Abstract
A few studies have suggested an association between maternal exposure to ambient air pollution from vehicular traffic and risk of congenital anomalies in the offspring, but epidemiologic evidence is neither strong nor entirely consistent.
In a population-based case-control study in a Northern Italy community encompassing 228 cases of birth defects and 228 referent newborns, we investigated if maternal exposure to PM10 and benzene from vehicular traffic during early pregnancy, as estimated through a dispersion model, was associated with excess teratogenic risk.
In conditional logistic regression analysis, and with adjustment for the other pollutant, we found that higher exposure to PM10 but not benzene was associated with increased risk of birth defects overall. Anomaly categories showing the strongest dose-response relation with PM10 exposure were musculoskeletal and chromosomal abnormalities but not cardiovascular defects, with Down syndrome being among the specific abnormalities showing the strongest association, though risk estimates particularly for the less frequent defects were statistically very unstable. Further adjustment in the regression model for potential confounders did not considerably alter the results. All the associations were stronger for average levels of PM10 than for their maximal level.
Findings of this study give some support for an excess teratogenic risk following maternal exposure during pregnancy to PM10, but not benzene. Such association appears to be limited to some birth defect categories.
Keywords: Birth defects, air pollution, PM10, benzene, epidemiology, case-control study
Background
During the last few years, a growing number of epidemiologic studies has addressed the possible teratogenic role of maternal exposure to ambient pollution from vehicular traffic and other sources, and such an association is supported by biological plausibility taking into account the toxic effects of the contaminants and their mixtures released by motorized sources (Farhi et al., 2014; Gianicolo et al., 2014; Liang et al., 2014; Lin et al., 2014; Schembari et al., 2014; Tanner et al., 2015; Vinikoor-Imler et al., 2015). Some epidemiologic investigations have found an increased risk of some categories of congenital anomalies or specific defects in the offspring of mothers exposed to high levels of air pollution, but not all studies are consistent and only some of the contaminants have been linked to excess prevalence at birth of malformations (Vrijheid et al., 2011; Chen et al., 2014). These inconsistencies may be due to methodological reasons, such as inadequate exposure assessment, or to unmeasured confounding, both common potential limitations of epidemiological studies on the health effect of air pollutants (Ritz and Wilhelm, 2008; Vrijheid et al., 2011; Chen et al., 2014; Filippini et al., 2015) and generally to observational studies. However, due to the growing number of observations showing a direct relation between maternal exposure to air toxics and risk of birth defects in the offspring, the teratogenic potential of some of the contaminants released by motorized traffic with associated widespread human exposure, and the substantial advancements of epidemiologic research on the assessment of exposure to outdoor air pollutants, such an association appears clearly worth further investigation. We sought to address this issue through a population-based case-control study in a northern Italian community.
Methods
Study population
We used a community-based series of birth defects and referent births to investigate the teratogenic risk of environmental exposures, whose selection methodology has been already described in detail (Vinceti et al., 2009; Malagoli et al., 2012). Briefly, we identified cases of congenital anomalies in the offspring or in aborted foetuses following voluntary pregnancy termination (induced abortions) of women residing in the Northern Italy Reggio Emilia municipality (around 150,000 inhabitants) from January 1, 1998 – December 31, 2006. We used two data sources to identify these cases, the IMER (Indagine sulle Malformazioni congenite in Emilia-Romagna) population-based registry of congenital malformations of the Emilia-Romagna region linked to the European network ‘Eurocat’ (Calzolari et al., 2003; Vinceti et al., 2014), and the Hospital Discharge Directory of Emilia-Romagna residents, available at the Regional Health Authority. Discharges reporting ICD9-CM codes from 740.0 to 759.9 were reviewed by a clinical geneticist (E.C.), and the cases of minor malformations according to the Eurocat guidelines were removed from further analysis.
Concerning control births, we randomly extracted from the regional Hospital Discharge Directory a series of control births among livebirths not reporting a diagnosis of malformation, matched 1:1 to the case mother for age and for hospital and year of delivery. We collected information about historical residence for both case and referent mothers within the 9 month period before parturition (or 3 months before induced abortion) as well as their educational attainment level and country of birth from the Reggio Emilia Municipality, along with the familial annual income from the national Revenue Agency. When the woman changed her residence during the above period, residence in the first three months after the estimated date of conception was used to assess exposure. For the three women who changed their residence during this early gestational period, we selected the longest period of residence within this time span to attribute exposure status. We geocoded maternal addresses using the database made available to us by the Reggio Emilia Province or, when unavailable through this source, by measuring it on site through a geographical positioning system device (Garmin GPSmap 60CSx, Garmin Int. Corp., Olathe, KS).
Exposure assessment
We modeled the estimated average and maximum hourly concentration exposure to two major outdoor air pollutants, particulate matter less than 10 micrometers in diameter (PM10) and benzene, at the geocoded maternal residence of interest within a Geographical Information System database. We reported in detail in a previous study (Vinceti et al., 2012) the methodology used to assess dispersion of the two pollutants, based on the CAlifornia Line Source Dispersion Model version 4 (Department of Transportation - Division of New Technology and Research, 1989). This model is a stationary plume dispersion model for roads and other linear sources, suitable for assessment of dispersion and deposition of air pollutants and particulate matter at predefined spatial receptors. We imputed in the model PM10 and benzene emissions from vehicular traffic based on traffic flows in 2005 on roads of the municipality accounting for nearly 90% of the overall vehicular traffic, applied over one year to take into account daily, weekly, and seasonal variation in weather and traffic conditions, as well as meteorological data. The model predicted hourly PM10 and benzene concentrations at maternal residence at a height of 2 meters. Traffic flow estimates were generated in a model which took into account demographic and occupational information for all municipal residents and personal mobility information collected by the National Institute of Statistics 2001 Census; this model was subsequently validated through surveys and with automatic vehicles counters by the Reggio Emilia municipality. The model was based on a matrix of vehicle movements for each road and the daily movements estimated for their residents taking into account their age, sex, family structure and occupation, further validated by a survey of randomly selected families and car drivers carried out in 2005 (Gandolfi et al., 2008). Emission factors for benzene, and specifically for light and heavy vehicles and for urban and suburban areas, were derived from a 1990–2007 national transport database developed by the Italian National Institute for Environmental Protection and Research (www.isprambiente.gov.it) and computed with the COPERT IV software developed by the Applied Thermodynamics Lab of the Thessaloniki University (http://emisia.com/sites/default/files/COPERT4_v7_0.pdf). Mean values of these emission factors (calculated from the number of vehicles registered and from the relative annual average mileage) were 23.5 mg/km and 0.82 mg/km for light and heavy vehicles, respectively, in the urban cycle, and 2.96 mg/km and 0.31 mg/km in the rural cycle. As suggested by the CALINE4 Technical Guide, we ran the model after increasing the road width by 3 meters to the right and left, to account for thermal and mechanical turbulence caused by vehicles. PM10 emission factors, which included both exhaust (emissions from tailpipes, obtained from ISPRA database using COPERT IV) and non-exhaust components (abrasion and resuspension processes, obtained from Gehrig determination), were 105.7 mg/km and 1054.6 mg/km for light and heavy vehicles, respectively, in urban areas, and 62.3 mg/km and 337.0 mg/km in rural areas. Meteorological data were obtained using the meteorological model CALMET of the Hydro Meteorological Service of the Emilia-Romagna environmental protection agency ARPA, which provided information on measured data temperature, wind speed and direction, stability class and height of the mixing layer (Vinceti et al., 2012). The model showed a strong variability of air levels of PM10 and benzene at the different children’s residences thus suggesting considerable within-municipality variability, as expected considering that the land use of the Reggio Emilia municipality was 75.3% rural and 23.1% urban according to the 2003 regional aerial mapping (Malagoli et al., 2015 (in press)). Finally, we validated our model by performing a comparison between the modeled and the measured average annual levels of the two pollutants at three air quality monitoring stations in Reggio Emilia and 4 available monitoring stations in the nearby Modena municipality (Vinceti et al., 2012). The Pearson correlation coefficient between the estimated and the measured yearly mean levels was 0.43 (95% CI −0.48–0.89) for benzene and for 0.64 (95% CI −0.21–0.94) for PM10.
Data analysis
We calculated the odds ratio (OR) estimating prevalence of congenital anomalies at birth or in the aborted foetus associated with maternal exposure to PM10 and benzene (expressed as average and maximum hourly concentration), along with its 95% confidence interval (CI), within a conditional logistic regression model. When this matched analysis did not yield a valid estimate, as occurred for single anomalies with very low prevalence, we used unconditional logistic regression adjusted for maternal age. Potentially predictive variables were modelled exposure to PM10 and benzene as well as residential exposure to magnetic fields from high-voltage power lines (Malagoli et al., 2012) and dioxin air levels from the municipal solid waste incinerator (Vinceti et al., 2009), as well as maternal educational attainment level, world area of birth, and family income (entered as continuous variable). For magnetic field exposure, we used the exposure categories of 0–0.1, 0.1–0.2, 0.2–0.4, >0.4 as described in detail elsewhere (Malagoli et al., 2012), while for dioxin exposure we attributed the exposure status according to the maternal residence in the low, intermediate and high exposure areas already defined using a priori specified cutpoints of exposure (Vinceti et al., 2012). We carried out analyses for overall congenital anomalies, single anomaly categories or specific birth defects.
For the birth defect categories found to be associated with PM10 levels, we also modeled the relation using restricted cubic spline regression analysis, implemented by the mkspline and xblc commands in Stata 13 (Orsini and Greenland, 2011) while simultaneously adjusting for benzene. Knots were placed at the 10th, 50th, and 90th percentiles.
Results
Overall, we identified 228 cases of congenital anomalies during the study period: 183 live- or stillbirths presenting with one or more defects or congenital syndromes and 45 induced abortions following antenatal detection of single or multiple anomalies. There were 191 cases with one birth defect identified, and 37 with multiple abnormalities. Overall, this number of major birth defects and their specific pattern for the Reggio Emilia community (characterized by 14,754 livebirths over the study period) were consistent with what observed over time and in the entire region according to the IMER Registry (Cocchi et al., 1996; Calzolari et al., 2003; Astolfi et al., 2013). Among cases, the maternal ages at birth or time of abortion ranged from 16 to 44 years; regarding educational attainment level, a similar proportion of case and referent women had rather similar educational attainment, since their upper level was elementary school for 19 case mothers (8.33%) and 9 for referents (3.95%), middle school 83 (36.40%) and 78 (34.21%) respectively, high school 90 (39.47%) and 104 (45.61%), and university degree 36 (15.79%) and 37 (16.23%) (chi-square P value=0.314). Cases and controls had rather comparable annual average family income, of 75,670 euros (standard deviation 171,417) and 63,737 euros (52,700) respectively (P-value of t-test for comparison= 0.440). An analysis of historical residence of these women, carried out in a random sample of 40, indicated that in the 12 months before the estimated conception date, 87.5% of them had resided at the same address throughout.
We report in Table 1 the exposure pattern in the study population, as estimated by our land-use regression analysis and Caline4 dispersion modeling. Using both a priori defined cutpoints of exposure and continuous levels of exposure and after adjusting for the other pollutant, we observed increased ORs of any birth defects with increased average and maximum PM10 exposure (Table 2), while no such relation emerged for average and maximal benzene exposure. The association between maximal PM10 exposure and overall risk of congenital anomalies was both weaker and more statistically unstable than that for corresponding average exposure. Results were similar when we simultaneously included in the model additional potential confounders, including maternal educational attainment and race, family income, estimated season of conception, season of delivery, exposure to magnetic fields from high-voltage power lines, and dioxin release from the local municipal solid waste incinerator, though the addition of these variables widened confidence intervals.
Table 1.
Distribution of annual average and maximum hourly pollutant concentration (µg/m3) in outdoor ambient air for the study population
| 5th | 50th | 95th | Mean (SD) | Min | Max | |
|---|---|---|---|---|---|---|
| Average PM10 level | ||||||
| Cases | 2.24 | 5.47 | 10.15 | 5.68 (2.48) | 1.68 | 16.04 |
| Controls | 2.09 | 5.40 | 9.08 | 5.46 (2.19) | 1.68 | 13.42 |
| Maximum PM10 level | ||||||
| Cases | 50.69 | 71.97 | 113.95 | 74.73 (17.02) | 27.88 | 145.12 |
| Controls | 44.82 | 70.91 | 112.78 | 74.21 (17.72) | 27.22 | 166.73 |
| Average benzene level | ||||||
| Cases | 0.15 | 0.57 | 1.19 | 0.62 (0.35) | 0.06 | 2.33 |
| Controls | 0.12 | 0.59 | 1.25 | 0.63 (0.35) | 0.10 | 1.79 |
| Maximum hourly benzene level | ||||||
| Cases | 3.39 | 6.24 | 9.57 | 6.45 (1.85) | 2.16 | 16.79 |
| Controls | 3.71 | 6.48 | 10.01 | 6.59 (1.66) | 2.13 | 13.59 |
Table 2.
Odds ratio (OR) with 95% confidence interval (95% CI) for any congenital anomaly associated with categories of maternal exposure to PM10 and benzene, simultaneously adjusting for the other pollutant1
| Exposure category µg/m3 | |||||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| Referent | OR (95%CI) | OR (95%CI) | OR (95%CI) | P trend2 | |
| Average PM10 | < 2.5 | 2.5 – < 5 | 5 – < 7.5 | ≥ 7.5 | |
| 15/24 | 81/75 | 92/91 | 40/38 | ||
| 1.0 | 2.10 (0.95–4.63) | 2.30 (0.95–5.58) | 2.65 (0.94–7.49) | 0.073 | |
| Maximum PM10 | < 50 | 50 – < 75 | 75 – < 100 | ≥ 100 | |
| 10/13 | 132/134 | 68/66 | 18/15 | ||
| 1.0 | 1.60 (0.65–3.93) | 1.85 (0.68–5.04) | 2.14 (0.66–7.01) | 0.456 | |
| Average benzene | < 0.10 | 0.10 – < 0.25 | 0.25 – < 0.50 | ≥ 0.50 | |
| 3/2 | 30/30 | 65/58 | 130/138 | ||
| 1.0 | 0.61 (0.10–4.00) | 0.63 (0.10–3.82) | 0.45 (0.07–2.83) | 0.128 | |
| Maximum benzene | < 4 | 4 – < 6 | 6 – < 8 | ≥ 8 | |
| 14/12 | 78/67 | 107/117 | 29/32 | ||
| 1.0 | 0.87 (0.36–2.08) | 0.67 (0.27–1.64) | 0.64 (0.23–1.78) | 0.275 | |
Based on a conditional logistic regression model, adjusting for the average and maximum hourly exposure to the other pollutant
P for linear trend using continuous level of pollutant exposure
Limiting the analysis to specific anomaly categories and using continuous values of average PM10 exposure, we observed a tendency towards an excess prevalence of chromosomal, eye and musculoskeletal anomalies and oral clefts in relation to higher exposure (Table 3). However, for eye malformations and clefts the risk estimates were extremely imprecise, as reflected by wide confidence intervals. On the converse, no evidence of increased risk emerged for defects of the nervous and the genitourinary system (with the partial exception of the highest exposure category), as well as for digestive, respiratory and skin anomalies, though for the latter defect categories numbers of cases were too low to yield valid or meaningful estimates. For cardiovascular disease, all exposure strata above the bottom one showed an OR considerably exceeding one, but no dose-response relation emerged in either category or trend analysis. On the converse, a dose-response monotonic relation could be detected for chromosomal defects and, in particular, for musculoskeletal anomalies. When we used maximum PM10 exposure instead of average PM10 to assess exposure, the ORs were generally lower compared with those associated with the corresponding averages. On the converse, as also shown in Table 2, little evidence of any increased prevalence for overall congenital anomalies emerged for benzene exposure after adjustment for PM10, and this was also true for the single defect categories (data not shown). Stratifying the analysis according to maternal age at delivery as shown in Table 4, evidence for a direct association between PM10 exposure and overall birth defect prevalence was much stronger for the younger age group, also due to an increased risk in this population of cardiovascular abnormalities, while this was not true for other birth defect categories such as musculoskeletal and chromosomal anomalies.
Table 3.
Odds ratio (OR) for congenital anomalies with 95% confidence intervals (95% CI) according to maternal exposure to average exposure to PM10 and benzene from vehicular traffic in Reggio Emilia, northern Italy, 1998–20061
| Average PM10 levels | Cases | Controls | OR (95% CI) | ||||
| n | (%) | n | (%) | ||||
| All abnormalities (N=228) | |||||||
| < 2.5 | 15 | (6.6) | 24 | (10.5) | 1.00 (referent) | ||
| 2.5 – < 5 | 81 | (35.5) | 75 | (35.9) | 2.10 (0.95–4.63) | ||
| 5 – < 7.5 | 92 | (40.4) | 91 | (39.9) | 2.30 (0.95–5.58) | ||
| ≥ 7.5 | 40 | (17.5) | 38 | (16.7) | 2.65 (0.94–7.49) | ||
| Continuous OR=1.16 (0.99–1.26), P trend=0.073 | |||||||
| Cardiovascular (N=96) | |||||||
| < 2.5 | 5 | (5.2) | 12 | (12.5) | 1.00 (referent) | ||
| 2.5 – < 5 | 38 | (39.6) | 30 | (31.3) | 4.63 (0.98–21.92) | ||
| 5 – < 7.5 | 41 | (42.7) | 37 | (38.5) | 4.78 (0.85–26.77) | ||
| ≥ 7.5 | 12 | (12.5) | 17 | (17.7) | 3.17 (0.45–22.24) | ||
| Continuous OR=1.05 (0.88–1.25), P trend=0.615 | |||||||
| Nervous system (N=23) | |||||||
| < 2.5 | 1 | (4.4) | 3 | (13.1) | 1.00 (referent) | ||
| 2.5 – < 5 | 9 | (39.1) | 8 | (34.8) | –2 | ||
| 5 – < 7.5 | 9 | (39.1) | 7 | (30.4) | –2 | ||
| ≥ 7.5 | 4 | (17.4) | 5 | (21.7) | –2 | ||
| Continuous OR=0.90 (0.60–1.36), P trend=0.626 | |||||||
| Chromosomal (N=41) | |||||||
| < 2.5 | 3 | (7.3) | 4 | (9.7) | 1.00 (referent) | ||
| 2.5 – < 5 | 11 | (26.8) | 17 | (41.5) | 2.61 (0.28–24.61) | ||
| 5 – < 7.5 | 21 | (51.2) | 13 | (31.7) | 11.59 (0.84–159.9) | ||
| ≥ 7.5 | 6 | (14.7) | 7 | (17.1) | 11.10 (0.57–214.8) | ||
| Continuous OR=1.52 (0.98–2.36), P trend=0.060 | |||||||
| Genitourinary (N=21) | |||||||
| < 2.5 | 3 | (14.3) | 1 | (4.8) | 1.00 (referent) | ||
| 2.5 – < 5 | 9 | (42.9) | 9 | (42.8) | 0.64 (0.04–12.54) | ||
| 5 – < 7.5 | 5 | (23.8) | 10 | (47.6) | 0.40 (0.28–5.65) | ||
| ≥ 7.5 | 4 | (19.0) | 1 | (4.8) | 2.90 (0.03–278.4) | ||
| Continuous OR=1.12 (0.60–2.10), P trend=0.724 | |||||||
| Musculoskeletal (N=39) | |||||||
| < 2.5 | 2 | (5.1) | 4 | (10.3) | 1.00 (referent) | ||
| 2.5 – < 5 | 14 | (35.9) | 13 | (33.3) | 4.90 (0.43–56.30) | ||
| 5 – < 7.5 | 14 | (35.9) | 14 | (35.9) | 8.64 (0.45–166.3) | ||
| ≥ 7.5 | 9 | (23.1) | 8 | (20.5) | 19.05 (0.49–739.7) | ||
| Continuous OR=1.34 (0.94–1.91), P trend=0.106 | |||||||
| Clefts (N=4) | |||||||
| < 2.5 | 1 | (25.0) | 0 | (0.0) | 1.00 (referent) | ||
| 2.5 – < 5 | 1 | (25.0) | 2 | (50.0) | –2 | ||
| 5 – < 7.5 | 0 | (0.0) | 2 | (50.0) | –2 | ||
| ≥ 7.5 | 2 | (50.0) | 0 | (0.0) | –2 | ||
| Continuous OR=1.88 (0.32–10.96), P trend=0.032 | |||||||
| Average benzene levels | Cases | Controls | OR (95% CI) | ||||
| n | (%) | n | (%) | ||||
| All abnormalities (N=228) | |||||||
| < 2.5 | 15 | (6.6) | 24 | (10.5) | 1.00 (referent) | ||
| 2.5 – < 5 | 81 | (35.5) | 75 | (35.9) | 0.61 (0.09–4.00) | ||
| 5 – < 7.5 | 92 | (40.4) | 91 | (39.9) | 0.63 (0.10–3.82) | ||
| ≥ 7.5 | 40 | (17.5) | 38 | (16.7) | 0.45 (0.07–2.83) | ||
| Continuous OR=0.55 (0.26–1.19), P trend=0.128 | |||||||
Computed with conditional logistic regression for categorical and for continuous exposure levels, adjusting for average levels of the other pollutants
No valid estimate could be computed
Table 4.
Age-specific odds ratios (OR) with 95% confidence intervals (95% CI) for congenital anomalies associated with average maternal exposure to PM10
| Maternal age (years) | OR (95% CI)1 | P trend1 |
|---|---|---|
| All abnormalities | ||
| <30 | 1.36 (1.04–1.81) | 0.027 |
| ≥30 | 1.02 (0.89–1.18) | 0.764 |
| Musculoskeletal | ||
| <30 | 1.12 (0.70–1.79) | 0.636 |
| ≥30 | 1.54 (0.87–2.72) | 0.142 |
| Chromosomal | ||
| <30 | 1.48 (0.73–2.93) | 0.276 |
| ≥30 | 1.60 (0.91–2.83) | 0.104 |
| Cardiovascular | ||
| <30 | 1.48 (0.90–2.41) | 0.121 |
| ≥30 | 0.86 (0.67–1.10) | 0.239 |
Linear trend estimates based on continuous level of PM10 exposure, adjusting for average benzene exposure
When we investigated in detail the risk of specific defects associated with pollutant exposure (Table 5), we found little evidence of an association with benzene exposure and in most cases also with PM10 exposure, though for most rare defects we could not compute valid estimates, and in addition estimates further decreased their statistical stability after the addition of potential confounders (dioxin exposure from municipal solid waste incinerator, magnetic fields from power lines, maternal education and race - data not shown). However, we observed an increased through statistically imprecise odds ratio for limb defects overall considered and for syndactyly, valgus deformity of the foot, Edward’s syndrome and particularly Down’s syndrome in relation to PM10 (Table 5), while benzene exposure was associated with somewhat increased but statistically unstable risk of spina bifida (4 cases, OR=5.05, 95% CI=0.01–4181, P=0.636), hydrocephalus (5 cases, 1728.3, 0.03-∞, 0.178), and hypospadias (3 cases, 48.68, 0.02–157736, 0.346), but not genitourinary anomalies.
Table 5.
Odds ratio (OR) with 95% confidence intervals (95% CI) for specific congenital anomalies according to maternal exposure to emissions to PM10 from vehicular traffic in Reggio Emilia, northern Italy, 1998–20061
| Average PM10(µg/m3)
Continuous exposure, 1 unit increase |
|||
|---|---|---|---|
| Cases | OR2(95%CI) | P | |
| Pulmonary valve stenosis | 5 | 0.04 (0.00–23.78) | 0.317 |
| Ventricular septal defect | 48 | 1.05 (0.84–1.32) | 0.670 |
| Atrial septal defect | 14 | 1.11 (0.66–1.86) | 0.688 |
| Esophageal atresia | 2 | 0.68 (0.12–3.73) | 0.657 |
| Varus deformity of foot | 11 | 1.02 (0.60–1.73) | 0.937 |
| Valgus deformity of foot | 4 | 2.10 (0.36–12.30) | 0.411 |
| Limb defects overall considered | 14 | 2.08 (0.78–5.56) | 0.143 |
| Reduction deformities of the limb | 3 | 0.08 (0.00–3.51) | 0.194 |
| Syndactyly, | 1 | 1.87(0.92–3.79) | 0.083 |
| Polydactyly | 8 | 1.11 (0.81–1.52) | 0.515 |
| Anomalies of abdominal wall | 4 | 1.24 (0.86–1.76) | 0.246 |
| Hypospadias | 3 | 0.56 (0.11–2.88) | 0.484 |
| Spina bifida | 4 | 0.73 (0.27–1.96) | 0.531 |
| Down’s syndrome | 21 | 2.30 (0.92–5.74) | 0.074 |
| Edward’s syndrome | 10 | 2.16 (0.42–11.11) | 0.357 |
Adjusting for average exposure to the other pollutant in conditional logistic regression, except for reduction deformities of the limb, syndactyly, polydactyly, anomalies of abdominal wall and hypospadias for which an unconditional logistic regression model adjusted for maternal age was used
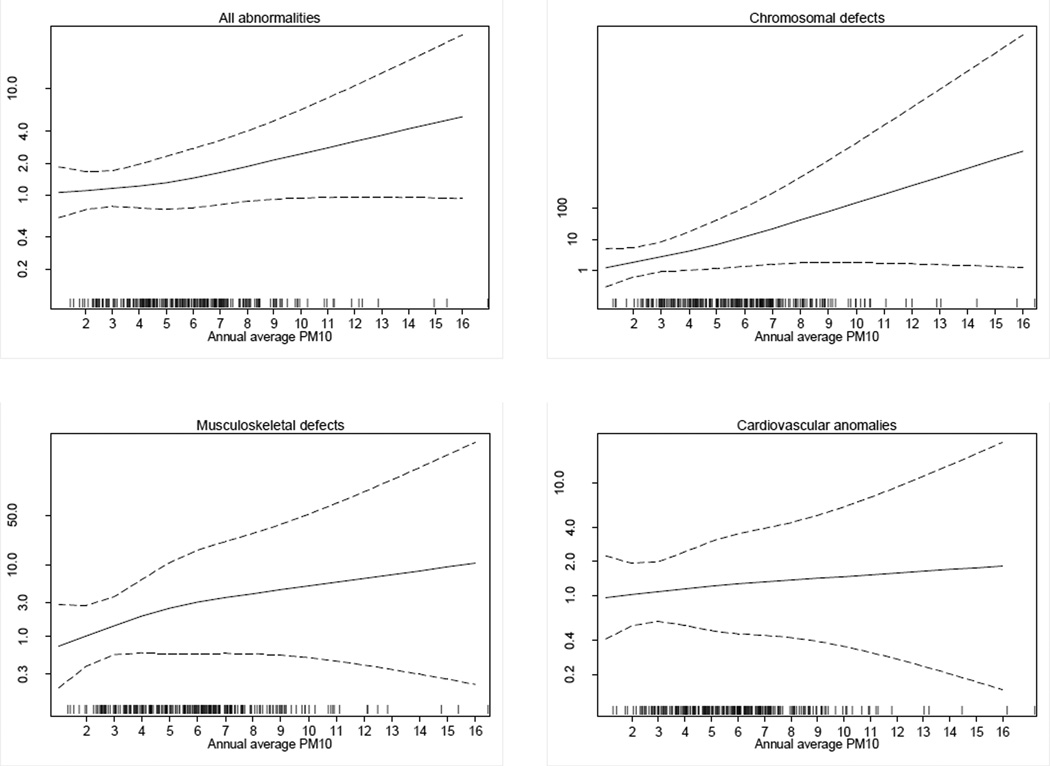
Plots estimating the relation between the odds of being a case and PM10 exposure for the relations identified above are provided in Figure 1. The natural cubic splines showed a direct relation between PM10 and risk of overall birth defects and chromosomal or musculoskeletal defects, while the relation with cardiovascular anomalies appeared to be much weaker.
Figure 1.
Natural cubic splines of the odds of being a case of birth defect according to PM10, Reggio Emilia municipality – Italy, 1998–2006 (dotted lines, 95% confidence limits), simultaneously adjusting for benzene
Discussion
Results of this study show a tendency towards an overall higher risk of congenital anomalies among women exposed during pregnancy to higher levels of a traffic pollutant, PM10, while no such association emerged for the other contaminant investigated, benzene. The involvement of particulate matter emerged only for its average outdoor values and not for its maximum levels, suggesting that time-integrated long-term exposure is more plausibly associated with disease risk compared with higher but more transient values. Our results are consistent with other studies which found direct associations between PM10 exposure and the risk of birth defects (Kim et al., 2007; Farhi et al., 2014; Liang et al., 2014), though others found little evidence of such an association (van den Hooven et al., 2009; Gianicolo et al., 2014). The increased risk for overall birth defects in relation to PM10 exposure in our study was mainly driven by an association with musculoskeletal anomalies and with chromosomal defects, with the former group showing a clear and strong dose-response relation across categories of exposure. Such relations are interesting, but their evaluation is not easy. In fact, musculoskeletal defects unfortunately have received little attention in epidemiologic research on the teratogenic effects of air pollution, with only one exception we are aware of which found no association with black smoke (particulate matter < 4µg/m3) exposure (Rankin et al., 2009). Therefore, this finding of the present study strongly calls for further research on this issue. Concerning chromosomal anomalies, a large investigation carried out in England has suggested an association of chromosomal defects and specifically Down syndrome with PM10 exposure in the 1991–1999 period (Dolk et al., 2010), while such an association was not detected in another smaller English study during 1985–90 in association to black smoke exposure (Rankin et al., 2009). Chromosomal defects were also found to be associated with first-trimester pregnancy modelled PM10 exposure in Israel, with a prevalence OR of 1.14 (95% CI 1.01–1.28) for 10 µg/m3 increase and 1.54 (95% CI 0.93–2.57) when comparing the highest vs. the lowest tertile of exposure (Farhi et al., 2014).
Our study gave some indication also for an association between PM10 exposure and risk of oral clefts, but the relative risk estimates were statistically very unstable due to the very low number of observed cases, and there has been limited support for such an association in the literature (Gilboa et al., 2005; Hwang and Jaakkola, 2008; Hansen et al., 2009; Dolk et al., 2010; Marshall et al., 2010) as recognized in recent reviews (Vrijheid et al., 2011; Chen et al., 2014). The excess prevalence of genitourinary defects in the highest exposure category is difficult to interpret, and conflicts with some other studies (Rankin et al., 2009; Padula et al., 2013c; Farhi et al., 2014; Schembari et al., 2014; Vinikoor-Imler et al., 2015) and lacks both statistical stability and a dose-response relation in our study population.
For cardiovascular defects, the results we obtained were rather equivocal and substantially null, since there was an excess risk in all exposure categories above the bottom one but no evidence of a dose-response relation, confirming the previous largely negative observations (Ritz et al., 2002; Hansen et al., 2009; Strickland et al., 2009; Dolk et al., 2010; Dadvand et al., 2011; Vrijheid et al., 2011; Gianicolo et al., 2014; Schembari et al., 2014; Vinikoor-Imler et al., 2015) with two exceptions (Agay-Shay et al., 2013; Farhi et al., 2014) and the recent report of an association with PM2.5 exposure (Tanner et al., 2015). Among specific cardiovascular anomalies, we found little evidence of increased risk for defect subtypes including atrial septal defects, a subgroup for which an association with maternal PM10 exposure was suggested by recent meta-analysis (Vrijheid et al., 2011) due to elevated estimates in several studies (Gilboa et al., 2005; Hansen et al., 2009; Strickland et al., 2009), and more recently confirmed in a study carried out in Israel for overall pregnancy exposure but not in the first trimester (Farhi et al., 2014). Our study also does not support previous occasional reports of excess risk for pulmonary valve stenosis or ventricular septal defects (Padula et al., 2013b; Farhi et al., 2014) nor of other cardiovascular defects recently found to be associated with maternal PM2.5 exposure in early pregnancy (Tanner et al., 2015).
Concerning other rare anomalies previously found to be associated with particulate matter exposure, little evidence has emerged apart from an increased risk of chromosomal anomalies such as Down and Edward’s syndrome, while other increased risks such as that for esophageal atresia were too statistically unstable to allow meaningful evaluation, despite a previous report of excess risk among exposed mothers (Padula et al., 2013c). The statistically imprecise but marked excess risk of limb defects, based on fourteen observed cases, is difficult to evaluate since previous studies yielded little evidence of any association but focused only on limb reduction (Dolk et al., 2010; Padula et al., 2013c; Schembari et al., 2014), with one exception (Lin et al., 2014). This excess risk of limb defects also contributed to a major finding of our study, the increased risk of musculoskeletal anomalies associated with higher particulate matter exposure.
A direct association between PM10 and risk of overall and category-specific birth defects is supported by the reliability of the model we used, as shown by the high correlation between measured and modelled values for the few air monitoring stations, with a Pearson coefficient of 0.64 (Vinceti et al., 2012) even if traffic emissions are not usually the only sources of such pollutant in urban areas. In addition, the population we studied were very residentially stable both across the gestational period and beforehand. This suggests that the differential in exposure status between case and control mothers, as estimated for the first trimester of pregnancy, was generally similar in the preconceptional period with little potential for differential exposure misclassification (Chen et al., 2010).
Clearly, no inference can be done on the specific biological and toxicological relation between PM10 exposure and teratogenic risk seen in the present study, since particulate matter may only be a proxy for exposure to one or more chemical contaminants which in turn are responsible for the increased birth defect prevalence.
Study results do not point to a major role of benzene exposure in enhancing teratogenic risk. Benzene levels in the study setting were lower than the national and European standard of 5 µg/m3, though they correlated with childhood leukemia risk in a previous investigation in the study area (Vinceti et al., 2012). Overall, the biological plausibility of an association between benzene and birth defects is also limited. It should however be noted that the correlation between measured and modelled values at air monitoring stations for benzene was slightly lower than that for PM10, thus raising some potential for non-differential exposure misclassification. In addition, the statistically imprecise indication of a possible higher risk of nervous system defects associated with benzene exposure may be worth further investigation, taking into consideration the direct association with neural tube defects in a previous study which specifically investigated the teratogenic effects of benzene exposure (Lupo et al., 2011) and the growing literature on traffic pollution and neurodevelopmental disorders (Newman et al., 2013; Kalkbrenner et al., 2015). It is worth noting that nervous system defects were not related with PM10 exposure in the present study, consistent with the results of a recent large study on neural tube defects (Padula et al., 2013a) but not with another study (Rankin et al., 2009). Unfortunately, our study was underpowered to assess the potential association between maternal benzene exposure and risk of oral clefts in the offspring, about which conflicting results have been reported by two recent studies carried out in Texas (Ramakrishnan et al., 2013) and Florida (Tanner et al., 2015).
A major limitation of our study was its size, which increased the statistical imprecision of risk estimates and precluded an reliable analysis of the most rare birth defects, which occasionally have been found to be associated with particulate matter exposure in previous investigations. In addition, we did not include in our air pollution model two potential sources of benzene and PM10 exposure, boilers for home heating and industrial sources. However, it is unlikely that these sources made a relevant contribution to outdoor benzene and PM10, since by far most home boilers in Reggio Emilia are powered by methane gas or gas liquid propane (over 97% according to data collected in 2010 by the Regional Environmental Protection Agency www.arpa.emr.it/cms3/documenti/_cerca_doc/meteo/ambiente/consumo-legna-er.pdf), which are not associated with emission of these two pollutants, and since the number of industries located in the region is extremely low and no heavy plant exists, with the possible exception of the former municipal waste incinerator whose emissions were taken into account in the multivariate model.
Another study limitation may have been unmeasured confounding. We did not assess exposure to other air pollutants such as carbon monoxide, nitrogen oxides and ozone, though their correlation with PM10 and benzene exposure may not have been very high due to the relevant role of domestic sources (heat boilers) in these emissions. Due to the study design we also lacked information on some lifestyle factors, such as smoking, which may well have acted as confounders in our analysis. However, when we adjusted our analysis for parental educational attainment and paternal income we did not observe major changes in risk estimates, suggesting that this type of unmeasured confounder was not strongly relevant. Smoking may not only have been a confounder in our study due to its independent teratogenicity, but it may also have induced some degree of exposure misclassification, being a major source of indoor PM10 release (Slezakova et al., 2014) and thus reducing the correlation between indoor and outdoor particulate matter (Happo et al., 2013). However, there is evidence that outdoor sources are significant independent contributors of indoor PM10, thus reducing the effects of such exposure misclassification (Gilboa et al., 2005; Custodio et al., 2014; Rovelli et al., 2014). Finally, we did not include in our study population the spontaneous abortions, and in addition the IMER Registry has always had serious concerns regarding its completeness of induced abortion coverage (E. Calzolari and G. Astolfi, unpublished data). This suggests that for some serious defects incompatible with pregnancy progression or frequently leading to its termination, such as neural tube defects, chromosomal anomalies, or severe cardiovascular abnormalities, our analysis was likely very underpowered.
In conclusion, results of the present study appear to support an association between PM10 pollution from vehicular traffic and overall risk of birth defects, mainly driven by an excess risk of musculoskeletal and chromosomal anomalies, while they offer little support for a teratogenic effect of benzene at the levels experienced in the study area. Though further investigations to validate such associations are clearly required, these findings add to the available epidemiologic evidence suggesting the opportunity to minimize exposure to particulate matter during and before pregnancy.
Acknowldgements
The study was supported by the Local Health Unit of Reggio Emilia and the Fondazione Pietro Manodori di Reggio Emilia (Dr. Vinceti), and the US National Institute of Health (grants R21CA175959, R03ES021643 to Dr. Heck).
References
- Agay-Shay K, Friger M, Linn S, Peled A, Amitai Y, Peretz C. Air pollution and congenital heart defects. Environ Res. 2013;124:28–34. doi: 10.1016/j.envres.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Astolfi G, Bianchi F, Lupi C, Napoli N, Neville A, Verdini E, et al. Using hospital discharge records, birth certificates and a birth defects registry for epidemiological and public health purposes: experience in Emilia-Romagna region (northern Italy) Epidemiol Prev. 2013;37:279–288. [PubMed] [Google Scholar]
- Calzolari E, Garani G, Cocchi G, Magnani C, Rivieri F, Neville A, et al. Congenital heart defects: 15 years of experience of the Emilia-Romagna Registry (Italy) Eur J Epidemiol. 2003;18:773–780. doi: 10.1023/a:1025312603880. [DOI] [PubMed] [Google Scholar]
- Chen EK, Zmirou-Navier D, Padilla C, Deguen S. Effects of air pollution on the risk of congenital anomalies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11:7642–7668. doi: 10.3390/ijerph110807642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110:162–168. doi: 10.1016/j.envres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cocchi G, Magnani C, Morini MS, Garani GP, Milan M, Calzolari E. Urinary tract abnormalities (UTA) and associated malformations: data of the Emilia-Romagna Registry. IMER Group. Emilia-Romagna Registry on Congenital Malformations. Eur J Epidemiol. 1996;12:493–497. doi: 10.1007/BF00144002. [DOI] [PubMed] [Google Scholar]
- Custodio D, Pinho I, Cerqueira M, Nunes T, Pio C. Indoor and outdoor suspended particulate matter and associated carbonaceous species at residential homes in northwestern Portugal. Sci Total Environ. 2014;473–474:72–76. doi: 10.1016/j.scitotenv.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Rankin J, Rushton S, Pless-Mulloli T. Ambient air pollution and congenital heart disease: a register-based study. Environ Res. 2011;111:435–441. doi: 10.1016/j.envres.2011.01.022. [DOI] [PubMed] [Google Scholar]
- A dispersion model for predicting air pollution concentration near roadways. Sacramento: State of California; 1989. Department of Transportation - Division of New Technology and Research. Caline 4. [Google Scholar]
- Dolk H, Armstrong B, Lachowycz K, Vrijheid M, Rankin J, Abramsky L, et al. Ambient air pollution and risk of congenital anomalies in England, 1991–1999. Occup Environ Med. 2010;67:223–227. doi: 10.1136/oem.2009.045997. [DOI] [PubMed] [Google Scholar]
- Farhi A, Boyko V, Almagor J, Benenson I, Segre E, Rudich Y, et al. The possible association between exposure to air pollution and the risk for congenital malformations. Environ Res. 2014;135:173–180. doi: 10.1016/j.envres.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Filippini T, Heck JE, Malagoli C, Giovane CD, Vinceti M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:36–66. doi: 10.1080/10590501.2015.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi P, Vittadini MR, Meggiato A, Gasparini G, Tupputi R, Montanari L, et al. Piano della mobilità di area vasta di Reggio Emilia. Comune di Reggio Emilia. 2008 [Google Scholar]
- Gianicolo EA, Mangia C, Cervino M, Bruni A, Andreassi MG, Latini G. Congenital anomalies among live births in a high environmental risk area--a case-control study in Brindisi (southern Italy) Environ Res. 2014;128:9–14. doi: 10.1016/j.envres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Gilboa SM, Mendola P, Olshan AF, Langlois PH, Savitz DA, Loomis D, et al. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am J Epidemiol. 2005;162:238–252. doi: 10.1093/aje/kwi189. [DOI] [PubMed] [Google Scholar]
- Hansen CA, Barnett AG, Jalaludin BB, Morgan GG. Ambient air pollution and birth defects in brisbane, australia. PLoS One. 2009;4:e5408. doi: 10.1371/journal.pone.0005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happo M, Markkanen A, Markkanen P, Jalava P, Kuuspalo K, Leskinen A, et al. Seasonal variation in the toxicological properties of size-segregated indoor and outdoor air particulate matter. Toxicol In Vitro. 2013;27:1550–1561. doi: 10.1016/j.tiv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Hwang BF, Jaakkola JJ. Ozone and other air pollutants and the risk of oral clefts. Environ Health Perspect. 2008;116:1411–1415. doi: 10.1289/ehp.11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26:30–42. doi: 10.1097/EDE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- Kim OJ, Ha EH, Kim BM, Seo JH, Park HS, Jung WJ, et al. PM10 and pregnancy outcomes: a hospital-based cohort study of pregnant women in Seoul. J Occup Environ Med. 2007;49:1394–1402. doi: 10.1097/JOM.0b013e3181594859. [DOI] [PubMed] [Google Scholar]
- Liang Z, Wu L, Fan L, Zhao Q. Ambient air pollution and birth defects in Haikou city, Hainan province. BMC Pediatr. 2014;14:283. doi: 10.1186/s12887-014-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Lee YL, Jung CR, Jaakkola JJ, Hwang BF. Air pollution and limb defects: a matched-pairs case-control study in Taiwan. Environ Res. 2014;132:273–280. doi: 10.1016/j.envres.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Lupo PJ, Symanski E, Waller DK, Chan W, Langlois PH, Canfield MA, et al. Maternal exposure to ambient levels of benzene and neural tube defects among offspring: Texas, 1999–2004. Environ Health Perspect. 2011;119:397–402. doi: 10.1289/ehp.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagoli C, Crespi CM, Rodolfi R, Signorelli C, Poli M, Zanichelli P, et al. Maternal exposure to magnetic fields from high-voltage power lines and the risk of birth defects. Bioelectromagnetics. 2012;33:405–409. doi: 10.1002/bem.21700. [DOI] [PubMed] [Google Scholar]
- Malagoli C, Malavolti M, Costanzini S, Fabbi S, Teggi S, Arcolin E, et al. Increased incidence of childhood leukemia in urban areas: a population-based case-control study. Epidemiol Prev. 2015 (in press). [PubMed] [Google Scholar]
- Marshall EG, Harris G, Wartenberg D. Oral cleft defects and maternal exposure to ambient air pollutants in New Jersey. Birth Defects Res A Clin Mol Teratol. 2010;88:205–215. doi: 10.1002/bdra.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman NC, Ryan P, Lemasters G, Levin L, Bernstein D, Hershey GK, et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect. 2013;121:731–736. doi: 10.1289/ehp.1205555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011;11:1–29. [Google Scholar]
- Padula AM, Tager IB, Carmichael SL, Hammond SK, Lurmann F, Shaw GM. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am J Epidemiol. 2013a;177:1074–1085. doi: 10.1093/aje/kws367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, Tager IB, Carmichael SL, Hammond SK, Yang W, Lurmann F, et al. Ambient air pollution and traffic exposures and congenital heart defects in the San Joaquin Valley of California. Paediatr Perinat Epidemiol. 2013b;27:329–339. doi: 10.1111/ppe.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, Tager IB, Carmichael SL, Hammond SK, Yang W, Lurmann FW, et al. Traffic-related air pollution and selected birth defects in the San Joaquin Valley of California. Birth Defects Res A Clin Mol Teratol. 2013c;97:730–735. doi: 10.1002/bdra.23175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan A, Lupo PJ, Agopian AJ, Linder SH, Stock TH, Langlois PH, et al. Evaluating the effects of maternal exposure to benzene, toluene, ethyl benzene, and xylene on oral clefts among offspring in Texas: 1999–2008. Birth Defects Res A Clin Mol Teratol. 2013;97:532–537. doi: 10.1002/bdra.23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Chadwick T, Natarajan M, Howel D, Pearce MS, Pless-Mulloli T. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environ Res. 2009;109:181–187. doi: 10.1016/j.envres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102:182–190. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002;155:17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- Rovelli S, Cattaneo A, Nuzzi CP, Spinazze A, Piazza S, Carrer P, et al. Airborne particulate matter in school classrooms of northern Italy. Int J Environ Res Public Health. 2014;11:1398–1421. doi: 10.3390/ijerph110201398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schembari A, Nieuwenhuijsen MJ, Salvador J, de Nazelle A, Cirach M, Dadvand P, et al. Traffic-related air pollution and congenital anomalies in Barcelona. Environ Health Perspect. 2014;122:317–323. doi: 10.1289/ehp.1306802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezakova K, Castro D, Delerue-Matos C, Morais S, Pereira Mdo C. Levels and risks of particulate-bound PAHs in indoor air influenced by tobacco smoke: a field measurement. Environ Sci Pollut Res Int. 2014;21:4492–4501. doi: 10.1007/s11356-013-2391-5. [DOI] [PubMed] [Google Scholar]
- Strickland MJ, Klein M, Correa A, Reller MD, Mahle WT, Riehle-Colarusso TJ, et al. Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. Am J Epidemiol. 2009;169:1004–1014. doi: 10.1093/aje/kwp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JP, Salemi JL, Stuart AL, Yu H, Jordan MM, DuClos C, et al. Associations between exposure to ambient benzene and PM during pregnancy and the risk of selected birth defects in offspring. Environ Res. 2015;142:345–353. doi: 10.1016/j.envres.2015.07.006. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, Jaddoe VW, de Kluizenaar Y, Hofman A, Mackenbach JP, Steegers EA, et al. Residential traffic exposure and pregnancy-related outcomes: a prospective birth cohort study. Environ Health. 2009;8:59. doi: 10.1186/1476-069X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Malagoli C, Fabbi S, Teggi S, Rodolfi R, Garavelli L, et al. Risk of congenital anomalies around a municipal solid waste incinerator: a GIS-based case-control study. Int J Health Geogr. 2009;8:8. doi: 10.1186/1476-072X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Malagoli C, Rothman KJ, Rodolfi R, Astolfi G, Calzolari E, et al. Risk of birth defects associated with maternal pregestational diabetes. Eur J Epidemiol. 2014;29:411–418. doi: 10.1007/s10654-014-9913-4. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Rothman KJ, Crespi CM, Sterni A, Cherubini A, Guerra L, et al. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case-control study in an Italian population. Eur J Epidemiol. 2012;27:781–790. doi: 10.1007/s10654-012-9727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor-Imler LC, Stewart TG, Luben TJ, Davis JA, Langlois PH. An exploratory analysis of the relationship between ambient ozone and particulate matter concentrations during early pregnancy and selected birth defects in Texas. Environ Pollut. 2015;202:1–6. doi: 10.1016/j.envpol.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J, et al. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect. 2011;119:598–606. doi: 10.1289/ehp.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]