Abstract
Cell-based therapies are emerging as a promising approach for various diseases. Their therapeutic efficacy depends on rational control and regulation of the functions and behaviors of cells during their treatment. Different from conventional regulatory strategy by chemical adjuvant or genetic engineering, which is restricted by limited synergistic regulatory efficiency or uncertain safety problems, a novel approach based on nanoscale artificial materials can be applied to modify living cells to endow them with novel functions and unique properties. Inspired by the natural “nano shell” and “nano compass” structures, cell nanomodification can be developed through both external and internal pathways. In this review, some novel cell surface engineering and intracellular nanoconjugation strategies are summarized. Their potential applications are also discussed, including cell protection, cell labeling, targeted delivery and in situ regulation. It is believed that these novel cell-material complexes can have great potentials for biomedical applications.
Keywords: cell-based therapy, cell functionalization, nanomodification, living-nonliving integration, material-based biological regulation
1. Introduction
Nowadays, cell-based therapies have attracted increasing attentions as a result of their large potentials for the treatment of various diseases [1–5]. For example, neural stem and progenitor cells have been utilized for the structure repair of human central nervous system due to their ability to produce neurons and glia [2]. Allogeneic haematopoietic stem cell transplantation (HSCT) has been applied as an important tool for the treatment of haematological malignancies, such as myelodysplastic syndromes and chronic lymphocytic leukemia, ascribing to their specific interactions with immune cells [6]. Besides that, mesenchymal stem cells (MSCs) [7], macrophages [8], erythrocytes [9] and some other therapeutic cells [10] have been well developed as cell-based drug carriers to deliver diverse genes or drugs to specific targets (tumor, lymph nodes, reticuloendothelial system (RES), etc.) by means of their natural biological tropisms. Collectively, cell-based therapies employing donor cells have become a promising strategy for various therapeutic applications.
It should be emphasized that the efficacy of cell-based therapies mainly depends on the rational control and regulation of cell functions and behaviors in physiological and pathological environment. In vivo behaviors of cells have traditionally been regulated by injecting exogenous adjuvant drugs simultaneously with donor cells [11–13], or tailoring the genetic programming to alter the biological properties of the cells [14, 15]. The first approach is limited by the distinct in vivo behaviors between therapeutic cells and adjuvant drugs. Many therapeutic cells have natural tropisms to certain tissues mediated by specific cytokines produced in associated microenvironments [7, 8, 16], while molecular adjuvant drugs have poor targeting property, exhibit no selective biodistribution, and are readily cleared or degraded from biological environment [17, 18]. Therefore, adjuvant drugs cannot effectively target to donor cell populations to alter their functions and phenotypes. Another regulatory pathway, gene engineering, can significantly tailor cells at genetic level to regulate their biological behaviors. However, such a genetic alternation is inheritable and irreversible, which may permanently impact the original genetic configuration of the modified cells. This may affect the intrinsic biological property and increase the risk of mutations, leading to potentially uncontrollable biosafety problems [19]. Therefore an efficient and biosecure strategy is desirable for cell behavior control.
Many living organisms can create various nanostructures to modify themselves with highly functionalized and biocompatible fashion to regulate their behaviors. For example, unicellular diatoms can catalyze the polymerization of silicon to silica during the cell wall synthesis [20]. This natural process may construct a nanosized silica shell to enhance the survivability of diatoms in harsh conditions by providing external protection [21]. Besides that, magnetotactic bacteria produce chain-arrangement magnetosome crystals inside the cells, which can act as a “nano compass” to help magnetotactic bacteria recognize the direction and swim along geomagnetic field lines [22, 23]. Inspired by the natural nanostructures, nanomodification of therapeutic cells can be achieved via both external and internal pathways using biomimetic materials, which can help functionalize the therapeutic cells to regulate their properties and behaviors in a biocompatible and desirable manner.
It should be noted that construction of therapeutic cell-biomaterial conjugates is a promising but challenging approach, as many therapeutic cells (e.g. MSCs, macrophages, etc.) cannot produce natural nanostructures spontaneously, and they are sensitive to nanomaterial interactions. During the nanomodification, many factors such as slight alternation of pH, temperature, ionic strength, osmolality and so on, may significantly influence cell viabilities and intrinsic functions [24, 25]. Thus strategies chosen for modifying cells should have minimal alternations to the biological properties of living cells. Only in this way, the modified cells can possess new functions conferred by synthetic materials and maintain their biological activities to the maximum extent. Besides, after treatment by synthetic materials, living cells tend to spontaneously weaken the influence of the exogenous materials by exocytosis, degradation and metabolism processes for self-protection [26, 27]. These cell responses may limit the stability of cell-conjugated materials. Therefore, rationally controlling the cell-material interactions to alleviate cellular response is of importance for cell modification. The biologically acceptable interactions, especially some biomimetic reactions (e.g. biotin and avidin interactions), are preferable for cell modification, as cells may recognize those reactions to be part of natural processes, showing less rejection and keeping the material for long time. Overall, the key to successfully modify living cells is to minimize alternations of the intrinsic properties of cells and lead to mild cellular rejection against material treatments. Herein, in this review we provide an overview about some recently developed cell modification strategies and their applications. In section 2, the approaches for cell surface engineering and intracellular nanoconjugation are analyzed, and in section 3, the therapeutic applications of modified cells are discussed. Moreover, we spell out some challenges and perspectives for the future development in this field.
2. Approaches for cell nanomodification
With the advancement of cell biology and material sciences, much has been learned about the mechanism of cell-material interactions, including the influences of bio-systems on artificial materials [28, 29] and effects of physicochemical properties of materials on living cells [27, 30]. Based on these understandings, various cytoreagents ranging from unicellular microorganisms to isolated mammalian cells have been successfully employed for material modification. The novel cell-material integrations maintain the original properties of biological species and possess new functions conferred by artificial nanomaterials, which may open a new window for improving traditional cell-based therapies. There are major nanomodification strategies, namely, cell surface engineering and intracellular nanomaterial conjugation, which may mimic natural extracellular and intracellular nano structures (“nano shell”, “nano compass”, etc.) to regulate cell functions and behaviors.
2.1 Cell surface engineering
2.1.1 Nanocoat modification
In nature, surface structure is quite important for a living cell to interact with its environment, which significantly influences its survivability and biological behaviors [31]. It is intriguing that many living organisms can spontaneously generate special outer surface material to help them adapt to the environment. For example, many bacteria can generate a thin (<100 nm) and tough proteinaceous coat on the surface in response to nutrient deficiency, to converse them to a non-dividing, dormant status [32]. The unicellular diatoms utilize silica as their inorganic coat to protect them from external aggressions and stimulations [20, 21]. However, most of the cells cannot form functional exterior shell structure on their surfaces due to the lack of suitable reaction pathways. Many biological reactions on cell surface can only proceed in specific bio-environment [33], which is not universal for all living organisms. Through the introduction of well-developed, artificially controllable physical or chemical reactions, scientists have succeeded in conjugating various designed structures onto the surfaces of the living cells to produce artificially functional exteriors.
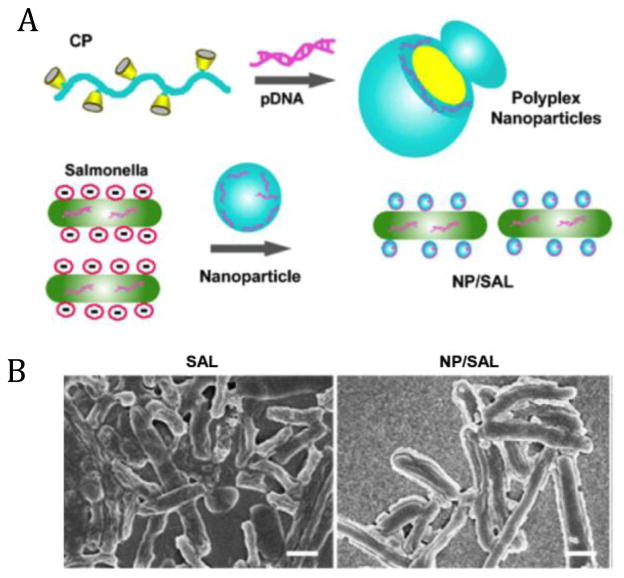
Live attenuated microorganisms such as salmonellae, have been developed as vaccines for a number of infectious diseases and several types of cancers, and were also exploited as potential vaccine vectors to deliver different types of antigenic messages for activating antitumor immune responses [35–37]. However, their applications are limited by poor bio-stability against physical or biological stimulations [34, 38]. An artificial material-based exterior seems to be capable of improving their survivability in detrimental environment by isolating enclosed living cells from external stimulations. The biological membrane of a cell is mainly composed of lipids and proteins, and about one third of the dry mass of the membrane is phospholipid, indicating a negatively charged surface [39–41]. Positively charged polymers can spontaneously bind to the outer-surface membrane of microorganism through electrostatic interactions [40, 41]. For example, a cationic polymer formed from host-guest supramolecular complexation between β-cyclodextrin-crosslinked polyethylenimine (PEI) and redox-sensitive poly(ethylene glycol) (PEG) [42] can be readily bioconjugated to the surface of salmonellae by simple co-incubation with cells for 20 min at room temperature (Fig. 1A). After surface modification, a dense coating layer over the rod-shaped Salmonella can be clearly observed as compared to native bacteria (Fig. 1B). This direct deposition approach for cell coating is quite simple and straightforward, which can provide potential protection and surface alternation to various therapeutic microorganisms [34, 40, 41].
Fig. 1.
(A) Schematic illustration of the construction of polyplex (complex of cationic polymer and pDNA), and the coating of polyplex nanoparticles on attenuated Salmonellae (NP/SAL). (B) Morphology of naked Salmonellae (SAL) (left) and coated Salmonellae by polyplex nanoparticles (right), as observed by scanning electron microscopy (SEM) (scale bar, 1 μm). Adapted from [34] with permission.
To construct more sophisticated surface structures, simple direct deposition is not sufficient without exquisitely controllable assembly. Therefore, a layer-by-layer (LbL) technique is further developed for cell surface modification, as it allows to fabricate diverse polyelectrolytes on various surfaces, even on living cells [45, 46]. After the interaction between negatively charged cell membrane and positively charged polyelectrolyte, the cell surface potential turns to be positive. Then a polyanion can be employed to anchor to the surface through electrostatic interactions, again inducing a negatively charged surface. After certain cycles of deposition and adsorption with oppositely charged polyelectrolytes, a thickness-tunable multilayer structure is generated (Fig. 2A), which serves as a “soft shell” to prevent undesirable aggressions and regulate the cell-environment interactions. This approach has been well developed for various microorganisms and isolated mammalian cells by using diverse natural macromolecules or artificial polymers (Table 1), suggesting a general cell surface engineering strategy. It should be mentioned that not only single cells can be engineered, but also living tissues such as pancreatic islets can be modified. For instance, alginate and poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG) multilayers can be effectively constructed on the surface of islets through LbL assembly (Fig. 2B), which may help alleviate the immune rejection for type 1 diabetes therapy [44, 47]. Besides the electrostatic interactions, many other assembly modes have also been applied to help build multilayer structures on cell exteriors. For example, Akashi et al. prepared fibronectin-based protein multilayers on cell surface by the interactions of gelatin and specific binding domains of fibronectin [48]. Tsukruk et al. developed a cross-linked poly(methacrylic acid)-co-NH2 multilayer by peptide bond formation between different layers [49]. These novel LbL approaches extend the choices of materials for cell surface modification, as neutral polymers or biomacromolecules can also be applied for cell coating while the deposition process is not restricted to electrostatic attractions. With the development of LbL technique, many more functional materials can be controllably constructed on cell surface with minimal disturbance to the cell and the interior. Notably, these layers can be well designed (molecular weight, charge, steric hindrance, etc.) to optimize their permeability, allowing waste or specific nutrients to transport between cell and its surroundings, effectively regulating cell-environment communications. Moreover, drugs, enzymes and certain proteins can be included within the LbL cell coat to locally affect cell behaviors and achieve desired therapeutic effects. Therefore, LbL technology for encapsulation of living cells can serve as a promising approach for improving diverse set of biomedical applications during cell-based therapies.
Fig. 2.
(A) Schematic illustration of layer-by-layer assembly of polyelectrolytes on living cells. Adapted from [43] with permission. (B) Schematic illustration of the structure of cell surface-modified multilayer polymer films on pancreatic islet. Adapted from [44] with permission.
Table 1.
Typical polyelectrolytes employed for LbL modification of living cells
| Type of Cells | Polycation | Polyanion | Reference |
|---|---|---|---|
| Yeast | Poly(allylamine hydrochloride) (PAH) Poly(diallyldimethylammonium chloride) (PDADMAC) |
Poly(styrene sulfonate) (PSS) Poly(acrylic acid) (PAA) |
[50–55] |
| Cyanobacteria | PDADMAC | PSS | [56] |
| Bacillus.subtilis | Poly-L-lysine (PLL) Poly(dimethyldiallylammonium chloride) (PDDA) |
Poly(glutamic acid) (PGA) PSS |
[57] |
| Allochromatium vinosum | PAH, PDDA | PAA, PSS, PGA | [58] |
| Red blood cell (RBC) | Chitosan-graft-phosphorylcholine (CH-PC) poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG) |
Alginate | [59] |
| MELN cell | PEI, PAH, Protamine sulfate (PS), PLL, PDADMAC | PSS | [60] |
| Mouse mesenchymal stem cell | PLL | Hyaluronic acid (HA) | [61] |
| Neural stem cell | Gelatin | Alginate | [62] |
| Pancreatic islet | PLL-g-PEG, PAH, PDADMAC | Alginate, PSS | [44, 47, 63] |
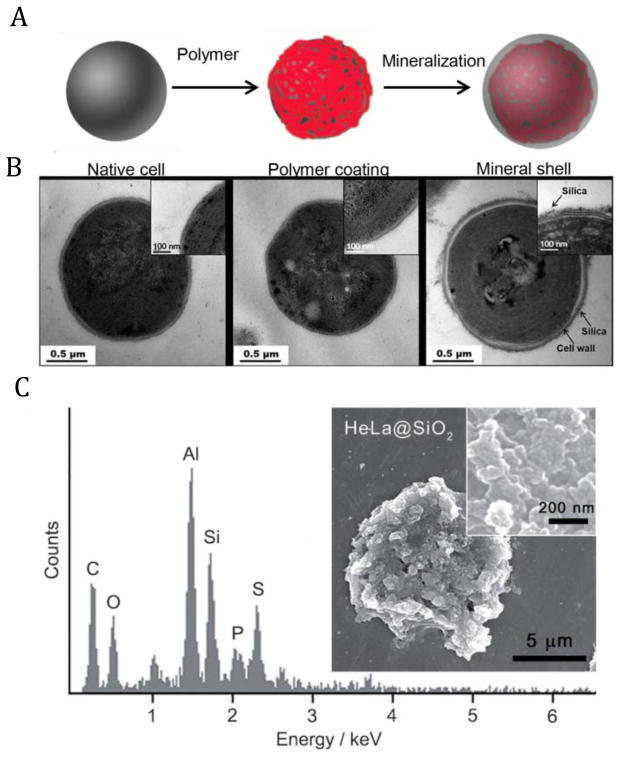
The polymer exterior formed via above strategies acts as a “soft shell”, which may significantly alter the properties of cell surface, but is not necessarily robust enough to fight against mechanical attacks, heat or radiant threats [43, 64–68]. It has been found that natural systems choose hard and tough shell structures [20, 21] to enhance their survivability under harsh conditions. Inspired by natural structures, artificial hard shells have been utilized to increase heat tolerance and radiation resistance of some single-cellular livings by means of their physicochemical durability and biological stability [43, 64, 65]. Microorganisms, such as cyanobacteria which are a general study model for cellular biology, can induce a uniform silica shell with the help of suitable polymer coating (Fig. 3A). During the LbL modification, the outmost layer of the surface can be controlled as a positively charged polymer, PDADMAC [56], which can mimic natural polycationic peptide-containing long-chain polyamines (LCPA) to catalyze in situ silicification reaction [69, 70]. It is of note that a thin polyelectrolyte film has undetectable influence on the cell surface structure, texture and morphology, but can effectively induce the subsequent mineralization reaction to form cell-material core-shell structures (Fig. 3B). After mineralization, cells exhibit obvious survival advantages against harsh conditions (e.g. intense light and high temperature) over the native ones [43]. Compared to microorganisms that have supportive cell wall structure, mineral-coating on mammalian cell is challenging as the hard shell materials may directly interact with cell membrane to affect its fluidity, and readily destroy its structure, leading to undesired cell toxicity [43, 68]. Despite the difficulties, researchers have made sustained efforts to achieve this challengeable goal (mammalian cell mineralization). For instance, Davis et al. have tried to directly coat MSCs with arginine-functionalized hydroxyapatite (positive charged particles). Although the differentiation behaviors of MSCs can be observed after mineralization, their long-term survivability is doubtful [71]. Choi et al. have attempted to take HeLa cells as a model to build silica shell on the cell surface (Fig. 3C). They firstly coated the cells with positive polymer PEI, and then utilized the hydrolysis of tetramethyl orthosilicate to in situ fabricate silica shell on cell surface. It is interesting that the mineral-coated cells showed certain resistance potential to enzyme attacks, but the cell viability still can not be maintained for long [72]. Although these initial attempts of mammalian cell mineralization are far from being mature, these approaches have emerged as potential strategies to remodel cell surface in a biomimetic way. It is believed that more biocompatible strategies for constructing artificial hard shell on mammalian cells can be well developed with the inspiration from natural processes, such as utilization of biomineralization-related proteins. These hard shells may act like natural structures (diatom coat or egg shell) to provide mechanical support and protection to enhance the survivability and efficiency of living species, benefiting their storage and applications during biomedical applications.
Fig. 3.
(A) Schematic illustration of artificial shell formation on living cells. (B) Transmission electron microscopy (TEM) micrographs of native cyanobacteria (left), polymer multilayer coated cyanobacteria (middle) and SiO2 coated cyanobacteria (right). Adapted from [56] with permission. (C) SEM micrographs and energy-dispersive X-ray spectrum of HeLa cells coated by SiO2. Adapted from [72] with permission.
2.1.2 Nanopatch modification
Uniform surface coating can provide a functional exterior, but it may prevent useful cell-environment interactions by occluding the cell surface. Therefore, modifying only a portion of cell surface using artificial nanomaterials (nanopatch structure) seems to be an alternative strategy to offer possibilities of tailoring the cell surface while not obviously influencing the communications and interactions of cells with the surrounding environment.
Aiming to form a nanopatch structure on cell surface, various specific materials with unique physicochemical properties have been introduced. However, most synthetic materials are easily internalized into the cell [73], followed by rapid degradation and clearance [27], limiting the nanopatch formation. Thus reducing cell internalization seems to be a key to construct durable and stable nanopatch structures on cell surface. It should be noted that physicochemical properties of artificial nanoparticles have profound influences on their fate during interaction with cells [30, 73]. Size is an important factor that affects cell internalization process. Chan et al. have revealed that Herceptin linked gold nanoparticles (Her-GNPs) with the size around 25–50 nm are preferably internalized by cells while larger (>70 nm) or smaller (<25 nm) ones can locate on cell surface for hours [74]. This may be ascribed to the fact that smaller or larger particles cannot provide suitable receptor-ligand interactions (Fig. 4A), which may not induce fast cell membrane wrapping to drive the particles into cells [74]. This principle may also be applied to explain the shape effect of nanoparticle internalization. The nanomaterials with large aspect ratio, such as a rod- or worm-like shapes can be durably attached to the cell surface (Fig. 4B) with longer time than the spherical ones [75, 76]. The contact mode of large-aspect-ratio materials with cells cannot provide the appropriate receptor-ligand interaction to induce quick membrane wrapping, delaying the internalization process [76–78]. Based on these understandings, controlling the size and shape of the materials seems to be a promising approach to construct stable nanopatch structures on cell surface with minimal undesired internalization.
Fig. 4.
Schematic illustration of nanopatch structures on living cells. (A) Top: illustrations of interaction of different sized Herceptin-gold nanoparticles (Her-GNPs) and their internalization efficiency; bottom: corresponding fluorescence images of ErbB2 receptor location after being treated with different sized Her-GNPs. Arrows indicate ErbB2 receptors (Scale bar, 10 μm). Adapted from [74] with permission. (B) Left: illustration of nanopatch structures from materials with large aspect ratio on cell surface, inset, confocal laser scanning microscopy (CLSM) image of large aspect ratio materials on CH27 B-lymphocyte (red fluorescence is from Cell-Tracker Red CMPTX and green fluorescence is from the FITC-Poly(allylamine hydrochloride), scale bar is 25 μm); middle: microscope image of polymer patch on the surface of a CH27 B-lymphocyte cell (scale bar, 10 μm); right: microscope image of polymer patch on the surface of a HuT 78 T-cell (scale bar, 10 μm). Adapted from [77] with permission. (C) Left: illustration of interaction between the functional groups from cell and nanoparticle surfaces; middle: microscope image of maleimide-functionalized nanopatches on T cell surface via the covalent reaction between maleimide head groups and thiols on membrane proteins (scale bar, 2 μm); right: microscope image of maleimide-functionalized nanopatches on hematopoietic stem cell surface (scale bar, 2 μm). Adapted from [79] with permission.
In addition to the geometry (size, shape, etc.) of nanomaterials, the connection mode of nanomaterials with cell surface also plays an important role in stabilizing nanopatch modification. In comparison to physical adsorption and receptor-ligand interactions (e.g. HA-CD44 interaction [77]), covalent linkage between reactive groups on cell and nanomaterial surfaces allows the particles to anchor to the cell surface, and this structure can be maintained for a rather long period of time. Interestingly, cell surface possesses reactive sites due to the containment of various proteins in the membrane. Among various reactive sites, the most used functional groups should be amino and thiol groups, which are mainly from lysine and cysteine residues of membrane proteins, respectively. Thiol groups are able to bind to maleimide groups to form stable thioether bonds, and this reaction is around 1000 times faster than the one between maleimide and amines under physiological pH condition [80]. This reaction has been well applied for nanopatch modification on therapeutic cells. A lipid or polymer nanoparticle with maleimide head groups has been conjugated to free thiols on the membrane protein of primary T cells or hematopoietic stem cells (HSCs) (Fig. 4C), leading to stable nanopatch structures for several days, even after cell division [79]. Amino groups have also been employed to help form nanopatches. For instance, N-hydroxysuccinimide biotin can readily form amide bonds with primary amine groups on membrane protein, generating a biotinylated surface [81]. Then mono-dispersed NeutrAvidin-coated polystyrene nanoparticles could be immobilized to the cell surface for at least 2 days, via the typical biorecognition reactions between biotin and avidin molecules [82]. Collectively, all these observations indicate that covalent linkage between cell surface and artificial materials based on natural functional groups on cell membrane serves as a controllable, biocompatible and effective strategy for durable cell surface modification with minimal internalization. In such a way, these partially surface-modified cells can maintain the activity of bio-surface without preventing any interactions with its environment. Moreover, nanopatch can be designed to assist cell therapy by conferring specific functions and properties (e.g. drug loading and release) [83]. Therefore, this kind of non-occluded modification helps cells possess both natural and functional exteriors, presenting both innately biological and manually operable therapeutic behaviors.
2.2 Intracellular nanomaterial conjugation
Many microorganisms such as bacteria and fungi are capable of fabricating intracellular nanoparticles [28, 29] to help them adapt to the environment and respond properly to the external stimulations. For instance, magnetotactic bacteria spontaneously generate magnetosome mineral crystals arranged in chains inside the cells, which may act as special “nano compass” to help the organisms recognize the direction of the geomagnetic field [22, 23]. This phenomenon suggests that nanosized structures inside cells can remarkably regulate the cell functions and behaviors by virtue of their unique physicochemical properties.
2.2.1 Nanomaterial internalization
It has been well documented that the internalization of nanoparticles by living cells is a frequently observed phenomenon in various systems, which can be applied to construct nanoparticle-conjugated cell systems [73]. The internalization of nanoparticles mainly involves clathrin-mediated endocytosis, caveolin-mediated endocytosis, macropinocytosis and some other pathways (Fig. 5). During the internalization process, nanoparticles are typically trapped within vesicle structures, which can be sorted, fused or dissociated, and mature into late endosomes and lysosomes. This maturation process is accompanied with rapid acidification in vesicles and recruitment of some degradative enzymes [84]. In this way, the materials inside vesicles can be easily degraded, preventing the formation of cell-material integrations. In order to escape from endosomal barrier, several strategies have been developed. One approach is to coat particles with membrane structures (polymer) that are able to fuse with endosomal membrane to improve the release of exogenous materials from endosomes or lysosomes [85]. Besides, nanomaterials can also be modified with pH-responsive biomolecules such as a glutamic acid-rich peptide, which can change the structure from random to helical form to promote its interaction with endosomal membranes, inducing membrane disruption and leakage of contents [86, 87]. After being released from the vesicle compartments, the nanomaterials will spontaneously interact with their intracellular targets such as various organelles or nucleus (Fig. 5). Notably, it has been shown that, upon cellular internalization, many nanoparticles are trafficked towards the perinuclear region (outside the cell nucleus) termed as the microtubule-organizing center (MTOC) [88], which might be the destination of many nanostructures. After reaching their intracellular targets, the nanoparticles are retained inside the cells for a period of time as most of the employed nanoparticles have been well designed with cytocompatible constituents, structures, and surface properties. Their internalization may not grossly disturb cellular metabolism processes, inducing negligible toxicity and cell rejection effect. In this way, nanoparticles can be tightly and securely combined with the living cells, providing an intracellularly nanoconjugated cell-material integration. Since nanoparticle internalization and intracellular transport can be investigated in various cells, this strategy has been extensively employed for diverse biological species (e.g. macrophages, MSCs, dendritic cells, etc.) [8, 89–93], suggesting a quite general cell modification strategy.
Fig. 5.
Schematic illustration of intracellular fate of nanomaterials. After internalization by various pathways, nanomaterials are trafficked along endolysosomal network within vesicle structures. Some of them may be eliminated from cells during exocytosis process and some may be transported to certain organelles or microtubule-organizing center (MTOC) regions. It should be noted that all the processes involve the escape of nanomaterials from endolysosomal network, which is the key for intracellular nanomaterial conjugation. Adapted from [27] with permission.
2.2.2 Intracellular in situ synthesis
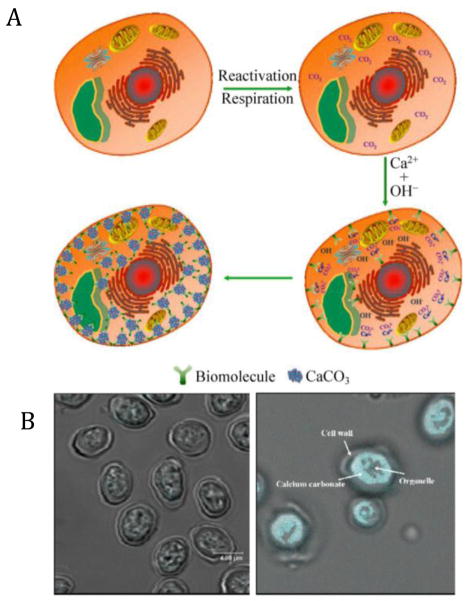
Loading nanomaterials inside cells can help regulate the functions and behaviors of living species, but many exogenous materials are not as compatible as endogenous ones. Thus some intracellular in situ synthetic nanosystems are developed, which can modify the living cells in a biomimetic way. Taking yeast as an example, excess amount of free calcium ions can break the ion balance and form CaCO3 nanoparticles intracellularly. Despite certain sophisticated factors that will influence the process, a simplified mechanism can be illustrated (Fig. 6A) to clarify the intracellular nanomaterial generation process. Firstly, after a certain period of time of cell culture, the cells gradually produce certain amount of carbon dioxide by respiration, which can easily dissolve to produce CO32− and HCO3− ions. Introduction of saturated solution of Ca(OH)2 leads to cell entry of free Ca2+ by a proton motivation force [94]. Meanwhile, biomolecules inside the cells such as acidic proteins and polysaccharides can specifically interact with Ca2+, providing some “nucleating sites”. As such, the CaCO3 nanoparticles can be generated and stabilized by the biomacromolecules (Fig. 6A). Intriguingly, the intracellular nanoparticles, which can be observed after tetracycline staining (Fig. 6B), present negligible influence on cell viability and metabolic status, suggesting superior cytocompatibility of these particles synthesized in situ [95].
Fig. 6.
(A) Schematic illustration of intracellular generation of CaCO3 nanoparticles. (B) Optical photos of control (left) and intracellular CaCO3 conjugated yeast cells (right, staining by tetracycline). Adapted from [95] with permission.
Intracellular in situ nanomaterial synthesis is not limited to microorganisms such as yeasts, many mammalian cells are also able to produce intracellular nanoparticles when encountering suitable reactive conditions. Although the precise mechanism of mammalian cell-based nanomaterial synthesis is not fully elucidated, it has been proven that this kind of synthesis is related to the reductive property of biomolecules inside the cells, especially in the case of intracellular metallic nanoparticle synthesis [28, 29]. For example, MCF10 epithelial cells have been chosen to study intracellular gold nanoparticle synthesis. After incubation with 1 mM Chloroauric acid in phosphate-buffered saline for 36 h, the cells showed pink color, indicating the reduction of gold ions and the formation of gold nanoparticles, which was further confirmed by biological TEM observation [96]. Interestingly, these intracellularly generated gold nanoparticles can serve as a probe for surface-enhanced Raman scattering (SERS), providing some specific characteristic signals of cell metabolism [96, 97]. Besides gold nanoparticles, silver nanoclusters can also be in situ produced inside NIH3T3 murine fibroblast cells, and even in the nucleus, which can be utilized to detect activity of cell proliferation by providing information of nucleolus organizing regions (NORs) [98]. Different from exogenous synthetic materials, the intracellularly generated nanoparticles are constructed directly under the same condition as their application environment, suggesting superb biocompatibility. Besides, these nanomaterials do not need to be transported along or escape from the endolysosomal network to reach intracellular targets, avoiding undesirable degradation or other side effects. Therefore, intracellular in situ synthesis is an ideal strategy to functionalize living cells with superior biocompatibility and biostability.
3 Therapeutic applications of nanomodified cells
3.1 Cell protection
Cell-based therapies are clinical procedures involving direct transplantation, injection or infusion of living cells to their in vivo targets to treat various diseases [4]. However, harsh biological or physicochemical environment such as acidic stomach condition (pH is around 2.0) is a hurdle for transfer of various therapeutic cells, especially for oral delivery [38]. Oral vaccination based on live attenuated strains of bacteria has been developed for certain infectious diseases and cancers [35–37]. Orally administered therapeutic bacteria (e.g. Salmonellae) are able to colonize the gut-associated lymphoid tissue through the microfold cells of Peyer’s patches, and then they are engulfed and processed by dendritic cells (DCs) or macrophages to induce a durable and strong immune response [99]. However, the oral infection efficiency of Salmonellae is not satisfactory due to the digestion of live attenuated salmonellae in the acidic stomach environment [38]. Interestingly, cationic nanoparticle-coating layer can help salmonellae resist to low pH condition by isolating the enclosed cells from the detrimental environment, ensuring the viability of the salmonellae during the delivery process [34]. Therefore, after dissemination of the bacteria into blood, the coated bacteria can maintain higher activity and induce a stronger immune reaction for cancer therapy than the native ones (Fig. 7). Besides acidic condition, enzymatic attack, heat, radiation, osmotic pressure and other factors may also negatively impact the viability of cytoreagents, limiting their storage and applications [43]. Take yeast as a model, which can be readily killed by lytic enzyme (zymolyase) as a result of cell wall digestion. A calcium phosphate shell can maintain the viability of the cells up to about 80% after zymolyase treatment by preventing the lytic enzyme from contacting with enclosed cells [53]. Similar protective effect can also be observed on mammalian cells. A silica shell can help HeLa cells resist trypsin attack which possesses strong nonspecific proteolytic activity to decrease cell viability after prolonged treatment [72]. Moreover, silica shell can absorb and retain water through hydrogen bonds between hydroxyl groups on silica surface and water molecules, so it can improve cell thermal stability by suppressing overall water loss to provide a fluid, water-rich microenvironment for the cells [100]. All these observations indicate that with the help of artificial coat formed on the cell surface, living systems can be more tolerant to harsh biological or physiochemical environments, which extend the range of conditions for their storage and applications. In such a way, therapeutic cytoreagents can be readily conserved, and may still efficiently exert their functions even after treatment in adverse environment.
Fig. 7.
(A) Schematic illustration of cationic nanoparticle-coated attenuated Salmonellae for improved antigen expression and tumor targeting immune responsive activation. Adapted from [34] with permission.
In addition to harsh biological or physicochemical environment that adversely affect cell viability, another hurdle for cell-based therapy is immune rejection after cytoreagent transplantation, especially for allogeneic species [101]. This undesired self-aggression by immune system limits the applications of cytoreagents, so exogenous cytoreagents will need additional “protection” to alleviate immune system aggression. This kind of “protection” may serve as a special regulatory strategy to improve the compatibility of donor cells to patients. For example, islet transplantation for the treatment of type 1 diabetes faces deleterious host responses, leading to poor clinical outcomes [101–103]. By introducing LbL assembled polyelectrolyte multilayers (PEM) on islet surface [44, 47, 63], the immunogenic surface antigens can be effectively camouflaged, potentially relieving undesired recognition and aggression. After labeling the polymer film with Cy3 or cyclooctyne-containing fluorescent molecules through biorecognition reaction or copper-free click chemistry (Fig. 8A), the PEM-coated islets can be directly visualized by following the fluorescent signals. Clearly, the fluorescence signal can be detected around individual islets distributed in the liver after transplantation through portal vein (Fig. 8B), suggesting that the PEM-coated islets maintained integrity against deleterious host recognition and aggression. More importantly, the insulin-release ability of islets in response to glucose stimulation will not be negatively influenced by film coating (Fig. 8C). As the PEM coating is not a complete dense film, it allows nutrients and functional molecules to selectively penetrate the multilayer structures [44, 47, 104, 105]. PEM-coated islets harvested from B10 mice can induce around 2-fold higher rate of recovery from a diabetic to euglycemic state than untreated islets after being intraportally transplanted into diabetic B6 mice [44], further confirming that transplanted islets can be effectively protected from immune rejection to exert their functions in vivo after PEM film coating.
Fig. 8.
(A) Surface structure of polyelectrolyte multilayer on islet and illustration of its labeling through biorecognition or copper-free click chemistry reaction (scale bar, 50 μm). (B) Micrographs of frozen section of liver (L) after transplantation of islets (I) that was coated by PEM film labeled with streptavidin-Cy3. Left: fluorescence image indicates the location of the transplanted islet (scale bar, 10 μm); right: merged image of fluorescence and bright-field photos. (C) Insulin secretion by PEM film (gray bar) coated islets and untreated islets (black bar) in response to glucose concentration change. Adapted from [44] with permission.
Collectively, proper cell nanomodification can mimic natural biological coat (protective shell or functional exterior) to provide an external defense for enclosed cells, which improves the cell viability in negative environment and reduces immune rejection by avoiding undesired aggressions. In such a way, various therapeutic living species are no longer inefficient and vulnerable, and may find more survival and applicable opportunities to expand their employments in diverse therapeutic practices.
3.2 Cell labeling
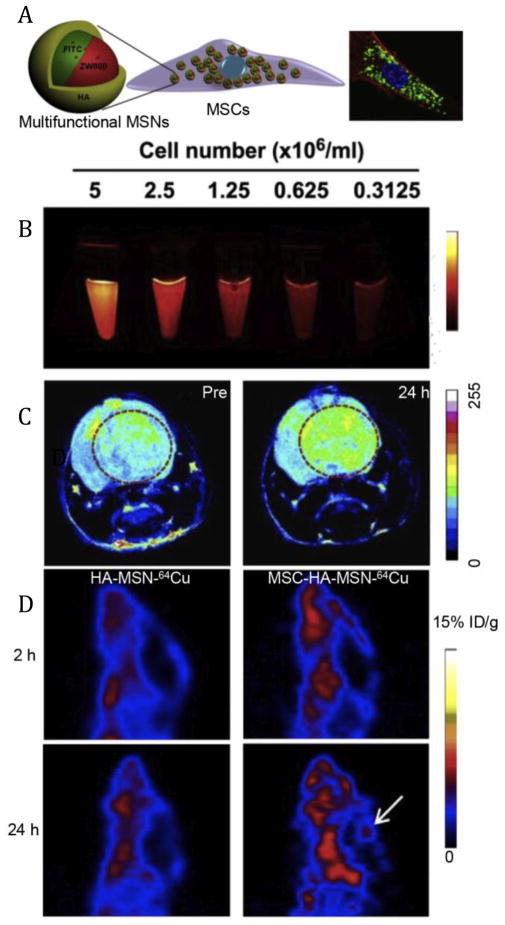
It has been well accepted that cell-based therapies have great potentials for the treatment of various diseases [4]. During this process, the therapeutic effects are largely associated with in vivo behaviors of cells. Therefore, it is critical to be able to track the behaviors of donor cells including survival, migration and differentiation is quite essential [106]. Aiming to track DCs which could present antigen to T cells within lymphoid organs to activate an adaptive immune response [107], Fe3O4-ZnO core-shell nanoparticles were loaded into DCs [92]. After ipsilateral footpad injection, the Fe3O4-ZnO loaded DCs showed obvious T2*-weighted signal in the draining lymph nodes while native particles failed to do so, suggesting that nanoparticle-labeled DCs migrated to their in vivo target to exert their natural functions and could be detected by magnetic resonance imaging (MRI) [92]. Similar findings were also observed using quantum dots (QDs) labeled DCs, which could track the migration of DCs into lymph nodes via near-infrared (NIR) fluorescence imaging [108]. In order to exhaustively monitor and analyze cell-based therapies [109–111], single imaging modality seemed to be insufficient to provide both high enough resolution and sensitivity [112]. A multimodality imaging strategy was used to improve imaging performance with the help of synergistic advantages of several imaging modalities. One typical example was the tracking of MSCs, which were typically used for cancer therapy and regenerative medicine [7, 113]. A hyaluronic acid (HA)-based polymer-coated multifunctional silica nanoparticles (HA-MSNs) loaded with fluorescein isothiocyanate (FITC), NIR dye ZW800, Gd3+ and 64Cu were successfully conjugated into MSCs (Fig. 9A) for optical, magnetic resonance and positron emission tomography (PET) imaging [93]. As shown in Fig. 9B, the signal of ZW800 dye could be clearly enhanced with increased cell number, confirming the success of cell labeling by the nanoprobes. For in vivo imaging, MRI showed that labeled MSCs gradually accumulated in tumor tissues within 24 h (Fig. 9C). The unique tumor-tropic properties of MSCs, was presumably due to the interactions of the tumor-related cytokines or chemokines with the corresponding receptors of MSCs [7]. PET imaging was further employed to quantify the distribution of cells after administration. Results clearly showed that the nanomaterial-cell platform had superior tumor targeting property over native nanoparticles with more intense PET signals (Fig. 9D), further confirming the tumor tropism of nanomodified cells. Besides MSCs and DCs, various other therapeutic cells such as macrophages [8] and embryonic stem cells [114] can also be conjugated with similar imaging nanosystems to follow their fate in vivo (migration, differentiation, etc.). It can be found that cell labeling with nanomaterials has emerged as a general strategy for imaging-guided cell behavior detection, which can provide abundant information about interactions of implanted cells with the host tissues.
Fig. 9.
(A) Schematic illustration of the construction of hyaluronic acid modified multifunctional silica nanoparticle (HA-MSN) for MSCs labeling. (B) The near-infrared fluorescence signal of MSCs labeled with HA-MSN. (C) MR enhancement at 24 h post-injection of nanoparticle-labeled MSCs. (D) PET imaging of the tumor accumulation of 64Cu-labeled HA-MSN and MSC-HA-MSN at the indicated time points. White arrow indicates the ventricle that contains glioblastoma inside. Adapted from [93] with permission.
3.3 Targeted delivery
One of the most promising advantages of cell-based therapies lies in the natural tropisms of therapeutic cells. For example, during tumor development, a number of tumor-derived cytokines (e.g. vascular endothelial cell growth factors) have been revealed to attract macrophages [8] and MSCs [7] to migrate to tumor sites, and the gradient of cytokines is correlated with the accumulation of those cells in tumors. In order to combine tumor-tropic cells and clinical drugs to achieve a synergistic and targeted therapy, various drug-loaded nanoparticles have been conjugated to the therapeutic cells [7, 90, 91, 115]. For instance, liposomal formula of doxorubicin (DOX) (LP-DOX) was successfully loaded into macrophages after a simple co-incubation (12 h), which would not significantly influence the viability of macrophages or the functions of DOX. The constructed macrophage-LP-DOX complex effectively targeted to the tumors and inhibited their growth after systemic administration [8], indicating the feasibility of utilizing nanodrug-conjugated tumor-tropic cells to achieve a synergistic and targeted therapy.
It should be noted that not only therapeutic cells can improve the targeting property of drug-loaded nanoparticles, but also nanostructures can enhance homing efficiency of the cells. For example, iron-based magnetic nanoparticles can actively augment chemokine receptor CXCR4 expression of MSCs to improve their homing efficiency to tumors via a HIF-1α up-regulation-related pathway [117]. Although the exact mechanism is still not very clear, this phenomenon suggests that nanoparticles can serve as nanoscale cytokines to regulate cell signaling pathways and their in vivo behaviors. In addition to molecular tumor targeting, many physical factors (e.g. magnetic field) are also introduced in biological systems to enhance targeting property [118], which do not require specific receptor-ligand interactions and thus can act as a general approach for regulating the behaviors of various therapeutic cells.
RBCs/erythrocytes have been proposed to be a potential candidate as a drug delivery system due to their inherent properties such as biocompatibility, flexibility, long circulation time and so on [9, 119]. However, when they are used for in vivo drug delivery, nonspecific distribution of RBCs limits their applications for targeted delivery [9, 120, 121]. Notably, magnetic materials have been applied for cell isolation and concentration by controlling cell motility in the external magnetic field [122]. Accordingly, magnetic iron oxide nanoparticles (IONPs) were employed to bind to the surface of RBCs via a biorecognition reaction between biotin and avidin molecules with the purpose of enhancing targeting property of RBCs (Fig. 10A) [116]. As shown in Fig. 10B, when Balb/c mice bearing two 4T1 tumors on both flanks were i.v. injected with RBC-IONP-chlorine e6 (Ce6)-PEG, and the left tumor was attached to a magnet while the right one was untreated as a control to detect the influence of magnetic field on RBC accumulation. Results clearly showed that magnet-attached tumors showed more accumulation of RBC-IONP-Ce6-PEG (according to Ce6 fluorescence signal) than the tumors without magnetic field treatment (Fig. 10C). Notably, free magnetic nanoparticles (IONP-Ce6-PEG) after intravenous injection did not have preferable tumor accumulation regardless of magnet attachment (Fig. 10C) due to the rapid clearance of the naked nanoparticles from circulation [123–125]. The results demonstrated that INOP-modified RBCs had unique advantages over native cells and artificial materials by simultaneously possessing the capabilities of long circulation and magnetic targeting. It should be emphasized that this kind of targeting strategy is independent of molecular recognition, which is not restricted to specific biological items. And the material-based targeting can be readily manipulated, just simply utilizing an external physical control (magnetic field). Therefore, it can serve as an effective and controllable strategy to potentially expand and improve the applications of some living species for selective and targeted therapies.
Fig. 10.
(A) Schematic illustration of RBC modification with IONPs, Ce6, DOX and PEG. (B) Illustration of modified RBC treatment and magnetic tumor targeting. (C) In vivo fluorescence signal (Ce6) of mice bearing two subcutaneous 4T1 tumors on opposite flanks, which are treated by i.v. injection of RBC-IONP-Ce6-PEG or an equivalent dose of free IONP-Ce6-PEG nanoparticles. White and green arrows indicate tumors with and without a magnet treatment, respectively. Adapted from [116] with permission.
3.4 In situ regulation
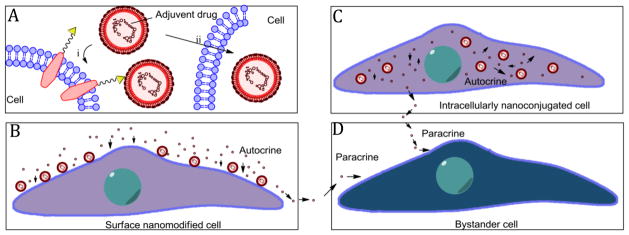
For cell-based therapy, in order to control and regulate cell functions and behaviors, adjuvant drugs are frequently used to target to donor cell population to alter their properties and phenotypes [11–13]. Unfortunately, most molecular adjuvant drugs have no selective distribution and are rapidly cleared or degraded from biological environment [17, 18]. Although some nanosystems have been designed for delivering adjuvant drugs to the therapeutic cells [126], it is still not effective enough because of the very different in vivo behaviors between biological cells and artificial nanovehicles. Moreover, many nanosystems cannot escape from the recognition and clearance by RES, limiting their ability to deliver adjuvant drugs to the specific cells [123–125]. Intriguingly, cell nanomodification provides a platform that integrates nanomaterials and donor cells (Fig. 11A). If those nanomaterials are exquisitely designed to load proper adjuvant drugs and can release them in a controllable manner, an in situ regulatory strategy can be achieved. Irvine et al. chose cytokines interleukin-15 (IL-15) and interleukin-21 (IL-21) as adjuvant drugs [79], which had been used as regulatory molecules to improve T cell expansion and effector functions [127]. Lipid nanoparticles were employed to encapsulate the cytokines and then bound to the surface of the T cells via the reactions of free thiol groups on cells and maleimide-groups on particles. This kind of nanopatch modification did not obvisouly influence cell functions, and could gradually release the cytokines for a week [79]. As such, the cytokines could be locally and sustainably released in an autocrine manner (Fig. 11B) to massively improve T cell expansion and effector function in vivo, largely improving cancer immunotherapy and completely clearing melanoma tumor burdens [33, 79]. Besides cell surface nanomodification, an intracellular nanoconjugation strategy has also been applied for in situ regulation (Fig. 11C). For example, Karp et al. developed dexamethasone-loaded particles to intracelluarly stimulate differentiation and control the secretome behavior of MSCs [89, 128]. Chen et al. loaded a polymer nanovehicle inside neural stem cells (NSCs) to gradually release morphogen in cytosol to consistently stimulate NSC differentiation into neurons for alleviating central nervous system damage and restoring brain functions from diseases or injuries [129]. It should be mentioned that during the in situ regulation, most adjuvant drug molecules released from cell-bound particles are prone to act on the carrier cell itself (antocrine stimulation), rather than bystander cells in the local microenvironment (paracrine stimulation) (Fig. 11D), presumably due to more chances of the released drug molecules to interact with cells. Thus a precise in situ regulation can be achieved with fewer side effects [79]. Collectively, all these observations confirm that cell-nanoparticle conjugations may act as an ideal system that can release adjuvant drug molecules locally and continuously to alter cell functions and phenotypes, which serves as a great way to control cell fate and behaviors temporally and spatially in vivo to effectively improve cell-based therapies.
Fig. 11.
(A) Schematic illustration of cell surface nanomodification (i) or intracellularly nanoconjugation (ii) by adjuvant drug-loaded nanoparticles. (B) Release of adjuvant drugs from surface-bounded nanoparticles for autocrine regulation. (C) Release of adjuvant drugs from intracellularly conjugated nanoparticles for autocrine regulation. (D) Bystander cells were influenced by released adjuvant drugs in a paracrine manner.
4 Challenges and future perspectives
Both extracellular and intracellular modifications of cells with nanomaterials are possible to control and regulate cell functions and behaviors. However, in order to translate these strategies into clinical practices, some challenges and concerns should be taken into account.
4.1 Avoid loss of cell viability
All the cell modification strategies developed for biomedical applications should avoid loss of cell viability to the maximum extent, which is the prerequisite for cell-based therapies. Although a lot of materials used for cell modification are considered to be biocompatible and bio-friendly, the modification process may involve some undesired alternations in the biological environment, including changes of pH, osmolality, ionic strength, temperature and so on. Even tiny changes in environmental factors might significantly and adversely impact cell viability [24, 25]. Considering the complexity and myriad functions of extracellular and intracellular biological structures, cell nanomodification should mimic natural processes as much as possible to avoid the loss of cell biological activities. For instance, during cell mineralization modification, biomineralization-related proteins seem to be better suited than artificial polymers to induce the mineral shell formation. In such a biomimetic way, all the reactions can proceed in biocompatible biological environment, which may not largely decrease cell survivability.
4.2 Maintain sufficient bio-stability
Another challenge that should be addressed is to maintain cell-material conjugate structures in physiological and pathological environment. Some nanostructures seem to be stable after conjugation with cells during in vitro treatment, but when cell-material conjugates encounter in vivo environment, many biological factors may challenge the stability of cell-material integrations. For example, after transplantation, injection or infusion, the nanomodified cells will be exposed to shear stress aroused from hemodynamic force [130, 131], and may undergo extensive transformation while migrating through tissues [132]. Those factors may break cell-material linkage that is not stable enough to detach the nanostructures (especially surface-conjugated materials) from cells. As such, the designed cell-material conjugates may be destructed, thus failing the originally designed material-based cell regulatory strategy. In order to improve the biostability of cell-material conjugates, the interaction mode is of importance. For example, in the case of cell surface modification, covalent linkage seems to be a better way than simple physical adsorption or some non-covalent interactions, as it will not be readily cleaved by regular biological stimulations (shear stress or cell reshaping). As the in vivo biological environment is rather sophisticated, various blood constituents or cellular factors may profoundly influence diverse cell-cell, cell-environment and cell-material interactions, the long-term stability of cell-material conjugate structures in complicated biological conditions is doubtful. Therefore, the robustness of nanomodified cell systems in various biological circumstances should be fully investigated before their clinical translation.
4.3 Ensure biosafety
The third challenge for the application of cell nanomodification lies in ensuring biosafety of cell-material integrations. Unlike gene engineering, material-based cell modification is not originally considered as an inheritable pathway to functionalize living cells. It just acts in a “throwaway” manner to tailor the therapeutic cells with no influence on their future generations. However, it has been recently observed that nanomaterials can serve as nanoscale cytokines to affect signaling pathways of living species, although the exactly mechanisms are still not clearly elucidated. For example, gold nanoparticles can remarkably alter the intracellular protein expressions that are related to cellular proliferation and survival [74], while iron-based magnetic nanoparticles can activate chemokine receptor CXCR4 of MSCs [117]. Moreover, such alternation can even occur at genetic levels. It has been found that 5 nm uncoated silver nanoparticles can lead to an obvious mutation in mouse lymphoma cells [133]. With increasing use of artificial materials for cell modification, some uncertain and uncontrollable biological alternations might be encountered. Therefore, in order to translate cell nanomodification strategies to clinical practices, the biological variation of nanomodified cells, especially at genetic level needs to be systemically evaluated to avoid some biosafety problems.
In summary, despite the challenges, living cell nanomodification has emerged as an effective and applicable strategy to improve cell-based therapies. Although not all the approaches are quite mature, some of them have potentials to be translated into clinic. With the rapid progress in biological and material sciences, many more new strategies of nanomodification strategies are under development. These novel cytoreagents have unique advantages over native cells or artificial nanomaterials alone by possessing both the functions of biological and artificial species. In this regard, we believe that cell nanomodification is a promising strategy for improving current and future cell-based therapeutic practices.
Acknowledgments
This work was supported in part, by the National High Technology Research and Development Program of China (863 Program) (No. 2012AA02A303), Chinese National Nature Sciences Foundation (No. 81473233), China Postdoctoral Science Foundation (2015M570745), and the Intramural Research Program, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Goldman S. Stem and progenitor cell–based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter MK, Frey-Vasconcells J, Rao MS. Developing safe therapies from human pluripotent stem cells. Nat Biotechnol. 2009;27:606–613. doi: 10.1038/nbt0709-606. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell stem cell. 2008;2:205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Jenq RR, Van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10:213–221. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Fu Y, Tabata Y, Gao J. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J Control Release. 2010;147:154–162. doi: 10.1016/j.jconrel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, Lee JS, Lee JS, Park HJ, Song SY. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195–4203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118:145–160. doi: 10.1016/j.jconrel.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg GL, Zakrzewski JL, Perales MA, van den Brink MR. Clinical strategies to enhance T cell reconstitution. Semin Immunol. 2007;19:289–296. doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, Riddell SR. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med. 2006;12:89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- 14.Palmqvist L, Pineault N, Wasslavik C, Humphries RK. Candidate genes for expansion and transformation of hematopoietic stem cells by NUP98-HOX fusion genes. PLoS One. 2007;2:e768. doi: 10.1371/journal.pone.0000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintarelli C, Savoldo B, Dotti G. Gene therapy to improve function of T cells for adoptive immunotherapy. Methods Mol Biol. 2010;651:119–130. doi: 10.1007/978-1-60761-786-0_8. [DOI] [PubMed] [Google Scholar]
- 16.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11:S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 18.Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425–446. doi: 10.1016/j.ejps.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Ho MW. Genetic engineering-dream or nightmare?: the brave new world of bad science and big business. Gateway Books; 1998. [Google Scholar]
- 20.Hildebrand M. Diatoms, biomineralization processes, and genomics. Chem Rev. 2008;108:4855–4874. doi: 10.1021/cr078253z. [DOI] [PubMed] [Google Scholar]
- 21.Hamm CE, Merkel R, Springer O, Jurkojc P, Maier C, Prechtel K, Smetacek V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature. 2003;421:841–843. doi: 10.1038/nature01416. [DOI] [PubMed] [Google Scholar]
- 22.Bazylinski DA, Frankel RB. Magnetosome formation in prokaryotes. Nat Rev Microbiol. 2004;2:217–230. doi: 10.1038/nrmicro842. [DOI] [PubMed] [Google Scholar]
- 23.Faivre D, Schüler D. Magnetotactic bacteria and magnetosomes. Chem Rev. 2008;108:4875–4898. doi: 10.1021/cr078258w. [DOI] [PubMed] [Google Scholar]
- 24.Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 2011;1810:361–373. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P. Biocompatibility of engineered nanoparticles for drug delivery. J Control Release. 2013;166:182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Sakhtianchi R, Minchin RF, Lee KB, Alkilany AM, Serpooshan V, Mahmoudi M. Exocytosis of nanoparticles from cells: role in cellular retention and toxicity. Adv Colloid Interfac. 2013;201:18–29. doi: 10.1016/j.cis.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Chou LYT, Ming K, Chan WCW. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 28.Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interfac. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biot. 2006;69:485–492. doi: 10.1007/s00253-005-0179-3. [DOI] [PubMed] [Google Scholar]
- 30.Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev. 2009;61:457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 32.Driks A. Maximum shields: the assembly and function of the bacterial spore coat. Trends Micobiol. 2002;10:251–254. doi: 10.1016/s0966-842x(02)02373-9. [DOI] [PubMed] [Google Scholar]
- 33.Stephan MT, Irvine DJ. Enhancing cell therapies from the outside in: cell surface engineering using synthetic nanomaterials. Nano today. 2011;6:309–325. doi: 10.1016/j.nantod.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Q, Wu M, Fang C, Cheng C, Zhao M, Fang W, Chu PK, Ping Y, Tang G. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Lett. 2015;15:2732–2739. doi: 10.1021/acs.nanolett.5b00570. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Hou J, Lin Z, Zhuo H, Chen D, Zhang X, Chen Y, Sun B. Attenuated Listeria monocytogenes as a cancer vaccine vector for the delivery of CD24, a biomarker for hepatic cancer stem cells. Cell Mol Immunol. 2014;11:184–196. doi: 10.1038/cmi.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardlik R, Fruehauf JH. Bacterial vectors and delivery systems in cancer therapy. IDrugs. 2010;13:701–706. [PubMed] [Google Scholar]
- 37.Toussaint B, Chauchet X, Wang Y, Polack B, Le Gouellec A. Live-attenuated bacteria as a cancer vaccine vector. Expert Rev Vaccines. 2013;12:1139–1154. doi: 10.1586/14760584.2013.836914. [DOI] [PubMed] [Google Scholar]
- 38.Gorden J, Small P. Acid resistance in enteric bacteria. Infect Immun. 1993;61:364–367. doi: 10.1128/iai.61.1.364-367.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, Bhunia AK, Mohammed S, Bashir R. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol. 2007;2:441–449. doi: 10.1038/nnano.2007.149. [DOI] [PubMed] [Google Scholar]
- 40.Feng X, Yang G, Liu L, Lv F, Yang Q, Wang S, Zhu D. A convenient preparation of multi-spectral microparticles by bacteria-mediated assemblies of conjugated polymer nanoparticles for cell imaging and barcoding. Adv Mater. 2012;24:637–641. doi: 10.1002/adma.201102026. [DOI] [PubMed] [Google Scholar]
- 41.Zhu C, Yang Q, Lv F, Liu L, Wang S. Conjugated polymer-coated bacteria for multimodal intracellular and extracellular anticancer activity. Adv Mater. 2013;25:1203–1208. doi: 10.1002/adma.201204550. [DOI] [PubMed] [Google Scholar]
- 42.Ping Y, Hu Q, Tang G, Li J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials. 2013;34:6482–6494. doi: 10.1016/j.biomaterials.2013.03.071. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Wang G, Tang R. Nanomodification of living organisms by biomimetic mineralization. Nano Res. 2014;7:1404–1428. [Google Scholar]
- 44.Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J. Cell surface engineering with polyelectrolyte multilayer thin films. J Am Chem Soc. 2011;133:7054–7064. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- 45.Tang Z, Wang Y, Podsiadlo P, Kotov NA. Biomedical applications of Layer-by-Layer assembly: from biomimetics to tissue engineering. Adv Mater. 2006;18:3203–3224. [Google Scholar]
- 46.Ai H, Fang M, Jones SA, Lvov YM. Electrostatic layer-by-layer nanoassembly on biological microtemplates: platelets. Biomacromolecules. 2002;3:560–564. doi: 10.1021/bm015659r. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JT, Krishnamurthy VR, Cui W, Qu Z, Chaikof EL. Noncovalent cell surface engineering with cationic graft copolymers. J Am Chem Soc. 2009;131:18228–18229. doi: 10.1021/ja908887v. [DOI] [PubMed] [Google Scholar]
- 48.Kadowaki K, Matsusaki M, Akashi M. Control of cell surface and functions by layer-by-layer nanofilms. Langmuir. 2010;26:5670–5678. doi: 10.1021/la903738n. [DOI] [PubMed] [Google Scholar]
- 49.Drachuk I, Shchepelina O, Lisunova M, Harbaugh S, Kelley-Loughnane N, Stone M, Tsukruk VV. pH-responsive layer-by-layer nanoshells for direct regulation of cell activity. ACS nano. 2012;6:4266–4278. doi: 10.1021/nn3008355. [DOI] [PubMed] [Google Scholar]
- 50.Krol S, Nolte M, Diaspro A, Mazza D, Magrassi R, Gliozzi A, Fery A. Encapsulated living cells on microstructured surfaces. Langmuir. 2005;21:705–709. doi: 10.1021/la047715q. [DOI] [PubMed] [Google Scholar]
- 51.Krol S, Cavalleri O, Ramoino P, Gliozzi A, Diaspro A. Encapsulated yeast cells inside Paramecium primaurelia: a model system for protection capability of polyelectrolyte shells. J Microsc. 2003;212:239–243. doi: 10.1111/j.1365-2818.2003.01251.x. [DOI] [PubMed] [Google Scholar]
- 52.Diaspro A, Silvano D, Krol S, Cavalleri O, Gliozzi A. Single living cell encapsulation in nano-organized polyelectrolyte shells. Langmuir. 2002;18:5047–5050. [Google Scholar]
- 53.Wang B, Liu P, Jiang W, Pan H, Xu X, Tang R. Yeast cells with an artificial mineral shell: protection and modification of living cells by biomimetic mineralization. Angew Chem Int Ed. 2008;47:3560–3564. doi: 10.1002/anie.200704718. [DOI] [PubMed] [Google Scholar]
- 54.Yang SH, Lee KB, Kong B, Kim JH, Kim HS, Choi IS. Biomimetic encapsulation of individual cells with silica. Angew Chem Int Ed. 2009;48:9160–9163. doi: 10.1002/anie.200903010. [DOI] [PubMed] [Google Scholar]
- 55.Svaldo-Lanero T, Krol S, Magrassi R, Diaspro A, Rolandi R, Gliozzi A, Cavalleri O. Morphology, mechanical properties and viability of encapsulated cells. Ultramicroscopy. 2007;107:913–921. doi: 10.1016/j.ultramic.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Xiong W, Yang Z, Zhai H, Wang G, Xu X, Ma W, Tang R. Alleviation of high light-induced photoinhibition in cyanobacteria by artificially conferred biosilica shells. Chem Comm. 2013;49:7525–7527. doi: 10.1039/c3cc42766h. [DOI] [PubMed] [Google Scholar]
- 57.Balkundi SS, Veerabadran NG, Eby DM, Johnson GR, Lvov YM. Encapsulation of bacterial spores in nanoorganized polyelectrolyte shells. Langmuir. 2009;25:14011–14016. doi: 10.1021/la900971h. [DOI] [PubMed] [Google Scholar]
- 58.Franz B, Balkundi SS, Dahl C, Lvov YM, Prange A. Layer-by-layer nano-encapsulation of microbes: controlled cell surface modification and investigation of substrate uptake in bacteria. Macromol Biosci. 2010;10:164–172. doi: 10.1002/mabi.200900142. [DOI] [PubMed] [Google Scholar]
- 59.Mansouri S, Merhi Y, Winnik FM, Tabrizian M. Investigation of layer-by-layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules. 2011;12:585–592. doi: 10.1021/bm101200c. [DOI] [PubMed] [Google Scholar]
- 60.Germain M, Balaguer P, Nicolas J, Claude, Lopez F, Esteve J, Pierre, Sukhorukov GB, Winterhalter M, Richard F, Hélène, Fournier D. Protection of mammalian cell used in biosensors by coating with a polyelectrolyte shell. Biosens Bioelectron. 2006;21:1566–1573. doi: 10.1016/j.bios.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Veerabadran NG, Goli PL, Stewart-Clark SS, Lvov YM, Mills DK. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol Biosci. 2007;7:877–882. doi: 10.1002/mabi.200700061. [DOI] [PubMed] [Google Scholar]
- 62.Li W, Guan T, Zhang X, Wang Z, Wang M, Zhong W, Feng H, Xing M, Kong J. The effect of layer-by-layer assembly coating on the proliferation and differentiation of neural stem cells. ACS Appl Mater Inter. 2015;7:3018–3029. doi: 10.1021/am504456t. [DOI] [PubMed] [Google Scholar]
- 63.Krol S, del Guerra S, Grupillo M, Diaspro A, Gliozzi A, Marchetti P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006;6:1933–1939. doi: 10.1021/nl061049r. [DOI] [PubMed] [Google Scholar]
- 64.Yang SH, Hong D, Lee J, Ko EH, Choi IS. Artificial spores: cytocompatible encapsulation of individual living cells within thin, tough artificial shells. Small. 2013;9:178–186. doi: 10.1002/smll.201202174. [DOI] [PubMed] [Google Scholar]
- 65.Park JH, Yang SH, Lee J, Ko EH, Hong D, Choi IS. Nanocoating of single cells: from maintenance of cell viability to manipulation of cellular activities. Adv Mater. 2014;26:2001–2010. doi: 10.1002/adma.201304568. [DOI] [PubMed] [Google Scholar]
- 66.Fakhrullin RF, Zamaleeva AI, Minullina RT, Konnova SA, Paunov VN. Cyborg cells: functionalisation of living cells with polymers and nanomaterials. Chem Soc Rev. 2012;41:4189–4206. doi: 10.1039/c2cs15264a. [DOI] [PubMed] [Google Scholar]
- 67.Wang S, Guo Z. Bio-inspired encapsulation and functionalization of living cells with artificial shells. Colloid Surface B. 2014;113:483–500. doi: 10.1016/j.colsurfb.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 68.Wang B, Liu P, Tang R. Cellular shellization: surface engineering gives cells an exterior. Bio Essays. 2010;32:698–708. doi: 10.1002/bies.200900120. [DOI] [PubMed] [Google Scholar]
- 69.Kröger N, Deutzmann R, Bergsdorf C, Sumper M. Species-specific polyamines from diatoms control silica morphology. Proc Natl Acad Sci USA. 2000;97:14133–14138. doi: 10.1073/pnas.260496497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pohnert G. Biomineralization in diatoms mediated through peptide- and polyamine-assisted condensation of silica. Angew Chem Int Ed. 2002;41:3167–3169. doi: 10.1002/1521-3773(20020902)41:17<3167::AID-ANIE3167>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez-McQuire R, Green DW, Partridge KA, Oreffo RO, Mann S, Davis SA. Coating of human mesenchymal cells in 3D culture with bioinorganic nanoparticles promotes osteoblastic differentiation and gene transfection. Adv Mater. 2007;19:2236–2240. [Google Scholar]
- 72.Lee J, Choi J, Park JH, Kim MH, Hong D, Cho H, Yang SH, Choi IS. Cytoprotective silica coating of individual mammalian cells through bioinspired silicification. Angew Chem Int Ed. 2014;53:8056–8059. doi: 10.1002/anie.201402280. [DOI] [PubMed] [Google Scholar]
- 73.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 75.Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 76.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swiston AJ, Cheng C, Um SH, Irvine DJ, Cohen RE, Rubner MF. Surface functionalization of living cells with multilayer patches. Nano Lett. 2008;8:4446–4453. doi: 10.1021/nl802404h. [DOI] [PubMed] [Google Scholar]
- 78.Swiston AJ, Gilbert JB, Irvine DJ, Cohen RE, Rubner MF. Freely suspended cellular “backpacks” lead to cell aggregate self-assembly. Biomacromolecules. 2010;11:1826–1832. doi: 10.1021/bm100305h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ravi S, Krishnamurthy VR, Caves JM, Haller CA, Chaikof EL. Maleimide–thiol coupling of a bioactive peptide to an elastin-like protein polymer. Acta Biomater. 2012;8:627–635. doi: 10.1016/j.actbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarkar D, Vemula PK, Teo GS, Spelke D, Karnik R, Wee le Y, Karp JM. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjugate Chem. 2008;19:2105–2109. doi: 10.1021/bc800345q. [DOI] [PubMed] [Google Scholar]
- 82.Cheng H, Kastrup CJ, Ramanathan R, Siegwart DJ, Ma M, Bogatyrev SR, Xu Q, Whitehead KA, Langer R, Anderson DG. Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS nano. 2010;4:625–631. doi: 10.1021/nn901319y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anselmo AC, Gilbert JB, Kumar S, Gupta V, Cohen RE, Rubner MF, Mitragotri S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: a generalized strategy to deliver drugs to treat inflammation. J Control Release. 2015;199:29–36. doi: 10.1016/j.jconrel.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 84.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol cell Bio. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 85.Akita H, Kudo A, Minoura A, Yamaguti M, Khalil IA, Moriguchi R, Masuda T, Danev R, Nagayama K, Kogure K. Multi-layered nanoparticles for penetrating the endosome and nuclear membrane via a step-wise membrane fusion process. Biomaterials. 2009;30:2940–2949. doi: 10.1016/j.biomaterials.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Hatakeyama H, Ito E, Akita H, Oishi M, Nagasaki Y, Futaki S, Harashima H. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J Control Release. 2009;139:127–132. doi: 10.1016/j.jconrel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi S, Nakase I, Kawabata N, Yu HH, Pujals S, Imanishi M, Giralt E, Futaki S. Cytosolic targeting of macromolecules using a pH-dependent fusogenic peptide in combination with cationic liposomes. Bioconjugate Chem. 2009;20:953–959. doi: 10.1021/bc800530v. [DOI] [PubMed] [Google Scholar]
- 88.Ruan G, Agrawal A, Marcus AI, Nie S. Imaging and tracking of tat peptide-conjugated quantum dots in living cells: new insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J Am Chem Soc. 2007;129:14759–14766. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- 89.Ankrum JA, Miranda OR, Ng KS, Sarkar D, Xu C, Karp JM. Engineering cells with intracellular agent–loaded microparticles to control cell phenotype. Nat Protoc. 2014;9:233–245. doi: 10.1038/nprot.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi M, Ran, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 91.Madsen SJ, Baek S, Kuk, Makkouk AR, Krasieva T, Hirschberg H. Macrophages as cell-based delivery systems for nanoshells in photothermal therapy. Ann Biomed Eng. 2012;40:507–515. doi: 10.1007/s10439-011-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D, Yang JS, Kim S, Kim YK, Seong SY. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6:675–682. doi: 10.1038/nnano.2011.149. [DOI] [PubMed] [Google Scholar]
- 93.Huang X, Zhang F, Wang H, Niu G, Choi KY, Swierczewska M, Zhang G, Gao H, Wang Z, Zhu L, Choi HS, Lee S, Chen X. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013;34:1772–1780. doi: 10.1016/j.biomaterials.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohsumi Y, Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983;258:5614–5617. [PubMed] [Google Scholar]
- 95.Ma X, Chen H, Yang L, Wang K, Guo Y, Yuan L. Construction and potential applications of a functionalized cell with an intracellular mineral scaffold. Angew Chem Int Ed. 2011;50:7414–7417. doi: 10.1002/anie.201100126. [DOI] [PubMed] [Google Scholar]
- 96.Shamsaie A, Jonczyk M, Sturgis J, Robinson JP, Irudayaraj J. Intracellularly grown gold nanoparticles as potential surface-enhanced Raman scattering probes. J Biomed Opt. 2007;12:020502. doi: 10.1117/1.2717549. [DOI] [PubMed] [Google Scholar]
- 97.Liu Z, Hu C, Li S, Zhang W, Guo Z. Rapid intracellular growth of gold nanostructures assisted by functionalized graphene oxide and its application for surface-enhanced Raman spectroscopy. Anal Chem. 2012;84:10338–10344. doi: 10.1021/ac3023907. [DOI] [PubMed] [Google Scholar]
- 98.Yu J, Patel SA, Dickson RM. In Vitro and Intracellular Production of Peptide-Encapsulated Fluorescent Silver Nanoclusters. Angew Chem Int Ed. 2007;119:2074–2076. doi: 10.1002/anie.200123456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones BD, Falkow S. SALMONELLOSIS: Host Immune Responses and Bacterial Virulence Determinants1. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 100.Wang G, Wang L, Liu P, Yan Y, Xu X, Tang R. Extracellular silica nanocoat confers thermotolerance on individual cells: a case study of material-based functionalization of living cells. Chem Bio Chem. 2010;11:2368–2373. doi: 10.1002/cbic.201000494. [DOI] [PubMed] [Google Scholar]
- 101.Bolton EM, Bradley JA. Avoiding immunological rejection in regenerative medicine. Regen Med. 2015;10:287–304. doi: 10.2217/rme.15.11. [DOI] [PubMed] [Google Scholar]
- 102.Evgenov NV, Medarova Z, Pratt J, Pantazopoulos P, Leyting S, Bonner-Weir S, Moore A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55:2419–2428. doi: 10.2337/db06-0484. [DOI] [PubMed] [Google Scholar]
- 103.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 104.Calafiore R, Basta G. Clinical application of microencapsulated islets: Actual prospectives on progress and challenges. Adv Drug Deliver Rev. 2014;67:84–92. doi: 10.1016/j.addr.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 105.Zhi Z, Singh J, Hope DCD, Peter JM. Assembly of bioactive multilayered nanocoatings on pancreatic islet cells: incorporation of α1-antitrypsin into the coating. Chem Comm. 2015:10652–10655. doi: 10.1039/c5cc02570b. [DOI] [PubMed] [Google Scholar]
- 106.Bhirde A, Xie J, Swierczewska M, Chen X. Nanoparticles for cell labeling. Nanoscale. 2011;3:142–153. doi: 10.1039/c0nr00493f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhargava A, Mishra D, Banerjee S, Mishra PK. Dendritic cell engineering for tumor immunotherapy: from biology to clinical translation. Immunotherapy. 2012;4:703–718. doi: 10.2217/imt.12.40. [DOI] [PubMed] [Google Scholar]
- 108.Noh YW, Lim YT, Chung BH. Noninvasive imaging of dendritic cell migration into lymph nodes using near-infrared fluorescent semiconductor nanocrystals. FASEB J. 2008;22:3908–3918. doi: 10.1096/fj.08-112896. [DOI] [PubMed] [Google Scholar]
- 109.Wang C, Irudayaraj J. Multifunctional magnetic®Coptical nanoparticle probes for simultaneous detection, separation, and thermal ablation of multiple pathogens. Small. 2010;6:283–289. doi: 10.1002/smll.200901596. [DOI] [PubMed] [Google Scholar]
- 110.Kim J, Piao Y, Hyeon T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem Soc Rev. 2009;38:372–390. doi: 10.1039/b709883a. [DOI] [PubMed] [Google Scholar]
- 111.Pichler BJ, Kolb A, gele TN, Schlemmer HP. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med. 2010;51:333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 112.Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110:3146–3195. doi: 10.1021/cr9003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y, Shao J, Xiang L, Dong X, Zhang G. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell B. 2008;40:815–820. doi: 10.1016/j.biocel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 114.Li Z, Suzuki Y, Huang M, Cao F, Xie X, Connolly AJ, Yang PC, Wu JC. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pierige F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliver Rev. 2008;60:286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 116.Wang C, Sun X, Cheng L, Yin S, Yang G, Li Y, Liu Z. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer. Adv Mater. 2014;26:4794–4802. doi: 10.1002/adma.201400158. [DOI] [PubMed] [Google Scholar]
- 117.Huang X, Zhang F, Wang Y, Sun X, Choi KY, Liu D, Choi J, Sil, Shin T, Hyun, Cheon J, Niu G, Chen X. Design considerations of iron-based nanoclusters for noninvasive tracking of mesenchymal stem cell homing. ACS nano. 2014;8:4403–4414. doi: 10.1021/nn4062726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mikhaylov G, Mikac U, Magaeva AA, Itin VI, Naiden EP, Psakhye I, Babes L, Reinheckel T, Peters C, Zeiser R. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat Nanotechnol. 2011;6:594–602. doi: 10.1038/nnano.2011.112. [DOI] [PubMed] [Google Scholar]
- 119.Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: Taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Millan CG, Marinero MLS, Castaneda AZ, Lanao JM. Drug, enzyme and peptide delivery using erythrocytes as carriers. J Control Release. 2004;95:27–49. doi: 10.1016/j.jconrel.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 121.Hu CMJ, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv Healthc Mater. 2012;1:537–547. doi: 10.1002/adhm.201200138. [DOI] [PubMed] [Google Scholar]
- 122.Schmitz B, Radbruch A, Kümmel T, Wickenhauser C, Korb H, Hansmann M, Thiele J, Fischer R. Magnetic activated cell sorting (MACS)—a new immunomagnetic method for megakaryocytic cell isolation: comparison of different separation techniques. Eur J Haematol. 1994;52:267–275. doi: 10.1111/j.1600-0609.1994.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 123.Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 124.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 125.Walkey CD, Chan WCW. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41:2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- 126.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliver Rev. 2011;63:943–955. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 127.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sarkar D, Ankrum JA, Teo GS, Carman CV, Karp JM. Cellular and extracellular programming of cell fate through engineered intracrine-, paracrine-, and endocrine-like mechanisms. Biomaterials. 2011;32:3053–3061. doi: 10.1016/j.biomaterials.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z, Wang Y, Wang Z, Zhao J, Gutkind JS, Srivatsan A, Zhang G, Liao HS, Fu X, Jin A, Tong X, Niu G, Chen X. Polymeric nanovehicle regulated spatiotemporal real-time imaging of the differentiation dynamics of transplanted neural stem cells after traumatic brain injury. ACS Nano. 2015;9:6683–6695. doi: 10.1021/acsnano.5b00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Katritsis D, Kaiktsis L, Chaniotis A, Pantos J, Efstathopoulos EP, Marmarelis V. Wall shear stress: theoretical considerations and methods of measurement. Prog Cardiovasc Dis. 2007;49:307–329. doi: 10.1016/j.pcad.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 131.Davies PF, Dewey CF, Jr, Bussolari SR, Gordon EJ, Gimbrone MA., Jr Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984;73:1121. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Mei N, Zhang Y, Chen Y, Guo X, Ding W, Ali SF, Biris AS, Rice P, Moore MM, Chen T. Silver nanoparticle-induced mutations and oxidative stress in mouse lymphoma cells. Environ Mol Mutagen. 2012;53:409–419. doi: 10.1002/em.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]