Abstract
One of the most challenging and clinically important goals in nanomedicine is to deliver imaging and therapeutic agents to solid tumors. Here we discuss the recent design and development of stimuli-responsive smart nanoparticles for targeting the common attributes of solid tumors such as their acidic and hypoxic microenvironments. This class of stimuli-responsive nanoparticles is inactive during blood circulation and under normal physiological conditions, but is activated by acidic pH, enzymatic up-regulation, or hypoxia once they extravasate into the tumor microenvironment. The nanoparticles are often designed to first “navigate” the body’s vascular system, “dock” at the tumor sites, and then “activate” for action inside the tumor interstitial space. They combine the favorable biodistribution and pharmacokinetic properties of nanodelivery vehicles and the rapid diffusion and penetration properties of smaller drug cargos. By targeting the broad tumor habitats rather than tumor-specific receptors, this strategy has the potential to overcome the tumor heterogeneity problem and could be used to design diagnostic and therapeutic nanoparticles for a broad range of solid tumors.
Keywords: Nanomedicine, tumor heterogeneity, tumor microenvironment, pH, hypoxia, matrix metalloproteinases
Graphical abstract
1. Introduction
The in-vivo delivery of imaging and therapeutic nanoparticles to solid tumors is one of the most important problems in cancer nanomedicine [1–6]. Current methods for systemic delivery are mainly based on an “active” mechanism and a “passive” mechanism. In the passive mode, nanoparticles without targeting ligands accumulate in the tumor interstitial space through the enhanced permeability and retention (EPR) effect resulting from the tumor’s leaky vasculature and impaired lymphatics [7–9]. The active mode adds molecular ligands to the particle surface such as antibodies, peptides, or small molecules which bind to specific receptors on the tumor cell membrane, often followed by internalization through receptor-mediated endocytosis [10–12]. In both mechanisms, the nanoparticles in the bloodstream must first move across the tumor blood vessels (usually leaky vasculatures), and then move out or extravasate into the tumor interstitial space. Recent advances have also developed stimuli-responsive nanoparticles to target the tumor habitats or microenvironment, which takes advantage of both passive and active targeting while overcoming their associated biological barriers [13–16]. As depicted in Figure 1, stimuli-triggered activation of nanoparticles in the tumor microenvironment can lead to accelerated drug release at the target site, improved cellular binding and internalization, and/or more efficient drug perfusion throughout the tumor volume.
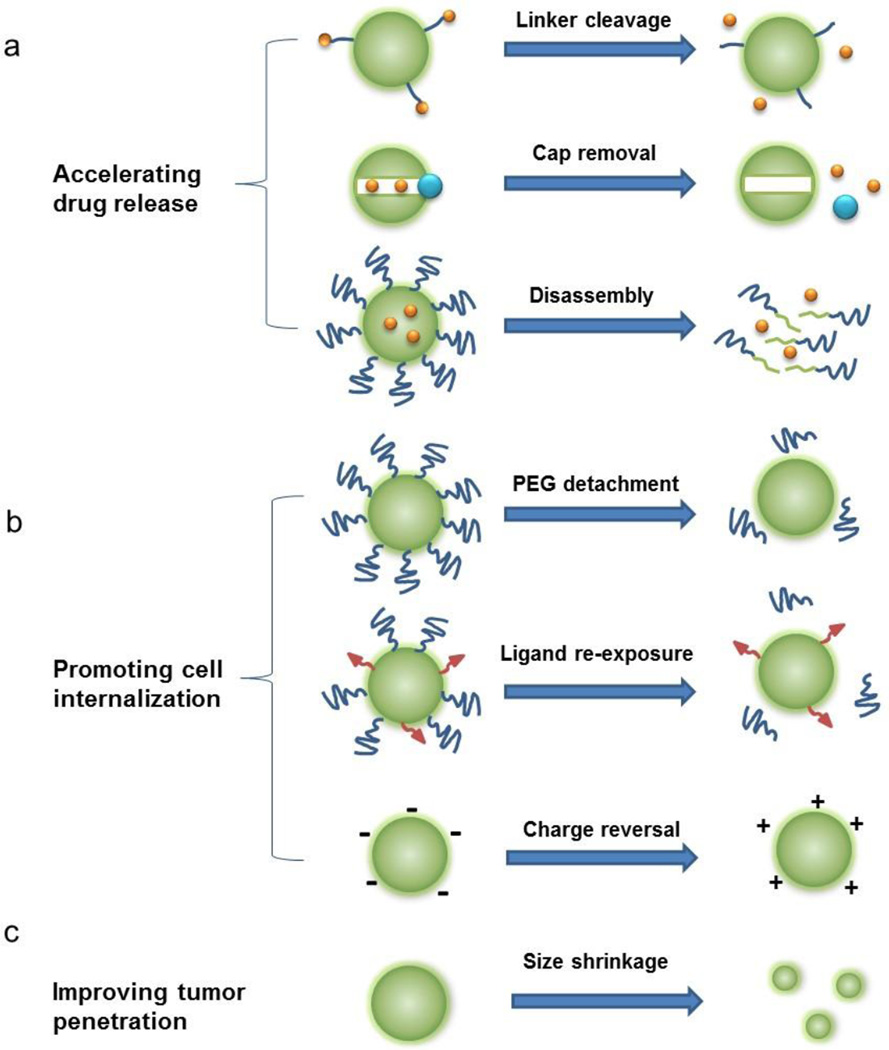
Figure 1.
Schematic illustration of stimuli-responsive nanoparticles for targeting the tumor microenvironment. Nanoparticle activation can lead to (a) accelerated drug release, (b) enhanced cellular binding and internalization, and / or (c) improved drug diffusion and tumor penetration.
It is well known that the tumor microenvironment has unique physiological characteristics such as acidic pH [17], hypoxia [18], and up-regulation of certain enzymes [19]. In particular, the extracellular pH (pHe) of solid tumors is more acidic (pH 6.5 to 6.8) than that of normal tissues because cancer cells rely heavily on glycolysis for energy consumption (rather than oxidative phosphorylation) to increase biosynthetic functions, leading to an increased rate of lactic acid production (also known as the Warburg effect) [20–22]. Hypoxia, or low oxygen supply, is typically a result of the aberrant vascular network not being able to deliver sufficient blood supply to all the cells in a tumor mass. The microenvironment also contains up-regulated enzymes such as matrix metalloproteinases that are involved in tumor development and progression [23]. These endogenous stimuli provide great opportunities for the development of activatable particles for targeted delivery and activation of imaging and therapeutic agents.
In comparison with conventional passive and active targeting strategies, the ability to target the tumor microenvironment has several important advantages. First, active targeting relies on specific interactions between the targeting motifs on the nanocarriers and the receptors on the tumor cells. However, the heterogeneous expression of membrane receptors among the various cancer cell populations limits its broad applicability [24]. In contrast, targeting the tumor microenvironment focuses on more general physiological features among all solid tumors offering a relatively universal approach for cancer imaging and treatment. Second, targeting ligands on the surface of nanoparticles can accelerate opsonization leading to increased nonspecific cell uptake and reduced binding affinity as a result of the adsorbed proteins blocking the ligand’s binding site [25–27]. Targeting the tumor microenvironment avoids this problem as the particles can be designed to be inert during circulation until they reach tumor tissues where they convert to actively targeted forms. Third, strong receptor-ligand interactions can hamper the penetration of actively targeted nanocarriers into the interstitial space, due to a phenomenon called the “binding site barrier” at the tumor periphery [28, 29]. Additionally, the sizes of particles optimal for EPR delivery have negligible diffusion within solid tumors. In the following, we discuss strategies and recent advances in designing activatable nanoparticles for targeting the tumor microenvironment.
2. Nanoparticle Activation by Acidic Tumor pH
As briefly noted above, solid tumors often have more acidic microenvironments than normal tissues due to the Warburg effect, which can be exploited for tumor specific delivery by several strategies. First, pH-sensitive nanocarriers have been designed by using molecular moieties with pKa values near the tumor interstitial pH. In this case, a small pH drop (more acidic) at the tumor site causes the protonation of multiple functional groups, disrupting the hydrophilic-hydrophobic equilibrium inside the nanoparticle and triggering a dramatic structural transformation. Typical examples of pH-sensitive groups include histidine-, tertiary amine-, and sulfonamide-containing groups [15, 30]. The second strategy is based on pH-sensitive linkages (chemical bonds) that are stable at neutral pH but are cleaved under acidic conditions. An example is the acid-catalyzed hydrolysis of the 2,3-dimethylmaleic amide bond [16]. The third strategy uses pH-responsive insertion peptides that have weak interactions with the cellular membrane at neutral pH, but can penetrate and form stable transmembrane complexes under slightly acidic environments [31].
Hydrophobic-hydrophilic transitions
A typical system based on this concept is that of poly-L-histidine polymeric micelles developed by Bae and coworkers [32–35]. The poly-Lhistidine (polyHis) has a pKa value near pH 7.0 and shows reversible hydrophilic to hydrophobic transitions in accordance with its protonated and deprotonated states. Bae and co-workers developed mixed micelles by blending polyHis-b-PEG diblock copolymer with poly(L-lactic acid) (PLLA)-b-PEG. The mixed micelles are stable at pH above 7.4, but becoming gradually destabilized below pH 7.0 due to the protonation of the polyHis block in the micelle core [32, 36]. The mixed micelles can encapsulate the anticancer drug doxorubicin (DOX) through hydrophobic interactions between DOX and deprotonated polyHis segments. At acidic tumor sites, the mixed micelles dissociate and selectively release the drug, leading to an improvement in antitumor efficacy as compared with their pH-insensitive counterparts [37]. PolyHis polymers have also been utilized for pH-triggered cellular binding and internalization [35, 38]. In this strategy, the targeting ligand is shielded at physiological pH from protein binding during blood circulation (stealth), but is exposed for cellular binding after transport into the acidic tumor microenvironment.
The polyHis-based system can also be modified to coordinate with inorganic nanoparticles such as iron oxide to fabricate pH-sensitive polymer-metal oxide hybrid superstructures, as reported by Hyeon and coworkers [39]. Specifically, the PEG-polyHis polymer is functionalized with catechol groups and chlorin e6 (Ce6), where the catechol groups anchor the polymers to the iron oxide surface, while Ce6 acts as a fluorescent probe to track the particles and as a photosensitizer for photodynamic therapy (PDT). At pH 7.4, the polyHis chains are hydrophobic and are entangled to form superstructures containing multiple iron oxide nanoparticles. The hybrid superstructures have a slightly negative charge, and both the fluorescence of Ce6 and the T1 relaxivity of iron oxide nanoparticles are suppressed. A drop in pH leads to increased protonation of the polyHis segments, reversing the charge from negative to positive. This charge reversal causes both particle swelling (leading to drug release) and cellular internalization. When the polymers are further protonated at endosomal pHs (5.5 – 6.0), the hydrophobic interactions between polymers are further diminished, resulting in a complete dissociation of the superparticles and a recovery of the fluorescence and T1 relaxivity. The recovery of Ce6 fluorescence simultaneously activates the production of cytotoxic singlet oxygen upon light irradiation, leading to superior efficacies in treating not only colorectal carcinoma xenografts but also highly heterogeneous drug-resistant tumors.
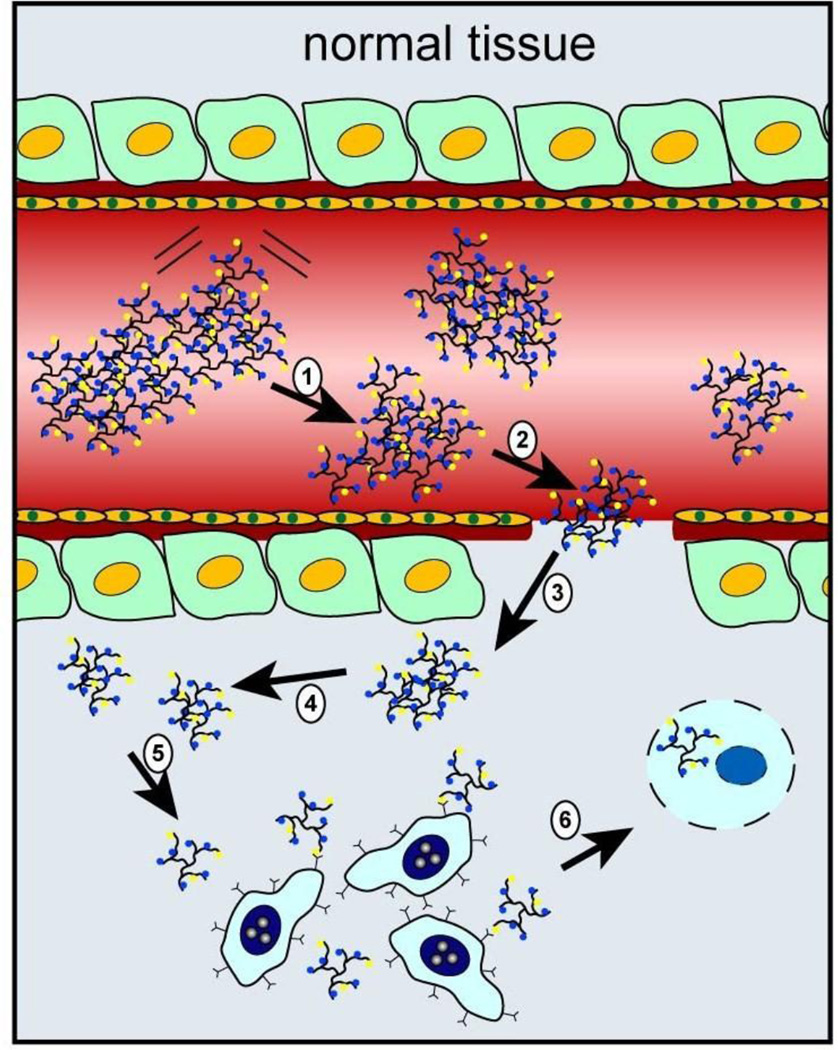
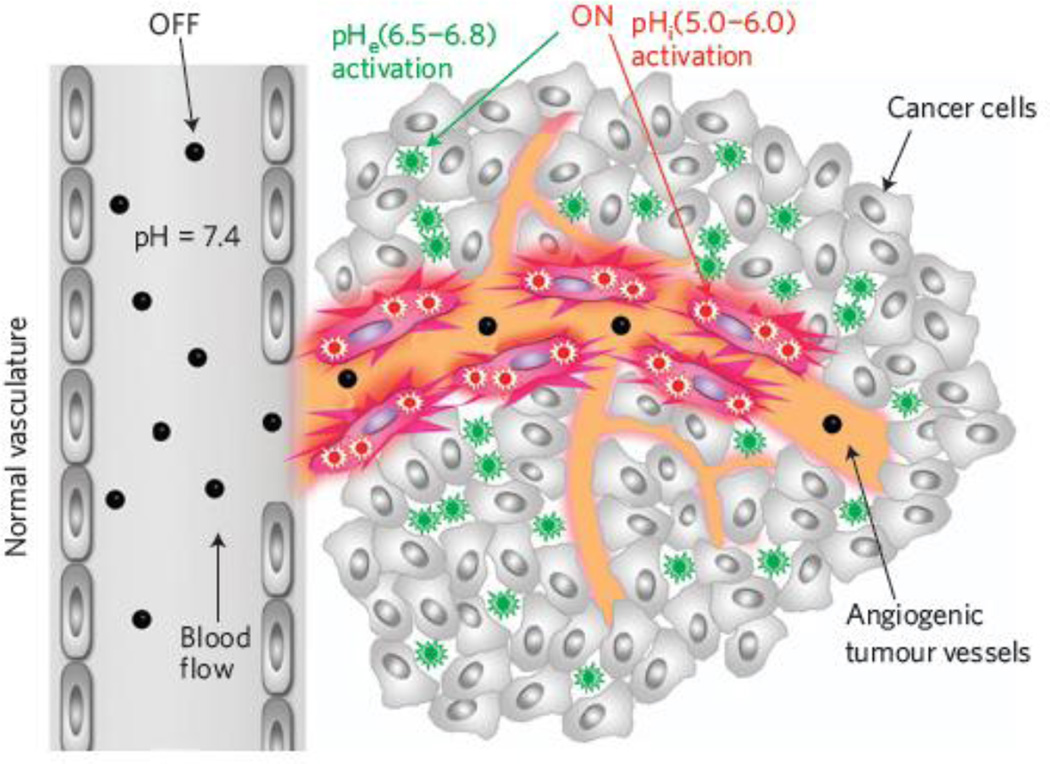
Another type of pH-sensitive nanoparticle is based on the use of copolymer materials with ionizable tertiary amine groups and covalently conjugated fluorescence dyes or encapsulated cancer drugs. In particular, Gao and coworkers have reported a class of ultra-pH-sensitive nanostructures that undergo a dramatic and sharp transition within the very narrow range of pH (often less than 0.2 pH units) [30, 40]. This pH-induced transition leads to rapid and complete dissociation of the nanomicelles, and as a result, the covalently linked dyes change from a self-quenched "off" state to a highly emissive bright "on" state. This super-sensitive and nonlinear response to external pH provides a new strategy in targeting acidic organelles in cancer cells as well as the acidic microenvironment in solid tumors (see Figure 2) [41]. Another feature is that detection sensitivity is significantly improved because each nanoparticle probe contains multiple copies of the dye, which are turned on (restored fluorescence) in an all-or-none fashion, leading to amplified fluorescence signals many times brighter than single dye molecules. A major limitation of optical imaging is that tissue penetration of the light beam is limited to a few millimeters due to mainly light scattering and absorption. However, this problem can be mitigated by adapting optical contrast agents and devices for endoscopic and image-guided surgery applications in which the light is brought to the tissue and tumor surfaces via an endoscope or a surgical incision. Overall, this class of pH-activated and super-sensitive polymeric micelles has demonstrated a new concept in designing novel nanoparticle probes and is expected to have broad applications in cancer biology, endoscopic cancer screening, and image-guided interventions.
Figure 2.
Schematic diagram showing nanoparticle fluorescence activation in the acidic tumor microenvironment (pH 6.5 – 6.8) and inside the more acidic organelles (pH 5.0 – 6.0). Adapted from Ref [41] with permission from Nature Publishing Group.
Sulfonamides have also been incorporated into polymers to develop pH-responsive nanomicelles because the secondary amine (–NH–) group linked to the sulfone group has a near neutral pKa. Thus, polymers of polysulfadimethoxine–PEG have been shown to associate with polycations at pH levels greater than 6.8 due to electrostatic interactions between the negatively charged sulfonamide and the positively charged polymer [42]. Once in acidic environments (below pH 6.8), the sulfonamides are no longer charged, thus eliminating the electrostatic association between polysulfadimethoxine–PEG polymers and polycations. This feature has been demonstrated to facilitate on-demand ligand exposure on the surface of nanocarriers. One example is a TAT peptide-decorated micelle with a pH triggered sheddable coating [43]. Another example is a zwitterionic gold nanoparticle with a pH-sensitive sulfonamide ligand layer [44]. At pH 7.4, this nanoparticle is neutral because of the zwitterionic property of the ligand. When the pH drops below 6.6, the sulfonamide loses its negative charge, breaking the charge balance and making the gold nanoparticle positively charged, which significantly increases the particle uptake and cytotoxicity.
Acid catalyzed bond cleavage
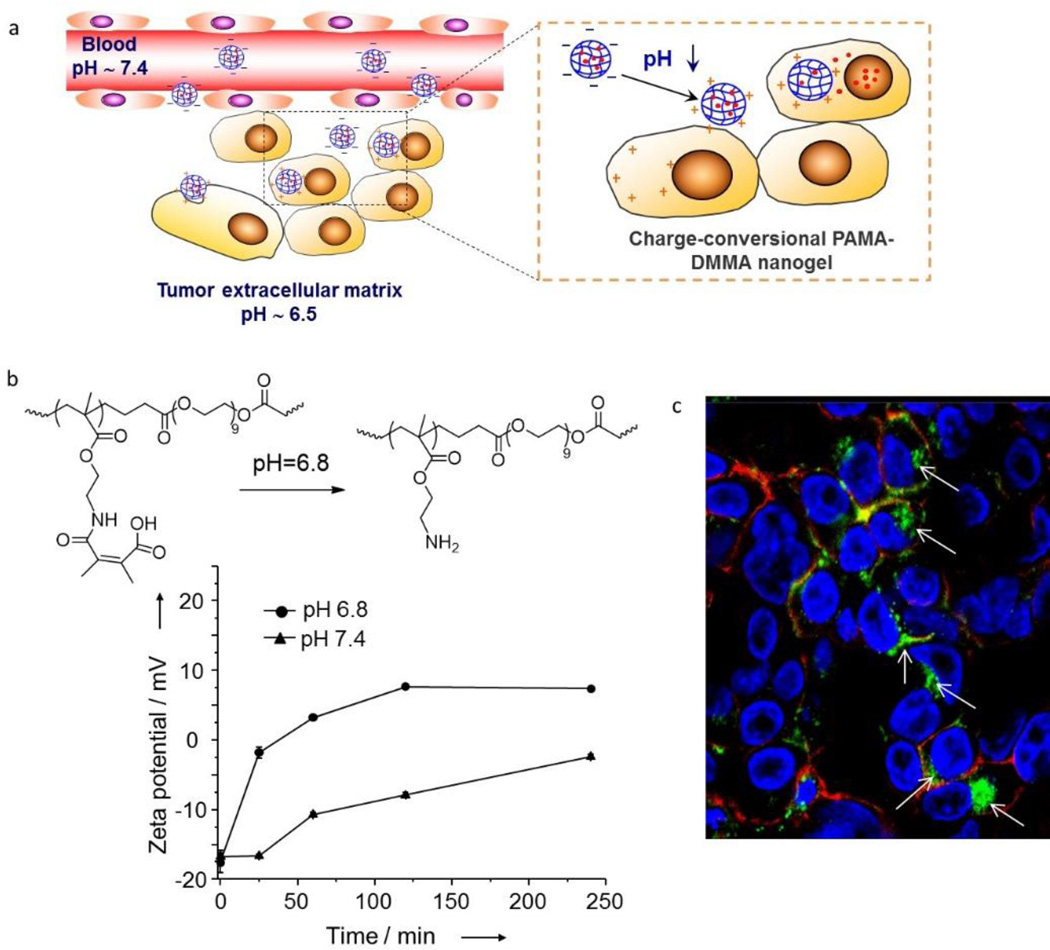
Although many acid-labile bonds have been exploited for pH-responsive drug delivery, most of them can only respond to the more acidic environments of lysosomes (pH 5.5-5.0) [45]. One exception is the 2,3-dimethylmaleic amide (DMMA) linkage that can be selectively cleaved at weakly acidic pHs of the tumor microenvironment [46]. Also, at neutral or basic pH, DMMA bears a negatively charged carboxylate group; upon cleavage of the amide bond at acidic pHs, an amine is left on the nanoparticle yielding a positive charge. This pH-activated charge reversal endows the nanoparticles with extra functions for drug delivery because surface charge plays an important role in determining the in vitro and in vivo fate of nanoparticles [47]. Compared with negatively charged nanoparticles, positively charged ones show higher affinity to negatively charged cell membranes, thus can be internalized by cells more efficiently [48, 49]. However, positively charged nanoparticles often have strong interactions with blood proteins, which causes aggregation and rapid elimination of the nanoparticles from the circulation [50]. Thus, it is highly desirable to fabricate nanoparticles that are resistant to non-specific protein adsorption in blood circulation, but alter their surface property to become recognizable by cancer cells after accumulation at the tumor target sites. This concept was first demonstrated by Wang and coworkers involving a cross-reacted nanogel of poly(2-aminoethyl methacrylate hydrochloride) and 2,3-dimethylmaleic anhydride (PAMA-DMMA) [46]. As shown in Figure 3, the nanogel has a negative charge under physiological conditions, but the charge changes to positive within just 1 hour incubation at pH 6.8, indicating effective cleavage of the amide bond. This charge reversal contributes significantly to the enhanced cellular uptake of the nanogel. In addition, the positively charged PAMA-DMMA nanogel can accelerate DOX release at acidic pH due to the increased repulsive force between the positively nanogel and DOX.
Figure 3.
(a) Schematic illustration of chemical bond cleavage and charge reversal in pH-sensitive nanogels. In the acidic tumor extracellular environment, the nanogel is activated to become positively charged and is efficiently internalized by tumor cells. (b) pH-activated chemical structure and zeta potential change of the nanogel. (c) Confocal fluorescence microscopy image showing the nanogel distribution in the tumor tissue following intratumoral injection. The white arrows indicate the locations of the nanogels. The nanogel was labeled with fluorescein isothiocyanate (FITC; green), while F-actin and nuclei of the cells were stained, respectively, with rhodamine phalloidin (red) and 4’,6-diamidino-2-phenylindole (DAPI; blue). Figure adapted from Ref [46] with permission from Wiley-VCH.
In addition to the enhanced cellular uptake of nanoparticles through negative-to-positive charge reversal, DMMA has been utilized to shed outer PEG layers from nanoparticles to promote nanoparticle-cell interactions. PEG is well known to prevent opsonization and to extend the circulation lifetime of intravenously administered nanoparticles. However, a PEG layer can hinder the uptake of the nanocarriers within their intended cellular targets. This situation has been referred to as the “PEG dilemma” [51, 52]. To overcome this problem, a sheddable nanoparticle system for siRNA delivery has been designed by attaching a pH-responsive PEGylated anionic polymer (mPEG-b-PAEP-Cya-DMMA) to the surface of positively charged ssPEI800/siRNA complexes through electrostatic interactions [53]. Under physiological conditions, the PEGylated complexes showed minimal nonspecific interactions with serum components and significantly improved their accumulation at tumor sites through the EPR effect. Once at the tumor sites, the acidic extracellular pH caused shedding of the PEGylated polymer and the siRNA-containing particles became positively charged. This pH-triggered charge reversal resulted in a 2.5-fold increase the siRNA tumor uptake and silencing efficacy in MDAMB- 231 tumor models.
Membrane insertion
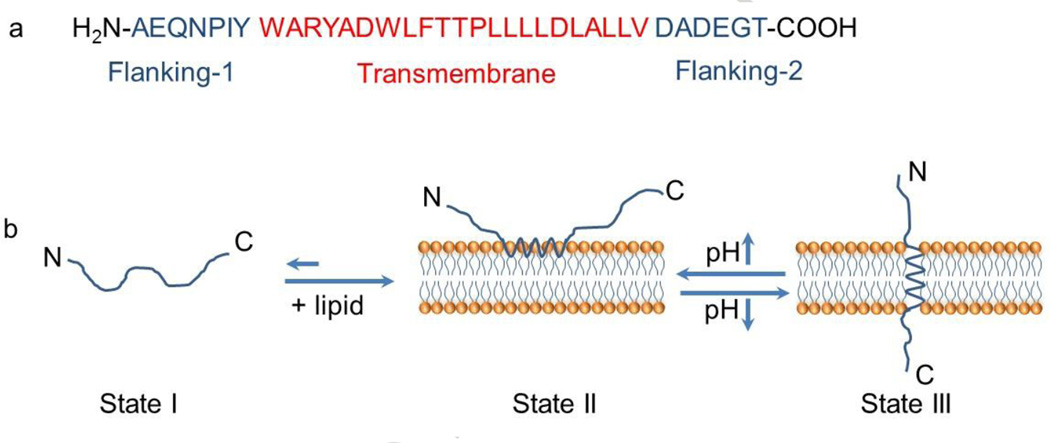
The acidic tumor microenvironment could also activate a class of peptides with specific sequences for insertion into cellular membranes [31, 54]. As shown in Figure 4, one common feature of the peptides is that they consist of two flanking sequences at each terminus and a transmembrane sequence in the middle. The flanking segments impart water solubility to the protein, while the transmembrane sequence consists of aspartate (Asp) and glutamate (Glu) residues which can become more hydrophobic at acidic pH values to increase interactions with membranes. At neutral or basic pHs, the peptides exist as largely unstructured monomers soluble in aqueous solution. In the presence of a lipid bilayer or a cellular membrane, they are associated reversibly with the outside surface of the membrane as monomers. When in acidic environments, the carboxyl groups of Aps and Glu residues from the transmembrane and the C-terminus become protonated, which increases their hydrophobicity. The protonation of the residues also triggers the formation of an interfacial helix which can insert itself within the hydrophobic bilayer of the cellular membrane. The insertion is predominantly unidirectional as usually the C-terminus propagates across the bilayer into the cytosol, while the N-terminus remains in the extracellular space [55, 56]. Studies have shown that the membrane insertion process is thermodynamically favorable and estimated that the bilayer affinity of the peptides is 30–50 times higher at low pH than at high pH. Using a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine vesicle as a model, the authors determined that the free energy difference of the free and membrane-bound peptides at low pH is -9 kcal/mol [57]. Additionally, the kinetics of the process is rather quick as the time from forming an interfacial helix to moving across the membrane can be finished within seconds to minutes [31, 58].
Figure 4.
(a) The amino acid sequence of a pH-responsive insertion peptide. (b) A schematic representation of the peptide in solution and interacting with a lipid bilayer at neutral (pH 7.0) and acidic pH (below pH 6.0). State I refers to the peptide in solution at normal and basic pHs. Upon addition of lipid, the unstructured peptide is adsorbed on the membrane surface (State II). The drop of pH leads to the protonation of Asp/Glu residues, increasing peptide hydrophobicity, and resulting in the insertion and formation of a transmembrane α-helix (State III). Adapted from Ref [59] with permission from the National Academy of Sciences.
The pH dependence of membrane insertion has promoted studies on the use of these peptides as cancer targeting agents. As a proof-of-concept study, near-infrared (NIR) fluorescent dyes were conjugated to a wild-type (WT)-peptide and intravenously injected into a breast adenocarcinoma mouse model [59]. The peptide-dye constructs were found to light up tumors of various sizes, even those too small to see without a fluorescent signal. The fluorescence signals achieved from tagged tumor tissues were approximately 5-fold higher than in healthy tissues and remained stable for more than 4 days. Furthermore, newly designed peptide variants also demonstrated excellent targeting of both metastatic 4T1 mammary tumors and spontaneous breast tumors in transgenic mice, whereas the staining of nonmalignant tissues in transgenic mice was minimal, demonstrating their superiority in selectively delivering fluorescent dyes to tumors [60]. Clinical imaging modalities such as PET (positron emission tomography) and SPECT (single-photon emission computed tomography) have also been delivered to tumor sites by using such peptides [61–63]. In addition, this pH-dependent insertion has been used for translocation of polar cargo molecules into the cytosol of cancer cells including phalloidin toxin [64], peptide nucleic acids, and microRNAs [65]. Such molecules can be conjugated to the peptide C-terminus via cleavable disulfide bond. The process for the cargo delivery is that the insertion peptides provide direct translocation of disulfide linked molecules across the plasma membrane, where subsequent cleavage of the disulfide bond in the cytoplasm’s reducing environment results in the intracellular release of the cargos.
In addition to the intracellular delivery of small molecules within cells, the insertion peptide technology can also assist in the tumor targeted delivery of nanoparticles. Yao et al. have used this class of peptides for transporting intravenously injected gold nanoparticles (1.4 nm) to tumor tissues and enhancing their perfusion throughout the entire tumor mass [66]. The same group also fabricated fusogenic peptide-coated liposomes and demonstrated that the presence of peptides on the surface of liposomes enhanced membrane fusion and lipid exchange in acidic tumor tissues which resulted in enhanced cellular internalization and intracellular drug concentration [67]. Using a similar concept, the Tan group has developed peptide-coated mesoporous silica nanoparticles (MSNs) as pH-targeted delivery of nanocarriers [68]. In their system, the peptides were conjugated onto the surface of MSNs with cleavable disulfide bonds. The peptides served two functions, acting as pH-targeting moieties and as a gatekeeper by blocking drug diffusion out of the nanoparticle pores. Once exposed to the tumor pH, the peptides rapidly inserted into the cancer cell membranes and translocated the nanoparticles into the cytoplasm. Inside the more reductive environment of the cytoplasm, the disulfide bonds are cleaved and trigger the detachment of peptides from the nanoparticle surface, allowing more efficient drug release from the particle pores.
A potential problem in using the insertion peptides to target the tumor microenvironment is that the transition pH of the wild-type peptide is around 6.1, which is lower than the typical pH ranges found at the tumor interstitium (pH 6.5–6.8). However improvements on the transition pH have been made as a result of an extensive structure–activity relationship study for a series of peptide variants performed by the An group [69]. They found that by replacing specific amino acid residues within the transmembrane domain, the transition pH can be tuned from 6.1 to 6.9. These new variants showed comparable activity to the WT peptide in their optimal conditions. Some specific modifications provided additional advantages such as imparting more sharp pH-dependent transition curves. Certain new variants showed improved insertion activity into the membrane at the tumor pH range of 6.5–7.0 than previously known insertion peptides. Using turn-on fluorescence assays and anti-proliferation studies in A549 cells with paclitaxel as a model drug, a variant with modifications at both 14 and 25 positions showed a considerable advantage over the WT peptide in cargo delivery at the tumor pH 6.6. This new progress further increased the usefulness of insertion peptides, with the potential to deliver more drugs into the cytoplasm of cancer cells via plasma membrane insertion.
3. Enzymatic Activation
In addition to acidic pH, altered expressions of enzymes in tumors have important implications for targeting the tumor microenvironment [70, 71]. Several enzymes within the protease and lipase families have been reported to be over-expressed by cancer cells, which can be exploited as endogenous triggers for cancer imaging and therapy [72, 73]. Having enzymes act as triggers has the advantage of being highly selective for specific substrates. Such specificity of enzymes for their substrates has sparked great interest in developing enzyme-responsive nanomaterials for tumor-specific drug delivery. In particular, matrix metalloproteinases (MMPs) are a class of proteases which break down components of the extracellular matrix. These enzymes are important in normal physiological processes such as tissue remodeling, inflammation, and angiogenesis. Additionally they are often overexpressed in the tumor microenvironment and facilitate the migration of cancer cells from the primary tumor leading to metastasis of other organs [70]. In particular, MMP2 and MMP9 are observed to be overexpressed in several different types of cancers including stomach, colorectal, breast, prostate, lung, and ovarian [74, 75]. Thus, MMP2 and MMP9 are the two most targeted types of MMPs for drug delivery.
MMP-mediated site-specific drug delivery can be classified into four general methods. First, anticancer drugs can be conjugated to either synthetic polymers or proteins through MMPs-specific peptide sequences that are recognized and cleaved by MMPs for release. For example, Mansour et al. developed a water-soluble derivative of doxorubicin incorporating a MMP2 specific peptide sequence (Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln) which was then conjugated conjugated to albumin. Once the protein-drug complex reached the tumor microenvironment, the cleavable peptide sequence was efficiently and specifically cleaved by MMP-2 to liberate a doxorubicin tetrapeptide to kill tumor cells [76]. Similar ideas have been expanded to other synthetic polymers like PEG [77] and poly(ethylene glycol)-b-poly(L-lysine) copolymer [78] with MMP2 triggered drug release at tumor sites. One shortcoming of this approach is that the released drugs are not the parent drugs as they will have a peptide sequence attached. This peptide sequence might compromise the drug’s efficacy in killing cancer cells.
A second delivery strategy is utilizing mesoporous silica nanoparticles (MSNs) as drug depots while introducing MMP cleavable biomacromolecules on the surface as gatekeepers [79–81]. MSNs possess a highly porous interior structure, and are therefore capable of loading a high dose of therapeutic drug, normally 200−300 mg, maximally about 600 mg drug per 1 g silica [82]. Under normal physiological conditions, the gatekeepers are maintained as caps over the pores of the MSNs preventing premature escape of the drugs contained within. Once the nanocarriers reach the tumor site, the heightened presence of MMPs digest the linkers and release the gatekeepers allowing drug release. In contrast to the first type of MMP targeting method mentioned above, this strategy is able to keep the parent drug structure intact, thus maintaining its anticancer efficacy.
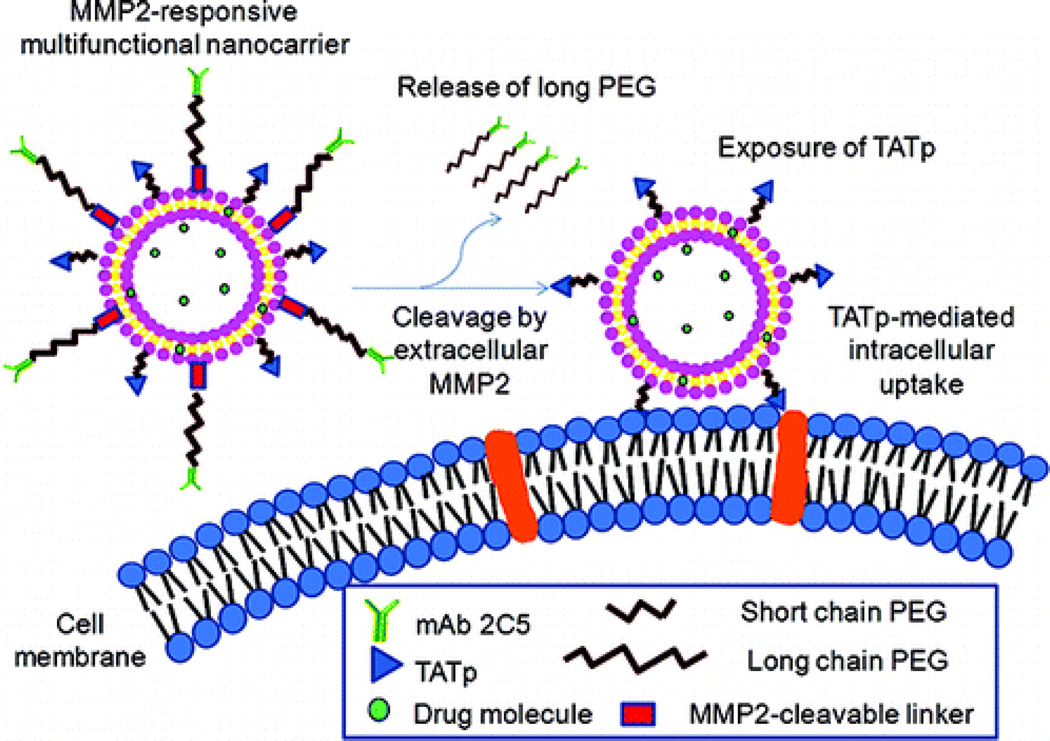
A third design is to use MMPs to detach PEGylation stealth layers from nanocarriers once they have reached the tumor sites. PEGylated nanoparticles, though having prolonged blood circulation, have impeded cellular uptake once at the intended target site. Using MMPs-activated deshielding of the outer PEG layer has the ability to gain the benefits of prolonged circulation and greater tumor site accumulation while also allowing activation for greater cell uptake. Such a nanocarrier has been developed by the Torchilin group [83]. In their design, the MMP2-cleavable octapeptide (Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln) was used as a linker to encapsulate a TAT targeted liposomal nanocarrier with an outer PEG layer. Using in vitro cultures of 4T1 breast tumor cells, the use of MMP2-treated particles led to a 2-fold increase in cellular internalization compared to non-treated controls (see Figure 5). The same group has also extended this concept to in vivo studies [84]. They synthesized a self-assembling drugpolymer conjugate, PEG2000-peptide-paclitaxel (PTX), using the MMP2-cleavable octapeptide as a linker between PEG and PTX. PEG2000-peptide-PTX was mixed with TAT-PEG1000-phosphoethanolamine (PE) (a cell-penetrating enhancer) and PEG1000-PE (a nanocarrier building block) to prepared mixed micelles in aqueous solution. Using the same concept, the mixed micelles expose the TAT peptide within the tumor site to enhance the uptake of PTX-containing nanocarriers by cancer cells and results in significantly improved efficacy in tumor suppression compared with the control group without MMP2 sensitivity. Another typical example is MMPs-sensitive multifunctional envelope-type nanodevice (MEND) for tumor-specific nuclei acid delivery developed by the Harashima group [85]. Other MMP sensitive polymeric micelles with MMP2 cleavable PEG deshielding have also been proposed for tumor-targeted siRNA delivery [86] or siRNA and hydrophobic drug co-delivery [87].
Figure 5.
MMP2-responsive multifunctional liposomal nanocarrier and its drug delivery strategy. The multifunctional liposomal nanocarriers are retained in the tumor site due to the EPR effect as well as the active targeting effect of mAb 2C5. The up-regulated MMP2 in the tumor microenvironment cleaves the MMP2-sensitive linker and removes the protective long-chain PEG, resulting in the exposure of TAT peptides (TATp) for the enhanced cellular internalization. Figure adapted from Ref [83] with permission from the American Chemical Society.
Also relying on enzymatic cleavage is a class of activatable cell penetrating peptides (ACPPs) reported by Tsien and coworkers [72]. In this approach, positively charged cell penetrating peptides (CPPs) were fused with a negatively charged peptide through an MMP cleavable linker. The negatively charged peptide inhibits the function of the cell penetrating peptide by charge neutralization, so the intact constructs show little or no cellular binding or internalization. However, when the linker is cleaved by up-regulated MMP enzymes at the tumor site, the inhibitory peptide is removed and the function of the cell penetration peptide is restored. For improved in-vivo circulation and tumor update, nanosized ACPPs have also been produced by conjugation to high-molecular-weight dendrimers [88, 89]. These peptide constructs can be further labeled with both a fluorescent dye and a gadolinium chelate for simultaneous optical and MR imaging. Upon enzymatic activation, the uptake of such nanoparticles in solid tumors was found to be 4-15 fold higher than original ACPPs (not conjugated to dendrimers), allowing residual tumor and metastases as small as 200 µm to be detected and resected under a fluorescence microscope.
MMP activity has also been utilized in the mothership nanoparticle delivery method where a larger nanoparticle encapsulates many smaller nanoparticles. Due to the limited vascular channels, elevated interstitial fluid pressure, and dense extracellular matrix in the tumor microenvironment, nanoparticles can only rely on slow diffusion for intratumoral transport [6]. Particle size plays an important role in the ability of nanoparticle penetration into tumor tissue as the diffusion rate scales inversely with particle size [90–92]. Smaller particles will have greater tumor penetration depths, but small nanoparticles (< 10 nm) are readily eliminated from blood circulation and have inefficient EPR delivery [93, 94]. Demonstrating the mothership concept which benefits from efficient EPR and greater tumor perfusion, Wong et al. developed nanocarriers which packaged 10 nm quantum dots inside 100 nm gelatin nanogels [95]. The larger gelatin nanoparticles accumulated at tumor site by the EPR effect, where the gelatin scaffold was then degraded by MMP2 and MMP9 present in the tumor microenvironment releasing the smaller quantum dots. This work offers a proof-of-concept demonstration of utilizing the tumor microenvironment to improve tumor transportation of nanocarriers. A shortcoming in the design, however, is that the released small particles do not have additional active targeting capability to promote cellular uptake. More studies along this direction can be performed to further optimize the properties of the released small particles, such as installing more cell-interactive moieties for cell entry or endowing the small nanoparticles with therapeutic functions.
In addition to proteases, certain lipases are also up-regulated in the tumor microenvironment, which can be exploited for nanoparticle activation as well. For example, phospholipase A2 (PLA2) is overexpressed in the extracellular matrix of cancerous and inflammatory tissue [96]. Andresen et al. have demonstrated long circulating liposomes which release their contents upon activation by extracellular PLA2 [97]. Antitumor ether lipids (AELs) are a class of potent anticancer drugs. However, their applications in cancer treatment are hampered by the severe hemolysis activity. Andresen group prepared masked AELs with a PLA2-activatable property. The masked AELs form liposomes in aqueous solution and can encapsulate water soluble anticancer drugs such as DOX. PLA2 hydrolyzes the ester bond of the masked AELs and leads to the rupture of the liposome, thereby releasing both activated AELs and the encapsulated drug. The AELs themselves are cytotoxic to cancer cells. Furthermore, the concomitantly generated fatty acids by PLA2 cleavage could function as permeability enhancers that promote drug uptake by the cancer cells [98]. They further applied their discovery to create a lipid-capsaicin prodrug. The prodrug was able to form small unilamellar vesicle (SUV), and the SUV was stable in the bloodstream, but could specifically respond to PLA2 to release the covalently attached anticancer drug by PLA2-promoted intramolecular cyclization [99].
Despite emerging progress made in enzyme-responsive drug delivery research, there are still challenges that need to be addressed. One major concern is the heterogeneous expression of a specific enzyme in different cancer types and even at different stages of one particular cancer. Investigations are needed to obtain precise information of the target enzyme levels at the specific tumor site. Furthermore, fundamental understanding of the spatial and temporal patterns of enzyme expression offers an essential foundation for designing more effective and precise delivery vehicles.
4. Hypoxic Activation
Hypoxia, a condition where the cells are deprived of oxygen, is another common feature of the tumor microenvironment [18]. The vascular networks within solid tumors are highly irregular and are unable to deliver sufficient oxygen and nutrients to all regions, resulting in groups of cancer cells that are chronically or transiently deprived of oxygen. The cancer cells in the hypoxic regions divide more slowly than their well oxygenated counterparts, making them less susceptible to conventional antiproliferative agents that target rapidly dividing cells [100]. Furthermore, hypoxic conditions can exacerbate the creation of cell variants with acquired resistance to traditional chemo- and radiotherapy, ultimately resulting in treatment failure and relapse [101]. The profound effect of hypoxia on cancer biology and therapy has led to increased interests in developing agents which can target hypoxic regions within the tumor tissue [100, 102]. A representative approach for hypoxia-targeted cancer therapy is use of hypoxia activated prodrugs (HAP). There are several HAP candidates in clinical trials, among which include TH-302 developed by Threshold Pharmaceuticals which is currently in Phase 3 clinical trials under a Special Protocol Assessment with the United State Food and Drug Administration [103, 104].
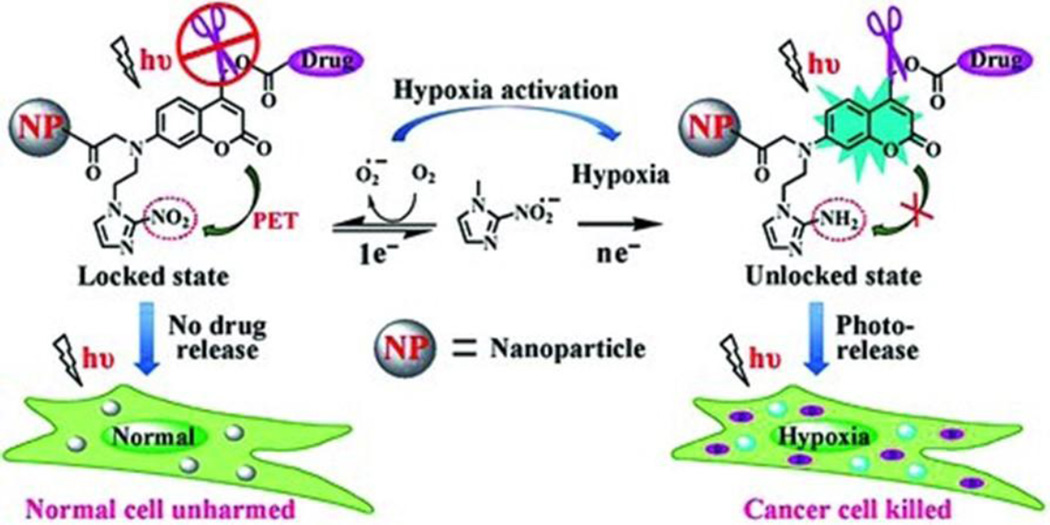
Recently, a few nanotherapeutic strategies have been developed for targeting the hypoxic environments within solid tumors. For example, Zhu et al. [105] constructed a hypoxia-activated phototriggered nanoparticles for highly selective release of anticancer drugs. The authors achieved the combinatory light and hypoxia activation by incorporating nitroimidazole, a hypoxia-responsive electron acceptor, and a coumarin-caged anticancer drug into glycol-chitosan nanoparticles (see Figure 6). In normal tissues, photoexcitation of the coumarin dyes were quenched via photoinduced electron transfer (PET) to the nitroimidazole electron acceptor, resulting in no photocleavage, thus no drug release. Within the anaerobic environment of solid tumors, the nitro group of the nitroimidazole is reduced to an amine which eliminates the PET quenching and activates the light induced cleavage of the drug-coumarin linker. Typically photoactivated prodrugs need UV to blue excitation sources which have little tissue depth penetration. However, the authors were able to demonstrate photolysis of the drug-coumarin linker by two-photon absorption using 800 nm wavelength excitation. Though two-photon adsorption is less effective for photocleavage than one-photon (λ = 400 nm) in direct excitation conditions, 800 nm excitation has significantly less attenuation and greater penetration depth within tissues. This strategy is an excellent example of highly localized treatment by combining physiological and user-controlled methods. Another nanoparticle delivery system based on nitroimidazole moieties focused on hypoxia induced disassembly of micelles [106]. In this system, the nitroimidazole derivative was covalently conjugated to water soluble carboxymethyldextran to generate an amphiphilic polymer, which could self-assemble into nanoparticles with the encapsulation of hydrophobic anticancer drugs. These carriers were stable in physiological conditions and capable of selectively releasing the hydrophobic drug under hypoxic conditions due to the hypoxia-triggered hydrophobic-to-hydrophilic transition and the resultant disassembly of the nanoparticles.
Figure 6.
Hypoxia-activated phototriggered nanoparticles specifically release drug to tumor cells. In this design, an electron acceptor, nitroimidazole, was incorporated into the coumarin phototrigger that has an intrinsic property of photo-S N 1-dependent cleavage and a sufficiently high two-photon absorption cross section. In normal tissues, photoexcitation of the coumarin dye relaxes via photoinduced electron transfer (PET) to the adjacent nitroimidazole group, resulting in the inactivation of fluorescence, photocleavage and drug release. In contrast, in the hypoxic environment of the solid tumors, the nitro group of the nitroimidazole is reduced to an amine, which activates the coumarin phototrigger by eliminating the PET process and leads to the recovery of fluorescence and the efficient drug release. Figure adapted from Ref [105] with permission from Wiley-VCH.
Biomacromolecules like small interfering RNA (siRNA) can also be delivered selectively to tumors through hypoxia-activated strategies. Torchilin et al. have reported siRNA nanocarriers that can be activated to disassemble within oxygen deprived environments by introducing an azobenzene group between PEG and polyethyleneimine (PEI) polymer segments [107]. When the particles entered the hypoxic tumor microenvironment, the azobenzene bond is cleaved which deshields the PEG coating. The remaining PEI/siRNA complex particles with their exposed positive charge are able to further facilitate cellular uptake. Enhanced silencing efficacy was observed with the responsive nanocarrier in both in vitro and in vivo experiments in comparison with its non-responsive counterpart.
Despite the advances in hypoxia-targeted strategies for tumor therapy, getting nanoparticles to these regions is quite challenging. The reason is that the hypoxic regions are typically distanced from the blood vessels, thus mass transport is limited to diffusion. For most nanoparticle systems, their diffusion rates will either be insufficient or practically nonexistent within solid tumors. Therefore, nanocarriers that can carry and release hypoxia-activated prodrugs within the tumor microenvironment could be a better option due to the higher diffusion rates of small molecules.
5. Concluding Remarks
The ability to target the tumor microenvironment provides an important strategy to overcome the problem of tumor heterogeneity and could be exploited to design diagnostic and therapeutic strategies for a broad range of solid tumors. This is most important for naturally occurring human tumors because they are especially complex and show a multitude of molecular and cellular heterogeneity [108]. At the molecular level, human cancer cells are heterogeneous both in their genetic mutations and in their phenotypic expression profiles. At the cellular level, malignant tumors are characterized by a complicated mix of benign cells, malignant cells, fibroblasts, and other stromal cells, vascular cells, and infiltrating inflammatory cells (such as macrophages and lymphocytes). Also, a small number of stem cells and progenitor cells are believed to be embedded in the perivascular region and could be responsible for tumor growth and recurrence [109–111]. To improve the tumor targeting specificity and sensitivity of nanoparticle systems, we believe that two directions are particularly promising in the near future. First, there is a need to develop ultrasensitive nanoparticles that can be activated for tumor imaging or therapeutic applications within a very narrow range of acidity or enzymatic activity. Pioneering work by Gao and coworkers has developed a new class of fluorescent nanoparticle probes that can be switched on and off by a pH change of only 0.1 – 0.2 units [40]. Second, more efforts should be made to improve the tumor penetration and cellular internalization of imaging and therapeutic agents. The abnormal vasculature and lack of functional lymphatics of tumor tissue provides an opportunity for nanocarriers to accumulate at tumor sites, but also lead to elevated levels of interstitial fluid pressure, which together with the dense extracellular matrix imposes tremendous obstacles for nanocarriers to transport to the poorly perfused regions. This is especially true for nanoparticle delivery systems, whose dimensions are often much larger than small molecules. Locally activated size-shrinkage system responding to the tumor microenvironment provides a solution to this problem, as reported by Wong et al [95]. Recent work in several groups [39, 112–114] has also demonstrated that the development of stimuli-responsive and multistage nanoparticles could ultimately lead to intelligent nanoparticle delivery systems with superior therapeutic and imaging results.
Acknowledgments
We acknowledge the National Institutes of Health for financial support (grants R01CA163256, RC2CA148265, and HHSN268201000043C to S.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
One of the authors (S.N.) is a scientific consultant of Spectropath Inc., a company to further develop and commercialize devices and contrast agents for image- guided cancer surgery.
References
- 1.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 2.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 3.Heath JR, Davis ME. Nanotechnology and cancer. Annu. Rev. Med. 2008;59:251–265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 5.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu. Rev. Biomed. Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 6.Lane LA, Qian X, Smith AM, Nie S. Physical chemistry of nanomedicine: understanding the complex behaviors of nanoparticles in vivo. Annu. Rev. Phys. Chem. 2015;66:521–547. doi: 10.1146/annurev-physchem-040513-103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 10.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012;4:128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 12.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gullotti E, Yeo Y. Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol. Pharm. 2009;6:1041–1051. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji T, Zhao Y, Ding Y, Nie G. Using functional nanomaterials to target and regulate the tumor microenvironment: diagnostic and therapeutic applications. Adv. Mater. 2013;25:3508–3525. doi: 10.1002/adma.201300299. [DOI] [PubMed] [Google Scholar]
- 15.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J. Control. Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du JZ, Mao CQ, Yuan YY, Yang XZ, Wang J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv. 2014;32:789–803. doi: 10.1016/j.biotechadv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 19.de la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 22.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nature Reviews Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 24.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 25.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin. Cancer Res. 2003;9:6551–6559. [PubMed] [Google Scholar]
- 26.Lazarovits J, Chen YY, Sykes EA, Chan WCW. Nanoparticle–blood interactions: the implications on solid tumour targeting. Chem. Commun. 2015;51:2756–2767. doi: 10.1039/c4cc07644c. [DOI] [PubMed] [Google Scholar]
- 27.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 28.Saga T, Neumann RD, Heya T, Sato J, Kinuya S, Le N, Paik CH, Weinstein JN. Targeting cancer micrometastases with monoclonal antibodies: a binding-site barrier. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8999–9003. doi: 10.1073/pnas.92.19.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, Weinstein JN. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52:5144–5153. [PubMed] [Google Scholar]
- 30.Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J. Tunable, ultrasensitive pHresponsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem. Int. Ed. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreev OA, Engelman DM, Reshetnyak YK. Targeting diseased tissues by pHLIP insertion at low cell surface pH. Front Physiol. 2014;5:97. doi: 10.3389/fphys.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin H, Lee ES, Kim D, Lee KH, Oh KT, Bae YH. Physicochemical characteristics of pH-sensitive poly(L-histidine)-b-poly(ethylene glycol)/poly(L-lactide)-b-poly(ethylene glycol) mixed micelles. J. Control. Release. 2008;126:130–138. doi: 10.1016/j.jconrel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 34.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 35.Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. Journal of Controlled Release. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. 2008;4:2043–2050. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- 39.Ling D, Park W, Park SJ, Lu Y, Kim KS, Hackett MJ, Kim BH, Yim H, Jeon YS, Na K, Hyeon T. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J. Am. Chem. Soc. 2014;136:5647–5655. doi: 10.1021/ja4108287. [DOI] [PubMed] [Google Scholar]
- 40.Zhou K, Liu H, Zhang S, Huang X, Wang Y, Huang G, Sumer BD, Gao J. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J. Am. Chem. Soc. 2012;134:7803–7811. doi: 10.1021/ja300176w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, Zhao T, Sumer BD, DeBerardinis RJ, Gao J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2014;13:204–212. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sethuraman VA, Na K, Bae YH. pH-responsive sulfonamide/PEI system for tumor specific gene delivery: an in vitro study. Biomacromolecules. 2006;7:64–70. doi: 10.1021/bm0503571. [DOI] [PubMed] [Google Scholar]
- 43.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control. Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuhara T, Saha K, Moyano DF, Kim CS, Yan B, Kim YK, Rotello VM. Acylsulfonamide-Functionalized Zwitterionic Gold Nanoparticles for Enhanced Cellular Uptake at Tumor pH. Angew. Chem. Int. Ed. 2015 doi: 10.1002/anie.201411615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong R, Tang L, Ma L, Tu C, Baumgartner R, Cheng J. Smart chemistry in polymeric nanomedicine. Chem. Soc. Rev. 2014;43:6982–7012. doi: 10.1039/c4cs00133h. [DOI] [PubMed] [Google Scholar]
- 46.Du JZ, Sun TM, Song WJ, Wu J, Wang J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew. Chem. Int. Ed. 2010;49:3621–3626. doi: 10.1002/anie.200907210. [DOI] [PubMed] [Google Scholar]
- 47.Mailander V, Landfester K. Interaction of Nanoparticles with Cells. Biomacromolecules. 2009;10:2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- 48.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, Rotello VM, Prakash YS, Mukherjee P. Effect of Nanoparticle Surface Charge at the Plasma Membrane and Beyond. Nano Lett. 2010;10:2543–2548. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oupicky D, Ogris M, Howard KA, Dash PR, Ulbrich K, Seymour LW. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol. Ther. 2002;5:463–472. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- 51.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 52.Hatakeyama H, Akita H, Kogure K, Oishi M, Nagasaki Y, Kihira Y, Ueno M, Kobayashi H, Kikuchi H, Harashima H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007;14:68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 53.Yang XZ, Du JZ, Dou S, Mao CQ, Long HY, Wang J. Sheddable ternary nanoparticles for tumor acidity-targeted siRNA delivery. ACS Nano. 2012;6:771–781. doi: 10.1021/nn204240b. [DOI] [PubMed] [Google Scholar]
- 54.Weerakkody D, Moshnikova A, Thakur MS, Moshnikova V, Daniels J, Engelman DM, Andreev OA, Reshetnyak YK. Family of pH (low) insertion peptides for tumor targeting. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5834–5839. doi: 10.1073/pnas.1303708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reshetnyak YK, Andreev OA, Lehnert U, Engelman DM. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6460–6465. doi: 10.1073/pnas.0601463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thevenin D, An M, Engelman DM. pHLIP-Mediated Translocation of Membrane-Impermeable Molecules into Cells. Chem. Biol. 2009;16:754–762. doi: 10.1016/j.chembiol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM. Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15340–15345. doi: 10.1073/pnas.0804746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreev OA, Karabadzhak AG, Weerakkody D, Andreev GO, Engelman DM, Reshetnyak YK. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4081–4086. doi: 10.1073/pnas.0914330107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andreev OA, Dupuy AD, Segala M, Sandugu S, Serra DA, Chichester CO, Engelman DM, Reshetnyak YK. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7893–7898. doi: 10.1073/pnas.0702439104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adochite RC, Moshnikova A, Carlin SD, Guerrieri RA, Andreev OA, Lewis JS, Reshetnyak YK. Targeting breast tumors with pH (low) insertion peptides. Mol. Pharm. 2014;11:2896–2905. doi: 10.1021/mp5002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macholl S, Morrison MS, Iveson P, Arbo BE, Andreev OA, Reshetnyak YK, Engelman DM, Johannesen E. In Vivo pH Imaging with Tc-99m-pHLIP. Molecular Imaging and Biology. 2012;14:725–734. doi: 10.1007/s11307-012-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daumar P, Wanger-Baumann CA, Pillarsetty N, Fabrizio L, Carlin SD, Andreev OA, Reshetnyak YK, Lewis JS. Efficient F-18-Labeling of Large 37-Amino-Acid pHLIP Peptide Analogues and Their Biological Evaluation. Bioconjugate Chem. 2012;23:1557–1566. doi: 10.1021/bc3000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vavere AL, Biddlecombe GB, Spees WM, Garbow JR, Wijesinghe D, Andreev OA, Engelman DM, Reshetnyak YK, Lewis JS. A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Res. 2009;69:4510–4516. doi: 10.1158/0008-5472.CAN-08-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.An M, Wijesinghe D, Andreev OA, Reshetnyak YK, Engelman DM. pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20246–20250. doi: 10.1073/pnas.1014403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM, Saltzman WM, Slack FJ. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA. pHLIP peptide targets nanogold particles to tumors. Proc. Natl. Acad. Sci. U. S. A. 2013;110:465–470. doi: 10.1073/pnas.1219665110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao L, Daniels J, Wijesinghe D, Andreev OA, Reshetnyak YK. pHLIP(R)-mediated delivery of PEGylated liposomes to cancer cells. J. Control. Release. 2013;167:228–237. doi: 10.1016/j.jconrel.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Z, Meng H, Wang N, Donovan MJ, Fu T, You M, Chen Z, Zhang X, Tan W. A controlled-release nanocarrier with extracellular pH value driven tumor targeting and translocation for drug delivery. Angew. Chem. Int. Ed. 2013;52:7487–7491. doi: 10.1002/anie.201302557. [DOI] [PubMed] [Google Scholar]
- 69.Onyango JO, Chung MS, Eng CH, Klees LM, Langenbacher R, Yao L, An M. Noncanonical amino acids to improve the pH response of pHLIP insertion at tumor acidity. Angew. Chem. Int. Ed. 2015;54:3658–3663. doi: 10.1002/anie.201409770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 71.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 74.Giambernardi TA, Grant GM, Taylor GP, Hay RJ, Maher VM, McCormick JJ, Klebe RJ. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998;16:483–496. doi: 10.1016/s0945-053x(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 75.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol. Rep. 2009;21:1323–1333. doi: 10.3892/or_00000358. [DOI] [PubMed] [Google Scholar]
- 76.Mansour AM, Drevs J, Esser N, Hamada FM, Badary OA, Unger C, Fichtner I, Kratz F. A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res. 2003;63:4062–4066. [PubMed] [Google Scholar]
- 77.Lee GY, Park K, Kim SY, Byun Y. MMPs-specific PEGylated peptide-DOX conjugate micelles that can contain free doxorubicin. Eur. J. Pharm. Biopharm. 2007;67:646–654. doi: 10.1016/j.ejpb.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 78.Chen WH, Luo GF, Lei Q, Jia HZ, Hong S, Wang QR, Zhuo RX, Zhang XZ. MMP-2 responsive polymeric micelles for cancer-targeted intracellular drug delivery. Chem. Commun. 2015;51:465–468. doi: 10.1039/c4cc07563c. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Zhang B, Luo Z, Ding X, Li J, Dai L, Zhou J, Zhao X, Ye J, Cai K. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale. 2015;7:3614–3626. doi: 10.1039/c5nr00072f. [DOI] [PubMed] [Google Scholar]
- 80.Zou Z, He X, He D, Wang K, Qing Z, Yang X, Wen L, Xiong J, Li L, Cai L. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials. 2015;58:35–45. doi: 10.1016/j.biomaterials.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 81.van Rijt SH, Bolukbas DA, Argyo C, Datz S, Lindner M, Eickelberg O, Konigshoff M, Bein T, Meiners S. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano. 2015;9:2377–2389. doi: 10.1021/nn5070343. [DOI] [PubMed] [Google Scholar]
- 82.He Q, Shi J. MSN anti-cancer nanomedicines: chemotherapy enhancement, overcoming of drug resistance, and metastasis inhibition. Advanced Materials. 2014;26:391–411. doi: 10.1002/adma.201303123. [DOI] [PubMed] [Google Scholar]
- 83.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17047–17052. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011;63:152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Wang HX, Yang XZ, Sun CY, Mao CQ, Zhu YH, Wang J. Matrix metalloproteinase 2-responsive micelle for siRNA delivery. Biomaterials. 2014;35:7622–7634. doi: 10.1016/j.biomaterials.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 87.Zhu L, Perche F, Wang T, Torchilin VP. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials. 2014;35:4213–4222. doi: 10.1016/j.biomaterials.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M, Ellies LG, Tsien RY. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 91.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 92.Tang L, Yang XJ, Yin Q, Cai KM, Wang H, Chaudhury I, Yao C, Zhou Q, Kwon M, Hartman JA, Dobrucki IT, Dobrucki LW, Borst LB, Lezmig S, Helferich WG, Ferguson AL, Fan TM, Cheng JJ. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15344–15349. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi HS, Liu WH, Liu FB, Nasr K, Misra P, Bawendi MG, Frangioni JV. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abe T, Sakamoto K, Kamohara H, Hirano Y, Kuwahara N, Ogawa M. Group II phospholipase A2 is increased in peritoneal and pleural effusions in patients with various types of cancer. Int. J. Cancer. 1997;74:245–250. doi: 10.1002/(sici)1097-0215(19970620)74:3<245::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 97.Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog. Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 98.Andresen TL, Davidsen J, Begtrup M, Mouritsen OG, Jorgensen K. Enzymatic release of antitumor ether lipids by specific phospholipase A2 activation of liposome-forming prodrugs. J. Med. Chem. 2004;47:1694–1703. doi: 10.1021/jm031029r. [DOI] [PubMed] [Google Scholar]
- 99.Linderoth L, Peters GH, Madsen R, Andresen TL. Drug delivery by an enzyme-mediated cyclization of a lipid prodrug with unique bilayer-formation properties. Angew. Chem. Int. Ed. 2009;48:1823–1826. doi: 10.1002/anie.200805241. [DOI] [PubMed] [Google Scholar]
- 100.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 101.Moyer MW. Targeting hypoxia brings breath of fresh air to cancer therapy. Nat. Med. 2012;18:636–637. doi: 10.1038/nm0512-636b. [DOI] [PubMed] [Google Scholar]
- 102.Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ. Targeting the metabolic microenvironment of tumors. Adv. Pharmacol. 2012;65:63–107. doi: 10.1016/B978-0-12-397927-8.00004-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kling J. Hypoxia-activated prodrugs forge ahead in cancer. Nat. Biotechnol. 2012;30:381. doi: 10.1038/nbt0512-381. [DOI] [PubMed] [Google Scholar]
- 104.Sun JD, Liu Q, Wang J, Ahluwalia D, Ferraro D, Wang Y, Duan JX, Ammons WS, Curd JG, Matteucci MD, Hart CP. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin. Cancer Res. 2012;18:758–770. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- 105.Lin Q, Bao C, Yang Y, Liang Q, Zhang D, Cheng S, Zhu L. Highly discriminating photorelease of anticancer drugs based on hypoxia activatable phototrigger conjugated chitosan nanoparticles. Adv. Mater. 2013;25:1981–1986. doi: 10.1002/adma.201204455. [DOI] [PubMed] [Google Scholar]
- 106.Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, Jo DG, Ahn CH, Suh YD, Kim K, Kwon IC, Lee DS, Park JH. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35:1735–1743. doi: 10.1016/j.biomaterials.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 107.Perche F, Biswas S, Wang T, Zhu L, Torchilin VP. Hypoxia-targeted siRNA delivery. Angew. Chem. Int. Ed. 2014;53:3362–3366. doi: 10.1002/anie.201308368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heppner GH, Miller BE. Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metast. Rev. 1983;2:5–23. doi: 10.1007/BF00046903. [DOI] [PubMed] [Google Scholar]
- 109.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895-1886. [DOI] [PubMed] [Google Scholar]
- 110.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 111.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 112.Sun Q, Sun X, Ma X, Zhou Z, Jin E, Zhang B, Shen Y, Van Kirk EA, Murdoch WJ, Lott JR, Lodge TP, Radosz M, Zhao Y. Integration of nanoassembly functions for an effective delivery cascade for cancer drugs. Adv. Mater. 2014;26:7615–7621. doi: 10.1002/adma.201401554. [DOI] [PubMed] [Google Scholar]
- 113.Li L, Sun W, Zhong JJ, Yang QQ, Zhu X, Zhou Z, Zhang ZR, Huang Y. Multistage Nanovehicle Delivery System Based on Stepwise Size Reduction and Charge Reversal for Programmed Nuclear Targeting of Systemically Administered Anticancer Drugs. Adv. Funct. Mater. 2015;25:4101–4113. [Google Scholar]
- 114.Yu HJ, Cui ZR, Yu PC, Guo CY, Feng B, Jiang TY, Wang SL, Yin Q, Zhong DF, Yang XL, Zhang ZW, Li YP. pH- and NIR Light-Responsive Micelles with Hyperthermia-Triggered Tumor Penetration and Cytoplasm Drug Release to Reverse Doxorubicin Resistance in Breast Cancer. Adv. Funct. Mater. 2015;25:2489–2500. [Google Scholar]