Abstract
Aspirin is chemopreventive; however, side effects preclude its long-term use. NOSH-aspirin (NBS-1120), a novel hybrid that releases nitric oxide and hydrogen sulfide, was designed to be a safer alternative. Here we compare the gastrointestinal safety, anti-inflammatory, analgesic, antipyretic, anti-platelet, and chemopreventive properties of aspirin and NBS-1120 administered orally to rats at equimolar doses. Gastrointestinal safety: 6h post-administration, the number and size of hemorrhagic lesions in stomachs were counted; tissue samples were frozen for PGE2, SOD, and MDA determination. Anti-inflammatory: 1h after drug administration, the volume of carrageenan-induced rat paw edemas was measured for 5h. Anti-pyretic: fever was induced by LPS (ip) an hour before administration of the test drugs, core body temperature was measured hourly for 5h. Analgesic: time-dependent analgesic effects were evaluated by carrageenan-induced hyperalgesia. Antiplatelet: anti-aggregatory effects were studied on collagen-induced platelet aggregation of human platelet-rich plasma. Chemoprevention: Nude mice were gavaged daily for 25 days with vehicle, aspirin or NBS-1120. After one week, each mouse was inoculated subcutaneously in the right flank with HT-29 human colon cancer cells. Both agents reduced PGE2 levels in stomach tissue; however, NBS-1120 did not cause any stomach ulcers, whereas aspirin caused significant bleeding. Lipid peroxidation induced by aspirin was higher than that exerted by NBS-1120. SOD activity was significantly inhibited by aspirin but increased by NBS-1120. Both agents showed similar anti-inflammatory, analgesic, anti-pyretic, and anti-platelet activities. Aspirin increased plasma TNFα more than NBS-1120-treated animals. NBS-1120 was better than aspirin as a chemopreventive agent; it dose-dependently inhibited tumor growth and tumor mass.
Keywords: Nitric oxide, hydrogen sulfide, aspirin, GI-sparing, chemopreventive
1. Introduction
Considerable body of evidence has established non-steroidal anti-inflammatory drugs (NSAIDs) in general, and aspirin in particular, as the prototypical chemopreventive agents against cancer [1–4]. Recently, Rothwell et al. [5] reported that daily aspirin use, whether regular strength or low dose, resulted in reductions in cancer incidence and mortality. Moreover, the same group has also reported that aspirin prevented distant metastasis [6]. However, long-term use of NSAIDs may lead to significant side effects, mainly gastrointestinal, cardiovascular, and renal. Amongst patients using NSAIDs, it is estimated that about 16,500 deaths occur every year in the United States. This figure is considerably greater than the number of deaths from multiple myeloma, asthma, cervical cancer, or Hodgkin’s disease [7]. Unfortunately, many physicians and most patients are unaware of the magnitude of the problem. The gastric damage is as a result of direct epithelial damage due to their acidic properties and also through the breakdown of mucosal defense mechanisms (leukocyte adherence, decreases in blood flow, bicarbonate and mucus secretions) due to a reduction of mucosal prostaglandin (PG) synthesis [8]. In our search for a “better aspirin” we recently developed NOSH-aspirin, a hybrid entity capable of releasing both nitric oxide (NO) and hydrogen sulfide (H2S), two gasotransmitters of physiological significance [9]. The rational for developing NOSH-aspirin was based on the observations that NO [10] and H2S [11] have some of the same properties as PGs within the gastric mucosa, thus modulating some components of the mucosal defense systems. Our preliminary results indicated that NOSH-aspirin was a potent anti-inflammatory agent devoid of cellular toxicity, which was also active against a variety of cancer lines in the nanomolar range; besides, NOSH-aspirin was efficacious against established tumors in a xenograft model of colon cancer [9, 12]. In the present study, we carried out a head-to-head comparison of the gastrointestinal safety, anti-inflammatory, analgesic, anti-pyretic, and anti-platelet, properties of aspirin with those of NOSH-aspirin. We also evaluated the effects of aspirin and NOSH-aspirin in a xenograft chemopreventive model of colon cancer.
2. Materials and methods
2.1. Chemicals
NOSH-aspirin (NBS-1120) 4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl 2-((4-(nitrooxy)butanoyl)oxy), benzoate was synthesized as described previously [9] and was a gift from Avicenna Pharmaceuticals Inc, (New York, NY). The structural components of the NOSH-aspirin are shown in Figure 1. Lipopolysaccharide (LPS) from E. coli, aspirin, and carrageenan were purchased from Sigma (St. Louis, MO, USA). The kits used for determination of PGE2, lipid peroxidation, and superoxide dismutase were purchased from Cayman Chemical (Ann Arbor, MI).
Figure 1.
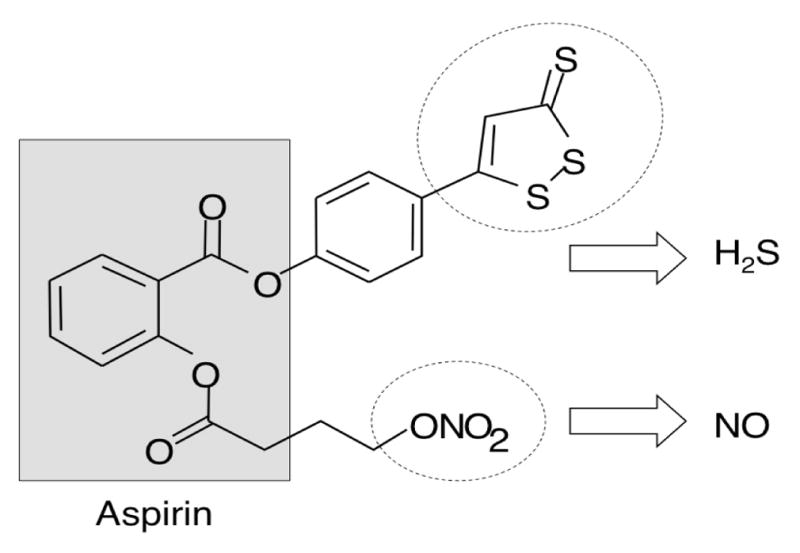
Structural components of NOSH-aspirin. The parent compound aspirin is shown in the shaded box. The parts of the molecule that releases NO and H2S are shown in the dotted ellipses.
2.2. Experimental groups and treatments
Animals
Our institutional animal care and research committee approved all experimental procedures described here. Male Wistar rats (5 per group) weighing 180–200 g were obtained from Charles River Laboratories International (Wilmington, MA). The rats were fed standard laboratory chow and water. Rats were fasted for 48 h with free access to drinking water. All test agents were administered orally by gavage, suspended in the vehicle (0.5% carboxymethylcellulose solution), at equimolar doses: ASA (180 mg/kg), and NOSH-ASA (477 mg/kg). Six hours post-administration, animals were euthanized by CO2; blood samples were drawn by cardiac puncture into heparin-containing vials and used for determination of plasma TNFα levels. Stomachs were then rapidly removed, cut along the greatest curvature, and rinsed with ice-cold distilled water. The ulcer index (UI) was determined as described by Best et al [13] and reported by us previously [14, 15]. Tissues from stomachs were excised and processed for measurement of Prostaglandin E2 (PGE2), malondialdehyde (MDA) and Superoxide dismutase (SOD) activity.
2.3
Determination of PGE2 levels: stomach tissue (~1 g) from each rat was placed in a test tube containing 5 mL of 0.1 M phosphate buffer (pH7.4), 1 mM EDTA, and 10 μM indomethacin. The tissue was homogenized and centrifuged for 10 min at 10,000 xg at 4°C. PGE2 content in supernatant was determined in duplicate by an enzyme immunoassay kit following the protocol described by the manufacturer (Cayman Chemical, Ann Arbor, MI) and as previously reported [14].
2.4. Index of lipid peroxidation
This was determined using a colorimetric kit from Cayman Chemical (Ann Arbor, MI) according to their prescribed protocol. In brief, stomach tissue (25 mg) was snap frozen and sonicated for 15 seconds at 40V over ice with 250 μL of radioimmunoprecipitation (RIPA) buffer (25 mM TrisHCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). Homogenates were centrifuged for 10 minutes at 200 xg at 4°C. Thiobarbituric acid reactant substances (TBARS) content which are produced when malondialdehyde (MDA) reacts with thiobarbituric acid (TBA) was then measured in the supernatant at 530–540nm.
2.5. Antioxidant enzymes
Superoxide dismutase (SOD) activity in the gastric mucosa was assayed using a colorimetric kit [14] following the protocol described by the manufacturer (Cayman Chemical, Ann Arbor, MI). Mucosal tissue (1 g) was homogenized with 5 mL of 20 mM HEPES buffer (pH 7.2) containing 1mM EGTA and 300 mM of sucrose solution. Homogenates were centrifuged at 1,500 r.p.m. for 10 minutes at 4°C. The supernatant was then removed and stored at −80°C until assayed.
2.6. Determination of plasma TNF-α
This was done by an enzyme immunoassay kit from R&D systems (Minneapolis, MN) following the protocol described by the manufacturer and reported by us previously [14, 15].
2.7. Anti-pyretic activity
To induce fever, LPS (50 μg/kg, Sigma, St. Louis, MO, USA) was administered intra-peritoneally to the animals an hour before the administration of test drugs as described previously [16]. Rectal temperature was measured by inserting a lubricated thermistor probe (external diameter: 3 mm) 2.8 cm into the rectum of the animal. The probe was linked to a digital reader, which displayed the temperature at the tip of the probe (± 0.1°C). The values displayed were manually recorded. Rectal temperatures were taken every hour for 5 h as previously reported [14].
2.8. Inflammatory Oedema Models
Carrageenan (1%, 100μL, suspended in sterile saline solution, type IV lambda, from Sigma Chemicals (St. Louis, MO) was subcutaneously injected into the plantar surface of the right hind paw in rats following the protocol described by Winter et al [17] and reported by us previously [14]. Paw volume was measured using a water displacement plethysmometer (Model 520, IITC/Life Sciences Instruments, Woodland Hills, CA) before carrageenan injection and thereafter at 1 h intervals for 5 h.
2.9. Induction and assessment of carrageenan-evoked hyperalgesia
Hindpaw inflammation was produced by intraplantar injection of carrageenan (100 μL of 1% carrageenan in sterile saline solution) into either hindpaw chosen at random. Suspensions of aspirin (180 mg/kg), NOSH-aspirin (477 mg/kg); or vehicle (carboxymethylcellulose, 0.5% w/v) were administered orally 1 h after carrageenan injection, and the mechanical nociceptive threshold determined 30 min after this and thereafter every h for 5 h. The paw hyperalgesia was measured with an electronic pressure-meter as reported earlier [14, 15].
2.10. Inhibition of human platelet aggregation in vitro
The anti-aggregatory effects of NOSH-aspirin and aspirin were studied on collagen-induced platelet aggregation of human platelet-rich plasma (PRP). It is known that collagen-induced aggregation occurs through a pathway dependent upon the arachidonic acid cascade [18]. Venous blood samples were obtained from healthy volunteers who had not taken any drugs for at least 2 weeks. PRP was prepared by centrifugation of citrated blood at 200 xg for 20 min. Aliquots (500 μL) of PRP were added into aggregometer cuvettes, and aggregation was recorded as increased light transmission under continuous stirring (1000 rpm) at 37 °C for 10 min after the addition of the stimulus. Collagen at submaximal concentrations (1.0 μg/mL) was used as the platelet activator in PRP. Aspirin or NOSH-aspirin were preincubated with PRP 10 min before the addition of collagen. Vehicle alone (0.5% DMSO) added to PRP did not affect platelet function in control samples. The anti-aggregatory activity of test compounds is evaluated as percent inhibition of platelet aggregation compared to control samples. IC50 values were calculated by nonlinear regression analysis.
2.11. Mouse xenograft model
Male athymic nude (NU/NU) mice (N = 20), age 5 weeks, were purchased from Charles River Laboratories, Inc., (Wilmington, MA). After one week of acclimation, the mice were randomly divided into 5 groups (N = 4/group). Each group was gavaged daily for 25 days with either vehicle (0.5% carboxy methylcelloluse) or aspirin (50 mg/kg) or NOSH-aspirin at 25 mg/kg, 50 mg/kg, or 100 mg/kg. After the first week of treatment, each mouse was inoculated subcutaneously in the right flank with HT-29 human colon cancer cells (3 × 106) suspended in 50% v/v Matrigel (BD Biosciences, San Jose, CA). In the vehicle-treated animals, small tumors were palpable after one week. Tumor size (Length and width) was measured at 3 day intervals with an electronic caliper from which tumor volume was calculated as length × width2/2. The mice were weighed every 3 days and were closely monitored for signs of toxicity.
2.12. Statistical Analysis
All data are presented as the mean ± SEM, with sample sizes of at least 5 rats/group (unless otherwise specified). Comparisons between groups were performed using a one-way analysis of variance followed by the Student-t test.
3. Results
3.1. Gastric mucosal lesions
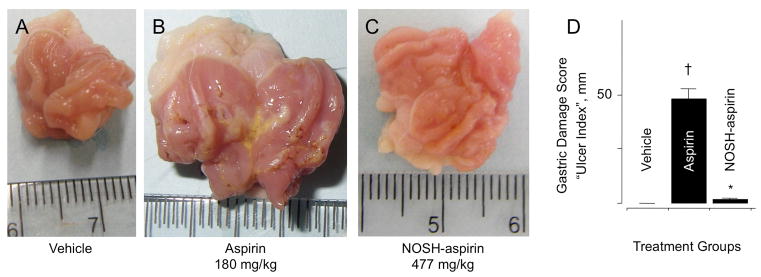
The rats receiving the vehicle (0.5% CMC solution) had a normal glandular region on the surface of their stomach, and no ulcerative damage was observed. For these rats, the gastric damage score (also described in the literature as “ulcer index”, or UI), was zero (UI = 0, Fig 2A). However, the administration of aspirin (180 mg/kg) resulted in extensive mucosal injury, UI = 48 to the glandular portion of the gastric fundus (Fig 2B). NOSH-aspirin at equimolar doses (477 mg/kg) did not produce any significant ulcerative damage (UI = 2), which represents a remarkable reduction (P < 0.01) in gastrointestinal toxicity (Fig 2C). The UIs are all quantified in Fig 2D.
Figure 2.
NOSH-aspirin does not cause gastric damage. Aspirin and NOSH-aspirin were administered orally at equimolar doses (1 mmol/kg) and effects on the stomach were evaluated 6 hrs post-administration as indicated in Section 2.2. Panel A, shows the stomach of a vehicle-treated rat; Panel B, stomach of an aspirin-treated rat showing ulceration and bleeding; Panel C, stomach of a NOSH-aspirin-treated rat which is devoid of ulcers. Panel D, gastric damage due to aspirin, UI = 48 ± 4 mm (†P < 0.01 compared to vehicle), whereas NOSH-aspirin was gastric damage-sparing, UI = 2 ± 0.5 mm (*P < 0.01 compared to aspirin).
3.2. Gastric mucosal and paw exudate prostaglandin E2 content
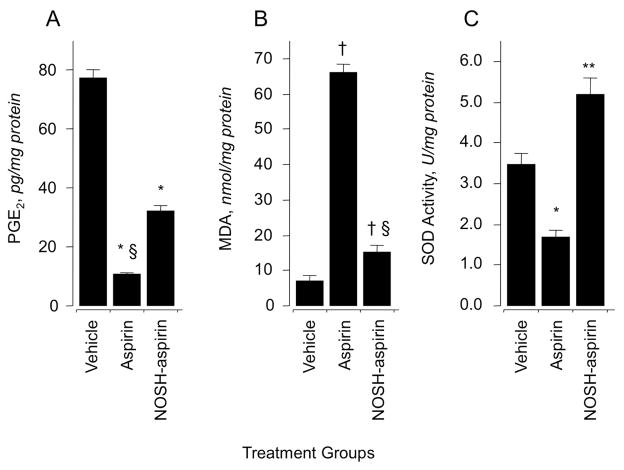
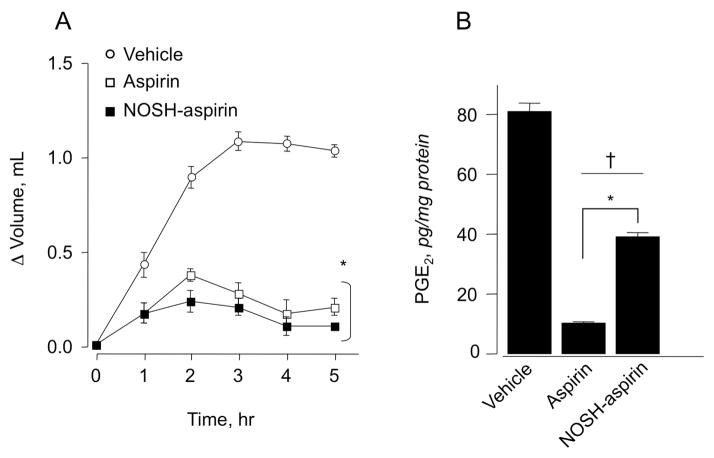
We investigated the effect of aspirin and NOSH-aspirin on prostaglandin E2 (PGE2) content in gastric mucous (Fig 3A) and paw exudates (Fig 4B). Animals treated with aspirin (180 mg/kg p.o.) produced about 85% less PGE2 than rats in the control group. NOSH-aspirin (477 mg/kg) also reduced PGE2 levels but not to the same extent as aspirin, the reduction being around 60% (Fig 3A). Prostaglandins are the main product of cyclooxygenase-mediated arachidonic acid metabolism in gastric mucosa, therefore, comparison of PGE2 content between control and drug-treated groups showed a clear and significant COX inhibition by both aspirin and NOSH-aspirin. Subsequently, we tested whether NOSH-aspirin exerted a similar decrease in PGE2 levels in the carrageenan-induced paw edema model in rats. In this assay as for the gastric mucosa above, similar results were obtained (Fig 4B).
Figure 3.
Effects of aspirin and NOSH-aspirin on gastric PGE2 level, lipid peroxidation (MDA) and superoxide dismutase (SOD). Three groups of rats were treated with vehicle, aspirin, and NOSH-aspirin for 6 hrs and their stomachs removed and processed as described in Sections 2.2–2.5. Aspirin, and NOSH-aspirin caused a significant reduction in gastric mucosal PGE2 levels (panel A). Results are mean ± SEM of 5 rats in each group, *P < 0.05 vs vehicle group, §P < 0.05 vs NOSH-aspirin group. Aspirin caused an almost 8-fold increase in MDA levels, for NOSH-aspirin-treated rats, MDA levels were about 2-fold higher (panel B). Results are mean ± SEM for 5 rats in each group, †P < 0.01 vs vehicle group, §P < 0.01 vs aspirin group. Aspirin caused a significant reduction in SOD activity, whereas NOSH-aspirin caused an increase (panel C). Results are mean ± SEM of 5 rats, *P < 0.05 vs vehicle group, **P < 0.01 vs aspirin group.
Figure 4.
Anti-inflammatory properties of aspirin and NOSH-aspirin. Rat paw edema was induced by carrageenan injection as described in Section 2.8. Aspirin and NOSH-aspirin caused a significant reduction in paw volume at all time points (panel A). Results are mean ± SEM of 5 rats in each group, *P < 0.05 vs vehicle treated rats at all time points. Aspirin and NOSH-aspirin also caused a significant reduction in PGE2 levels in the paw exudate (panel B). Results are mean ± SEM for 5 rats in each group, †P< 0.01 vs vehicle, *P <0.05 vs NOSH-aspirin.
3.3. Lipid peroxidation
Oxidative stress in gastric tissue was assessed by measuring the concentration of MDA in intact mucosa 6 h post administration of drugs at the doses indicated above. MDA levels were 7 ± 2 nmol/mg protein for vehicle (Fig 3B), and this increased to 65 ± 3 nmol/mg protein for the aspirin treated animals but was significantly lower for the NOSH-aspirin treated animals, 14 ± 2 nmol/mg protein (Fig 3B).
3.4. SOD activity
In intact gastric mucosal (control group) SOD activity was 3.5 ± 0.4 U/mg protein. Following administration of aspirin, a significant decrease in SOD activity (1.7 ± 0.3 U/mg protein) was observed. Treatment with NOSH-aspirin increased SOD activity to 5.2 ± 0.5 U/mg protein (Fig 3C).
3.5. Carrageenan-induced paw swelling
The most common use for NSAIDs including aspirin is the treatment of inflammatory conditions. We wanted to compare the COX-dependent anti-inflammatory activity of aspirin to that obtained with NOSH-aspirin. After inducing inflammation, animals receiving vehicle showed a fast time-dependent increase in paw volume (ΔV = 0.8 mL) after 2 h, and gradual increase to 1.1 mL over the course of the experiment (5 h) (Fig 4 A). In contrast, animals receiving aspirin showed a weak inflammatory response (ΔV =0.3 mL by 2h), which decreased over the next 3h (Fig 4A). The anti-inflammatory effect registered in animals dosed with NOSH-aspirin was similar to that of aspirin or may be even better at times (Fig 4A). These results are similar to our earlier observation [9] and were repeated here for comparison reasons in a head-to-head evaluation of all the classic pharmacological properties NOSH-aspirin to that of aspirin.
3.6. Plasma TNFα levels
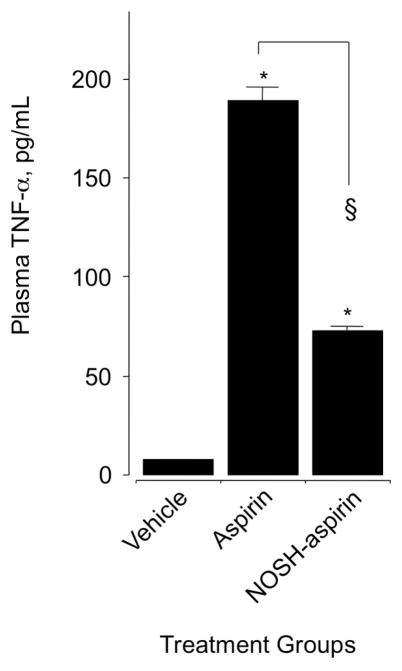
We determined the inhibitory effect of aspirin and NOSH-aspirin on proinflammatory cytokine tumor necrosis factor-α (TNFα) in plasma obtained from control and drug-treated animals. Administration of ASA (1 mmol/kg) increased TNFα concentration by about 15-fold (12 ± 0.4 control and 180 ± 3 pg/mL), however, this rise was considerably lower in the NOSH-aspirin-treated animals, the value being 75 ± 1 pg/mL (Fig 5).
Figure 5.
Effect of aspirin and NOSH-aspirin on plasma TNF-α. Rats were treated with equimolar amounts (1 mmol/kg) of aspirin and NOSH-aspirin and plasma TNF-α was measured as described in Section 2.6. Aspirin caused a significant rise in plasma TNF-α, however, this rise was significantly less in the NOSH-aspirin-treated rats. Results are mean ± SEM for 5 rats in each group, *P < 0.001 vs vehicle, §P < 0.01 vs aspirin.
3.7. Antipyretic activity
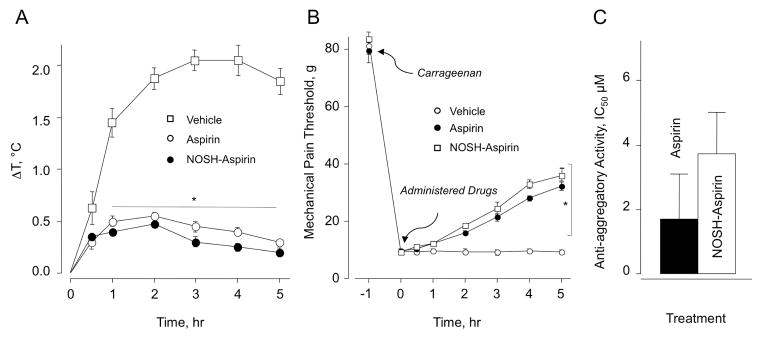
NSAIDs exert a moderate antipyretic effect when administered orally; therefore, we wanted to determine the decrease in body temperature induced by NOSH-aspirin compared to that obtained with aspirin. Experimental drugs at the doses indicated above were administered (po) 30 minutes before injecting LPS (50 μg/kg ip) in experimental animals. Control animals showed a time-dependent increase in body temperature (ΔT = 2 °C) up to 3 h and essentially maintained it until the end of the experiment (5 h). However, aspirin and NOSH-aspirin-treated animals showed only about half degree increase in body temperature 1 h after LPS injection and preserved it within this range (0.2–0.5 °C) throughout the experiment (Fig 6A).
Figure 6.
Aspirin and NOSH-aspirin reduce LPS-induced fever, raise the threshold for hyperalgesia, and show anti-platelet activity. Panel A: LPS (50 μg/kg, ip) was administered to the rats one hour before administration aspirin and NOSH-aspirin as described in Section 2.7. Core body temperature was recorded at 30 min and thereafter hourly for 5 h. Results are mean ± SEM for 5 rats in each group, *P < 0.01 vs vehicle for both aspirin and NOSH-aspirin from 1–5 h.
Panel B: Mechanical pain threshold was increased in a time-dependent manner by aspirin and NOSH-aspirin as detailed in Section 2.9. Results are mean ± SEM for 5 rats in each group. *P < 0.05 vs vehicle for aspirin and NOSH-aspirin 2–5 h.
Panel C: Aspirin and NOSH-aspirin were equally effective in inhibiting human platelet aggregation, protocol as described in Section 2.10. Results are mean ± range for two individuals.
3.8. Carrageenan-induced mechanical hyperalgesia
The ability of a test drug to reverse hyperalgesia, that is decreased threshold to a painful stimuli, is measured by this assay. Mechanical pain threshold was increased as a function of time by both aspirin and NOSH-aspirin (Fig 6B). Pain threshold was markedly reduced from about 80g to about 10g in animals receiving vehicle (control group), indicating a higher sensitivity to mechanical stimuli (non-painful at normal conditions). Hyperalgesia was decreased in animals receiving aspirin or NOSH-aspirin to the same extent, mechanical pain threshold reduced to about 30–35 g (~45% reduction compared to the initial response).
3.9. Platelet anti-aggregatory Activity
Anti-aggregatory effects of aspirin and NOSH-aspirin were studied on collagen-induced platelet aggregation of human platelet-rich plasma (PRP). The results expressed as IC50 are shown in Fig 6C. Analysis of the data does not show any statistical differences between aspirin and NOSH-aspirin.
3.10. Effect of NOSH-aspirin on tumor growth in a xenograft model
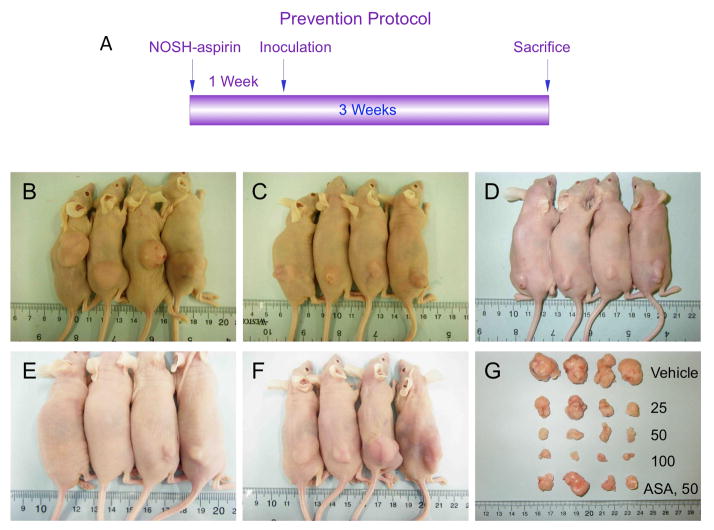
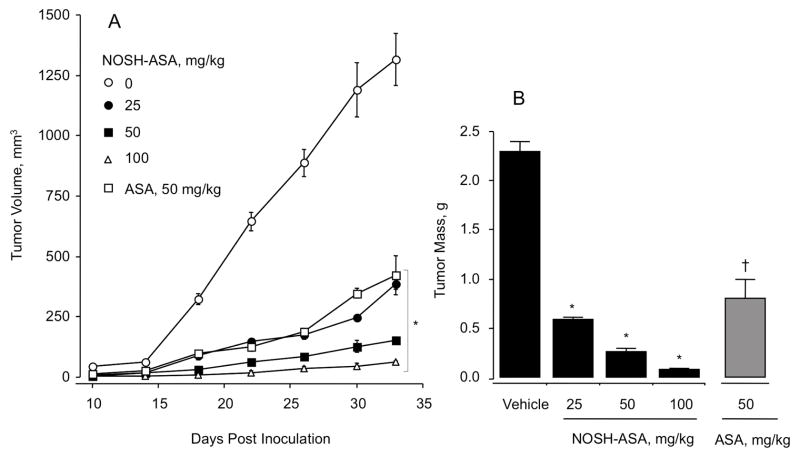
Athymic nude (nu/nu) male mice were treated for 7 days with either vehicle (0.5% carboxy methylcelloluse), or aspirin (50 mg/kg) or different doses of NOSH-aspirin (25 mg/kg, 50 mg/kg, or 100 mg/kg) after which they were injected subcutaneously with HT-29 cells in the right flank. This treatment regimen continued for another 18 days (Fig 7A). The first measurements of tumor volume were made 10 days post inoculation which showed that tumor volume in the control group was ~50 mm3 but was not detectable or around 10–30 mm3 in the pretreated groups (Fig 8A). At the end of the study, NOSH-aspirin-treated mice showed a dose-dependent inhibition of tumor growth compared with vehicle-treated mice (Fig 7B–E, Fig 8A). Compared with the vehicle-treated group with a mean tumor volume of 1420 ± 114 mm3, NOSH-aspirin-treated animals had tumor volumes to 385 ± 20 mm3, 152 ± 14 mm3, and 61 ± 5 mm3 at 25 mg/kg, 50 mg/kg, and 100 mg/kg, respectively. This is equivalent to a mean reduction of 73%, 89%, and 96% at 25 mg/kg, 50 mg/kg, and 100 mg/kg, respectively. Compared to the vehicle-treated group with average tumor mass of 2.3 ± 0.4 g, NOSH-aspirin reduced the tumor mass to 0.59 ± 0.02 g (74% reduction), 0.26 ± 0.03 g (89% reduction), and 0.08 ± 0.007 g (97% reduction), at 25 mg/kg, 50 mg/kg, and 100 mg/kg, respectively which was consistent with continued regression of tumor volume over the same treatment period (Fig 7G, Fig 8B). Aspirin treatment of the mice at 50 mg/kg also caused a reduction in tumor volume (Fig 7F, Fig 8A) and tumor mass (Fig 7G, Fig 8B). The mean tumor volume in the aspirin-treated group was 422 ± 79 mm3 (70% reduction) and the tumor mass was reduced to 0.8 ± 0.2 g (65% reduction). However, although at this dose the stomach’s of the aspirin-treated mice did show any ulcers (UI = 0), their stomach’s did show signs of erosion (EI = 9) as we have previously reported [14] which could eventually lead to bleeding ulcers. The stomachs of NOSH-aspirin treated mice were totally devoid of erosions (EI = 0). It should also be noted that aspirin at 50 mg/kg has 1.3-times the aspirin content of NOSH-aspirin at 100 mg/kg.
Figure 7.
NOSH-aspirin prevents tumor xenograft growth. Panel A: Study protocol. Athymic nude male mice were treated for 7 days via gavage with vehicle (Panel B), or NOSH-aspirin (25, 50, 100 mg/kg, Panels C–E), or aspirin (50 mg/kg, Panel F) and then injected subcutaneously with HT-29 cells for the development of subcutaneous tumors as described in Section 2.11. Daily administration of the test agents continued to the end of the experiment. Tumor sizes for untreated and treated mice are shown in Panel G.
Figure 8.
NOSH-aspirin and aspirin reduce tumor growth and tumor mass. Animals were treated as depicted in Fig 7. Average tumor volume as function of time and tumor mass at sacrifice are shown in Panels A and B, respectively. NOSH-aspirin dose-dependently and aspirin at 50 mg/kg significantly reduced tumor growth from day 18 post inoculation to sacrifice, *P < 0.01. Tumor mass was also significantly and dose-dependently reduced by NOSH-aspirin treatment, *P < 0.01 and aspirin at 50 mg/kg (†P < 0.05).
4. Discussion
In the United States over 30 million people use NSAIDs on a daily basis [19], and therefore a considerable number of these people are potentially at risk of developing gastrointestinal, cardiovascular, and renal side effects [20, 21]. Hence, the need for better NSAIDs that preferably do not have or at least have less serious side effects. NOSH-aspirin (NBS-1120) belongs to a new class of NSAIDs [9, 22] that appear to have enhanced safety. This hybrid molecule was designed to be a safer aspirin by releasing nitric oxide and hydrogen sulfide, two gasotransmitters of physiological relevance that have been shown to have some of the same properties as prostaglandins within the gastric mucosa [10, 11], thus enhancing the local mucosal defense systems. Here we conducted an extensive head-to-head comparison between NOSH-aspirin and its parent molecule, aspirin.
Initially, we compared the extent of gastric damage exerted by the two compounds. NOSH-aspirin was significantly less toxic than aspirin, which is consistent with prior evidence describing the role of NO [23] and H2S [24] in reducing the gastric damage produced by NSAIDs. In the gastric mucosa, both aspirin and NOSH-aspirin reduced PGE2 content indicating significant COX inhibition. This is inline with our previous report indicating preferential dose-dependent inhibition of pure ovine COX-1 vs COX-2 enzymatic activity by NOSH-aspirin [12]. However, it should be noted that although NOSH-aspirin inhibits COX-1 more than COX-2 in vitro (COX-1 selectivity), in the present study we have demonstrated that it inhibits platelet aggregation, a COX-1 mediated effect; effectively reduces LPS-induced fever, a COX-2 mediated effect, and has excellent anti-inflammatory properties using the carrageenan paw oedema model representing a COX-2 mediated effect. Thus, these results underscore the importance of being cautious when applying in vitro data to any in vivo settings. Also, in the present study, NOSH-aspirin and aspirin had similar anti-platelet effects. In this regard, aspirin is known to acetylate Ser530 of COX-1 binding site. Using molecular docking studies, we recently reported that NOSH-aspirin interacted with most of the residues that line the COX-1 enzymatic binding site but had no direct interaction with Ser530 [25]. This may explain the weaker inhibition of COX-1 by NOSH-aspirin vs aspirin reported previously [12]. However, NOSH-aspirin is capable of forming a number of metabolites [25] that could potentially inhibit COX-1 enzyme activity, thus contributing to the overall anti-platelet effects observed here.
In samples of gastric tissue, MDA and index of lipid peroxidation was increased 9-fold by aspirin but only by about 2-fold by NOSH-aspirin, that is aspirin caused 4.5-fold more oxidative stress compared to NOSH-aspirin. This observation correlates well with the SOD enzymatic activity, which is an antioxidative marker. SOD activity was significantly lower in the aspirin-treated rats than that observed in the NOSH-aspirin-treated animals. Therefore, some if not all of the changes in the gastric mucosal tissue may be the result of the antioxidative effects exerted by NOSH-aspirin. Furthermore, we observed that aspirin caused an upregulation of the proinflammatory mediator TNF-α, which has been reported to trigger the adherence and activation of leukocytes, leading to the release of oxygen-derived free radicals (oxygen superoxide) and proteases, producing epithelial injury [26]. NOSH-aspirin also induced an increase in TNF-α in vivo α but this change was significantly lower than that observed with the parent drug, aspirin. This result is in agreement with one of our previous reports [9].
Aspirin is the prototypical agent against that is most often used in treating inflammation. We therefore compared the anti-inflammatory profile of NOSH-aspirin to that of aspirin using the in vivo carrageenan-induced rat paw edema model. As reported previously [9] and confirmed here, both aspirin and NOSH-aspirin showed significant anti-inflammatory activity by decreasing the inflammatory response (paw volume) and reducing the amount of PGE2 in the paw exudate. Although aspirin and NOSH-aspirin were equivalent in their anti-inflammatory activity, aspirin reduced PGE2 levels to greater extent compared to NOSH-aspirin. This response is preferentially mediated by COX-2, which is inhibited to lesser extent in vitro by NOSH-aspirin [12]. Nevertheless, since the anti-inflammatory activity of the two agents appear to be equivalent, this would strongly suggest a role for NO and H2S in such a setting (reviewed in [27, 28]).
NSAIDs in general, and aspirin in particular, are the mainstay in the treatment of pain. In that respect, we compared the analgesic effect of NOSH-aspirin to that of aspirin in an in vivo model of hyperalgesia. Both agents were equally effective in increasing the threshold response to painful stimuli. Recently, we reported that NOSH-aspirin was more potent than aspirin, and presented a long-lasting effect in producing analgesia in different experimental models of inflammatory pain [29]. NOSH-aspirin’s enhanced antinociceptive effect was suggested to be due to its ability to reduce the production of IL-1α and restore neuronal sensitization caused by PGE2 through upregulation of KATP channels, which was not observed with aspirin.
Regular use of aspirin has been correlated with a lower incidence of colon and other cancers [2–5, 30]. We had previously reported that NOSH-aspirin was orders of magnitude more potent than aspirin in inhibiting the growth of HT-29 human colon cancer cells over a 24–72 h time period [12]. This growth inhibition was as a result of reductions in cell proliferation, G0/G1 cell cycle arrest, leading to increased apoptosis. In the present study, we compared the efficacy of NOSH-aspirin at different concentrations to that of aspirin using an in vivo xenograft mouse model of colon cancer chemoprevention. NOSH-aspirin dose-dependently inhibited tumor growth and tumor mass. At the highest dose of 100 mg/kg (0.21 mmol/kg), the reduction in tumor growth and mass were 95% and 97%, respectively. On the other hand, aspirin at 50 mg/kg (0.28 mmol/kg), that is at 1.3-fold the aspirin content of NOSH-aspirin at 100 mg/kg, only had a modest effect, tumor growth and mass were reduced by 70% and 65%, respectively. The results are even more impressive if we look at effects on tumor growth and tumor mass which were approximately in the same ballpark at 50 mg/kg (0.28 mmol/kg) aspirin and 25 mg/kg (0.052 mmol/kg) NOSH-aspirin. Comparison in terms of mmol/kg indicates that NOSH-aspirin was ~5-fold more potent than aspirin. Furthermore, the stomachs of the aspirin-treated mice exhibited erosions consistent with pre-ulcer development [14]. Clearly, as a chemo-preventive agent, NOSH-aspirin is superior to aspirin both in terms of efficacy and safety. These results further strengthen our original hypothesis [9] that incorporating NO and H2S-releasing moieties within the aspirin molecule would enhance both its activity/potency and safety profiles.
In summary our data shows that NOSH–aspirin maintains all the classic therapeutic indications of its parent NSAID, aspirin: a potent anti-inflammatory and analgesic that has anti-pyretic and anti-platelet activity without any GI toxicity. It should be noted that aspirin is used prophylactically for prevention of primary and secondary cardiovascular disease because of its anti-platelet activity [31, 32]. Based on our results presented here, NOSH-aspirin may prove to be a suitable replacement. In addition to its GI safety, NOSH-aspirin might also prove to have enhanced cardiovascular and renal safety profiles. This is because NO and H2S have protective roles in the cardiovascular and renal systems [33–35]. Safety and efficacy are the most important aspects of any chemopreventive agent. From the limited data available, it appears that NOSH-aspirin is a good candidate for further evaluation as a chemopreventive agent. Current work is directed towards unraveling the molecular targets of NOSH-aspirin in different in vivo models of cancer and further evaluating its safety profile.
Acknowledgments
This work was supported in part by NIH grant R24 DA018055. The funding agency had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Abbreviations
- NO
nitric oxide
- H2S
hydrogen sulfide
- NOSH
nitric oxide-hydrogen sulfide
- NSAIDs
nonsteroidal anti-inflammatory drugs
- ASA
aspirin
- LPS
Lipopolysaccharide
- PGE2
Prostaglandin E2
- MDA
malondialdehyde
- SOD
Superoxide dismutase
Footnotes
Conflict of interest
The authors have nothing to disclose except for KK, who has an equity position in Avicenna Pharmaceuticals, Inc. the supplier of NOSH-aspirin used in these studies and to which NBS-1120 is licensed.
Authorship Contributions
Participated in research design: Kashfi, Chattopadhyay, Kodela.
Conducted experiments: Kodela, Chattopadhyay, Velázquez-Martínez.
Performed data analysis: Kashfi, Chattopadhyay, Velázquez-Martínez.
Wrote or contributed to the writing of the manuscript: Kashfi, Chattopadhyay, Velázquez-Martínez.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kune G, Kune S, Watson L. Colorectal cancer risk, chronic illnesses, operations, and medications: case-control results from the Melbourne Colorectal Cancer Study. Cancer Research. 1988;48:4399–404. [PubMed] [Google Scholar]
- 2.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 3.Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–36. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 4.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 8.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547–65. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 9.Kodela R, Chattopadhyay M, Kashfi K. NOSH-Aspirin: A Novel Nitric Oxide-Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. ACS Med Chem Lett. 2012;3:257–62. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–20. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 11.Fiorucci S. Prevention of nonsteroidal anti-inflammatory drug-induced ulcer: looking to the future. Gastroenterology clinics of North America. 2009;38:315–32. doi: 10.1016/j.gtc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem Biophys Res Commun. 2012;419:523–8. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 13.Best R, Lewis DA, Nasser N. The anti-ulcerogenic activity of the unripe plantain banana (Musa species) Br J Pharmacol. 1984;82:107–16. doi: 10.1111/j.1476-5381.1984.tb16447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chattopadhyay M, Velazquez CA, Pruski A, Nia KV, Abdellatif KR, Keefer LK, et al. Comparison between 3-Nitrooxyphenyl acetylsalicylate (NO-ASA) and O2-(acetylsalicyloxymethyl)-1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (NONO-ASA) as safe anti-inflammatory, analgesic, antipyretic, antioxidant prodrugs. J Pharmacol Exp Ther. 2010;335:443–50. doi: 10.1124/jpet.110.171017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodela R, Chattopadhyay M, Goswami S, Gan ZY, Rao PPN, Nia KV, et al. Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: Differences in mode of cyclooxygenase inhibition. J Pharmacol Exp Ther. 2013 doi: 10.1124/jpet.112.201970. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto L, Borreli F, Bomberdelli E, Cristonic A, Capasso F. Antiinflammatory, analgesis and antipyretic effects of glaucine in rats and mice. Pharm Pharmacol Communication. 1998;4:502–5. [Google Scholar]
- 17.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 18.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–61. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 19.American-Gastroenterological-Association. Study shows long-term use of NSAIDs causes severe intestinal damage. ScienceDaily. 2005 http://www.sciencedaily.com/releases/2005/01/50111123706.htm.
- 20.Patricio JP, Barbosa JP, Ramos RM, Antunes NF, de Melo PC. Relative cardiovascular and gastrointestinal safety of non-selective non-steroidal anti-inflammatory drugs versus cyclo-oxygenase-2 inhibitors: implications for clinical practice. Clinical drug investigation. 2013;33:167–83. doi: 10.1007/s40261-013-0052-6. [DOI] [PubMed] [Google Scholar]
- 21.Ejaz P, Bhojani K, Joshi VR. NSAIDs and kidney. The Journal of the Association of Physicians of India. 2004;52:632–40. [PubMed] [Google Scholar]
- 22.Kodela R, Chattopadhyay M, Kashfi K. Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. Med Chem Commun. 2013;4:1472–81. doi: 10.1039/C3MD00185G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorucci S, Del Soldato P. NO-aspirin: mechanism of action and gastrointestinal safety. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2003;35 (Suppl 2):S9–19. doi: 10.1016/s1590-8658(03)00047-1. [DOI] [PubMed] [Google Scholar]
- 24.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–71. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Vannini F, Chattopadhyay M, Kodela R, Rao PPN, Kashfi K. Positional isomerism markedly Affects the growth inhibition of colon Cancer Cells by NOSH-Aspirin: COX Inhibition and Modeling. Redox iology. 2015 doi: 10.1016/j.redox.2015.08.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perini R, Fiorucci S, Wallace JL. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal injury and repair: a window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2004;18:229–36. doi: 10.1155/2004/890585. [DOI] [PubMed] [Google Scholar]
- 27.Kashfi K. Anti-inflammatory agents as cancer therapeutics. Advances in pharmacology (San Diego, Calif) 2009;57:31–89. doi: 10.1016/S1054-3589(08)57002-5. [DOI] [PubMed] [Google Scholar]
- 28.Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonesca MD, Cunha FQ, Kashfi K, Cunha TM. NOSH-aspirin (NBS-1120), a dual nitric oxide and hydrogen sulfide-releasing hybrid, reduces inflammatory pain. Pharmacol Res Perspect. 2015;3:1–12. doi: 10.1002/prp2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. Journal of the National Cancer Institute. 2009;101:256–66. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schror K. Why we should not skip aspirin in cardiovascular prevention. Hamostaseologie. 2015:35. doi: 10.5482/hamo-14-10-0048. [DOI] [PubMed] [Google Scholar]
- 32.Stegeman I, Bossuyt PM, Yu T, Boyd C, Puhan MA. Aspirin for Primary Prevention of Cardiovascular Disease and Cancer. A Benefit and Harm Analysis. PLoS One. 2015;10:e0127194. doi: 10.1371/journal.pone.0127194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nature reviews Drug discovery. 2002;1:375–82. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- 34.Huledal G, Jonzon B, Malmenas M, Hedman A, Andersson LI, Odlind B, et al. Renal effects of the cyclooxygenase-inhibiting nitric oxide donator AZD3582 compared with rofecoxib and naproxen during normal and low sodium intake. Clinical pharmacology and therapeutics. 2005;77:437–50. doi: 10.1016/j.clpt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Rossoni G, Manfredi B, Tazzari V, Sparatore A, Trivulzio S, Del Soldato P, et al. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. European journal of pharmacology. 2010;648:139–45. doi: 10.1016/j.ejphar.2010.08.039. [DOI] [PubMed] [Google Scholar]