Abstract
The modern day drug delivery technology is only 60 years old. During this period numerous drug delivery systems have been developed. The first generation (1950–1980) has been very productive in developing many oral and transdermal controlled release formulations for clinical applications. On the other hand, the second generation (1980–2010) has not been as successful in generating clinical products. This is in large part due to the nature of the problems to overcome. The first generation of drug delivery technologies dealt with physicochemical problems, while the second struggled with biological barriers. Controlled drug delivery systems can be made with controllable physicochemical properties, but they cannot overcome the biological barriers. The third generation (from 2010) drug delivery systems need to overcome both physicochemical and biological barriers. The physicochemical problems stem from poor water solubility of drugs, large molecular weight of peptide and protein drugs, and difficulty of controlling drug release kinetics. The biological barriers to overcome include distribution of drug delivery systems by the body rather than by formulation properties, limiting delivery to a specific target in the body. In addition, the body's reaction to formulations limits their functions in vivo. The prosperous future of drug delivery systems depends on whether new delivery systems can overcome limits set by human physiology, and the development process can be accelerated with new ways of thinking.
Keywords: drug delivery, history, physicochemical barriers, biological barriers
Graphical abstract
1. Drugs and Drug Delivery Systems
Drug delivery systems exist to provide a more effective way to deliver drugs. The most important ingredient in any formulation is the drug. All other ingredients, collectively known as excipients, in a formulation are used to make the drug more effective. Once in a while, a newly developed drug becomes a blockbuster drug, i.e., the annual sales exceed $1 billion. The blockbuster drugs during the last few years include those treating hypercholesterolemia (e.g., Lipitor and Crestor), acid reflux (e.g., Nexium), arthritis (e.g., Humira, Enbrel, and Remicade), depression (Seroquel, Cymbalta, and Zyprexa), and asthma (Advair and Singular). Of these, Seroquelis unique in formulation as it employs a sustained release technology for once-a-day delivery of quetiapine. Quite often, sustained release versions of drug formulations are developed for product lifecycle management[1]. Thus, the sustained release technology is important to make existing drugs more effective.
When a new drug is developed, it is usually formulated into a simplest possible dosage form that is effective in treating the intended disease. Different drugs have different physicochemical and biological properties, necessitating different formulations. This point is made here by comparing oral and parenteral routes of administration. Table 1 lists some of the drug properties that need to be considered for finding suitable delivery systems. Since oral delivery is the most convenient and widely used route of drug administration, it is the first to consider. Some drugs, however, have very poor water solubility or very poor permeability across the cells, making it difficult to develop oral formulations. In addition, a recent breed of biotech drugs, such as peptides, proteins, and nucleic acids, are much larger than the traditional small molecular drugs. They are usually delivered by parenteral routes due to their large size, limited stability, and short half-life.
Table 1.
Drugs with different properties requiring different delivery systems.
| Drugs | |||||||
|---|---|---|---|---|---|---|---|
| Small Molecules | Large Molecules | ||||||
| BCS Class I |
BCS Class II |
BCS Class III |
BCS Class IV |
Peptides | Proteins | Nucleic Acids |
|
| Molecular weight | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ |
| Water solubility | ↑ | ↓ | ↑ | ↓ | ↔ | ↔ | ↔ |
| Cell permeability | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Half life | ↕ | ↕ | ↕ | ↕ | ↓ | ↓ | ↓ |
| Main delivery route | Oral | Oral | Oral | Oral | Parenteral | Parenteral | Parenteral |
BCS: Biopharmaceutics Classification System
| ↓: Low | ↑: high | ↔: Acceptable | ↕: Individual variation from low to high |
2. History of drug delivery technologies
Before 1950, all drugs were made into pill or capsule formulations that released the loaded drug immediately upon contact with water without any ability to control the drug release kinetics. In 1952, Smith Klein Beecham introduced the first sustained release formulation that was able to control the drug release kinetics and achieve 12-hour efficacy[2]. The technology, known as the Spansule technology, allowed control of the drug release kinetics at a predetermined rate. In the early days when the new controlled drug delivery technology began, various terms were introduced to describe newer formulations having minor differences each other. Controlled release formulations included those with sustained release, timed release, extended release, and others. Of these, the term “sustained release” has been used more widely than any other names. These terms, however, are used interchangeably nowadays. After several decades of advances in drug delivery technologies, the small differences in the functions that different names entail have become unnecessary.
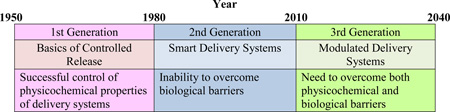
The history of controlled drug delivery field is described in Table 2. Most of the fundamental understanding on the drug release mechanisms, especially oral and transdermal dosage forms, was obtained during the first generation (1G) of development from 1950 to 1980. This period identified four drug release mechanisms that accelerated development of numerous oral and transdermal controlled release formulations. The most widely used mechanisms were dissolution-controlled and diffusion-controlled systems. Osmosis-based formulations gained a transient popularity, but the number of products based on osmosis is orders of magnitude smaller than those with the other two. The ion-exchange mechanism distinguishes itself from the others, but it has not been useful without combining with diffusion-controlled mechanism. Even today, many oral once-a-day formulations are developed based on the dissolution- or diffusion-controlled mechanism. Since oral delivery is the most convenient mode of drug administration, oral sustained release formulations will continue to flourish.
Table 2.
History of drug delivery technology from 1950 to the present and the technology necessary for the future.
| Year | ||
|---|---|---|
| 1950 1980 2010 2040 | ||
| 1st Generation | 2nd Generation | 3rd Generation |
| Basics of Controlled Release | Smart Delivery Systems | Modulated Delivery Systems |
Oral delivery
|
Zero-order release
|
Poorly soluble drug delivery
|
Transdermal delivery
|
Peptide & protein delivery
|
Peptide & protein delivery
|
Drug release mechanisms
|
Smart polymers & hydrogels
|
Smart polymers & hydrogels
|
Nanoparticles
|
Targeted drug delivery
|
|
| Successful control of physicochemical properties of delivery systems | Inability to overcome biological barriers | Need to overcome both physicochemical and biological barriers |
Unlike 1G drug delivery formulations, the second generation (2G) technologies have been less successful, as measured by the number of clinical products produced. One of the reasons for this is that the 2G technologies deal with more difficult formulations. For example, injectable depot formulations made of biodegradable poly(lactic-co-glycolic acid) (PLGA) are designed to deliver peptide and protein drugs for a month or longer. Most depot formulations have a difficult time controlling the initial burst release, which often releases 50% of the total drug in the first day or two[3]. During the 2G period, pulmonary delivery systems for insulin have been also developed. Pulmonary insulin delivery system was developed, but its lower bioavailability required delivery of several times more drug than required by parenteral injection. This, in turn, resulted in unexpected side effects that, along with other factors, caused withdrawal of the product from the market[4]. In an alternative approach, various self-regulated insulin delivery systems have been developed over the years[5–8]. Self-regulated insulin delivery systems work reasonably well in the laboratory setting, but they lose the function soon after implanted in vivo. The last decade of the 2G period (i.e., 2000~2010)has focused on tumor-targeted drug delivery using nanoparticles. The seemingly promising nanoparticle approaches based on small animal models have not been successful in numerous clinical trials[9, 10]. The limited successes of the 2G technologies need careful analysis to make the current 3G technologies prepared for eventual clinical applications.
3. Differences between 1G and 2G Drug Delivery Technologies
Development of more clinical products based on the 3G technologies, which are still under development, requires understanding why most of the 2G technologies have not been translated into clinical products. Huge successes of the 1G technology are mainly based on the oral and transdermal drug delivery systems. In these formulations, adjusting in vitro drug release kinetics has a direct effect on the in vivo pharmacokinetics. For oral and transdermal systems, the relationships between in vitro drug release kinetics and in vivo bioavailability are fairly well understood. Once the in vitro-in vivo correlation (IVIVC) of a formulation is established, other formulations using different mechanisms can be easily produced with an expectation that the new systems will be as effective as the reference formulation [11, 12]. For most drug delivery systems developed in the 1G period, mainly for oral and transdermal delivery, understanding the physicochemical properties (e.g., in vitro drug release kinetics) was enough for developing clinically useful formulations. No particular biological barriers were identified for those formulations, except for the inability to overcome the limited gastrointestinal (GI) transit time and the different absorption properties by different segments in the GI tract (i.e., absorption window) of oral formulations.
The drug delivery systems developed during the 2G period dealt with more difficult problems. The technologies developed during the 2G period are listed in Table 2. Various oral controlled release formulations were developed to achieve zero-order release, but the zero-order release achieved in various in vitro dissolution systems did not result in maintenance of the constant drug concentration in vivo, mainly due to the variations in the drug absorption properties along the GI tract. Drug absorption is controlled by the biological barrier, in addition to the drug release kinetics from oral formulations. More importantly, maintaining the constant drug concentration in the blood is not necessary, as long as the drug concentration is above the minimal therapeutically effective concentration [13]. The 2G period also introduced sustained release formulations of peptide/protein drugs after implantation in the body[14, 15]. The drug release from a formulation in vivo depends not only on the formulation properties, but also on the biological environment surrounding the implanted formulation. This makes prediction of the drug release kinetics in vivo, and thus, bioavailability, more difficult. Simply put, IVIVC has not been found for most parenteral formulations of biotech drugs, making it difficult to predict the in vivo bioavailability from the in vitro release profiles, especially for long-term depot formulations [16]. Furthermore, there are no standard in vitro drug release test methods that can reliably predict in vivo pharmacokinetic profiles[17]. The difficulty of predicting in vivo behavior of drug delivery systems is aggravated for self-regulated insulin delivery systems. Upon introduction to the body, modulated insulin delivery systems fail to function after a day or two due to the interference with proteins and cells present in the body[18]. Recent uses of nanotechnology for tumor-targeted drug delivery is another casualty of inadequate understanding of the effects of the body on drug delivery systems[13]. In short, the difficulty faced by the 2G drug delivery systems is mainly due to the inability of the drug delivery systems to overcome biological barriers.
4. The 3G Drug Delivery Technologies
The limited success of the 2G drug delivery technologies is, in large part, due to their inability to overcome the body responses after drug delivery systems are administered by parenteral route. The current drug delivery systems, however smart they may have been constructed, are not able to deal with challenges posed by the biological environment which is not-well understood and unpredictable. For the 1G formulations, controlling physicochemical properties, such as water solubility and cell permeability, were adequate enough to establish IVIVC. The 3G drug delivery technologies will have to be advanced much beyond the 2G technologies to overcome both physicochemical and biological barriers. As a brief review of the 2G technologies above indicates, understanding and overcoming the biological barriers, in addition to physicochemical barriers, is the key for success. Some of the barriers to overcome for developing successful 3G drug delivery systems are listed in Table 3. There are many other drug delivery systems that need to be developed during the 3G period. The four areas in Table 3 are discussed here solely to emphasize the importance of understanding and overcoming biological barriers.
Table 3.
Barriers to overcome by the 3G drug delivery systems.
| Delivery Technology | Formulation Barriers | Biological Barriers |
|---|---|---|
| Poorly water-soluble drug delivery |
|
|
| Peptide/protein/nucleic acid delivery |
|
|
| Targeted drug delivery using nanoparticles |
|
|
| Self-regulated drug delivery |
|
|
4.1. Delivery of poorly water-soluble drugs
Poor water solubility of drugs was one of the most important problems in drug development, and it still remains to be true today. Discussion on poorly soluble drugs requires understanding of the meaning of drug solubility. Table 4 shows the descriptive terms used in U.S. Pharmacopeial and National Formulary to indicate approximate drug solubilities in water. The term “poorly soluble” is commonly used to describe drugs that belong to the “practically insoluble” category. For these drugs the aqueous solubility is 0.1 mg/mL or less, i.e., 100 µg/mL or less. Many new drug candidates are poorly water soluble, and thus, a large portion of the candidate drugs are not translated into clinically useful formulations. Analysis of 200 orally administered drug products showed that practically insoluble drugs account for almost 40% of the total drugs [19]. Delivering these drugs effectively through the GI tract for therapeutically effective bioavailability remains an important issue. The dissolution rate of practically insoluble drugs may be so slow that dissolution takes longer than the GI transit time resulting in therapeutically unacceptable bioavailability [20].
Table 4.
Solubility Definitions.
| Descriptive Terms | Parts of Solvent Required for 1 Part of Solute |
Solubility Range | |
|---|---|---|---|
| mg/mL | % | ||
| Very soluble | Less than 1 | > 1,000 | >100 |
| Freely soluble | From 1 to 10 | 100 ~ 1,000 | 10 ~ 100 |
| Soluble | From 10 to 30 | 33 ~ 100 | 3.3 ~ 10 |
| Sparingly soluble | From 30 to 100 | 10 ~ 33 | 1 ~ 3.3 |
| Slightly soluble | From 100 to 1,000 | 1 ~ 10 | 0.1 ~ 1 |
| Very slightly soluble | From 1,000 to 10,000 | 0.1 ~ 1 | 0.01 ~ 0.1 |
| Practically insoluble | 10,000 and over | ≤0.1 | ≤0.01 |
Technologies to dissolve poorly soluble drugs in water have been studied for decades, and some of the methods are listed in Table 5. Poorly soluble drugs have inherently low water solubility, and thus, suitable excipients are added to increase the solubility by using surfactants, polymer micelles, hydrotropic agents, complexing agents (e.g., cyclodextrins and proteins), cosolvents, and lipid formulations (e.g., self-emulsifying systems)[21–23]. For weakly acidic or basic drugs, pH can be controlled to increase the drug solubility. Alternative to increasing the drug solubility, drug dissolution kinetics can be enhanced through selecting appropriate polymorph, making solid dispersions (i.e., maintaining amorphous structure of the drug using polymers), reducing drug particle size, and increasing wetting with surfactants. Of these, the solid dispersion approach has been widely used for its ease of preparation and efficacy [24–26]. Making drug nanocrystals has also been frequently used, as the increase in bioavailability by increasing the drug crystal surface resulted in improved bioavailability [23]. The surface area increases proportionally as the decrease in the size of drug particles. The drug solubility is an inherent property and so it should not change as the dissolution kinetics increases. But increasing the dissolution kinetics can result in improved bioavailability of oral formulations. Enhanced dissolution of the drug can produce the dissolved drug in sufficient quantity fast enough to replace those drugs that have been absorbed from the GI tract, thereby improving bioavailability.
Table 5.
Methods to improve drug dissolution.
| Enhancing Drug Solubility | Enhancing Dissolution Kinetics |
|---|---|
| Using surfactant micelles | Selecting appropriate polymorph |
| Using polymer micelles | Making amorphous forms (solid dispersions) |
| Using hydrotropic agents | Reducing particle size (nanocrystals) |
| Using complexing agents | Adding surfactant for better wetting |
| Using cosolvents | |
| Using self-emulsifying systems | |
| Controlling pH |
The problem of poor water solubility becomes even more serious for intravenous formulations. For example, many anticancer drugs are extremely poorly water soluble, e.g., <1 µg/mL, and thus they are usually dissolved in organic solvents. Paclitaxel and docetaxel are good examples of poorly soluble drugs making injectable formulations difficult. There are various injectable formulations of paclitaxel: Taxol utilizing Cremophor® EL [27], Abraxane® based on paclitaxel-albumin complex[23, 28, 29], and Genexol® utilizing PEG-PLA polymer micelle [30]. Taxotere, delivering docetaxel, a derivative of paclitaxel, is dissolved in polysorbate 80 which is suspected to cause hypersensitivity [31, 32]. Cremophor EL, an excipient used to increase the solubility of paclitaxel, can cause serious hypersensitivity reactions and kill patients if the patient is not properly preconditioned[27]. Development of new drug delivery systems for poorly soluble drugs without using organic solvent is important for bringing promising new drug candidates to clinical applications and more effective use of existing drugs.
4.2. Peptide/protein/nucleic acid delivery
Macromolecular drugs, such as peptides, proteins, and nucleic acids, are usually delivered by parenteral administration. They are too big to cross the intestinal epithelium, i.e., to be absorbed from the GI tract[33]. A number of attempts have been made to protect them from the harsh acidic condition of the stomach by enteric coating, and from enzymatic degradation by adding enzyme inhibitors. These attempts, however, do not address the real issue that proteins cannot be absorbed without enzymatic degradation into small molecules[34, 35]. It has been suggested that nanoparticles can be translocated across M cells in Peyer’s patches and enterocytes in the villus part of the intestine, but the extent of particle absorption has been controversial [36]. The absorbed amount is too low and too irreproducible to have therapeutic significance. Thus, these macromolecular drugs are mainly delivered by parenteral routes. Recently, new approaches have been attempted to deliver them by non-invasive, or minimally-invasive means, such as pulmonary, nasal, and transdermal delivery [37].
Macromolecular drugs usually have very short half-lives, ranging from minutes to hours, and thus, sustained release for months requires depot formulations. There are more than a dozen depot formulations that are administered by parenteral routes. They include Zoladex® Depot (goserelin acetate), Lupron Depot® (leuprolide acetate), Sandostatin LAR® Depot (octreotide acetate), Nutropin Depot® (somatropin), Trelstar® (triptorelin pamoate), Suprefact® Depot (Buserelin acetate), Somatuline® Depot (lanreotide), Arestin® (minocycline HCl), Eliaard (leuprolide acetate), Risperdal® CONSTA® (risperidone), Vivitrol® (naltrexone), Ozurdex® (dexamethasone), and Bydureon® (exenatide). The fact that there are only a handful of depot formulations, as compared with thousands of oral sustained release formulations, indicates the difficulty associated with developing parenteral depot formulations. The majority of these formulations have the initial burst release, resulting in the initial peak blood concentration much larger (up to 100 times) than the therapeutically effective concentration at the steady state (i.e., after drug concentration at the steady state after the initial peak). Thus, it is urgently required to improve the technology of controlling the drug release profiles. The ability of controlling drug release kinetics becomes even more important as the drug loading increases. Depot formulations designed to have longer duration need higher drug loading. Thus, patient-friendly depot formulations must have higher drug loading with controllable drug release kinetics for a long-period of time, up to 1 year, or even longer.
4.2.1. The Initial Burst Release from PLGA Depot Formulations
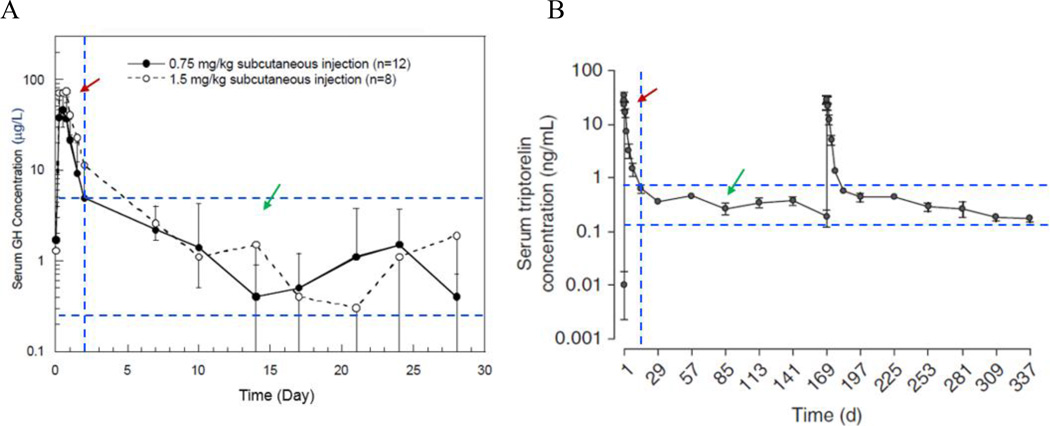
Examples of pharmacokinetic profiles of two clinically used depot products are shown in Figure 1. Each PK profile can be divided into two regions: the initial burst release region (red arrows in Figure 1) and therapeutically effective region (green arrows in Figure 1). The Y axis in Figure 1 is in the log scale, and the peak concentration in the initial burst region is about 100 times larger than the concentrations that are in the therapeutically effective range. This observation brings a few questions. First, is it really necessary to have 100 times higher drug concentration in the first day or two than the known therapeutically effective drug concentration? Second, does the initial burst release play any role in the efficacy of the drug at the steady state? There is no scientific reason to justify that the initial burst release is necessary for the therapeutic effect. The initial burst release is simply an outcome of the emulsion methods for microparticle production available a few decades ago. Some may argue that the initial peak concentration in blood may be necessary for therapeutic efficacy. This, however, cannot be true, because it implies that daily injection of the same drug without the peak concentration should not work. This, of course, is not the case. It is the drug concentrations in the therapeutically effective region that is important. Controlling the initial burst release is still not easy, but improved understanding on the emulsion methods and recent development of new microfabrication processes have made it possible to reduce or eliminate the initial burst release.
Figure 1.
Examples of pharmacokinetic profiles of Nutropin Depot (A) and Trelstar (B) (obtained from the packaging inserts). The red arrow indicates the PK region resulting from the initial burst release of a drug, and the green arrow indicates the PK region of the therapeutically effective drug concentrations.
4.3. Targeted drug delivery using nanoparticles
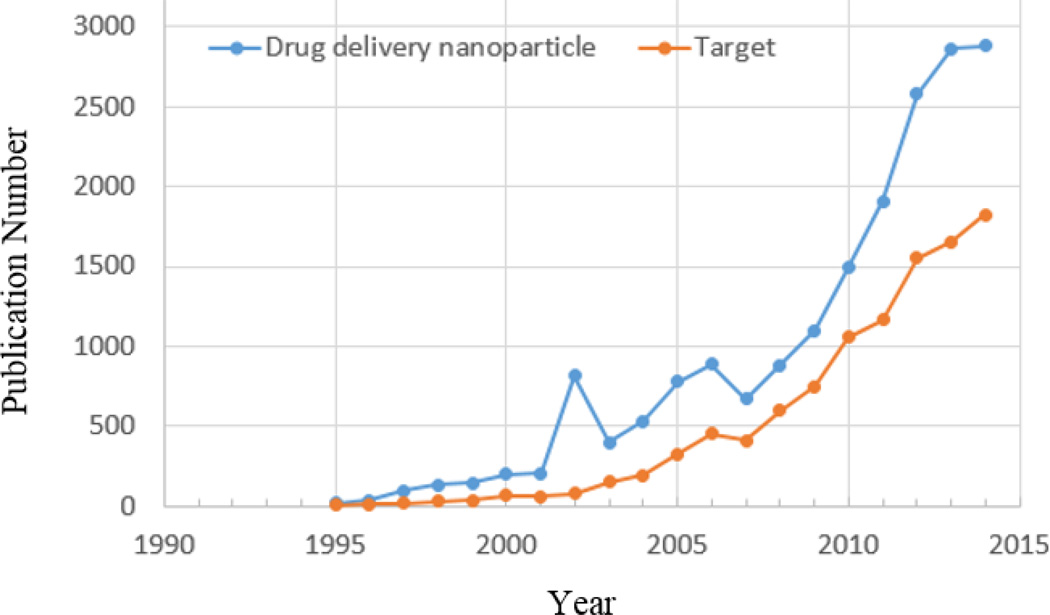
Nanoparticle-based drug delivery systems have been used extensively for the last few decades. A search in SciFinder using “drug delivery nanoparticle” resulted in 19,950 references during 1995–2014 (Figure 2). Of these, 57% are associated with the term “target” for targeted drug delivery or targeting. Clearly, the majority of the studies on nanoparticle-based drug delivery has been focused on targeted drug delivery, mainly on tumor-targeted drug delivery.
Figure 2.
The number of articles on nanoparticle drug delivery systems published from 1995 to 2014. In SciFinder, the research topic of “drug delivery nanoparticle” was used for the initial search to find more than 30,000 references containing the concept. The search was further refined using the research topic of “target”.
The initial excitement on nanoparticulate drug delivery systems arose from the ability of producing nanoparticles in various size and shape, and the ability to control the physicochemical and surface properties to make smart nanoparticles. Many of these systems have worked well in the laboratory where cell culture systems were used for testing drug delivery. The systems also worked reasonably well in small animal models, mostly xenograft mouse models. The nanoparticle systems showing promising results in those models have not been translated into clinical studies[38, 39]. The current nanoparticles cannot control their fate after intravenous administration. The so-called “targeting” by nanoparticles is a misleading concept, because the current nanoparticles cannot find their way to an intended target, but are simply distributed throughout the body by the blood circulation[40]. Only a very small fraction of the total administered nanoparticles end up at the target site, mostly by chance. The concept of the enhanced permeability and retention (EPR) effect is frequently cited whenever nanoparticles are used for drug delivery to tumors. However, most studies have not quantitatively measured the actual amount of drugs reaching the target tumor, and thus, there is no quantitative information on the role of the EPR effect in targeted drug delivery. The tumors grown in mice are usually 1~2 mm which are similar in size as the liver, but only a small fraction, in the range of about 1% of the total administered dose, of the so-called targeted nanoparticles end up at the tumors, while the majority ends up at the liver [41]. For nanoparticle systems to become a clinically effective tool for targeted drug delivery, they may have to be designed differently from those showing potential in small animal studies. The observations made in mice, which have only a few milliliters of blood, may not be extended to human with 5 liters of blood. Furthermore, the size ratio of a tumor in a mouse is usually much larger than that of a tumor in a human. This massive scale differences need to be considered when experimental animal models are used and their data are analyzed.
Nanoparticles may have unexpected benefits, even though the anticipated targeting has not been observed yet. The nanoparticles, with suitable surface modification, may alter the biodistribution, which in turn, may alter the toxicity profiles of the same drug. In fact, reducing the toxicity, or the side effects, of the drug through engineering nanoparticle formulations may be a better way of utilizing the unique properties of nanoparticles. Doxil®, the PEGylated liposome formulation, is a case in point. It was approved by the U.S. Food and Drug Administration not because of its improved drug efficacy, but because of its reduced cardiotoxicity [42]. Considering the difficulties in translating the targeting ability observed in mouse models to clinical applications, one could consider utilizing nanoparticle formulations for reducing the toxicity. This can be achieved not only by altering the biodistribution, but also by increasing the water-solubility without using toxic organic solvents. Good examples of this approach are Abraxane® and Genexol® as described above. Formulations without organic solvents, such as Cremophor EL or polysorbate, are certainly more desirable, especially when the resulting therapeutic efficacy is about the same [43].
4.4. Self-regulated drug delivery
Self-regulated drug delivery, in particular, self-regulated insulin delivery, remains one of the most important technologies to develop. Imagine that millions of diabetes patients can take care of their glucose level for months with one injection of self-regulated insulin delivery system, instead of multiple daily injections of insulin. There are several self-regulated insulin delivery systems developed over the years which work well in the laboratory setting[5–8, 44, 45]. As soon as they are introduced inside the body, however, their function decreases by hours. The glucose sensor, which is essential in detecting the varying glucose level, becomes less efficient due to protein adsorption and cell adhesion, and the insulin delivery module becomes less efficient after each cycle[18, 46, 47]. It has been several decades since the concept of self-regulated insulin delivery started, but the progress has been slow. This is also mainly due to the biological barriers that the body poses to the implanted device[48]. Unless the biological barriers are understood and the new delivery systems are designed to overcome those, development of self-regulated insulin delivery system will remain as a concept for a while. The biological barriers to overcome include maintaining glucose sensor specificity and sensitivity in the biological milieu. Another key requirement is to build an actuator that releases a right amount insulin fast with automatic turn-off function[13].
5. Perspective of the Future
Significant advances in drug development, along with better and early diagnostics for preventive medicine, have helped extend human life expectancy. This, in turn, requires development of more drugs for various diseases, such as coronary artery disease, diabetes mellitus, chronic pain, chronic lower respiratory disease, Alzheimer’s disease, and Parkinson’s disease. Finding drugs for these diseases is the first and most important step. The drug delivery systems can make drug candidates with poor water solubility into therapeutically effective drug formulation, and drug candidates with short half-lives into sustained release formulations. The drug delivery technologies will have valuable contributions to the development of new drugs. Various drug delivery systems need to be developed for delivering drugs with various different properties.
Advances in drug delivery systems are the results of numerous trials and errors, i.e., results of an evolutionary process. Many different drug delivery systems need to be tried, and variations of the systems with most potential need to be repeated. This process will have to continue until a proper solution is found for a disease. Trying many different approaches requires diverse ideas, instead of the same approach that others have tried for a decade or longer. For example, a large number of nanoparticle-based drug delivery systems have been developed, but they are pretty much the same approach with only minute differences. Thus, it is not surprising to see the absence of any progress by this approach. If nanoparticles are such a great tool for delivering drugs to target sites, why is it that almost all nanoparticle systems are developed for targeted delivery to tumors? There are so many other important diseases, but only a few of them were dealt with nanoparticle formulations. This is why we have to expand our imagination outside the box. If the next generation of scientists are all bound inside the current box of nanotechnology, creating new drug delivery systems will take longer. It is time to try different ideas and approaches for various diseases.
It is emphasized again that the goal of studying drug delivery systems is to develop clinically useful formulations for patients. Development of effective drug delivery systems requires clear goals. Making a new delivery system alone is not enough. It has to work in the human body, i.e., safe and effective. The goal of clinical applications poses certain restrictions, and overcoming those from the early stage of the development is critical. Developing clinically useful drug delivery systems, which many incorrectly call solving practical problems, is based on understanding properties of the drug delivery systems as well as biological barriers. There is no so-called basic study versus practical study in drug delivery. There is only the study of developing clinical formulations treating various diseases.
Acknowledgment
This work was supported by the Showalter Research Trust Fund and the National Institute of Health through CA129287 and GM095879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davar N, Ghosh S. Oral controlled release-based products for life cycle management. In: Wen H, Park K, editors. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice. New York, NY: John Wiley & Sons; 2010. pp. 305–320. [Google Scholar]
- 2.Lee PI, Li J-X. Evolution of oral controlled release dosage forms. In: Wen H, Park K, editors. Oral Controlled Release Formulation Design and Drug Delivery. Hoboken, NJ: John Wiley & Sons, Inc.; 2010. pp. 21–31. [Google Scholar]
- 3.Ye M, Kim SW, Park K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release. 2010;156:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann L. The failure of Exubera: Are we beating a dead horse? Journal of Diabetes Science and Technology (Online) 2008;2:518–529. doi: 10.1177/193229680800200325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung Wan K, Chaul Min P, Kimiko M, Seminoff LA, Holmberg DL, Gleeson JM, Wilson DE, Mack EJ. Self-regulated glycosylated insulin delivery. Journal of Controlled Release. 1990;11:193–201. [Google Scholar]
- 6.Klumb LA, Horbett TA. Design of insulin delivery devices based on glucose sensitive membranes. Journal of Controlled Release. 1992;18:59–80. [Google Scholar]
- 7.Kim JJ, Park K. Modulated insulin delivery from glucose-sensitive hydrogel dosage forms. Journal of Controlled Release. 2001;77:39–47. doi: 10.1016/s0168-3659(01)00447-3. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RA. Stimuli sensitive polymers and self regulated drug delivery systems: A very partial review. Journal of Controlled Release. 2014;190:337–351. doi: 10.1016/j.jconrel.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BK, Yun YH, Park K. Smart nanoparticles for drug delivery: Boundaries and opportunities. Chemical Engineering Science. 2015;125:158–164. doi: 10.1016/j.ces.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo MX. Dissolution testing: In vitro characterization of oral controlled release dosage forms. In: Wen H, Park K, editors. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice. New York, NY: John Wiley & Sons; 2010. pp. 245–256. [Google Scholar]
- 12.Lu Y, Kim SW, Park K. In vitro-in vivo correlation: perspectives on model development. Int. J. Pharm. 2011;418:142–148. doi: 10.1016/j.ijpharm.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park K. Controlled drug delivery systems: Past forward and future back. Journal of Controlled Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada H. Depot injectable microcapsules of leuprorelin acetate (Lupron Depot) In: Morishita M, Park K, editors. Biodrug Delivery Systems: Fundamentals, Applications and Clinical Development. Boca Raton, FL: CRC Press; 2009. pp. 370–383. [Google Scholar]
- 15.Rawat A, Burgess DJ. Parenteral delivery of peptides and proteins. In: Morishita M, Park K, editors. Biodrug Delivery Systems: Fundamentals, Applications and Clinical Development. Boca Raton, FL: CRC Press; 2009. pp. 50–68. [Google Scholar]
- 16.Cadot J-M. In vitro-in vivo correlation: Application to biotech product development. In: Morishita M, Park K, editors. Biodrug Delivery Systems: Fundamentals, Applications and Clinical Development. Boca Raton, FL: CRC Press; 2009. pp. 341–356. [Google Scholar]
- 17.Martinez M, Rathbone M, Burgess D, Huynh M. In vitro and in vivo considerations associated with parenteral sustained release products: A review based upon information presented and points expressed at the 2007 Controlled Release Society Annual Meeting. Journal of Controlled Release. 2008;129:79–87. doi: 10.1016/j.jconrel.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: Current methods and recommendations. Biomaterials. 2007;28:3687–3703. doi: 10.1016/j.biomaterials.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A Provisional Biopharmaceutical Classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Molecular Pharmaceutics. 2006;3:631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 20.Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract1. Advanced Drug Delivery Reviews. 2001;46:75–87. doi: 10.1016/s0169-409x(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 21.Pouton CW. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. European Journal of Pharmaceutical Sciences. 2006;29:278–287. doi: 10.1016/j.ejps.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. International Journal of Pharmaceutics. 2011;420:1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013;453:198–214. doi: 10.1016/j.ijpharm.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paudel A, Worku ZA, Meeus J, Guns S, Van den Mooter G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. International Journal of Pharmaceutics. 2013;453:253–284. doi: 10.1016/j.ijpharm.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Sotthivirat S, McKelvey C, Moser J, Rege B, Xu W, Zhang D. Development of amorphous solid dispersion formulations of a poorly water-soluble drug, MK-0364. International Journal of Pharmaceutics. 2013;452:73–81. doi: 10.1016/j.ijpharm.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Sun DD, Lee PI. Probing the mechanisms of drug release from amorphous solid dispersions in medium-soluble and medium-insoluble carriers. Journal of Controlled Release. 2015;211:85–93. doi: 10.1016/j.jconrel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 28.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. Journal of Controlled Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Yardley DA. nab-Paclitaxel mechanisms of action and delivery. Journal of Controlled Release. 2013;170:365–372. doi: 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, Kim NK, Bang YJ. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 31.Engels FK, Mathot RA, Verweij J. Alternative drug formulations of docetaxel: a review. Anticancer Drugs. 2007;18:95–103. doi: 10.1097/CAD.0b013e3280113338. [DOI] [PubMed] [Google Scholar]
- 32.Norris LB, Qureshi ZP, Bookstaver PB, Raisch DW, Oliver Sartor M, Chen H, Chen F, Bennett CL. Polysorbate 80 hypersensitivity reactions: a renewed call to action. Community Oncology. 2010;7:425–428. [Google Scholar]
- 33.Vander AJ, Luciano D, Sherman J. Human Physiology: The Mechanism of Body Function. Eighth Edition. Ch. 17. Boston, MA: McGraw–Hill; 2001. p. 803. [Google Scholar]
- 34.Shen W-C. Oral peptide and protein delivery: unfulfilled promises? Drug Discovery Today. 2003;8:607–608. doi: 10.1016/s1359-6446(03)02692-8. [DOI] [PubMed] [Google Scholar]
- 35.Yun Y, Cho YW, Park K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Advanced Drug Delivery Reviews. 2013;65:822–832. doi: 10.1016/j.addr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieux Ad, Fievez V, Garinot M, Schneider Y-J, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Walle CFVD. Peptide and Protein Delivery. Burlington MA: Academic Press; 2011. p. 360. [Google Scholar]
- 38.Park K. Lessons learned from thermosensitive liposomes for improved chemotherapy. J. Control. Release. 2014;174:219. doi: 10.1016/j.jconrel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Park K. Translation from mouse to human: Time to think in new boxes. Journal of Controlled Release. 2014;189:187. doi: 10.1016/j.jconrel.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 40.Nichols JW, Bae YH. EPR: Evidence and fallacy. Journal of Controlled Release. 2014;190:451–464. doi: 10.1016/j.jconrel.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 41.Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the EPR effect and image-guided drug delivery. J. Control. Release. 2013;172:12–21. doi: 10.1016/j.jconrel.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barenholz Y. Doxil® - The first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Stirland DL, Nichols JW, Miura S, Bae YH. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release. 2013;172:1045–1064. doi: 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitano S, Koyama Y, Kataoka K, Okano T, Sakurai Y. A novel drug delivery system utilizing a glucose responsive polymer complex between poly (vinyl alcohol) and poly (N-vinyl-2-pyrrolidone) with a phenylboronic acid moiety. Journal of Controlled Release. 1992;19:161–170. [Google Scholar]
- 45.Peppas NA. Is there a future in glucose-sensitive, responsive insulin delivery systems? Journal of Drug Delivery Science and Technology. 2004;14:247–256. [Google Scholar]
- 46.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17:882–887. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 47.Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids and Surfaces B: Biointerfaces. 2000;18:197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 48.Abel PU, von Woedtke T. Biosensors for in vivo glucose measurement: can we cross the experimental stage. Biosensors and Bioelectronics. 2002;17:1059–1070. doi: 10.1016/s0956-5663(02)00099-4. [DOI] [PubMed] [Google Scholar]