Abstract
Objective
To determine the association between serum autoantibodies and survival in patients with incident systemic sclerosis (SSc)-pulmonary arterial hypertension (PAH) enrolled in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) Registry.
Methods
Patients with definite PAH diagnosed by right heart catheterization within 6 months of registry enrollment were studied. Serum autoantibodies were assayed at each participating institution’s clinical laboratory. Mortality data were collected from electronic medical records and/or the Social Security Death Index. Kaplan-Meier survival estimates were reported for 5 autoantibody groups (anti-centromere/AC, nucleolar ANA/NUC, anti-topoisomerase/Scl-70, overlapping or non-specific autoantibodies/other, and a combined group with similar survival consisting of RNA polymerase III, U1RNP, and autoantibody negative patients). Cox proportional hazards models permitted examination of the association between autoantibody groups and overall survival, controlling for age, sex, race, and SSc disease duration.
Results
162 subjects had PAH, and serum autoantibody and survival information. Sixty (37%) had AC, 39 (24%) NUC, 11 (7%) Scl-70, 28 (17%) had other, 9 (6%) RNA pol, 8 (5%) U1RNP autoantibodies, and 7 (4%) had negative antibodies. Thirty-two (20%) subjects died over a median follow-up time of 2.1 years (range 0.01-6.8). One- and three-year survival estimates were 94% and 78% for AC; 94% and 72% for NUC; 89% and 63% for Scl-70; 92% and 79% for other group; and 100% and 93% for the combined group. Unadjusted and adjusted hazard ratios revealed no statistically significant association between risk of death and autoantibodies.
Conclusion
Anti-centromere and NUC autoantibodies are prevalent in SSc-PAH patients. An association between serum autoantibodies and survival in patients with SSc-PAH was not identified in the PHAROS cohort.
Keywords: Mortality, Pulmonary hypertension, Pulmonary arterial hypertension, Risk factors, Systemic scleroderma, Serum autoantibody
Pulmonary arterial hypertension (PAH) is one of the most serious complications occurring in systemic sclerosis (SSc), with a cumulative incidence of 15% over 15 years of follow-up(1). Patients with SSc-PAH have worse outcomes than patients with idiopathic PAH and other connective tissue disease associated PAH(2). Anti-centromere and antinuclear antibodies (ANA) with a nucleolar pattern (anti-Th/To antibodies, anti-U3-ribonucleoprotein and anti-B23) have been associated with an increased risk for the development of PAH in SSc patients, but the mortality risk associated with specific serum autoantibodies in patients with definite SSc-PAH is unknown(3).
SSc-specific autoantibodies are directed against ubiquitously expressed antigens and yet are associated with unique clinical phenotypes including PAH. Studies suggest that the pathogenesis of SSc-associated complications such as PAH may involve a complex interplay between target tissue damage (to release and/or modify intercellular antigens) and autoimmune responses (antigen-specific cytotoxic T-lymphocyte expansion)(4). Numerous data have shown that SSc-specific autoantigens including CENPs B and C (centromere antigens) that are associated with SSc-PAH undergo structural changes during T-lymphocyte-mediated immune responses(4). We postulate that SSc patients who develop PAH incur pulmonary vasculature endothelial cell damage that leads to the presentation of centromere antigens to activated immune cells. Thus, we hypothesize that patients expressing specific serum autoantibodies, such as anticentromere antibodies, may demonstrate unique clinical features or experience higher mortality rates.
A revised pulmonary hypertension (PH) classification scheme was published in 2009(5). Patients with SSc are at increased risk for developing World Health Organization (WHO) Group 1 PAH, Group 2 pulmonary hypertension secondary to left-sided heart disease, and Group 3 pulmonary hypertension due to interstitial lung disease and/or hypoxemia(6). The multi-center, observational Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) patient registry was created in 2006 in order to prospectively follow SSc patients at high risk for developing or with newly diagnosed SSc-associated PH(6). Three-year survival of this cohort of patients was recently reported to be 75% which is better than other historical cohorts of SSc-PAH patients(7). Factors associated with poor survival in the PHAROS cohort include New York Heart Association (NYHA) Functional Class IV status at PAH diagnosis, male gender, diffusing capacity of carbon monoxide (DLCO)<39% predicted and age >60 years(8). The purpose of this study was to examine whether specific serum autoantibodies were associated with worse survival in patients enrolled in the PHAROS registry with right heart catheterization (RHC)-confirmed SSc-PAH. The ability to identify SSc-PAH patients at highest risk for death will help inform development of rational PAH-specific treatment protocols.
PATIENTS AND METHODS
The Institutional Review Board at each of the 22 participating US centers approved the PHAROS protocol and patients provided written informed consent prior to enrollment in the study. All subjects fulfilled American College of Rheumatology criteria for SSc or the LeRoy definitions of limited or diffuse cutaneous SSc(6). Only patients with incident WHO Group 1 PAH were included in the analysis. Specifically, patients had to have undergone a RHC within 6 months of registry enrollment that demonstrated an elevated mean pulmonary artery pressure (mPAP) ≥ 25mm Hg and a normal pulmonary capillary wedge pressure (PCWP) ≤ 15mm Hg. Subjects with significant pulmonary fibrosis were excluded.
Clinical features and laboratory assessments including autoantibody profiles were performed as previously described(6). High-resolution thoracic computed tomography (HRCT) scans, RHC, pulmonary function tests (PFTs), 2-dimensional echocardiograms with tissue Doppler, and 6-minute walk distance were performed at baseline and repeated annually or as clinically indicated as determined by the site investigator. Local electronic health records or the Social Security Death Index were queried to determine dates and causes of death. Each participating site reported subjects’ vital status immediately prior to these analyses.
Baseline characteristics were assessed by descriptive statistics. Continuous variables were summarized by mean ± standard deviation and compared using t-tests or non-parametric equivalent. Categorical variables were compared using the chi-squared statistic or Fisher’s exact test. A two-sided p-value < 0.05 was considered statistically significant. Kaplan-Meier survival curves were generated for patients in the seven autoantibody categories (anti-centromere/AC, ANA with an isolated nucleolar pattern/NUC, anti-topoisomerase I/Scl-70, other non specific positive ANAs or overlapping antibodies/Other, anti-RNA polymerase III/RNA pol, anti-U1RNP and negative ANA/negative). Because patients with RNA pol, U1RNP and negative antibodies had similar survival rates and small numbers, we collapsed them into one group termed the combined group. The time from the first RHC when PAH was diagnosed to the last follow-up visit or death was used. Cox proportional hazards models were used to evaluate the association between serum autoantibodies and overall survival. Univariate Cox models were used to examine associations between potential covariates and overall survival. A multivariable Cox model in which serum autoantibody was the main predictor was performed adjusting for age, sex, race, and SSc disease duration (defined as duration since first Raynaud symptom). Adjustments for forced vital capacity (FVC) % predicted and modified Rodnan skin score (mRSS), that may be part of the causal pathway, were not made. The proportionality assumption for these models was confirmed by testing for interactions with time for the covariates used in the model. Differences in Kaplan-Meier survival curves were assessed by the log rank test. SAS version 9.4 (Cary, NC) was used for all statistical analyses.
RESULTS
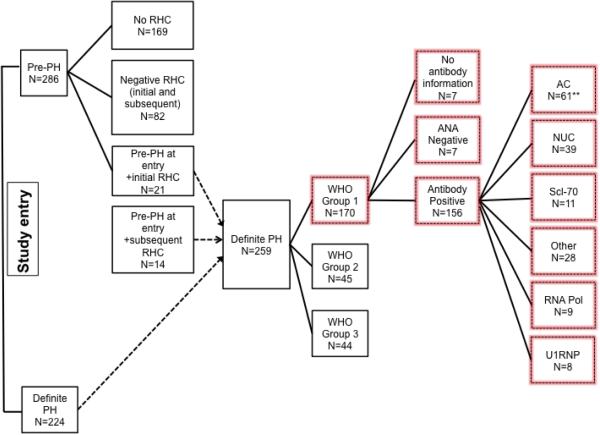
At the time of this analysis, 286 subjects were enrolled in the pre-PAH group (fulfilled one of three criteria at study entry: (1) DLCO < 55% predicted without severe interstitial lung disease (ILD); severe ILD was defined by FVC < 65% predicted and/or a thoracic HRCT scan that showed moderate to severe ILD according to the local radiologist; or (2) FVC %predicted/DLCO% predicted ratio ≥ 1.6; or (3) systolic pulmonary artery pressure (sPAP) > 40mm Hg obtained by Doppler echocardiography), and 224 subjects met criteria for definite PH (Figure 1). Thirty-five subjects in the pre-PAH group subsequently fulfilled PH criteria and were included in the definite PH category. One hundred seventy out of 259 PHAROS subjects with PH met RHC criteria for Group 1 PAH. Table 1 summarizes the clinical features of 162 patients (7 missing autoantibody and 1 missing time to death information). The mean age of the cohort was 59.7±10.7 years, and the majority of the group was female (86%), Caucasian (80%), and had limited cutaneous SSc (72%). The mean disease duration was 14.7±12.0 years from the onset of Raynaud phenomenon and 10.7±9.4 years from the onset of the first non-Raynaud symptom. The mean mPAP was 36.9±10.2 mmHg and the mean PCWP was 10.0±3.3 mmHg, consistent with the classification of PAH. The mean FVC was 79.8±22.8% predicted, while the mean DLCO was 42.7±17.2% predicted.
Figure 1.
Flow diagram of PHAROS cohort. Data from subjects within the highlighted boxes were included in the analysis. **One subject with +AC serum autoantibodies lacked survival information. PH=pulmonary hypertension; RHC=right heart catheterization; ANA=anti-nuclear antibody; AC=anticentromere; NUC= nucleolar ANA; Scl-70=anti-topoisomerase; Other=non-specific antinuclear or overlapping autoantibodies, RNA pol= RNA polymerase III.
Table 1.
Clinical Characteristics for World Health Organization Group 1 (PAH) Patients (N=162) with Survival Information
| Mean (SD) or as indicated |
Overall N=162 (100%) |
AC N=60 (37%) |
NUC N=39 (24%) |
Scl-70 N=11 (7%) |
Other N=28 (17%) |
RNA pol** N=9 (6%) |
U1RNP**
N=8 (5%) |
Negative**
N=7 (4%) |
p- value * |
|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 59.8(10.7 ) |
62.6(9.0) | 55.7(10.5 ) |
57.0(7.8) | 62.2(11.9 ) |
61.7(11.8 ) |
49.1(13.0 ) |
61.1(6.2) | 0.001 |
| Sex, n (% female) |
139(86) | 56(93) | 32(82) | 9(82) | 24(86) | 6(67) | 6(75) | 6(86) |
0.33 |
| Race, n (% Caucasian) |
129(80) | 50(83) | 26(67) | 8(73) | 24(86) | 7(78) | 7(88) | 7(100) |
0.22 |
|
SSc disease
duration: (y) From Raynaud onset From non- Raynaud onset |
14.7(12.0 ) 10.7(9.4) |
18.8(13.1 ) 13.5(11.2 ) |
12.2(9.8) 7.6(6.1) |
8.8(10.2) 8.4(10.2) |
13.8(12.5 ) 9.6(9.0) |
10.6(9.2) 10.5(8.9) |
11.7(10.6 ) 10.2(9.5) |
10.5(4.1) 11.2(3.8) |
0.02 0.13 |
|
SSc subtype:
(%) Limited cutaneous Diffuse cutaneous Unclassified |
116(72) 41(25) 5(3) |
58(97) 2(3) 0(0) |
28(72) 11(28) 0(0) |
5(45) 6(55) 0(0) |
15(53) 10(36) 3(11) |
2(22) 7(78) 0(0) |
5(63) 1(12) 2(25) |
3(43) 4(57) 0(0) |
<.0001 |
| mRSS | 8.6(9.0) | 5.7(4.8) | 10.3(11.8 ) |
13.9(10.9 ) |
7.2(6.6) | 18.1(13.7 ) |
8.1(7.5) | 7.8(6.4) | 0.001 |
| 6MWD (m) | 339.3(125.6) | 311.2(122.0) | 358.1(125.3) | 338.1(159.4) | 363.0(111.9) | 314.4(104.6) | 323.8(203.9) | 439.3(70.6) | 0.38 |
|
Echocardiogra
m SPAP (mmHg) |
60.5(21.1 ) |
61.0(20.5 ) |
62.1(22.8 ) |
55.1(17.0 ) |
58.0(20.8 ) |
57.4(13.0 ) |
68(30.4) |
48.3(11.3) |
0.71 |
|
Heart
catheterization mPAP (mmHg) PCWP (mmHg) |
36.9(10.2 ) 10.0(3.3) |
37.6(10.4 ) 9.9(3.4) |
38.6(11.4 ) 10.3(3.56 ) |
33.3(10.1 ) 11.3(2.6) |
35.2(9.0) 9.3(2.9) |
32.4(5.5) 11.3(3.5) |
42.4(10.0 ) 8.6(2.4) |
31.4(7.1) 10.3(3.8) |
0.13 0.41 |
|
Pulmonary
function tests FVC % Predicted DLCO % Predicted |
79.8(22.8 ) 42.7(17.2 ) |
85.1(14.9 ) 44.4(17.2 ) |
75.9(38.0 ) 41.6(18.4 ) |
75.1(10.2 ) 37.6(11.4 ) |
76.6(14.5 ) 41.4(15.0 ) |
69.9(12.7 ) 33.5(11.7 ) |
80.8(24.6 ) 50.3(23.9 ) |
87.5(16.2) 53.8(23.5) |
0.27 0.27 |
|
% Survival Rate
1-year 2-year 3-year |
94% 88% 78% |
94% 89% 72% |
94% 88% 79% |
89% 63% 63% |
92% 88% 79% |
100% 100% 100% |
100% 100% 86% |
100% 100% 100% |
0.59*** |
SSc=systemic sclerosis, mRSS=modified Rodnan skin score, 6MWD=6-minute walk distance, SPAP=systolic pulmonary artery pressure, mPAP=mean pulmonary artery pressure, PCWP=pulmonary capillary wedge pressure, DLCO=diffusion capacity for carbon monoxide, FVC=forced vital capacity, AC=anti-centromere, NUC=nucleolar, Scl-70=anti-topoisomerase, Other=other nonspecific positive ANAs, RNA pol=RNA polymerase III.
Comparing seven antibody groups. SSc subtype only compared limited and diffuse cutaneous.
Three groups (RNA pol, U1RNP, Negative) were collapsed into one ‘combined group’ for Kaplan Meier survival analyses due to similar survival rates.
Log-rank test for equality of survival curves over five antibody groups (AC, NUC, Scl-70, Combined, Other). One subject with autoantibody data was missing survival data.
Sixty (37%) subjects had AC, 39 (24%) isolated NUC pattern, 11 (7%) Scl-70, 28 (17%) had Other, 9 (6%) had RNA pol, 8 (5%) had U1RNP, and 7 (4%) were negative (Figure 1). The only clinical characteristics that were significantly different among the seven autoantibody groups were age, SSc disease duration from onset of Raynaud phenomenon (SSc duration), SSc subtype, and mRSS (Table 1). The mean SSc disease duration at PAH diagnosis was longest for AC (18.8±13.1 years) and shortest for NUC patients (12.2±9.8 years), (p=0.01 comparing +NUC to +AC). Patients with NUC, Scl-70 and RNA pol were more likely to have diffuse cutaneous disease.
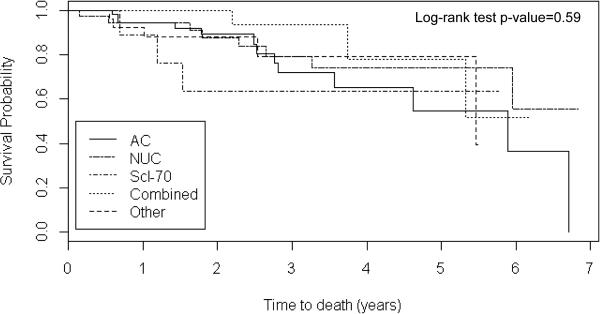
Thirty-two (20%) subjects died over a mean (SD) follow-up time of 2.4 years (1.7). The 1- and 3-year survival in the overall cohort was 94% and 78% (Table 1). For AC, the 1- and 3-year estimates were 94% and 72%; for NUC, 94% and 79%; for Scl-70, 89% and 63%. For patients in the other group, the survival rates were 92% and 79%. Survival was similar for the RNA pol, U1RNP and negative autoantibody groups with few deaths thus, these patients were collapsed into the “combined group” for Kaplan-Meier survival analyses. There were neither significant survival differences comparing the 5 autoantibody groups (Figure 2), nor between patients with and without each of the 5 different autoantibodies (data not shown).
Figure 2.
Kaplan-Meier survival analyses for subjects in the five serum autoantibody groups. The Combined group consisted of patients with RNA pol, U1RNP and negative autoantibodies who had similar survival. The Other group consisted of patients with non-specific antinuclear or overlapping antibodies. One hundred thirty subjects were censored. The median (range) follow-up time was 2.1 years (0.01-6.8). AC=anticentromere, NUC= nucleolar ANA, Scl-70=anti-topoisomerase I, Combined= RNA polymerase III/U1RNP/Negative.
Multivariable Cox regression analyses were performed to assess risk of death in each autoantibody group, controlling for age, sex, race and SSc duration (Table 2). There were no differences in survival in the adjusted model for patients in the different autoantibody groups.
Table 2.
Unadjusted and Adjusted Hazard Ratios (95% Confidence Intervals) for Mortality (N=162)
| Risk Factor | Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| AC + vs. − | 1.36 (0.67, 2.76) |
1.53 (0.68, 3.44) |
- | ||||
| NUC + vs. − | 0.71 (0.32,1.61) |
0.62 (0.25, 1.54) |
- | ||||
| Scl-70 + vs. − | 1.85 (0.56, 6.12) |
1.27 (0.29, 5.52) |
- | ||||
| Other + vs. − | 1.16 (0.44, 3.06) |
1.40 (0.52, 3.76) |
- | ||||
| RNA pol + vs. − | NA* | - | |||||
| U1RNP +vs. − | 0.98 (0.23, 4.12) |
1.41 (0.29, 6.88) |
- | ||||
| Negative + vs. − | 0.38 (0.06, 3.03) |
- | - | - | - | - | 0.44 (0.06, 3.28) |
| Age, y | 1.02 (0.98, 1.05) |
1.03 (0.99, 1.08) |
1.03 (0.99, 1.07) |
1.04 (0.99, 1.08) |
1.03 (0.99, 1.08) |
1.04 (1.00, 1.08) |
1.04 (1.00, 1.08) |
| Sex, male vs. female |
1.92 (0.72, 5.08) |
1.79 (0.58, 5.49) |
1.69 (0.56, 5.05) |
1.61 (0.54, 4.81) |
1.55 (0.52, 4.61) |
1.48 (0.47, 4.59) |
1.52 (0.51, 4.51) |
| Race, non-white vs. white |
1.46 (0.65, 3.27) |
1.59 (0.64, 3.95) |
1.77 (0.70, 4.49) |
1.64 (0.66, 4.10) |
1.64 (0.65, 4.10) |
1.67 (0.67, 4.21) |
1.52 (0.61, 3.82) |
| SSc disease duration, y |
1.00 (0.96, 1.03) |
0.99 (0.95, 1.03) |
0.99 (0.96, 1.03) |
1.00 (0.96, 1.04) |
1.00 (0.96, 1.04) |
1.00 (0.96, 1.03) |
1.00 (0.61, 1.03) |
AC=anticentromere, NUC=nucleolar ANA, Scl-70=anti-topoisomerase I, Other=other non-specific positive ANAs, overlapping antibodies, RNA pol=RNA polymerase III.
No PHAROS patients with +RNA pol died during the follow-up period.
DISCUSSION
Serum autoantibody assessment is useful in establishing an SSc diagnosis and predicting the risk for SSc-associated internal organ complications(9). Further, there is a growing body of evidence that implicates perturbations in immune system responses to ubiquitously expressed antigens in the pathogenesis of PAH(4). Serum autoantibodies that have been previously shown to be associated with SSc-PAH include AC, NUC, and U1RNP while Scl-70 is uncommonly seen in patients with SSc-PAH(3). Because PAH is one of the leading causes of death in patients with SSc, and autoantibody tests are routinely obtained during clinical practice, we sought to determine whether specific serum autoantibodies were associated with increased mortality in a clinically well-characterized cohort of SSc-PAH patients(2, 10). Our results did not demonstrate an association between specific serum autoantibodies and survival in SSc-PAH patients, but we did find that patients with AC antibodies had the longest, and those with NUC antibodies had the shortest, SSc disease duration at the time of PAH diagnosis.
Using the PHAROS cohort of 131 SSc patients with incident PAH, we previously identified age>60 years, male gender, NYHA functional class IV status, DLCO<39% predicted and diffuse cutaneous disease as poor prognostic factors(11). Although univariate analyses of 161 PHAROS subjects in the present analysis did not identify an association between male sex or older age and mortality, we included these variables in our multivariate models because of our previous results(11).
Pulmonary arterial hypertension registry data demonstrate that functional class at the time of PAH diagnosis impacts survival. Overall one and three-year survival for SSc-PAH patients in the Registry to EValuate Early And Long-term (REVEAL) PAH disease management, a registry developed by pulmonologists, was 78% and 54%(11). In the REVEAL registry, the majority of patients were functional class III and IV at diagnosis(3). Overall one and three-year survival for SSc-PAH patients in PHAROS, a registry developed by rheumatologists dedicated to early SSc-PAH detection, was 94% and 78%(12). Hazard ratios (95% CI) for death based upon NYHA functional class at study entry in our cohort was 1.24 (0.29, 5.32) for Class II, 1.98 (0.53, 7.39) for Class III and 6.87 (1.19, 37.7) for Class IV. Although lead-time bias may partially explain these results, earlier diagnosis and initiation of treatment appear to improve survival in SSc-PAH(8).
Serum biomarkers that identify SSc-PAH patients at high risk for death would be useful to enable closer monitoring and more aggressive treatment. In our study 61% of the patients had AC or NUC antibodies that are known to be associated with an increased risk for PAH. This is particularly important because an isolated NUC antibody is normally seen in only 15% of SSc patients(3). Unlike previous studies, we did not find an association between U1RNP antibodies and SSc-PAH, but the number of patients with +U1RNP antibodies was small. Our data demonstrate that although antibodies are associated with PAH development and time to PAH diagnosis, none of the SSc-specific serum autoantibodies were predictive of mortality. Brain natriuretic peptide or NT-proBNP may be useful biomarkers to identify SSc patients at increased risk for PAH development as well as patients with incident PAH at increased risk for death(13). We are currently evaluating the prognostic utility of these biomarkers in the PHAROS cohort.
The study strengths include a multicenter study design, recruitment of patients with incident SSc-PAH earlier in SSc disease course (10.7y from first SSc non-Raynaud symptom) and with better functional status at diagnosis that permits examination and follow-up of a different cohort than previous studies. Another strength is that serum autoantibody testing was completed at local laboratories thus increasing the generalizability of the study results. Although overall survival has improved with PAH-specific therapies, SSc-PAH patients have worse outcomes compared to idiopathic PAH patients(12). This may be partially due to delayed SSc-PAH diagnosis, a problem we attempted to overcome with our study design.
There are also limitations. The lack of centralized testing for autoantibodies that may have resulted in subject misclassification is a study weakness, just as it is a strength. Another study limitation is the relatively short duration of follow-up. Data collection including vital status is ongoing for the PHAROS registry patients that will permit additional longitudinal studies.
In summary our data highlight the need for identification of biomarkers that can predict which patients with SSc-PAH are at highest risk of death. Unfortunately, although commercially available autoantibodies do seem to predict the development of PAH, they did not predict survival difference in SSc patients with incident PAH in our cohort. Although the timing of PAH development was different between patients with AC and nucleolar pattern ANAs (the two most prevalent antibodies in patients with SSc-PAH), survival was not different between the autoantibody groups. Larger patient cohorts may be required to detect significant associations. Future survival studies in SSc-PAH patients should be designed to include robust measurements of serum autoantibodies and other potential biomarkers to permit more in-depth analyses of the association between serum factors and survival.
Acknowledgments
Grants and Financial Support: PHAROS Registry funded by Gilead Sciences and Actelion Pharmaceuticals. This work was supported in part by the NIH K23 AR059763 (MH), by a research award from the Scleroderma Research Foundation (MH, LC) and NIH NIAMS P60AR064464 (JL, RWC). The project was supported, in part, by the Northwestern University Clinical and Translational Science Institute, Grant Number UL1TR000150 from the National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Clinical and Translational Science Award (CTSA) is a registered trademark of the U.S. Department of Health and Human Services (DHHS).
The Pulmonary Hypertension Assessment and Recognition of Outcomes (PHAROS) Registry is supported by industry (Gilead Sciences). Virginia Steen, MD receives compensation from Gilead Sciences and Actelion for her participation in a speaker’s bureau and research as well as from United Therapeutics for research and consulting. Each academic center enrolling patients into the PHAROS registry receives funding from Gilead Sciences to support clinical coordinator salaries.
Abbreviations
- PAH
pulmonary arterial hypertension
- SSc
systemic sclerosis
- ANA
antinuclear antibodies
- PH
pulmonary hypertension
- WHO
World Health Organization
- PHAROS
Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma
- NYHA
New York Heart Association
- DLCO
diffusion capacity for carbon monoxide
- RHC
right heart catheterization
- mPAP
mean pulmonary artery pressure
- PCWP
pulmonary capillary wedge pressure
- HRCT
high-resolution computed tomography of the lungs
- PFT
pulmonary function tests
- AC
anticentromere
- NUC
isolated nucleolar ANA
- Scl-70
anti-topoisomerase I
- RNA pol
RNA polymerase III
- FVC
forced vital capacity % predicted
- mRSS
modified Rodnan skin score
- ILD
interstitial lung disease
- sPAP
systolic pulmonary artery pressure
- HR
hazard ratio
- CI
95% confidence interval
- REVEAL
Registry to Evaluate Early and Long-term Disease Management
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors claim sole responsibility for the manuscript content.
REFERENCES
- 1.Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long term survival in systemic sclerosis. Arthritis & rheumatology (Hoboken, NJ) 2014 doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 2.Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138(6):6–1383. doi: 10.1378/chest.10-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35(1):1–35. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Doering K, Rosen A. Autoantibodies in Pathogenesis. In: Varga J, Denton C, Wigley F, editors. Scleroderma: From Pathogenesis to Comprehensive Management. Springer; New York, Dordrecht, Heidelberg, London: 2012. pp. 199–208. [Google Scholar]
- 5.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):6–1219. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 6.Hinchcliff M, Fischer A, Schiopu E, Steen VD, Investigators P. Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS): baseline characteristics and description of study population. J Rheumatol. 2011;38(10):10–2172. doi: 10.3899/jrheum.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48(2):2–516. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 8.Chung L, Domsic RT, Lingala B, Alkassab F, Bolster M, Csuka ME, et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res (Hoboken) 2014;66(3):3–489. doi: 10.1002/acr.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaqub A, Chung L. Epidemiology and risk factors for pulmonary hypertension in systemic sclerosis. Current rheumatology reports. 2013;15(1):302. doi: 10.1007/s11926-012-0302-2. [DOI] [PubMed] [Google Scholar]
- 10.Nikpour M, Baron M. Mortality in systemic sclerosis: lessons learned from population-based and observational cohort studies. Curr Opin Rheumatol. 2014;26(2):2–131. doi: 10.1097/BOR.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 11.Chung L, Farber HW, Benza R, Miller DP, Parsons L, Hassoun PM, et al. Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest. 2014;146(6):6–1494. doi: 10.1378/chest.13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung L, Farber HW, Benza R, Miller DP, Parsons L, Hassoun PM, et al. Unique Predictors of Mortality in Patients With Pulmonary Arterial Hypertension Associated With Systemic Sclerosis in the Reveal Registry. Chest. 2014 doi: 10.1378/chest.13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27(12):12–1485. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]