly diminished cell proliferation, cell migration and augmented caspase activity are observed. This process leads to enhanced apoptosis of human breast adenocarcinoma cells (T47D) and prostate cancer cells (PC-3) (Tables 6 and 7). These results indicate enhanced activity of chemotherapeutics in the presence of ritonavir. In summary, combination therapy of anticancer drug with ritonavir may overcome drug resistance by deactivating the overexpression of efflux transporters and metabolizing enzymes and reprograming cell death. Therefore, drug regimens containing ritonavir may enhance therapeutic exposure of cancer cells to anticancer agents, potentially improving chemotherapeutic efficacy with lower resistance development.
Table 6.
Summary of activity changes (folds) of chemotherapeutics in presence of ritonavir in T47D cells
| Drug | Cell Proliferation (IC50) 
|
Migration 
|
Apoptosis 
|
|---|---|---|---|
| Doxorubicin | 2.86 | 2.08 | 1.49 |
| Paclitaxel | 3.66 | 2.08 | 1.53 |
| Tamoxifen | 3.18 | 1.41 | 1.41 |
| Vinblastin | 7.71 | 1.52 | 1.78 |
Table 7.
Summary of activity changes (folds) of chemotherapeutics in presence of ritonavir in PC-3 cells
| Drug | Cell Proliferation (IC50) 
|
Migration 
|
Apoptosis 
|
|---|---|---|---|
| Doxorubicin | 3.91 | 2.96 | 2.79 |
| Paclitaxel | 4.32 | 3.63 | 2.01 |
| Tamoxifen | 2.98 | 2.18 | 2.47 |
| Vinblastin | 3.17 | 2.51 | 3.17 |
10. CONCLUSION
Cancer has emerged as a leading cause of death worldwide. Although conventional chemotherapy has been the keystone to combat cancer, it is associated with normal cell toxicity. Due to lack of specificity, conventional cancer treatments often cause severe side effects and toxicities. Severity of cancer makes it imperative to develop novel approaches to treat such diseases. Current challenges of anticancer drug development include the site specific delivery with low systemic toxicity. An ebbing tumor represents a dynamic environment with changes in its angiogenic status, cell mass, and extracellular matrix composition, among other factors. With recent advancements and progress in drug delivery approaches, preventive interventions are being identified. New chemopreventive agents may be delivered through novel cell targeting approaches. Focus of such novel approaches is based on cancer treatment with innovative methods. Consequently, these promising technologies offer new opportunities for cancer prevention and treatment with minimal/lower toxicity to normal cells which can be realized in the clinic in the very near future. In this review, we discussed various novel approaches such as ligand and receptor based targeting, triggered release methods, gene delivery, prodrug approach and analogue/chemical conjugation to treat cancer cells. This comprehensive review paper has discussed many recent approaches such as intracellular drug targeting, cell penetrating peptides, aptamer based targeting, cancer stem cells therapy, cancer immunotherapy, magnetic drug targeting and ultrasound-mediated drug therapy to deliver cancer therapeutics. Such approaches have been investigated to overcome the limitations of conventional therapy. This article covers various aspects of multidrug resistance (MDR), metabolizing enzymes and consequently response to chemotherapy.
AKNOWLEDGMENTS
This article has been supported by CTSA Grant from NCI through University of Kansas Cancer Center and NIH grant R01 AI071199.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Authors do not have any conflict of interest.
REFERENCES
- 1.Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, Liang XJ. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnology advances. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 2.de la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Advanced drug delivery reviews. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics. 2014, CA: a cancer journal for clinicians. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Talekar M, Tran TH, Amiji M. Translational Nano-Medicines: Targeted Therapeutic Delivery for Cancer and Inflammatory Diseases. The AAPS journal. 2015;17:813–827. doi: 10.1208/s12248-015-9772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MD, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SEER Training Modules, Cancer Registration & Surveillance Modules. U. S. National Institutes of Health; 2015. [Google Scholar]
- 8.Portenoy RK. Postherpetic neuralgia: a workable treatment plan. Geriatrics. 1986;41:34–36. 41-33, 47-38. [PubMed] [Google Scholar]
- 9.Youm I, Agrahari V, Murowchick JB, Youan BB. Uptake and cytotoxicity of docetaxel-loaded hyaluronic acid-grafted oily core nanocapsules in MDA-MB 231 cancer cells. Pharmaceutical research. 2014;31:2439–2452. doi: 10.1007/s11095-014-1339-x. [DOI] [PubMed] [Google Scholar]
- 10.Traynor K. Ado-trastuzumab emtansine approved for advanced breast cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70:562. doi: 10.2146/news130023. [DOI] [PubMed] [Google Scholar]
- 11.Goncalves A, Tredan O, Villanueva C, Dumontet C. [Antibody-drug conjugates in oncology: from the concept to trastuzumab emtansine (T-DM1)] Bulletin du cancer. 2012;99:1183–1191. doi: 10.1684/bdc.2012.1669. [DOI] [PubMed] [Google Scholar]
- 12.Teicher BA, Doroshow JH. The promise of antibody-drug conjugates. The New England journal of medicine. 2012;367:1847–1848. doi: 10.1056/NEJMe1211736. [DOI] [PubMed] [Google Scholar]
- 13.Ansell SM. Brentuximab vedotin. Blood. 2014;124:3197–3200. doi: 10.1182/blood-2014-06-537514. [DOI] [PubMed] [Google Scholar]
- 14.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Yang Y, Sievers EL, Kennedy DA, Shustov A. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, Spitzer G, Palanca-Wessels MC, Kennedy DA, Levine P, Yang J, Bartlett NL. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125:1394–1402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- 16.Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annual review of medicine. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 17.Bunka DH, Stockley PG. Aptamers come of age - at last. Nature reviews. Microbiology. 2006;4:588–596. doi: 10.1038/nrmicro1458. [DOI] [PubMed] [Google Scholar]
- 18.Lao YH, Phua KK, Leong KW. Aptamer nanomedicine for cancer therapeutics: barriers and potential for translation. ACS nano. 2015;9:2235–2254. doi: 10.1021/nn507494p. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Dai A, Sun J, Li X, Gao F, Wu L, Fang Y, Yang H, An L, Wu H, Yang S. Aptamer-conjugated Mn3O4@SiO2 core-shell nanoprobes for targeted magnetic resonance imaging. Nanoscale. 2013;5:10447–10454. doi: 10.1039/c3nr03490a. [DOI] [PubMed] [Google Scholar]
- 20.Radom F, Jurek PM, Mazurek MP, Otlewski J, Jelen F. Aptamers: molecules of great potential. Biotechnology advances. 2013;31:1260–1274. doi: 10.1016/j.biotechadv.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Meng L, Yang L, Zhao X, Zhang L, Zhu H, Liu C, Tan W. Targeted delivery of chemotherapy agents using a liver cancer-specific aptamer. PloS one. 2012;7:e33434. doi: 10.1371/journal.pone.0033434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shangguan D, Meng L, Cao ZC, Xiao Z, Fang X, Li Y, Cardona D, Witek RP, Liu C, Tan W. Identification of liver cancer-specific aptamers using whole live cells. Analytical chemistry. 2008;80:721–728. doi: 10.1021/ac701962v. [DOI] [PubMed] [Google Scholar]
- 23.Cao HY, Yuan AH, Chen W, Shi XS, Miao Y. A DNA aptamer with high affinity and specificity for molecular recognition and targeting therapy of gastric cancer. BMC cancer. 2014;14:699. doi: 10.1186/1471-2407-14-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong HY, Byun J. Screening and characterization of a novel RNA aptamer that specifically binds to human prostatic acid phosphatase and human prostate cancer cells. Molecules and cells. 2015;38:171–179. doi: 10.14348/molcells.2015.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He XW, Liu T, Chen YX, Cheng DJ, Li XR, Xiao Y, Feng YL. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer gene therapy. 2008;15:193–202. doi: 10.1038/sj.cgt.7701122. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Xu Z, Guo S, Zhang L, Sharma A, Robertson GP, Huang L. Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:1919–1929. doi: 10.1038/mt.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Hao Y, Li H, Zhao Y, Meng D, Li D, Shi J, Zhang H, Zhang Z, Zhang Y. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. Journal of drug targeting. 2015:1–15. doi: 10.3109/1061186X.2015.1025077. [DOI] [PubMed] [Google Scholar]
- 28.Jacoby DB, Dyskin E, Yalcin M, Kesavan K, Dahlberg W, Ratliff J, Johnson EW, Mousa SA. Potent pleiotropic anti-angiogenic effects of TM601, a synthetic chlorotoxin peptide. Anticancer research. 2010;30:39–46. [PubMed] [Google Scholar]
- 29.Rjeibi I, Mabrouk K, Mosrati H, Berenguer C, Mejdoub H, Villard C, Laffitte D, Bertin D, Ouafik L, Luis J, Elayeb M, Srairi-Abid N. Purification, synthesis and characterization of AaCtx, the first chlorotoxin-like peptide from Androctonus australis scorpion venom. Peptides. 2011;32:656–663. doi: 10.1016/j.peptides.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 30.McGuire MJ, Gray BP, Li S, Cupka D, Byers LA, Wu L, Rezaie S, Liu YH, Pattisapu N, Issac J, Oyama T, Diao L, Heymach JV, Xie XJ, Minna JD, Brown KC. Identification and characterization of a suite of tumor targeting peptides for non-small cell lung cancer. Scientific reports. 2014;4:4480. doi: 10.1038/srep04480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XY, Lu WY. Recent advances in lymphatic targeted drug delivery system for tumor metastasis. Cancer biology & medicine. 2014;11:247–254. doi: 10.7497/j.issn.2095-3941.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Advanced drug delivery reviews. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. Journal of controlled release : official journal of the Controlled Release Society. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Noberini R, Stebbins JL, Das S, Zhang Z, Wu B, Mitra S, Billet S, Fernandez A, Bhowmick NA, Kitada S, Pasquale EB, Fisher PB, Pellecchia M. Targeted delivery of paclitaxel to EphA2-expressing cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:128–137. doi: 10.1158/1078-0432.CCR-12-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao W, Xiang B, Meng TT, Liu F, Qi XR. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials. 2013;34:4137–4149. doi: 10.1016/j.biomaterials.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Tarek PMF, Fahmy M, Amit Goyal, Mark Saltzman W. Targeted for drug delivery. Materials Today. 2005;8:18–26. [Google Scholar]
- 37.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular pharmaceutics. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano letters. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 39.Regberg J, Srimanee A, Langel U. Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals. 2012;5:991–1007. doi: 10.3390/ph5090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lelle M, Frick SU, Steinbrink K, Peneva K. Novel cleavable cell-penetrating peptide-drug conjugates: synthesis and characterization. Journal of peptide science : an official publication of the European Peptide Society. 2014;20:323–333. doi: 10.1002/psc.2617. [DOI] [PubMed] [Google Scholar]
- 41.Soler M, Gonzalez-Bartulos M, Figueras E, Ribas X, Costas M, Massaguer A, Planas M, Feliu L. Enzyme-triggered delivery of chlorambucil from conjugates based on the cell-penetrating peptide BP16. Organic & biomolecular chemistry. 2015;13:1470–1480. doi: 10.1039/c4ob01875c. [DOI] [PubMed] [Google Scholar]
- 42.Alvisi G, Poon IK, Jans DA. Tumor-specific nuclear targeting: promises for anti-cancer therapy? Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2006;9:40–50. doi: 10.1016/j.drup.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Current medicinal chemistry. 2008;15:2648–2655. doi: 10.2174/092986708786242859. [DOI] [PubMed] [Google Scholar]
- 44.Sui M, Liu W, Shen Y. Nuclear drug delivery for cancer chemotherapy. Journal of controlled release : official journal of the Controlled Release Society. 2011;155:227–236. doi: 10.1016/j.jconrel.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 45.Wagstaff KM, Fan JY, De Jesus MA, Tremethick DJ, Jans DA. Efficient gene delivery using reconstituted chromatin enhanced for nuclear targeting. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:2232–2242. doi: 10.1096/fj.07-099911. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Martin C, Krapcho K, White R. Isolation and mapping of a polymorphic DNA sequence (pCMM8.1) on chromosome 1p [D1S63] Nucleic acids research. 1988;16:9370. doi: 10.1093/nar/16.19.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lahtinen U, Honsho M, Parton RG, Simons K, Verkade P. Involvement of caveolin-2 in caveolar biogenesis in MDCK cells. FEBS letters. 2003;538:85–88. doi: 10.1016/s0014-5793(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 48.Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochemical and biophysical research communications. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 49.Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008;9:435–443. doi: 10.1021/bm700535p. [DOI] [PubMed] [Google Scholar]
- 50.Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Molecular cell. 2004;16:319–330. doi: 10.1016/j.molcel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 51.Ryan KJ, Wente SR. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Current opinion in cell biology. 2000;12:361–371. doi: 10.1016/s0955-0674(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 52.Ribbeck K, Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. The EMBO journal. 2001;20:1320–1330. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohr D, Frey S, Fischer T, Guttler T, Gorlich D. Characterisation of the passive permeability barrier of nuclear pore complexes. The EMBO journal. 2009;28:2541–2553. doi: 10.1038/emboj.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei W, Ma GH, Hu G, Yu D, McLeish T, Su ZG, Shen ZY. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. Journal of the American Chemical Society. 2008;130:15808–15810. doi: 10.1021/ja8039585. [DOI] [PubMed] [Google Scholar]
- 55.Tagliazucchi M, Peleg O, Kroger M, Rabin Y, Szleifer I. Effect of charge, hydrophobicity, and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3363–3368. doi: 10.1073/pnas.1212909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 57.Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M, Altmann A, Haberkorn U, Mier W. The pharmacokinetics of cell-penetrating peptides. Molecular pharmaceutics. 2010;7:2224–2231. doi: 10.1021/mp100223d. [DOI] [PubMed] [Google Scholar]
- 58.Hoang B, Ekdawi SN, Reilly RM, Allen C. Active targeting of block copolymer micelles with trastuzumab Fab fragments and nuclear localization signal leads to increased tumor uptake and nuclear localization in HER2-overexpressing xenografts. Molecular pharmaceutics. 2013;10:4229–4241. doi: 10.1021/mp400315p. [DOI] [PubMed] [Google Scholar]
- 59.Yuan W, Kuai R, Ran R, Fu L, Yang Y, Qin Y, Liu Y, Tang J, Fu H, Zhang Q, Yuan M, Zhang Z, Gao F, He Q. Increased delivery of doxorubicin into tumor cells using extracellularly activated TAT functionalized liposomes: in vitro and in vivo study. Journal of biomedical nanotechnology. 2014;10:1563–1573. doi: 10.1166/jbn.2014.1837. [DOI] [PubMed] [Google Scholar]
- 60.Zhu JY, Lei Q, Yang B, Jia HZ, Qiu WX, Wang X, Zeng X, Zhuo RX, Feng J, Zhang XZ. Efficient nuclear drug translocation and improved drug efficacy mediated by acidity-responsive boronate-linked dextran/cholesterol nanoassembly. Biomaterials. 2015;52:281–290. doi: 10.1016/j.biomaterials.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 61.Swift LP, Rephaeli A, Nudelman A, Phillips DR, Cutts SM. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer research. 2006;66:4863–4871. doi: 10.1158/0008-5472.CAN-05-3410. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Wu C, Zhou X, Han T, Xin X, Wu J, Zhang J, Guo S. Enhancing cell nucleus accumulation and DNA cleavage activity of anti-cancer drug via graphene quantum dots. Scientific reports. 2013;3:2852. doi: 10.1038/srep02852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Misra R, Sahoo SK. Intracellular trafficking of nuclear localization signal conjugated nanoparticles for cancer therapy. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2010;39:152–163. doi: 10.1016/j.ejps.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Aronov O, Horowitz AT, Gabizon A, Fuertes MA, Perez JM, Gibson D. Nuclear localization signal-targeted poly(ethylene glycol) conjugates as potential carriers and nuclear localizing agents for carboplatin analogues. Bioconjugate chemistry. 2004;15:814–823. doi: 10.1021/bc0499331. [DOI] [PubMed] [Google Scholar]
- 65.Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. Journal of controlled release : official journal of the Controlled Release Society. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen Y, Zhou Z, Sui M, Tang J, Xu P, Van Kirk EA, Murdoch WJ, Fan M, Radosz M. Charge-reversal polyamidoamine dendrimer for cascade nuclear drug delivery. Nanomedicine. 2010;5:1205–1217. doi: 10.2217/nnm.10.86. [DOI] [PubMed] [Google Scholar]
- 67.Xu P, Van Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angewandte Chemie. 2007;46:4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- 68.Cao S, Cripps A, Wei MQ. New strategies for cancer gene therapy: progress and opportunities. Clinical and experimental pharmacology & physiology. 2010;37:108–114. doi: 10.1111/j.1440-1681.2009.05268.x. [DOI] [PubMed] [Google Scholar]
- 69.Alexandrova R. Experimental strategies in gene therapy of cancer. Journal of B.U.ON. : official journal of the Balkan Union of Oncology. 2009;14(Suppl 1):S23–S32. [PubMed] [Google Scholar]
- 70.Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biology direct. 2006;1:29. doi: 10.1186/1745-6150-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibraheem D, Elaissari A, Fessi H. Gene therapy and DNA delivery systems. International journal of pharmaceutics. 2014;459:70–83. doi: 10.1016/j.ijpharm.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 72.Castro MG, Candolfi M, Wilson TJ, Calinescu A, Paran C, Kamran N, Koschmann C, Moreno-Ayala MA, Assi H, Lowenstein PR. Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert opinion on biological therapy. 2014;14:1241–1257. doi: 10.1517/14712598.2014.915307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukazawa T, Matsuoka J, Yamatsuji T, Maeda Y, Durbin ML, Naomoto Y. Adenovirus-mediated cancer gene therapy and virotherapy (Review) International journal of molecular medicine. 2010;25:3–10. [PubMed] [Google Scholar]
- 74.Yao X, Yoshioka Y, Morishige T, Eto Y, Watanabe H, Okada Y, Mizuguchi H, Mukai Y, Okada N, Nakagawa S. Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis. Gene therapy. 2009;16:1395–1404. doi: 10.1038/gt.2009.95. [DOI] [PubMed] [Google Scholar]
- 75.Yao X, Yoshioka Y, Morishige T, Eto Y, Narimatsu S, Kawai Y, Mizuguchi H, Gao JQ, Mukai Y, Okada N, Nakagawa S. Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:1619–1625. doi: 10.1038/mt.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma G, Shimada H, Hiroshima K, Tada Y, Suzuki N, Tagawa M. Gene medicine for cancer treatment: commercially available medicine and accumulated clinical data in China. Drug design, development and therapy. 2009;2:115–122. doi: 10.2147/dddt.s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo J, Luo Y, Sun J, Zhou Y, Zhang Y, Yang X. Adeno-associated virus-mediated cancer gene therapy: current status. Cancer letters. 2015;356:347–356. doi: 10.1016/j.canlet.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiu TL, Peng CW, Wang MJ. Enhanced anti-glioblastoma activity of microglia by AAV2-mediated IL-12 through TRAIL and phagocytosis in vitro. Oncology reports. 2011;25:1373–1380. doi: 10.3892/or.2011.1213. [DOI] [PubMed] [Google Scholar]
- 79.Han T, Abdel-Motal UM, Chang DK, Sui J, Muvaffak A, Campbell J, Zhu Q, Kupper TS, Marasco WA. Human anti-CCR4 minibody gene transfer for the treatment of cutaneous T-cell lymphoma. PloS one. 2012;7:e44455. doi: 10.1371/journal.pone.0044455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan J, Zhang Q, Zhou J, Ma D, Xiao X, Wang DW. Recombinant adeno-associated virus encoding Epstein-Barr virus latent membrane proteins fused with heat shock protein as a potential vaccine for nasopharyngeal carcinoma. Molecular cancer therapeutics. 2009;8:2754–2761. doi: 10.1158/1535-7163.MCT-08-1176. [DOI] [PubMed] [Google Scholar]
- 81.He SS, Shi HS, Yin T, Li YX, Luo ST, Wu QJ, Lu L, Wei YQ, Yang L. AAV-mediated gene transfer of human pigment epithelium-derived factor inhibits Lewis lung carcinoma growth in mice. Oncology reports. 2012;27:1142–1148. doi: 10.3892/or.2012.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di L, Zhu Y, Jia J, Yu J, Song G, Zhang J, Che L, Yang H, Han Y, Ma B, Zhang C, Yuan Y, You M, Wan F, Wang X, Zhou X, Ren J. Clinical safety of induced CTL infusion through recombinant adeno-associated virus-transfected dendritic cell vaccination in Chinese cancer patients. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2012;14:675–681. doi: 10.1007/s12094-012-0854-7. [DOI] [PubMed] [Google Scholar]
- 83.Xie Y, Hicks MJ, Kaminsky SM, Moore MA, Crystal RG, Rafii A. AAV-mediated persistent bevacizumab therapy suppresses tumor growth of ovarian cancer. Gynecologic oncology. 2014;135:325–332. doi: 10.1016/j.ygyno.2014.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alam S, Bowser BS, Israr M, Conway MJ, Meyers C. Adeno-associated virus type 2 infection of nude mouse human breast cancer xenograft induces necrotic death and inhibits tumor growth. Cancer biology & therapy. 2014;15:1013–1028. doi: 10.4161/cbt.29172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yla-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1831–1832. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Passer BJ, Cheema T, Wu S, Wu CL, Rabkin SD, Martuza RL. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer gene therapy. 2013;20:17–24. doi: 10.1038/cgt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng WG, Li JJ, Hu P, Lei L, Wang JN, Liu RB. An oncolytic herpes simplex virus vector, G47Delta, synergizes with paclitaxel in the treatment of breast cancer. Oncology reports. 2013;29:2355–2361. doi: 10.3892/or.2013.2359. [DOI] [PubMed] [Google Scholar]
- 88.Goshima F, Esaki S, Luo C, Kamakura M, Kimura H, Nishiyama Y. Oncolytic viral therapy with a combination of HF10, a herpes simplex virus type 1 variant and granulocyte-macrophage colony-stimulating factor for murine ovarian cancer, International journal of cancer. Journal international du cancer. 2014;134:2865–2877. doi: 10.1002/ijc.28631. [DOI] [PubMed] [Google Scholar]
- 89.Breckpot K, Aerts JL, Thielemans K. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene therapy. 2007;14:847–862. doi: 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- 90.Wang W, Zhu H, Zhang H, Zhang L, Ding Q, Jiang H. Targeting PPM1D by lentivirus-mediated RNA interference inhibits the tumorigenicity of bladder cancer cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.] 2014;47:1044–1049. doi: 10.1590/1414-431X20143645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang ZD, Li Y, Fan Q, Zhao B, Tan B, Zhao XF. Annexin A2 is implicated in multi-drug-resistance in gastric cancer through p38MAPK and AKT pathway. Neoplasma. 2014;61:627–637. doi: 10.4149/neo_2014_078. [DOI] [PubMed] [Google Scholar]
- 92.Zhang C, Chen Y, Wang M, Chen X, Li Y, Song E, Liu X, Kim S, Peng H. PPM1D silencing by RNA interference inhibits the proliferation of lung cancer cells. World journal of surgical oncology. 2014;12:258. doi: 10.1186/1477-7819-12-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin H, Yan Z, Liang Y, Liu B, Su Q. Knockdown of protein phosphatase magnesium-dependent 1 (PPM1D) through lentivirus-mediated RNA silencing inhibits colorectal carcinoma cell proliferation. Technology in cancer research & treatment. 2013;12:537–543. doi: 10.7785/tcrt.2012.500349. [DOI] [PubMed] [Google Scholar]
- 94.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nature reviews. Genetics. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 95.Guo X, Huang L. Recent advances in nonviral vectors for gene delivery. Accounts of chemical research. 2012;45:971–979. doi: 10.1021/ar200151m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fioretti D, Iurescia S, Rinaldi M. Recent advances in design of immunogenic and effective naked DNA vaccines against cancer. Recent patents on anti-cancer drug discovery. 2014;9:66–82. doi: 10.2174/1574891x113089990037. [DOI] [PubMed] [Google Scholar]
- 97.Pharmaceuticals A. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2015 July 13]. Phase 2 Study of ALN-RSV01 in Lung Transplant Patients Infected With Respiratory Syncytial Virus (RSV) [Google Scholar]
- 98.Pharmaceuticals Q. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2015 July 13]. I5NP for Prophylaxis of Delayed Graft Function in Kidney Transplantation. [Google Scholar]
- 99.Mazda O, Kishida T. Molecular therapeutics of cancer by means of electroporation-based transfer of siRNAs and EBV-based expression vectors. Frontiers in bioscience. 2009;1:316–331. doi: 10.2741/E31. [DOI] [PubMed] [Google Scholar]
- 100.Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annual review of biomedical engineering. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]
- 101.Heller R, Heller LC. Gene electrotransfer clinical trials. Advances in genetics. 2015;89:235–262. doi: 10.1016/bs.adgen.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 102.Bosnjak M, Prosen L, Dolinsek T, Blagus T, Markelc B, Cemazar M, Bouquet C, Sersa G. Biological properties of melanoma and endothelial cells after plasmid AMEP gene electrotransfer depend on integrin quantity on cells. The Journal of membrane biology. 2013;246:803–819. doi: 10.1007/s00232-013-9550-y. [DOI] [PubMed] [Google Scholar]
- 103.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cha E, Daud A. Plasmid IL-12 electroporation in melanoma. Human vaccines & immunotherapeutics. 2012;8:1734–1738. doi: 10.4161/hv.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun Y, Peng S, Qiu J, Miao J, Yang B, Jeang J, Hung CF, Wu TC. Intravaginal HPV DNA vaccination with electroporation induces local CD8+ T-cell immune responses and antitumor effects against cervicovaginal tumors. Gene therapy. 2015;22:528–535. doi: 10.1038/gt.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonamassa B, Hai L, Liu D. Hydrodynamic gene delivery and its applications in pharmaceutical research. Pharmaceutical research. 2011;28:694–701. doi: 10.1007/s11095-010-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene therapy. 2007;14:99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- 108.Yazawa H, Murakami T, Li HM, Back T, Kurosaka K, Suzuki Y, Shorts L, Akiyama Y, Maruyama K, Parsoneault E, Wiltrout RH, Watanabe M. Hydrodynamics-based gene delivery of naked DNA encoding fetal liver kinase-1 gene effectively suppresses the growth of pre-existing tumors. Cancer gene therapy. 2006;13:993–1001. doi: 10.1038/sj.cgt.7700970. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Li H, Sun J, Gao J, Liu W, Li B, Guo Y, Chen J. DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. International journal of pharmaceutics. 2010;390:198–207. doi: 10.1016/j.ijpharm.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 110.Qin F, Zhou Y, Shi J, Zhang Y. A DNA transporter based on mesoporous silica nanospheres mediated with polycation poly(allylamine hydrochloride) coating on mesopore surface. Journal of biomedical materials research. Part A. 2009;90:333–338. doi: 10.1002/jbm.a.31923. [DOI] [PubMed] [Google Scholar]
- 111.Qureshi HY, Ahmad R, Zafarullah M. High-efficiency transfection of nucleic acids by the modified calcium phosphate precipitation method in chondrocytes. Analytical biochemistry. 2008;382:138–140. doi: 10.1016/j.ab.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 112.Lu W, Zhang G, Zhang R, Flores LG, 2nd, Huang Q, Gelovani JG, Li C. Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer research. 2010;70:3177–3188. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saccardo P, Villaverde A, Gonzalez-Montalban N. Peptide-mediated DNA condensation for non-viral gene therapy. Biotechnology advances. 2009;27:432–438. doi: 10.1016/j.biotechadv.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 114.Fang S, Wu L, Li M, Yi H, Gao G, Sheng Z, Gong P, Ma Y, Cai L. ZEB1 knockdown mediated using polypeptide cationic micelles inhibits metastasis and effects sensitization to a chemotherapeutic drug for cancer therapy. Nanoscale. 2014;6:10084–10094. doi: 10.1039/c4nr01518e. [DOI] [PubMed] [Google Scholar]
- 115.Ikegami S, Yamakami K, Ono T, Sato M, Suzuki S, Yoshimura I, Asano T, Hayakawa M, Tadakuma T. Targeting gene therapy for prostate cancer cells by liposomes complexed with anti-prostate-specific membrane antigen monoclonal antibody. Human gene therapy. 2006;17:997–1005. doi: 10.1089/hum.2006.17.997. [DOI] [PubMed] [Google Scholar]
- 116.Costa PM, Cardoso AL, Mendonca LS, Serani A, Custodia C, Conceicao M, Simoes S, Moreira JN, Pereira de Almeida L, Pedroso de Lima MC. Tumor-targeted Chlorotoxin-coupled Nanoparticles for Nucleic Acid Delivery to Glioblastoma Cells: A Promising System for Glioblastoma Treatment. Molecular therapy. Nucleic acids. 2013;2:e100. doi: 10.1038/mtna.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Costa PM, Cardoso AL, Custodia C, Cunha P, Pereira de Almeida L, Pedroso de Lima MC. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. Journal of controlled release : official journal of the Controlled Release Society. 2015;207:31–39. doi: 10.1016/j.jconrel.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 118.Chen Y, Wang X, Liu T, Zhang DS, Wang Y, Gu H, Di W. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. International journal of nanomedicine. 2015;10:2579–2594. doi: 10.2147/IJN.S78774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y, Gu H, Zhang DS, Li F, Liu T, Xia W. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials. 2014;35:10058–10069. doi: 10.1016/j.biomaterials.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Yang H, Li Y, Li T, Xu M, Chen Y, Wu C, Dang X, Liu Y. Multifunctional core/shell nanoparticles cross-linked polyetherimide-folic acid as efficient Notch-1 siRNA carrier for targeted killing of breast cancer. Scientific reports. 2014;4:7072. doi: 10.1038/srep07072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ekin A, Karatas OF, Culha M, Ozen M. Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. The journal of gene medicine. 2014;16:331–335. doi: 10.1002/jgm.2810. [DOI] [PubMed] [Google Scholar]
- 122.Kim HJ, Takemoto H, Yi Y, Zheng M, Maeda Y, Chaya H, Hayashi K, Mi P, Pittella F, Christie RJ, Toh K, Matsumoto Y, Nishiyama N, Miyata K, Kataoka K. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS nano. 2014;8:8979–8991. doi: 10.1021/nn502125h. [DOI] [PubMed] [Google Scholar]
- 123.Mitra M, Kandalam M, Rangasamy J, Shankar B, Maheswari UK, Swaminathan S, Krishnakumar S. Novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold nanoparticles for enhanced and targeted small interfering RNA delivery to retinoblastoma cells. Molecular vision. 2013;19:1029–1038. [PMC free article] [PubMed] [Google Scholar]
- 124.Ki MH, Kim JE, Lee YN, Noh SM, An SW, Cho HJ, Kim DD. Chitosan-based hybrid nanocomplex for siRNA delivery and its application for cancer therapy. Pharmaceutical research. 2014;31:3323–3334. doi: 10.1007/s11095-014-1422-3. [DOI] [PubMed] [Google Scholar]
- 125.Li TS, Yawata T, Honke K. Efficient siRNA delivery and tumor accumulation mediated by ionically cross-linked folic acid-poly(ethylene glycol)-chitosan oligosaccharide lactate nanoparticles: for the potential targeted ovarian cancer gene therapy. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2014;52:48–61. doi: 10.1016/j.ejps.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 126.Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta pharmacologica Sinica. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kunnimalaiyaan S, Trevino J, Tsai S, Gamblin TC, Kunnimalaiyaan M. Xanthohumol-Mediated Suppression of Notch1 Signaling Is Associated with Antitumor Activity in Human Pancreatic Cancer Cells. Molecular cancer therapeutics. 2015;14:1395–1403. doi: 10.1158/1535-7163.MCT-14-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ihle MA, Trautmann M, Kuenstlinger H, Huss S, Heydt C, Fassunke J, Wardelmann E, Bauer S, Schildhaus HU, Buettner R, Merkelbach-Bruse S. miRNA-221 and miRNA-222 induce apoptosis via the KIT/AKT signalling pathway in gastrointestinal stromal tumours. Molecular oncology. 2015 doi: 10.1016/j.molonc.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ho-Yen CM, Jones JL, Kermorgant S. The clinical and functional significance of c-Met in breast cancer: a review. Breast cancer research : BCR. 2015;17:52. doi: 10.1186/s13058-015-0547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brinkhof B, van Tol HT, Groot Koerkamp MJ, Riemers FM, SG IJ, Mashayekhi K, Haagsman HP, Roelen BA. A mRNA landscape of bovine embryos after standard and MAPK-inhibited culture conditions: a comparative analysis. BMC genomics. 2015;16:277. doi: 10.1186/s12864-015-1448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zoni E, van der Pluijm G, Gray PC, Kruithof-de Julio M. Epithelial Plasticity in Cancer: Unmasking a MicroRNA Network for TGF-beta-, Notch-, and Wnt-Mediated EMT. Journal of oncology. 2015;2015:198967. doi: 10.1155/2015/198967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noort AR, van Zoest KP, Weijers EM, Koolwijk P, Maracle CX, Novack DV, Siemerink MJ, Schlingemann RO, Tak PP, Tas SW. NF-kappaB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. The Journal of pathology. 2014;234:375–385. doi: 10.1002/path.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature reviews. Clinical oncology. 2015 doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan AN, Kolomeyevskaya N, Singel KL, Grimm MJ, Moysich KB, Daudi S, Grzankowski KS, Lele S, Ylagan L, Webster GA, Abrams SI, Odunsi K, Segal BH. Targeting myeloid cells in the tumor microenvironment enhances vaccine efficacy in murine epithelial ovarian cancer. Oncotarget. 2015;6:11310–11326. doi: 10.18632/oncotarget.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Msaouel P, Galeas JN, Boiles AR, Ruiz RR, Koutsilieris M. Targeting the Bone Microenvironment in Metastatic Castration-Resistant Prostate Cancer. Current drug targets. 2015 doi: 10.2174/1389450116666150420143932. [DOI] [PubMed] [Google Scholar]
- 137.Wang Z, Li Z, Wang Y, Cao D, Wang X, Jiang M, Li M, Yan X, Li Y, Liu Y, Luo F. Versican silencing improves the antitumor efficacy of endostatin by alleviating its induced inflammatory and immunosuppressive changes in the tumor microenvironment. Oncology reports. 2015;33:2981–2991. doi: 10.3892/or.2015.3903. [DOI] [PubMed] [Google Scholar]
- 138.Kashyap MK, Kumar D, Villa R, La Clair JJ, Benner C, Sasik R, Jones H, Ghia EM, Rassenti LZ, Kipps TJ, Burkart MD, Castro JE. Targeting the spliceosome in chronic lymphocytic leukemia with the macrolides FD-895 and pladienolide-B. Haematologica. 2015;100:945–954. doi: 10.3324/haematol.2014.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martner A, Wiktorin HG, Lenox B, Ewald Sander F, Aydin E, Aurelius J, Thoren FB, Stahlberg A, Hermodsson S, Hellstrand K. Histamine promotes the development of monocyte-derived dendritic cells and reduces tumor growth by targeting the myeloid NADPH oxidase. Journal of immunology. 2015;194:5014–5021. doi: 10.4049/jimmunol.1402991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nature reviews. Clinical oncology. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 142.Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnology journal. 2006;1:138–147. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- 143.Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, Sanmamed MF, Melero I. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Current opinion in immunology. 2014;27:89–97. doi: 10.1016/j.coi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 144.Hoffman AS. “Intelligent” polymers in medicine and biotechnology. Artificial organs. 1995;19:458–467. doi: 10.1111/j.1525-1594.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 145.Xu D, Wang L, Gourevich D, Kabha E, Arditti F, Athamna M, Cochran S, Melzer A, Gnaim JM. Synthesis and inclusion study of a novel gamma-cyclodextrin derivative as a potential thermo-sensitive carrier for doxorubicin. Chemical & pharmaceutical bulletin. 2014;62:627–635. doi: 10.1248/cpb.c13-00950. [DOI] [PubMed] [Google Scholar]
- 146.Wadajkar AS, Bhavsar Z, Ko CY, Koppolu B, Cui W, Tang L, Nguyen KT. Multifunctional particles for melanoma-targeted drug delivery. Acta biomaterialia. 2012;8:2996–3004. doi: 10.1016/j.actbio.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim DH, Guo Y, Zhang Z, Procissi D, Nicolai J, Omary RA, Larson AC. Temperature-sensitive magnetic drug carriers for concurrent gemcitabine chemohyperthermia. Advanced healthcare materials. 2014;3:714–724. doi: 10.1002/adhm.201300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Agrahari V, Zhang C, Zhang T, Li W, Gounev TK, Oyler NA, Youan BB. Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro. The AAPS journal. 2014;16:181–193. doi: 10.1208/s12248-013-9546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Progress in lipid research. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 150.Minelli C, Lowe SB, Stevens MM. Engineering nanocomposite materials for cancer therapy. Small. 2010;6:2336–2357. doi: 10.1002/smll.201000523. [DOI] [PubMed] [Google Scholar]
- 151.Nazli C, Demirer GS, Yar Y, Acar HY, Kizilel S. Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs), Colloids and surfaces. B. Biointerfaces. 2014;122:674–683. doi: 10.1016/j.colsurfb.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 152.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD, Extracellular pH distribution in human tumours. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology. North American Hyperthermia Group. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 153.Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Molecular medicine today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 154.Stuart MA, Huck WT, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nature materials. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 155.Liu J, Ma H, Wei T, Liang XJ. CO2 gas induced drug release from pH-sensitive liposome to circumvent doxorubicin resistant cells. Chemical communications. 2012;48:4869–4871. doi: 10.1039/c2cc31697h. [DOI] [PubMed] [Google Scholar]
- 156.Han Y, Chen W, Kuang Y, Sun H, Wang Z, Peng X. UV-Induced DNA Interstrand Cross-Linking and Direct Strand Breaks from a New Type of Binitroimidazole Analogue. Chemical research in toxicology. 2015;28:919–926. doi: 10.1021/tx500522r. [DOI] [PubMed] [Google Scholar]
- 157.Skarbek C, Lesueur LL, Chapuis H, Deroussent A, Pioche Durieu C, Daville A, Caron J, Rivard M, Martens T, Bertrand JR, Le Cam E, Vassal G, Couvreur P, Desmaele D, Paci A. Preactivated oxazaphosphorines designed for isophosphoramide mustard delivery as bulk form or nanoassemblies: synthesis and proof of concept. Journal of medicinal chemistry. 2015;58:705–717. doi: 10.1021/jm501224x. [DOI] [PubMed] [Google Scholar]
- 158.Johnson KM, Parsons ZD, Barnes CL, Gates KS. Toward hypoxia-selective DNA-alkylating agents built by grafting nitrogen mustards onto the bioreductively activated, hypoxia-selective DNA-oxidizing agent 3-amino-1,2,4-benzotriazine 1,4-dioxide (tirapazamine) The Journal of organic chemistry. 2014;79:7520–7531. doi: 10.1021/jo501252p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen W, Balakrishnan K, Kuang Y, Han Y, Fu M, Gandhi V, Peng X. Reactive oxygen species (ROS) inducible DNA cross-linking agents and their effect on cancer cells and normal lymphocytes. Journal of medicinal chemistry. 2014;57:4498–4510. doi: 10.1021/jm401349g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cao S, Wang Y, Peng X. The leaving group strongly affects H(2)O(2)-induced DNA cross-linking by arylboronates. The Journal of organic chemistry. 2014;79:501–508. doi: 10.1021/jo401901x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cao S, Christiansen R, Peng X. Substituent effects on oxidation-induced formation of quinone methides from arylboronic ester precursors. Chemistry. 2013;19:9050–9058. doi: 10.1002/chem.201300539. [DOI] [PubMed] [Google Scholar]
- 162.Strese S, Wickstrom M, Fuchs PF, Fryknas M, Gerwins P, Dale T, Larsson R, Gullbo J. The novel alkylating prodrug melflufen (J1) inhibits angiogenesis in vitro and in vivo. Biochemical pharmacology. 2013;86:888–895. doi: 10.1016/j.bcp.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 163.Cytarska J, Misiura K, Filip-Psurska B, Wietrzyk J. Acyloxymethyl esters of isophosphoramide mustard as new anticancer prodrugs. Acta poloniae pharmaceutica. 2013;70:481–487. [PubMed] [Google Scholar]
- 164.Wolfe AL, Duncan KK, Parelkar NK, Brown D, Vielhauer GA, Boger DL. Efficacious cyclic N-acyl O-amino phenol duocarmycin prodrugs. Journal of medicinal chemistry. 2013;56:4104–4115. doi: 10.1021/jm400413r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McCrane MP, Hutchinson MA, Ad O, Rokita SE. Oxidative quenching of quinone methide adducts reveals transient products of reversible alkylation in duplex DNA. Chemical research in toxicology. 2014;27:1282–1293. doi: 10.1021/tx500152d. [DOI] [PubMed] [Google Scholar]
- 166.Johnstone TC, Alexander SM, Wilson JJ, Lippard SJ. Oxidative halogenation of cisplatin and carboplatin: synthesis, spectroscopy, and crystal and molecular structures of Pt(IV) prodrugs. Dalton transactions. 2015;44:119–129. doi: 10.1039/c4dt02627f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zheng YR, Suntharalingam K, Johnstone TC, Yoo H, Lin W, Brooks JG, Lippard SJ. Pt(IV) prodrugs designed to bind non-covalently to human serum albumin for drug delivery. Journal of the American Chemical Society. 2014;136:8790–8798. doi: 10.1021/ja5038269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Novohradsky V, Zerzankova L, Stepankova J, Vrana O, Raveendran R, Gibson D, Kasparkova J, Brabec V. Antitumor platinum(IV) derivatives of oxaliplatin with axial valproato ligands. Journal of inorganic biochemistry. 2014;140:72–79. doi: 10.1016/j.jinorgbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 169.Neumann W, Crews BC, Sarosi MB, Daniel CM, Ghebreselasie K, Scholz MS, Marnett LJ, Hey-Hawkins E. Conjugation of cisplatin analogues and cyclooxygenase inhibitors to overcome cisplatin resistance. ChemMedChem. 2015;10:183–192. doi: 10.1002/cmdc.201402353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Neumann W, Crews BC, Marnett LJ, Hey-Hawkins E. Conjugates of cisplatin and cyclooxygenase inhibitors as potent antitumor agents overcoming cisplatin resistance. ChemMedChem. 2014;9:1150–1153. doi: 10.1002/cmdc.201402074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zanellato I, Bonarrigo I, Colangelo D, Gabano E, Ravera M, Alessio M, Osella D. Biological activity of a series of cisplatin-based aliphatic bis(carboxylato) Pt(IV) prodrugs: how long the organic chain should be? Journal of inorganic biochemistry. 2014;140:219–227. doi: 10.1016/j.jinorgbio.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 172.Xiao H, Noble GT, Stefanick JF, Qi R, Kiziltepe T, Jing X, Bilgicer B. Photosensitive Pt(IV)-azide prodrug-loaded nanoparticles exhibit controlled drug release and enhanced efficacy in vivo. Journal of controlled release : official journal of the Controlled Release Society. 2013 [PubMed] [Google Scholar]
- 173.Pathak RK, Marrache S, Choi JH, Berding TB, Dhar S. The prodrug platin-A: simultaneous release of cisplatin and aspirin. Angewandte Chemie. 2014;53:1963–1967. doi: 10.1002/anie.201308899. [DOI] [PubMed] [Google Scholar]
- 174.Min Y, Li J, Liu F, Yeow EK, Xing B. Near-infrared light-mediated photoactivation of a platinum antitumor prodrug and simultaneous cellular apoptosis imaging by upconversion-luminescent nanoparticles. Angewandte Chemie. 2014;53:1012–1016. doi: 10.1002/anie.201308834. [DOI] [PubMed] [Google Scholar]
- 175.Yang XZ, Du XJ, Liu Y, Zhu YH, Liu YZ, Li YP, Wang J. Rational design of polyion complex nanoparticles to overcome cisplatin resistance in cancer therapy. Advanced materials. 2014;26:931–936. doi: 10.1002/adma.201303360. [DOI] [PubMed] [Google Scholar]
- 176.Yang Y, Zhang YM, Chen Y, Chen JT, Liu Y. Targeted polysaccharide nanoparticle for adamplatin prodrug delivery. Journal of medicinal chemistry. 2013;56:9725–9736. doi: 10.1021/jm4014168. [DOI] [PubMed] [Google Scholar]
- 177.Yoong SL, Wong BS, Zhou QL, Chin CF, Li J, Venkatesan T, Ho HK, Yu V, Ang WH, Pastorin G. Enhanced cytotoxicity to cancer cells by mitochondria-targeting MWCNTs containing platinum(IV) prodrug of cisplatin. Biomaterials. 2014;35:748–759. doi: 10.1016/j.biomaterials.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 178.Hou J, Shang J, Jiao C, Jiang P, Xiao H, Luo L, Liu T. A core cross-linked polymeric micellar platium(IV) prodrug with enhanced anticancer efficiency. Macromolecular bioscience. 2013;13:954–965. doi: 10.1002/mabi.201300057. [DOI] [PubMed] [Google Scholar]
- 179.Johnstone TC, Wilson JJ, Lippard SJ. Monofunctional and higher-valent platinum anticancer agents. Inorganic chemistry. 2013;52:12234–12249. doi: 10.1021/ic400538c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao Y, Woods JA, Farrer NJ, Robinson KS, Pracharova J, Kasparkova J, Novakova O, Li H, Salassa L, Pizarro AM, Clarkson GJ, Song L, Brabec V, Sadler PJ. Diazido mixed-amine platinum(IV) anticancer complexes activatable by visible-light form novel DNA adducts. Chemistry. 2013;19:9578–9591. doi: 10.1002/chem.201300374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mi Y, Zhao J, Feng SS, Targeted co-delivery of docetaxel. cisplatin and herceptin by vitamin E TPGS-cisplatin prodrug nanoparticles for multimodality treatment of cancer. Journal of controlled release : official journal of the Controlled Release Society. 2013;169:185–192. doi: 10.1016/j.jconrel.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 182.Valiahdi SM, Egger AE, Miklos W, Jungwirth U, Meelich K, Nock P, Berger W, Hartinger CG, Galanski M, Jakupec MA, Keppler BK. Influence of extracellular pH on the cytotoxicity, cellular accumulation, and DNA interaction of novel pH-sensitive 2-aminoalcoholatoplatinum(II) complexes. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2013;18:249–260. doi: 10.1007/s00775-012-0970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fujimoto K, Takematsu YK, Shigeno A, Furusawa M, Sakamoto T. Short oligonucleotide prodrug having 5-fluoro and 5-iodouracil inhibits the proliferation of cancer cells in a photo-responsive manner. Bioorganic & medicinal chemistry letters. 2014;24:3736–3738. doi: 10.1016/j.bmcl.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 184.Weiss JT, Dawson JC, Fraser C, Rybski W, Torres-Sanchez C, Bradley M, Patton EE, Carragher NO, Unciti-Broceta A. Development and bioorthogonal activation of palladium-labile prodrugs of gemcitabine. Journal of medicinal chemistry. 2014;57:5395–5404. doi: 10.1021/jm500531z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui SX, Zhang HL, Xu WF, Qu XJ. 13F-1, a novel 5-fluorouracil prodrug containing an Asn-Gly-Arg (NO2) COOCH3 tripeptide, inhibits human colonic carcinoma growth by targeting Aminopeptidase N (APN/CD13) European journal of pharmacology. 2014;734:50–59. doi: 10.1016/j.ejphar.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 186.Baraniak J, Pietkiewicz A, Kaczmarek R, Radzikowska E, Kulik K, Krolewska K, Cieslak M, Krakowiak A, Nawrot B. N-Acyl-phosphoramidates as potential novel form of gemcitabine prodrugs. Bioorganic & medicinal chemistry. 2014;22:2133–2140. doi: 10.1016/j.bmc.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 187.Slusarczyk M, Lopez MH, Balzarini J, Mason M, Jiang WG, Blagden S, Thompson E, Ghazaly E, McGuigan C. Application of ProTide technology to gemcitabine: a successful approach to overcome the key cancer resistance mechanisms leads to a new agent (NUC-1031) in clinical development. Journal of medicinal chemistry. 2014;57:1531–1542. doi: 10.1021/jm401853a. [DOI] [PubMed] [Google Scholar]
- 188.Zhao J, Zhou R, Fu X, Ren W, Ma L, Li R, Zhao Y, Guo L. Cell-penetrable lysine dendrimers for anti-cancer drug delivery: synthesis and preliminary biological evaluation. Archiv der Pharmazie. 2014;347:469–477. doi: 10.1002/ardp.201300415. [DOI] [PubMed] [Google Scholar]
- 189.Bekkara-Aounallah F, Ambike A, Gref R, Couvreur P, Rosilio V. Interfacial behavior of PEGylated lipids and their effect on the stability of squalenoyl-drug nanoassemblies. International journal of pharmaceutics. 2014;471:75–82. doi: 10.1016/j.ijpharm.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 190.Daman Z, Ostad S, Amini M, Gilani K. Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: a comparison with its self-assembled nanoparticles. International journal of pharmaceutics. 2014;468:142–151. doi: 10.1016/j.ijpharm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 191.Kim S, Lee DJ, Kwag DS, Lee UY, Youn YS, Lee ES. Acid pH-activated glycol chitosan/fullerene nanogels for efficient tumor therapy. Carbohydrate polymers. 2014;101:692–698. doi: 10.1016/j.carbpol.2013.09.108. [DOI] [PubMed] [Google Scholar]
- 192.Ueki N, Lee S, Sampson NS, Hayman MJ. Selective cancer targeting with prodrugs activated by histone deacetylases and a tumour-associated protease. Nature communications. 2013;4:2735. doi: 10.1038/ncomms3735. [DOI] [PubMed] [Google Scholar]
- 193.Bui DT, Nicolas J, Maksimenko A, Desmaele D, Couvreur P. Multifunctional squalene-based prodrug nanoparticles for targeted cancer therapy. Chemical communications. 2014;50:5336–5338. doi: 10.1039/c3cc47427e. [DOI] [PubMed] [Google Scholar]
- 194.Jin Y, Yang F, Du L. Nanoassemblies containing a fluorouracil/zidovudine glyceryl prodrug with phospholipase A2-triggered drug release for cancer treatment, Colloids and surfaces. B. Biointerfaces. 2013;112:421–428. doi: 10.1016/j.colsurfb.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 195.Tsybulskaya I, Kulak T, Baranovsky A, Golubeva M, Kuzmitsky B, Kalinichenko E. Synthesis and in vitro cytostatic activity of 1,2- and 1,3-diacylglycerophosphates of clofarabine. Bioorganic & medicinal chemistry. 2013;21:5414–5419. doi: 10.1016/j.bmc.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 196.Lee BS, Cho YW, Kim GC, Lee do H, Kim CJ, Kil HS, Chi DY, Byun Y, Yuk SH, Kim K, Kim IS, Kwon IC, Kim SY. Induced phenotype targeted therapy: radiation-induced apoptosis-targeted chemotherapy. Journal of the National Cancer Institute. 2015;107 doi: 10.1093/jnci/dju403. [DOI] [PubMed] [Google Scholar]
- 197.Soudy R, Chen C, Kaur K. Novel peptide-doxorubucin conjugates for targeting breast cancer cells including the multidrug resistant cells. Journal of medicinal chemistry. 2013;56:7564–7573. doi: 10.1021/jm400647r. [DOI] [PubMed] [Google Scholar]
- 198.Tang L, Duan R, Zhong YJ, Firestone RA, Hong YP, Li JG, Xin YC, Wu HL, Li Y. Synthesis, identification and in vivo studies of tumor-targeting agent peptide doxorubicin (PDOX) to treat peritoneal carcinomatosis of gastric cancer with similar efficacy but reduced toxicity. Molecular cancer. 2014;13:44. doi: 10.1186/1476-4598-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tarasenko N, Cutts SM, Phillips DR, Berkovitch-Luria G, Bardugo-Nissim E, Weitman M, Nudelman A, Rephaeli A. A novel valproic acid prodrug as an anticancer agent that enhances doxorubicin anticancer activity and protects normal cells against its toxicity in vitro and in vivo. Biochemical pharmacology. 2014;88:158–168. doi: 10.1016/j.bcp.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 200.Duhem N, Danhier F, Pourcelle V, Schumers JM, Bertrand O, Leduff CS, Hoeppener S, Schubert US, Gohy JF, Marchand-Brynaert J, Preat V. Self-assembling doxorubicin-tocopherol succinate prodrug as a new drug delivery system: synthesis, characterization, and in vitro and in vivo anticancer activity. Bioconjugate chemistry. 2014;25:72–81. doi: 10.1021/bc400326y. [DOI] [PubMed] [Google Scholar]
- 201.Oommen OP, Garousi J, Sloff M, Varghese OP. Tailored doxorubicin-hyaluronan conjugate as a potent anticancer glyco-drug: an alternative to prodrug approach. Macromolecular bioscience. 2014;14:327–333. doi: 10.1002/mabi.201300383. [DOI] [PubMed] [Google Scholar]
- 202.Wang Q, Zhong YJ, Yuan JP, Shao LH, Zhang J, Tang L, Liu SP, Hong YP, Firestone RA, Li Y. Targeting therapy of hepatocellular carcinoma with doxorubicin prodrug PDOX increases anti-metastatic effect and reduces toxicity: a preclinical study. Journal of translational medicine. 2013;11:192. doi: 10.1186/1479-5876-11-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Samuelson LE, Scherer RL, Matrisian LM, McIntyre JO, Bornhop DJ. Synthesis and in vitro efficacy of MMP9-activated NanoDendrons. Molecular pharmaceutics. 2013;10:3164–3174. doi: 10.1021/mp4002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guo X, Shi C, Wang J, Di S, Zhou S. pH-triggered intracellular release from actively targeting polymer micelles. Biomaterials. 2013;34:4544–4554. doi: 10.1016/j.biomaterials.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 205.Nasrolahi Shirazi A, Tiwari R, Chhikara BS, Mandal D, Parang K. Design and biological evaluation of cell-penetrating peptide-doxorubicin conjugates as prodrugs. Molecular pharmaceutics. 2013;10:488–499. doi: 10.1021/mp3004034. [DOI] [PubMed] [Google Scholar]
- 206.Girotti AW, Minotti G. Development of a tumor-specific photoactivatable doxorubicin prodrug. Photochemistry and photobiology. 2013;89:1009–1010. doi: 10.1111/php.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang Y, Wang H, Chen Y, Liu X, Jin Q, Ji J. pH and hydrogen peroxide dual responsive supramolecular prodrug system for controlled release of bioactive molecules, Colloids and surfaces. B. Biointerfaces. 2014;121:189–195. doi: 10.1016/j.colsurfb.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 208.Ding J, Li D, Zhuang X, Chen X. Self-assemblies of pH-activatable PEGylated multiarm poly(lactic acid-co-glycolic acid)-doxorubicin prodrugs with improved long-term antitumor efficacies. Macromolecular bioscience. 2013;13:1300–1307. doi: 10.1002/mabi.201300160. [DOI] [PubMed] [Google Scholar]
- 209.Du C, Deng D, Shan L, Wan S, Cao J, Tian J, Achilefu S, Gu Y. A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials. 2013;34:3087–3097. doi: 10.1016/j.biomaterials.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 210.Shanmugam V, Chien YH, Cheng YS, Liu TY, Huang CC, Su CH, Chen YS, Kumar U, Hsu HF, Yeh CS. Oligonucleotides--assembled Au nanorod-assisted cancer photothermal ablation and combination chemotherapy with targeted dual-drug delivery of Doxorubicin and Cisplatin prodrug. ACS applied materials & interfaces. 2014;6:4382–4393. doi: 10.1021/am5000905. [DOI] [PubMed] [Google Scholar]
- 211.Zhang X, Achazi K, Steinhilber D, Kratz F, Dernedde J, Haag R. A facile approach for dual-responsive prodrug nanogels based on dendritic polyglycerols with minimal leaching. Journal of controlled release : official journal of the Controlled Release Society. 2014;174:209–216. doi: 10.1016/j.jconrel.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 212.Bhuniya S, Maiti S, Kim EJ, Lee H, Sessler JL, Hong KS, Kim JS. An activatable theranostic for targeted cancer therapy and imaging. Angewandte Chemie. 2014;53:4469–4474. doi: 10.1002/anie.201311133. [DOI] [PubMed] [Google Scholar]
- 213.Santi DV, Schneider EL, Ashley GW. Macromolecular prodrug that provides the irinotecan (CPT-11) active-metabolite SN-38 with ultralong half-life, low C(max), and low glucuronide formation. Journal of medicinal chemistry. 2014;57:2303–2314. doi: 10.1021/jm401644v. [DOI] [PubMed] [Google Scholar]
- 214.Xu Z, Zheng W, Yin Z. Synthesis and optimization of a bifunctional hyaluronan-based camptothecin prodrug. Archiv der Pharmazie. 2014;347:240–246. doi: 10.1002/ardp.201300177. [DOI] [PubMed] [Google Scholar]
- 215.Christodoulou MS, Zunino F, Zuco V, Borrelli S, Comi D, Fontana G, Martinelli M, Lorens JB, Evensen L, Sironi M, Pieraccini S, Dalla Via L, Gia OM, Passarella D. Camptothecin-7-yl-methanthiole: semisynthesis and biological evaluation. ChemMedChem. 2012;7:2134–2143. doi: 10.1002/cmdc.201200322. [DOI] [PubMed] [Google Scholar]
- 216.Henne WA, Kularatne SA, Hakenjos J, Carron JD, Henne KL. Synthesis and activity of a folate targeted monodisperse PEG camptothecin conjugate. Bioorganic & medicinal chemistry letters. 2013;23:5810–5813. doi: 10.1016/j.bmcl.2013.08.113. [DOI] [PubMed] [Google Scholar]
- 217.Wang J, Sun X, Mao W, Sun W, Tang J, Sui M, Shen Y, Gu Z. Tumor redox heterogeneity-responsive prodrug nanocapsules for cancer chemotherapy. Advanced materials. 2013;25:3670–3676. doi: 10.1002/adma.201300929. [DOI] [PubMed] [Google Scholar]
- 218.Xu Z, Wang D, Xu S, Liu X, Zhang X, Zhang H, Preparation of a camptothecin prodrug with glutathione-responsive disulfide linker for anticancer drug delivery. Chemistry. an Asian journal. 2014;9:199–205. doi: 10.1002/asia.201301030. [DOI] [PubMed] [Google Scholar]
- 219.McRae Page S, Martorella M, Parelkar S, Kosif I, Emrick T. Disulfide cross-linked phosphorylcholine micelles for triggered release of camptothecin. Molecular pharmaceutics. 2013;10:2684–2692. doi: 10.1021/mp400114n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wu Q, Du F, Luo Y, Lu W, Huang J, Yu J, Liu S. Poly(ethylene glycol) shell-sheddable nanomicelle prodrug of camptothecin with enhanced cellular uptake, Colloids and surfaces. B. Biointerfaces. 2013;105:294–302. doi: 10.1016/j.colsurfb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 221.Tang Q, Cao B, Cheng G. Co-delivery of small interfering RNA using a camptothecin prodrug as the carrier. Chemical communications. 2014;50:1323–1325. doi: 10.1039/c3cc47970f. [DOI] [PubMed] [Google Scholar]
- 222.Satsangi A, Roy SS, Satsangi RK, Vadlamudi RK, Ong JL. Design of a paclitaxel prodrug conjugate for active targeting of an enzyme upregulated in breast cancer cells. Molecular pharmaceutics. 2014;11:1906–1918. doi: 10.1021/mp500128k. [DOI] [PubMed] [Google Scholar]
- 223.Mura S, Zouhiri F, Lerondel S, Maksimenko A, Mougin J, Gueutin C, Brambilla D, Caron J, Sliwinski E, Lepape A, Desmaele D, Couvreur P. Novel isoprenoyl nanoassembled prodrug for paclitaxel delivery. Bioconjugate chemistry. 2013;24:1840–1849. doi: 10.1021/bc400210x. [DOI] [PubMed] [Google Scholar]
- 224.Arpicco S, Stella B, Schiavon O, Milla P, Zonari D, Cattel L. Preparation and characterization of novel poly(ethylene glycol) paclitaxel derivatives. International journal of pharmaceutics. 2013;454:653–659. doi: 10.1016/j.ijpharm.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 225.Yuan L, Chen W, Hu J, Zhang JZ, Yang D. Mechanistic study of the covalent loading of paclitaxel via disulfide linkers for controlled drug release. Langmuir : the ACS journal of surfaces and colloids. 2013;29:734–743. doi: 10.1021/la304324r. [DOI] [PubMed] [Google Scholar]
- 226.Caron J, Maksimenko A, Wack S, Lepeltier E, Bourgaux C, Morvan E, Leblanc K, Couvreur P, Desmaele D. Improving the antitumor activity of squalenoyl-paclitaxel conjugate nanoassemblies by manipulating the linker between paclitaxel and squalene. Advanced healthcare materials. 2013;2:172–185. doi: 10.1002/adhm.201200099. [DOI] [PubMed] [Google Scholar]
- 227.Wohl AR, Michel AR, Kalscheuer S, Macosko CW, Panyam J, Hoye TR. Silicate esters of paclitaxel and docetaxel: synthesis, hydrophobicity, hydrolytic stability, cytotoxicity, and prodrug potential. Journal of medicinal chemistry. 2014;57:2368–2379. doi: 10.1021/jm401708f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Goodarzi N, Ghahremani MH, Amini M, Atyabi F, Ostad SN, Shabani Ravari N, Nateghian N, Dinarvand R. CD44-targeted docetaxel conjugate for cancer cells and cancer stem-like cells: a novel hyaluronic acid-based drug delivery system. Chemical biology & drug design. 2014;83:741–752. doi: 10.1111/cbdd.12288. [DOI] [PubMed] [Google Scholar]
- 229.Xie C, Chang J, Hao XD, Yu JM, Liu HR, Sun X. Mitochondrial-targeted prodrug cancer therapy using a rhodamine B labeled fluorinated docetaxel. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2013;85:541–549. doi: 10.1016/j.ejpb.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 230.Kuznetsova NR, Svirshchevskaya EV, Sitnikov NS, Abodo L, Sutorius H, Zapke J, Velder J, Thomopoulou P, Oschkinat H, Prokop A, Schmalz HG, Fedorov AY, Vodovozova EL. Lipophilic prodrugs of a triazole-containing colchicine analogue in liposomes: biological effects on human tumor cells. Bioorganicheskaia khimiia. 2013;39:609–618. [PubMed] [Google Scholar]
- 231.Kambhampati S, Rajewski RA, Tanol M, Haque I, Das A, Banerjee S, Jha S, Burns D, Borrego-Diaz E, Van Veldhuizen PJ, Banerjee SK. A second-generation 2-Methoxyestradiol prodrug is effective against Barrett’s adenocarcinoma in a mouse xenograft model. Molecular cancer therapeutics. 2013;12:255–263. doi: 10.1158/1535-7163.MCT-12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bio M, Rajaputra P, Nkepang G, You Y. Far-red light activatable, multifunctional prodrug for fluorescence optical imaging and combinational treatment. Journal of medicinal chemistry. 2014;57:3401–3409. doi: 10.1021/jm5000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.dos Santos Edos A, Hamel E, Bai R, Burnett JC, Tozatti CS, Bogo D, Perdomo RT, Antunes AM, Marques MM, Matos Mde F, de Lima DP. Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorganic & medicinal chemistry letters. 2013;23:4669–4673. doi: 10.1016/j.bmcl.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ghinet A, Tourteau A, Rigo B, Stocker V, Leman M, Farce A, Dubois J, Gautret P. Synthesis and biological evaluation of fluoro analogues of antimitotic phenstatin. Bioorganic & medicinal chemistry. 2013;21:2932–2940. doi: 10.1016/j.bmc.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 235.Marquez Ruiz JF, Kedziora K, Pigott M, Keogh B, Windle H, Gavin J, Kelleher DP, Gilmer JF. A nitrophenyl-based prodrug type for colorectal targeting of prednisolone, budesonide and celecoxib. Bioorganic & medicinal chemistry letters. 2013;23:1693–1698. doi: 10.1016/j.bmcl.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 236.Prijic S, Scancar J, Romih R, Cemazar M, Bregar VB, Znidarsic A, Sersa G. Increased cellular uptake of biocompatible superparamagnetic iron oxide nanoparticles into malignant cells by an external magnetic field. The Journal of membrane biology. 2010;236:167–179. doi: 10.1007/s00232-010-9271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 238.Wilson MW, Kerlan RK, Jr, Fidelman NA, Venook AP, LaBerge JM, Koda J, Gordon RL. Hepatocellular carcinoma: regional therapy with a magnetic targeted carrier bound to doxorubicin in a dual MR imaging/ conventional angiography suite--initial experience with four patients. Radiology. 2004;230:287–293. doi: 10.1148/radiol.2301021493. [DOI] [PubMed] [Google Scholar]
- 239.Semelka RC, Helmberger TKG. Contrast Agents for MR Imaging of the Liver. Radiology. 2001;218:27–38. doi: 10.1148/radiology.218.1.r01ja2427. [DOI] [PubMed] [Google Scholar]
- 240.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Licciardi M, Scialabba C, Cavallaro G, Sangregorio C, Fantechi E, Giammona G. Cell uptake enhancement of folate targeted polymer coated magnetic nanoparticles. Journal of biomedical nanotechnology. 2013;9:949–964. doi: 10.1166/jbn.2013.1606. [DOI] [PubMed] [Google Scholar]
- 242.Yoo H, Moon SK, Hwang T, Kim YS, Kim JH, Choi SW, Kim JH. Multifunctional magnetic nanoparticles modified with polyethylenimine and folic acid for biomedical theranostics. Langmuir : the ACS journal of surfaces and colloids. 2013;29:5962–5967. doi: 10.1021/la3051302. [DOI] [PubMed] [Google Scholar]
- 243.Jiang QL, Zheng SW, Hong RY, Deng SM, Guo L, Hu RL, Gao B, Huang M, Cheng LF, Liu GH, Wang YQ. Folic acid-conjugated Fe3O4 magnetic nanoparticles for hyperthermia and MRI in vitro and in vivo. Applied Surface Science. 2014;307:224–233. [Google Scholar]
- 244.Jang M, Yoon YI, Kwon YS, Yoon TJ, Lee HJ, Hwang SI, Yun BL, Kim SM. Trastuzumab-conjugated liposome-coated fluorescent magnetic nanoparticles to target breast cancer. Korean journal of radiology. 2014;15:411–422. doi: 10.3348/kjr.2014.15.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang HM, Park CW, Woo MA, Kim MI, Jo YM, Park HG, Kim JD. HER2/neu antibody conjugated poly(amino acid)-coated iron oxide nanoparticles for breast cancer MR imaging. Biomacromolecules. 2010;11:2866–2872. doi: 10.1021/bm100560m. [DOI] [PubMed] [Google Scholar]
- 246.Shamsipour F, Zarnani AH, Ghods R, Chamankhah M, Forouzesh F, Vafaei S, Bayat AA, Akhondi MM, Ali Oghabian M, Jeddi-Tehrani M. Conjugation of Monoclonal Antibodies to Super Paramagnetic Iron Oxide Nanoparticles for Detection of her2/neu Antigen on Breast Cancer Cell Lines. Avicenna journal of medical biotechnology. 2009;1:27–31. [PMC free article] [PubMed] [Google Scholar]
- 247.Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, Duan H, Ni C, Yuan Q, Adams G, Smith MQ, Wood WC, Gao X, Nie S. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5:235–243. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bharde AA, Palankar R, Fritsch C, Klaver A, Kanger JS, Jovin TM, Arndt-Jovin DJ. Magnetic nanoparticles as mediators of ligand-free activation of EGFR signaling. PloS one. 2013;8:e68879. doi: 10.1371/journal.pone.0068879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lin CW, Wei KC, Liao SS, Huang CY, Sun CL, Wu PJ, Lu YJ, Yang HW, Ma CC. A reusable magnetic graphene oxide-modified biosensor for vascular endothelial growth factor detection in cancer diagnosis. Biosensors & bioelectronics. 2015;67:431–437. doi: 10.1016/j.bios.2014.08.080. [DOI] [PubMed] [Google Scholar]
- 250.Hsieh WJ, Liang CJ, Chieh JJ, Wang SH, Lai IR, Chen JH, Chang FH, Tseng WK, Yang SY, Wu CC, Chen YL. In vivo tumor targeting and imaging with anti-vascular endothelial growth factor antibody-conjugated dextran-coated iron oxide nanoparticles. International journal of nanomedicine. 2012;7:2833–2842. doi: 10.2147/IJN.S32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wu S, Duan N, Wang Z, Wang H. Aptamer-functionalized magnetic nanoparticle-based bioassay for the detection of ochratoxin A using upconversion nanoparticles as labels. The Analyst. 2011;136:2306–2314. doi: 10.1039/c0an00735h. [DOI] [PubMed] [Google Scholar]
- 252.Aravind A, Nair R, Raveendran S, Veeranarayanan S, Nagaoka Y, Fukuda T, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Sakthi Kumar D. Aptamer conjugated paclitaxel and magnetic fluid loaded fluorescently tagged PLGA nanoparticles for targeted cancer therapy. Journal of Magnetism and Magnetic Materials. 2013;344:116–123. [Google Scholar]
- 253.Fang C, Veiseh O, Kievit F, Bhattarai N, Wang F, Stephen Z, Li C, Lee D, Ellenbogen RG, Zhang M. Functionalization of iron oxide magnetic nanoparticles with targeting ligands: their physicochemical properties and in vivo behavior. Nanomedicine. 2010;5:1357–1369. doi: 10.2217/nnm.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jafari A, Salouti M, Shayesteh SF, Heidari Z, Rajabi AB, Boustani K, Nahardani A. Synthesis and characterization of Bombesin-superparamagnetic iron oxide nanoparticles as a targeted contrast agent for imaging of breast cancer using MRI. Nanotechnology. 2015;26:075101. doi: 10.1088/0957-4484/26/7/075101. [DOI] [PubMed] [Google Scholar]
- 255.Martin AL, Hickey JL, Ablack AL, Lewis JD, Luyt LG, Gillies ER. Synthesis of bombesin-functionalized iron oxide nanoparticles and their specific uptake in prostate cancer cells. Journal of nanoparticle research : an interdisciplinary forum for nanoscale science and technology. 2009;12:1599–1608. doi: 10.1007/s11051-009-9681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Obayemi JD, Dozie-Nwachukwu S, Danyuo Y, Odusanya OS, Anuku N, Malatesta K, Soboyejo WO. Biosynthesis and the conjugation of magnetite nanoparticles with luteinizing hormone releasing hormone (LHRH), Materials science & engineering. C. Materials for biological applications. 2015;46:482–496. doi: 10.1016/j.msec.2014.10.081. [DOI] [PubMed] [Google Scholar]
- 257.Leuschner C, Kumar CS, Hansel W, Soboyejo W, Zhou J, Hormes J. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast cancer research and treatment. 2006;99:163–176. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 258.Babic M, Horak D, Trchova M, Jendelova P, Glogarova K, Lesny P, Herynek V, Hajek M, Sykova E. Poly(L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjugate chemistry. 2008;19:740–750. doi: 10.1021/bc700410z. [DOI] [PubMed] [Google Scholar]
- 259.Kumar M, Medarova Z, Pantazopoulos P, Dai G, Moore A. Novel membrane-permeable contrast agent for brain tumor detection by MRI. Magnetic resonance in medicine. 2010;63:617–624. doi: 10.1002/mrm.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Medarova Z, Kumar M, Ng SW, Moore A. Development and application of a dual-purpose nanoparticle platform for delivery and imaging of siRNA in tumors. Methods in molecular biology. 2009;555:1–13. doi: 10.1007/978-1-60327-295-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nature biotechnology. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 262.Lin J, Li Y, Li Y, Wu H, Yu F, Zhou S, Xie L, Luo F, Lin C, Hou Z. Drug/Dye-Loaded, Multifunctional PEG-Chitosan-Iron Oxide Nanocomposites for Methotraxate Synergistically Self-Targeted Cancer Therapy and Dual Model Imaging. ACS applied materials & interfaces. 2015;7:11908–11920. doi: 10.1021/acsami.5b01685. [DOI] [PubMed] [Google Scholar]
- 263.Cassim SM, Giustini AJ, Baker I, Hoopes PJ. Development of Novel Magnetic Nanoparticles for Hyperthermia Cancer Therapy. Proceedings of SPIE--the International Society for Optical Engineering. 2011;7901:790115. doi: 10.1117/12.876514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Purushotham S, Ramanujan RV. Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy. Acta biomaterialia. 2010;6:502–510. doi: 10.1016/j.actbio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 265.Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Advanced drug delivery reviews. 2011;63:789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wong JE, Krishnakumar Gaharwar A, Müller-Schulte D, Bahadur D, Richtering W. Layer-by-layer assembly of a magnetic nanoparticle shell on a thermoresponsive microgel core. Journal of Magnetism and Magnetic Materials. 2007;311:219–223. [Google Scholar]
- 267.Satarkar NS, Hilt JZ. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. Journal of controlled release : official journal of the Controlled Release Society. 2008;130:246–251. doi: 10.1016/j.jconrel.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 268.Derfus Geoffrey AMvM, Harris Todd J, Duza Tasmia, Vecchio Kenneth S, Ruoslahti Erkki, Bhatia Sangeeta N. Remotely Triggered Release from Magnetic Nanoparticles. Advanced materials. 2007;19:3932–3936. [Google Scholar]
- 269.Liu L, Chang S, Sun J, Zhu S, Yin M, Zhu Y, Wang Z, Xu RX. Ultrasound-mediated destruction of paclitaxel and oxygen loaded lipid microbubbles for combination therapy in ovarian cancer xenografts. Cancer letters. 2015;361:147–154. doi: 10.1016/j.canlet.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 270.Deng Z, Yan F, Jin Q, Li F, Wu J, Liu X, Zheng H. Reversal of multidrug resistance phenotype in human breast cancer cells using doxorubicin-liposome-microbubble complexes assisted by ultrasound. Journal of controlled release : official journal of the Controlled Release Society. 2014;174:109–116. doi: 10.1016/j.jconrel.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 271.Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug design, development and therapy. 2013;7:375–388. doi: 10.2147/DDDT.S31564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Timko BP, Dvir T, Kohane DS. Remotely triggerable drug delivery systems. Advanced materials. 2010;22:4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 273.Ikeda-Dantsuji Y, Feril LB, Jr, Tachibana K, Ogawa K, Endo H, Harada Y, Suzuki R, Maruyama K. Synergistic effect of ultrasound and antibiotics against Chlamydia trachomatis-infected human epithelial cells in vitro. Ultrasonics sonochemistry. 2011;18:425–430. doi: 10.1016/j.ultsonch.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 274.Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. Journal of the National Cancer Institute. 2007;99:1095–1106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 275.Solorio L, Exner AA. Applications of ultrasound for image-guided drug delivery in cancer chemotherapy. Therapeutic delivery. 2013;4:785–789. doi: 10.4155/tde.13.49. [DOI] [PubMed] [Google Scholar]
- 276.Zhou Y. Ultrasound-mediated drug/gene delivery in solid tumor treatment. Journal of healthcare engineering. 2013;4:223–254. doi: 10.1260/2040-2295.4.2.223. [DOI] [PubMed] [Google Scholar]
- 277.Panje CM, Wang DS, Willmann JK. Ultrasound and microbubble-mediated gene delivery in cancer: progress and perspectives. Investigative radiology. 2013;48:755–769. doi: 10.1097/RLI.0b013e3182982cc1. [DOI] [PubMed] [Google Scholar]
- 278.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. Journal of the National Cancer Institute. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 279.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer research. 2000;60:4440–4445. [PubMed] [Google Scholar]
- 280.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer research. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 281.Nishita T. Heat-sensitive liposomes containing cisplatin and localized hyperthermia in treatment of murine tumor. Osaka city medical journal. 1998;44:73–83. [PubMed] [Google Scholar]
- 282.Weinstein JN, Magin RL, Cysyk RL, Zaharko DS. Treatment of solid L1210 murine tumors with local hyperthermia and temperature-sensitive liposomes containing methotrexate. Cancer research. 1980;40:1388–1395. [PubMed] [Google Scholar]
- 283.Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, Pruitt AF, Klein A, Case B, Thrall DE, Needham D, Dewhirst MW. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:4004–4010. doi: 10.1158/1078-0432.CCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 284.Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: extravasation and release of contents in tumor microvascular networks. International journal of radiation oncology, biology, physics. 1996;36:1177–1187. doi: 10.1016/s0360-3016(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 285.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti SK, Li KC, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Advanced drug delivery reviews. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Paparel P, Curiel L, Chesnais S, Ecochard R, Chapelon JY, Gelet A. Synergistic inhibitory effect of high-intensity focused ultrasound combined with chemotherapy on Dunning adenocarcinoma. BJU international. 2005;95:881–885. doi: 10.1111/j.1464-410X.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 288.Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound in medicine & biology. 2004;30:519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 289.Prat F, Chapelon JY, el Fadil FA, Theillere Y, Ponchon T, Cathignol D. In vivo effects of cavitation alone or in combination with chemotherapy in a peritoneal carcinomatosis in the rat. British journal of cancer. 1993;68:13–17. doi: 10.1038/bjc.1993.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free radical biology & medicine. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 291.Bommannan D, Menon GK, Okuyama H, Elias PM, Guy RH. Sonophoresis. II. Examination of the mechanism(s) of ultrasound-enhanced transdermal drug delivery. Pharmaceutical research. 1992;9:1043–1047. doi: 10.1023/a:1015806528336. [DOI] [PubMed] [Google Scholar]
- 292.Yudina A, Lepetit-Coiffe M, Moonen CT. Evaluation of the temporal window for drug delivery following ultrasound-mediated membrane permeability enhancement. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2011;13:239–249. doi: 10.1007/s11307-010-0346-5. [DOI] [PubMed] [Google Scholar]
- 293.Meijering BD, Juffermans LJ, van Wamel A, Henning RH, Zuhorn IS, Emmer M, Versteilen AM, Paulus WJ, van Gilst WH, Kooiman K, de Jong N, Musters RJ, Deelman LE, Kamp O. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circulation research. 2009;104:679–687. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- 294.van Wamel A, Kooiman K, Emmer M, ten Cate FJ, Versluis M, de Jong N. Ultrasound microbubble induced endothelial cell permeability. Journal of controlled release : official journal of the Controlled Release Society. 2006;116:e100–e102. doi: 10.1016/j.jconrel.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 295.Saito M, Mazda O, Takahashi KA, Arai Y, Kishida T, Shin-Ya M, Inoue A, Tonomura H, Sakao K, Morihara T, Imanishi J, Kawata M, Kubo T. Sonoporation mediated transduction of pDNA/siRNA into joint synovium in vivo. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25:1308–1316. doi: 10.1002/jor.20392. [DOI] [PubMed] [Google Scholar]
- 296.Kheirolomoom A, Mahakian LM, Lai CY, Lindfors HA, Seo JW, Paoli EE, Watson KD, Haynam EM, Ingham ES, Xing L, Cheng RH, Borowsky AD, Cardiff RD, Ferrara KW. Copper-doxorubicin as a nanoparticle cargo retains efficacy with minimal toxicity. Molecular pharmaceutics. 2010;7:1948–1958. doi: 10.1021/mp100245u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Frulio N, Trillaud H, Deckers R, Lepreux S, Moonen C, Quesson B. Influence of ultrasound induced cavitation on magnetic resonance imaging contrast in the rat liver in the presence of macromolecular contrast agent. Investigative radiology. 2010;45:282–287. doi: 10.1097/RLI.0b013e3181dac2a7. [DOI] [PubMed] [Google Scholar]
- 298.Paoli EE, Kruse DE, Seo JW, Zhang H, Kheirolomoom A, Watson KD, Chiu P, Stahlberg H, Ferrara KW. An optical and microPET assessment of thermally-sensitive liposome biodistribution in the Met-1 tumor model: Importance of formulation. Journal of controlled release : official journal of the Controlled Release Society. 2010;143:13–22. doi: 10.1016/j.jconrel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Advanced drug delivery reviews. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Guenther F, von zur Muhlen C, Ferrante EA, Grundmann S, Bode C, Klibanov AL. An ultrasound contrast agent targeted to P-selectin detects activated platelets at supra-arterial shear flow conditions. Investigative radiology. 2010;45:586–591. doi: 10.1097/RLI.0b013e3181ed1b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Phillips LC, Klibanov AL, Wamhoff BR, Hossack JA. Targeted gene transfection from microbubbles into vascular smooth muscle cells using focused, ultrasound-mediated delivery. Ultrasound in medicine & biology. 2010;36:1470–1480. doi: 10.1016/j.ultrasmedbio.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Patil AV, Rychak JJ, Allen JS, Klibanov AL, Hossack JA. Dual frequency method for simultaneous translation and real-time imaging of ultrasound contrast agents within large blood vessels. Ultrasound in medicine & biology. 2009;35:2021–2030. doi: 10.1016/j.ultrasmedbio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Xuan JW, Bygrave M, Valiyeva F, Moussa M, Izawa JI, Bauman GS, Klibanov A, Wang F, Greenberg NM, Fenster A. Molecular targeted enhanced ultrasound imaging of flk1 reveals diagnosis and prognosis potential in a genetically engineered mouse prostate cancer model. Molecular imaging. 2009;8:209–220. [PubMed] [Google Scholar]
- 304.Bohmer MR, Klibanov AL, Tiemann K, Hall CS, Gruell H, Steinbach OC. Ultrasound triggered image-guided drug delivery. European journal of radiology. 2009;70:242–253. doi: 10.1016/j.ejrad.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 305.Chappell JC, Song J, Burke CW, Klibanov AL, Price RJ. Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small. 2008;4:1769–1777. doi: 10.1002/smll.200800806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hollander D, Adelman LS. Eosinophilia-myalgia syndrome associated with ingestion of L-tryptophan: muscle biopsy findings in 4 patients. Neurology. 1991;41:319–321. doi: 10.1212/wnl.41.2_part_1.319. [DOI] [PubMed] [Google Scholar]
- 307.Ninomiya K, Yamashita T, Kawabata S, Shimizu N. Targeted and ultrasound-triggered drug delivery using liposomes co-modified with cancer cell-targeting aptamers and a thermosensitive polymer. Ultrasonics sonochemistry. 2014;21:1482–1488. doi: 10.1016/j.ultsonch.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 308.Mo S, Coussios CC, Seymour L, Carlisle R. Ultrasound-enhanced drug delivery for cancer. Expert opinion on drug delivery. 2012;9:1525–1538. doi: 10.1517/17425247.2012.739603. [DOI] [PubMed] [Google Scholar]
- 309.Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. M. Bioeffects Committee of the American Institute of Ultrasound in, Overview of therapeutic ultrasound applications and safety considerations. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2012;31:623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Frenkel V, Gurka R, Liberzon A, Shavit U, Kimmel E. Preliminary investigations of ultrasound induced acoustic streaming using particle image velocimetry. Ultrasonics. 2001;39:153–156. doi: 10.1016/s0041-624x(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 311.Frenkel V, Kimmel E, Iger Y. Ultrasound-facilitated transport of silver chloride (AgCl) particles in fish skin. Journal of controlled release : official journal of the Controlled Release Society. 2000;68:251–261. doi: 10.1016/s0168-3659(00)00264-9. [DOI] [PubMed] [Google Scholar]
- 312.Eggen S, Fagerland SM, Morch Y, Hansen R, Sovik K, Berg S, Furu H, Bohn AD, Lilledahl MB, Angelsen A, Angelsen B, de Lange Davies C. Ultrasound-enhanced drug delivery in prostate cancer xenografts by nanoparticles stabilizing microbubbles. Journal of controlled release : official journal of the Controlled Release Society. 2014;187:39–49. doi: 10.1016/j.jconrel.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 313.Holland CK, McPherson DD. Echogenic Lipsomes for Targeted Drug Delivery. Proceedings / IEEE International Symposium on Biomedical Imaging: from nano to macro. IEEE International Symposium on Biomedical Imaging. 2009;2009:755–758. [PMC free article] [PubMed] [Google Scholar]
- 314.Ferrara KW. Driving delivery vehicles with ultrasound. Advanced drug delivery reviews. 2008;60:1097–1102. doi: 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Stieger SM, Caskey CF, Adamson RH, Qin S, Curry FR, Wisner ER, Ferrara KW. Enhancement of vascular permeability with low-frequency contrast-enhanced ultrasound in the chorioallantoic membrane model. Radiology. 2007;243:112–121. doi: 10.1148/radiol.2431060167. [DOI] [PubMed] [Google Scholar]
- 316.Dayton PA, Zhao S, Bloch SH, Schumann P, Penrose K, Matsunaga TO, Zutshi R, Doinikov A, Ferrara KW. Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy. Molecular imaging. 2006;5:160–174. [PMC free article] [PubMed] [Google Scholar]
- 317.Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound in medicine & biology. 1999;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 318.Lum AF, Borden MA, Dayton PA, Kruse DE, Simon SI, Ferrara KW. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. Journal of controlled release : official journal of the Controlled Release Society. 2006;111:128–134. doi: 10.1016/j.jconrel.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rapoport N, Nam KH, Gupta R, Gao Z, Mohan P, Payne A, Todd N, Liu X, Kim T, Shea J, Scaife C, Parker DL, Jeong EK, Kennedy AM. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. Journal of controlled release : official journal of the Controlled Release Society. 2011;153:4–15. doi: 10.1016/j.jconrel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rapoport N, Kennedy AM, Shea JE, Scaife CL, Nam KH. Ultrasonic nanotherapy of pancreatic cancer: lessons from ultrasound imaging. Molecular pharmaceutics. 2010;7:22–31. doi: 10.1021/mp900128x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam KH. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. Journal of controlled release : official journal of the Controlled Release Society. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics. 2008;48:279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound in medicine & biology. 2008;34:930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. International journal of cancer. Journal international du cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 325.Vykhodtseva N, McDannold N, Hynynen K. Induction of apoptosis in vivo in the rabbit brain with focused ultrasound and Optison. Ultrasound in medicine & biology. 2006;32:1923–1929. doi: 10.1016/j.ultrasmedbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 326.Lu P, Zhu XQ, Xu ZL, Zhou Q, Zhang J, Wu F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery. 2009;145:286–293. doi: 10.1016/j.surg.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 327.Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, Lyerly HK, Clay TM, Zhong P. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. Journal of translational medicine. 2007;5:34. doi: 10.1186/1479-5876-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation, International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology. North American Hyperthermia Group. 2007;23:165–171. doi: 10.1080/02656730701206638. [DOI] [PubMed] [Google Scholar]
- 329.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature reviews. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 330.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochimica et biophysica acta. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Frontiers in pharmacology. 2013;4:28. doi: 10.3389/fphar.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Vadlapudi AD, Vadlapatla RK, Kwatra D, Earla R, Samanta SK, Pal D, Mitra AK. Targeted lipid based drug conjugates: a novel strategy for drug delivery. International journal of pharmaceutics. 2012;434:315–324. doi: 10.1016/j.ijpharm.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wilson AK, Latham JR, Steinbrecher RA. Transformation-induced mutations in transgenic plants: analysis and biosafety implications. Biotechnology & genetic engineering reviews. 2006;23:209–237. doi: 10.1080/02648725.2006.10648085. [DOI] [PubMed] [Google Scholar]
- 334.Gottesman MM. Mechanisms of cancer drug resistance. Annual review of medicine. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 335.International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nature reviews. Drug discovery. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 337.Biedler JL, Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer research. 1970;30:1174–1184. [PubMed] [Google Scholar]
- 338.Higgins CF. ABC transporters: from microorganisms to man. Annual review of cell biology. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 339.Locher KP, Borths E. ABC transporter architecture and mechanism: implications from the crystal structures of BtuCD and BtuF. FEBS letters. 2004;564:264–268. doi: 10.1016/S0014-5793(04)00289-3. [DOI] [PubMed] [Google Scholar]
- 340.Wang Z, Pal D, Patel A, Kwatra D, Mitra AK. Influence of overexpression of efflux proteins on the function and gene expression of endogenous peptide transporters in MDR-transfected MDCKII cell lines. International journal of pharmaceutics. 2013;441:40–49. doi: 10.1016/j.ijpharm.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Khurana V, Minocha M, Pal D, Mitra AK. Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors. Drug metabolism and drug interactions. 2014;29:179–190. doi: 10.1515/dmdi-2013-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK. Mechanisms of drug resistance in cancer chemotherapy: coordinated role and regulation of efflux transporters and metabolizing enzymes. Current pharmaceutical design. 2013;19:7126–7140. doi: 10.2174/13816128113199990493. [DOI] [PubMed] [Google Scholar]
- 343.Harker WG, Bauer D, Etiz BB, Newman RA, Sikic BI. Verapamil-mediated sensitization of doxorubicin-selected pleiotropic resistance in human sarcoma cells: selectivity for drugs which produce DNA scission. Cancer research. 1986;46:2369–2373. [PubMed] [Google Scholar]
- 344.Inaba M, Kobayashi H, Sakurai Y, Johnson RK. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer research. 1979;39:2200–2203. [PubMed] [Google Scholar]
- 345.Ozols RF, Cunnion RE, Klecker RW, Jr, Hamilton TC, Ostchega Y, Parrillo JE, Young RC. Verapamil and adriamycin in the treatment of drug-resistant ovarian cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1987;5:641–647. doi: 10.1200/JCO.1987.5.4.641. [DOI] [PubMed] [Google Scholar]
- 346.Jonsson B, Nilsson K, Nygren P, Larsson R. SDZ PSC-833--a novel potent in vitro chemosensitizer in multiple myeloma. Anti-cancer drugs. 1992;3:641–646. doi: 10.1097/00001813-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 347.Pirker R, FitzGerald DJ, Raschack M, Frank Z, Willingham MC, Pastan I. Enhancement of the activity of immunotoxins by analogues of verapamil. Cancer research. 1989;49:4791–4795. [PubMed] [Google Scholar]
- 348.Giaccone G, Linn SC, Welink J, Catimel G, Stieltjes H, van der Vijgh WJ, Eeltink C, Vermorken JB, Pinedo HM. A dose-finding and pharmacokinetic study of reversal of multidrug resistance with SDZ PSC 833 in combination with doxorubicin in patients with solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 1997;3:2005–2015. [PubMed] [Google Scholar]
- 349.Wilson WH, Jamis-Dow C, Bryant G, Balis FM, Klecker RW, Bates SE, Chabner BA, Steinberg SM, Kohler DR, Wittes RE. Phase I and pharmacokinetic study of the multidrug resistance modulator dexverapamil with EPOCH chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13:1985–1994. doi: 10.1200/JCO.1995.13.8.1985. [DOI] [PubMed] [Google Scholar]
- 350.Lum BL, Gosland MP. MDR expression in normal tissues. Pharmacologic implications for the clinical use of P-glycoprotein inhibitors. Hematology/oncology clinics of North America. 1995;9:319–336. [PubMed] [Google Scholar]
- 351.Dantzig AH, Shepard RL, Cao J, Law KL, Ehlhardt WJ, Baughman TM, Bumol TF, Starling JJ, Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator. LY335979. Cancer research. 1996;56:4171–4179. [PubMed] [Google Scholar]
- 352.Dale IL, Tuffley W, Callaghan R, Holmes JA, Martin K, Luscombe M, Mistry P, Ryder H, Stewart AJ, Charlton P, Twentyman PR, Bevan P, Reversal of P-glycoprotein-mediated multidrug resistance by XR9051. a novel diketopiperazine derivative. British journal of cancer. 1998;78:885–892. doi: 10.1038/bjc.1998.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer research. 1993;53:4595–4602. [PubMed] [Google Scholar]
- 354.Dantzig AH, Law KL, Cao J, Starling JJ. Reversal of multidrug resistance by the P-glycoprotein modulator, LY335979, from the bench to the clinic. Current medicinal chemistry. 2001;8:39–50. doi: 10.2174/0929867013373903. [DOI] [PubMed] [Google Scholar]
- 355.Ruff P, Vorobiof DA, Jordaan JP, Demetriou GS, Moodley SD, Nosworthy AL, Werner ID, Raats J, Burgess LJ. A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer chemotherapy and pharmacology. 2009;64:763–768. doi: 10.1007/s00280-009-0925-9. [DOI] [PubMed] [Google Scholar]
- 356.Lancet JE, Baer MR, Duran GE, List AF, Fielding R, Marcelletti JF, Multani PS, Sikic BI. A phase I trial of continuous infusion of the multidrug resistance inhibitor zosuquidar with daunorubicin and cytarabine in acute myeloid leukemia. Leukemia research. 2009;33:1055–1061. doi: 10.1016/j.leukres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 357.Morschhauser F, Zinzani PL, Burgess M, Sloots L, Bouafia F, Dumontet C. Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leukemia & lymphoma. 2007;48:708–715. doi: 10.1080/10428190701190169. [DOI] [PubMed] [Google Scholar]
- 358.Sandler A, Gordon M, De Alwis DP, Pouliquen I, Green L, Marder P, Chaudhary A, Fife K, Battiato L, Sweeney C, Jordan C, Burgess M, Slapak CA. A Phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride (LY335979), administered intravenously in combination with doxorubicin in patients with advanced malignancy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:3265–3272. doi: 10.1158/1078-0432.CCR-03-0644. [DOI] [PubMed] [Google Scholar]
- 359.Kathawala RJ, Wang YJ, Shukla S, Zhang YK, Alqahtani S, Kaddoumi A, Ambudkar SV, Ashby CR, Jr, Chen ZS. ATP-binding cassette subfamily B member 1 (ABCB1) and subfamily C member 10 (ABCC10) are not primary resistance factors for cabazitaxel. Chinese journal of cancer. 2015;34:115–120. doi: 10.1186/s40880-015-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bardenheuer W, Lehmberg K, Rattmann I, Brueckner A, Schneider A, Sorg UR, Seeber S, Moritz T, Flasshove M. Resistance to cytarabine and gemcitabine and in vitro selection of transduced cells after retroviral expression of cytidine deaminase in human hematopoietic progenitor cells. Leukemia. 2005;19:2281–2288. doi: 10.1038/sj.leu.2403977. [DOI] [PubMed] [Google Scholar]
- 361.Sampath D, Cortes J, Estrov Z, Du M, Shi Z, Andreeff M, Gandhi V, Plunkett W. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood. 2006;107:2517–2524. doi: 10.1182/blood-2005-08-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]