Abstract
The oral route of drug administration is most preferred due to its ease of use, low cost, and high patient compliance. However, the oral uptake of many small molecule drugs and biotherapeutics is limited by various physiological barriers, and, as a result, drugs suffer from issues with low solubility, low permeability, and degradation following oral administration. The flexibility of micro- and nanofabrication techniques has been used to create drug delivery platforms designed to address these barriers to oral drug uptake. Specifically, micro/nanofabricated devices have been designed with planar, asymmetric geometries to promote device adhesion and unidirectional drug release toward epithelial tissue, thereby prolonging drug exposure and increasing drug permeation. Furthermore, surface functionalization, nanotopography, responsive drug release, motion-based responses, and permeation enhancers have been incorporated into such platforms to further enhance drug uptake. This review will outline the application of micro/nanotechnology to specifically address the physiological barriers to oral drug delivery and highlight technologies that may be incorporated into these oral drug delivery systems to further enhance drug uptake.
Keywords: Microfabrication, nanofabrication, oral drug delivery, microdevice, nanotopography, targeted delivery
Graphical Abstract
1. Introduction
Oral drug administration is the most preferred and common route. As opposed to parenteral administration, the oral route typically causes neither tissue damage nor pain and requires less patient supervision, resulting in high patient compliance and decreased cost of care [1]. Oral drug formulations may also provide advantages over intravenous drug formulations, which can involve injection of solubilizing excipients associated with toxicity and/or altered disposition of coadministered drugs [2–4]. In addition to being the primary route for systemic drug administration, oral administration allows for localized drug treatment of gastrointestinal (GI) tissue. However, there is currently a lack of approaches to target diseased tissue [5, 6]. Therefore, diseases of the GI tract are often treated through formulations designed for systemic administration, resulting in system-wide side effects [5, 7].
While oral administration is most preferred, approximately 50% of active pharmaceutical agents suffer from limited oral uptake [8, 9]. The oral route is associated with issues with 1) drug degradation, 2) low drug solubility, and 3) low drug permeability, preventing uptake of intact drug into the bloodstream [10]. Current approaches to improve drug uptake include permeation enhancers, excipients to enhance drug solubility or provide sustained drug release, micro- and nanoparticulate systems, drug conjugation and modification, enteric coating, metabolic and transporter protein inhibitors, and bioadhesive polymers and ligands, which have been reviewed in detail [6, 11–19]. While these approaches allow for control over many properties of drug delivery systems, they do not typically provide precise geometric control, which can be used to facilitate interaction with the micro- and nanoscale features of GI tract physiology for increased adhesion and tissue permeability [20, 21].
Photolithography, soft lithography, and nanofabrication approaches can be used to fabricate oral drug delivery systems with precise control over feature geometry, symmetry, dimensions, material composition, and surface modification, allowing for design of microscale devices that specifically address physiological barriers of the GI tract. These fabrication technologies have also been reviewed in detail previously [6, 22–25]. Application of these approaches to oral drug delivery has been expanding to utilize biocompatible and bioadhesive polymers, asymmetric geometries, nanotopographical features, and materials that respond to environmental cues to improve drug uptake. This review will highlight recent advances in the application of micro/nanotechnology to oral drug delivery and predict how current and developing technologies may be incorporated into these micro/nanofabricated platforms to improve bioavailabilities of a wide range of drugs and biotherapeutics.
2. Physiological barriers to oral drug uptake
2.1 Physiological considerations for oral drug delivery
The physiology of the human digestive tract presents significant barriers to oral drug delivery. Among these challenges are poor drug stability, poor drug solubility, and mucosal barriers that limit the application of current oral dosing strategies to drugs that possess good bioavailability. In general, the digestive tract is composed of a series of compartments that work together using mechanical and chemical processes to help convert food into energy and nutrients for the body. Beginning with the oral cavity, multiple accessory organs (i.e. teeth, salivary glands, tongue) break down food and secrete lubricants to help food pass into the pharynx, a tube that connects the mouth and the esophagus. Passing through the esophagus, the food reaches the stomach where the low pH and digestive enzymes help break down the food. The digested food moves to the small intestine for further digestion and absorption of nutrients and finally moves to the large intestine where water is absorbed and microflora futher breaks down waste products prior to excretion.
The physiology throughout the digestive tract can differ significantly in the form of gastric mucosa, organization of the epithelium, and chemical microenvironment. Therefore, the physiological characteristics of the targeted region and all preceding regions must be considered when designing drug delivery platforms. The following sections will provide the reader with a general understanding of the different targets for oral drug delivery and some of the benefits and challenges posed by each target.
2.2 Buccal cavity
Delivery to the oral cavity is sometimes preferred over the conventional oral route because it avoids pre-systemic clearance by the liver and physiological challenges related to membrane permeability and absorption found in the gastrointestinal tract. Buccal mucosa includes a stratified squamous epithelium that comprises the upper and lower lips as well as the lining of the cheek and is richly vascularized. As such, buccal administration is an attractive target due to easy accessibility, mild pH microenvironment, and direct access to systemic circulation thereby avoiding the hepatic “first-pass” effect [26]. However, drawbacks include the smaller surface area of membranes, about 170 cm2, of which ~50 cm2 of non-keratinized buccal tissue is available for drug absorption [27]. In addition, saliva is constantly secreted in the oral cavity and can dilute and/or degrade labile drugs susceptible to enzymatic degradation [28]. The reader is referred to the following reviews for more in-depth information regarding buccal anatomy and conventional drug delivery approaches [29, 30]. To bypass these physiological barriers, drug delivery platforms can be designed to prolong residence time with the mucosa, enhance drug permeation, and protect the drug from degradation.
2.3 Esophagus
The esophagus is a site not often utilized for drug delivery due to presence of a stratified, squamous epithelial layer that is known for its low permeability and very short residence time [31]. For example, a 10 mL bolus has less than 16 s of residence time in the esophagus before reaching the stomach [32]. Additionally, poor blood supply makes esophageal delivery less ideal for systemic delivery. Certain local diseases such as esophageal cancer, bacterial infection, and Barrett’s esophagus can be targeted specifically through the use of bioadhesives to improve local delivery [33]. However, some solid dose formulations may become lodged in the esophagus, which is a concern. In this scenario, a high concentration of drug is delivered to a localized area, potentially causing damage to the tissue. As such, drug delivery to the esophagus must focus on devices that can improve device residence time while preventing permanent lodging of the device.
2.4 Stomach
The stomach is responsible for storing food temporarily and slowly releasing it into the small intestine. Within the stomach is a harsh microenvironment with a pH range of 1.0–2.5 that acts as a chemical factory for breaking down food and harmful pathogens [34]. To prevent self-digestion, the stomach possesses the extrinsic gastrointestinal barrier, a layer of mucus spanning 40–450 μm and bicarbonate ions secreted by surface epithelial cells [35, 36]. Much of the mature epithelial cells have high turnover rate and die within a few days. Beneath this layer is the intrinsic barrier, which is composed of epithelial cells that seal the paracellular space with tight junctions. With a small surface area, there is little absorption that takes place making delivery difficult [37]. Drug delivery systems that target diseases like peptic ulcer disease in the stomach must seek out methods to increase gastroretention time to avoid gastric emptying. This is primarily achieved by means of high and low density, bioadhesive, swellable, expandable, or raft floating drug delivery systems for which the reader is referred to the following review for further detail [38].
2.5 Small intestine
Digested food from the stomach moves into the small intestine for further digestion and processing. The small intestine can be further divided into three structural parts: duodenum, jejunum, and ileum. The duodenum is a short structure that receives the gastric chyme from the stomach and secretes an alkaline solution of bicarbonate that neutralizes the stomach pH. The pH changes rapidly to about pH 6 in the duodenum and will rise to about 7.4 in the terminal ileum [39]. The jejunum connects the duodenum to the ileum and possesses villi that increase its surface area for absorption of nutrients. The ileum is the final section that possesses similar physiological structure to the jejunum and absorbs remaining nutrients not absorbed by the jejunum.
Greater than 99% of the epithelial tissue is covered with enterocytes to form the villi, finger-like projections 0.5–1 mm in length [40]. The role of villi is to use their apical projections, called microvilli, to increase surface area for greater absorption [41]. The total surface area is approximately 400 m2, although this varies with the individual, with an average measurable microvilli height and diameter of 0.67–1.36 μm and 0.08–0.15 μm, respectively [42]. Covering the villi are follicle associated epithelium, M-cells, and mucus secreting goblet cells, which play a role in protecting the epithelium. The high surface area and numerous transport mechanisms make the small intestine a prime target for oral drug delivery [43].
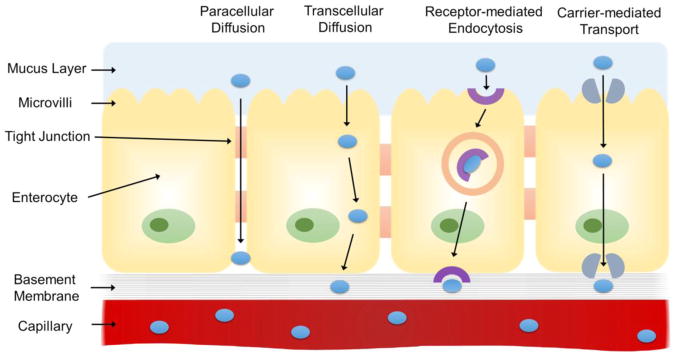
However, there are multiple challenges that arise from its physiology. Drugs must survive the severe chemical microenvironment in the stomach prior to entering the small intestine. Once in the intestine, drugs are then exposed to numerous pancreatic enzymes and bile salts that can degrade drugs. In addition, the presence of a mucosal layer results in low drug bioavailability of most water-soluble macromolecules due to their low mucus permeability. Drugs must be able to cross the mucosal layer followed by a submucosal and areolar cell barrier where they interact with a plethora of transport pathways including transcellular transport, paracellular transport, or receptor/carrier-mediated processes to enter systemic circulation (Figure 1) [13]. Various microdevices and nanotechnology-based approaches propose to take advantage of the physiology of the small intestine [42, 44]. Ability to control device dimensions in micro- and nanoscale allows targeting of specific villi and microvilli dimensions, leading to increased retention of the device, which may increase overall bioavailability [23]. Furthermore, microfabrication can also incorporate permeation enhancers that promote absorption of therapeutics that have traditionally been difficult to use for oral drug delivery. This will be explored in further detail in later sections.
Figure 1.
Schematic representing the routes of drug transport through the physiological barriers of the small intestine. Drug molecules can be transported passively via paracellular or transcellular diffusion or actively via receptor-mediated endocytosis and carrier-mediated transport.
2.6 Colon
Digesting food moves from the small intestine and into the large intestine, or colon, which is the final site of the digestive system before excretion. Compared to other oral drug delivery sites, the colon is host to lower digestive enzyme activity, higher pH compared to the stomach and small intestine, and a long residence time of up to 5 days [45, 46]. Furthermore, the gut microflora can carry out chemical reactions that may metabolize a number of different drugs. Delivery to the colon is important for treatment of bowel diseases including Crohn’s disease, ulcerative colitis, and colorectal cancer. Specific targeting of these diseases would benefit from local delivery by using less drug and can reduce side effects that arise from systemic delivery. As the colon is the most distal target, device design must ensure that drug release and absorption do not occur in the stomach or small intestine while protecting the drug against degradation in the variable microenvironments encountered. However, a major drawback to targeting the colon is the inherent variability of the patient’s pH levels, gastric emptying times, and differing microflora [47, 48]. As a result, the unique microenvironment found in each patient leads to variable drug release and poor bioavailability, which makes it challenging to deliver consistent, therapeutic doses. Therefore, device design must take these physiological challenges into consideration.
2.7 Concluding remarks for physiology
There are a number of physiological barriers that must be considered in the design of new devices to transport and protect drugs with subsequent delivery to targeted tissue. Therefore, it is important to design systems capable of taking advantage of biology by means of increased adhesion to promote retention time, response to stimuli in the local microenvironment, and enhanced permeation at target sites while being capable of delivering a significant dose of drug by efficient loading. The following sections will describe in detail how micro/nanofabrication present approaches to drug delivery that specifically take advantage of physiology in device design.
3. Strategies to increase micro/nanofabricated oral drug delivery system adhesion
3.1 Rationale for promoting adhesion
The GI tract presents unique barriers and micro/nanoscale features that can be addressed by oral drug delivery systems. Micro- and nanofabrication approaches provide precise control over device geometry, surface modification, material composition, symmetry, and size, all of which can be used to design drug delivery systems for specific interactions with GI tract tissue. One particular interaction that is advantageous for oral drug delivery is bioadhesion, as adhesion to GI tract enhances drug uptake by 1) prolonging device residence time and drug exposure and 2) allowing for release of drug in high concentrations proximally to epithelial tissue for enhanced permeation effects [20, 21]. Drug delivery platforms can be fabricated to adhere to the lining of the GI tract via geometric, mechanical, biochemical, nanotopographical and/or motion-based approaches [49, 50]. To promote adhesion to specific regions of the GI tract, these approaches may utilize bioresponsive “smart” materials or be used in combination with other targeting technologies such as enteric coating.
3.2 Geometry-mediated adhesion
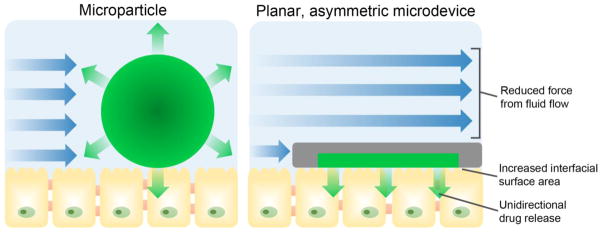
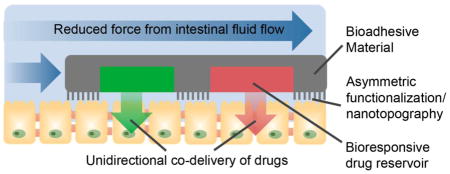
Microscale drug delivery systems are capable of enhanced adhesion over macroscale drug delivery systems as a result of their high surface-area-to-mass ratio and ability to become entrapped within the microscale villi [6]. Geometry-based approaches can further promote device adhesion by utilizing a flat or planar device shape that is typical of microdevices for oral drug delivery [21, 51–56]. As shown in Figure 2, a planar geometry promotes adhesion by 1) increasing the contact area available for interaction with the epithelial lining of the GI tract and 2) decreasing the force exerted on the devices from the fluid flow in the GI tract [20, 21]. Furthermore, microdevices can be fabricated asymmetrically with a drug reservoir on only one side of the device, providing unidirectional drug release to create a steep concentration gradient to increase drug permeation. Tao et al. investigated the effect of device geometry on adhesion by incubating planar, asymmetric devices with dimensions of 150 × 150 × 5 μm over a monolayer of Caco-2 intestinal epithelial cells and exposing the devices to multiple washing steps [52]. After washing, 68% of the planar microdevices remained adhered while 17% of poly(methyl methacrylate) (PMMA) microspheres of similar surface area remained adhered. When loaded with the model drug fluorescein and added to a Caco-2 monolayer under flow conditions, these devices increased permeation of drug 10-fold over that of a bolus dose [55]. Furthermore, Chirra et al. demonstrated the effect of planar device geometry on adhesion in vivo [53]. When PMMA microdevices 200 μm in diameter and 8 μm in thickness (Figure 3 A) were administered to mice, they showed 27% retention in the proximal small intestine after 2 hours while PMMA microspheres of similar surface area demonstrated 12% retention. When loaded with drug, the planar PMMA microdevices provided a fourfold increase in oral bioavailability of acyclovir, a Biopharmaceutics Classification System (BCS) class III poorly permeable drug, relative to that of a bolus dose.
Figure 2.
In contrast to spherical microparticles, planar, asymmetric microdevices provide proximal, unidirectional drug release and increased residence time in the GI tract. A planar microdevice shape reduces the force experienced from intestinal fluid flow (blue arrows) and increases surface area available for binding to epithelial tissue, increasing device adhesion to the lining of the GI tract and prolonging drug exposure. Devices can be asymmetrical fabricated with a drug reservoir on one side of the device, allowing for proximal, unidirectional release of drug (green) toward epithelial tissue.
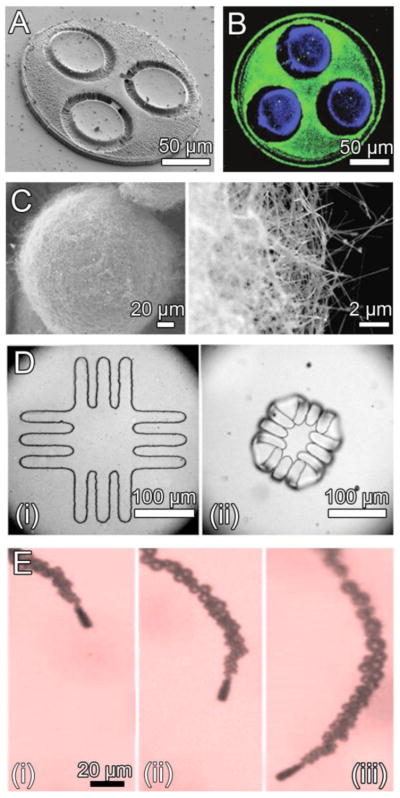
Figure 3.
Micro- and nanofabrication-based approaches to enhance bioadhesion. A. A planar device geometry for increased surface area available for interaction with epithelial tissue and decreased force from intestinal fluid flow [53]. B. Lectin (green) surface modification to promote bioadhesion of the side of devices with drug reservoirs (blue) for unidirectional drug release [54]. C. Silica nanowires coating silicon microparticles provide increased surface area, promoting muco- and cytoadhesion [57]. D. Bilayered microdevices before (i) and after (ii) exposure to water. Microdevice folding is designed for mechanical attachment to intestinal mucosa [58]. E. Micromotors consisting of a zinc core encased within a polymeric microtube react with gastric acid, propelling the micromotors for entrapment within the stomach lining (1 s intervals, i–iii) [50]. Images reproduced with permission.
3.3 Biochemical surface modifications to enhance adhesion
In addition to providing geometry-mediated enhancement in bioadhesion, micro and nanofabricated oral drug delivery platforms can be surface modified with bioadhesive compounds to promote adhesion. Microdevices are typically fabricated on a silicon wafer or other substrates, facilitating asymmetric functionalization of exposed device regions [22]. This asymmetric surface modification can be used to promote binding of the drug-releasing side of the device, providing unidirectional drug release toward epithelial tissue [20, 21]. Lectins, carbohydrate-binding proteins capable of binding to glycosylated proteins and cell membrane components to provide muco- and cytoadhesion [14], have been functionalized onto drug delivery systems to promote adhesion to the lining of the GI tract [6, 51–53, 59–61]. PMMA microdevices modified with tomato lectin (Figure 3 B), which binds selectively to the epithelium of the small intestine [62], demonstrated 92 ± 4% retention in an in vitro Caco-2 adhesion assay, whereas devices lacking modification showed 29 ± 9% retention [51]. In vivo, lectin-conjugated PMMA microdevices showed 41% retention in the proximal small intestine of mice two hours following oral administration as opposed to 27% for bare devices [53]. Biochemical adhesion utilizing high-affinity interactions between a targeting ligand and specific moieties can provide highly specific binding to the small intestine or diseased tissue. However, one drawback to the use of biomolecules and other surface modifications to promote adhesion is degradation as a result of the low pH of the stomach and proteolytic and metabolic enzymes throughout the GI tract [63]. Therefore, molecular stability must be considered for surface modification of oral drug delivery platforms.
3.4 Micro- and nanotopography-mediated adhesion
Topography-mediated adhesion presents an alternative approach to promote bioadhesion that is dependent upon geometry rather than degradable surface modifications. By increasing surface area, micro- and nanofeatures increase the interfacial surface adhesion [64–67]. Cylindrical pills coated with microneedles designed for physical penetration of epithelial tissue to increase drug permeation are also likely to provide the additional benefit of increased adhesion to the GI tract [68]. As with asymmetric surface functionalization, asymmetric topographical modifications have potential to promote unidirectional drug release toward epithelial tissue. In an example of hierarchical microdevice structure, multi-layer fabrication was employed to modify one surface of 150 × 150 μm microdevices with microposts 10 μm in diameter [69]. In an alternate approach, bottom-up nanofabrication approaches have been employed to create nanoengineered microparticles (NEMPs) consisting of silicon oxide nanowire-coated silicon microparticles for oral drug delivery (Figure 3 C) [57, 70–73]. Following contact with an epithelial layer, the nanowire coating of these microparticles interdigitated with the microvilli on the surface of the epithelial cells [73]. The NEMPs showed a 100-fold increase in required lift-off force from an in vitro epithelial monolayer relative to unmodified microparticles [73]. In vivo, the retention time of the NEMPs in the GI tract following oral administration was 10-fold that of bare microparticles [57]. While NEMPs can be fabricated with a relatively planar shape for enhanced adhesion [71], the fabrication approaches used for NEMP fabrication do not allow for asymmetric nanowire functionalization for unidirectional drug release or use of highly biocompatible polymers. However, the techniques of photolithography and nanotemplating were recently combined to asymmetrically coat PMMA microstructures with polycaprolactone (PCL) nanowires [74]. While not yet applied to oral drug delivery, this approach has potential to combine the benefits of asymmetric, planar microdevices with nanowire-mediated adhesion while utilizing polymers with FDA approval for medical applications [75, 76].
3.5 Mucoadhesive bulk materials
As an alternative to surface modification, a number of mucoadhesive materials including alginate [77, 78], chitosan and chitosan derivatives [79–81], hyaluronic acid [82, 83], gelatin [84, 85], cellulose derivatives [86, 87], and a number of synthetic polymers [88] are available for use as a bulk material in fabrication of oral drug delivery systems. Among these materials, chitosan has been highly utilized in a number of oral drug delivery systems, including chitosan-based micro- and nanoparticulate drug delivery systems [89–94], chitosan-drug conjugates [95, 96], and chitosan macroscale patches [97, 98]. Chitosan is an attractive material for micro/nanofabricated platforms as it is compatible with a number of microfabrication approaches [99, 100], is stable through pH values relevant to GI physiology [101], and has been utilized in microfabricated oral drug delivery systems [58, 102].
3.6 Mechanical and motion-based adhesion
While device surface modifications including biochemical and nanotopographical cues are capable of interacting with epithelial tissue to enhance cytoadhesion and drug permeability, the mucus layer may prevent direct interaction between nanofeatures and epithelial cells. Mechanical and motion-based adhesion approaches may provide a mechanism for microscale devices to penetrate through the mucus layer and directly contact epithelial tissue.
Microscale drug delivery systems can be designed to mechanically respond to the environment of the GI tract to promote adhesion. Self-folding devices have been developed to respond to solvent exposure [58, 102], temperature [103, 104], pH [102], and ionic strength [105]. Self-folding properties have been incorporated into microscale oral drug delivery systems to promote mechanical attachment to the lining of the GI tract. For example, Guan et al. fabricated bilayered devices consisting of chitosan and a copolymer of poly(ethylene glycol) methacrylate (PEGMA) and poly(ethylene glycol) dimethacrylate (PEGDMA) which used differential swelling to fold upon exposure to water (Figure 3 D) [102]. Similar bilayered devices composed of crosslinked poly(methacrylic acid) (PMAA) and poly(hydroxyethyl methacrylate) (PHEMA) were capable of mechanical attachment to excised pig intestinal mucosa [58], and demonstrated enhanced mucoadhesion, lower drug leakage into luminal space, and improved unidirectional delivery, resulting in improved drug transport across excised porcine mucosal epithelium [49]. While these self-folding devices have not demonstrated specificity in binding to the small intestine or other specific regions of the GI tract, alternative bioresponsive materials could be utilized to respond to pH or other cues for more specific targeting. Alternatively, these self-folding devices could be combined with other targeting technologies such as enteric capsules to release these devices at the desired region of the GI tract.
In an alternate approach involving motion-based adhesion, a number of technologies for chemically induced locomotion have been developed [106–111]. Gao et al. applied this technology to oral drug delivery systems by developing a pH-responsive micromotor approach to enhance device adhesion and payload delivery to the lining of the mouse stomach (Figure 3 E) [50]. Their design of micromotors consisting of a zinc core encased within a poly(3,4-ethylenedioxythiophene) (PEDOT) microtube was then tested in mice by oral administration. Upon exposure to the low pH of the stomach, the zinc core reacted to form hydrogen gas, propelling the micromotors into the lining of the stomach, enhancing binding and retention of the devices and delivery of gold nanoparticles to the stomach wall. The reaction of these micromotors to low pH environments makes them ideal for promotion of stomach-specific adhesion. However, different compounds will need to be incorporated into the micromotor core to improve adhesion in other regions of the GI tract with higher pH values.
4. Loading of novel micro- and nanoscale oral drug delivery platforms
Ideally, the loading of drugs into carrier systems should be 1) efficient, minimizing the amount of drug wasted during the loading process, 2) high-throughput, allowing for scalability in production, and 3) able to maintain drug integrity under loading and storage conditions. The versatility of using polymers in combination with MEMS technology has led to several innovative ideas of loading novel drug delivery carriers for controlled release applications [112–114]. Most of these innovative carriers load and release drugs using responsive polymeric hydrogels, degradable polymer coatings, dissolvable thin metal films, or capillary action.
The most common and successful microelectromechanical systems (MEMS) technique to load miniaturized carriers with a variety of drugs is by using photolithography. Photolithographic crosslinking of polymers in the presence of a photoinitiator proves useful in tailoring specific material properties such as hydrophobicity, biodegradability, and biocompatibility that play a role in drug release kinetics, cellular interaction, and immunogenicity. These properties can also be modified by varying the chemical structure/functionality of the monomer used, molecular weight, and/or crosslinking density [49, 58, 115, 116]. The process involves spin casting a photoinitiator mixed pre-polymer solution as shown in Figure 4 A. Polymerization is then carried out through the localization of light using an appropriate photomask. The drug is loaded inside the polymer matrix either by mixing it with the pre-polymer solution or via responsive swelling-diffusion-collapse method [117–120]. While spin casting is rapid in loading microreservoirs with drug solutions, a significant amount of drug is lost during the spin-casting and development steps, which makes the platform not viable for expensive drugs. Furthermore, drugs loaded by photolithographic approaches must be able to remain intact following UV exposure.
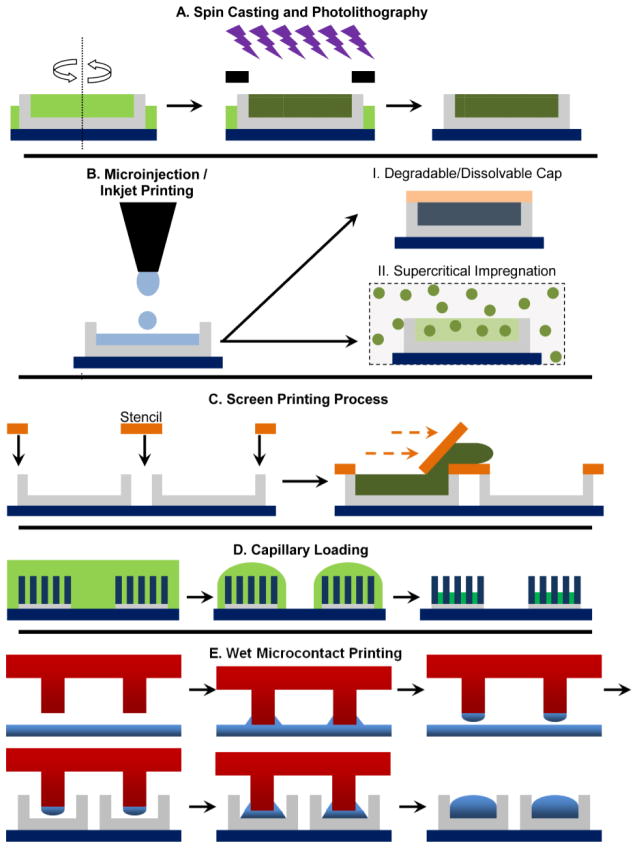
Figure 4.
Schematic representation of loading drugs into microdevice platforms. A. Rapid loading of reservoirs via spin casting followed by drug entrapment inside a photopolymerized polymer matrix. B. Precise loading of microreservoirs using microinjection/inkjet printing and then entrapping the drug within the reservoir via (i) introduction of a cap layer on top or (ii) supercritical impregnation. C. A high-throughput screen printing method involves the use of a transparent stencil that aligns with the microdevices to the reservoirs. D. Drug loading via capillary action of drug solution during solvent evaporation. E. Novel soft lithography methods of wet microcontact printing or microtransfer printing to load drugs into multiple devices simultaneously.
A more precise low-wastage drug loading method can be achieved by individually loading each reservoir with the appropriate volume of drug-polymer solution. Boisen and co-workers recently employed an inkjet printer to load a drug solution into microdevice reservoirs [121, 122], which is a quasi-no-waste performance technique as shown in Figure 4 B. They also loaded microdevice reservoirs with hydrophobic drugs in the absence of toxic organics by incorporating supercritical fluid impregnation with inkjet printing [123]. In this method, well defined quantities of poly(vinyl pyrolidone) (PVP) were dispensed into microcontainers/microreservoirs. Then the poorly soluble drug ketoprofen was impregnated into the polymer matrix using supercritical carbon dioxide as the loading medium (Figure 4 B(ii)). The amount of drug loaded or dosage achieved in microcontainers is tuned by varying the impregnation parameters. Compared to solid dispersions of the same drug, supercritical impregnated microdevices exhibit more reproducible drug loading and faster dissolution of drug, which allows for the modulation of drug release. While this method is capable of precise, relatively zero-waste performance, the sequential loading of each microdevice in a semi-automated manner makes it a low-throughput technique relative to spin casting. In their current state, microinjection/inkjet printing methods are not ideal for mass manufacturing production [21].
Simpler techniques for loading drugs into reservoirs have been utilized to overcome the low-throughput issue associated with microinjection/inkjet printing and high-wastage issue involved with spin casting. Nielsen et al. used a modified screen printing technique that involves the use of a stencil mask pre-fabricated by laser machining that aligns on top of microdevices for accessing the vacant reservoirs [124]. The stencil has pores/holes matching the microdevice reservoirs with a high level of precision. Once the stencil is aligned using an optical microscope, powdered amorphous drug is pressed through the stencil into the microreservoirs (Figure 4 C). Any excess powdered drug not located within the microwells is removed along with the stencil and is reused for loading more devices, thereby reducing drug waste. An alternative wet loading technique developed by Guan and co-workers uses a discontinuous de-wetting technique to collect and crystallize model drugs via solvent evaporation into device reservoirs to provide a high throughput drug loading method [58]. A similar high-throughput and rapid approach to loading microdevices with drug is achieved by harnessing the phenomenon of capillary action. Several researchers have fabricated nanowire-coated oral microdevices and used the high surface area of nanowires to effectively load both aqueous and non-aqueous drugs via capillary action. After solvent evaporation, the drug crystallizes over the microdevice surface at the base of the nanowires (Figure 4 D) [71, 72, 74]. Because solvents of drug solutions can evaporate in a manner of minutes with >90% of drug collecting over nanowire-coated microdevices [74], loading via capillary action is efficient in both throughput and minimizing drug waste. However, because the drug is surface-loaded rather than loaded within a matrix with tunable properties, this approach may present challenges in tuning release rates.
A more controlled approach to loading drugs into oral devices involves using modified soft lithography techniques. Soft lithography is a well-known tool used in the patterning of hydrophilic or hydrophobic molecules or polymers, polysaccharides, stimuli-sensitive and responsive materials, proteins, and growth factors over a wide variety of surfaces [125–128]. Micromolding in capillaries (MIMIC) uses a polydimethylsiloxane (PDMS) mold that comes into contact with the substrate surface. A low-viscosity prepolymer solution is then placed at the open end of the channel, wherein the solution is transferred to specific locations on the substrate by fluid flow or capillary action [129, 130]. A modified version of MIMIC is currently being studied to load drugs into microdevice reservoirs. Lee et al. loaded small amounts of model drugs methylene blue and tetracycline hydrochloride into microreservoirs using wet microcontact printing method [131]. In this process, a liquid drug-carrier solvent mixture was transferred to the reservoir by contact printing process as shown in Figure 4 E. By prolonging the time of contact printing in the presence of a non-volatile drug carrier solvent such as poly(ethylene glycol) (PEG), a higher dosage of drug was loaded into the microreservoirs. Moreover, unlike conventional PDMS stamps used for microcontact printing, the use of an active polymeric composite membrane fabricated by Ahmed and group is able to enhance the rate of microreservoir loading [132]. These smart microtransfer stamps can locally sense and actuate during wetting and printing process, thereby reducing drug wastage and increasing rate of manufacturing. Although research on loading drugs into oral drug delivery microplatforms is at its relative inception, well established semi-conductor industry fabrication techniques combined with polymer technology is bound to improve the mass fabrication of novel microplatforms loaded with a variety of oral drugs.
5. Incorporation of tunable and bioresponsive smart materials for sustained and controlled drug release
Ideally, oral administration should deliver drug to a specific target of the intestine at a required concentration within the therapeutic window. In addition, the drug should be delivered at the right time in a safe and reproducible manner. Currently, with a variety of loading techniques available for introduction of drugs into oral microplatforms, the future of oral microdevices involves the appropriate selection of polymer systems and other materials that can be tuned to modulate the release kinetics of entrapped drug. Multiple drugs can be dispensed from the microarray devices at specific locations in the intestine in a pulsatile fashion using rapid responsive or dissolving materials to treat localized intestinal pathologies. The high drug concentrations achieved during pulsatile release may provide therapeutic drug concentrations in local drug delivery to the GI tract or allow for saturation of metabolic or proteolytic enzymes to enhance systemic drug uptake. In the case of drugs that are sensitive to the harsh GI environment, including macromolecules and proteins, co-delivery of enzyme inhibitors and the therapeutic drug is beneficial. For this, a multi-reservoir microdevice can be loaded with multiple entities for simultaneous release at different rates.
The development of such devices is made possible through use of different polymeric matrices that entrap the drugs of interest. Polymers have varying chain length, hydrophilicity, and ionic charge, granting ample control when tailoring drug release kinetics. Additionally, advances in the field of polymer science have fostered the design and preparation of polymers with sensitivity to pH, temperature, and a variety of additional stimuli, which confers added specificity. Use of these materials would allow for targeted and independent release of multiple drugs, resulting in the widespread application of miniaturized oral platforms that release a wide array of drugs including sensitive drugs and macromolecules that are currently administered non-orally. Here, we summarize both well investigated, as well as novel ‘smart’ drug release materials that are promising for oral administration.
5.1 Multi-drug release
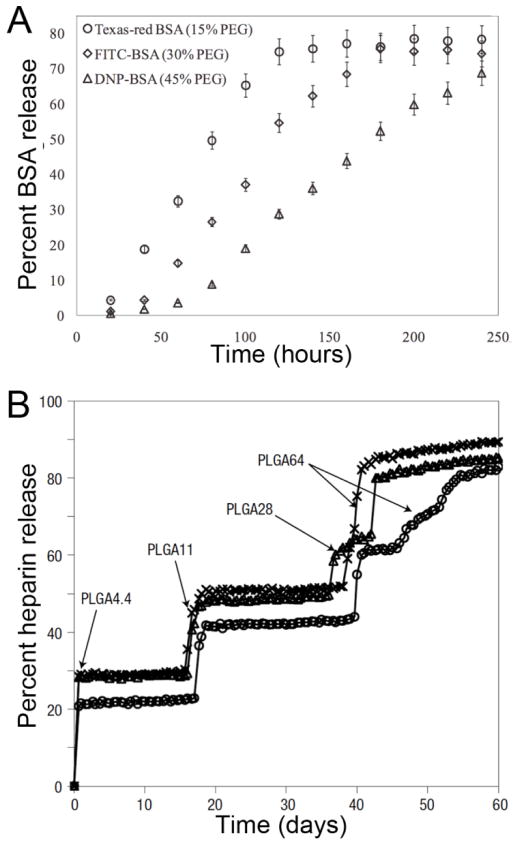
Combination therapies and co-delivery of permeation enhancers with the therapeutic drug of intertest may be enhanced by localized co-delivery by micro/nanofabricated platforms. Towards the goal of multi-drug release with separate release profiles, Ainslie et al. loaded single-reservoir microdevices with multiple drugs via a sequential layer-by-layer photolithographic process in combination with spin casting [20]. While the sequential loading of drugs into the same reservoir is useful for achieving sequential drug release kinetics, the release profile of each drug is dependent upon the property of all hydrogel layers. Alternatively, independent loading and release of multiple drugs can be achieved by using multiple reservoirs in the same microdevice. Chirra et al. entrapped three different model drugs via photopolymerization within separate reservoirs to provide simultaneous release [54]. The release profile of each drug loaded within the device was thereby solely dependent upon the property of the respective encompassing polymer/hydrogel matrix (Figure 5 A). This custom-release microdevice may be effectively used to deliver multiple drugs at different rates in localized regions to treat intestinal diseases including inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) or for co-delivery of permeation enhancers.
Figure 5.
Tunable multi-drug and pulsatile release in microfabricated drug delivery devices. A. Multi-reservoir microdevices loaded with different BSA-fluorophore conjugates demonstrated independent release at specific rates determined by drug matrix crosslinking density [54]. B. Microarray devices using multiple reservoirs coated with PLGA of varying molecular weights delivered heparin in a stepwise fashion [133]. Figures reproduced with permission.
5.2 MEMS-based drug release
The earliest MEMS device developed for controlled drug delivery included the GI microcapsule and the ChipRx smart pill. Unlike what the name suggests, the GI microcapsule is a 1 × 3 cm tube that carries out real-time drug release and gastrointestinal fluid sampling, while passing through the intestine [134]. This remote-controlled polycarbonate capsule has a location monitoring unit, a receiver, a driving device, reservoirs for drug and sampling, and a battery. The ChipRx smart pill is an implantable, single reservoir device equipped with a sensor to detect changes in the tissue environment [134]. After a builtin chip processes the sensor’s signal, a drug-releasing signal is sent, actuating a polymer membrane that covers the reservoir to release a specific amount of drug. A similar ‘smart’ system that caters to the variations in patient physiology and allows the user to deliver drugs on demand is the Philips IntelliCap technology [135]. It uses a telemetric capsule that delivers a drug at a certain place with a programmable release profile, which is realized by a miniaturized pumping system. Other pursuits concern the use of flexible, modularized systems where individual modules have its own drug and release kinetics. For example, Dome Matrix® technology, which is based on the concept of module assembly, has been used as an antimalarial drug delivery system that releases artesunate and clindamycin with different release profiles [136].
A more actively smart microdevice system that releases drug on cue is developed by MicroCHIPS Inc. Herein, a programmable microdevice made of an array of pyramid-shaped microreservoirs is used. The microarray of reservoirs is loaded with drug of interest using a microinjection method as shown in Figure 4 B. The loaded reservoirs are then capped with a thin gold film, as shown in Figure 4 B(i) that serves as an impermeable anode which readily dissolves to release the drug once triggered by a wireless electrochemical potential. Recently, a modified version of the microdevice was tested in clinical trials for delivering parathyroid hormone to humans [137]. Using the MicroCHIPs device, both in vitro and in vivo pulsatile release of drugs has been well demonstrated [138–141]. In pursuit of a biodegradable platform, MicroCHIPs used a resorbable platform consisting of an array of microreservoirs capped with biodegradable poly (lactic-co-glycolic acid) (PLGA) membranes instead of a dissolving metal film [133, 142]. The degradable PLGA membranes were synthesized using various ratios of lactic acid to glycolic acid, different molecular weights, and different thicknesses to provide multi-pulse drug release (Figure 5B). Even though the MicroCHIPS platform was not used for oral drug delivery, similar devices with multiple drug reservoirs can be fabricated with polymer films with faster degradation rates to provide pulsatile drug release at rates relevant to oral drug delivery. Furthermore, while this platform utilized a single drug of interest, multiple drugs could be incorporated for sequential, pulsatile release.
5.3 Swellable polymers
The first systems developed for oral drug delivery were based on hydrophilic polymers that swelled in the presence of water to allow release of drug [143, 144]. Such systems were appealing due to previously demonstrated material biocompatibility and FDA approval, and as such, currently form the bulk of oral drug delivery strategies. However, a variety of complications limit the effectiveness of these systems for oral drug delivery. Due to the swelling-based mechanism of drug release, these systems often experience an initial burst release of drug before the drug delivery system reaches its intended site of action. Additionally, such systems are susceptible to bulk degradation by hydrolysis, altering drug release kinetics and creating another challenge in controlling drug release. The use of hydrophobic materials circumvents several of these issues, as they primarily degrade via surface erosion, maintaining drug release kinetics [143]. However, poor efficacy due to limited specificity and low bioavailability remain significant challenges in these systems.
5.4 Stimuli-responsive materials
Stimuli-responsive materials are promising for controlled drug release because they specifically regulate drug release by actively sensing and responding to external conditions. When exposed to a specific stimulus, including temperature, pH, light, electric field, magnetic field, ultrasound and binding of biomolecules [145–147], these materials undergo conformational changes that alter their hydrophilicity, affecting bulk matrix properties that modify drug release. Of these, pH- and enzyme-sensitive systems are the most relevant for oral drug delivery, as they detect and respond to signals along the GI tract without the need for external intervention.
pH-responsive hydrogels are a class of stimuli-responsive systems that have great potential in oral drug delivery due to dynamic changes in pH along the GI tract. Researchers have taken advantage of this variability in pH to design drug delivery systems that release therapeutic payloads specifically in the stomach, intestine, or colon. These systems are generally made of synthetic polyacids or polybases [148–151], although naturally occurring pH-sensitive polymers (e.g., alginate, chitosan, hyaluronan) have also been investigated [145, 146, 148–151].
In addition to environmental pH, biochemical cues can be used to impart control over drug release. Generally, these materials modulate drug release via swelling or shrinking after binding a target biomolecule or enzyme-mediated degradation. Researchers have developed materials that can be degraded by microbial enzymes found in the intestine and colon [152–155]. Early strategies involved prodrugs and hydrogels containing azoaromatic linkages for the treatment of IBS and other colon-related diseases [155]. These materials are protected from the harsh conditions of the upper GI tract, but are degraded by azoreductases found in the colon, triggering release of active drug. Another promising material for colonic drug delivery is pectin. Pectin is resistant to proteases and amylase in the upper intestine but is digested by enzymes in the colon [153]. Dextranase-, chymotrypsin-, and papain-sensitive hydrogels have also been investigated for enzyme-sensitive drug release [156].
Hydrogels that swell/shrink in response to a biological cue have also been developed. For example, glucose-responsive hydrogels containing phenylboronic acid or concanavalin A (ConA) are able to bind glucose and swell to release entrapped insulin [157]. These moieties generate a closed-feedback loop that senses and responds to the demands of the environment. While these systems are appealing, such biological interactions are not always easily found and incorporated into drug delivery systems. To this end, antibody- and aptamer-containing hydrogels have been engineered to respond to a multitude of biological stimuli [158, 159].
5.5 Molecularly imprinted polymers
Additionally, molecularly imprinted polymers (MIPs) have recently gained much attention as materials that can be designed to respond to virtually any substrate of interest [160]. MIPs are prepared by allowing a template molecule to form either covalent or non-covalent interactions with a monomer of interest, and then crosslinking the monomers to fix the interaction in place. The template molecule is then removed to leave a cavity that is specific for the desired target or a structurally similar molecule. Due to this increased affinity, these systems have been shown to have a higher drug loading capacity and slower drug release compared to non-imprinted matrices. External stimuli can be used to disrupt the binding interaction between the cavity and the drug to initiate drug release. While these systems have not been widely investigated for oral drug delivery, their use in ocular drug delivery devices is promising [144].
5.6 Multi-stimuli-responsive polymers
One major obstacle to oral drug delivery is the large variation in gastric retention time and GI pH between patients. In order to address this, numerous materials have been developed to exhibit responsiveness to two or more stimuli, enabling tighter control over delivery of a drug payload [145]. Most relevant to oral drug delivery are pH- and enzyme-sensitive systems. He et al. developed poly(ester amide) microspheres for oral insulin delivery using a dual-controlled system. The polymeric microspheres collapse at gastric pH levels, protecting insulin from degradation in the harsh conditions of the upper GI tract. Upon reaching the small intestine, the pH significantly increases, allowing the microspheres to swell and begin releasing insulin. Additionally, the poly(ester amide) microspheres can be degraded by elastases and chymotrypsin found in the small intestine, facilitating drug release and particle degradation [161]. Similarly, Popat et al. developed mesoporous silica nanoparticles that are coated with a pH- and pancreatin-responsive polymer shell. As it reaches the small intestine, the polymer shell expands and slowly releases a prodrug containing an azoaromatic linkage, which is selectively activated in the colon [152].
5.7 Incorporating “smart” polymers into micro- and nanofabricated oral drug delivery systems
While many of these materials are promising for oral drug delivery, relatively few have been used in combination with micro- and nanofabrication technologies to combine the chemical and biological responsiveness with micro/nanotopography and other forms of geometric engineering. By incorporating these “smart” materials into these microscale devices, it may be possible to tailor the extent and specificity of drug loading and drug release for oral drug administration. The most facile method for achieving this is to use a matrix system where drugs of interest are entrapped in these materials and then tune the release rates at the relevant physiological conditions. Additionally, it is possible to use a reservoir system where these polymers can be used as a cap that will either control diffusion for the lifetime of the device or block drug release until it is dissolved in the presence of low pH or gastrointestinal enzymes. This ability to design microdevices on a molecular scale as well as on the micro- and nanoscale enables precise control over where and how drugs are delivered to the GI tract, suggesting the great potential of microdevices as oral drug delivery platforms.
6. Epithelial permeation enhancement
6.1 Advantages and risks of epithelial permeation enhancement
While polymer chemistry allows us to tailor drug release kinetics with significant control, there remain barriers to drug uptake once the drug leaves the microdevices, such as the existence of a thick mucus layer and the presence of tight junction proteins between epithelial cells. In order to address the physiological barriers posed by the intestinal epithelial layer, chemical and nanotopographical permeation enhancers have been explored in the field of drug delivery. Permeation enhancers temporarily disturb epithelial tightness, allowing greater transepithelial transport of therapeutics. With proper use, permeation enhancers can greatly increase bioavailability of orally administered drugs and allow more efficient delivery of therapeutics. However, since a normal epithelial layer serves as a natural barrier to harmful foreign agents, disruption of the membrane may have potentially dangerous side effects. Therefore, it is important to ensure that the effects of permeation enhancers are reversible and do not cause any permanent cellular damage.
6.2 Mechanisms of permeation enhancement
While many different mechanisms may be utilized to achieve increased permeation, one of the most common modes by which permeation enhancers function is through modulation of tight junctions. Tight junctional complexes greatly inhibit paracellular drug permeation, lowering bioavailability, and often serve as the rate-limiting barrier to various hydrophilic, large therapeutic agents. Another common mode of permeation enhancement includes modulation of transcellular drug transport by disruption of the cell membrane using excipients, such as fatty acids. In addition, some studies have utilized efflux inhibitors in order to reduce efflux of therapeutics by membrane transporters, such as p-glycoprotein [162–164]. We refer to the reviews by Aungst [165, 166] for more detailed information regarding various types of permeation enhancers that have been used as excipients in clinical trials as well as their mechanism of action. In this review, we focus on the incorporation of chemical enhancers as well as permeation enhancing topography into micro/nanofabricated oral drug delivery systems.
6.3 Co-delivery of chemical permeation enhancers
Drug release profiles of co-delivered drugs from micro/nanofabricated drug delivery systems can be individually tailored to facilitate optimal chemical permeation enhancement. For example, as mentioned earlier, work by Chirra et al. enabled co-delivery of several therapeutics by loading them into multi-reservoir bioadhesive microdevices [54]. Using this approach, permeation enhancers described above, such as efflux inhibitors, can be co-delivered with the therapeutic drug of interest in order to provide localized permeation enhancement at the site of delivery. This localized co-delivery of permeation enhancer may potentially reduce side effects associated with universal enhancement of transepithelial permeation along the intestinal lining. In addition, since the release of drugs from each reservoir is independent of each other, this technology allows the therapeutic to be delivered at a different rate than the permeation enhancer, potentially providing epithelial disruption prior to the release of the bulk of the therapeutic drug. This approach can also be used with the design of multilayered polymeric microdevice by Ainslie et al. [56]. Their study showed that their microdevices can provide simultaneous, unidirectional release of multiple therapeutics as well as achieve a significant increase in transepithelial permeation. By adding a layer that simultaneously releases a permeation enhancer along with the therapeutic of interest, localized permeation enhancement and consequent reduction in side effects may be possible.
6.4 Topographical permeation enhancement
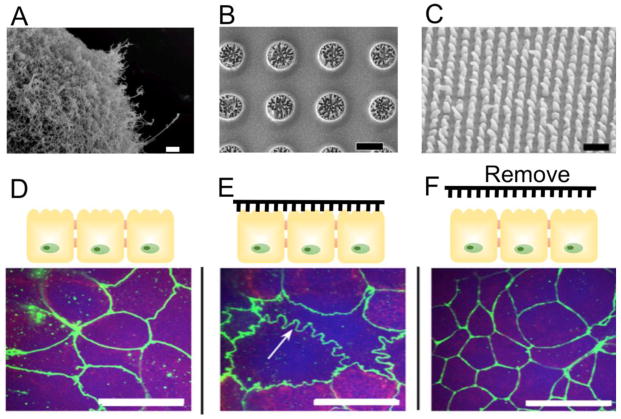
Similarly to a subset of chemical permeation enhancers that rely on perturbation of tight junctional proteins, micro- and nanotopography have been shown to achieve tight junctional reorganization, which resulted in enhanced transepithelial permeability in cell culture models [70, 71, 167]. For instance, studies by Uskokovic et al showed that silica microparticles covered with PEGylated silicon nanowires (Figure 6 A) are able to increase transport of fluorescein-Na across a layer of Caco-2 cells compared to bare particles. Moreover, the nanowire-coated particles were able to decrease transepithelial electrical resistance (TEER), which indicates epithelial tightness, to a greater extent than bare particles when tested on Caco-2 cells [70]. This platform has further advanced to the form of planar nanowired microdevices that are made with biodegradable polymers for enhanced biocompatibility in oral drug delivery applications (Figure 6 B) [74].
Figure 6.
A–C. Scanning electron microscope (SEM) images of (A) nanowire-covered silica microparticles [71], (B) planar nanowired microdevices [74], and (C) nanostructured polypropylene film [167]. D–F. Immunohistochemical staining (green) of zonula occluden (ZO-1) in Caco-2 cells (D) before nanostructure contact, (E) after 2 hours of nanostructure contact and (F) 24 hours after removal of nanostructured film [167]. Upon contact with nanostructured film, a ruffling pattern of ZO-1 staining, which indicates loosening of epithelial barrier, is observed. ZO-1 morphology reverts to a similar morphology observed in (D) upon removal of nanostructure contact, which indicates reversibility of the permeation enhancement. Scale bars are (A) 2 μm, (C) 3 μm, and (B, D–F) 20 μm. Figures reproduced with permission.
Additional studies have focused on the role of nanostructured polymeric films in increasing transepithelial transport [167]. Unlike the nanoengineered microparticles described above, this study focused on the effects of nanotopography in the form of nanostructured thin films that were relatively large (>2 mm in dimension) compared to the microparticles. Nanostructured polypropylene thin films (Figure 6 C) were fabricated using nanoimprint lithography and placed on top of Caco-2 cells that were cultured in transwell inserts. In line with work by Uskokovic et al [70], this study showed a significant decrease in TEER with nanostructure contact, which was reversible upon removal of the film. As mentioned earlier, the transient nature of TEER reduction upon nanostructure contact is noteworthy, as it is important to ensure lack of long-term disruption of the epithelial layer. The reversibility of tight junction modulation was also evident in immunohistochemical staining images (Figure 6 D–F), which show ruffled ZO-1 pattern upon nanostructure contact (Figure 6 E), which was reversible upon removal of the film (Figure 6 F). Moreover, the study noted a significant increase in transport of large molecules, such as etanercept (MW = 150 kDa), across a Caco-2 layer upon contact with nanostructured films. While its underlying mechanism has yet been fully elucidated, the notable increase in transport is thought to be due to active modulation of tight junctional complexes by formation of focal adhesion complexes at the site of nanostructure-epithelial cell contact [167, 168].
While nanotopography-mediated modulation of tight junctional complexes introduced an interesting new mode of permeation enhancement, methods that utilize physical penetration of the epithelial membrane using micro- or nanoneedles have also shown great promise. While microneedles have been more widely explored in drug delivery across the skin, the idea of physically puncturing GI tissue using microneedle pills have been recently presented in the field [68]. This proof-of-concept study showed safety and feasibility in evaluation of the prototype in vivo by delivering insulin through the oral route.
In addition to microneedles, nanoneedles have recently gained considerable interest in the field for their ability to achieve highly efficient drug delivery to living cells [169–176]. While free-floating nanotubes, such as single-walled carbon nanotubes, can be utilized as delivery vehicles in suspension [172, 173], more recent studies have focused on using vertical nanowire arrays for direct intracellular access. The nanoneedle platform involves vertical nanowires on a flat surface that puncture the cell membrane in order to deliver therapeutics of interest directly to the cell cytosol [170]. Various types of cells have been cultured on top of nanowired platforms to gain better access to the cell interior without significantly affecting their viability [171, 176]. The nanoneedle platform has expanded into various designs in the last decade, including a nanotemplated fluidic platform that allows hollow nanostraws to pierce the cell membrane and provide continuous intracellular access [169] and a nanowire-cell sandwich assay that allows enzymatic probing [176]. While the vertical nanoneedle approach has not been actively explored for direct cellular access in oral drug delivery applications, this direct mode of delivery may provide additional advantages over permeation enhancers that aim to increase transcellular drug transport. For instance, for drugs that require entrance into cells that are present along the digestive tract, microparticles covered with vertical nanowires may be able to achieve localized membrane penetration at the target site, allowing efficient intracellular drug delivery and reducing unnecessary drug distribution in the non-target sites.
7. Conclusion
The GI tract presents a complex set of physiological barriers that limit drug uptake. Micro- and nanotechnology provide flexibility in microdevice design, allowing for fabrication of drug delivery platforms that specifically address these barriers. The efficacy of micro/nanofabricated oral drug delivery systems may be enhanced by incorporating 1) tunable and/or responsive drug reservoir polymers for targeted release of intact drug, 2) adhesive polymers, surface modifications, and topographies to enhance adhesion, and 3) chemical and topographical permeation enhancers to increase drug permeability. With recent success in vivo, these technologies show promise for clinical trials. However, many of the top-down approaches used to fabricate these platforms for proof of concept are low-throughput and expensive relative to bottom-up fabrication techniques. To scale these technologies to the clinic, efficient, low-cost fabrication and drug loading approaches are being developed. Furthermore, to maximize cost-efficiency, these platforms may be used with highly potent drugs to minimize the number of devices required per dosage. As micro- and nanofabrication approaches continue to incorporate new technologies, future micro/nanofabricated oral drug delivery systems may combine smart materials, bioadhesive functionalization, nanotopography, planar shape, asymmetric design, and/or motion-based responses to address the many barriers to oral drug uptake in a manner not possible with conventional technologies.
Acknowledgments
This work was partially funded by the National Institutes of Health. CBF was supported by NIH Training Grant 5T32GM007175-37 and an ARCS Fellowship. JK and LVL were supported by NIH Training Grant T32GM008155-29. CLN was supported by an NSF Graduate Research Fellowship. HDC was supported by the Z-Cube Zambon Research Venture.
Abbreviations
- BCS
Biopharmaceutics Classification System
- ConA
concanavalin A
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- MEMS
microelectromechanical systems
- MIMIC
micromolding in capillaries
- MIPs
molecularly imprinted polymers
- NEMPs
nanoengineered microparticles
- PCL
polycaprolactone
- PDMS
polydimethylsiloxane
- PEDOT
poly(3,4-ethylenedioxythiophene)
- PEG
poly(ethylene glycol)
- PEGDMA
poly(ethylene glycol) dimethacrylate
- PEGMA
poly(ethylene glycol) methacrylate
- PHEMA
poly(hydroxyethyl methacrylate)
- PLGA
poly (lactic-co-glycolic acid)
- PMAA
poly(methacrylic acid)
- PMMA
poly(methyl methacrylate)
- PVP
poly(vinyl pyrolidone)
- TEER
transepithelial electrical resistance
Footnotes
Conflicts of interests
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moeller EH, Jorgensen L. Alternative routes of administration for systemic delivery of protein pharmaceuticals. Drug Discov Today Technol. 2008;5:e89–94. doi: 10.1016/j.ddtec.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Garrido-Siles M, Arenas-Villafranca JJ, Perez-Ruiz E, de Linares Fernandez MF, Tortajada B, Rivas-Ruiz F, Faus V, Rueda A. New cutaneous toxicities with generic docetaxel: are the excipients guilty? Support Care Cancer. 2015;23:1917–1923. doi: 10.1007/s00520-014-2499-2. [DOI] [PubMed] [Google Scholar]
- 3.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 4.Thiel G, Hermle M, Brunner FP. Acutely impaired renal function during intravenous administration of cyclosporine A: a cremophore side-effect. Clin Nephrol. 1986;25(Suppl 1):S40–42. [PubMed] [Google Scholar]
- 5.Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine. 2015 doi: 10.1016/j.nano.2015.02.018. [DOI] [PubMed]
- 6.Chirra HD, Desai TA. Emerging microtechnologies for the development of oral drug delivery devices. Adv Drug Deliv Rev. 2012;64:1569–1578. doi: 10.1016/j.addr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keely S, Ryan SM, Haddleton DM, Limer A, Mantovani G, Murphy EP, Colgan SP, Brayden DJ. Dexamethasone-pDMAEMA polymeric conjugates reduce inflammatory biomarkers in human intestinal epithelial monolayers. J Control Release. 2009;135:35–43. doi: 10.1016/j.jconrel.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stegemann S, Leveiller F, Franchi D, de Jong H, Linden H. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci. 2007;31:249–261. doi: 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Zhao L. Developing early formulations: practice and perspective. Int J Pharm. 2007;341:1–19. doi: 10.1016/j.ijpharm.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal U, Sharma R, Gupta M, Vyas SP. Is nanotechnology a boon for oral drug delivery? Drug Discov Today. 2014;19:1530–1546. doi: 10.1016/j.drudis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz UI, Gramatte T, Krappweis J, Oertel R, Kirch W. P-glycoprotein inhibitor erythromycin increases oral bioavailability of talinolol in humans. Int J Clin Pharmacol Ther. 2000;38:161–167. doi: 10.5414/cpp38161. [DOI] [PubMed] [Google Scholar]
- 12.Ponchel G, Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34:191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 13.Pawar VK, Meher JG, Singh Y, Chaurasia M, Surendar Reddy B, Chourasia MK. Targeting of gastrointestinal tract for amended delivery of protein/peptide therapeutics: strategies and industrial perspectives. J Control Release. 2014;196:168–183. doi: 10.1016/j.jconrel.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Lehr CM. Lectin-mediated drug delivery: the second generation of bioadhesives. J Control Release. 2000;65:19–29. doi: 10.1016/s0168-3659(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 15.Hunter AC, Elsom J, Wibroe PP, Moghimi SM. Polymeric particulate technologies for oral drug delivery and targeting: a pathophysiological perspective. Nanomedicine. 2012;8(Suppl 1):S5–20. doi: 10.1016/j.nano.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Hassan N, Ahad A, Ali M, Ali J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin Drug Deliv. 2010;7:97–112. doi: 10.1517/17425240903338758. [DOI] [PubMed] [Google Scholar]
- 17.Felton LA, Porter SC. An update on pharmaceutical film coating for drug delivery. Expert Opin Drug Deliv. 2013;10:421–435. doi: 10.1517/17425247.2013.763792. [DOI] [PubMed] [Google Scholar]
- 18.Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99:1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernkop-Schnurch A, Kast CE, Guggi D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems. J Control Release. 2003;93:95–103. doi: 10.1016/j.jconrel.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Ainslie KM, Desai TA. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip. 2008;8:1864–1878. doi: 10.1039/b806446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed A, Bonner C, Desai TA. Bioadhesive microdevices with multiple reservoirs: a new platform for oral drug delivery. J Control Release. 2002;81:291–306. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 22.Siegel RA, Gu Y, Lei M, Baldi A, Nuxoll EE, Ziaie B. Hard and soft micro- and nanofabrication: An integrated approach to hydrogel-based biosensing and drug delivery. J Control Release. 2010;141:303–313. doi: 10.1016/j.jconrel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sant S, Tao SL, Fisher OZ, Xu Q, Peppas NA, Khademhosseini A. Microfabrication technologies for oral drug delivery. Adv Drug Deliv Rev. 2012;64:496–507. doi: 10.1016/j.addr.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuxoll E. BioMEMS in drug delivery. Adv Drug Deliv Rev. 2013;65:1611–1625. doi: 10.1016/j.addr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen NT, Shaegh SA, Kashaninejad N, Phan DT. Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv Drug Deliv Rev. 2013;65:1403–1419. doi: 10.1016/j.addr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Chinna Reddy P, Chaitanya KS, Madhusudan Rao Y. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. Daru. 2011;19:385–403. [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: the next generation. J Pharm Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Rathbone MJ, Tucker IG. Mechanisms, Barriers and Pathways of Oral Mucosal Drug Permeation. Advanced Drug Delivery Reviews. 1993;12:41–60. [Google Scholar]
- 29.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Batchelor H. Bioadhesive dosage forms for esophageal drug delivery. Pharm Res. 2005;22:175–181. doi: 10.1007/s11095-004-1183-5. [DOI] [PubMed] [Google Scholar]
- 32.Russell CO, Hill LD, Holmes ER, 3rd, Hull DA, Gannon R, Pope CE., 2nd Radionuclide transit: a sensitive screening test for esophageal dysfunction. Gastroenterology. 1981;80:887–892. [PubMed] [Google Scholar]
- 33.Zhang L, Russell D, Conway BR, Batchelor H. Strategies and therapeutic opportunities for the delivery of drugs to the esophagus. Crit Rev Ther Drug Carrier Syst. 2008;25:259–304. doi: 10.1615/critrevtherdrugcarriersyst.v25.i3.20. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Lai TC, Kwon GS, Sako K. pH- and ion-sensitive polymers for drug delivery. Expert Opin Drug Deliv. 2013;10:1497–1513. doi: 10.1517/17425247.2013.821978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68–77. doi: 10.1097/00054725-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Garg T, Kumar A, Rath G, Goyal AK. Gastroretentive Drug Delivery Systems for Therapeutic Management of Peptic Ulcer. Critical Reviews in Therapeutic Drug Carrier Systems. 2014;31:531–557. doi: 10.1615/critrevtherdrugcarriersyst.2014011104. [DOI] [PubMed] [Google Scholar]
- 38.Prajapati VD, Jani GK, Khutliwala TA, Zala BS. Raft forming system-an upcoming approach of gastroretentive drug delivery system. J Control Release. 2013;168:151–165. doi: 10.1016/j.jconrel.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46:183–196. [PubMed] [Google Scholar]
- 40.Lim YF, Williams MA, Lentle RG, Janssen PW, Mansel BW, Keen SA, Chambers P. An exploration of the microrheological environment around the distal ileal villi and proximal colonic mucosa of the possum (Trichosurus vulpecula) J R Soc Interface. 2013;10:20121008. doi: 10.1098/rsif.2012.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappenheimer JR, Michel CC. Role of villus microcirculation in intestinal absorption of glucose: coupling of epithelial with endothelial transport. J Physiol. 2003;553:561–574. doi: 10.1113/jphysiol.2003.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown AL., Jr Microvilli of the human jejunal epithelial cell. J Cell Biol. 1962;12:623–627. doi: 10.1083/jcb.12.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 44.Mei L, Zhang Z, Zhao L, Huang L, Yang XL, Tang J, Feng SS. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Deliv Rev. 2013;65:880–890. doi: 10.1016/j.addr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Philip AK, Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J. 2010;25:79–87. doi: 10.5001/omj.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11:1117–1132. doi: 10.1016/j.nano.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Chu JS, Fix JA. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm. 2002;235:1–15. doi: 10.1016/s0378-5173(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 49.He H, Guan J, Lee JL. An oral delivery device based on self-folding hydrogels. J Control Release. 2006;110:339–346. doi: 10.1016/j.jconrel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Gao W, Dong R, Thamphiwatana S, Li J, Gao W, Zhang L, Wang J. Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano. 2015;9:117–123. doi: 10.1021/nn507097k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao SL, Lubeley MW, Desai TA. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. J Control Release. 2003;88:215–228. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 52.Tao SL, Desai TA. Micromachined devices: the impact of controlled geometry from cell-targeting to bioavailability. J Control Release. 2005;109:127–138. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Chirra HD, Shao L, Ciaccio N, Fox CB, Wade JM, Ma A, Desai TA. Planar microdevices for enhanced in vivo retention and oral bioavailability of poorly permeable drugs. Adv Healthc Mater. 2014;3:1648–1654. doi: 10.1002/adhm.201300676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chirra HD, Desai TA. Multi-reservoir bioadhesive microdevices for independent rate-controlled delivery of multiple drugs. Small. 2012;8:3839–3846. doi: 10.1002/smll.201201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ainslie KM, Lowe RD, Beaudette TT, Petty L, Bachelder EM, Desai TA. Microfabricated devices for enhanced bioadhesive drug delivery: attachment to and small-molecule release through a cell monolayer under flow. Small. 2009;5:2857–2863. doi: 10.1002/smll.200901254. [DOI] [PubMed] [Google Scholar]
- 56.Ainslie KM, Kraning CM, Desai TA. Microfabrication of an asymmetric, multi-layered microdevice for controlled release of orally delivered therapeutics. Lab Chip. 2008;8:1042–1047. doi: 10.1039/b800604k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer KE, Nagaraj G, Hugh Daniels R, Li E, Cowles VE, Miller JL, Bunger MD, Desai TA. Hierarchical nanoengineered surfaces for enhanced cytoadhesion and drug delivery. Biomaterials. 2011;32:3499–3506. doi: 10.1016/j.biomaterials.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan J, He H, Lee LJ, Hansford DJ. Fabrication of particulate reservoir-containing, capsulelike, and self-folding polymer microstructures for drug delivery. Small. 2007;3:412–418. doi: 10.1002/smll.200600240. [DOI] [PubMed] [Google Scholar]
- 59.Wood KM, Stone GM, Peppas NA. The effect of complexation hydrogels on insulin transport in intestinal epithelial cell models. Acta Biomater. 2010;6:48–56. doi: 10.1016/j.actbio.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood KM, Stone GM, Peppas NA. Wheat germ agglutinin functionalized complexation hydrogels for oral insulin delivery. Biomacromolecules. 2008;9:1293–1298. doi: 10.1021/bm701274p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood KM, Stone G, Peppas NA. Lectin functionalized complexation hydrogels for oral protein delivery. J Control Release. 2006;116:e66–68. doi: 10.1016/j.jconrel.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 62.Carreno-Gomez B, Woodley JF, Florence AT. Studies on the uptake of tomato lectin nanoparticles in everted gut sacs. Int J Pharm. 1999;183:7–11. doi: 10.1016/s0378-5173(99)00050-2. [DOI] [PubMed] [Google Scholar]
- 63.Fox CB, Chirra HD, Desai TA. Planar bioadhesive microdevices: a new technology for oral drug delivery. Curr Pharm Biotechnol. 2014;15:673–683. doi: 10.2174/1389201015666140915152706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pennisi E. Biomechanics. Geckos climb by the hairs of their toes. Science. 2000;288:1717–1718. doi: 10.1126/science.288.5472.1717a. [DOI] [PubMed] [Google Scholar]
- 65.Crosby AJ, Hageman M, Duncan A. Controlling polymer adhesion with “pancakes”. Langmuir. 2005;21:11738–11743. doi: 10.1021/la051721k. [DOI] [PubMed] [Google Scholar]
- 66.Autumn K, Sitti M, Liang YA, Peattie AM, Hansen WR, Sponberg S, Kenny TW, Fearing R, Israelachvili JN, Full RJ. Evidence for van der Waals adhesion in gecko setae. Proc Natl Acad Sci U S A. 2002;99:12252–12256. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Autumn K, Liang YA, Hsieh ST, Zesch W, Chan WP, Kenny TW, Fearing R, Full RJ. Adhesive force of a single gecko foot-hair. Nature. 2000;405:681–685. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- 68.Traverso G, Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Polat BE, Anderson DG, Blankschtein D, Langer R. Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci. 2015;104:362–367. doi: 10.1002/jps.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tao SL, Desai TA. Microfabrication of multilayer, asymmetric, polymeric devices for drug delivery. Advanced Materials. 2005;17:1625. [Google Scholar]
- 70.Uskokovic V, Lee PP, Walsh LA, Fischer KE, Desai TA. PEGylated silicon nanowire coated silica microparticles for drug delivery across intestinal epithelium. Biomaterials. 2012;33:1663–1672. doi: 10.1016/j.biomaterials.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uskokovic V, Lee K, Lee PP, Fischer KE, Desai TA. Shape effect in the design of nanowire-coated microparticles as transepithelial drug delivery devices. ACS Nano. 2012;6:7832–7841. doi: 10.1021/nn3019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer KE, Jayagopal A, Nagaraj G, Daniels RH, Li EM, Silvestrini MT, Desai TA. Nanoengineered surfaces enhance drug loading and adhesion. Nano Lett. 2011;11:1076–1081. doi: 10.1021/nl103951e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fischer KE, Aleman BJ, Tao SL, Hugh Daniels R, Li EM, Bunger MD, Nagaraj G, Singh P, Zettl A, Desai TA. Biomimetic nanowire coatings for next generation adhesive drug delivery systems. Nano Lett. 2009;9:716–720. doi: 10.1021/nl803219f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox CB, Kim J, Schlesinger EB, Chirra HD, Desai TA. Fabrication of micropatterned polymeric nanowire arrays for high-resolution reagent localization and topographical cellular control. Nano Lett. 2015;15:1540–1546. doi: 10.1021/nl503872p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimko DA, Nauman EA. Development and characterization of a porous poly(methyl methacrylate) scaffold with controllable modulus and permeability. J Biomed Mater Res B Appl Biomater. 2007;80:360–369. doi: 10.1002/jbm.b.30605. [DOI] [PubMed] [Google Scholar]
- 76.Rohner D, Hutmacher DW, Cheng TK, Oberholzer M, Hammer B. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J Biomed Mater Res B Appl Biomater. 2003;66:574–580. doi: 10.1002/jbm.b.10037. [DOI] [PubMed] [Google Scholar]
- 77.Patil SB, Kaul A, Babbar A, Mathur R, Mishra A, Sawant KK. In vivo evaluation of alginate microspheres of carvedilol for nasal delivery. J Biomed Mater Res B Appl Biomater. 2012;100:249–255. doi: 10.1002/jbm.b.31947. [DOI] [PubMed] [Google Scholar]
- 78.Pal D, Nayak AK. Novel tamarind seed polysaccharide-alginate mucoadhesive microspheres for oral gliclazide delivery: in vitro-in vivo evaluation. Drug Deliv. 2012;19:123–131. doi: 10.3109/10717544.2012.657717. [DOI] [PubMed] [Google Scholar]
- 79.Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52:117–126. doi: 10.1016/s0169-409x(01)00231-9. [DOI] [PubMed] [Google Scholar]
- 80.Sogias IA, Williams AC, Khutoryanskiy VV. Why is chitosan mucoadhesive? Biomacromolecules. 2008;9:1837–1842. doi: 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- 81.Chen MC, Mi FL, Liao ZX, Hsiao CW, Sonaje K, Chung MF, Hsu LW, Sung HW. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliv Rev. 2013;65:865–879. doi: 10.1016/j.addr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 82.Uccello-Barretta G, Balzano F, Vanni L, Sanso M. Mucoadhesive properties of tamarind-seed polysaccharide/hyaluronic acid mixtures: A nuclear magnetic resonance spectroscopy investigation. Carbohydr Polym. 2013;91:568–572. doi: 10.1016/j.carbpol.2012.07.085. [DOI] [PubMed] [Google Scholar]
- 83.Sandri G, Rossi S, Ferrari F, Bonferoni MC, Zerrouk N, Caramella C. Mucoadhesive and penetration enhancement properties of three grades of hyaluronic acid using porcine buccal and vaginal tissue, Caco-2 cell lines, and rat jejunum. J Pharm Pharmacol. 2004;56:1083–1090. doi: 10.1211/0022357044085. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Tauchi Y, Deguchi Y, Morimoto K, Tabata Y, Ikada Y. Positively charged gelatin microspheres as gastric mucoadhesive drug delivery system for eradication of H. pylori. Drug Deliv. 2000;7:237–243. doi: 10.1080/107175400455173. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Tabata Y, Bi D, Morimoto K. Evaluation of gastric mucoadhesive properties of aminated gelatin microspheres. J Control Release. 2001;73:223–231. doi: 10.1016/s0168-3659(01)00288-7. [DOI] [PubMed] [Google Scholar]
- 86.Rahmat D, Muller C, Barthelmes J, Shahnaz G, Martien R, Bernkop-Schnurch A. Thiolated hydroxyethyl cellulose: design and in vitro evaluation of mucoadhesive and permeation enhancing nanoparticles. Eur J Pharm Biopharm. 2013;83:149–155. doi: 10.1016/j.ejpb.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Mazoniene E, Joceviciute S, Kazlauske J, Niemeyer B, Liesiene J. Interaction of cellulose-based cationic polyelectrolytes with mucin. Colloids Surf B Biointerfaces. 2011;83:160–164. doi: 10.1016/j.colsurfb.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 88.Sosnik A, das Neves J, Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Progress in Polymer Science. 2014;39:2030–2075. [Google Scholar]
- 89.Shrestha N, Shahbazi MA, Araujo F, Zhang H, Makila EM, Kauppila J, Sarmento B, Salonen JJ, Hirvonen JT, Santos HA. Chitosan-modified porous silicon microparticles for enhanced permeability of insulin across intestinal cell monolayers. Biomaterials. 2014;35:7172–7179. doi: 10.1016/j.biomaterials.2014.04.104. [DOI] [PubMed] [Google Scholar]
- 90.Sajeesh S, Bouchemal K, Marsaud V, Vauthier C, Sharma CP. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: An oral delivery system for insulin. J Control Release. 2010;147:377–384. doi: 10.1016/j.jconrel.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 91.Liao ZX, Chuang EY, Hsiao CW, Sung HW. 21. pH-sensitive chitosan-based nanoparticles for protein drug delivery: oral approaches: Original research article: a novel pH-sensitive hydrogel composed of carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery, 2004. J Control Release. 2014;190:68–70. [PubMed] [Google Scholar]
- 92.Gradauer K, Barthelmes J, Vonach C, Almer G, Mangge H, Teubl B, Roblegg E, Dunnhaupt S, Frohlich E, Bernkop-Schnurch A, Prassl R. Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J Control Release. 2013;172:872–878. doi: 10.1016/j.jconrel.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gadalla HH, Soliman GM, Mohammed FA, El-Sayed AM. Development and in vitro/in vivo evaluation of Zn-pectinate microparticles reinforced with chitosan for the colonic delivery of progesterone. Drug Deliv. 2015:1–14. doi: 10.3109/10717544.2015.1028602. [DOI] [PubMed] [Google Scholar]
- 94.Fan B, Xing Y, Zheng Y, Sun C, Liang G. pH-responsive thiolated chitosan nanoparticles for oral low-molecular weight heparin delivery: in vitro and in vivo evaluation. Drug Deliv. 2014:1–10. doi: 10.3109/10717544.2014.909908. [DOI] [PubMed] [Google Scholar]
- 95.Dunnhaupt S, Barthelmes J, Iqbal J, Perera G, Thurner CC, Friedl H, Bernkop-Schnurch A. In vivo evaluation of an oral drug delivery system for peptides based on S-protected thiolated chitosan. J Control Release. 2012;160:477–485. doi: 10.1016/j.jconrel.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 96.Ahn S, Lee IH, Lee E, Kim H, Kim YC, Jon S. Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J Control Release. 2013;170:226–232. doi: 10.1016/j.jconrel.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 97.Xu J, Strandman S, Zhu JX, Barralet J, Cerruti M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials. 2015;37:395–404. doi: 10.1016/j.biomaterials.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 98.Tang C, Guan YX, Yao SJ, Zhu ZQ. Preparation of ibuprofen-loaded chitosan films for oral mucosal drug delivery using supercritical solution impregnation. Int J Pharm. 2014;473:434–441. doi: 10.1016/j.ijpharm.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 99.Liang Y, Liu W, Han B, Yang C, Ma Q, Zhao W, Rong M, Li H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J Mater Sci Mater Med. 2011;22:175–183. doi: 10.1007/s10856-010-4190-6. [DOI] [PubMed] [Google Scholar]
- 100.Koev ST, Dykstra PH, Luo X, Rubloff GW, Bentley WE, Payne GF, Ghodssi R. Chitosan: an integrative biomaterial for lab-on-a-chip devices. Lab Chip. 2010;10:3026–3042. doi: 10.1039/c0lc00047g. [DOI] [PubMed] [Google Scholar]
- 101.Lopez-Leon T, Carvalho EL, Seijo B, Ortega-Vinuesa JL, Bastos-Gonzalez D. Physicochemical characterization of chitosan nanoparticles: electrokinetic and stability behavior. J Colloid Interface Sci. 2005;283:344–351. doi: 10.1016/j.jcis.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 102.Guan J, He H, Hansford DJ, Lee LJ. Self-folding of three-dimensional hydrogel microstructures. J Phys Chem B. 2005;109:23134–23137. doi: 10.1021/jp054341g. [DOI] [PubMed] [Google Scholar]
- 103.Zakharchenko S, Ionov L. Anisotropic Liquid Microcapsules from Biomimetic Self-Folding Polymer Films. ACS Appl Mater Interfaces. 2015 doi: 10.1021/am505755j. [DOI] [PubMed] [Google Scholar]
- 104.Stoychev G, Puretskiy N, Ionov L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter. 2011;7:3277–3279. [Google Scholar]
- 105.Bassik N, Abebe BT, Laflin KE, Gracias DH. Photolithographically patterned smart hydrogel based bilayer actuators. Polymer. 2010;51:6093–6098. [Google Scholar]
- 106.Wu Z, Wu Y, He W, Lin X, Sun J, He Q. Self-propelled polymer-based multilayer nanorockets for transportation and drug release. Angew Chem Int Ed Engl. 2013;52:7000–7003. doi: 10.1002/anie.201301643. [DOI] [PubMed] [Google Scholar]
- 107.Wilson DA, Nolte RJ, van Hest JC. Autonomous movement of platinum-loaded stomatocytes. Nat Chem. 2012;4:268–274. doi: 10.1038/nchem.1281. [DOI] [PubMed] [Google Scholar]
- 108.Paxton WF, Kistler KC, Olmeda CC, Sen A, St Angelo SK, Cao Y, Mallouk TE, Lammert PE, Crespi VH. Catalytic nanomotors: autonomous movement of striped nanorods. J Am Chem Soc. 2004;126:13424–13431. doi: 10.1021/ja047697z. [DOI] [PubMed] [Google Scholar]
- 109.Mirkovic T, Zacharia NS, Scholes GD, Ozin GA. Fuel for thought: chemically powered nanomotors out-swim nature’s flagellated bacteria. ACS Nano. 2010;4:1782–1789. doi: 10.1021/nn100669h. [DOI] [PubMed] [Google Scholar]
- 110.Guix M, Mayorga-Martinez CC, Merkoci A. Nano/micromotors in (bio)chemical science applications. Chem Rev. 2014;114:6285–6322. doi: 10.1021/cr400273r. [DOI] [PubMed] [Google Scholar]
- 111.Gao W, Uygun A, Wang J. Hydrogen-bubble-propelled zinc-based microrockets in strongly acidic media. J Am Chem Soc. 2012;134:897–900. doi: 10.1021/ja210874s. [DOI] [PubMed] [Google Scholar]
- 112.Grayson ACR, Shawgo RS, Johnson AM, Flynn NT, Li YW, Cima MJ, Langer R. A BioMEMS review: MEMS technology for physiologically integrated devices. Proceedings of the Ieee. 2004;92:6–21. [Google Scholar]
- 113.LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 114.Amer S, Badawy W. An integrated platform for bio-analysis and drug delivery. Curr Pharm Biotechnol. 2005;6:57–64. doi: 10.2174/1389201053167220. [DOI] [PubMed] [Google Scholar]
- 115.Ito Y, Hasuda H, Morimatsu M, Takagi N, Hirai Y. A microfabrication method of a biodegradable polymer chip for a controlled release system. J Biomater Sci Polym Ed. 2005;16:949–955. doi: 10.1163/1568562054414621. [DOI] [PubMed] [Google Scholar]
- 116.He H, Cao X, Lee LJ. Design of a novel hydrogel-based intelligent system for controlled drug release. J Control Release. 2004;95:391–402. doi: 10.1016/j.jconrel.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 117.Sharpe LA, Daily AM, Horava SD, Peppas NA. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin Drug Deliv. 2014;11:901–915. doi: 10.1517/17425247.2014.902047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koetting MC, Peppas NA. pH-Responsive poly(itaconic acid-co-N-vinylpyrrolidone) hydrogels with reduced ionic strength loading solutions offer improved oral delivery potential for high isoelectric point-exhibiting therapeutic proteins. Int J Pharm. 2014;471:83–91. doi: 10.1016/j.ijpharm.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knipe JM, Chen F, Peppas NA. Enzymatic biodegradation of hydrogels for protein delivery targeted to the small intestine. Biomacromolecules. 2015;16:962–972. doi: 10.1021/bm501871a. [DOI] [PubMed] [Google Scholar]
- 120.Duran-Lobato M, Carrillo-Conde B, Khairandish Y, Peppas NA. Surface-modified P(HEMA-co-MAA) nanogel carriers for oral vaccine delivery: design, characterization, and in vitro targeting evaluation. Biomacromolecules. 2014;15:2725–2734. doi: 10.1021/bm500588x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petersen RS, Keller SS, Boisen A. Hot punching of high-aspect-ratio 3D polymeric microstructures for drug delivery. Lab Chip. 2015;15:2576–2579. doi: 10.1039/c5lc00372e. [DOI] [PubMed] [Google Scholar]
- 122.Marizza P, Keller SS, Boisen A. Inkjet printing as a technique for filling of micro-wells with biocompatible polymers. Microelectronic Engineering. 2013;111:391–395. [Google Scholar]
- 123.Marizza P, Keller SS, Mullertz A, Boisen A. Polymer-filled microcontainers for oral delivery loaded using supercritical impregnation. J Control Release. 2014;173:1–9. doi: 10.1016/j.jconrel.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 124.Nielsen LH, Nagstrup J, Gordon S, Keller SS, Ostergaard J, Rades T, Mullertz A, Boisen A. pH-triggered drug release from biodegradable microwells for oral drug delivery. Biomed Microdevices. 2015;17:9958. doi: 10.1007/s10544-015-9958-5. [DOI] [PubMed] [Google Scholar]
- 125.Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23:561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 126.Bos GW, Scharenborg NM, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Proliferation of endothelial cells on surface-immobilized albumin-heparin conjugate loaded with basic fibroblast growth factor. J Biomed Mater Res. 1999;44:330–340. doi: 10.1002/(sici)1097-4636(19990305)44:3<330::aid-jbm12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 127.Blawas AS, Reichert WM. Protein patterning. Biomaterials. 1998;19:595–609. doi: 10.1016/s0142-9612(97)00218-4. [DOI] [PubMed] [Google Scholar]
- 128.Tan JL, Tien J, Chen CS. Microcontact printing of proteins on mixed self-assembled monolayers. Langmuir. 2002;18:519–523. [Google Scholar]
- 129.Kim E, Xia YN, Whitesides GM. Polymer Microstructures Formed by Molding in Capillaries. Nature. 1995;376:581–584. [Google Scholar]
- 130.Jo WJ, Baek SK, Park JH. A wireless actuating drug delivery system. Journal of Micromechanics and Microengineering. 2015;25 [Google Scholar]
- 131.Lee HP, Ryu W. Wet microcontact printing (microCP) for micro-reservoir drug delivery systems. Biofabrication. 2013;5:025011. doi: 10.1088/1758-5082/5/2/025011. [DOI] [PubMed] [Google Scholar]
- 132.Ahmed N, Dagdeviren C, Rogers JA, Ferreira PM. Active Polymeric Composite Membranes for Localized Actuation and Sensing in Microtransfer Printing. J Microelectromechanical Systems. 2014:1. [Google Scholar]
- 133.Richards Grayson AC, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. 2003;2:767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 134.Cui J, Zheng X, Hou W, Zhuang Y, Pi X, Yang J. The study of a remote-controlled gastrointestinal drug delivery and sampling system. Telemed J E Health. 2008;14:715–719. doi: 10.1089/tmj.2007.0118. [DOI] [PubMed] [Google Scholar]
- 135.Koziolek M, Grimm M, Becker D, Iordanov V, Zou H, Shimizu J, Wanke C, Garbacz G, Weitschies W. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap System. J Pharm Sci. 2014 doi: 10.1002/jps.24274. [DOI] [PubMed] [Google Scholar]
- 136.Strusi OL, Barata P, Traini D, Young PM, Mercuri S, Colombo G, Sonvico F, Bettini R, Colombo P. Artesunate-clindamycin multi-kinetics and site-specific oral delivery system for antimalaric combination products. J Control Release. 2010;146:54–60. doi: 10.1016/j.jconrel.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 137.Farra R, Sheppard NF, Jr, McCabe L, Neer RM, Anderson JM, Santini JT, Jr, Cima MJ, Langer R. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4:122ra121. doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]
- 138.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Jr, Maloney JM, Coppeta J, Yomtov B, Staples MA, Santini JT., Jr Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat Biotechnol. 2006;24:437–438. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 139.Maloney JM, Uhland SA, Polito BF, Sheppard NF, Jr, Pelta CM, Santini JT., Jr Electrothermally activated microchips for implantable drug delivery and biosensing. J Control Release. 2005;109:244–255. doi: 10.1016/j.jconrel.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Shawgo RS, Tyler B, Henderson PT, Vogel JS, Rosenberg A, Storm PB, Langer R, Brem H, Cima MJ. In vivo release from a drug delivery MEMS device. J Control Release. 2004;100:211–219. doi: 10.1016/j.jconrel.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 141.Li Y, Ho Duc HL, Tyler B, Williams T, Tupper M, Langer R, Brem H, Cima MJ. In vivo delivery of BCNU from a MEMS device to a tumor model. J Control Release. 2005;106:138–145. doi: 10.1016/j.jconrel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 142.Richards Grayson AC, Cima MJ, Langer R. Molecular release from a polymeric microreservoir device: Influence of chemistry, polymer swelling, and loading on device performance. J Biomed Mater Res A. 2004;69:502–512. doi: 10.1002/jbm.a.30019. [DOI] [PubMed] [Google Scholar]
- 143.Tiwari SB, Rajabi-Siahboomi AR. Extended-release oral drug delivery technologies: monolithic matrix systems. Methods Mol Biol. 2008;437:217–243. doi: 10.1007/978-1-59745-210-6_11. [DOI] [PubMed] [Google Scholar]
- 144.Tieppo A, White CJ, Paine AC, Voyles ML, McBride MK, Byrne ME. Sustained in vivo release from imprinted therapeutic contact lenses. J Control Release. 2012;157:391–397. doi: 10.1016/j.jconrel.2011.09.087. [DOI] [PubMed] [Google Scholar]
- 145.Schattling P, Jochum FD, Theato P. Multi-stimuli responsive polymers - the all-in-one talents. Polymer Chemistry. 2014;5:25–36. [Google Scholar]
- 146.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 147.Cabane E, Zhang X, Langowska K, Palivan CG, Meier W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases. 2012;7:9. doi: 10.1007/s13758-011-0009-3. [DOI] [PubMed] [Google Scholar]
- 148.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 149.Gao X, Cao Y, Song X, Zhang Z, Zhuang X, He C, Chen X. Biodegradable, pH-responsive carboxymethyl cellulose/poly(acrylic acid) hydrogels for oral insulin delivery. Macromol Biosci. 2014;14:565–575. doi: 10.1002/mabi.201300384. [DOI] [PubMed] [Google Scholar]
- 150.Foss AC, Goto T, Morishita M, Peppas NA. Development of acrylic-based copolymers for oral insulin delivery. Eur J Pharm Biopharm. 2004;57:163–169. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 151.Bai Y, Zhang Z, Zhang A, Chen L, He C, Zhuang X, Chen X. Novel thermo- and pH-responsive hydroxypropyl cellulose- and poly (L-glutamic acid)-based microgels for oral insulin controlled release. Carbohydr Polym. 2012;89:1207–1214. doi: 10.1016/j.carbpol.2012.03.095. [DOI] [PubMed] [Google Scholar]
- 152.Popat A, Jambhrunkar S, Zhang J, Yang J, Zhang HW, Meka A, Yu CZ. Programmable drug release using bioresponsive mesoporous silica nanoparticles for site-specific oral drug delivery. Chemical Communications. 2014;50:5547–5550. doi: 10.1039/c4cc00620h. [DOI] [PubMed] [Google Scholar]
- 153.Wong TW, Colombo G, Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech. 2011;12:201–214. doi: 10.1208/s12249-010-9564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vulic K, Shoichet MS. Affinity-based drug delivery systems for tissue repair and regeneration. Biomacromolecules. 2014;15:3867–3880. doi: 10.1021/bm501084u. [DOI] [PubMed] [Google Scholar]
- 155.Van den Mooter G, Maris B, Samyn C, Augustijns P, Kinget R. Use of azo polymers for colon-specific drug delivery. J Pharm Sci. 1997;86:1321–1327. doi: 10.1021/js9702630. [DOI] [PubMed] [Google Scholar]
- 156.Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;224:19–38. doi: 10.1016/s0378-5173(01)00720-7. [DOI] [PubMed] [Google Scholar]
- 157.Wu W, Zhou S. Responsive materials for self-regulated insulin delivery. Macromol Biosci. 2013;13:1464–1477. doi: 10.1002/mabi.201300120. [DOI] [PubMed] [Google Scholar]
- 158.Yang H, Liu H, Kang H, Tan W. Engineering target-responsive hydrogels based on aptamer-target interactions. J Am Chem Soc. 2008;130:6320–6321. doi: 10.1021/ja801339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Miyata T, Asami N, Uragami T. Preparation of an antigen-sensitive hydrogel using antigen-antibody bindings. Macromolecules. 1999;32:2082–2084. [Google Scholar]
- 160.Lulinski P. Molecularly imprinted polymers as the future drug delivery devices. Acta Pol Pharm. 2013;70:601–609. [PubMed] [Google Scholar]
- 161.He P, Tang Z, Lin L, Deng M, Pang X, Zhuang X, Chen X. Novel biodegradable and pH-sensitive poly(ester amide) microspheres for oral insulin delivery. Macromol Biosci. 2012;12:547–556. doi: 10.1002/mabi.201100358. [DOI] [PubMed] [Google Scholar]
- 162.Woo JS, Lee CH, Shim CK, Hwang SJ. Enhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031. Pharm Res. 2003;20:24–30. doi: 10.1023/a:1022286422439. [DOI] [PubMed] [Google Scholar]
- 163.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 164.Akhtar N, Ahad A, Khar RK, Jaggi M, Aqil M, Iqbal Z, Ahmad FJ, Talegaonkar S. The emerging role of P-glycoprotein inhibitors in drug delivery: a patent review. Expert Opin Ther Pat. 2011;21:561–576. doi: 10.1517/13543776.2011.561784. [DOI] [PubMed] [Google Scholar]
- 165.Aungst BJ. Absorption enhancers: applications and advances. AAPS J. 2012;14:10–18. doi: 10.1208/s12248-011-9307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aungst BJ. Intestinal permeation enhancers. J Pharm Sci. 2000;89:429–442. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 167.Kam KR, Walsh LA, Bock SM, Koval M, Fischer KE, Ross RF, Desai TA. Nanostructure-mediated transport of biologics across epithelial tissue: enhancing permeability via nanotopography. Nano Lett. 2013;13:164–171. doi: 10.1021/nl3037799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walsh L, Ryu J, Bock S, Koval M, Mauro T, Ross R, Desai T. Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism. Nano Lett. 2015;15:2434–2441. doi: 10.1021/nl504829f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.VanDersarl JJ, Xu AM, Melosh NA. Nanostraws for direct fluidic intracellular access. Nano Lett. 2012;12:3881–3886. doi: 10.1021/nl204051v. [DOI] [PubMed] [Google Scholar]
- 170.Shalek AK, Robinson JT, Karp ES, Lee JS, Ahn DR, Yoon MH, Sutton A, Jorgolli M, Gertner RS, Gujral TS, MacBeath G, Yang EG, Park H. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc Natl Acad Sci U S A. 2010;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shalek AK, Gaublomme JT, Wang L, Yosef N, Chevrier N, Andersen MS, Robinson JT, Pochet N, Neuberg D, Gertner RS, Amit I, Brown JR, Hacohen N, Regev A, Wu CJ, Park H. Nanowire-mediated delivery enables functional interrogation of primary immune cells: application to the analysis of chronic lymphocytic leukemia. Nano Lett. 2012;12:6498–6504. doi: 10.1021/nl3042917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Z, Winters M, Holodniy M, Dai H. siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew Chem Int Ed Engl. 2007;46:2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- 173.Cai D, Mataraza JM, Qin ZH, Huang Z, Huang J, Chiles TC, Carnahan D, Kempa K, Ren Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat Methods. 2005;2:449–454. doi: 10.1038/nmeth761. [DOI] [PubMed] [Google Scholar]
- 174.Xu AM, Aalipour A, Leal-Ortiz S, Mekhdjian AH, Xie X, Dunn AR, Garner CC, Melosh NA. Quantification of nanowire penetration into living cells. Nat Commun. 2014;5:3613. doi: 10.1038/ncomms4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xie X, Xu AM, Angle MR, Tayebi N, Verma P, Melosh NA. Mechanical model of vertical nanowire cell penetration. Nano Lett. 2013;13:6002–6008. doi: 10.1021/nl403201a. [DOI] [PubMed] [Google Scholar]
- 176.Na YR, Kim SY, Gaublomme JT, Shalek AK, Jorgolli M, Park H, Yang EG. Probing enzymatic activity inside living cells using a nanowire-cell “sandwich” assay. Nano Lett. 2013;13:153–158. doi: 10.1021/nl3037068. [DOI] [PMC free article] [PubMed] [Google Scholar]