Abstract
Background
The incidence of myocardial inflammation in patients with unexplained cardiomyopathy referred for ventricular arrhythmias (VA) is unknown.
Objective
To report fasting PET scan findings in consecutive patients referred with unexplained cardiomyopathy and VA.
Methods
18-FDG PET/CT scans with a >16 hour fasting protocol were prospectively ordered for patients referred for VA and unexplained cardiomyopathy (EF<55%). Patients with focal myocardial FDG uptake were labeled as arrhythmogenic inflammatory cardiomyopathy (AIC) and classified into four groups based on the presence of lymph node uptake (AIC+) and perfusion abnormalities (early vs late stage).
Results
Over a 3-year period, 103 PET scan were performed with 49% (AIC+=17, AIC=33) exhibiting focal FDG uptake. The mean age was 52±12 years with an EF of 36±16%. Patients with AIC were more likely to have a history of pacemaker (32% vs 6%, p=0.002) compared to those with normal PET. When biopsy was performed, histologic diagnosis revealed non-granulomatous inflammation in 6 patients and sarcoidosis in 18 patients. 90% of patients with AIC/AIC+ were prescribed immunosuppressive therapy and 58% underwent ablation. Correlation between areas of perfusion abnormalities and FDG uptake with electro-anatomic mapping was observed in 79% patients and MRI findings matched in only 33%.
Conclusions
Nearly 50% of patients referred with unexplained cardiomyopathy and VA demonstrate ongoing focal myocardial inflammation on FDG PET. These data suggests that a significant proportion of patients labeled “idiopathic” may have occult arrhythmogenic inflammatory cardiomyopathy, which may benefit from early detection and immunosuppressive medical therapy.
Keywords: cardiomyopathy, positron emission tomography, inflammation, ventricular arrhythmias
INTRODUCTION
Among patients referred for treatment and ablation of ventricular arrhythmias (VA), nonischemic cardiomyopathy (NICM) etiologies represent an increasing proportion.1 Idiopathic dilated cardiomyopathy represents the largest subgroup of such patients with NICM, although the etiology remains unknown. Additionally, NICM includes heterogeneous substrates that result from postinfectious, autoimmune, and infiltrative etiologies.2 Cardiac sarcoidosis is one particular form of idiopathic chronic granulomatous inflammation that can mimic other forms of NICM.3 Disease-specific therapy with immunosuppressive and immunomodulatory medications with adjunctive catheter ablation has been shown to be effective in reducing recurrent VA in patients with granulomatous inflammation.4
We hypothesized that inflammation is clinically under-recognized and may play a role in patients who present with arrhythmias and unexplained cardiomyopathy. The aim of this study was to evaluate the incidence of occult inflammation with 18-FDG PET/CT imaging in a consecutive cohort of patients referred for management of VA in the setting of “idiopathic” NICM.
METHODS
Study Population
FDG PET/CT was ordered in patients with NICM referred for VA management to the UCLA Cardiac Arrhythmia Center from 1/2012 to 1/2015. Inclusion criteria consisted of patients with EF<55% presenting with monomorphic VT, polymorphic VT, and/or premature ventricular contractions (PVC). For patients that presented with sudden cardiac death from primary VF (n=5), a specific ejection fraction cutoff was not required for inclusion. Patients were excluded if they had any history of myocardial infarction, history of revascularization, or evidence of flow-limiting coronary artery disease (>50% stenosis) on invasive coronary angiography or CT angiography. Retrospective review of electronic medical record information for this study was authorized by the Institutional Review Board (IRB) of the UCLA Office of Human Research Protection Program.
PET/CT Imaging: Assessment of Myocardial Perfusion and FDG uptake
Patients were referred to nuclear medicine for routine clinical evaluation of systemic and cardiac sarcoidosis. Patients were asked to fast for at least 16 hours to induce myocardial free fatty acid metabolism. Myocardial perfusion at rest was assessed with intravenous N-13 ammonia (20 mCi) and PET/CT (Siemens, Erlangen, Germany), followed by intravenous administration of FDG (10 mCi) and, one hour later, by acquisition of whole body and dedicated myocardial FDG uptake images. When N-13 ammonia was unavailable, myocardial perfusion at rest was evaluated with Tc-99m tetrofosmin (15 mCi) and SPECT, either several hours prior to the PET/CT study or the following day.
Images were analyzed by two experienced nuclear medicine physicians. The whole body FDG images were evaluated for systemic sarcoidosis whereas the myocardial perfusion and FDG images were analyzed for regional perfusion defects and for focal FDG uptake. FDG activity in normal myocardium was assessed on axial FDG and was graded relative to the blood pool activity as absent (less or equal to that in blood), faint diffuse uptake (slightly above blood pool activity) and normal homogeneous uptake.
The myocardial FDG uptake was reported as either none, focal, diffuse, or focal on diffuse uptake.5 Perfusion imaging was reported as normal or abnormal with an abnormal study further classified as either matched or mismatched with FDG uptake. Whole body PET/CT images were evaluated for extra-cardiac FDG uptake; location and intensity (SUVmax) of abnormal focal FDG were noted. For the present analysis, only patients with focal or focal on diffuse patterns of myocardial FDG uptake were considered as PET positive to improve specificity; diffuse myocardial FDG uptake patterns were attributed to inadequate FDG suppression and were not included in the analysis.
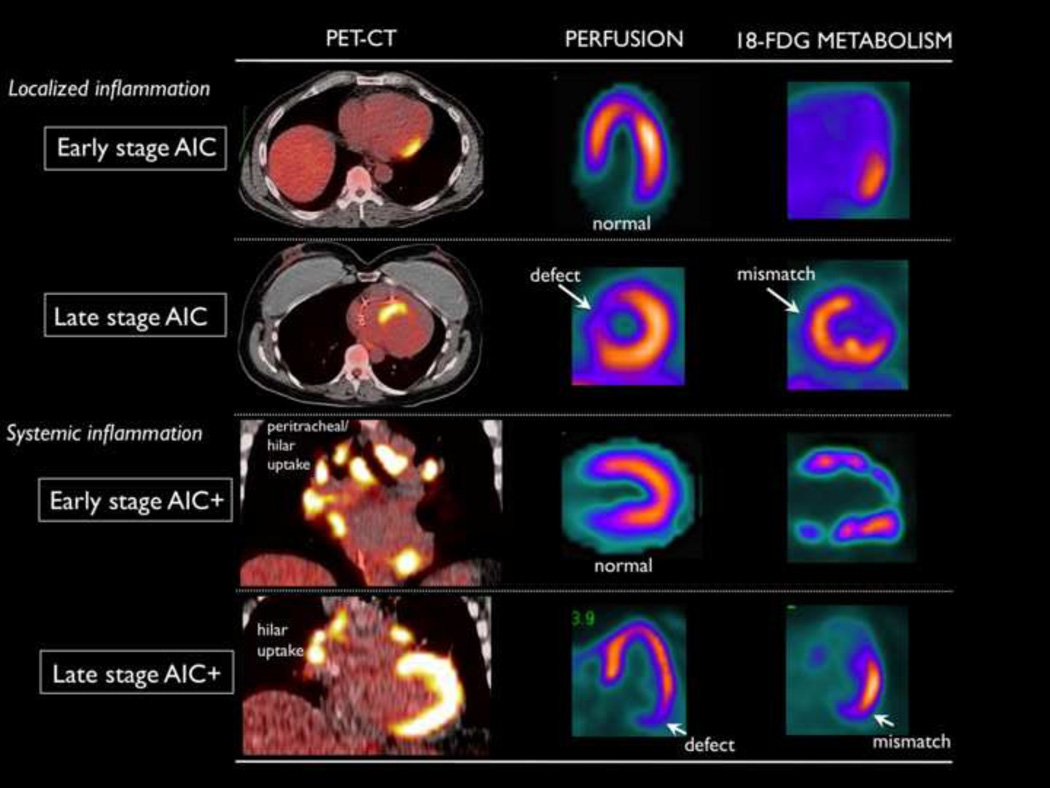
Patients with isolated myocardial FDG uptake with a focal pattern were categorized as arrhythmogenic inflammatory cardiomyopathy (AIC) and those with cardiac and extra-cardiac FDG uptake were categorized as arrhythmogenic inflammatory cardiomyopathy plus systemic inflammation (AIC+). Early stage AIC was defined as the absence of perfusion abnormalities and late stage required the presence of focal perfusion defects (FIGURE 1).
FIGURE 1.
Classification of patients with abnormal 18-FDG uptake. The presence of perfusion defects differentiates late stage from early stage disease and the increased FDG uptake in the lymph nodes indicates systemic disease (AIC+).
Electroanatomic Mapping and Ablation
Mapping and catheter ablation was performed at the discretion of the treating physician in a subgroup of patients in those who were felt to have 1) inactive disease with predominantly post-inflammatory scarring and/or 2) poor correlation between the presenting VA morphology and pattern of PET uptake. The approach and strategy for ablation of scar-mediated VT at our center has been previously reported.6 When ICD was present, non-invasive programed stimulation was performed under light sedation to assess the morphology of VT and hemodynamic tolerance. Programmed stimulation was performed at two drive cycle lengths (400–600ms) with up to triple extra-stimuli down to 200ms or ventricular refractoriness. High-density electroanatomic maps were created in sinus rhythm using CARTO (Biosense Webster, Diamond Bar, CA) or NAVX (St. Jude Medical, Minneapolis, MN) with standard low voltage settings (0.5–1.5mV). The low voltage area characterized by electroanatomic mapping were correlated with focal FDG uptake regions on PET/CT.
Cardiac Magnetic Resonance Imaging
In patients that also underwent cardiac MRI (Siemens Avanto 1.5T), multiplanar truFISP, truFISP cine, time-resolved and 3D contrast-enhanced high-resolution MRA of the vessels of the chest and upper abdomen were obtained, with administration of gadolinium contrast agent (Magnevist). Supplemental multiplanar inversion recovery images were obtained at 10 minutes post contrast administration to assess for delayed myocardial enhancement. The routine protocol at UCLA did not include T2 weighted imaging unless acute myocarditis was suspected. Post-processing, including maximum intensity projection, was performed inline on the MRI workstation console and all images were reviewed on a GE PACS workstation. Images were reviewed by two experienced cardiac MR radiologists and the locations of delayed contrast enhancement were correlated with regions that demonstrated FDG avidity.
Histologic diagnosis
Endomyocardial biopsy was performed at the discretion of the treating physician in a subgroup of PET-CT positive patients for histologic diagnosis. Written informed consent was obtained from all patients. Venous access was obtained via right internal jugular or femoral approach with insertion of a venous sheath. A bioptome was passed through sheath with acquisition of minimum of three specimens from the RV septum sent for histopathologic assessment. Left ventricular biopsy was not performed. Analysis was performed by the pathology department and confirmed by two cardiac pathologists. In patients with AIC+ demonstrating extra-cardiac involvement, lymph node biopsy was performed under CT-guidance or video-assisted thoracoscopic approach.
Immunosuppressive Therapy
IST was initiated after careful review of the PET scans was completed between nuclear cardiology and the treating physicians. Focal FDG uptake interpreted as pathologic was correlation between the aggregate of the 12-lead morphology of the clinical arrhythmia and other imaging studies (abnormal wall motion or thinning on echocardiography or MRI wall motion abnormality±delayed enhancement). All patients with AIC+ were treated with IST.
Assessment of clinical outcomes was completed after a six-month trial of IST in AIC patients receiving corticosteroid therapy (initial dose Prednisone 40 mg daily). For patients that presented with VT storm, IV steroid was administered (Solumedrol 1g × 2) prior to to Prednisone. Repeat PET/CT imaging after a minimum of 8 weeks of IST was performed to assess for improvement or resolution of myocardial FDG uptake. Concomitant use of antiarrhythmic medications were left to the discretion of the treating physician. If the arrhythmia burden decreased and the repeat PET showed partial or complete resolution of FDG uptake, a steroid-sparing immunomodulatory agent was started to allow for a variable monthly tapering of Prednisone (10mg every 1–2 months). Steroid-sparing agents utilized were azathioprine, methotrexate, and hydroxychloroquine for long-term IST. Improvement in VA burden was determined based upon baseline and post IST review of ICD interrogation data in which total VA, antitachycardia pacing, and shocks were recorded. In a subgroup of PET-CT positive patients, catheter ablation was deferred in favor of primary immunosuppressive therapy (IST).
Statistical Analysis
Results are reported as the means ± standard deviation. Comparison of nominal data sets including PET-CT imaging, EAM, and cardiac MRI was completed using the McNemar statistical test method with differences reported including p value and 95% confidence interval (95% CI). For all of the statistical analyses, a value of p ≤ .05 was considered to indicate significance. All of the statistical analysis was completed on the computer software SPSS (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc).
RESULTS
The baseline characteristics of the entire study population are shown Table 1, stratified by FDG uptake. The cohort was 75% male with a mean age of 55±12 years. 85% were Caucasian, 7% were African-American, and 8% were of Asian descent. The mean ejection fraction was 37±15% overall and was similar in patients with positive and normal PET. The presenting VA was PVC in 14%, monomorphic VT in 64%, and polymorphic VT/VF in 22%.
TABLE 1.
Patient characteristics by PET scan result
| Overall (n=103) | PET positive (n=50) | PET negative (n=53) |
p value | ||
|---|---|---|---|---|---|
| Age | 53±13 | 52±12 | 54±14 | 0.31 | |
| Ethnicity | |||||
| Caucasian | 88 (85% | 40 (80%) | 48 (90%) | ||
| African-American | 7 (7%) | 4 (8%) | 3 (6%) | ||
| Asian | 8 (8%) | 6 (12%) | 2 (4%) | ||
| Male | 77 (75%) | 36 (72%) | 41 (77%) | 0.47 | |
| HTN | 39 (38%) | 15 (30%) | 24 (45%) | 0.19 | |
| DM | 11 (11%) | 4 (8%) | 7 (13%) | 0.29 | |
| Pulmonary disease | 5 (5%) | 2 (4%) | 3 (6%) | 0.62 | |
| Ejection Fraction | 37±15 | 36±16 | 37±15 | 0.96 | |
| NYHA class (n=75) | |||||
| I | 40 (39%) | 19 (38%) | 21 (40%) | 0.78 | |
| II | 21 (20%) | 9 (18%) | 12 (23%) | ||
| III | 12 (12%) | 6 (12%) | 6 (11%) | ||
| IV | 2 (2%) | 1 (2%) | 1 (2%) | ||
| Duration of cardiomyopathy (yrs) | 6.1±5.9 | 6.5±6.1 | 5.8±5.1 | 0.58 | |
| Ventricular arrhythmia | |||||
| PVC | 14 (14%) | 8 (16%) | 6 (11%) | 0.56 | |
| Monomorphic VT | 66 (64%) | 30 (60%) | 36 (68%) | 0.38 | |
| Polymorphic/VF | 23 (22%) | 12 (24%) | 11 (21%) | 0.77 | |
| Sick sinus syndrome | 4 (4%) | 2 (4%) | 2 (4%) | 0.98 | |
| AV block | 19 (18%) | 16 (32%) | 3 (6%) | 0.002 | |
| Fascicular block | 11 (11%) | 7 (14%) | 4 (8%) | 0.15 | |
| LBBB | 7 (7%) | 3 (6%) | 4 (8%) | 0.64 | |
| RBBB | 10 (10%) | 5 (10%) | 5 (9%) | 0.81 | |
| Pacemaker | 19 (18%) | 16 (32%) | 3 (6%) | 0.002 | |
| ICD | 52 (50%) | 28 (56%) | 24 (45%) | 0.43 | |
| CRT | 25 (24%) | 17 (34%) | 8 (15%) | 0.12 | |
| Beta-blocker | 90 (87%) | 46 (92%) | 44 (83%) | 0.37 | |
| Amiodarone | 45 (44%) | 22 (44%) | 23 (43%) | 0.88 | |
| ACE level | 38.8±19.5 | 51.1±29.3 | 18.4±10.1 | 0.01 | |
The average duration from the original diagnosis of cardiomyopathy was 6.1±5.9 years and there was no difference between patients with positive PET and negative PET (6.5±6.1 vs. 5.8±5.1 years, p=0.58). Overall, amiodarone usage was 44% and 50% of patients had an ICD. Differences that were noted to be statistically significant between the PET positive and PET negative groups included the presence of AV block and a permanent pacemaker (32% vs 6%, p=0.002) as well as the serum level of angiotensin converting enzyme (ACE) (51±29 vs 18±10, p=0.01).
FDG PET/CT imaging findings
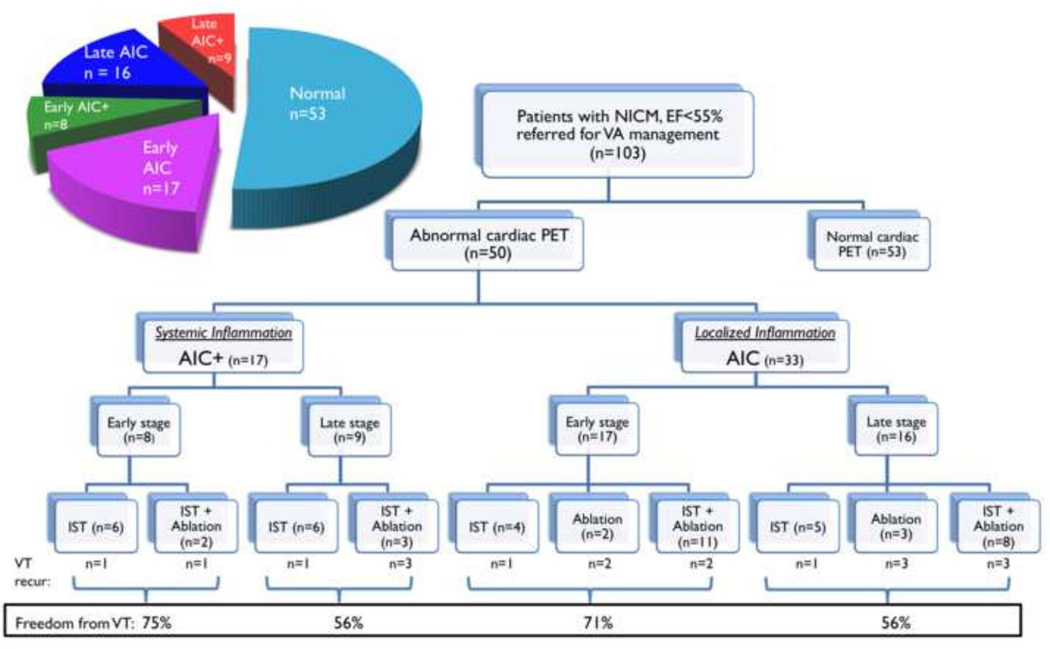
Among 103 patients with unexplained cardiomyopathy and VA, 49% (n=50) had focal FDG uptake on PET. (FIGURE 2) Systemic inflammation with lymph node FDG uptake was observed in 17 patients with AIC+, and isolated cardiac involvement was seen in 33 patients with AIC. The distribution of FDG uptake sites was: anterior 52%, inferior 52%, lateral 56%, septum 44%, and right ventricle 12%. (Supplemental Figure 1) Perfusion defects were present in 52% (9/17) of patients with AIC+ and 48% (16/33) of patients with AIC.
FIGURE 2.
Flowchart of patients included in cohort for analysis.
Electroanatomic mapping and Ablation
Among patients with abnormal PET, 29 patients underwent electrophysiological study and catheter ablation. Five patients had catheter ablation performed at an outside hospital and 5 did not have voltage mapping performed in the region of the PET abnormality for correlation due to an activation mapping strategy or mapping performed in the other ventricle. Only one patient had ablation at our institution prior to an abnormal PET scan.
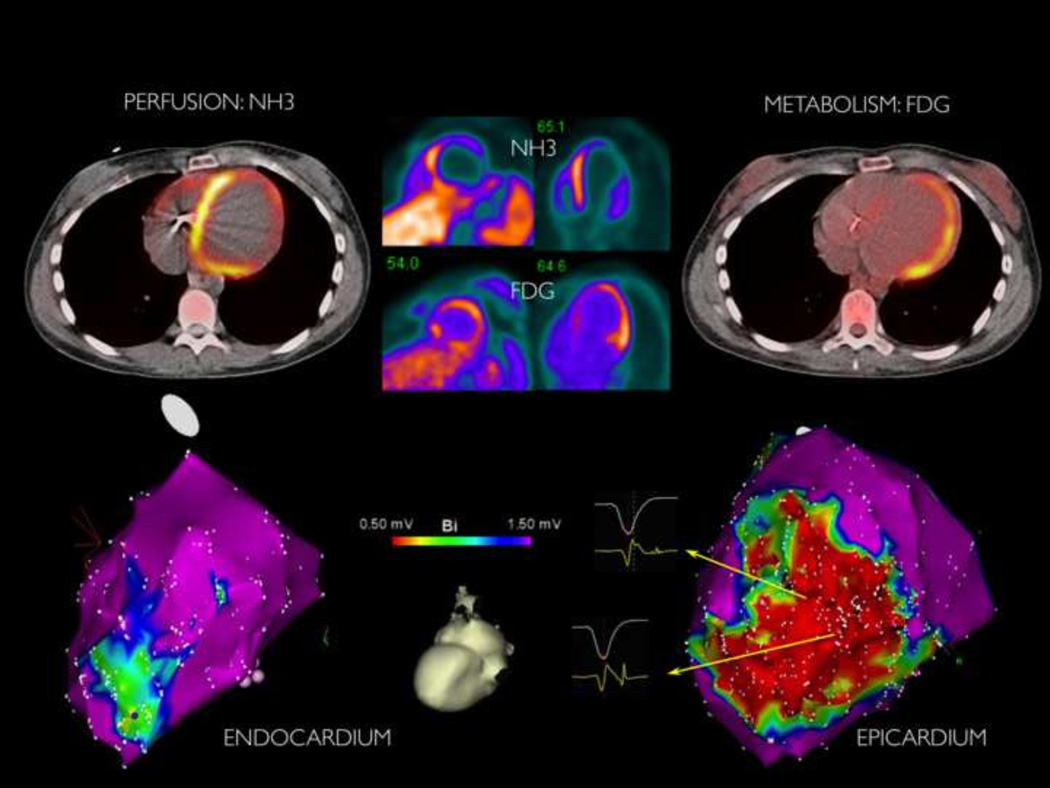
19 patients that had mapping in the region of PET abnormalities, endocardial mapping (RV=5, LV=16) and epicardial mapping (n=14) was performed at our institution. 74% (14/19) of patients demonstrated correlation between low voltage regions on EAM with FDG uptake. (FIGURE 3) Perfusion abnormalities matched EAM low voltage regions in 6 patients. A median of 2 VTs (range 0–5) were induced with tachycardia cycle length of 375ms (range 210–620ms). (TABLE 2)
FIGURE 3.
Example of patient with late AIC with correlation between perfusion-metabolism mismatch in region of epicardial lateral scar. This patient was diagnosed with presumed post-viral nonischemic cardiomyopathy five years prior to PET scan.
TABLE 2.
Electrophysiology Study Findings and Correlation between Electroanatomic Mapping and PET
| Patient | Epicardial | VTs | TCL | VT Morphology |
EAM | Perfusion defect | 18-FDG uptake |
|---|---|---|---|---|---|---|---|
| EAM/PET Concordance | |||||||
| 1 | yes | 1 | 430 | RBI | anteroseptal epi | anteroseptal, inferoseptal | anteroseptal, inferoseptal |
| 2 | yes | 4 | 410,390,370,250 | RBS (3), LBI | lateral, septal | lateral, inferolateral | inferior |
| 3 | no | 0 | anterolateral | anterior | inferolateral, anterolateral | ||
| 4 | yes | 5 | 480,420,410,360,350 | RBI (2), RBS (2) | lateral | septal | lateral |
| 5 | no | 5 | 490,490,410,370,270 | LBI (2), RBI (2), LBS | anteroseptal, RV | normal | anteroseptal |
| 6 | yes | 3 | 620,500,420 | RB (2), RB | lateral, septal | normal | anterolateral |
| 7 | no | 2 | PVC | LBI | RVOT epi | normal | RVOT |
| 8 | yes | 0 | inferoseptal, inferior | normal | inferior, inferoseptal | ||
| 9 | yes | 4 | 450,420,410,390 | RBS (3), LBI | inferior | normal | inferolateral |
| 10 | no | 0 | basal lateral | apex, inferolateral, anterolateral | anterolateral, inferolateral | ||
| 11 | yes | 6 | 500,380,375,340,330,300 | LBS (4), LBI | RV free wall, RV septum | anteroseptal | RV free wall |
| 12 | no | 0 | septum | septum | septum, basal lateral | ||
| 13 | yes | 2 | 310,280 | RBI, LBI | anterolateral epi | normal | lateral, apex, septum |
| 14 | yes | 0 | lateral, RVOT | inferior | inferolateral, septum | ||
| EAM/PET Discordance | |||||||
| 15 | yes | 2 | 310,210 | RB, LB | basal lateral | normal | septum |
| 16 | yes | 4 | 280,280,270,255 | LBI (2), RBI (2) | basal anterolateral | normal | anteroapical, inferior |
| 17 | yes | 1 | 390 | RBS | basal inferoseptum | anterior | anteroseptal |
| 18 | yes | 1 | 330 | LBI | RV | anterolateral | anterolateral |
| 19 | yes | 1 | 250 | RBI | anterolateral | anteroseptal, inferior | RV |
Magnetic Resonance Imaging
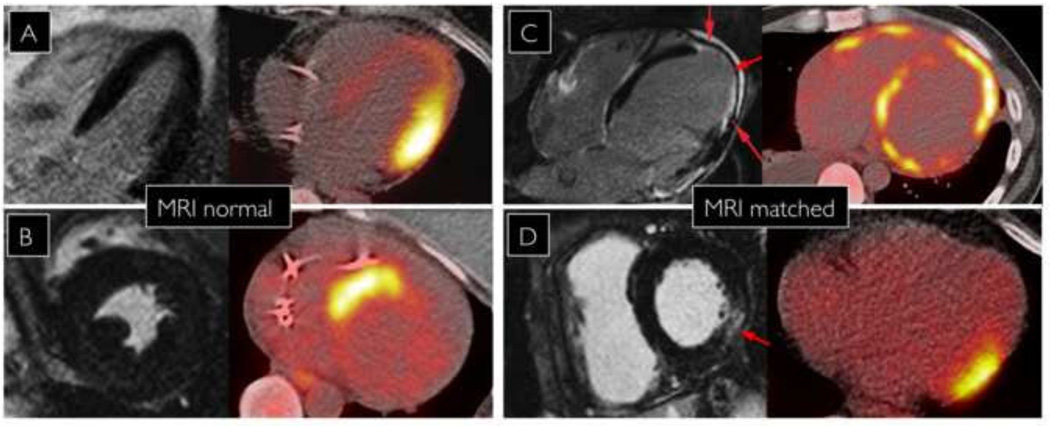
Cardiac MRI was performed in 60% (30/50) of patients with abnormal PET. Areas of delayed gadolinium myocardial enhancement on cardiac MRI were matched with FDG uptake in 40% (12/30). Concordant findings were observed in 38% (6/16) with early AIC/AIC+ and 43% (6/14) with late AIC/AIC+. Amongst patients with discordant findings between MRI and PET, 80% (early AIC/AIC+=9, late AIC/AIC+=7) had no evidence of delayed enhancement on MRI. (FIGURE 4)
FIGURE 4.
Variable correlation between regions of FDG uptake and MRI delayed enhancement. Patients A and B have normal MRI and abnormal PET. Patients C and D have matched findings on MRI and FDG uptake on PET.
In the two patients with mismatch between abnormal MRI and PET, one patient with late AIC demonstrated anterolateral FDG uptake with septal delayed enhancement. However, a septal perfusion defect was observed. The other patient with early AIC had increased FDG uptake in the distal anterior and basal inferolateral walls with MRI enhancement seen only in the RV.
Histologic Diagnosis
Tissue biopsy was performed in 50% (25/50) of patients with abnormal PET scans, with EMBx completed in 20% (10/50) and extra-cardiac lymph node biopsy in 30% (15/50). The histopathologic features in the patients who underwent EMBx were: 30% (3/10) with non-necrotizing granulomatous inflammation, 60% (6/10) with chronic lymphocytic inflammation in absence of granuloma formation, and 10% (1/10) without notable inflammatory infiltrate. In the patients who underwent extra-cardiac lymph node biopsy, 100% (15/15) demonstrated non-necrotizing granulomatous inflammation. Among the entire study cohort (n=103), 17% of patients had a histologic diagnosis of cardiac sarcoidosis.
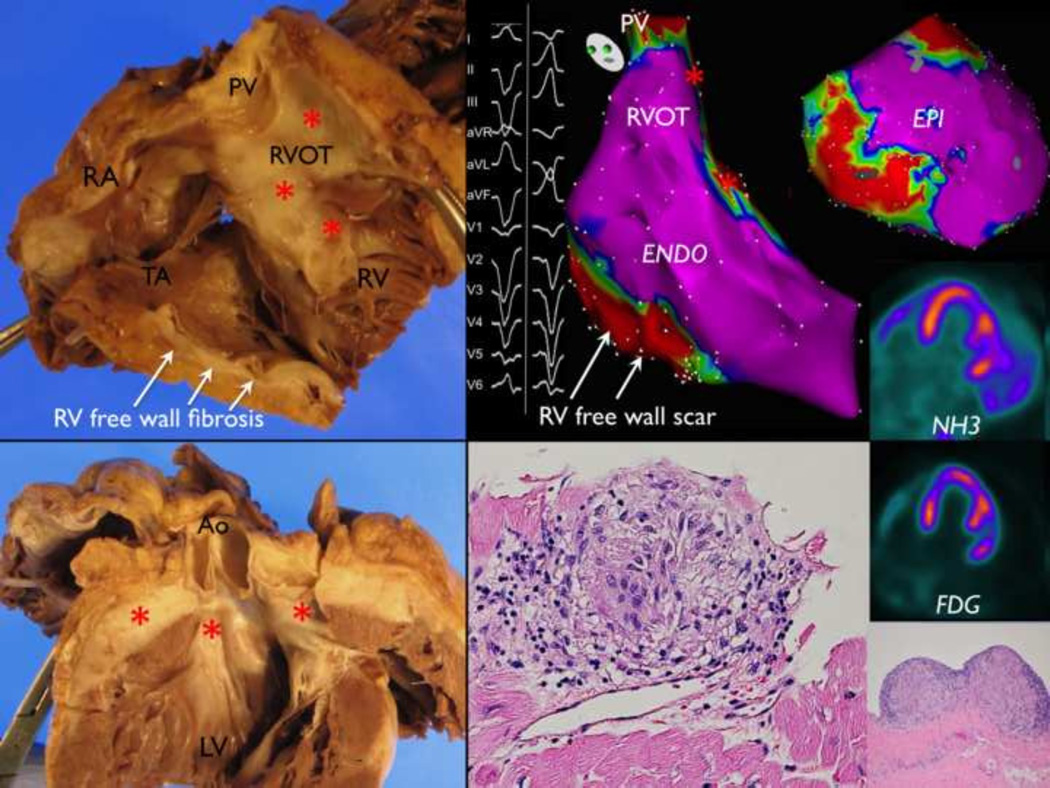
One patient underwent cardiac transplantation and pathologic examination of the explanted heart revealed three focal non-necrotizing granulomas (papillary muscle and subepicardial basal anterolateral). This patient had two negative biopsies and was classified as late AIC by PET. Mapping and ablation was performed twice in this patient after PET and fibrosis correlated well with the low voltage areas found in the RVOT and RV free wall. (FIGURE 5)
FIGURE 5.
Gross and histopathologic examination of a patient with late AIC that underwent transplantation with a history of ablation in the RV. Isolated cardiac sarcoidosis was suspected but could not be proven as two biopsies were negative. At autopsy, three non-necrotizing granulomas were seen on the anterolateral epicardium and anterolateral papillary muscle after steroid therapy. (red asterisks indicate fibrosis)
Clinical Follow-up
IST was initiated in 90% (45/50) of patients with AIC/AIC+ and 42% (21/50) had catheter ablation deferred in favor of IST as primary monotherapy. At 9±3 months, 53% of patients prescribed IST were also started on a single steroid-sparing agent during the steroid taper at physician discretion.
In 17 patients with AIC+, all patients were treated with IST. 29% of patients also underwent catheter ablation. Patients with early stage disease had a trend toward greater freedom from VA recurrence compared to those with late disease (75% at 11 month median follow-up vs. 56% at 9 month median followup, p=0.6). In 33 patients with AIC, 27% were treated with IST monotherapy, 15% with ablation, and 58% were treated with a combination of IST with ablation. Patients with early stage disease had a trend toward greater freedom from VA recurrence compared to those with late disease (71% at 9 month median follow-up vs. 56% at 8 month median follow-up, p=0.6). (FIGURE 2)
Follow up PET scans were available for review in all 21 patients treated with IST monotherapy patients and 25% (6/24) of patients with IST and ablation. In patients with IST monotherapy, improvement or resolution of cardiac PET uptake was seen in 100% (4/4) of early AIC, 83% (5/6) of early AIC+ (FIGURE 6). 60% (3/5) of late AIC, and 50% (3/6) of late AIC+. In patients treated with IST and ablation, improvement or resolution of cardiac PET uptake was seen in 100% (2/2) of early AIC, 66% (2/3) of late AIC, and 0% (0/1) of late AIC+. Values of SUVmax in patients with follow-up PET are shown in the supplemental table.
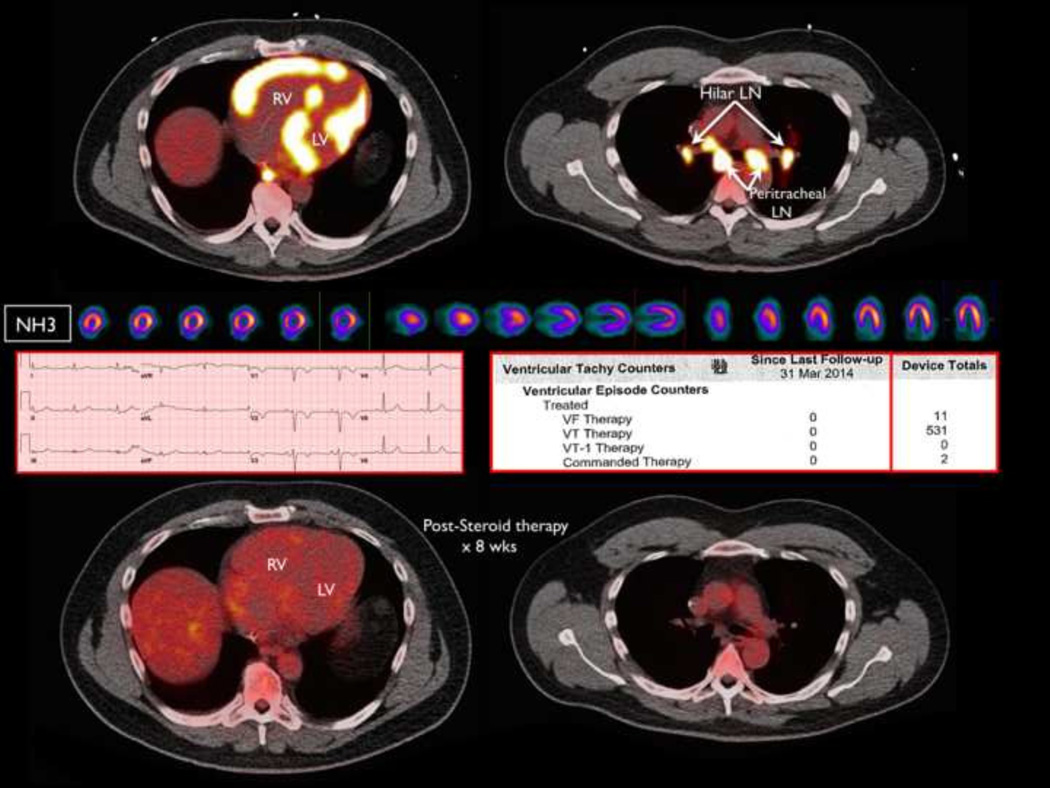
FIGURE 6.
Patient with presumed ARVC (RV dysfunction, left bundle branch VT, and precordial T wave inversions) referred for VT ablation that demonstrated focal biventricular FDG uptake and peri-hilar lymphadenopathy consistent with AIC+ by PET. Complete resolution of inflammation was seen on repeat PET after 8 weeks of primary immunosuppressive therapy with elimination of recurrent VT on ICD counter.
Freedom from recurrent VT was 81% (17/21) in patients that were treated with IST monotherapy. (Supplemental Table 1) In this group, concomitant treatment with antiarrhythmics was prescribed in 38% of patients. In patients that underwent catheter ablation, freedom from recurrent VT was 52% (15/29). Patients with early stage AIC/AIC+ had improved freedom from VT compared to those with late state AIC/AIC+ (72% (18/25) vs 56% (14/25), p=0.031)
In patients treated with IST monotherapy, improvement in ejection fraction (EF) occurred in 80% (8/10) of early stage AIC/AIC+ compared to 55% (6/11) of late stage AIC/AIC+. Mean EF improvement was 19±7% in early stage disease in contrast to 5±3% in late stage disease (p=0.045)
DISCUSSION
The major findings of the present study are:
In patients with unexplained cardiomyopathy presenting with VAs, nearly 50% demonstrate abnormalities in myocardial metabolism with evidence of ongoing inflammation on FDG-PET scan.
Immunosuppressive therapy may have a primary therapeutic role in patients with AIC and early detection of inflammation may portend a better prognosis.
The pathogenesis of dilated cardiomyopathy has long been presumed to be “idiopathic” in etiology or the result of post-viral myocarditis and in patients with longstanding diagnosis, diagnostic testing is often not sought after coronary artery disease and reversible causes are excluded. Although biopsy proven myocarditis has been reported to be 9–16% in patients with unexplained cardiomyopathy7, 8, biopsy is infrequently performed and is poorly sensitive due to sampling error.2 Therefore, the vast majority of patients with DCM in clinincal practice elude etiologic diagnosis. The present findings, to the best of our knowledge, are the first to suggest that inflammation identified by PET may have a significant role in the arrhythmic presentation of patients with “idiopathic” cardiomyopathy. Fasting PET has not been systematically studied in a population with unexplained cardiomyopathy with VAs.
Multiple etiologies of chronic myocarditis have been reported, including autoreactive, immune-mediated, and infectious myocarditis.9 The present data supports that NICM represents a confluence of etiologies, where various types of inflammation, including granulomatous, are present in nearly half of the patients in the cohort. Although post-viral immune and autoimmune activation occurs in the sub-acute phase of acute myocarditis (weeks to several months), persistent cytokine and antibody activation has been related to further cardiac injury and structural remodeling in the chronic phase of dilated cardiomyopathy.10, 11 The findings of ongoing and occult inflammation on PET supports a pathophysiologic stage of extended chronic immune activation after an initial insult, as the mean time from diagnosis was over 6 years.
Among patients with AIC and AIC+, cardiac sarcoidosis was confirmed histologically in 36% of patients in this cohort. Cardiac sarcoidosis has been reported to represent 5–10% of NICM patients referred for catheter ablation of VA.3, 12 The diagnostic challenge of sarcoidosis is the requirement of both clinical criteria and histologic confirmation. Due to the biopsy sampling error (<25% sensitivity) many patients in clinical practice have only “suspected” sarcoidosis.13 While the present data does not assert that all patients with FDG avidity have cardiac sarcoidosis, it is probable that many patients with AIC may have unconfirmed sarcoidosis, as inclusion criteria of this cohort consists of 2 potential major criteria (positive PET as surrogate for gallium and depressed ejection fraction) and 1 minor criteria (VAs). The patient that underwent transplantation with gross pathology correlation (FIGURE 6) consistent with treated isolated cardiac sarcoidosis highlights the diagnostic frustration that is frequently encountered in these patients. Other conditions that could present with cardiac and systemic inflammation include tuberculosis, scleroderma, and lupus.
Sarcoidosis has protean manifestations and the incidence of cardiac involvement and isolated cardiac sarcoidosis has been thought to be uncommon. However, in autopsy studies, 20–70% of patients with extracardiac sarcoid have subclinical cardiac involvement. The number of AIC patients with isolated cardiac inflammation was nearly double those with systemic FDG (AIC+) uptake in this cohort. The scar pattern of sarcoidosis has many similiarities with ARVC and dilated cardiomyopathy with a predilection for the right ventricular free wall, outflow tract, septum, basal lateral wall, and epicardial involvement14–17 These similarities may result in misdiagnosis and under diagnosis of sarcoidosis.18, 19
While PET scans after glucose loading have been performed in patients with dilated cardiomyopathy20, studies in prolonged fasting patients with dilated cardiomyopathy are limited.21 Fasting PET imaging has the ability to provide functional imaging of inflammatory disease activity, where activated macrophages demonstrate higher glucose utilization.22 Although not incorporated in the updated guidelines for cardiac sarcoidosis, studies have demonstrated high sensitivity and specificity when compared with gallium-67 SPECT imaging.23 In patients with sarcoidosis, the quantification of FDG uptake has also been shown to be a prognostic indicator for clinical events as well as a marker of disease activity to assess response to immunosuppression.24, 25
The role of adjunctive catheter ablation with immunosuppression in patients with cardiac sarcoidosis has been demonstrated in observational studies.4, 26 Patients treated with methotrexate did not demonstrate any clinical benefit in the Myocarditis Treatment Trial, although prednisone was associated with improvement in ejection fraction in patients with dilated cardiomyopathy and ongoing inflammation.8, 27 In a single center randomized trial (TIMIC study), immunosuppression was shown to improve LV function and remodeling in chronic, virus-negative myocarditis of >6 months duration.28 However, the specific role of immunosuppressive therapy in patients with AIC has not be systematically evaluated, although small studies have shown improvement in heart function in early phase sarcoidosis.29 Prospective multi-center studies are necessary and are currently being initiated.
Patients with nonischemic cardiomyopathy represent a challenge with catheter ablation due to a relative paucity of late potentials and variable scar transmurality. Outcomes have been shown to be inferior compared to patients with ischemic cardiomyopathy (ICM).30 Progression of substrate has been proposed a potential factor that may contribute to higher recurrence rate in NICM patients.31 Chronic untreated inflammation may be the underlying pathophysiologic basis for expansion of scar substrates and may be underrecognized with current diagnostic and treatment paradigms.
LIMITATIONS
Although PET scans were routinely ordered at our center during the study period for diagnostic work-up, treatment and follow-up of patients with AIC was not protocol-mandated with discretionary treatment strategies including deferring ablation, antiarrhythmics, and dose and duration of IST. In part, this heterogeneity is due to the lack of established guidelines that would dictate a standard approach for this new potential disease entity. Additionally, histologic confirmation was not available for all patients, although biopsy yield has low sensitivity, highlighting an important limitation for patients with suspected isolated cardiac sarcoidosis in real-world practice. The numbers within each subgroup of AIC and AIC+ patients with respect to treatment strategy are small, which limits the power to draw conclusions based on this observational cohort. The referred population in this cohort represent only patients referred for management of VAs and therefore, the present findings cannot be generalized across the spectrum of NICM patients without arrhythmias.
Due to a high variability of normal fasting cardiac FDG uptake patterns that can be seen even with prolonged fasting, PET may not entirely specific and the rate of false positives findings are not known in this clinical context. Dedicated T2 edema imaging, which is not fully standardized, may enhance the sensitivity of MRI to detect early AIC/edema. However, delayed enhancement is is an established clinical criteria for sarcoidosis. Further, due to the fact MRI has better detection for advanced disease, the limited correlation between these imaging modalities has been previously described.32
To avoid overestimation of the incidence of abnormal PET in this cohort, we specifically excluded patients with homogeneous FDG uptake as this may result from inadequate suppression of normal myocardial FDG uptake. Cases of diffuse FDG uptake are not included in our classification to increase specificity, although focal inflammation that is obscured by background FDG uptake cannot be excluded. However, FDG uptake with differential uptake in the posterolateral wall relative to the anterior wall and septum has been previously described as a normal physiologic pattern, which may confound interpretation.33, 34
Referral bias is present when generalizing the true incidence of AIC and sarcoidosis. As illustrated recently by Kandolin et al35, where the annual incidence of cardiac sarcoidosis increased >20 fold over a 25 year period, the detection rate is likely a function of clinical awareness and increased diagnostic accuracy. The observations made in the present cohort reflect the experience of a tertiary academic center with specialization in VA management and those referred for PET already had “idiopathic” cardiomyopathy and VAs, which may have enriched the prevalence of AIC and/or cardiac sarcoidosis. However, a reverse referral bias may be present in patients typically evaluated for cardiac sarcoidosis with documented systemic or pulmonary disease. Isolated cardiac sarcoidosis is likely to be overestimated in the former, and underestimated in the latter.13 It is not known if the present findings are
In this study, specific serologic markers and autoantibodies were not systemically tested. Viral serologies were not routinely ordered in this cohort as the mean time to diagnosis was 6 years and acute myocarditis was not suspected in these patients. Additionally, the high prevalence of viral antibodies in the normal population, reactivation, and cross reactivity all limit the diagnostic specificity of viral testing.2
CONCLUSIONS
Nearly 50% of patients referred with unexplained cardiomyopathy and VA demonstrate ongoing focal myocardial inflammation on FDG PET. This data suggests that a significant proportion of patients labeled “idiopathic” may have occult arrhythmogenic inflammatory cardiomyopathy, which may benefit from early detection and immunosuppressive medical therapy. Further prospective studies are warranted as this may represent an opportunity to identify and prevent progression of disease.
Supplementary Material
CLINICAL PERSPECTIVES.
Patients with idiopathic nonischemic cardiomyopathy represent a growing population referred for management of ventricular arrhythmias. As the etiology of nonischemic cardiomyopathy is heterogeneous and largely unknown, the identification of myocardial inflammation by fasting PET scan may be helpful for diagnosis and therapy. The present study highlights the potential ongoing clinical underappreciation and diagnostic underdetection of inflammation that underlies the arrhythmic presentation of nearly 50% of patients with unexplained cardiomyopathy. These data suggest that a fasting PET scan should be considered in such patients as the finding of focal FDG uptake may justify further histologic diagnostic testing to enable identification of myocardial inflammation at an early stage and has implications for a potential role of immunosuppressive therapy. A prospective, multi-center, randomized trial is necessary and currently being planned to explore the role of fasting PET imaging in nonischemic cardiomyopathy for diagnosis, prognosis, and treatment.
Abbreviations list
- NICM
nonischemic cardiomyopathy
- VA
ventricular arrhythmias
- PVC
premature ventricular contraction
- PET
positron emission tomography
- AIC
arrhythmogenic inflammatory cardiomyopathy
- IST
immunosuppressive therapy
- ICD
implantable cardioverter defibrillator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
REFERENCES
- 1.Sacher F, Tedrow UB, Field ME, Raymond JM, Koplan BA, Epstein LM, Stevenson WG. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythm Electrophysiol. 2008;1:153–161. doi: 10.1161/CIRCEP.108.769471. [DOI] [PubMed] [Google Scholar]
- 2.Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Bohm M. Update on myocarditis. J Am Coll Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 3.Koplan BA, Soejima K, Baughman K, Epstein LM, Stevenson WG. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3:924–929. doi: 10.1016/j.hrthm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Thachil A, Christopher J, Sastry BK, Reddy KN, Tourani VK, Hassan A, Raju BS, Narasimhan C. Monomorphic ventricular tachycardia and mediastinal adenopathy due to granulomatous infiltration in patients with preserved ventricular function. J Am Coll Cardiol. 2011;58:48–55. doi: 10.1016/j.jacc.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, Ito N, Ohira H, Ikeda D, Tamaki N, Nishimura M. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–1543. doi: 10.1093/eurheartj/ehi180. [DOI] [PubMed] [Google Scholar]
- 6.Tung R, Mathuria N, Michowitz Y, Yu R, Buch E, Bradfield J, Mandapati R, Wiener I, Boyle N, Shivkumar K. Functional pace-mapping responses for identification of targets for catheter ablation of scar-mediated ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;5:264–272. doi: 10.1161/CIRCEP.111.967976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore) 1999;78:270–283. doi: 10.1097/00005792-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 9.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. 2648a–2648d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 10.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 11.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–1100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Barbhaiya C, Nagashima K, et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol. 2015;8:87–93. doi: 10.1161/CIRCEP.114.002145. [DOI] [PubMed] [Google Scholar]
- 13.Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivisto SM, Kupari M. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–468. doi: 10.1111/j.1365-2796.2011.02396.x. [DOI] [PubMed] [Google Scholar]
- 14.Jefic D, Joel B, Good E, Morady F, Rosman H, Knight B, Bogun F. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6:189–195. doi: 10.1016/j.hrthm.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Manabe O, Yoshinaga K, Ohira H, Sato T, Tsujino I, Yamada A, Oyama-Manabe N, Masuda A, Magota K, Nishimura M, Tamaki N. Right ventricular (18)F-FDG uptake is an important indicator for cardiac involvement in patients with suspected cardiac sarcoidosis. Ann Nucl Med. 2014;28:656–663. doi: 10.1007/s12149-014-0860-7. [DOI] [PubMed] [Google Scholar]
- 16.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 17.Ichinose A, Otani H, Oikawa M, Takase K, Saito H, Shimokawa H, Takahashi S. MRI of cardiac sarcoidosis: basal and subepicardial localization of myocardial lesions and their effect on left ventricular function. AJR Am J Roentgenol. 2008;191:862–869. doi: 10.2214/AJR.07.3089. [DOI] [PubMed] [Google Scholar]
- 18.Dechering DG, Kochhauser S, Wasmer K, et al. Electrophysiological characteristics of ventricular tachyarrhythmias in cardiac sarcoidosis versus arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2013;10:158–164. doi: 10.1016/j.hrthm.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Philips B, Madhavan S, James CA, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis: distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol. 2014;7:230–236. doi: 10.1161/CIRCEP.113.000932. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa S, Kusuoka H, Maruyama K, Nishimura T, Hori M, Hatazawa J. Myocardial positron emission computed tomographic images obtained with fluorine-18 fluoro-2-deoxyglucose predict the response of idiopathic dilated cardiomyopathy patients to beta-blockers. J Am Coll Cardiol. 2004;43:224–233. doi: 10.1016/j.jacc.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H, Hasegawa S, Yoshioka J, Uehara T, Hashimoto K, Kusuoka H, Kuzuya T, Hori M, Nishimura T. Characteristics of myocardial 18F-fluorodeoxyglucose positron emission computed tomography in dilated cardiomyopathy and ischemic cardiomyopathy. Ann Nucl Med. 2000;14:33–38. doi: 10.1007/BF02990476. [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, Yoshikawa J. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–1036. [PubMed] [Google Scholar]
- 23.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 24.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahara N, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, Baba K, Ishibashi M, Hayabuchi N, Narula J, Imaizumi T. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–1228. doi: 10.1016/j.jcmg.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Naruse Y, Sekiguchi Y, Nogami A, et al. Systematic treatment approach to ventricular tachycardia in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2014;7:407–413. doi: 10.1161/CIRCEP.113.000734. [DOI] [PubMed] [Google Scholar]
- 27.Parrillo JE, Cunnion RE, Epstein SE, Parker MM, Suffredini AF, Brenner M, Schaer GL, Palmeri ST, Cannon RO, 3rd, Alling D, et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–1068. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 28.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30:1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 29.Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 30.Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch E, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liuba I, Frankel DS, Riley MP, et al. Scar progression in patients with nonischemic cardiomyopathy and ventricular arrhythmias. Heart Rhythm. 2014;11:755–762. doi: 10.1016/j.hrthm.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, Miura M, Sakaue S, Tamaki N, Nishimura M. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. doi: 10.1007/s00259-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 33.Choi Y, Brunken RC, Hawkins RA, Huang SC, Buxton DB, Hoh CK, Phelps ME, Schelbert HR. Factors affecting myocardial 2-[F-18]fluoro-2-deoxy-D-glucose uptake in positron emission tomography studies of normal humans. Eur J Nucl Med. 1993;20:308–318. doi: 10.1007/BF00169806. [DOI] [PubMed] [Google Scholar]
- 34.Iozzo P, Chareonthaitawee P, Di Terlizzi M, Betteridge DJ, Ferrannini E, Camici PG. Regional myocardial blood flow and glucose utilization during fasting and physiological hyperinsulinemia in humans. Am J Physiol Endocrinol Metab. 2002;282:E1163–E1171. doi: 10.1152/ajpendo.00386.2001. [DOI] [PubMed] [Google Scholar]
- 35.Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.