Abstract
Nanoparticle based delivery formulations have become a leading delivery strategy for cancer imaging and therapy. The success of nanoparticle-based therapy relies heavily on their ability to utilize the enhanced permeability and retention (EPR) effect and active targeting moieties to their advantage. However, these methods often fail to enable a uniform NP distribution across the tumor, and lead to insufficient local concentrations of drug. Oftentimes, this heterogeneous drug distribution is one of the primary reasons for suboptimal treatment efficacy in NP delivery platforms. Herein, we seek to examine the biophysical causes of heterogeneous NP distribution in stroma-rich desmoplastic tumors; namely the abnormal tumor vasculature, deregulated extracellular matrix and high interstitial hypertension associated with these tumors. It is suggested that these factors help explain the discrepancy between promising outlooks for many NP formulations in preclinical studies, but suboptimal clinical outcomes for most FDA approved nanoformulations. Furthermore, examination into the role of the physicochemical properties of NPs on successful drug delivery was conducted in this review. In light of the many formidable barriers against successful NP drug delivery, we provided possible approaches to mitigate delivery issues from the perspective of stromal remodeling and NP design. In all, this review seeks to provide guidelines for optimizing nanoparticle-based cancer drug delivery through both modified nanoparticle design and alleviation of biological barriers to successful therapy.
Keywords: Nanoparticle, Tumor microenvironment, Extracellular matrix, Cancer associated fibroblasts, Abnormal Vasculature
1. Introduction
The field of nanomedicine has recently attracted tremendous attention, particularly for applications in cancer drug delivery. Owing to the advancements in material science and new manufacturing methods, nanoparticle (NP) drug delivery platforms can now be fabricated to an almost unlimited number of configurations with respect to size, shape, and payload, allowing for versatile applications for the detection, prevention and treatment in oncology. Nanotherapeutics such as liposomes, polymeric micelles and inorganic NP possess a distinct functional advantage over conventional small molecule chemotherapy regimens by overcoming severe systemic toxicities that limit the clinical application of most chemotherapy drugs [19]. Furthermore, nanoparticle drug delivery platforms permit significantly prolonged circulation when compared to small molecule drugs alone. Most importantly, the leakiness of vessels established during angiogenesis and the impairment of lymphatic drainage, constituting the so called EPR effect, provides the primary driving force for the extravasation of NP, improving the intratumoral accumulation and distribution and resulting in enhanced therapeutic outcome [4]. Examples of applications include both the preclinically investigated nanoformulation (i.e. liposomes, polymeric micelles) and FDA approved NP of liposomal doxorubicin (Doxil®) and albumin-bound paclitaxel (Abraxane®). Despite demonstrating outstanding antitumor efficacy in preclinical studies and promising outlooks for multifunctional use in clinical trials, nanoparticulate anti-cancer therapeutics have only provided modest survival benefits overall [34]. Factors such as the fundamental design of the NP, abnormal tumor microenvironment, and the heterogeneity across tumors can compromise the EPR effect and lead to treatment failure. Clinically, the tortuous tumor vasculature and abnormal basement membrane limit both the trans-vascular and interstitial transport of NP. Additionally, the high level of extracellular molecules, increased solid stress and high interstitial pressure limit successful NP extravasation. In addition, off-targeted distribution to non-tumor stromal cells induced by the heterogeneity of the tumor microenvironment (TME) result in diminished tumor cell accumulation, induced drug resistance and compromised clinical outcomes [39]. These serve to explain the discrepancies between the promising results obtained from preclinical studies, and the subpar performance in clinical trials.
Herein, we first present mathematical models for intratumoral NP transport to achieve a better understanding of this complex process. We then discuss the key physiological barriers for NP transport, and analyze the design of NP for enhanced intratumoral transport. Finally, we summarize the strategies to overcome delivery barriers through remodeling the TME and designing the TME-responsive NP.
2. Mathematical Modeling and In Vitro Models of NP’s Intratumoral Distribution
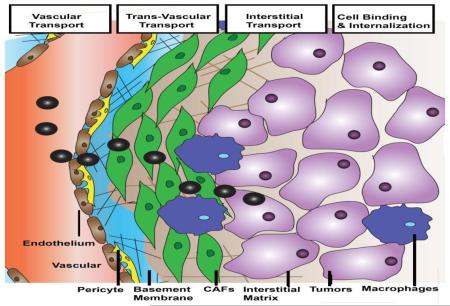
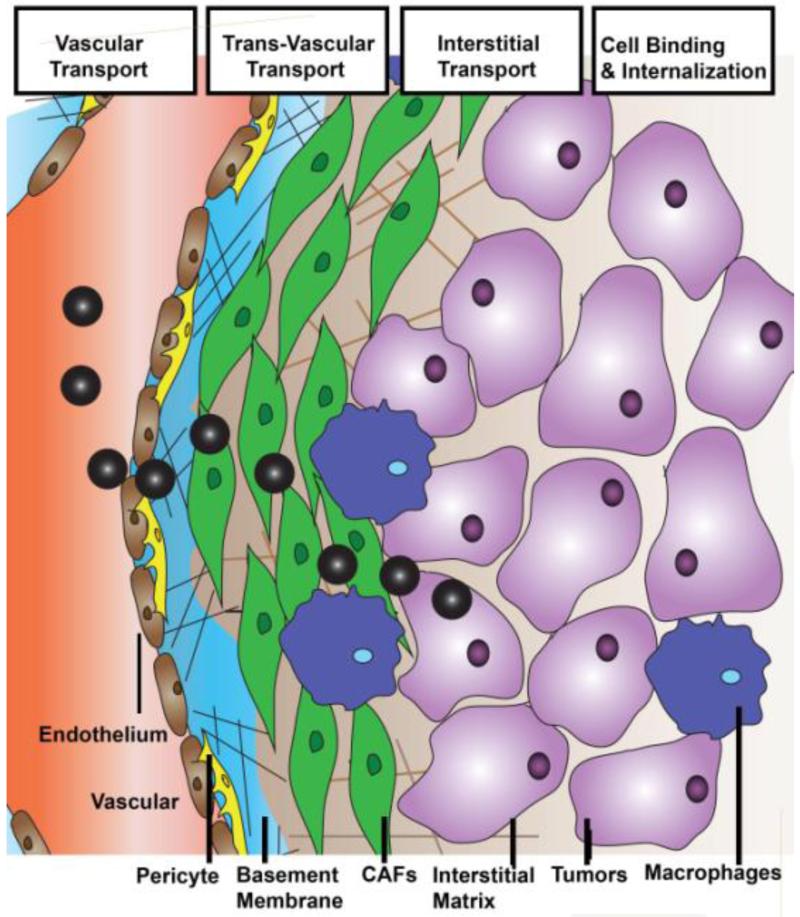
The intratumoral delivery of macromolecules and NP requires several steps in transport, including vascular transport, transvascular transport, interstitial transport, cellular binding, internalization and metabolism (Figure 1) [19]. All these steps are generally limited by pathophysiology of tumors. To better understand the biophysical underpinnings of these transport barriers, Jain and his colleagues have developed several mathematical models to simulate the intratumoral behaviors of NPs [47-49].
Figure 1. Scheme of NP intratumoral transport.
For the modeling of vessel and trans-vasculature transport, the tumor vasculature was represented by a two-dimensional percolation network with one inlet and one outlet that resembles the vascular structure and function of tumors [50]. Vessel transport is mainly dominated by convection (flow rate governed by pressure gradient) and is quantified based on the perfusion rate of blood flow (Q). Poiseuilles’s law was used to simulate vessel transport, suggesting that blood flow is proportional to the vascular pressure gradient and blood viscosity [50]. The transvascular flow was set proportional to hydraulic conductivity of the vessel wall, the surface area of the vessel and also the influence of interstitial fluid pressure. All parameters can be measured using standard intravital microscopy, multiphoton microscopy and optical frequency domain imaging [19, 51-54]. This modeling formula emphasizes the potential influence of blood vessel area, pore size and interstitial fluid pressure (IFP) on NP transport.
Interstitial transport, mainly indicating the diffusion of NP through the extracellular matrix (ECM) toward tumor cell targets, is another significant step in determining NP penetration. Interstitial transport follows the Darcy’s theory, which requires calculation of the diffusion coefficients (Deff) of NP in the ECM [50, 55]. Diffusivity of NP in the ECM was modeled in vitro using matrigel or collagen confined diffusion chamber models. Diffusion coefficients were determined using these in vitro ECM models by non-linear fits of intensity gradients to a diffusion model (e.g. Fickian model) [34, 55, 56]. Diffusion coefficients of macromolecules and liposomes can also be quantified in vivo using either single-photon fluorescence recovery after photobleaching or two-photon fluorescence correlation microscopy. These measurements are more clinically relevant, but limited by equipment requirements and cost [56, 59]. A recent report by Lu et al. indicated that cellular density is another factor affecting interstitial transport [60]. Densely packed tumor cells induce solid stress and reduce the interstitial space for NP transport. Therefore, parameters including cell volume, density and spacing should be taken into consideration when modeling the sophisticated interstitial transport.
For the majority of the aforementioned short time scale transvascular transport models or long-term NP penetration models, neither the binding of NP to cancer cells, nor cell uptake was included [61]. However, these two factors play a substantial role in the process of NP transport. In light of this, Mok et al. are credited for the development of mathematical models considering rapid cell surface binding, internalization and degradation through an intratumorally infused Herpes Simplex Virus (HSV). In addition to diffusion coefficient, second-order binding rate constants, first-order dissociation constants and internalization constants were included in the differential equation to distinguish the free interstitial virus, bound virus and internalized virus respectively [61, 62]. Using a similar mathematical model, Kim et al discriminated the interstitial diffusion, cellular uptake and intracellular release of fluorescein-labelled gold NP (6 nm overall with ligand) on a three-dimensional multicellular tumor cylindroid model [63]. The intracellular release kinetics of fluorescein from NP were further included into the differential equation. However, these mathematical equations are all confined to in vitro 3D tumor models or intratumoral injection of NP, and do not consider the effect of vascular transport and plasma clearance. In another study, Schmidt et al. modeled the cellular binding affinity of the targeted molecules along with the dynamic plasma clearance of macromolecules into the mechanistic compartmental model, and avidly studied the effect of molecular size and binding affinity on tumor targeting [64].
Although, most of the mathematical and in vitro models are based on assumptions and limitations, the overall modeling of intratumoral transport of NP still provides a semi-quantitative method for the extrapolation of parameters such as NP physicochemical properties and tumoral barriers on NP transport. This can then be extended so as to predict dynamics of NP transport and the therapeutic outcomes of NP delivering chemotherapy and gene therapy.
3. Enhanced Permeability and Retention Effect and Anti-cancer NP in the Clinical Trials
The mathematical models discussed in the previous section emphasize the importance of the tumor vasculature on vascular and transvasculature transport of NP. New vessels formed during angiogenesis are known to have a leaky and tortuous morphology, permitting NP extravasation [1]. On a different note, rapid unconstrained proliferation is coupled to an IFP and solid stress, which results in the compression of lymphatic vessels and impairs NP clearance [65]. Together, these characteristics comprise the enhanced permeability and retention (EPR) effect. The EPR effect describes how the leaky vasculature of tumors permits enhanced NP permeability, while the lack of a functioning lymphatic network promotes NP retention in the tumor. The EPR effect states perhaps one of the most fundamental advantages for NP-based drug delivery. Clinically, NP have been applied to treat a broad range of cancers. Abraxane is an albumin-stabilized NP designed for the delivery of paclitaxel [66]. Along with other platforms such as a PEGylated liposome based doxorubicin delivery system (Doxil®), both share the ability to exhibit enhanced tumor localization through the EPR effect.
4. Tumor Microenvironment Barriers for Intratumoral NP Distribution
Ironically enough, the mechanistic basis for the EPR effect also comprises one of the primary barriers to NP delivery. Namely, the elevated IFP [67] and increased solid stress act to inhibit successful NP extravasation into the tumor. This paradoxical observation explains the discrepancy between promising preclinical research and the subpar clinical outcomes for NP application. Therefore, successful NP drug delivery relies heavily on the balance of these two competing aims.
4.1 Abnormal Tumor Vasculature Plays Paradoxical Roles in NP-based Delivery
Tumor vessels are known to be heterogeneous and dilated, leaving avascular spaces of various sizes. In addition, abnormal vessel-wall structures with heterogeneous basement membranes, wide inter-endothelial junctions and large pore sizes contribute to the irregularity of the tumor vasculature [4, 68-71]. These factors therefore compromise NP transport and undermines the efficacy of therapeutic agents. The dynamic formation process of abnormal tumor vasculature structure and its role in compromising NP delivery was discussed in details as follows:
Genetic and epigenetic changes drive tumor and mesenchymal cells to produce pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) [72]. Due to over-activation of these pro-angiogenic pathways, tumor vessels become tortuous and leaky. As mentioned in the previous section, leaky vasculature then facilitates transient NP extravasation through the EPR effect. However, the mechanistic basis behind the EPR effect also contributes to excessive fluid extravasation, inducing increased IFP, fluid viscosity and therefore impairs NP vascular transport [73]. The tortuous nature of tumor vessels further contributes to this elevated geometric resistance through decreasing blood flow [1, 74]. Meanwhile, solid stress resulting from unconstrained tumor growth and an abnormal ECM, further compresses vessels, blocking blood flow and leading to vessel collapse [1]. The decrease in blood flow, in turn, has a great effect on the viscous resistance of the blood. Slow blood-flow rates and high blood viscosities govern the vascular and transvascular transport of small molecules, macromolecules and NP. Furthermore, unlike normal tissues, blood velocity in tumors is independent of vessel diameter and unevenly distributed. The poorly perfused or even unperfused blood supply leads to hypoxia and acidic conditions, which bolsters drug resistance and further limits NP diffusion [4]. Aside from blood vessel constriction, the lymphatic vessels in the tumor are also compressed by proliferating cancer cells, causing collapse. The inefficient drainage of fluid from the tumor center coupled with fluid leakage from tumor vessels contributes to interstitial hypertension, which further limits NP perfusion deep into the tumor core [39].
4.2 Acidic and Hypoxia Limit Nanotherapeutic Approaches to Necrotic Areas
Acidic and hypoxic conditions are distinctive features of most solid tumors. Acidification of tumor microenvironments primarily arises from the Warburg effect, which describes the tumor’s shift in energy metabolism from pyruvate oxidation to glycolysis. The excess lactic acid produced through this pathway remains in the tumor due to poor lymphatic drainage as described previously, a characteristic of most solid tumors [75]. Acidic conditions lead to the ionization of weakly basic drugs and compounds, limiting their ability to diffuse in the ECM [76]. On the other hand, hypoxic conditions primarily arise due to unconstrained cell proliferation that outgrows the blood supply in the tumor. As a result, it is well documented that hypoxic regions most commonly surround the necrotic center of the tumor [77]. Due to the altered blood-flow in hypoxic regions, particles often fail to localize in regions of hypoxia [78]. Even if delivery of NP is successful to hypoxic regions, cells deficient of oxygen show surprising resilience to chemotherapy. As hypoxia reduces cell proliferation, chemotherapy drugs targeting rapidly proliferating cells are rendered useless in these regions [79]. Furthermore, hypoxic regions are hotspots of hypoxia-inducible factor (HIF) production. The stabilization of HIF-1A, a normally labile transcription factor, is known to induce drug resistance through up-regulation of genes such as MDR1 [79] [80]. Therefore, hypoxic and acidic environments are a barrier to nanoparticle-based chemotherapy options from both a delivery and therapeutic standpoint. However, many novel treatment regimens seek hypoxic and acidic environments as a tumor drug target. Specifically, some treatments use the acidic environment to induce drug release from polymeric nanoparticles [81].
4.3 High Interstitial Fluid Pressure Limits NP Convection and Accumulation
Another notable contradiction that arises when discussing the mechanism behind the EPR effect is the elevated IFP. The balance between elevated IFP and the increased NP uptake via EPR effect influences successful NP delivery. High IFP is known to be the result of a variety of factors. Firstly, the dense surrounding collagen matrix of the TME is rich in cancer associated fibroblasts (CAF), which contract and tighten the collagen network by secreting ECM associated molecules and integrin dependent binding [67, 82]. This first barrier physically limits the expansion of the tumor cavity in response to growth. Continuous unregulated tumor proliferation in this enclosed space then compresses blood and lymphatic vessels due to growth induced solid stress, which in turn prevents the efficient discharge both NP and interstitial fluid from the tumor [1, 83, 84]. An elevated IFP is known to be particularly detrimental for large molecule/nanoparticle delivery, which rely primarily on convection for their extravasation [70]. High IFP acts against convection and force NP to enter via passive diffusion, a kinetically slower process. Furthermore, IFP induced vessel constriction has been shown to cause tumor hypoxia, where the increased precedent of angiogenic and growth factors contribute to lymph node metastasis and drug resistance. These secreted factors are then relocated from the tumor periphery toward the outer invasive front due to the IFP gradient where they communicate with fibroblasts to induce resistance and metastasis [85-87]. A high IFP therefore obstructs the therapeutic efficacy of NPs and leads to heterogeneous drug distribution in the tumor stroma.
4.4 High Tumor Cell Density Influences the Interstitial Diffusion of NP
While aforementioned discussions outlined the role of IFP modulation on convective transport, a study conducted by Kuh et al. demonstrated the influence of high tumor cell density on NP diffusion into the tumors [88]. The study performed in vitro using tumor fragments in the absence of a functioning blood supply avoided the impact of IFP and convection on NP transport. It therefore demonstrated that high tumor cell density is a key determinant for the diffusion process. This observation was further confirmed in vivo by Lu et al, showing that cell density was crucial for both convection and diffusion of NP to the tumor [60]. As discussed earlier in this review, rapid tumor cell growth in a limited area imposes solid stress and compresses blood vessels, thus, limiting convection [1]. High cellular density also reduces the interstitial space and limits NP diffusion. Further coherent studies using apoptosis-inducing drugs (paclitaxel and doxorubicin) to reduce tumor cell density led to increased NP perfusion and highlight the importance of cell density on the modulation of NP interstitial transport [89].
4.5 Abnormal Extracellular Matrix Interferes with NP-based Drug Delivery
The non-cellular components of the tumor ECM is another key element that imposes solid stress [1]. Particularly, the high cellularity of tumors compresses the interconnected collagen network and space-filling hydrogel-like glycosaminoglycans (GAG), the two major components of the ECM. The compressed network in turn results in the accumulation of solid stress and dictates interstitial transport [90]. At the cellular level, ECM is localized at two different intratumoral sites, the basement membrane (BM) and the interstitial matrix.
The BM functions as a scaffold for endothelial and mural cells. In particular, the matrix of the BM contains highly compact, sheet-like dispositions formed from fibronectin, laminin and type IV collagen, linked via nidogen and heparin sulfates [91, 92]. More than 99% of non-cancerous blood vessels are covered by a thin layer of BM, which regulates vessel development through paracrine secretion of pro-inflammatory and pro-angiogenic cytokines [93]; Whereas the BM of tumor microvessels are primarily continuous but conspicuously abnormal [93]. Heterogeneous BM morphologies were observed in different tumors or different regions of the same tumor. MCa-IV breast carcinomas and 4T1 breast cancer are characterized by a lesser amount of collagen in the vessel wall, which are loosely associated with the underlying endothelia [16]. Separately, breast cancer 3LLL and pancreatic cancer BxPC3 are distinguished by a second type of BM, with a distribution of more condensed collagen nodules overlapped with the capillary. The BM does not induce the elevation of IFP, yet functions as a sieve to modulate extravasation of free drug and NP from capillaries into the tumor microenvironment. Free DOX, FITC-tagged dextran and 80 nm PEGylated, DOX loaded liposomes (DOX-PLD) were used as tracers to evaluate the extravasation of small molecules and large NP into the tumor microenvironment [16]. Yokoi et al’s study showed that the extravasation pattern of small molecules (DOX, FITC-tagged dextran) in the type I BM model 3LLL and type II BM Model 4T1 were comparable [16]. However, the extravasation of NP (PLD) from the same two tumor types was very different, as suggested by their in vivo NP diffusion model and in vitro collagen-sleeve model. Diffusion based transport of NP was severely hindered by the thickness of collagen fibers, fiber mesh pore size and fiber density. Moreover, the BM is not static, but dynamic as angiogenesis of blood vessels requires degradation of collagen IV by matrix metalloproteases MMP2 and MMP9 [94, 95]. Therefore, degradation of collagen IV provides a transient niche with leaky tumor vasculature and a low/thin BM, a beneficial window for NP delivery. Identification of this window is thus, important to achieve improved NP extravasation. Besides the collagen meshwork, some earlier works also proposed that the extensively charged heparan sulfate chain, which is attached to the laminin/collagen IV network, is essential for the microscopic filtering of positive charged particles. Simultaneously, the nidogen molecules and the protein core of the perlecan complex geometrically hinder the negatively charged particles [18]. Overall, BM characteristics such as the matrix density, presence of proteoglycans and angiogenesis mediated BM remodeling limit the extravasation of NP from blood vessels into the interstitium of tumors.
While the interstitium is also rich with ECM, it limits NP penetration differently from the BM in three ways. Firstly, high stromal fraction and large matrix molecules are restricted to a limited interstitial volume, compressing the matrix into a dense network with increased solid stress and IFP; limiting convection of NP. Secondly, fibrillar structure, mesh size and collagen thickness directly limit the diffusion of NP. Since the molecular components of interstitial ECM are more confluent than BM, the interactions between these parameters are both more pronounced and more complicated. The tortuous nature of the interstitial space is the third primary barrier for NP since it elongates the diffusion path of both NP and macromolecules from blood vessels to target cells. In tumors with a lower amount of interstitial matrix, such as melanoma and colorectal cancers, NP can easily diffuse across the interstitial barrier, access tumor cells and therefore induce growth inhibition [6]. However, this process proves more difficult for tumors with a thick interstitial matrix. Collagen content is the major determinant of interstitial transport [4]. Unlike BM, collagen I rather than collagen V is the major component of the collagen matrix [96]. The fibril orientation of collagen I influences particle diffusion rates. During cancer development, collagen-remodeling enzymes convert the orientation of collagen scaffolds from thin and relaxed collagens (curly fibrils) to thick, aligned fibrils. A study done by Stylianopoulos et al. suggested that this alignment of the collagen fibrils stiffens the ECM, narrows the inter-fiber spacing, and retards the movement of particles [23, 97]. The crosslinking of collagen fibers also causes disparate NP diffusion. Crosslinking of collagen via lysyl oxidase (LOX), regulated by fibronectin and organized by SPARC (secreted protein acidic and rich in cysteine) increased the stiffness of collagen fibers [97, 98]. These molecules are therefore target candidates to inhibit stromal stiffness and improve delivery. With this aim, Kanapathipillai et al. designed a PLGA loaded LOX inhibitory antibodies to decrease collagen crosslinking and improve therapy [99].
The contribution of interstitial GAGs toward macromolecular diffusion is controversial. As one of the major non-sulfated GAG, hyaluronan is a linear polysaccharide with repeating disaccharide units of β-d-glucuronic acid and N-acetyl-β-D-glucosamine [23]. In most cases, the elimination of hyaluronan inhibits nanoparticle transport, opposite to the effect of eliminating collagen [59]. The polymerization of HA has been shown to partition the collagen matrix into aqueous and viscous compartments. Hyaluronidase treatment increased the proportion of slow-diffusing compartments and decreased the diffusion rate for NP transport [17, 100]. On the other hand, for certain tumors such as pancreatic cancer, in which more than 70% ECM consists of HA, the degradation of HA resulted in increased drug diffusion [24]. The sulfated glycosaminoglycan, similar to that in the BM, carries a highly negative charge, which can inhibit the transport of macromolecules or NP by forming aggregates [4, 101].
4.6 Stromal Cells Regulate the Interstitial Distribution of NP
Tumor stroma contains modified ECM attached to multifaceted stromal cells, including fibroblasts/myofibroblasts (carcinoma-associated fibroblasts), mesenchymal cells such as pericytes/mural cells, endothelial cells and immune cells [1, 39]. Apart from the unconstrained proliferation of tumor cells, the high cellular density of stromal cells also contribute to the solid stress, compressing the matrix into a disorganized network and limiting NP penetration. On the other hand, stromal cells compromise the internalization of therapeutic NP in cancer cells through nonspecific depletion of NP that extravasate from adjacent microvessels.
Cancer associated fibroblasts are a major player of tumor fibrosis. CAF inhibit the interstitial transport of NP via the secretion of ECM, construction and stiffening the fibrillar structure and also secretion of paracrine growth factors for tumor resistance and metastasis. For example, lysyl oxidase (LOX) synthesized by CAF could be stimulated via the TGF-β signaling pathway and contributed to increased stromal stiffness through crosslinked collagen fibers [102]. As a major stromal cellular component, CAF also nonspecifically internalizes therapeutic NP and compromises NP’s association with tumor cells in turn; resulting in sub-optimal clinical outcomes [103]. The ratio of off-target NP distribution into fibroblasts is regulated by the spatial distribution of blood vessels in relation to tumor cells and other stromal components (i.e. fibroblasts) [104]. Two dominant phenotypes based on tumor stromal architecture delineate tumor types into either a tumor vessel phenotype (with vessels embedded throughout tumor cells) or a stromal vessel phenotype (vessels distributed in proximity to fibroblasts). While the tumor vessel phenotype is commonly applied in xenograft models, the stromal vessels are more clinically relevant and seen in orthotopic tumors (4T1 breast cancer or pancreatic cancer) and other primary cancers. Off-target distribution of NP into CAF is in fact, more prominent in the stromal vessel type. This was demonstrated in an experiment with 120 nm Docetaxel-conjugated NP which distributed to fibroblasts in 4T1 and MDA-MB-231 orthotopic breast cancer to deplete CAF content by 70% [28]. Given that the tumor-fibroblast transition is a major source of CAF, surface receptors that are highly expressed in tumor cells are not surprisingly also found on respective fibroblasts [105]. Because of the increased binding affinity between fibroblasts and targeted NP, the binding site barrier of CAF is stronger for the targeted NP, rendering off-target distribution more likely. Although therapeutic NP delivered off-target to fibroblasts could deplete fibroblasts and synergize with chemotherapy in some tumor models [27], the therapeutic effect of anti-cancer therapies are also likely to significantly deviate from initial predictions in CAF, due to the different sensitivities and resistance mechanisms of benign fibroblasts and tumor cells [104]. For example, CAF attacked with chemotherapy could secrete survival factors such as Wnt16 that induced the formation of resistant phenotypes of prostate cancer [103]. Overall, the off-target delivery of NP in CAF severely hinders NP penetration and induced convoluted anti-tumor efficacy.
Immune cells, including B cells, T cells, granulocytes, dendritic cells, myeloid derived suppressor cells and macrophages, are indispensable constituents of the TME that modulate the intra-tumoral immune response [106, 107]. Owing to the partial peri-vasculature localization of infiltrated immune cells (other immune cells are likely to distribute in inflammatory hypoxia and necrosis area)[108] and phagocytic properties of some of the immune cells (e.g. macrophages) [109], off-target internalization of NP is inevitable. Using an orthotopic model of melanoma and fluorescently labeled PRINT nanoparticles, Roode et al demonstrated that association between tumor-associated macrophages (TAM) and NP were 4-fold greater than that of cancer cells despite TAM constituting only 1% of all cells in tumors [110]. In another study, the correlation between increased delivery and release of CKD-602 from S-CKD602 liposomes, and increased expression of CD11c-positive dendritic cells in a SKOV3 ovarian xenograft suggested that the NP disposition may be associated with the phagocytic cells (i.e. DC and TAM) [111, 112]. Although, the off-target association of NP in leukocytes and the general immune compartment may modulate the immune pathway (i.e. Stat-3, ERK) and modify the suppressive tumor microenvironment to synergistically improve cancer vaccines (Data not shown), direct phagocytosis of NP by phagocytic cells may deplete the NP and will limit accumulation into the tumor. Overall, the cellular components of the tumor stroma deplete NPs through multiple mechanisms and interfere with the therapeutic outcome of anti-cancer agents.
Pericytes are another group of stromal cells that regulate intratumoral NP transport. Pericytes are primarily characterized as periendothelial mesenchymal cells embedded within the vascular basement membrane[113], which can be identified by pericyte markers such as alpha smooth muscle actin (α-SMA), PDGFR-β, NG2, RGS5 and XlacZ4 [114]. The establishment of pericytes starts with recruitment, which is primarily mediated by endothelial cells through PDGF-β /PDGFR-β signaling during physiological angiogenesis [113]. On the other hand, VEGF-α acts as a negative regulator of pericyte function and vessel maturation [115, 116]. The structure of newly established pericytes is loosely associated with the endothelial cells, and has cytoplasmic processes that penetrate deep into the tumor parenchyma. However, heterogeneity of tumors determines the spatial arrangement pericytes. Work by Kano et al recently classified cancers into two categories based on pericytes coverage: those with less coverage of tumor neovasculature (e.g. colon cancer, CT26) by pericytes and those with more (e.g. pancreatic cancer and diffuse-type gastric cancer) [31]. Unlike the previously discussed stromal cells which compromise NP delivery by depletion, pericytes influence NP transport by regulating blood flow along with controlling the stabilization and maturation of tumor vasculature. Neither leaky, immature blood vessels with little coverage, nor over-matured vessels with abundant pericyte coverage are suitable for NP delivery and should be optimized for better NP perfusion [2]. For example, NP extravasation was severely impeded in the high pericyte coverage (BxPC3) model. Yet, treatment by TGF-β inhibitors or PDGF-β inhibitors could slow down pericyte recruitment, inhibit endothelial pericyte associations and therefore, improve NP extravasation. While the low pericyte subtype showed better NP perfusion, modulation of the pericytes still offered an improvement in NP perfusion with respect to the unmodified group. Specifically, through diminishing non-functional microvessels with low pericyte coverage while increasing pericyte coverage in the normalized tumor vasculature [2]. For example, the VEGF inhibitor, Sorafenib, which negatively controls the regulations of pericytes, increased extravasation of 2 MDa dextran in the CT26 model [5].
5. Physicochemical Properties of NP influences NP transport in Stroma-rich Tumors
Though the biophysical properties of tumors present prominent barriers for successful NP delivery, the physicochemical properties of NP govern the extent and limitations of such barriers. Steric small particles (with size <50 nm), such as PEGylated NP (i.e. polymeric micelles, gold nanoparticles or quantum dots), can penetrate poorly permeable hypovascular tumors such as BxPC3, better than the larger NP (> 50 nm) [6]. The inverse relationship between diffusion rate and NP size was also observed in vitro on the multicellular spheroid models [117]. One potential explanation is that the vascular pore size and the cross-linked collagen fiber mesh form pores that are in between the size of the large and small particles. Therefore, both transvascular and interstitial transport of smaller NP occur rapidly [118]. However, one should note that NP smaller than 10 nm are likely to be excreted from the kidney, at least partially. Therefore, NP below the 10 nm limit exhibit compromised pharmacokinetic profiles and an increase in collateral damage toward normal organs. Increasing the size of NP will provide selectivity, but at the cost of limiting extravasation and diffusion.
Aside from particle size, surface charge also affects intratumoral transport by regulating NP’s diffusive mobility in the ECM. Both PEGylated NP and neutrally charged liposomes exhibit quasi-free diffusive motion in ECM hydrogel and have the advantage of deep penetration into tumors. Cationic NP (e.g. DOTAP liposomes), on the other hand, were entrapped in the hydrogel [18]. However, cationic NP have been shown to exhibit optimized transvascular transport by preferential targeting to the tumor endothelial cells and electrostatic attraction with the negatively-charged vessel pores [119, 120]. Furthermore, positively charged NP are more likely to be taken up by proliferating cells (e.g. tumor cells) compared to neutral and negatively charged NP, which is an additional advantage for effective drug delivery [63].
As far as the shape of NP is concerned, research has shown that NP or macromolecules with linear, rod-like semi-flexible configurations diffuse and penetrate more efficiently into the interstitial matrix compared with solid spherical particles of similar size. The shape of therapeutic NP also affects their circulation time in the blood stream. For example, rod-shaped micelles have a circulation lifetime ten times longer than their spherical counterparts [4, 47, 121].
Surface modification of NP with targeting ligands is another concern for enhancing NP intra-tumoral transport. High binding affinities between NP and the target site are generally seen as an advantage by increasing the internalization of NP. However, the use of targeted NP with high binding affinity may elicit a binding site barrier. This regards a phenomenon where NP binding to target cells paradoxically reduces diffusion deep into tumors. The binding site barrier was first observed during antibody delivery into tumors and later found to be present for NP based delivery as well. High avidity may also comprise the selectivity, since particles may also inadvertently bind to non-tumor cells expressing low levels of tumor specific determinant and depleted accordingly. After all, targeting ligands exclusive to tumor cells are unlikely to exist [122, 123].
Though surface charges and targeting moieties modulated intra-tumoral NP distribution, a growing field of evidence suggests that nanomaterials interacting with biological fluids (e.g. plasma) are likely to adopt a “new” identity through acquiring a surface corona of biomolecules (such as lipids and proteins). This phenomenon, in turn, may comprise the influence of surface properties and dictate the in-vivo biodistribution of NP [124]. The composition of this dynamic corona reflects properties of the nanomaterial such as size, surface curvature and hydrophobicity [125]. Among the identified compositions, opsonins (including immunoglobulin (IgG), complementary factors and fibrinogen) were reported to promote receptor mediated phagocytosis, leading to rapid clearance and limited tumor accumulation of NP [124]. The attachment of antifouling polymers, in particular PEG, has widely been used to prevent protein binding, and consequently modulate the pharmacokinetic profiles of NP [125]. On the other hand, strongly bound monolayer bio-corona could be exploited to prolong NP circulation. For example, albumin complexes with negatively charged NP to promote prolonged circulation times. Abraxane™, the FDA approved albumin-bound form of paclitaxel, is a prime example of using an albumin based drug carrier for efficient anti-tumor therapy [126]. Furthermore, one may consider exploiting the bio-corona for targeting purposes [124]. Kim et al demonstrated specific ApoE binding to NP for delivery across the BBB into the brain [127]. The aforementioned study focused on bio-corona formed in plasma and discussed the pros and cons of this bio-corona for NP delivery. However, NPs approaching the tumor cells must first pass through a dense stromal matrix. How the bio-corona coat interacts with the stroma and whether covered biomolecules will exchange with the stroma are key in determining the ultimate fate of NP disposition. This poorly understood phenomenon requires further investigation into the optimal use of bio-corona coated nanoparticles.
Overall, the physiological properties of NP greatly influence the pharmacokinetics, transvascular transport, intratumoral penetration and cellular internalization in paradoxical manners. This helps to explain the inconsistency between preclinical animal studies and clinical outcomes and therefore, physicochemical properties of NP need to be optimized for each tumor.
6. Strategies to Improve NP extravasation and Penetration
Examination of the barriers that hinder NP delivery has opened doors for new treatment regimens that seek to mediate these factors. Generally, these approaches involve restoring the abnormal tumor vasculature and interstitial stress towards that of normal tissue, and modifying NP with environmentally responsive modifications to enhance delivery (Table 1).
Table 1.
Summary of Stromal Barriers and Strategies
| Major Barriers |
Type | Barriers Constituent |
Barriers Mechanism | Strategy Mechanisms |
Strategy Agents |
Applied Tumor Models |
Ref |
|---|---|---|---|---|---|---|---|
|
Vascula
ture |
Angiogenesis Tortuosity Low blood flow; |
IFP increase Solid stress increase EPR effect decrease |
Normalization | DC101 (VEGF-R mAb); bevacizumab, ranibizumab (VEGF mAb); SST0001 (Heparanase inhibitor); Trastuzumab (HER2-R mAb) |
Hypervasculature: colon carcinoma, myeloma, melanoma |
[1-4] | |
| High viscosity | Leakiness Improvement |
Imatinib (PDGF antagonist); LY364947 (TGF-β inhibitor); Thrombin (vasoactive agents) Angiotensin II (transient vessels pressure raising agent) |
Hypovasculature: pancreatic, lung and breast carcinoma |
[2, 5, 6] |
|||
|
| |||||||
| Stroma | BM | Collagen IV | Thickness, mesh size, orientation, and density limit NP penetration |
Degradation | Collagenase; MMPs | Lung and breast carcinoma |
[16] |
| Nidogen Perlecan |
Crosslinking Collagen Network, and hinder the anionic NP |
Modulating the surface charge and antifouling effect of NP |
[4, 17, 18] |
||||
| GAG | Trap the cationic NP | - | - | ||||
| ECM | Collagen I | Same as Collagen IV | Degradation | Collagenase; Relaxin | Lung carcinoma, melanoma |
[20- 22] |
|
| HA | Partitioning collagen matrix into aqueous and viscous compartments |
Degradation (Paradox) |
Hyaluronidase; PEGPH20 | Osteosarcoma, PDA | [23, 24] |
||
|
| |||||||
|
Stromal
Cells |
CAF | Secrete ECM molecules, stiffen ECM |
Reprogram to Normal Fibroblasts | Losartan (inhibit collagen synthesis); VDR inhibitor (inhibit CAFs activation) |
Breast carcinoma, PDA |
[25, 26] |
|
| Off target depletion NP | CAF Depletion (Paradox) |
cisplatin NP and Doxectaxel conjugates; pFAP (anti-FAP vaccine); FAP substrate-drug Conjugates; FAP targeted liposomes |
Stroma Rich Bladder Cancer; 4T1 breast cancer; colon |
[27 30] |
|||
| Pericytes | Regulate vasculature maturation and limit NP penetration |
TGF beta inhibitors PDGF-β inhibitors VEGF inhibitors |
Pancreatic and breast carcinoma |
[5, 31, 32] |
|||
Note: MMP, matrix metalloproteinase; PDA, pancreatic ductal adenocarcinoma
6.1. Normalization of Tumor Vasculature Benefits NP Extravasation
To counter the detrimental effects of abnormal tumor vasculature on NP transport, strategies to restore normal vasculature have been proposed [31, 123]. Since irregular BM and pericyte coverage hinders the maturation of tumor vasculatures, promoting BM and pericyte recruitment has been proposed to normalize blood vessels [31] Collagen IV, the major constituent of BM, have been shown to be degraded by MMPs. Therefore, the application of MMP inhibitors such as the peptide inhibitor TIMP-1, or a non-peptide inhibitor, AG3340, could inhibit BM remodeling and is considered a promising method for vessel normalization [128]. Another method involves the knockdown of the VEGF signaling pathway. VEGF receptor-2 blocking antibody DC101 can prune immature vessels and recruit pericytes. Proangiogenic molecules, including VEGF, fibroblast growth factor (FGF) and PDGF are overexpressed in tumors and favor angiogenesis [4]. Therefore, VEGF inhibitors can also be applied as an anti-angiogenic agent to revert the vasculature toward a more normal phenotype. A variety of monoclonal antibodies (mAb) against VEGF and other angiogenic signaling factors have been designed. For example, bevacizumab (Avastin), the first approved anti-angiogenic mAb, and its derivative, ranibizumab have been applied in the treatment of metastatic colorectal cancer. Furthermore, the inhibition of heparanase, which plays a major role in angiogenesis, has also been considered as a promising tumor priming strategy [3]. The resulting modifications reduced size of pores in the vessel walls and decreased IFP, allowing NP extravasation to occur through convection rather than diffusion, a much faster process for free molecules and small NP (<12 nm) [50].
Despite these improvements, the smaller vessel pores established through normalization may compromise the advantages gained from enhanced convection for large NP, since the increased steric and hydrodynamic hindrance inhibit the extravasation process [48, 50]. As an additional consideration, the anticancer agents should be administered during the window of normalization to obtain improved delivery, since vascular normalization is a transient process. Furthermore, this strategy may not lead to desirable results against cancer with compressed and less permeable tumors as seen in the case of pancreatic tumor BxPC3 and breast cancer 4T1. Owing to the thick pericyte coverage that limits diffusion of NP in these compressed hypovasculature tumor models, strategies to inhibit angiogenesis may lead to diminished NP accumulation. While somewhat counterintuitive, tumors of the hypovasculature phenotype should be remodeled to improve the leakiness of blood vessels by decreasing pericyte coverage and BM thickness. TGF-β receptor antagonists are the most frequently used therapeutic agents, since low dose TGF-β inhibitor inhibits pericyte recruitment without affecting the function of tumor cells and endothelial cells. Consistent with this finding, various types of TGF-β inhibitors, including small kinase inhibitor and its nano-formulation in addition to siRNA and antibodies, have been used to improve the intratumoral penetration of sub-100 nm NP, such as liposomes, polymeric micelles and PEI-PEG-coated mesoporous silica nanoparticles (MSNP) in hypovasculature tumor models [2, 32, 129]. Another approach to improve NP penetration in the hypovascular models involves the administration of vasoactive agents such as thrombin, lipopolysaccharide endoxin, to initiate a cascade of cellular events that lead to the disruption of endothelial cell junction and increase vascular permeability. Transiently raising the systemic pressure by infusing vasoconstrictors (e.g., angiotensin II) can also increase the vascular permeability and consequently increase NP extravasation. A combined treatment might also beneficial for hypovasculature model, with one treatment to alleviate solid stress through depletion of stromal cells or extracellular matrix, and a subsequent or concurrent vascular normalization treatment to improve perfusion [130]. Overall, the approaches for remodeling tumor vasculatures to improve NP delivery vary with regards to vasculature contents and abnormalities, as well as the size of the therapeutic NP. One should be cautious when choosing strategies and agents since the efficacy of treatment depends largely on the nature of each specific tumor [2].
6.2 Normalization of the Extracellular Matrix Improves NP Penetration
Apart from activation of chemical signaling, interstitial stromal barriers in desmoplastic tumors physically restrict diffusion of macromolecules and nanotherapeutics within the tumor parenchyma. Attesting to this, Dellian et al observed heterogeneous distribution of stealth liposomal-clusters in the perivascular ECM of a xenograft tumor, indicating the role of ECM in limiting the particle diffusion [131]. In another study, Eikenes et al. showed that the degradation of the structural collagen network is more important than the degradation of the GAG and hyaluronan when attempting to increase diffusion of NP and macromolecules [17]. Jain and his colleagues further confirmed this finding [59, 132, 133]. Based on these findings, numerous studies have focused on the subsequent or concurrent delivery of collagenase alongside nanotherapeutics to enhance the intratumoral transport of NP. For example, co-intratumoral injection of oncolytic HSV vector along with bacterial collagenase increased the viral vector distribution by nearly a 3-fold difference in a human melanoma xenograft [20]. In another example, intravenous injection of collagenase-I resulted in higher gene expression of lipoplex in xenograft tumors, further confirming the usefulness of collagen degradation in improving NP distribution [22]. Enhanced diffusion after modification of collagen production was also demonstrated by the use of hormone relaxin, which was reported to both stimulate collagenase synthesis and down-regulate collagen production [21, 134]. Additionally, relaxin is safer compared with bacterial collagenase for in vivo application and proposed for long term use [1].
Similar to collagen, GAG are also key matrix element that induces vascular collapse; among which, hyaluronan (HA) is a major component [133]. HA polymerizes into cage-like structures, partitioning the interstitial space into aqueous and viscous compartments as previously investigated. The use of hyaluronidase to improve the tumor permeability is controversial. High doses of hyaluronidase collapse the HA-based water swelling cage, increase the ECM viscosity and thereby reduces the diffusion coefficient of NP [135]. Notably, elevated expression of tumor-derived hyaluronidase has been used as a diagnostic cue for high-grade bladder cancer and limited perfusion, suggesting the negative outcome of combining hyaluronidase with therapeutic NP [100, 136]. However, in pancreatic ductal carcinoma (PDA), with HA overpowering collagen and constituting 70% of the ECM, the opposite is observed [23]. In a genetically engineered mouse model of PDA, PEG-PH20, a PEGylated recombinant human PH20 hyaluronidase, can effectively improve vascular perfusion of doxorubicin and gemcitabine [23]. Intratumoral administration of bovine hyaluronidases has also shown promise in several xenograft models [137, 138]. A recent study in a human osteosarcoma xenograft model indicated that hyaluronidase induced a 4-fold increase in the distribution of liposomal doxorubicin [24]. Therefore, the application of HA is not limited to small molecules and can also be a promising combinatory component to improve the delivery of NP with larger particle size [23].
However, the aforementioned ECM modifiers may produce collateral damage in healthy tissues (e.g., bacterial collagenase) or increase the risk of tumor progression (e.g., relaxin, matrix metalloproteinases, and hyaluronidase) [25]. Therefore, rather than systemic delivery of these modifiers, site-specific degradation of ECM was preferred and conducted by coating NP with specific ECM enzymes [139, 140]. Goodman et al indicated that collagenase coated 100 nm gold NP showed a 4-fold increase in the number of particles delivered to the spheroid core compared with normal stealth particles [139]. The major concern for enzyme coated NP is maintaining the enzymatic activity during, and after conjugation with the NP. Also, the pharmacokinetic profile of the coated NP proves important as well. Recent work by Ji et al examined these major concerns with enzyme coated NP platforms and confirmed the applicability of the design for improved NP diffusion [141].
6.3 Disruption of Stromal Cells Improves the Intratumoral NP Delivery
In addition to high ECM concentrations, desmoplastic tumors are usually characterized with high levels of stromal cell density. As discussed earlier in this review, this abundance of stromal cells secretes interstitial matrix molecules (i.e. collagen, fibronectin, etc.) that constituent the high interstitial solid stress. Simultaneously, they act as strong binding site barriers for interstitial NP delivery. CAF has been considered as the major component of tumor stroma. The dense ECM associated with CAF also obstructs the intratumoral vasculature, preventing small molecule and NP delivery. These findings suggest that CAF represents a potential target for both therapeutic efficacy and NP delivery. Directly eliminating fibroblasts from the tumor can increase the interstitial transport and distribution of nanotherapeutics by decreasing the tortuosity of the interstitium. For example, off-target distribution of therapeutic NP through platforms such as cisplatin NP and Docetaxel conjugates greatly improved the outcome of stroma rich bladder and breast cancer models through depletion of fibroblasts [27, 28]. To improve the therapeutic outcome, targeted depletion of fibroblasts was investigated in detail. One example is the oral DNA vaccine that targets fibroblast activation protein (FAP), which is commonly overexpressed on CAF [142]. The DNA vaccine specifically depleted fibroblasts and improved the delivery of the therapeutic agents [142]. Later on, CAF targeted NP, such as FAP antibody conjugated immuno-liposomes and FAP substrate conjugated drugs, were designed to specifically deplete CAF [29, 30]. Though depletion of CAF undoubtedly induced an increased vessel perfusion and enhanced NP diffusion, two recent studies also suggest that eliminating stroma by targeted deletion of fibroblasts results in undifferentiated progression of aggressive pancreatic cancer, suggesting the paradoxical effect of CAF depletion [143]. One explanation for the paradox is that these depletion strategies run the risk of eliminating key stromal components needed for tissue homeostasis. Furthermore, the off-target distribution of chemotherapy in fibroblasts may induce senescence in CAF, a procedure that will induce the growth and resistance of neighboring tumor cells and ultimately stiffen ECM, limiting NP perfusion in the long run [144]. To prevent this paradox, alternative approaches were adopted to remodel fibroblasts and ECM. Note that resting fibroblasts (normal fibroblasts) can be transdifferentiated into CAF in response to cytokines (i.e. TGF-β, PDGF and sonic hedgehog (SHH) [145-147]), growth factors, oxidative or metabolic stress, and synthesize abundant ECM proteins to support tumor proliferation. Therefore, the pharmacological means to reprogram CAF back to this quiescent state would be a promising strategy to inhibit tumor growth and improve NP delivery [26, 148]. Recent studies by Diop-Frimpong et al indicated the downregulation of TGF-β signaling in CAF by an angiotensin receptor inhibitor, Losartan, could reprogram and reduce the activity of CAF, decreasing the synthesis of collagen I and thus improving the intratumoral penetration of NP [25]. Success from this formulation has led to a phase II clinical trial of Losartan along with FOLFIRINOX in patients [123, 149]. Vitamin D Receptor (VDR) has also been shown to be overexpressed in the pancreatic stellate cells (PSC), an activated form of CAF in PDA. Sherman et al suggested that the VDR ligand acts as a master transcriptional regulator of PSC to reprise the quiescent stroma, reduce the fibrotic content and increase intratumoral vasculatures; which is promising for enhancing NP delivery [26]. Evidence presented above emphasizes the advantages of stromal cell reprogramming over depletion. Despite diluting the ECM components for increased NP perfusion in the reprogramming strategy, high cell densities still act as a binding site barrier for NP with high cell binding affinity, which limits the outcome of this strategy. Considering the spatial intratumoral distribution of CAF and blood vessels as discussed in the earlier sections, fibroblasts are a more likely target for NP and regarded as a therapeutic target. Instead of depletion and transdifferentiation back to noncancerous fibroblasts, CAF can be reprogrammed into killer cells that secrete toxins or other factors that inhibit the proliferation of neighboring tumor cells. Fibroblasts can be engineered into macrophage like cells, providing us hope for in situ engineering using NP delivered agents to convert fibroblasts into natural killers [150].
6.4 Design of Extracellular Matrix Responsive NP
Specific characteristics of the TME can be utilized to design the stimuli-responsive NP to improve intratumoral NP drug delivery (Table 2). Strategies can be divided into two categories. In terms of the first strategy, stimuli-responsive NP can be designed to release the free drug in the tumor interstitium. This method is advantageous as it overcomes the limitation of barriers in the ECM for large sized NP. Stable hydrophobic drugs with membrane permeability could be used as candidate drugs, for example, doxorubicin (DOX). The hydrophobic drug could be directly conjugated to a hydrophilic polymer through an ECM cleavable linkage, or the segments of block copolymers could be connected by stimuli-cleavable linkers and further assembled into micellar NP [10]. Another strategy is based on the premise that the binding site barrier hindered NP distribution. To improve the penetration, stealth materials, such as PEG, were linked to the NP with stimuli-responsive linkers as a shielding layer. The degradation and cleavage of this shield triggered by the TME resulted in the exposure of targeting ligands, which lead to the efficient internalization by neighboring cells [151]. The aforementioned ECM stimuli, distinct from intracellular stimuli, are usually referred to as extracellular stimuli, including temperature, acidic pH and enzymes. When designing the responsive structure, the structure should be smart enough to distinguish the extracellular signals from intracellular signals, and thus achieve the original designed purposes.
Table 2. Design of ECM targeted NP.
| Stimuli Factors |
Major Stimuli Structure |
Formulation | Materials | Stimuli Criteria |
Disease Model (Cell line) |
Drug |
In Vitro&
In Vivo |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
Thermo
Sensitive |
PNIPAAm | Polymeric Micelle |
PNIPAAm-b-PLA | LCST 36°~40° |
- | Dox | in vitro | [7] |
| P(NIPAAm-co-NDAPM)-b- PCL |
LCST 36.5° | - | Prednisone Acetate |
in vitro | [8] | |||
| PNAS-b-PNIPAAm-b-PCL | LCST 36.5° | Hela | Dox | in vitro | [9] | |||
| DHBCS, PEG-b-PNIPAM | LCST 32.0° | PKH26 | Dox | in vitro | [10] | |||
| PNVCL | Chitosan-g-PNVCL | LCST 38.0° | L929, MCF7, PC3, KB | Curcumin | in vitro | [11, 12] |
||
| P(mNVCL)-co-PNVCL | LCST1 20~24°; LCST2 30~42° |
B16-F10 melanoma | Dox | in vitro | [13] | |||
| DPPC, DSPC | Liposome | DPPC, DSPC, CHOL, DSPE- PEG2000 |
Tm 41° | SK-BR-3,MDA-MB-435 breast cancer; U87-MG glioma; B16F10 |
Dox |
in vivo
& in vitro (external) |
[14, 15] |
|
|
Leucine Zipper
Peptide |
Lipid-peptide NP |
Leucine zipper peptide;DPPC, DSPC,MSPC, HSPC |
Tm 40° | B16F10, SW480 | Dox |
in vivo
& in vitro |
[33] | |
|
Elastin &
Elastin mimetic |
NP | Elastin/DNA aggregation | Tm 50-60° | - | - | - | [35] | |
|
| ||||||||
|
pH
Sensitive |
PHis | Micelle | PEG-PHis-PLL or PLL-b-PEG and PHis-b-PEG Mixed |
pKa ~6.5; dissembling |
4T1 breast cancer | Dox | in vivo | [8, 36, 37] |
| PHEMA-b-PHis | HCT116 human colon | Dox |
in vivo
& in vitro |
[38] | ||||
| PAE | PEG-b-(PLA-co-PAE) | pKa ~7.0 | HepG2 | Dox | in vitro | [40] | ||
| PEG-PAE | BT-20, B16F10 melanoma | Paclitaxel | in vitro | [41] | ||||
| PC7A | PEG-b-PC7A | pKa ~6.9 | A549 | Fluorescen ce |
in vivo | [42 44] |
||
| PSD | Polyplex | PEG-PSD/PEI NP | pKa ~7.0 | A2780, human ovarian carcinoma |
Gene | in vitro | [45] | |
| Chitosan | NP | Chitosan-silica nanospheres |
pKa~6.3; swelling |
MCF-7 Breast cancer | TNFα |
in vitro &
in vivo |
[46] | |
|
| ||||||||
|
Enzyme
Sensitive |
MMP2-
cleavable octapeptide gelatin |
liposome | PEG-MMP cleavable peptide-PE (Gly-Pro-Leu- Gly-Ile-Ala-Gly-Gln) |
MMP | 4T1 breast cancer | Fluorescen t probes |
in vitro &
in vivo |
[57] |
| NP | gelatin-gold/quantum dots fabricated multistage NP |
C6 glioma cells | Gold NP, quantum dots |
in vitro
& in vivo |
[34, 58] |
|||
|
GPA peptide
sequence |
Drug Conjugate |
Peptide-FAP cleavable substrate-promelittin protoxin | FAP | MCF7 breast cancer | promelittin protoxin |
in vitro &
in vivo |
[29] | |
Note: LCST, the lower critical solution temperature; PNIPAAm, poly(N-isopropylacrylamide); NDAPM, N-(3-(dimethylamino)propyl)methacrylamide; PNAS, poly(N-acroyloxysuccinimide); PNVCL, poly(N-vinylcaprolactam); DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPC, 1. 1,2-distearoyl-sn-glycero-3-phosphocholine; PAE, polyacrylic ester; PSD, polysulfonamide
6.4.1 Temperature Responsive NP
Temperature is originally considered as an external stimuli generated using external forces [152]. Temperature responsive materials was first found during hyperthermia treatment of tumors, dated back to 1970s, and lately became a major constituent of chemotherapy and used as a complementary strategy for hyperthermia treatment [152, 153]. However, in early 1990s, studies revealed that the core temperature of breast cancer was higher than the peripheral tissues, suggesting that temperature is not only applied as an external force but also an internal physicochemical feature of the ECM [154]. Abnormal temperature gradients were also observed later in brain tumors and melanoma [155, 156]. It is suspected that the elevated temperature in tumor regions is due to glycolysis degradation and reaction, energy exhaustion [157]. Temperature dependent response is usually governed by a sharp nonlinear change in the conformation and physicochemical properties of at least one component of the nanoformulations across their phase transition temperature. The sharp response triggers the release of free drugs from the cargos. Ideally, materials exhibiting relatively sharp thermal phase transitions around body temperature would be utilized to form the thermo-response NP [152]. The commonly used chemicals are summarized in Table 2, among which the thermo-responsive polymer, including poly(N-isopropylacrylamide) (PNIPAM), poly(vinyl chloride) (PVC) and their derivatives were most widely investigated. Thermo-responsive NP were designed according to the aforementioned two strategies. Besides polymer micelles, liposome and peptide conjugates were also designed with thermal responsive components [158]. For liposomes, temperature responsiveness usually arises from a phase transition of the constituent lipids and the associated conformational variations in the lipid bilayers [152]. However, since the tumor temperature is hard to detect, most of the studies in this field focus on the in vitro characterization.
6.4.2 Tumor acidity
Due to the production of lactic acid converted from glucose during the aerobic glycolysis (also known as the Warburg effect, the hallmark of tumors), the extracellular pH of most tumor tissues is mildly acidic in the range of 6.5-6.8, which is in between that of blood (7.4) and lysosome pH (6.4) [159, 160]. pH variations between pathological situations and the normal tissues have been exploited to trigger the release of therapeutic drugs and imaging agents [10]. Notably, pH-dependent delivery systems designed on the basis of this subtle pH difference require that the systems possesses ultrasensitive pH-responsiveness [44]. In the past decades, pH sensitive polymers that are synthesized to meet the requirement include tertiary amine-containing methacrylate copolymers, poly(β-amino ester) (PAE) and poly(sulfadimethoxine) (PSD), as well as those containing pH-sensitive chemical bonds (e.g., imidazole functionality of histidine, 2,3-dimethylmaleic anhydride (DMMA) modified amine moieties) (Table 2) [10]. Accordingly, designs of nanoformulations can be classified into two categories. One is the use of polyacids or polybase with ionizable groups that undergo conformational changes in response to environmental pH variations; the other is the design of polymeric systems with acid-sensitive bounds whose cleavage enables the release of molecules anchored at the polymer backbone. For example, a series of micellar NP possessing ultrasensitive pH-activatable fluorescence emission were formulated by Gao’s group [42, 44]. The pH-activatable nano-probe was composed of a PEG conjugated poly(2-(hexamethyleneimino)ethyl methacrylate) copolymer and showed a micellization-disintegration transition within a narrow pH range of 0.25 units. The release of fluorescent dye from micelles in response to extracellular pH can nonlinearly amplify tumor microenvironment specific signals for imaging purposes [44].
6.4.3 Extracellular Enzymes
In the tumor tissue microenvironment, specific enzymes, including MMPs, phospholipases, glycosidases and esterases, etc., are present with high concentrations and abnormally high activity [10]. Therefore, they have been extensively exploited to modulate targeted drug delivery and release. The first approach is the direct conjugation of lipophilic drugs to a hydrophilic polymer via enzyme cleavable linkage, and further self-assembled into micelles for both anti-tumor drug delivery and tumor imaging. One such linker is the MMPs specific cleavable peptide Gly-Pro-Leu-Gly-Val (GPLGV) [161]. The PEG-peptide-DOX micelle formulation improves the release and delivery of DOX both in vitro and in vivo [161, 162]. The second approach is to use the enzyme-cleavable linkers to link segments of polymers or to use enzyme-degradable materials to loaded chemotherapeutic drugs or imaging agents and release them via enzyme degradation. Enzyme-cleavable linkers crosslinked with hydrogel is among one of the most commonly used formulations. For example, cisplatin was loaded in PEG-diacrylate hydrogel wafers linked with an MMP substrate. The controlled release of cisplatin from the hydrogel upon MMP degradation improved the therapeutic outcome for the treatment of Glioblastoma multiform (GBM) [163, 164]. Gelatin is another frequently used nanocarrier that can be degraded by MMP-2, which is overexpressed in the ECM. A recent study by Wong et al. indicated that a multistage NP composed of 100 nm gelatin core covered with 10 nm quantum dots showed deep tumor diffusion. The triggered release of smaller NP upon MMP degradation lowered the diffusional hindrance in the interstitial matrix compared to larger NP. In the meantime, the lymphatic clearance rate for 10 nm NP was significantly lower in comparison with free small molecular drugs [34, 61].
7. Conclusions and Future Perspectives
The treatment of tumors requires efficient delivery of therapeutic agents to the target cells through a series of transport steps, including vascular transport, transvascular transport and interstitial transport. The dense ECM structure and aberrant tumor vasculature constitute the physiological barriers that hinder the transport of NP through these steps, and subsequently limit therapeutic outcome. The physicochemical properties of NP, including size, shape, surface properties would also regulate the intratumoral NP distribution. In this review, we summarized the barriers and provided strategies to overcome these barriers for improved NP delivery.
However, penetration alone is not the only criteria to evaluate therapeutic response. Firstly, the approaches to remodel the TME for enhanced NP transport may controversially promote tumor cells migration and metastasis, compromising or even reversing the therapeutic effects. As seen, degradation of collagen improves the NP diffusion, but also increases the risk of tumor cell migration by eliminating barriers that usually prevent it. Using cell-based delivery systems such as stem cells with homing properties, may overcome the delivery barriers without adversely changing the TME. Secondly, heterogeneous distribution of NP in the interstitium and disparate internalization of NP to stromal cells may result in acquired resistance from TME and eventually lead to the treatment failure. Therefore, the current challenge is to design multifunctional NP to target both tumor and stroma cells, blocking the resistant tumor-stroma crosstalk [165]. Thirdly, the dynamic change of TME during cancer development or during the remodeling process using priming agents require the efficient nanotherapeutics delivery within the optimal modeling “window”. Therefore, time-dependent dosing in accordance with time-dependent TME changes are beneficial for enhanced NP delivery. To tackle this problem, time-dependent TME change should be first monitored either using imaging tools to visualize the TME or monitoring the levels of certain secreted factors in the blood that could change in response to the TME modeling process. Upon determining the therapeutic window based on monitoring TME changes using the aforementioned methods, therapeutic strategies described in the review can be applied to each specific situation. This monitoring followed by therapy strategy could also provide guidance for the possibility of individualized therapy, considering the heterogeneity of the TME across different cancers or even individuals is a prime deterrent to successful therapy. Fourthly, in addition to delivering therapeutic and diagnostic NP to solid tumors, the delivery of NP to metastatic sites for cancer therapy is even more difficult considering the difference in TME between primary tumors and the metastatic niche. Recently, Swami et al. approached this challenge by formulating NP to target myeloma and the bone metastatic microenvironments [166]. Furthermore, a recent study on the relationship of melanoma-derived exosomes, which induce vascular leakiness at pre-metastatic sites, may provide a means of passively targeting NP to metastatic sites [167]. More systemic cancer treatments may require the combination of immune therapy, and other methods.
Overall, the advancement of NP mediated cancer drug delivery has been fueled by discoveries in material science and understandings in the pathology of tumor TME. This knowledge allows the development of nanotherapeutics that can cater to a seemingly endless number of situations. As examined, this versatility is crucial considering the differences between each tumor and across different patients. As each specific case demands its own specific treatment approach, the diversity of investigated and available nanotherapeutics proves promising to the advancement of cancer treatment.
Acknowledgements
The work was supported by NIH grants CA149363, CA149387, CA151652, and DK100664. The authors thank Karen Bulaklak’s assistance in manuscript preparation.
Abbreviations
- α-SMA
alpha smooth muscle actin
- BM
basement membrane
- CAFs
cancer associated fibroblasts
- ECM
extracellular matrix
- EPR
enhanced permeability and retention
- FAP
fibroblast activation protein
- GAG
glycosaminoglycans
- HSV
herpes simplex virus
- IFP
interstitial fluid pressure
- TAM
tumor associated macrophages
- MMP
matrix metalloproteinase
- MSNP
mesoporous silica nanoparticles
- NP
nanoparticles
- TME
tumor microenvironment
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. Journal of controlled release : official journal of the Controlled Release Society. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- [2].Kano MR, Komuta Y, Iwata C, Oka M, Shirai YT, Morishita Y, Ouchi Y, Kataoka K, Miyazono K. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer science. 2009;100:173–180. doi: 10.1111/j.1349-7006.2008.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pisano C, Vlodavsky I, Ilan N, Zunino F. The potential of heparanase as a therapeutic target in cancer. Biochemical pharmacology. 2014;89:12–19. doi: 10.1016/j.bcp.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nature reviews. Clinical oncology. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, Hirakawa K, Ouchi Y, Nishiyama N, Kataoka K, Miyazono K. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nature nanotechnology. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- [7].Xu FY, Luo T. Synthesis and Micellization of Thermosensitive PNIPAAm-b-PLA Amphiphilic Block Copolymers Based on a Bifunctional Initiator. Macromolecular Research. 2011;19:9. Y. [Google Scholar]
- [8].Li YY, Cheng H, Zhang ZG, Wang C, Zhu JL, Liang Y, Zhang KL, Cheng SX, Zhang XZ, Zhuo RX. Cellular internalization and in vivo tracking of thermosensitive luminescent micelles based on luminescent lanthanide chelate. ACS Nano. 2008;2:125–133. doi: 10.1021/nn700145v. [DOI] [PubMed] [Google Scholar]
- [9].Quan CW, Chang D, Zhang C, Cheng G, Zhang S, Zhuo X. Synthesis of Thermo-Sensitive Micellar Aggregates Self-Assembled from Biotinylated PNAS-b-PNIPAAm-b-PCL Triblock Copolymers for Tumor Targeting. J. Phys. Chem. C. 2009;113:6. R. [Google Scholar]
- [10].Ge Z, Liu S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chemical Society reviews. 2013;42:7289–7325. doi: 10.1039/c3cs60048c. [DOI] [PubMed] [Google Scholar]
- [11].Rejinold NS, Muthunarayanan M, Divyarani VV, Sreerekha PR, Chennazhi KP, Nair SV, Tamura H, Jayakumar R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. Journal of colloid and interface science. 2011;360:39–51. doi: 10.1016/j.jcis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- [12].Rejinold NS, Sreerekha PR, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. International journal of biological macromolecules. 2011;49:161–172. doi: 10.1016/j.ijbiomac.2011.04.008. [DOI] [PubMed] [Google Scholar]
- [13].Levacheva I, Samsonova O, Tazina E, Beck-Broichsitter M, Levachev S, Strehlow B, Baryshnikova M, Oborotova N, Baryshnikov A, Bakowsky U. Optimized thermosensitive liposomes for selective doxorubicin delivery: formulation development, quality analysis and bioactivity proof. Colloids and surfaces. B, Biointerfaces. 2014;121:248–256. doi: 10.1016/j.colsurfb.2014.02.028. [DOI] [PubMed] [Google Scholar]
- [14].Al-Ahmady ZS, Chaloin O, Kostarelos K. Monoclonal antibody-targeted, temperature-sensitive liposomes: in vivo tumor chemotherapeutics in combination with mild hyperthermia. Journal of controlled release : official journal of the Controlled Release Society. 2014;196:332–343. doi: 10.1016/j.jconrel.2014.10.013. [DOI] [PubMed] [Google Scholar]
- [15].Dicheva BM, ten Hagen TL, Schipper D, Seynhaeve AL, van Rhoon GC, Eggermont AM, Koning GA. Targeted and heat-triggered doxorubicin delivery to tumors by dual targeted cationic thermosensitive liposomes. Journal of controlled release : official journal of the Controlled Release Society. 2014;195:37–48. doi: 10.1016/j.jconrel.2014.07.058. [DOI] [PubMed] [Google Scholar]
- [16].Yokoi K, Kojic M, Milosevic M, Tanei T, Ferrari M, Ziemys A. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer research. 2014;74:4239–4246. doi: 10.1158/0008-5472.CAN-13-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eikenes L, Tufto I, Schnell EA, Bjorkoy A, De Lange Davies C. Effect of collagenase and hyaluronidase on free and anomalous diffusion in multicellular spheroids and xenografts. Anticancer research. 2010;30:359–368. [PubMed] [Google Scholar]
- [18].Lieleg O, Baumgartel RM, Bausch AR. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophysical journal. 2009;97:1569–1577. doi: 10.1016/j.bpj.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annual review of chemical and biomolecular engineering. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- [20].McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer research. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- [21].Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nature medicine. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- [22].Kato M, Hattori Y, Kubo M, Maitani Y. Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex. International journal of pharmaceutics. 2012;423:428–434. doi: 10.1016/j.ijpharm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- [23].Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eikenes L, Tari M, Tufto I, Bruland OS, de Lange Davies C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. British journal of cancer. 2005;93:81–88. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O’Dwyer PJ, Liddle C, Tuveson DA, Downes M, Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang J, Miao L, Guo S, Zhang Y, Zhang L, Satterlee A, Kim WY, Huang L. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. Journal of controlled release : official journal of the Controlled Release Society. 2014;182:90–96. doi: 10.1016/j.jconrel.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer research. 2013;73:4862–4871. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- [29].LeBeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Molecular cancer therapeutics. 2009;8:1378–1386. doi: 10.1158/1535-7163.MCT-08-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baum P, Muller D, Ruger R, Kontermann RE. Single-chain Fv immunoliposomes for the targeting of fibroblast activation protein-expressing tumor stromal cells. Journal of drug targeting. 2007;15:399–406. doi: 10.1080/10611860701453034. [DOI] [PubMed] [Google Scholar]
- [31].Zhang L, Nishihara H, Kano MR. Pericyte-coverage of human tumor vasculature and nanoparticle permeability. Biological & pharmaceutical bulletin. 2012;35:761–766. doi: 10.1248/bpb.35.761. [DOI] [PubMed] [Google Scholar]
- [32].Meng H, Zhao Y, Dong J, Xue M, Lin YS, Ji Z, Mai WX, Zhang H, Chang CH, Brinker CJ, Zink JI, Nel AE. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS nano. 2013;7:10048–10065. doi: 10.1021/nn404083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Al-Ahmady ZS, Scudamore CL, Kostarelos K. Triggered doxorubicin release in solid tumors from thermosensitive liposome-peptide hybrids: Critical parameters and therapeutic efficacy, International journal of cancer. Journal international du cancer. 2015;137:731–743. doi: 10.1002/ijc.29430. [DOI] [PubMed] [Google Scholar]
- [34].Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin-mimetic polypeptide sequences. Advanced drug delivery reviews. 2002;54:1057–1073. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- [36].Gao GH, Li Y, Lee DS. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. Journal of controlled release : official journal of the Controlled Release Society. 2013;169:180–184. doi: 10.1016/j.jconrel.2012.11.012. [DOI] [PubMed] [Google Scholar]
- [37].Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. Journal of controlled release : official journal of the Controlled Release Society. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Meng F, Zhong Y, Cheng R, Deng C, Zhong Z. pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: concept and recent advances. Nanomedicine (Lond) 2014;9:487–499. doi: 10.2217/nnm.13.212. [DOI] [PubMed] [Google Scholar]
- [39].Li Y, Wang J, Wientjes MG, Au JL. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Advanced drug delivery reviews. 2012;64:29–39. doi: 10.1016/j.addr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang CY, Yang YQ, Huang TX, Zhao B, Guo XD, Wang JF, Zhang LJ. Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials. 2012;33:6273–6283. doi: 10.1016/j.biomaterials.2012.05.025. [DOI] [PubMed] [Google Scholar]
- [41].Potineni A, Lynn DM, Langer R, Amiji MM. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery. Journal of controlled release : official journal of the Controlled Release Society. 2003;86:223–234. doi: 10.1016/s0168-3659(02)00374-7. [DOI] [PubMed] [Google Scholar]
- [42].Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew Chem Int Ed Engl. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou K, Liu H, Zhang S, Huang X, Wang Y, Huang G, Sumer BD, Gao J. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. Journal of the American Chemical Society. 2012;134:7803–7811. doi: 10.1021/ja300176w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, Zhao T, Sumer BD, DeBerardinis RJ, Gao J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nature materials. 2014;13:204–212. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sethuraman VA, Na K, Bae YH. pH-responsive sulfonamide/PEI system for tumor specific gene delivery: an in vitro study. Biomacromolecules. 2006;7:64–70. doi: 10.1021/bm0503571. [DOI] [PubMed] [Google Scholar]
- [46].Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK. Hollow chitosan-silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials. 2011;32:4976–4986. doi: 10.1016/j.biomaterials.2011.03.050. [DOI] [PubMed] [Google Scholar]
- [47].Pluen A, Netti PA, Jain RK, Berk DA. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophysical journal. 1999;77:542–552. doi: 10.1016/S0006-3495(99)76911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- [49].Stylianopoulos T, Diop-Frimpong B, Munn LL, Jain RK. Diffusion anisotropy in collagen gels and tumors: the effect of fiber network orientation. Biophysical journal. 2010;99:3119–3128. doi: 10.1016/j.bpj.2010.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nature nanotechnology. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kamoun WS, Chae SS, Lacorre DA, Tyrrell JA, Mitre M, Gillissen MA, Fukumura D, Jain RK, Munn LL. Simultaneous measurement of RBC velocity, flux, hematocrit and shear rate in vascular networks. Nature methods. 2010;7:655–660. doi: 10.1038/nmeth.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Leunig M, Yuan F, Menger MD, Boucher Y, Goetz AE, Messmer K, Jain RK. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer research. 1992;52:6553–6560. [PubMed] [Google Scholar]
- [53].Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nature medicine. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- [54].Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nature medicine. 2009;15:1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chauhan VP, Popovic Z, Chen O, Cui J, Fukumura D, Bawendi MG, Jain RK. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed Engl. 2011;50:11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhu S, Niu M, O’Mary H, Cui Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Molecular pharmaceutics. 2013;10:3525–3530. doi: 10.1021/mp400216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ruan S, He Q, Gao H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale. 2015;7:9487–9496. doi: 10.1039/c5nr01408e. [DOI] [PubMed] [Google Scholar]
- [59].Alexandrakis G, Brown EB, Tong RT, McKee TD, Campbell RB, Boucher Y, Jain RK. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nature medicine. 2004;10:203–207. doi: 10.1038/nm981. [DOI] [PubMed] [Google Scholar]
- [60].Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. The Journal of pharmacology and experimental therapeutics. 2007;322:80–88. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- [61].Stylianopoulos T, Economides EA, Baish JW, Fukumura D, Jain RK. Towards Optimal Design of Cancer Nanomedicines: Multi-stage Nanoparticles for the Treatment of Solid Tumors. Annals of biomedical engineering. 2015 doi: 10.1007/s10439-015-1276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mok W, Stylianopoulos T, Boucher Y, Jain RK. Mathematical modeling of herpes simplex virus distribution in solid tumors: implications for cancer gene therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2352–2360. doi: 10.1158/1078-0432.CCR-08-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nature nanotechnology. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Molecular cancer therapeutics. 2009;8:2861–2871. doi: 10.1158/1535-7163.MCT-09-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kharaishvili G, Simkova D, Bouchalova K, Gachechiladze M, Narsia N, Bouchal J. The role of cancer-associated fibroblasts, solid stress and other microenvironmental factors in tumor progression and therapy resistance. Cancer Cell Int. 2014;14:41. doi: 10.1186/1475-2867-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. International journal of nanomedicine. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Torosean S, Flynn B, Axelsson J, Gunn J, Samkoe KS, Hasan T, Doyley MM, Pogue BW. Nanoparticle uptake in tumors is mediated by the interplay of vascular and collagen density with interstitial pressure. Nanomedicine : nanotechnology, biology, and medicine. 2013;9:151–158. doi: 10.1016/j.nano.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Seo BR, Delnero P, Fischbach C. In vitro models of tumor vessels and matrix: engineering approaches to investigate transport limitations and drug delivery in cancer. Advanced drug delivery reviews. 2014;69-70:205–216. doi: 10.1016/j.addr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jain RK. Transport of molecules across tumor vasculature. Cancer metastasis reviews. 1987;6:559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- [71].Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer research. 1997;57:765–772. [PubMed] [Google Scholar]
- [72].Dvorak HF, Detmar M, Claffey KP, Nagy JA, van de Water L, Senger DR. Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. International archives of allergy and immunology. 1995;107:233–235. doi: 10.1159/000236988. [DOI] [PubMed] [Google Scholar]
- [73].Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Developmental cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sevick EM, Jain RK. Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure. Cancer research. 1989;49:3506–3512. [PubMed] [Google Scholar]
- [75].Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, ten Hagen TL, Needham D, Dewhirst MW. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer research. 2012;72:5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat. 2000;3:39–47. doi: 10.1054/drup.2000.0119. [DOI] [PubMed] [Google Scholar]
- [77].Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med (Berl) 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- [78].Devadasu VR, Wadsworth RM, Ravi Kumar MN. Tissue localization of nanoparticles is altered due to hypoxia resulting in poor efficacy of curcumin nanoparticles in pulmonary hypertension. Eur J Pharm Biopharm. 2012;80:578–584. doi: 10.1016/j.ejpb.2011.12.008. [DOI] [PubMed] [Google Scholar]
- [79].Kirtane AR, Kalscheuer SM, Panyam J. Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities. Advanced drug delivery reviews. 2013;65:1731–1747. doi: 10.1016/j.addr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- [81].Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, Jo DG, Ahn CH, Suh YD, Kim K, Kwon IC, Lee DS, Park JH. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35:1735–1743. doi: 10.1016/j.biomaterials.2013.11.022. [DOI] [PubMed] [Google Scholar]
- [82].Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- [83].Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- [84].Nehoff H, Parayath NN, Domanovitch L, Taurin S, Greish K. Nanomedicine for drug targeting: strategies beyond the enhanced permeability and retention effect. International journal of nanomedicine. 2014;9:2539–2555. doi: 10.2147/IJN.S47129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Guo S, Lin CM, Xu Z, Miao L, Wang Y, Huang L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano. 2014;8:4996–5009. doi: 10.1021/nn5010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rofstad EK, Galappathi K, Mathiesen BS. Tumor interstitial fluid pressure-a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia. 2014;16:586–594. doi: 10.1016/j.neo.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- [88].Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL. Determinants of paclitaxel penetration and accumulation in human solid tumor. The Journal of pharmacology and experimental therapeutics. 1999;290:871–880. [PubMed] [Google Scholar]
- [89].Jang SH, Wientjes MG, Au JL. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: effect of treatment schedule. The Journal of pharmacology and experimental therapeutics. 2001;296:1035–1042. [PubMed] [Google Scholar]
- [90].Chauhan VP, Lanning RM, Diop-Frimpong B, Mok W, Brown EB, Padera TP, Boucher Y, Jain RK. Multiscale measurements distinguish cellular and interstitial hindrances to diffusion in vivo. Biophysical journal. 2009;97:330–336. doi: 10.1016/j.bpj.2009.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Inoue S. Ultrastructure of basement membranes. International review of cytology. 1989;117:57–98. doi: 10.1016/s0074-7696(08)61334-0. [DOI] [PubMed] [Google Scholar]
- [92].Yurchenco PD, Ruben GC. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. The Journal of cell biology. 1987;105:2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. The American journal of pathology. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochimica et biophysica acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- [95].Mammoto T, Jiang A, Jiang E, Panigrahy D, Kieran MW, Mammoto A. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. The American journal of pathology. 2013;183:1293–1305. doi: 10.1016/j.ajpath.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- [97].Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Current opinion in cell biology. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kanapathipillai M, Mammoto A, Mammoto T, Kang JH, Jiang E, Ghosh K, Korin N, Gibbs A, Mannix R, Ingber DE. Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano letters. 2012;12:3213–3217. doi: 10.1021/nl301206p. [DOI] [PubMed] [Google Scholar]
- [99].Kanapathipillai M, Brock A, Ingber DE. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Advanced drug delivery reviews. 2014 doi: 10.1016/j.addr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- [100].Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer discovery. 2011;1:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Thorne RG, Lakkaraju A, Rodriguez-Boulan E, Nicholson C. In vivo diffusion of lactoferrin in brain extracellular space is regulated by interactions with heparan sulfate. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8416–8421. doi: 10.1073/pnas.0711345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. The Journal of cell biology. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature medicine. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Smith NR, Baker D, Farren M, Pommier A, Swann R, Wang X, Mistry S, McDaid K, Kendrew J, Womack C, Wedge SR, Barry ST. Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6943–6956. doi: 10.1158/1078-0432.CCR-13-1637. [DOI] [PubMed] [Google Scholar]
- [105].Lee H, Fonge H, Hoang B, Reilly RM, Allen C. The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Molecular pharmaceutics. 2010;7:1195–1208. doi: 10.1021/mp100038h. [DOI] [PubMed] [Google Scholar]
- [106].Almeida JP, Lin AY, Langsner RJ, Eckels P, Foster AE, Drezek RA. In vivo immune cell distribution of gold nanoparticles in naive and tumor bearing mice. Small. 2014;10:812–819. doi: 10.1002/smll.201301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor microenvironment. J Immunother. 2001;24:392–407. [PubMed] [Google Scholar]
- [108].Casazza A, Mazzone M. Altering the intratumoral localization of macrophages to inhibit cancer progression. Oncoimmunology. 2014;3:e27872. doi: 10.4161/onci.27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- [110].Roode LB, Perry T, Luft J, Bear C, Davis J, DeSimone I. Investigation of sub-tumor accumulation of PRINT nanoparticles reveals dose and route of administration dependence on particle association. Cancer research. 2014:1. J. [Google Scholar]
- [111].Zamboni WC, Strychor S, Joseph E, Walsh DR, Zamboni BA, Parise RA, Tonda ME, Yu NY, Engbers C, Eiseman JL. Plasma, tumor, and tissue disposition of STEALTH liposomal CKD-602 (S-CKD602) and nonliposomal CKD-602 in mice bearing A375 human melanoma xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:7217–7223. doi: 10.1158/1078-0432.CCR-07-1035. [DOI] [PubMed] [Google Scholar]
- [112].Song G, Darr DB, Santos CM, Ross M, Valdivia A, Jordan JL, Midkiff BR, Cohen S, Nikolaishvili-Feinberg N, Miller CR, Tarrant TK, Rogers AB, Dudley AC, Perou CM, Zamboni WC. Effects of tumor microenvironment heterogeneity on nanoparticle disposition and efficacy in breast cancer tumor models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:6083–6095. doi: 10.1158/1078-0432.CCR-14-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- [114].Kano MR. Nanotechnology and tumor microcirculation. Advanced drug delivery reviews. 2014;74:2–11. doi: 10.1016/j.addr.2013.08.010. [DOI] [PubMed] [Google Scholar]
- [115].Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes & development. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Huang K, Ma H, Liu J, Huo S, Kumar A, Wei T, Zhang X, Jin S, Gan Y, Wang PC, He S, Zhang X, Liang XJ. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Stylianopoulos T, Poh MZ, Insin N, Bawendi MG, Fukumura D, Munn LL, Jain RK. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophysical journal. 2010;99:1342–1349. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Stylianopoulos T, Soteriou K, Fukumura D, Jain RK. Cationic nanoparticles have superior transvascular flux into solid tumors: insights from a mathematical model. Annals of biomedical engineering. 2013;41:68–77. doi: 10.1007/s10439-012-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, Hanahan D, McDonald DM. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. The Journal of clinical investigation. 1998;101:1401–1413. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annual review of biomedical engineering. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- [122].Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annual review of biomedical engineering. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Fadeel B, Feliu N, Vogt C, Abdelmonem AM, Parak WJ. Bridge over troubled waters: understanding the synthetic and biological identities of engineered nanomaterials, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2013;5:111–129. doi: 10.1002/wnan.1206. [DOI] [PubMed] [Google Scholar]
- [125].Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Advanced drug delivery reviews. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. Journal of controlled release : official journal of the Controlled Release Society. 2012;157:168–182. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- [127].Goppert TM, Muller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. Journal of drug targeting. 2005;13:179–187. doi: 10.1080/10611860500071292. [DOI] [PubMed] [Google Scholar]
- [128].Hoekstra R, Eskens FA, Verweij J. Matrix metalloproteinase inhibitors: current developments and future perspectives. The oncologist. 2001;6:415–427. doi: 10.1634/theoncologist.6-5-415. [DOI] [PubMed] [Google Scholar]
- [129].Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16618–16623. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Dellian M, Yuan F, Trubetskoy VS, Torchilin VP, Jain RK. Vascular permeability in a human tumour xenograft: molecular charge dependence. British journal of cancer. 2000;82:1513–1518. doi: 10.1054/bjoc.1999.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer research. 2000;60:2497–2503. [PubMed] [Google Scholar]
- [133].Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophysical journal. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990;265:10681–10685. [PubMed] [Google Scholar]
- [135].Qiu XL, Brown LV, Parameswaran S, Marek VW, Ibbott GS, Lai-Fook SJ. Effect of hyaluronidase on albumin diffusion in lung interstitium. Lung. 1999;177:273–288. doi: 10.1007/pl00007647. [DOI] [PubMed] [Google Scholar]
- [136].Pham HT, Block NL, Lokeshwar VB. Tumor-derived hyaluronidase: a diagnostic urine marker for high-grade bladder cancer. Cancer research. 1997;57:778–783. [PubMed] [Google Scholar]
- [137].Beckenlehner K, Bannke S, Spruss T, Bernhardt G, Schonenberg H, Schiess W. Hyaluronidase enhances the activity of adriamycin in breast cancer models in vitro and in vivo. Journal of cancer research and clinical oncology. 1992;118:591–596. doi: 10.1007/BF01211802. [DOI] [PubMed] [Google Scholar]
- [138].Brekken C, de Lange Davies C. Hyaluronidase reduces the interstitial fluid pressure in solid tumours in a non-linear concentration-dependent manner. Cancer letters. 1998;131:65–70. doi: 10.1016/s0304-3835(98)00202-x. [DOI] [PubMed] [Google Scholar]
- [139].Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. International journal of nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- [140].Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, Yazdi IK, Corbo C, Palomba R, Khaled SZ, Martinez JO, Brown BS, Isenhart L, Tasciotti E. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8:9874–9883. doi: 10.1021/nn502807n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Murty SG, Qiao T, Tabtieng P, Higbee T, Zaki E, Pure A, Tsourkas E. Nanoparticles functionalized with collagenase exhibit improved tumor accumulation in a murine xenograft model. Particle & Particle Systems Characterization. 2014;31:6. doi: 10.1002/ppsc.201400169. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. The Journal of clinical investigation. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Capparelli C, Guido C, Whitaker-Menezes D, Bonuccelli G, Balliet R, Pestell TG, Goldberg AF, Pestell RG, Howell A, Sneddon S, Birbe R, Tsirigos A, Martinez-Outschoorn U, Sotgia F, Lisanti MP. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle. 2012;11:2285–2302. doi: 10.4161/cc.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Frontiers in physiology. 2012;3:344. doi: 10.3389/fphys.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, Ando S, Martinez-Outschoorn U, Sotgia F, Lisanti MP. Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: connecting TGF-beta signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–3035. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. Journal of gastroenterology. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- [149].Bild W, Hritcu L, Stefanescu C, Ciobica A. Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Progress in neuro-psychopharmacology & biological psychiatry. 2013;43:79–88. doi: 10.1016/j.pnpbp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- [150].Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F, Stanley ER, Graf T. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Poon Z, Chang D, Zhao X, Hammond PT. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5:4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Advanced drug delivery reviews. 2012;64:866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [153].Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- [154].Yahara T, Koga T, Yoshida S, Nakagawa S, Deguchi H, Shirouzu K. Relationship between microvessel density and thermographic hot areas in breast cancer. Surgery today. 2003;33:243–248. doi: 10.1007/s005950300055. [DOI] [PubMed] [Google Scholar]
- [155].Gateff E, Loffler T, Wismar J. A temperature-sensitive brain tumor suppressor mutation of Drosophila melanogaster: developmental studies and molecular localization of the gene. Mechanisms of development. 1993;41:15–31. doi: 10.1016/0925-4773(93)90052-y. [DOI] [PubMed] [Google Scholar]
- [156].Parry D, Peters G. Temperature-sensitive mutants of p16CDKN2 associated with familial melanoma. Molecular and cellular biology. 1996;16:3844–3852. doi: 10.1128/mcb.16.7.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer research. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- [158].Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ, Kostarelos K. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano. 2012;6:9335–9346. doi: 10.1021/nn302148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- [160].Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer research. 1996;56:1194–1198. [PubMed] [Google Scholar]
- [161].Bae M, Cho S, Song J, Lee GY, Kim K, Yang J, Cho K, Kim SY, Byun Y. Metalloprotease-specific poly(ethylene glycol) methyl ether-peptide-doxorubicin conjugate for targeting anticancer drug delivery based on angiogenesis. Drugs under experimental and clinical research. 2003;29:15–23. [PubMed] [Google Scholar]
- [162].Lee GY, Park K, Kim SY, Byun Y. MMPs-specific PEGylated peptide-DOX conjugate micelles that can contain free doxorubicin. Eur J Pharm Biopharm. 2007;67:646–654. doi: 10.1016/j.ejpb.2007.03.023. [DOI] [PubMed] [Google Scholar]
- [163].Tauro JR, Gemeinhart RA. Matrix metalloprotease triggered delivery of cancer chemotherapeutics from hydrogel matrixes. Bioconjugate chemistry. 2005;16:1133–1139. doi: 10.1021/bc0501303. [DOI] [PubMed] [Google Scholar]
- [164].Kim JK, Anderson J, Jun HW, Repka MA, Jo S. Self-assembling peptide amphiphile-based nanofiber gel for bioresponsive cisplatin delivery. Molecular pharmaceutics. 2009;6:978–985. doi: 10.1021/mp900009n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Miao L, Huang L. Exploring the Tumor Microenvironment with Nanoparticles. In: Mirkin CA, Meade TJ, Petrosko SH, Stegh AH, editors. Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer. Springer International Publishing; 2015. pp. 193–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, Zhang S, Moschetta M, Seevaratnam D, Zhang Y, Liu J, Memarzadeh M, Wu J, Manier S, Shi J, Bertrand N, Lu ZN, Nagano K, Baron R, Sacco A, Roccaro AM, Farokhzad OC, Ghobrial IM. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10287–10292. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]