Abstract
Sugar-based polymers have been extensively explored as a means to increase drug delivery systems’ biocompatibility and biodegradation. Here, we review the use of sugar-based polymers for drug delivery applications, with a particular focus on the utility of the sugar component(s) to provide benefits for drug targeting and stimuli-responsive systems. Specifically, numerous synthetic methods have been developed to reliably modify naturally-occurring polysaccharides, conjugate sugar moieties to synthetic polymer scaffolds to generate glycopolymers, and utilize sugars as a multifunctional building block to develop sugar-linked polymers. The design of sugar-based polymer systems has tremendous implications on both the physiological and biological properties imparted by the saccharide units and are unique from synthetic polymers. These features include the ability of glycopolymers to preferentially target various cell types and tissues through receptor interactions, exhibit bioadhesion for prolonged residence time, and be rapidly recognized and internalized by cancer cells. Also discussed are the distinct stimuli-sensitive properties of saccharide-modified polymers to mediate drug release under desired conditions. Saccharide-based systems with inherent pH- and temperature-sensitive properties, as well as enzyme-cleavable polysaccharides for targeted bioactive delivery, are covered. Overall, this work emphasizes inherent benefits of sugar-containing polymer systems for bioactive delivery.
Keywords: Carbohydrate, Glycopolymer, Polymer architecture, Targeted drug delivery, Stimuli-responsive, Self-assembled carriers
Graphical Abstract
1. Introduction
Great advancements in polymer synthetic methods in the past few decades have led to a new generation of sugar-based polymers with complex architectures, compositions, and well-defined molecular weights. All of these characteristics greatly enrich polymer diversity and enable a range of functions in biomaterials and biomedicine [1]. Compared with other synthetic polymers, sugar-based biomaterials have the advantages of being biocompatible, biodegradable, and non-immunogenic, making them particularly suitable for in vivo therapeutic applications. Sugar-based polymers have attracted substantial interest for biomedical applications, including drug, gene, protein, and antigen delivery, as well as diagnostic devices. As such, they represent one of most promising delivery vehicles and can be easily fabricated into different formulations, such as nanoparticles, micelles, and hydrogels, to encapsulate bioactive agents with varying hydrophobicity [2].
Sugars, as an essential component of the human body, are integral in several biological processes. Rapidly-growing interdisciplinary research provides insight into incorporating sugars, such as mannose, lactose, or galactose, into polymers as targeting ligands due to the high specificity of sugar-protein interactions [3]. Sugar-based conjugates have shown success as multifaceted carriers by exhibiting inherent bioactivity through competitive receptor inhibition in addition to mediating delivery of bioactive agents [4]. While various strategies such as introducing pH-sensitive bonds into polymers have been well-documented elsewhere, the use of sugar-based polymers (e.g., polysaccharides) with intrinsic stimuli-responsiveness is highlighted herein.
This review presents the preparation and development of tunable and versatile sugar-based polymers as targeted carriers and stimuli-responsive controlled release systems for bioactives over the past decade, highlighting the benefits of using sugar-based polymers over other synthetic polymers. It describes the design and functionalization strategies of sugar-based systems to improve carrier targeting specificity and stimuli-triggered localized drug release.
2. Benefits of sugar-based polymeric delivery systems
In recent years, sugars (i.e., saccharides) have attracted increased interest in the field of drug delivery due to their inherent biocompatible, biodegradable, and bioadhesive merits [5]. Furthermore, they are derived from abundant natural resources, exist in various repeat units (i.e., monosaccharides, oligosaccharides, and polysaccharides), and possess functional groups amenable to a wide range of chemical modifications. These features make them especially suitable structural building blocks for bioactive carriers. Sugars also play essential roles in many biological processes, such as molecular recognition, adhesion, and inflammation [6], motivating the development of sugar-comprised delivery systems to mimic natural biological processes. Sugar conjugation and modification also enable numerous desirable properties, such as reducing toxicity and immunogenicity [7, 8], improving serum stability, depressing freezing point [9], and promoting bioadhesion [10].
Sugars, such as chitosan or starch, are rich in hydrophilic functional groups, which allow for interactions with biological tissues. Carriers made of sugars have prolonged residence time in certain tissues, thus increasing absorbance of loaded drugs [11]. While synthetic polymers such as poly(ethylene glycol) (PEG) effectively shield carriers from uptake by the reticuloendothelial system, and thus extend half-life, they also retard cellular internalization due to decreased interactions between carriers and cellular membranes. Alternatively, sugar functionalization can camouflage delivery systems, increasing circulation time in the bloodstream, while also enabling cellular entry, which is highly desirable for delivery applications [12]. Polymeric delivery systems bearing pendant sugars with appropriate spatial arrangements can induce remarkable binding affinity enhancement for proteins due to multivalent interactions, known as cluster glycoside effects [1], which is one of the underlying merits of sugars as active targeting ligands (see Section 4 for more detailed discussion).
Recent innovation in synthetic approaches to generate sugar-based polymeric delivery systems [13] enables their construction in a controlled manner and assists the understanding of structure-activity relationships in extensive detail [14, 15]. These well-defined polymers are highly tunable and can be manipulated into different formulations (e.g., micelles, nanoparticles, vesicles, hydrogels), presenting a versatile platform for bioactive delivery. As such, sugar-based polymers have become increasingly prevalent in the broad portfolio of biomedicine and biomaterial applications [16].
3. Sugar-based polymers
Synthesis of polymeric materials has attracted tremendous interest over past few decades due to the precise control over architecture, stereochemistry, and composition. Compared to synthetic building blocks, sugar units have inherent tunability, chirality, and unique degradation properties in vivo. Linear sugars have varying numbers of hydroxyl groups, which can be used as conjugation sites for polymer decoration, and the differences in relative orientation of hydroxyl groups (i.e., stereochemistry) renders specific 3D configuration and spatial arrangement of conjugated moieties. More interestingly, as the human body can only metabolize the dextrorotary (D) form of sugars, the ratio of D and levorotary (L) sugar enantiomers sugars can be altered to tune the polymer degradation profile.
3.1 Synthetic approaches for sugar-based polymers
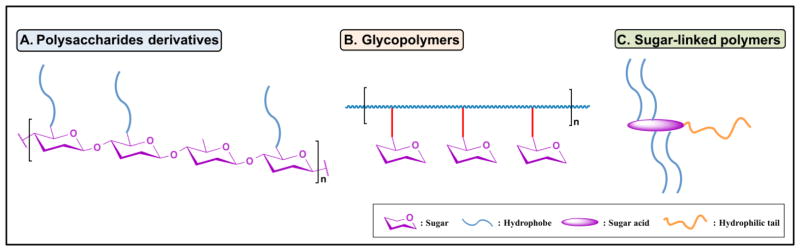
Sugar-based polymeric delivery systems can be categorized into three major types according to the roles of the sugar entities: (i) polysaccharide derivatives, where the sugar is the bulk polymer composition; (ii) sugar-functionalized polymers (i.e., glycopolymers), where sugar moieties are conjugated as pendent groups, and (iii) sugar-linked polymers, where a sugar is used as a branch site or backbone (Fig. 1).
Fig. 1.
Schematic representation and classification of sugar-based polymeric delivery systems.
Natural polysaccharides can be easily modified due to their various functional groups, such as hydroxyl, carboxyl, and amino groups. Liu et al. have written an excellent review on polysaccharide derivatives in 2008 [5]. Various conjugation methods have been well-established to accommodate different reactive groups, such as carbodiimide-mediated and alkoxychloro 1,3,5-triazine-mediated coupling for ester and amide formation respectively [17, 18], leading to comprehensive libraries of hydrophobically- and hydrophilically-modified polysaccharides. One example of a synthetic approach particularly amenable to sugar-based polymers is the use of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride as a coupling reagent for polysaccharide modification, due to its ability to selectively promote amide formation in the presence of unprotected hydroxyl groups [19]. When necessary, linker molecules are used to enable conjugation and present alternative functional groups, such as a succinic [20] or less frequently used formaldehyde linker [21].
Synthetic glycopolymers can be synthesized either by polymerization of glycosylated monomers or by post-functionalization of pre-synthesized polymer scaffolds. Considerable efforts have focused on the controlled synthesis of well-defined glycopolymers [22] by controlled/living radical polymerization, such as nitroxide mediated polymerization (NMP) [23], atom transfer radical polymerization (ATRP) [24], and reversible addition fragmentation chain transfer (RAFT) [15] polymerization. These synthetic methods enable facile preparation of polymers with low polydispersity indices and well-controlled molecular weights and monomer sequences, giving rise to precisely prepared bioactive carriers. Conventional free radical polymerizations, (e.g., ring-opening polymerization (ROP) [14]) commonly require protected sugar-containing monomers. This necessity limits the applicability of ROP, as sequential deprotection of the sugars involves the use of strong alkaline or acidic conditions that may not be compatible with the existing polymer backbone. Combination of multiple polymerization methods is a robust approach to prepare hybrid polymers, such as ROP/ATRP [25]. A less common, but still effective method, is the use of enzyme-mediated polymerization, which is also under investigation as an environmentally-friendly alternative to conventional polymerizations [24]. Another complementary strategy that has also been widely used is post-functionalization of polymeric scaffolds for ease of purification and presentation of protruding sugar moieties, including free-standing polymers or preformed formulation surfaces [26, 27]. The latter approach ensures the presence of sugar moieties on the formulation (e.g., nanoparticles) surface to enable cellular interaction, making it especially suitable for targeted delivery. Glycosylation can be chemically achieved through reductive amination [12], click chemistry for alkyne bearing polymers [28, 29], coupling reactions for conjugation via ester or amide [26, 30], and enzymatic transglycosylation [12]. Click chemistry is beneficial for quantitative carrier glycosylation [29], as it allows for precise control over the degree of glycosylation.
Sugar linked-polymers usually use the multiple hydroxyl groups of sugars as attachment sites. Uhrich et al. developed several series of sugar-based amphiphilic polymers (SBAPs) by acylating or alkylating different sugar acids (e.g., mucic acid, galacturonic acid) to form a branched hydrophobic domain, followed by conjugation to a hydrophilic PEG tail [31–33]. The branched domain could be further functionalized with various ethyleneimines to prepare gene delivery vehicles [34]. Similarly, Synatschke et al. used a core-first approach with functionalized sugars with multiple initiating sites for sequential polymerization by ATRP [35].
3.2. Sugar-based polymers design criteria and strategies
3.2.1. Polysaccharide derivatives
Numerous polysaccharide derivatives have been synthesized to investigate the influence of polymer structure variations on materials effectiveness, such as degree of substitution (DS), substitution linkage types, grafting density, etc. The polysaccharide DS has been well explored to modulate drug loading, encapsulation efficiency, and release profiles [17, 36]. Higher degree of hydrophobic substitution is generally associated with more compact particles, leading to higher drug loading and encapsulation efficiency, along with extended and sustained cargo release [37]. This effect is likely due to stronger intra- and/or intermolecular hydrophobic interactions under aqueous conditions. It should be noted that excessive substitution may lead to limited polymer solubility in water, compromising translational capabilities as drug carriers [36].
Substitution linkage types also play a critical role in adjusting physicochemical properties of delivery systems. Vallée et al. showed that amide derivatives of alginate hydrogels had strongly reduced solubility and better degradation stability compared to ester counterparts [38]. In addition, grafting density is a robust tool to fine-tune hydrogel properties. Mundargi et al. reported that by increasing the acrylamide grafting ratio onto xanthangum, prolonged drug release and reduced swelling were achieved [39].
Tailoring the molecular weight and DS can modulate polymer-nucleic acid complex stability, unpacking, and transfection efficacy for nucleic acid delivery systems. A proper molecular weight and/or DS are required for efficient nucleic acid delivery due to the subtle balance between formulation stability and nucleic acid release. By fine-tuning the molecular weight of cationic chitosan and molar ratio of uncharged oligosaccharide, Strand et al. successfully identified the optimal chain length and substitution degree of glycosylated chitosan that would provide sufficient DNA protection and balanced electrostatic interactions with DNA to facilitate release upon cellular uptake [40].
3.2.2 Glycopolymers
Glycopolymers with different architectures (e.g., star-shaped, linear, and dendritic) have been extensively explored and characterized, providing insight into the rational design of sugar-functionalized delivery vehicles. Aggregates assembled from star-shaped polymers typically demonstrate better thermodynamic stability compared to linear counterparts. Dai et al. reported the synthesis of star-shaped poly(ε-caprolactone)-b-glycopolymer from the controlled ROP of a ε-caprolactone monomer, followed by direct ATRP of unprotected glycomonomer [25, 41]. Through systematic investigation, the star macromolecular architecture was shown to be critical in improving the thermodynamic stability of aggregates. In fact, dendritic copolymers had an order of magnitude lower critical aggregation concentration compared to linear analogues. In addition, by adjusting the weight fraction of hydrophilic glucose-bearing segments, the self-assembly properties and aggregate morphologies of amphiphilic copolymers in aqueous solutions were significantly changed, which can be attributed to the hydrophilic-lipophilic balance (HLB). Morphologies including micelles, vesicles, and large compound aggregates can be fabricated from sugar-decorated polymers with varying HLB, presenting a range of sugar densities and spatial arrangements [42]. For example, giant polymersomes (i.e., vesicles) with 20–25 μm diameters were prepared with glycosylated polyethylene-block-poly(ethylene glycol) (PE-b-PEG) with appropriate HLB values, capable of hydrophobic dye encapsulation [43].
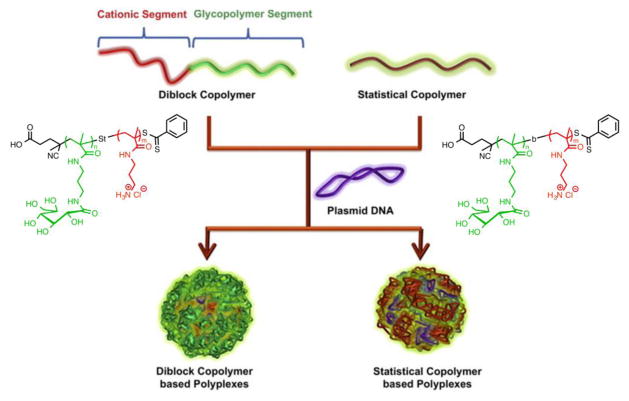
To better understand the influence of architecture on cationic nucleic acid delivery vectors, Ahmed et al. synthesized a library of cationic glycopolymers containing pendant sugars of different architectures (block vs. random) via RAFT [15]. Interestingly, the random copolymers demonstrated greater cell viability and higher transfection efficiency in both serum-free and serum-containing conditions in comparison with corresponding diblock copolymers. These results indicate that the sugar residues were less able to mask the toxicity of the cationic segment in the block configuration. It was likely that block glycopolymers form core-shell structures with a sugar-modified corona, as opposed to random copolymers with a more accessible surface charge (Fig. 2). However, polyplex aggregation induced by serum proteins was considered a major drawback as compared to block copolymers for in vivo applications [15]. Built upon aforementioned findings, Ahmed et al. further developed cationic “block-random” copolymers. To prepare these cationic vectors with block-random configurations, homopolymers of glycomonomer were first synthesized using RAFT polymerization and sequentially used as macro chain transfer agents for copolymerization with the cationic monomer. In contrast to statistical analogues, sugar-based block-random copolymers showed higher gene expression and lower toxicity, in addition to improved stability in physiological conditions [44].
Fig. 2.
Schematic illustration of polyplexes prepared from dib lock and random copolymers using plasmid DNA (adapted from [15] with permission).
Molecular weight is a major factor in cytotoxicity and gene transfection efficiency, which is consistent with trends observed for a broad range of nucleic acid delivery vehicles [45]. High molecular weight cationic glycopolymers (Fig. 2) were found to show comparable gene expression to the poly(ethyleneimine) (PEI) control with significantly lower toxicity [15].
Variations in hydrophobicity and saccharide length should also be considered when optimizing delivery properties. For example, Bhatia et al. developed biocompatible sugar-PEG-based drug carriers by lipase-mediated copolymerization of PEG dimethyl ester and hydrophobically modified sugar monomers. The hydrophobicity of the monomers was modulated to promote aggregation and achieve higher micellar drug loading [24]. Höbel et al. varied the length of oligosaccharides used for preparing grafted PEI from maltose, maltotriose, to maltoheptaose and established the influence of sugar length on physicochemical and biological properties of corresponding nucleic acid complexes. The longer oligomaltose further shielded the surface charge after complexation with siRNA as compared to shorter oligomaltoses, resulting in altered uptake and biodistribution [7].
Sugar-functionalized polymers prepared by post-functionalization from a pre-formed polymer scaffold typically include optimizing sugar conjugation location sites. Kim et al. modified branched PEI (25 kDa) with PEG and mannose as siRNA delivery systems. To characterize the effect of mannose ligand location on cellular uptake and gene silencing efficiency, the mannosylated PEGylated PEI delivery systems targeting macrophages were constructed by either directly conjugating mannose and PEG to the PEI backbone (Man-PEI-PEG) or conjugating mannose to PEI via a PEG spacer (PEI-PEG-Man). The PEI-PEG-Man/siRNA polyplexes, which had mannose moieties exposed on the surface, exhibited faster endocytosis and higher knockdown efficiency than Man-PEI-PEG [46]. The study implies that sugar moieties should be incorporated onto carrier surfaces so that they are available for sugar-cell interactions.
3.2.3 Sugar-linked polymers
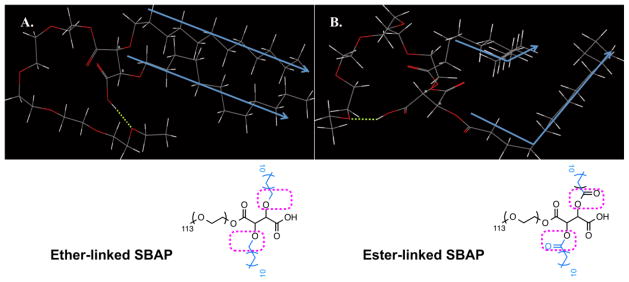
Although sugars used as backbones or branch points are less exploited, a few research groups have explored the structure-activity relationship of sugar-linked polymers for bioactive delivery. Particularly, Uhrich et al. developed a series of SBAPs with extremely low critical micelle concentrations (CMCs) (10−7–10−4 M) as stabilized micellar delivery systems [31, 47, 48]. Unlike conventional diblock copolymers, SBAPs have a branched hydrophobic domain, where the degree of branching [31, 49], linkage type [32], and stereochemistry [50] of the hydrophobic domain were systematically analyzed to determine structure-property relationships. By changing the sugar acid backbone (e.g., tartaric acid vs. mucic acid), the degree of branching, and thus the size of the hydrophobic domain, the structure was conveniently tuned for the desired applications [47, 49]. Interestingly, the conjugation chemistry (e.g., ester linkage versus ether linkage) between alkyl arms and sugar backbone appeared to contribute significantly to solution properties of polymeric micelles. Ether-linked SBAPs had CMC values one or two orders of magnitude lower than corresponding ester-linked SBAPs, correlating well with molecular modeling observations. Better alignment and packing within the hydrophobic domain could be achieved by replacing the relatively rigid ester bond with more flexible ether bond (Fig. 3), leading to more compact aggregates and enhanced colloidal stability [33].
Fig. 3.
Equilibrium (lo w-energy) conformation of SBAPs with tartaric acid backbone (Left: Ether-linked, right: Ester-linked SBAP) in aqueous condition from dynamic simulation. Atoms are color-coded: C(gray), H(white), O(red). The blue arrows depict the orientation of alkyl arms (adapted from [33] with permission).
Evidence has accumulated that non-viral gene delivery systems with nonlinear architecture (e.g., star-shaped polycations) manifest better transfection efficacy than linear polycations [51]. By polymerizing poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) from functionalized sugars (glucose and saccharose) with multiple initiating sites as the central core, Synatschke et al. generated two star-shaped PDMAEMA polymers containing 3-arm stars and 5-arm stars via ATRP. Despite the high general toxicity of polycations, 5-arm stars afforded lower cytotoxicity compared to 3-arm stars with a similar molecular weight, suggesting that cytotoxicity decreased with increased branching degree [35]. While the potential mechanism remains unclear, it was hypothesized that the internal positively charged nitrogens of the star polymers were sterically hindered from interacting with cell membranes, improving cytocompatibility.
4. Sugar-based carriers for targeted drug delivery
Key concerns in the field of drug delivery include systemic toxicity, low drug bioavailability, and rapid drug clearance. Recently, targeted drug delivery systems have gained interest as a way to increase therapeutic efficacy and address these issues by delivering bioactive agents to specific areas of the body. Of the potential targeting strategies, glycosylation targets specific locations in the body, while improving carrier biocompatibility and circulation time [2]. In addition, the bioadhesive properties of glycosylated delivery systems allows for alternative, non-invasive administration routes [52]. Sugar-based polymers can act as carriers for both active and passive targeting. Active targeting provides specific delivery to sugar receptors and reaches the target site with high efficacy, while passive targeting relies on the carrier’s physicochemical properties for localized drug accumulation. This section highlights recent advances in sugar-based drug targeting technologies, focusing on their unique ability to provide cell- and tissue-specific interactions, adhere to and absorb through biological tissues, and be selectively metabolized by cancer cells.
4.1 Passive targeting with sugar-based carriers
Passive targeting with glycosylated nanoassemblies is a common approach, especially for tumors [53]. In cancer progression, partial angiogenesis leads to leaky vasculature and enlarged gap junctions (100 nm to 2 μm), allowing macromolecular carriers to enter the tumor interstitial space and remain for extended time periods by the enhanced permeability and retention effect (EPR) [54].
A number of groups have used sugar-based polymers to locally deliver drugs by mimicking proteins’ physicochemical properties (e.g., surface charge) involved with biological processes at target sites [4, 55]. Additionally, these systems are found to exhibit inherent bioactivity by competitively inhibiting ligands from binding to their receptors and interrupting the normal ligand-activated pathways, such as intracellular signaling, immune response, and other disease cascades. For example, several research groups have exploited the ability of ionic micelles, liposomes, or nanoparticles to preferentially accumulate in macrophages and repress atherogenesis [55–59]. Cardiovascular research by Moghe and Uhrich et al. has demonstrated that by tailoring polymer charge, anionic nanoparticles comprised of SBAPs with a sugar-based backbone and PEG tail target the lesion sites and competitively bind macrophage and smooth muscle cell scavenger receptors to limit cholesterol accumulation in atherosclerosis and neointima hyperplasia in restenosis, respectively [57]. Another example of passive targeting is the use of sulfated ester nanoparticles with a lactose core, which demonstrate high selectin binding efficacy on leukocytes and platelets in blood. For instance, dendritic β-lactose-PEG glycopolymers with terminal sulfate moieties competitively bind leukocyte-selectin receptors to block chemokines from binding to the epithelium, thereby providing anti-inflammatory effects. The authors suggested this effect was likely due to multivalent properties of the glycopolymers, where multiple sulfate moieties associated with leukocyte- and platelet-selectins through electrostatic interactions [60, 61]. Sulfated esters also bind to other selectins, such as glyCAM-1 on endothelial cells, despite the fact that the polymers lack the typical fucose and sialic acid residues required for ligand-receptor binding [62, 63]. While great advancements continue to be made in passive targeting research, the remainder of this section will highlight current strategies for designing particles that promote active targeting specificity.
4.2 Active targeting with sugar-based carriers
Active targeting has gained significant attention as a method to deliver bioactives to specific cell or tissue types and with minimal effects to other areas of the body. In contrast to passive targeting, where sugars are usually present in the macromolecular backbone, active targeting requires the bioactive (e.g., sugar, antibody, etc.) to be displayed on the outside of the nanoassemblies to promote receptor binding.
Carbohydrate receptor-mediated delivery systems are favorable since they are easy, cost-effective, and have well-established synthetic, fabrication, and characterization methods. Specific sugar molecules, such as galactose and mannose, are recognized by carbohydrate-binding proteins (i.e., lectins) present on a variety of cell surfaces [64]. These interactions can be utilized for sugar-mediated targeting by decorating the carrier surface with sugars, known as glycosylation. Once glycosylated particles are bound to the lectins or vice versa, receptor-mediated endocytosis occurs, where the particles are internalized by the cell [64]. The process of endocytosis begins with invagination of the cellular membrane which results in particle uptake, internal vesicle (i.e., endosome) formation, and internalization of extracellular particles.
Alternative common targeting ligands to sugars include small molecules (e.g., folic acid, biotin, etc.), peptides/proteins, nucleic acids, aptamers, and antibodies [65]. Small targeting molecules are favorable due to their efficacy in targeting cancer cell receptors [66], facile preparation, and inexpensive particle conjugation with good efficacy [65]. However, many normal cell types, such as the placenta and kidneys, express the same receptors, so high specificity is often difficult to achieve [67]. Peptide ligands have good targeting specificity [68], small size, high stability, and relative scalability for large-scale synthesis. Nucleic acid aptamers can recognize proteins, phospholipids, sugars, and nucleic acids with high affinity and specificity, and have cost-effective production with good reproducibility and lower immunogenicity and smaller size than antibodies to allow for specific receptor-target interactions [65]. While antibodies provide high specificity and a range of binding affinities, the associated immunogenicity and the logistical and cost challenges of biologic therapeutics has limited its clinical success.
As a result, hybrid technologies have emerged, such as the development of antibodies glycosylated with oligosaccharides to target sugar receptor-bearing cells [69]. Although these antibody therapies were designed to have reduced immunogenicity and improved physiological retention time, studies have demonstrated mixed results. As some glycosylated antibodies have a comparable or shorter half-life than the natural glycoforms of IgG Fc, additional research is needed to provide a therapeutic benefit [70, 71].
4.2.1 Sugar receptor-mediated active targeting
Numerous glycosylated polymeric delivery systems take advantage of active targeting via sugar receptors. A critical aspect of glycopolymer development is the selection of an appropriate targeting ligand, where the ligand physicochemical properties, biocompatibility, cell specificity, binding affinity, and ease of conjugation and processing affect ligand suitability for a particular application [72]. As targeting ability is primarily dependent on the interactions between glycosylated material and lectin, much research has focused on understanding protein-polymer interactions. Additionally, some receptors, such as the mannose receptor, are present on multiple types of cells or tissues. Several types of intermolecular forces exist between the lectin receptor and the glycosylated polymer, and are specific to the structural conformations of each entity. Sugars primarily interact with receptors through H-bonding between sugar hydroxyls and amides or acid groups of the receptor’s amino acids and the protein hydration shell [73]. If the hydroxyl is not available to hydrogen bond with amino acids, such as upon ester or amide formation during sugar modification, the binding affinity will be reduced, resulting in a relatively high ligand concentration required to achieve the maximum physiological response. C-H-π stacking also exists, where aliphatic C-H groups interact with aromatic residues in the binding pocket, giving rise to vander Waals forces [74]. While significant progress has been made to understand these interactions using computational and/or experimental methods, sugar structure diversity and the wide range of lectins makes it difficult to precisely elucidate structure-activity relationships.
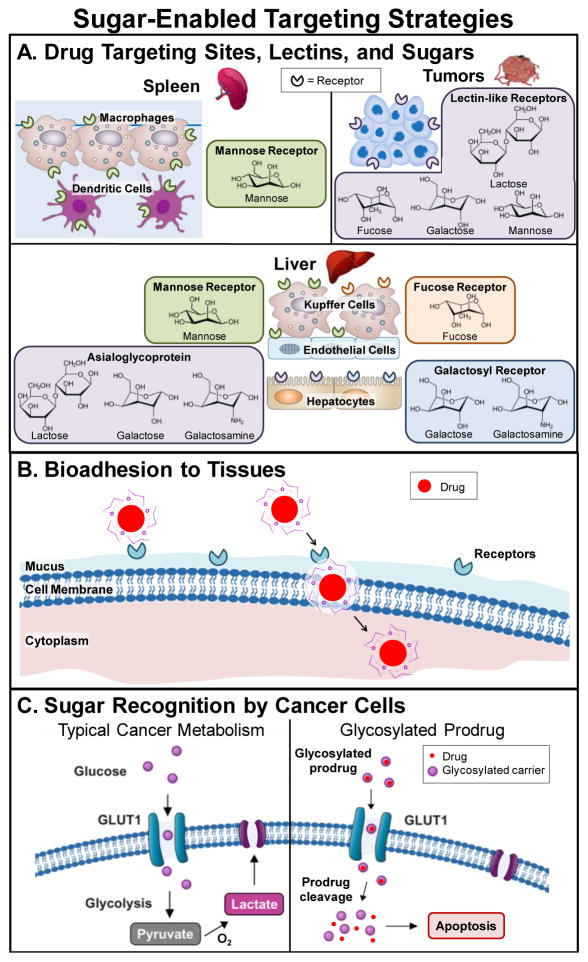
A vast array of cellular lectins have been identified (Fig. 4). A prevalent family of receptors, C-type lectin receptors, facilitate a range of cellular functions, such as cell-cell adhesion, immune response to pathogens, and apoptosis [75]. The most common C-type receptors are the mannose receptor, asialoglycoprotein, liver- and dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN), and selectins [76]. More information on sugar receptors and their targeting ligands can be found in Table 1.
Fig. 4.
Glycosylation enables a range of targeting strategies including glycosylated drug carriers for tissue - and cell-specific interactions (A), bioadhesion and/or mucoadhesion to tissues (B), and recognition as nutrients for cancer cell internalization (C).
Table 1.
Common receptors and corresponding ligands for a range of bio medical applications
| Organ or Location | Cell Type | Receptor | Ligand(s) | Common Targeting Applications | Ref. |
|---|---|---|---|---|---|
| Liver | Hepatocytes, cancer cells | Asialoglyco-protein | Galactose Galactosamine Lactose |
Cancer, Wilson’s disease, hemochromatosis | [21, 82] |
| Liver | Endothelial cells and Kupffer cells | Mannose receptor (C-type lectin receptor) | Mannose | Bacterial infections, inflammation, cardiovascular disease, cancer, cerebral ischaemia/stroke, genetic metabolic diseases, Leishmaniasis | [83] |
| Liver Liver |
Hepatocytes Kupffer cells |
Galactosyl receptor Fucose receptor, Lectin B |
Galactose Fucose |
Blood filtration Tissue binding, biofilm formation |
[84] |
| Liver, spleen, brain, bone marrow, and lungs | Macrophages | Mannose receptor (C-type lectin receptor) | Mannose | Bacterial infections, inflammation, cardiovascular disease, cancer, cerebral ischaemia/stroke, genetic metabolic diseases, Leishmaniasis | [83] |
| Spleen, lymph nodes, blood, skin | Dendritic cells | Mannose receptor (C-type lectin receptor) | Mannose | Adaptive immune response/vaccination | [76, 85] |
| Tumor sites | Malignant cells | Lectin-like receptors (e.g., galectins) | Galactose, lactose, mannose, fucose, Sialic Lewisx glycosides | Anti-proliferation | [86] |
| Oral cavity | Buccal cells | Lectin receptors | Lactose and mannose | Bioadhesion | [87] |
| Eye | Corneal/ conjunctival cells | Lectin receptors | Glucose and galactose, chitosan | Conjunctivitis | [88] |
| Colon | Epithelial cells | Galectin | Galactose | Cancer, bioadhesion | [89] |
| Lung | Pulmonary cells | Lectin receptors | Galactose | Cystic fibrosis, lung cancer, and pulmonary tuberculosis | [90] |
| Brain | Neurons | Anionic membranes and lectins | Trimethylated chitosan | Crossing the blood brain barrier, memory impairment | [91] |
Various research groups evaluated the influence of conjugation variables, including sugar type, degree of glycosylation, and the number of sugar repeat units on lectin binding. For enhanced binding, some groups evaluated the targeting ligand density by grafting multiple sugars to one grafting site for cooperative multivalent interactions [77, 78]. A fundamental study evaluating the role of sugar type (i.e., galactose and lactose) and sugar valency in the recognition of hydroxypropyl methacrylamide copolymers by colon adenocarcinoma cells demonstrated that binding was dependent on the number of sugar residues at a single grafting site [77]. In many cases, multiple sugars at one grafting site increases the binding affinity and leads to increased targeting efficacy via the glycoside cluster effect [77, 78]. To overcome weak binding affinity, multiple sugars can be conjugated to the polymer surface to enhance lectin interactions [67, 78].
The impact of polymer molecular weight and DS on tumor targeting characteristics of nanosized drug delivery systems was investigated by Kwon et al. [79, 80]. They prepared nanoparticles from hydrophobically modified glycol chitosan with different molecular weights (20 kDa, 100 kDa, and 250 kDa), yet a similar degree of hydrophobic substitution. Despite different molecular weights, all three glycol chitosan nanoparticles exhibited similar particle size, surface charge, and in vitro stability. In vivo tissue distribution and tumor accumulation revealed that high molecular weight glycol chitosan nanoparticles displayed prolonged blood circulation time and enhanced tumor accumulation. They also identified that the hydrophobic DS greatly influenced stability and deformability of resulting nanoparticles, thus affecting tumor targeting efficiency in vivo [80]. A proper DS was required to balance stability and deformability to reach optimal blood circulation time and tumor-targeting efficiency through the EPR effect [81].
In addition to monosaccharide conjugation, oligosaccharides have been used to decorate the nanoassembly exterior. For example, trimethylated chitosan-conjugated poly(lactic-co-glycolic acid) nanoparticles demonstrated successful coumarin delivery to the brain by targeting extracellular lectins and negatively charged membranes [91]. Another example of oligosaccharide ligands is the use of hyaluronan technologies to target CD44 receptors overexpressed on several tumor cell surfaces and provide delivery of cancer therapeutics [92, 93].
4.2.2. Active targeting by bioadhesion
Due to their tissue adhesive nature, glycosylated polymers can enable alternative dosage forms by adhering to tissues to prolong localized delivery [52]. Sugar-mediated mucoadhesion and cytoadhesion allows for enhanced bioavailability, local retention, and efficacy, and alternative administration routes, such as nasal or pulmonary delivery [52]. Delivery to the oral, nasal, or pulmonary mucosa is common, because they have relatively permeable membrane structures. The oral mucosa is frequently targeted due to the ability to bypass significant liver metabolism to increase drug bioavailability, unidirectional drug flux, and ease of accessibility and patient compliance. Moreover, many oral drugs are designed to be absorbed by the gastrointestinal (GI) system, but have limitations including high liver metabolism, enzymatic drug degradation during absorption, and high mucus turnover. Bioadhesive carriers are particularly useful in applications where fast clearance by EPR is an issue. Some notable examples of bioadhesive carriers include inhalable mannosylated liposomes for lung infections [83] and bioadhesive mannosylated NPs for oral retention [87].
For mucosal peptide or protein delivery, a number of chitosan-based carriers have been developed to deliver insulin [94, 95], plasmid DNA [96], or siRNA [97] by encapsulation [94–96] or self-assembly [98]. A common mucoadhesive moiety, chitosan, is a biocompatible, biodegradable, and cationic polysaccharide, which are favorable properties for mucoadhesion [99]. While chitosan alone can be used to form mucoadhesive carrier systems, co-incorporation of high charge density anionic polymers, such as dextran-sulfate or alginate in an ionic gelation processes can produce nanospheres capable of high insulin encapsulation efficiency (~49–96%) and controlled drug release [95, 100]. Mucoadhesive systems release insulin by dissociation as the pH changes. In these cases, mucoadhesion allows for a highly specific targeted and localized release. Another notable application is to improve the gene transfection efficiency of polylactic acid (PLA), a biocompatible and biodegradable non-viral gene delivery vector, by copolymerizing methoxypoly(ethylene glycol)-PLA and chitosan [101]. The in vivo results in mice demonstrated that localized polymer retention led to higher gene expression in the stomach and intestine compared to co-blended PLA-chitosan/DNA and lipofectamine/DNA complexes. To improve chitosan carriers, trimethyl chitosan-cysteine (TMC) conjugates were evaluated. Contrary to the low permeability of insoluble chitosan, TMC is a quaternized cationic derivative with good solubility and enhanced permeation. Combining TMC with thiolated polymers imparted increased mucoadhesion compared to non-thiolated nanoparticles and resulted in up to a 12-fold increase in insulin transport through rat intestines and 13-fold increase in Caco-2 cell internalization [98].
Targeting mucous layers is of particular interest for drugs that cannot be orally delivered due to acidic degradation or significant liver metabolism. Many absorptive epithelial mucosa, such as those found in the intestines, oral cavity, and airway epithelium, are covered with a highly glycosylated mucin-based layer that is anionically charged [102]. Under the mucosal layer is the glycocalyx, comprised of proteoglycans, glycoproteins, and glycolipids in the cell membrane. An alternative strategy of using sugars to target the mucosal surfaces is to decorate the delivery system exterior with lectins to interact with glycosylated surfaces, such as mucosal surfaces [103]. Studies have evaluated lectinized liposomes in binding to alveolar type II epithelial cells [104] or to intestinal mucosa for oral delivery [105].
4.2.3. Active targeting via sugar recognition by cancer cells
The Warburg effect, where malignant cells have an accelerated metabolism and high glucose requirements compared to non-malignant cells, has been well-established in cancer pathology [106]. Glucose and other glucose transporter substrate sugars are internalized by the tumor cells via the glucose transporter protein GLUT, which is overexpressed on human cancer cells [107, 108]. GLUT is correlated to poor cancer prognosis [109, 110], and glucose is utilized as a nutrient source to increase cell proliferation, leading to rapid tumor growth [111]. Thus, sugar-based therapeutics have been investigated for higher uptake rates by tumor cells compared to normal basal cells. Much of this work was inspired by early research where Som and coworkers successfully employed fluorescently-labeled glucose analogues to deliver the fluorescent dye to tumor cells for subsequent imaging with positron emission tomography [112]. More recently, fluorescently-labeled glucose carriers have been applied to deliver and track the anti-cancer therapeutic cisplatin [113].
A number of glucose-based drug delivery technologies provide enhanced solubility of hydrophobic actives and target lung, breast, colorectal, endometrial carcinomas, bone and soft-tissue sarcomas, and a number of lymphomas [106]. A common strategy is the use of glycan-conjugated prodrugs, which target cancer cell uptake via the GLUT transporter and release the active compound upon hydrolysis [114]. As cancer cells rapidly internalize glucose, the glucosylated carriers promote malignant cell apoptosis by upregulating GLUT in a positive feedback mechanism, causing an increased uptake of the glycan-prodrug polymer [115]. For example, glucosylated paclitaxel demonstrated high aqueous solubility and cytotoxicity against cancer cells, but limited toxicity towards normal cell lines or cells with low GLUT receptors levels [114]. Another glucose analogue, 2-deoxy-D-glucose (2-DG), has met great success in inhibiting glucose metabolism. 2-DG is recognized and internalized by tumor cells, in a similar manner to glucose, but cannot be converted to adenosine triphosphate to provide energy for cells [115]. When 2-DG is conjugated to anti-cancer therapeutics, the glycosylated shell enhances drug solubility, promotes cellular internalization of the prodrug, and upregulates GLUT expression. After the drug is internalized by the cell, hydrolysis of the anti-cancer prodrug leads to apoptosis, quickly killing tumor cells (Fig. 4) [114]. One example is to conjugate monosaccharides to DNA alkylators, which are used in chemotherapy to damage the DNA of cancer cells [116, 117]. Reux et al. demonstrated high specificity of 2-fluorodeoxyglucose-conjugated chlorambucil towards cancer cells in a mouse leukemia model. The glycoconjugated anti-cancer drug demonstrated increased cytotoxicity in vivo compared to the unacetylated sugar analog and the unconjugated chlorambucil in human cancer cell lines [116]. For targeted brain delivery, 2-DG-modified PEG-poly(trimethylene carbonate) nanoparticles were used as a dual-delivery system to enhance the blood-brain barrier penetration and glial drug accumulation by GLUT-mediated transcytosis and endocytosis, respectively [118]. Fluorescent images demonstrated high specificity and efficiency of these particles in an in vivo intracranial tumor mouse model with no acute toxicity to other organs after one week, representing a promising technology for brain glioma.
5. Stimuli-responsive sugar-based polymers
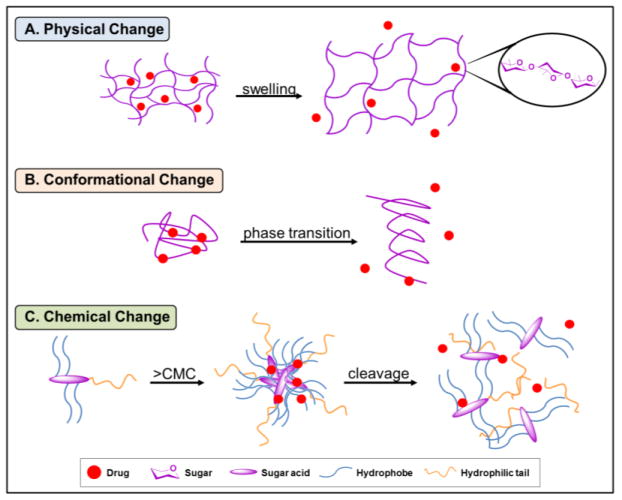
Stimuli-responsive delivery systems are designed to release bioactive agents at a desired location in response to physical or chemical changes. The stimuli can be biological or applied from an external source, and includes changes in pH or ionic strength in different tissues or cellular compartments [119, 120], the presence of tissue-specific enzymes [121], or external heat, ultrasound, or infrared irradiation application. Upon exposure to stimuli, a change in the polymer system occurs, resulting in bioactive release. This response can occur via chemical composition changes of the polymer [122, 123] or alterations in the polymer’s physical or conformational properties (Fig. 5) [123]. Some notable examples are that changes in chemical composition may be induced by conjugating the polymer to a bioactive, similar to a prodrug, by a stimuli-cleavable bond for local release of the free drug upon stimuli exposure. This stimulus-induced change can include cleavage via enzymes or pH changes [124, 125]. Physical changes include stimuli-triggered changes in hydrogel swelling properties [122], polymer folding conformation [126], or aggregate assembly morphology.
Fig. 5.
Schematic representation of select examples of stimuli-triggered responses to mediate drug release
Stimuli-triggered release systems offer several advantages, including reduced toxicity associated with systemic exposure by releasing the bioactive at the desired location. This approach reduces the amount of drug required during administration to elicit a therapeutic effect, as the localized release mechanism causes a higher concentration at the target site. While a vast number of polymer systems exhibit stimuli-dependent differential release, several sugar-based polymers inherently enable stimuli-triggered release [127, 128]. This section will focus on sugar-based systems where stimuli-responsive properties are a direct result of the sugar components. For delivery systems that incorporate sugars, but where the stimuli-responsiveness is not necessarily attributed to the sugar, readers are referred to a few excellent reviews [122, 123].
5.1 pH-responsive sugar-based polymers
One of the most commonly used triggers is pH. When the desired drug release location is intracellular, the pH of cellular compartments associated with uptake mechanisms, such as endocytosis, is utilized. As endocytosis progresses, the endosome pH decreases, from pH 5–6 in the early endosome, to pH 4–5 when the endosome fuses with the lysosome [120]. Drug release prior to lysosomal fusion is favorable to avoid cargo degradation. Additionally, certain tissues differ in pH compared to circulation; cancerous tumors have a slightly more acidic extracellular pH (pH 6–7) than normal tissues [119]. This difference is primarily due to the Warburg effect and increased glycolysis rate, which exports acidic molecules to the extracellular environment (Fig. 4C) [129]. Alternatively, the pathway taken by oral delivery systems experiences a gradual pH increase, culminating in the basic environment of the colon (pH 7.4–8.0).
As the pH of different cellular compartments varies, polymer systems with acid-labile bonds or protonatable functionalities can be utilized to facilitate endosomal drug release by increasing hydrolysis rates [130] or changing the polymer protonation [94]. Several sugars have pKa values close to physiological conditions, such as chitosan. This feature results in protonation differences under the different pH of intracellular vesicles and tumor tissues and leads to a change in biological or polymer conditions to release entrapped cargo. Ionic and/or reactive functional groups, such as those in chitosan and alginate, further allow for conjugation to other materials to impart pH sensitivity by chemical modification via pH-sensitive bonds [124, 130] or stimuli-responsive bulk polymer systems (e.g., poly(acrylic acid)) [131].
Many sugar-based polymers naturally exhibit pH sensitivity and can be utilized for controlled bioactive release in response to pH changes. Chitosan bears primary amines with a pKa of approximately 6.2 resulting in more protonated amines under acidic conditions, promoting endosomal escape of polysaccharide-drug complexes into the intracellular environment through the proton sponge effect [132]. An excellent review of chitosan-based hydrogel delivery systems has recently been reported [127]. An alternative approach to impart pH sensitivity into chitosan is to use the free carboxylates as crosslinking sites to generate sugar-based hydrogels [133]. The crosslinking agents can be naturally derived, such as genipin [134] and alginate [135], or the hydrogels can be blended with hydrophilic polymers, such as poly(vinyl alcohol) to increase swelling responses to pH changes[134]. Alternatively, negatively charged sugars such as alginate can be crosslinked with divalent cations to generate pH sensitive hydrogel systems for controlled release [136, 137]. Notably, Zhuo et al. designed Ca2+ crosslinked alginate, chitosan, and pectin composite beads that were subsequently released as microparticles for oral protein delivery [137]. Composite systems exhibited enhanced pH-sensitive protein release profiles under basic conditions, while protecting the protein from degradation in acidic conditions.
Systems have also been developed to release cargo under neutral or basic conditions for GI delivery. Wang et al. generated semi-interpenetrating network hydrogels of carboxymethyl chitosan crosslinked with alginate for pH-sensitive protein delivery to the colon and intestines. Under neutral and basic conditions, the hydrogels have decreased swelling volumes, resulting in bioactive release [135]. Similarly, chitosan particles designed to complex oligonucleotides, have been prepared by reverse microemulsion techniques and also demonstrated an increase in burst release under neutral and basic conditions compared to acidic conditions [138]. At elevated pH, the net cationic charge of chitosan is decreased, likely resulting in decreased oligonucleotide affinity. Alternative formulations, such as microcapsules [139] or nanoparticles [94] of chitosan-derived materials, also exhibit pH sensitivity and controlled release of encapsulated drugs and peptides. Of particular interest are chitosan-coated alginate nanoparticles designed by Kundu et al. to encapsulate insulin for oral delivery [140]. The alginate interior exhibits a collapsed structure under acidic conditions, protecting insulin under simulated stomach acid conditions. Upon neutralization, mimicking entry into the GI tract, insulin release increases as the polymer returns to an un-collapsed state. The chitosan-coated alginate nanoparticles demonstrate promise in protecting insulin during oral delivery to aid intestinal adsorption.
Heparin, a highly sulfated glycosaminoglycan, interacts with tumor-related factors and reduces tumor growth and metastasis [141]. It can be utilized to elicit synergistic effects with tumor drugs for cancer treatment. Gu et al. conjugated doxorubicin to dendronized heparin via a pH-sensitive hydrazone bond for tumor-specific release, which led to a significantly reduced tumor volume in mice [142]. The numerous functional groups on different polysaccharides provide sites for direct drug conjugation via a variety of pH-sensitive linkages, such as oxime, hydrazone, imine, and acetal bonds[124]. Examples of linear sugar-based polymers conjugated to anti-cancer drugs or PEG through a hydrazone linkage exhibit enhanced drug loading in micellar aggregates and pH sensitivity [125, 130]. The Uhrich group utilized a hydrazone linkage to conjugate a hydrophobically modified sugar backbone to PEG to generate SBAPs that self-assemble into micelles under neutral and basic conditions [125]. At acidic pH, the labile hydrazone is cleaved, separating the hydrophilic and hydrophobic domains, resulting in aggregate assembly disruption and subsequent drug release.
5.2. Enzyme-specific cleavage of sugar-based polymers
A particularly unique feature of sugar-based polymers is their recognition as natural substrates by naturally-occurring human or bacterial enzymes, allowing for the use of polysaccharides for colon specific delivery [143]. Due to the differential enzyme expression, or habitable environments of naturally-occurring bacteria, drugs can be physically encapsulated in sugar-based polymer networks and controllably released at target locations upon glycoside linkage cleavage. A natural gradient exists in the number of bacterial colonies within the digestive system: the small intestine has 103–104 colony forming units (CFUs) while there are approximately 1011–1012 CFU/mL in the colon [121]. These naturally-occurring colonic microflora express enzymes that break down plant carbohydrates to facilitate digestion [144]. The specific recognition of particular polysaccharides by such enzymes facilitates greater specificity and localized release compared to analogous biodegradable polymer systems that rely on non-specific esterases or amidases for degradation-mediated release.
Konjac glucomannan (KGM) [128] and pectin [145] are two naturally-occurring polysaccharides that are cleaved by colonic bacterial enzymes, such as β-mannanase, β-D-glucosidase, β-D-galactosidase, etc., and are utilized to facilitate specific drug release in the colon for GI disorder treatment. These polysaccharides are commonly formulated into hydrogels [145, 146] and engineered as nano- or micro-particles [147–149] for drug encapsulation. Generally, higher degrees of crosslinking result in extended release by limiting the drugs’ ability to diffuse out of the hydrogel, and lower crosslinking density results in burst release [148, 150].
As these polysaccharides can be naturally derived, their specific chemical properties, often based on their isolation source, influencing delivery properties [145]. Martínez-Pacheco et al. explored the effect of varying acetylation degrees between KGM of different origins on particle size, swelling properties, and degradation rates in a mixed hydrogel. KGM and xantham gum were formulated as thermoreversible gels for diltiazem delivery to the colon. Studies revealed that the drug was fully released in 24 h in the presence of β-mannanase for highly acetylated Japanese KGM, but only 60% released under identical conditions from American KGM-based hydrogels. These results highlight the potential tunability of combining various ratios of highly acetylated and deacetylated KGM in hydrogel formulations for colon specific delivery [151]. KGM has been incorporated into other formulations for colon-targeted delivery, such as a plug for a rapid dissolving capsule [147], and cationic KGM in combination with phytagel has been used for siRNA delivery against TNF-α for inflammatory bowel disease [146].
Pectin is a natural polysaccharide that can be enzymatically degraded by colonic microflora. Pectin varies by its esterification degree, and low-methoxyl pectin, with a higher degree of esterification is highly soluble in stomach and upper GI tract fluids, where drug delivery and adsorption are not beneficial, thus limiting use as an oral drug delivery matrix [152]. However, the carboxylates of pectin can be crosslinked by divalent cations, such as Zn2+ and Ca2+, resulting in solubility characteristics amenable to oral delivery [145]. Organic molecules bearing cationic functionalities, such as chitosan, can also be used for cross linking [150]. Ng et al. developed Zn-pectin-chitosan composite nanoparticles that demonstrated colon-specific release in vitro following pre-exposure to acidic conditions, such as in the stomach, and in vivo in rats. They concluded that higher incorporation of chitosan increased the lag time preceding drug release, allowing the drugs to reach the intended site [150]. Previously described composite microparticles developed by Zhuo et al. further benefit from inclusion of pectin. In addition to the pH-sensitive release, enhanced specificity was demonstrated by the faster release profile of encapsulated protein with pectinase present [137].
Additionally, there are examples of sugar-based systems that do not use enzyme-susceptible polysaccharides as the bulk component. Rather, they utilize an oligosaccharide shell that is enzymatically degradable to promote drug release [153, 154]. Notably, Amorós et al. developed saccharide- (e.g., starch, lactose, etc.) capped silica nanoparticles, where the saccharide acts as shell and enzymatic cleavage, by enzymes such as pancreatin or β-D-galactosidase, release drugs by a gate-controlled mechanism at the target location (Fig. 6) [154].
Fig. 6.
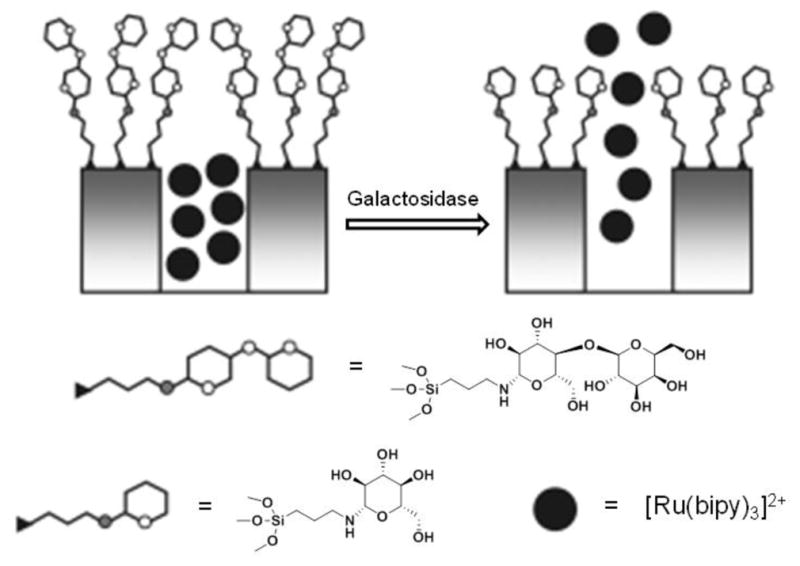
Schematic representation of saccharide-capped silica nanoparticles that release drugs using a gate-controlled mechanism for enzyme-dependent release profiles (adapted from [153] with permission).
5.3 Temperature-sensitive sugar-based polymers
Temperature-triggered release can be beneficial for localized therapeutic delivery by external heat. A few sugars inherently possess temperature sensitivity. Gellangum and xanthangum are two polysaccharides that exhibit conformational transitions from ordered helical structures to random coiled structures at elevated temperatures [155, 156]. In addition, several saccharide-containing polymers have a lower critical solution temperature (LCST), and sugar addition can modulate the LCST of synthetic polymers by altering the hydrophilicity or H-bonding capabilities of the polymer [157]. Above the LCST, the polymer adopts gel-like properties, allowing for small molecule encapsulation. Below the LCST, these sugar-based polymers are free flowing solutions, allowing for ease of handling and injection for translational applications [123].
The majority of sugar-based systems with temperature-sensitive properties utilize polysaccharides as the bulk sugar components. For example, when crosslinked with aspartic acid, xanthangum hydrogels exhibited reversible thermo-responsive swelling properties that are dependent on the sugar to amino acid ratio [158]. It is also noteworthy that the combination of these two naturally-occurring materials provided additional pH- and ionic strength-dependent swelling properties. Temperature-sensitive properties must be able to be fine-tuned, as highly elevated temperatures will cause irreparable cell damage. An example of a highly tunable system is pullalan-g-poly(L-lactide) nanogels developed by Na et al. that exhibited temperature sensitivity just above physiological conditions (37°C-42°C). This temperature range is amenable to external heat application but within the temperature range that will not significantly damage cells. A notable increase in drug release was observed at high temperatures, and the grafted L-lactide density had a strong influence on the temperature at which release occurred [159].
Alternatively, saccharides can be utilized as a cross-linking agent or additive to fine tune the temperature that a physical change in pre-synthesized polymers occurs. Although not inherently temperature-sensitive, addition of sodium alginate to semi-interpenetrating network hydrogels of PEG-co-poly(ε-caprolactone) macromer and N-isopropylacrylamide increased temperature sensitivity. The addition of alginate further contributed to increased mechanical properties and higher total protein release from the network [160]. Similarly, upon addition of dextran maleic anhydride to poly(N-isopropylacrylamide) (PNIPAm) hydrogels, the LCST of the synthetic polymer system could be fine-tuned from 35 to 39 °C, depending on the amount of incorporated dextran, and higher sugar content increased the LCST [161].
Semi-interpenetrating polymer networks of PNIPAm with gellangum were developed by Aminabhavi et al. and formulated into microspheres for controlled delivery of atenalol. The formulations exhibited pulsatile release, with rapid release at 25°C and nearly no release at 37°C. The ratio of polymer to saccharide had a drastic influence on particle size, drug loading, and release profiles of atenalol [162]. This example has direct implications for delivery of highly toxic drugs that must only be released at the intended site.
The addition of non-conjugated saccharides to polymers with an LCST also influences phase transition, as sugars influence the solution hydrophilicity and compete with water molecules to interact with the polymer. Recently, Le Cerf et al. investigated the role of sugar length and structure on LCST decreases of a PEG and poly(propylene oxide) polymer using differential scanning calorimetry, nuclear magnetic resonance spectroscopy, and cloud point measurements [163]. As the length of the saccharide chain increased, the effect on LCST was less pronounced. For example, tri-saccharides exhibited minimal influence on LCST, whereas the same concentration of mono-saccharides significantly altered the phase transition temperature. They further identified structural parameters that influence the sugar ‘s ability to interact with the polymer, particularly the hydroxyl at the C4-position and the linkage type between disaccharide monomers.
6. Conclusions and future perspectives
A variety of sugar-based systems have been explored for bioactive delivery. Among these systems, sugar-based polymers have drawn considerable attention due to high composition diversity, architecture, molecular weight, and functionalization offered by advanced synthetic approaches. The utilization of sugar-based polymeric delivery systems has provided pharmaceutical benefits including improved in vivo stability, prolonged circulation time, reduced toxicity of carriers, localized and sustained release, and improved pharmacokinetic profiles and superior tissue distribution since the advent of targeting and stimuli-responsiveness. Despite their clear promise, few examples exist of their translation to clinical practice and development. For instance, PK2, a galactosamine polymer doxorubicin conjugate, rapidly cleared from circulation in Phase I clinical trials despite encouraging results acquired in a rat model [3].
As such, increasing efforts have been devoted to designing formulations with optimized properties, such as batch-to-batch consistency, increased circulation half-lives, and improved tissue distribution [164]. Although still in their early stages, formulation technologies are rapidly emerging in targeted drug delivery and controlled drug release that incorporate multiple features to improve specific release of bioactives. For example, Kranning et al. generated glucopolymers that adopt a helical conformation under acidic conditions [126]. Under basic and neutral pH, the polymer transitions to a random coil conformation to present glucose functionalities and demonstrated enhanced selective binding to legume lectin, concanavalin, compared to non-glucosylated co-poly(L-glutamate)s. Two complimentary mechanisms, receptor targeting and stimuli-responsiveness, can be used to develop smart carrier systems for targeted drug release by combining active release features into the sugar-based polymer systems. Similar dual-targeting delivery approaches will create new opportunities for exciting, cutting-edge research on bioactive delivery to translate from the laboratory to clinical trials.
Acknowledgments
The authors gratefully acknowledge support from the National Institutes of Health (NIH R01 HL107913) and the National Science Foundation Graduate Research Fellowship (JWC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miura Y. Synthesis and biological application of glycopolymers. Journal of Polymer Science Part A: Polymer Chemistry. 2007;45:5031–5036. [Google Scholar]
- 2.Jain K, Kesharwani P, Gupta U, Jain NK. A review of glycosylated carriers for drug delivery. Biomaterials. 2012;33:4166–4186. doi: 10.1016/j.biomaterials.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Spain SG, Cameron NR. A spoonful of sugar: the application of glycopolymers in therapeutics. Polymer Chemistry. 2011;2:60–68. [Google Scholar]
- 4.Gu L, Faig A, Abdelhamid D, Uhrich K. Sugar-Based Amphiphilic Polymers for Biomedical Applications: From Nanocarriers to Therapeutics. Accounts of chemical research. 2014;47:2867–2877. doi: 10.1021/ar4003009. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Advanced Drug Delivery Reviews. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Fazio F, Bryan MC, Blixt O, Paulson JC, Wong CH. Synthesis of Sugar Arrays in Microtiter Plate. Journal of the American Chemical Society. 2002;124:14397–14402. doi: 10.1021/ja020887u. [DOI] [PubMed] [Google Scholar]
- 7.Höbel S, Loos A, Appelhans D, Schwarz S, Seidel J, Voit B, Aigner A. Maltose- and maltotriose-modified, hyperbranched poly(ethylene imine)s (OM-PEIs): Physicochemical and biological properties of DNA and siRNA complexes. Journal of Controlled Release. 2011;149:146–158. doi: 10.1016/j.jconrel.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal P, Gupta U, Jain NK. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28:3349–3359. doi: 10.1016/j.biomaterials.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Smith AE, Sizovs A, Grandinetti G, Xue L, Reineke TM. Diblock Glycopolymers Promote Colloidal Stability of Polyplexes and Effective pDNA and siRNA Delivery under Physiological Salt and Serum Conditions. Biomacromolecules. 2011;12:3015–3022. doi: 10.1021/bm200643c. [DOI] [PubMed] [Google Scholar]
- 10.Pan Y, Li Y-j, Zhao H-y, Zheng J-m, Xu H, Wei G, Hao J-s, Cui F-d. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. International Journal of Pharmaceutics. 2002;249:139–147. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 11.Makhlof A, Werle M, Tozuka Y, Takeuchi H. Nanoparticles of glycol chitosan and its thiolated derivative significantly improved the pulmonary delivery of calcitonin. International journal of pharmaceutics. 2010;397:92–95. doi: 10.1016/j.ijpharm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Morille M, Passirani C, Letrou-Bonneval E, Benoit JP, Pitard B. Galactosylated DNA lipid nanocapsules for efficient hepatocyte targeting. International Journal of Pharmaceutics. 2009;379:293–300. doi: 10.1016/j.ijpharm.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 13.Murphy JJ, Furusho H, Paton RM, Nomura K. Precise Synthesis of Poly(macromonomer)s Containing Sugars by Repetitive ROMP and Their Attachments to Poly(ethylene glycol): Synthesis, TEM Analysis and Their Properties as Amphiphilic Block Fragments. Chemistry A European Journal. 2007;13:8985–8997. doi: 10.1002/chem.200700291. [DOI] [PubMed] [Google Scholar]
- 14.Suriano F, Pratt R, Tan JPK, Wiradharma N, Nelson A, Yang YY, Dubois P, Hedrick JL. Synthesis of a family of amphiphilic glycopolymers via controlled ring-opening polymerization of functionalized cyclic carbonates and their application in drug delivery. Biomaterials. 2010;31:2637–2645. doi: 10.1016/j.biomaterials.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed M, Narain R. The effect of polymer architecture, composition, and molecular weight on the properties of glycopolymer-based non-viral gene delivery systems. Biomaterials. 2011;32:5279–5290. doi: 10.1016/j.biomaterials.2011.03.082. [DOI] [PubMed] [Google Scholar]
- 16.Borase T, Ninjbadgar T, Kapetanakis A, Roche S, O’Connor R, Kerskens C, Heise A, Brougham DF. Stable Aqueous Dispersions of Glycopeptide-Grafted Selectably Functionalized Magnetic Nanoparticles. Angewandte Chemie International Edition. 2013;52:3164–3167. doi: 10.1002/anie.201208099. [DOI] [PubMed] [Google Scholar]
- 17.Tan Y-l, Liu C-G. Self-aggregated nanoparticles from linoleic acid modified carboxymethyl chitosan: Synthesis, characterization and application in vitro. Colloids and Surfaces B: Biointerfaces. 2009;69:178–182. doi: 10.1016/j.colsurfb.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Chan P, Kurisawa M, Chung JE, Yang YY. Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials. 2007;28:540–549. doi: 10.1016/j.biomaterials.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 19.Farkaš P, Bystrický S. Efficient activation of carboxyl polysaccharides for the preparation of conjugates. Carbohydrate Polymers. 2007;68:187–190. [Google Scholar]
- 20.Yu JM, Li YJ, Qiu LY, Jin Y. Self-aggregated nanoparticles of cholesterol-modified glycol chitosan conjugate: Preparation, characterization, and preliminary assessment as a new drug delivery carrier. European Polymer Journal. 2008;44:555–565. [Google Scholar]
- 21.Yang X, Zhang Q, Wang Y, Chen H, Zhang H, Gao F, Liu L. Self-aggregated nanoparticles from methoxy poly(ethylene glycol)-modified chitosan: Synthesis; characterization; aggregation and methotrexate release in vitro. Colloids and Surfaces B: Biointerfaces. 2008;61:125–131. doi: 10.1016/j.colsurfb.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Voit B, Appelhans D. Glycopolymers of various architectures more than mimicking nature. Macromolecular Chemistry and Physics. 2010;211:727–735. [Google Scholar]
- 23.Ting SRS, Min EH, Escalé P, Save M, Billon L, Stenzel MH. Lectin Recognizable Biomaterials Synthesized via Nitroxide-Mediated Polymerization of a Methacryloyl Galactose Monomer. Macromolecules. 2009;42:9422–9434. [Google Scholar]
- 24.Bhatia S, Mohr A, Mathur D, Parmar VS, Haag R, Prasad AK. Biocatalytic route to sugar-PEG-based polymers for drug delivery applications. Biomacromolecules. 2011;12:3487–3498. doi: 10.1021/bm200647a. [DOI] [PubMed] [Google Scholar]
- 25.Dai X-H, Dong C-M. Synthesis self-assembly and recognition properties of biomimetic star-shaped poly(ε-caprolactone)-b-glycopolymer block copolymers. Journal of Polymer Science Part A: Polymer Chemistry. 2008;46:817–829. [Google Scholar]
- 26.Carrillo-Conde B, Song EH, Chavez-Santoscoy A, Phanse Y, Ramer-Tait AE, Pohl NLB, Wannemuehler MJ, Bellaire BH, Narasimhan B. Mannose-Functionalized “Pathogen-like” Polyanhydride Nanoparticles Target C-Type Lectin Receptors on Dendritic Cells. Molecular Pharmaceutics. 2011;8:1877–1886. doi: 10.1021/mp200213r. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed M, Jiang X, Deng Z, Narain R. Cationic Glyco-Functionalized Single-Walled Carbon Nanotubes as Efficient Gene Delivery Vehicles. Bioconjugate Chemistry. 2009;20:2017–2022. doi: 10.1021/bc900229v. [DOI] [PubMed] [Google Scholar]
- 28.Richards SJ, Jones MW, Hunaban M, Haddleton DM, Gibson MI. Probing Bacterial-Toxin Inhibition with Synthetic Glycopolymers Prepared by Tandem Post-Polymerization Modification: Role of Linker Length and Carbohydrate Density. Angewandte Chemie International Edition. 2012;51:7812–7816. doi: 10.1002/anie.201202945. [DOI] [PubMed] [Google Scholar]
- 29.Semsarilar M, Ladmiral V, Perrier S. Highly Branched and Hyperbranched Glycopolymers via Reversible Addition Fragmentation Chain Transfer Polymerization and Click Chemistry. Macromolecules. 2010;43:1438–1443. [Google Scholar]
- 30.Perdih P, Čebašek S, Možir A, Žagar E. Post-Polymerization Modification of Poly (l-glutamic acid) with d-(+)-Glucosamine. Molecules. 2014;19:19751–19768. doi: 10.3390/molecules191219751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian L, Yam L, Zhou N, Tat H, Uhrich KE. Amphiphilic Scorpion-like Macromolecules: Design, Synthesis, and Characterization. Macromolecules. 2004;37:538–543. [Google Scholar]
- 32.Abdelhamid D, Arslan H, Zhang Y, Uhrich KE. Role of branching of hydrophilic domain on physicochemical properties of amphiphilic macromolecules. Polymer Chemistry. 2014;5:1457–1462. doi: 10.1039/C3PY01072D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelhamid DS, Zhang Y, Lewis DR, Moghe PV, Welsh WJ, Uhrich KE. Tartaric acid-based amphiphilic macromolecules with ether linkages exhibit enhanced repression of oxidized low density lipoprotein uptake. Biomaterials. 2015;53:32–39. doi: 10.1016/j.biomaterials.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparks SM, Waite CL, Harmon AM, Nusblat LM, Roth CM, Uhrich KE. Efficient Intracellular siRNA Delivery by Ethyleneimine-Modified Amphiphilic Macromolecules. Macromolecular Bioscience. 2011;11:1192–1200. doi: 10.1002/mabi.201100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Synatschke CV, Schallon A, Jérôme V, Freitag R, Müller AHE. Influence of Polymer Architecture and Molecular Weight of Poly(2-(dimethylamino)ethyl methacrylate) Polycations on Transfection Efficiency and Cell Viability in Gene Delivery. Biomacromolecules. 2011;12:4247–4255. doi: 10.1021/bm201111d. [DOI] [PubMed] [Google Scholar]
- 36.Zhu JY, Lei Q, Yang B, Jia HZ, Qiu WX, Wang X, Zeng X, Zhuo RX, Feng J, Zhang XZ. Efficient nuclear drug translocation and improved drug efficacy mediated by acidity-responsive boronate-linked dextran/cholesterol nanoassembly. Biomaterials. 2015;52:281–290. doi: 10.1016/j.biomaterials.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 37.Yao B, Ni C, Xiong C, Zhu C, Huang B. Hydrophobic modification of sodium alginate and its application in drug controlled release. Bioprocess Biosyst Eng. 2010;33:457–463. doi: 10.1007/s00449-009-0349-2. [DOI] [PubMed] [Google Scholar]
- 38.Vallée F, Müller C, Durand A, Schimchowitsch S, Dellacherie E, Kelche C, Cassel JC, Leonard M. Synthesis and rheological properties of hydrogels based on amphiphilic alginate-amide derivatives. Carbohydrate Research. 2009;344:223–228. doi: 10.1016/j.carres.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Mundargi RC, Patil SA, Aminabhavi TM. Evaluation of acrylamide-grafted-xanthangum copolymer matrix tablets for oral controlled delivery of antihypertensive drugs. Carbohydrate Polymers. 2007;69:130–141. [Google Scholar]
- 40.Strand SP, Lelu S, Reitan NK, de Lange Davies C, Artursson P, Vårum KM. Molecular design of chitosan gene delivery systems with an optimized balance between polyplex stability and polyplex unpacking. Biomaterials. 2010;31:975–987. doi: 10.1016/j.biomaterials.2009.09.102. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W, Dai XH, Dong CM. Biodegradable and Biomimetic Poly(ε-caprolactone)/Poly(lactobionamidoethyl methacrylate) Biohybrids: Synthesis, Lactose-Installed Nanoparticles and Recognition Properties. Macromolecular Bioscience. 2008;8:268–278. doi: 10.1002/mabi.200700131. [DOI] [PubMed] [Google Scholar]
- 42.Petrova KT, Dey SS, Barros MT. Formation of spherical and core shell polymeric microparticles from glycopolymers. Carbohydrate Polymers. 2015;125:281–287. doi: 10.1016/j.carbpol.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 43.Eissa AM, Smith MJP, Kubilis A, Mosely JA, Cameron NR. Polymersome-forming amphiphilic glycosylated polymers: Synthesis and characterization. Journal of Polymer Science Part A: Polymer Chemistry. 2013;51:5184–5193. [Google Scholar]
- 44.Ahmed M, Jawanda M, Ishihara K, Narain R. Impact of the nature, size and chain topologies of carbohydrate phosphorylcholine polymeric gene delivery systems. Biomaterials. 2012;33:7858–7870. doi: 10.1016/j.biomaterials.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Chu DSH, Schellinger JG, Shi J, Convertine AJ, Stayton PS, Pun SH. Application of Living Free Radical Polymerization for Nucleic Acid Delivery. Accounts of Chemical Research. 2012;45:1089–1099. doi: 10.1021/ar200242z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim N, Jiang D, Jacobi AM, Lennox KA, Rose SD, Behlke MA, Salem AK. Synthesis and characterization of mannosylated pegylated polyethylenimine as a carrier for siRNA. International journal of pharmaceutics. 2012;427:123–133. doi: 10.1016/j.ijpharm.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao L, Uhrich KE. Novel amphiphilic macromolecules and their in vitro characterization as stabilized micellar drug delivery systems. Journal of Colloid and Interface Science. 2006;298:102–110. doi: 10.1016/j.jcis.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Djordjevic J, Barch M, Uhrich KE. Polymeric Micelles Based on Amphiphilic Scorpion-like Macromolecules: Novel Carriers for Water-Insoluble Drugs. Pharmaceutical Research. 2005;22:24–32. doi: 10.1007/s11095-004-9005-3. [DOI] [PubMed] [Google Scholar]
- 49.Tao L, Chan JW, Uhrich KE. Drug Loading and Release Kinetics in Polymeric Micelles: Comparing Dynamic vs. Unimolecular Sugar-based Micelles for Controlled Release. Journal of Bioactive and Compatible Polymers. 2015 In press. [Google Scholar]
- 50.Poree DE, Zablocki K, Faig A, Moghe PV, Uhrich KE. Nanoscale Amphiphilic Macromolecules with Variable Lipophilicity and Stereochemistry Modulate Inhibition of Oxidized Low-Density Lipoprotein Uptake. Biomacromolecules. 2013;14:2463–2469. doi: 10.1021/bm400537w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schallon A, Jérôme V, Walther A, Synatschke CV, Müller AHE, Freitag R. Performance of three PDMAEMA-based polycation architectures as gene delivery agents in comparison to linear and branched PEI. Reactive and Functional Polymers. 2010;70:1–10. [Google Scholar]
- 52.Kammona O, Kiparissides C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. Journal of Controlled Release. 2012;161:781–794. doi: 10.1016/j.jconrel.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 53.Torchilin V. Passive and Active Drug Targeting: Drug Delivery to Tumors as an Example. In: Schäfer-Korting M, editor. Drug Delivery. Springer; Berlin Heidelberg: 2010. pp. 3–53. [DOI] [PubMed] [Google Scholar]
- 54.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced Drug Delivery Reviews. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 55.York AW, Zablocki KR, Lewis DR, Gu L, Uhrich KE, Prud’homme RK, Moghe PV. Kinetically Assembled Nanoparticles of Bioactive Macromolecules Exhibit Enhanced Stability and Cell-Targeted Biological Efficacy. Advanced Materials. 2012;24:733–739. doi: 10.1002/adma.201103348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chono S, Tauchi Y, Deguchi Y, Morimoto K. Efficient drug delivery to atherosclerotic lesions and the antiatherosclerotic effect by dexamethasone incorporated into liposomes in atherogenic mice. Journal of drug targeting. 2005;13:267–276. doi: 10.1080/10611860500159030. [DOI] [PubMed] [Google Scholar]
- 57.Lewis DR, Kholodovych V, Tomasini MD, Abdelhamid D, Petersen LK, Welsh WJ, Uhrich KE, Moghe PV. In silico design of anti-atherogenic biomaterials. Biomaterials. 2013;34:7950–7959. doi: 10.1016/j.biomaterials.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen LK, York AW, Lewis DR, Ahuja S, Uhrich KE, Prud’homme RK, Moghe PV. Amphiphilic Nanoparticles Repress Macrophage Atherogenesis: Novel Core/Shell Designs for Scavenger Receptor Targeting and Down-Regulation. Molecular Pharmaceutics. 2014;11:2815–2824. doi: 10.1021/mp500188g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plourde NM, Kortagere S, Welsh W, Moghe PV. Structure Activity Relations of Nanolipoblockers with the Atherogenic Domain of Human Macrophage Scavenger Receptor A. Biomacromolecules. 2009;10:1381–1391. doi: 10.1021/bm8014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rele SM, Cui W, Wang L, Hou S, Barr-Zarse G, Tatton D, Gnanou Y, Esko JD, Chaikof EL. Dendrimer-like PEO glycopolymers exhibit anti-inflammatory properties. Journal of the American Chemical Society. 2005;127:10132–10133. doi: 10.1021/ja0511974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinhart M, Gröger D, Enders S, Riese SB, Dernedde J, Kainthan RK, Brooks DE, Haag R. The Role of Dimension in Multivalent Binding Events: Structure Activity Relationship of Dendritic Polyglycerol Sulfate Binding to L-Selectin in Correlation with Size and Surface Charge Density. Macromolecular Bioscience. 2011;11:1088–1098. doi: 10.1002/mabi.201100051. [DOI] [PubMed] [Google Scholar]
- 62.Bruehl RE, Bertozzi CR, Rosen SD. Minimal sulfated carbohydrates for recognition by L-selectin and the MECA-79 antibody. Journal of Biological Chemistry. 2000;275:32642–32648. doi: 10.1074/jbc.M001703200. [DOI] [PubMed] [Google Scholar]
- 63.Galustian C, Lubineau A, le Narvor C, Kiso M, Brown G, Feizi T. L-selectin interactions with novel mono-and multisulfated Lewisx sequences in comparison with the potent ligand 3′-sulfated Lewisa. Journal of Biological Chemistry. 1999;274:18213–18217. doi: 10.1074/jbc.274.26.18213. [DOI] [PubMed] [Google Scholar]
- 64.Wiederschain GY. Essentials of glycobiology. Biochemistry (Moscow) 2009;74:1056–1056. [Google Scholar]
- 65.Swami A, Shi J, Gadde S, Votruba AR, Kolishetti N, Farokhzad OC. Multifunctional Nanoparticles for Drug Delivery Applications. Springer; 2012. Nanoparticles for targeted and temporally controlled drug delivery; pp. 9–29. [Google Scholar]
- 66.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Accounts of chemical research. 2007;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 67.Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee T-Y, Lin C-T, Kuo S-Y, Chang D-K, Wu H-C. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer research. 2007;67:10958–10965. doi: 10.1158/0008-5472.CAN-07-2233. [DOI] [PubMed] [Google Scholar]
- 69.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nature reviews Drug discovery. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 70.Jones AJ, Papac DI, Chin EH, Keck R, Baughman SA, Lin YS, Kneer J, Battersby JE. Selective clearance of glycoforms of a complex glycoprotein pharmaceutical caused by terminal N-acetylglucosamine is similar in humans and cynomolgus monkeys. Glycobiology. 2007;17:529–540. doi: 10.1093/glycob/cwm017. [DOI] [PubMed] [Google Scholar]
- 71.Keck R, Nayak N, Lerner L, Raju S, Ma S, Schreitmueller T, Chamow S, Moorhouse K, Kotts C, Jones A. Characterization of a complex glycoprotein whose variable metabolic clearance in humans is dependent on terminal N-acetylglucosamine content. Biologicals. 2008;36:49–60. doi: 10.1016/j.biologicals.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nature Reviews Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 73.Freichels H, Jérôme R, Jérôme C. Sugar-labeled and PEGylated (bio)degradable polymers intended for targeted drug delivery systems. Carbohydrate Polymers. 2011;86:1093–1106. [Google Scholar]
- 74.Solís D, Jiménez-Barbero J, Kaltner H, Romero A, Siebert H-C, Wvd Lieth C, Gabius H-J. Towards defining the role of glycans as hardware in information storage and transfer: basic principles, experimental approaches and recent progress. Cells Tissues Organs. 2001;168:5–23. doi: 10.1159/000016802. [DOI] [PubMed] [Google Scholar]
- 75.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nature Reviews Immunology. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irache JM, Salman HH, Gamazo C, Espuelas S. Mannose-targeted systems for the delivery of therapeutics. 2008. [DOI] [PubMed] [Google Scholar]
- 77.David A, Kopecková P, Kopecek J, Rubinstein A. The Role of Galactose, Lactose, and Galactose Valency in the Biorecognition of N-(2-Hydroxypropyl)Methacrylamide Copolymers by Human Colon Adenocarcinoma Cells. Pharm Res. 2002;19:1114–1122. doi: 10.1023/a:1019885807067. [DOI] [PubMed] [Google Scholar]
- 78.Managit C, Kawakami S, Nishikawa M, Yamashita F, Hashida M. Targeted and sustained drug delivery using PEGylated galactosylated liposomes. International journal of pharmaceutics. 2003;266:77–84. doi: 10.1016/s0378-5173(03)00383-1. [DOI] [PubMed] [Google Scholar]
- 79.Park K, Kim J-H, Nam YS, Lee S, Nam HY, Kim K, Park JH, Kim I-S, Choi K, Kim SY, Kwon IC. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. Journal of Controlled Release. 2007;122:305–314. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Yhee JY, Son S, Kim SH, Park K, Choi K, Kwon IC. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. Journal of Controlled Release. 2014;193:202–213. doi: 10.1016/j.jconrel.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Na JH, Lee S-Y, Lee S, Koo H, Min KH, Jeong SY, Yuk SH, Kim K, Kwon IC. Effect of the stability and deformability of self-assembled glycol chitosan nanoparticles on tumor-targeting efficiency. Journal of Controlled Release. 2012;163:2–9. doi: 10.1016/j.jconrel.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 82.Fiume L, Di Stefano G. Lactosaminated human albumin, a hepatotropic carrier of drugs. European Journal of Pharmaceutical Sciences. 2010;40:253–262. doi: 10.1016/j.ejps.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 83.Chono S, Tanino T, Seki T, Morimoto K. Efficient drug targeting to rat alveolar macrophages by pulmonary administration of ciprofloxacin incorporated into mannosylated liposomes for treatment of respiratory intracellular parasitic infections. Journal of Controlled Release. 2008;127:50–58. doi: 10.1016/j.jconrel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 84.Fiete D, Srivastava V, Hindsgaul O, Baenziger JU. A hepatic reticuloendothelial cell receptor specific for SO 4-4GalNAcβ1, 4GlcNAcβ1, 2Manα that mediates rapid clearance of lutropin. Cell. 1991;67:1103–1110. doi: 10.1016/0092-8674(91)90287-9. [DOI] [PubMed] [Google Scholar]
- 85.Jiang H-L, Kang ML, Quan J-S, Kang SG, Akaike T, Yoo HS, Cho C-S. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials. 2008;29:1931–1939. doi: 10.1016/j.biomaterials.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida M, Takimoto R, Murase K, Sato Y, Hirakawa M, Tamura F, Sato T, Iyama S, Osuga T, Miyanishi K. Targeting anticancer drug delivery to pancreatic cancer cells using a fucose-bound nanoparticle approach. PloS one. 2012;7:e39545. doi: 10.1371/journal.pone.0039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fievez V, Plapied L, des Rieux A, Pourcelle V, Freichels H, Wascotte V, Vanderhaeghen M-L, Jerôme C, Vanderplasschen A, Marchand-Brynaert J, Schneider Y-J, Préat V. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. European Journal of Pharmaceutics and Biopharmaceutics. 2009;73:16–24. doi: 10.1016/j.ejpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Diebold Y, Jarrín M, Sáez V, Carvalho ELS, Orea M, Calonge M, Seijo B, Alonso MJ. Ocular drug delivery by liposome chitosan nanoparticle complexes (LCS-NP) Biomaterials. 2007;28:1553–1564. doi: 10.1016/j.biomaterials.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 89.Shukla RK, Tiwari A. Carbohydrate polymers: Applications and recent advances in delivering drugs to the colon. Carbohydrate Polymers. 2012;88:399–416. [Google Scholar]
- 90.Chen J, Gao X, Hu K, Pang Z, Cai J, Li J, Wu H, Jiang X. Galactose-poly(ethylene glycol)-polyethylenimine for improved lung gene transfer. Biochemical and Biophysical Research Communications. 2008;375:378–383. doi: 10.1016/j.bbrc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Wang ZH, Wang ZY, Sun CS, Wang CY, Jiang TY, Wang SL. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials. 2010;31:908–915. doi: 10.1016/j.biomaterials.2009.09.104. [DOI] [PubMed] [Google Scholar]
- 92.Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maiolino S, Russo A, Pagliara V, Conte C, Ungaro F, Russo G, Quaglia F. Biodegradable nanoparticles sequentially decorated with Polyethyleneimine and Hyaluronan for the targeted delivery of docetaxel to airway cancer cells. Journal of Nanobiotechnology. 2015;13:29. doi: 10.1186/s12951-015-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Makhlof A, Tozuka Y, Takeuchi H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. European journal of pharmaceutical sciences. 2011;42:445–451. doi: 10.1016/j.ejps.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 95.Sarmento B, Ribeiro A, Veiga F, Ferreira D. Development and character ization of new insulin containing polysaccharide nanoparticles. Colloids and Surfaces B: Biointerfaces. 2006;53:193–202. doi: 10.1016/j.colsurfb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Zheng F, Shi X-W, Yang G-F, Gong L-L, Yuan H-Y, Cui Y-J, Wang Y, Du Y-M, Li Y. Chitosan nanoparticle as gene therapy vector via gastrointestinal mucosa administration: results of an in vitro and in vivo study. Life sciences. 2007;80:388–396. doi: 10.1016/j.lfs.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 97.Jere D, Jiang H-L, Kim Y-K, Arote R, Choi Y-J, Yun C-H, Cho M-H, Cho C-S. Chitosan-graft-polyethylenimine for Akt1 siRNA delivery to lung cancer cells. International Journal of Pharmaceutics. 2009;378:194–200. doi: 10.1016/j.ijpharm.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 98.Yin L, Ding J, He C, Cui L, Tang C, Yin C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 2009;30:5691–5700. doi: 10.1016/j.biomaterials.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 99.Lai SK, Wang Y-Y, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced Drug Delivery Reviews. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarmento B, Ribeiro A, Veiga F, Ferreira D, Neufeld R. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. Journal of nanoscience and nanotechnology. 2007;7:2833–2841. doi: 10.1166/jnn.2007.609. [DOI] [PubMed] [Google Scholar]
- 101.Chen J, Tian B, Yin X, Zhang Y, Hu D, Hu Z, Liu M, Pan Y, Zhao J, Li H, Hou C, Wang J, Zhang Y. Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems. Journal of Biotechnology. 2007;130:107–113. doi: 10.1016/j.jbiotec.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 102.Smart JD. Lectin-mediated drug delivery in the oral cavity. Advanced drug delivery reviews. 2004;56:481–489. doi: 10.1016/j.addr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 103.Sosnik A, Menaker Raskin M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnology Advances. doi: 10.1016/j.biotechadv.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Murata M, Yonamine T, Tanaka S, Tahara K, Tozuka Y, Takeuchi H. Surface modification of liposomes using polymer wheat germ agglutinin conjugates to improve the absorption of peptide drugs by pulmonary administration. Journal of Pharmaceutical Sciences. 2013;102:1281–1289. doi: 10.1002/jps.23463. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y, Wang P, Sun C, Zhao J, Du Y, Shi F, Feng N. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. International Journal of Pharmaceutics. 2011;419:260–265. doi: 10.1016/j.ijpharm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 106.Calvaresi EC, Hergenrother PJ. Glucose conjugation for the specific targeting and treatment of cancer. Chemical Science. 2013;4:2319–2333. doi: 10.1039/C3SC22205E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ciampi R, Vivaldi A, Romei C, Del Guerra A, Salvadori P, Cosci B, Pinchera A, Elisei R. Expression analysis of facilitative glucose transporters (GLUTs) in human thyroid carcinoma cell lines and primary tumors. Molecular and cellular endocrinology. 2008;291:57–62. doi: 10.1016/j.mce.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 108.Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich J, Oefner PJ. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. The American journal of pathology. 2009;174:1544–1552. doi: 10.2353/ajpath.2009.080596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kunkel M, Moergel M, Stockinger M, Jeong J-H, Fritz G, Lehr H-A, Whiteside TL. Overexpression of GLUT-1 is associated with resistance to radiotherapy and adverse prognosis in squamous cell carcinoma of the oral cavity. Oral oncology. 2007;43:796–803. doi: 10.1016/j.oraloncology.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 110.Ohba S, Fujii H, Ito S, Fujimaki M, Matsumoto F, Furukawa M, Yokoyama J, Kusunoki T, Ikeda K, Hino O. Overexpression of GLUT-1 in the invasion front is associated with depth of oral squamous cell carcinoma and prognosis. Journal of oral pathology & medicine. 2010;39:74–78. doi: 10.1111/j.1600-0714.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 111.Shaw RJ. Glucose metabolism and cancer. Current opinion in cell biology. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 112.Som P, Atkins H, Bandoypadhyay D, Fowler J, MacGregor R, Matsui K, Oster Z, Sacker D, Shiue C, Turner H. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980;21:670–675. [PubMed] [Google Scholar]
- 113.Li X, Li R, Qian X, Ding Y, Tu Y, Guo R, Hu Y, Jiang X, Guo W, Liu B. Superior antitumor efficiency of cisplatin-loaded nanoparticles by intratumoral delivery with decreased tumor metabolism rate. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70:726–734. doi: 10.1016/j.ejpb.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 114.Lin Y-S, Tungpradit R, Sinchaikul S, An F-M, Liu D-Z, Phutrakul S, Chen S-T. Targeting the Delivery of Glycan-Based Paclitaxel Prodrugs to Cancer Cells via Glucose Transporters. Journal of Medicinal Chemistry. 2008;51:7428–7441. doi: 10.1021/jm8006257. [DOI] [PubMed] [Google Scholar]
- 115.Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 0000;87:805–812. doi: 10.1038/sj.bjc.6600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miot-Noirault E, Reux B, Debiton E, Madelmont J-C, Chezal J-M, Coudert P, Weber V. Preclinical investigation of tolerance and antitumour activity of new fluorodeoxyglucose-coupled chlorambucil alkylating agents. Investigational new drugs. 2011;29:424–433. doi: 10.1007/s10637-009-9371-0. [DOI] [PubMed] [Google Scholar]
- 117.Reux B, Weber V, Galmier M-J, Borel M, Madesclaire M, Madelmont J-C, Debiton E, Coudert P. Synthesis and cytotoxic properties of new fluorodeoxyglucose-coupled chlorambucil derivatives. Bioorganic & medicinal chemistry. 2008;16:5004–5020. doi: 10.1016/j.bmc.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 118.Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F, Feng C, Xie Y, Sha X, Fang X. Nanoparticles of 2-deoxy-D-glucose functionalized poly (ethylene glycol)-co-poly (trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials. 2014;35:518–529. doi: 10.1016/j.biomaterials.2013.09.094. [DOI] [PubMed] [Google Scholar]
- 119.Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. Journal of Nuclear Medicine. 2010;51:1167–1170. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiological reviews. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 121.Berg RD. The indigenous gastrointestinal microflora. Trends in Microbiology. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 122.Alvarez-Lorenzo C, Blanco-Fernandez B, Puga AM, Concheiro A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Advanced Drug Delivery Reviews. 2013;65:1148–1171. doi: 10.1016/j.addr.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 123.Schmaljohann D. Thermo-and pH-responsive polymers in drug delivery. Advanced drug delivery reviews. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 124.Xu W, Ding J, Xiao C, Li L, Zhuang X, Chen X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials. 2015;54:72–86. doi: 10.1016/j.biomaterials.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 125.Gu L. PH-responsive amphiphilic macromolecules for anticancer drug and siRNA delivery. Rutgers University-Graduate School; New Brunswick: 2014. [Google Scholar]
- 126.Krannig K-S, Schlaad H. pH-Responsive Bioactive Glycopolypeptides with Enhanced Helicity and Solubility in Aqueous Solution. Journal of the American Chemical Society. 2012;134:18542–18545. doi: 10.1021/ja308772d. [DOI] [PubMed] [Google Scholar]
- 127.Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Advanced drug delivery reviews. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 128.Zhang C, Chen J-d, Yang F-q. Konjac glucomannan, a promising polysaccharide for OCDDS. Carbohydrate Polymers. 2014;104:175–181. doi: 10.1016/j.carbpol.2013.12.081. [DOI] [PubMed] [Google Scholar]
- 129.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.del Rosario LS, Demirdirek B, Harmon A, Orban D, Uhrich KE. Micellar Nanocarriers Assembled from Doxorubicin-Conjugated Amphiphilic Macromolecules (DOX AM) Macromolecular bioscience. 2010;10:415–423. doi: 10.1002/mabi.200900335. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Zhang X, Han Y, Cheng C, Li C. pH-and glucose-sensitive glycopolymer nanoparticles based on phenylboronic acid for triggered release of insulin. Carbohydrate polymers. 2012;89:124–131. doi: 10.1016/j.carbpol.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 132.Richard I, Thibault M, De Crescenzo G, Buschmann MD, Lavertu M. Ionization behavior of chitosan and chitosan DNA polyplexes indicate that chitosan has a similar capability to induce a proton-sponge effect as PEI. Biomacromolecules. 2013;14:1732–1740. doi: 10.1021/bm4000713. [DOI] [PubMed] [Google Scholar]
- 133.Jahren SL, Butler MF, Adams S, Cameron RE. Swelling and Viscoelastic Characterisation of pH-Responsive Chitosan Hydrogels for Targeted Drug Delivery. Macromolecular Chemistry and Physics. 2010;211:644–650. [Google Scholar]
- 134.Nand AV, Rohindra DR, Khurma JR. Characterization of genipin crosslinked hydrogels composed of chitosan and partially hydrolyzed poly (vinyl alcohol) e-Polymers. 2007;7:402–410. [Google Scholar]
- 135.Yang J, Chen J, Pan D, Wan Y, Wang Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydrate Polymers. 2013;92:719–725. doi: 10.1016/j.carbpol.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 136.Abd El-Ghaffar MA, Hashem MS, El-Awady MK, Rabie AM. pH-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydrate Polymers. 2012;89:667–675. doi: 10.1016/j.carbpol.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 137.Yu C-Y, Yin B-C, Zhang W, Cheng S-X, Zhang X-Z, Zhuo R-X. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloids and Surfaces B: Biointerfaces. 2009;68:245–249. doi: 10.1016/j.colsurfb.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 138.Manchanda R, Nimesh S. Controlled size chitosan nanoparticles as an efficient, biocompatible oligonucleotides delivery system. Journal of applied polymer science. 2010;118:2071–2077. [Google Scholar]
- 139.Liu L, Yang J-P, Ju X-J, Xie R, Liu Y-M, Wang W, Zhang J-J, Niu CH, Chu L-Y. Monodisperse core-shell chitosan microcapsules for pH-responsive burst release of hydrophobic drugs. Soft Matter. 2011;7:4821–4827. [Google Scholar]
- 140.Mukhopadhyay P, Chakraborty S, Bhattacharya S, Mishra R, Kundu P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. International journal of biological macromolecules. 2015;72:640–648. doi: 10.1016/j.ijbiomac.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 141.Park K, Kim Y-S, Lee GY, Park R-W, Kim I-S, Kim SY, Byun Y. Tumor endothelial cell targeted cyclic RGD-modified heparin derivative: inhibition of angiogenesis and tumor growth. Pharm Res. 2008;25:2786–2798. doi: 10.1007/s11095-008-9643-y. [DOI] [PubMed] [Google Scholar]
- 142.She W, Li N, Luo K, Guo C, Wang G, Geng Y, Gu Z. Dendronized heparin-doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials. 2013;34:2252–2264. doi: 10.1016/j.biomaterials.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 143.Chourasia M, Jain S. Polysaccharides for colon targeted drug delivery. Drug Delivery. 2004;11:129–148. doi: 10.1080/10717540490280778. [DOI] [PubMed] [Google Scholar]
- 144.Nakajima N, Matsuura Y. Purification and characterization of konjac glucomannan degrading enzyme from anaerobic human intestinal bacterium, Clostridium butyricum-Clostridium beijerinckii group. Bioscience, biotechnology, and biochemistry. 1997;61:1739–1742. doi: 10.1271/bbb.61.1739. [DOI] [PubMed] [Google Scholar]
- 145.Munarin F, Tanzi M, Petrini P. Advances in biomedical applications of pectin gels. International journal of biological macromolecules. 2012;51:681–689. doi: 10.1016/j.ijbiomac.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 146.Huang Z, Gan J, Jia L, Guo G, Wang C, Zang Y, Ding Z, Chen J, Zhang J, Dong L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials. 2015;48:26–36. doi: 10.1016/j.biomaterials.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 147.Liu J, Zhang L, Hu W, Tian R, Teng Y, Wang C. Preparation of konjac glucomannan-based pulsatile capsule for colonic drug delivery system and its evaluation in vitro and in vivo. Carbohydrate Polymers. 2012;87:377–382. doi: 10.1016/j.carbpol.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 148.Jung J, Arnold RD, Wicker L. Pectin and charge modified pectin hydrogel beads as a colon-targeted drug delivery carrier. Colloids and Surfaces B: Biointerfaces. 2013;104:116–121. doi: 10.1016/j.colsurfb.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 149.Ganguly K, Chaturvedi K, More UA, Nadagouda MN, Aminabhavi TM. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. Journal of Controlled Release. 2014;193:162–173. doi: 10.1016/j.jconrel.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 150.Das S, Chaudhury A, Ng K-Y. Preparation and evaluation of zinc pectin chitosan composite particles for drug delivery to the colon: role of chitosan in modifying in vitro and in vivo drug release. International Journal of Pharmaceutics. 2011;406:11–20. doi: 10.1016/j.ijpharm.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 151.Alvarez-Manceñido F, Landin M, Martinez-Pacheco R. Konjac glucomannan/xanthan gum enzyme sensitive binary mixtures for colonic drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69:573–581. doi: 10.1016/j.ejpb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 152.Sriamornsak P. Application of pectin in oral drug delivery. Expert opinion on drug delivery. 2011;8:1009–1023. doi: 10.1517/17425247.2011.584867. [DOI] [PubMed] [Google Scholar]
- 153.Bernardos A, Aznar E, Marcos MD, Martínez-Máñez R, Sancenón F, Soto J, Barat JM, Amorós P. Enzyme-Responsive Controlled Release Using Mesoporous Silica Supports Capped with Lactose. Angewandte Chemie. 2009;121:5998–6001. doi: 10.1002/anie.200900880. [DOI] [PubMed] [Google Scholar]
- 154.Bernardos A, Mondragón L, Aznar E, Marcos MD, Martínez-Máñez R, Sancenón F, Soto J, Barat JM, Pérez-Payá E, Guillem C, Amorós P. Enzyme-Responsive Intracellular Controlled Release Using Nanometric Silica Mesoporous Supports Capped with “Saccharides”. ACS Nano. 2010;4:6353–6368. doi: 10.1021/nn101499d. [DOI] [PubMed] [Google Scholar]
- 155.Milas M, Rinaudo M. Properties of xanthan gum in aqueous solutions: Role of the conformational transition. Carbohydrate Research. 1986;158:191–204. [Google Scholar]
- 156.Milas M, Shi X, Rinaudo M. On the physicochemical properties of gellan gum. Biopolymers. 1990;30:451–464. doi: 10.1002/bip.360300322. [DOI] [PubMed] [Google Scholar]
- 157.Kim Y-H, Kwon IC, Bae YH, Kim SW. Saccharide effect on the lower critical solution temperature of thermosensitive polymers. Macromolecules. 1995;28:939–944. [Google Scholar]
- 158.Yang J, Fang L, Wang F, Tan T. Preparation and characterization of a novel pH-, thermo-, and ionic strength-responsive hydrogels based on xanthan gum poly(aspartic acid) Journal of Applied Polymer Science. 2007;105:539–546. [Google Scholar]
- 159.Seo S, Lee C-S, Jung Y-S, Na K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly (l-lactide) copolymers. Carbohydrate Polymers. 2012;87:1105–1111. [Google Scholar]
- 160.Zhao S, Cao M, Li H, Li L, Xu W. Synthesis and characterization of thermo-sensitive semi-IPN hydrogels based on poly (ethylene glycol)-co-poly (ε-caprolactone) macromer, N-isopropylacrylamide, and sodium alginate. Carbohydrate research. 2010;345:425–431. doi: 10.1016/j.carres.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 161.Zhang X, Wu D, Chu CC. Synthesis and characterization of partially biodegradable, temperature and pH sensitive Dex MA/PNIPAAm hydrogels. Biomaterials. 2004;25:4719–4730. doi: 10.1016/j.biomaterials.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 162.Mundargi RC, Shelke NB, Babu VR, Patel P, Rangaswamy V, Aminabhavi TM. Novel thermo-responsive semi-interpenetrating network microspheres of gellan gum-poly (N-isopropylacrylamide) for controlled release of atenolol. Journal of applied polymer science. 2010;116:1832–1841. [Google Scholar]
- 163.Belbekhouche S, Dulong V, Picton L, Le Cerf D. Saccharide effect on the LCST property of a polyether: Influence of structure and length. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2013;428:25–31. [Google Scholar]
- 164.Mishra B, Patel BB, Tiwari S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2010;6:9–24. doi: 10.1016/j.nano.2009.04.008. [DOI] [PubMed] [Google Scholar]