Abstract
Chronic pain is a significant healthcare problem, ineffectively treated due to its unclear etiology and heterogeneous clinical presentation. Emerging evidence demonstrates that microRNAs regulate the expression of pain-relevant genes, yet little is known about their role in chronic pain. Here, we evaluate the relationship between pain, psychological characteristics, plasma cytokines and whole blood microRNAs in 22 healthy controls (HC); 33 subjects with chronic pelvic pain (vestibulodynia: VBD); and 23 subjects with VBD and irritable bowel syndrome (VBD+IBS). VBD subjects were similar to HCs in self-reported pain, psychological profiles and remote bodily pain. VBD+IBS subjects reported decreased health and function; and an increase in headaches, somatization and remote bodily pain. Furthermore, VBD subjects exhibited a balance in pro- and anti-inflammatory cytokines, while VBD+IBS subjects failed to exhibit a compensatory increase in anti-inflammatory cytokines. VBD subjects differed from controls in expression of 10 microRNAs of predicted importance for pain and estrogen signaling. VBD+IBS subjects differed from controls in expression of 11 microRNAs of predicted importance for pain, cell physiology and insulin signaling. MicroRNA expression was correlated with pain-relevant phenotypes and cytokine levels. These results suggest microRNAs represent a valuable tool for differentiating VBD subtypes (localized pain with apparent peripheral neurosensory disruption versus widespread pain with a central sensory contribution) that may require different treatment approaches.
Introduction
Vestibulodynia (VBD) is a complex chronic pain condition (CCPC), characterized by entry dyspareunia, tenderness to touch and presence of erythema on the vulvar vestibule, that affects 10–15% of women in the United States.1, 2 VBD often co-occurs with other CCPCs including irritable bowel syndrome (IBS; 35%)3, temporomandibular disorder (TMD; 78%)1 and fibromyalgia (FMS; 17%).3 VBD and related CCPCs represent a significant healthcare problem, together affecting up to one billion adults worldwide.4 Current treatment regimens remain ineffective due to the conditions’ uncertain etiology and heterogeneous clinical manifestation.1 To understand the nature of these complex conditions and improve standards of care, the identification of unique biological signatures and pathways that map onto distinguishing clinical features is required.
While clinical manifestations are heterogeneous, VBD and related CCPCs are associated with a state of pain amplification, psychological distress and enhanced inflammation.5 In VBD, pain is localized to the pelvis, possibly due to altered permeability or cellular composition of the mucosa.1 Women with VBD demonstrate higher levels of anxiety and somatization as well as enhanced production of pro-inflammatory cytokines.2 Compared to individuals with one CCPC, those with co-occurring CCPCs exhibit increased psychological distress1 and imbalances in pro- and anti-inflammatory mediators possibly indicative of abnormalities in central pain processing.6
MicroRNAs (miRNAs) represent biological determinants of pain, mood and inflammation. miRNAs are small, noncoding pieces of RNA that control gene expression by inhibiting protein translation or degrading downstream target mRNAs.7 Aberrant miRNA profiles have been associated with several animal models of pain and inflammation as well as painful conditions in humans.8 Further, miRNA profiling represents a novel and clinically-relevant approach for patient stratification of pain-related conditions.9 Emerging evidence also suggests a role for miRNAs in psychological conditions such as depression and anxiety.10 Lastly, miRNAs regulate genes involved in activation of immune cells and secretion of inflammatory cytokines.11 This suggests that miRNAs are key contributors to the pain amplification, psychological distress and enhanced inflammation characteristic of CCPCs such as VBD.
We elucidate novel clinical features and biological pathways unique to women with VBD and to those with VBD plus a commonly co-occurring CCPC, IBS (VBD+IBS). Specifically, those with VBD have pain localized to the pelvis, normal self-reported pain and psychological profiles, increased levels of pro- and anti-inflammatory cytokines and dysregulation of miRNAs predicted to be involved in pain processing and estrogen signaling. Those with VBD+IBS have greater pain sensitivity at remote bodily sites, enhanced self-reported pain and somatization, imbalanced pro- and anti-inflammatory responses and dysregulation of miRNAs predicted to be involved in pain processing, cellular physiology and central sensory pathways. Collectively, these results suggest miRNA profiles may be useful for understanding the shared and unique mechanisms of localized versus widespread pain conditions.
Materials and Methods
Subject Consent and Enrollment
All subjects were enrolled after giving informed consent as approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill (UNC).
Inclusion and Exclusion Criteria
This study utilized data from 78 women (33 VBD, 23 VBD+IBS and 22 HC). Subjects in VBD and VBD+IBS groups were recruited at the UNC Pelvic Pain Clinic and subjects in the HC group were recruited through fliers placed on campus and in the local community between August 2008 and August 2010. Sample sizes were based upon prior miRNA studies.9, 12–14 Preliminary eligibility assessment of interested subjects was conducted via a phone interview. Subjects were excluded for a positive response to any of the following criteria: 1) age <21 or >45; 2) breastfeeding, pregnant or menopausal; 3) significant medical conditions such as seizure disorder, diabetes or thyroid disorder; and 4) known diagnosis of comorbid urogenital pain conditions such as interstitial cystitis. Subjects without vulvovaginal complaints were recruited as potential controls.
Subjects were then clinically assessed during a standardized gynecological exam during which they rated pain using the modified Gracely Pain Scale.15 VBD was diagnosed in women who reported a history of pain during intercourse or tampon insertion and who experienced tenderness to touch upon palpation of the vestibule with a cotton swab during the exam.16 The threshold for pain history was a rating of 3 or more using the Gracely Pain Unpleasantness Scale. During the clinical exam, subjects with suspected dermatological disorders (e.g. lichen sclerosis, contact dermatitis) and vaginismus were excluded. All exams were performed by a single examiner.
Subjects were also assessed for other pain disorders including IBS, FM, TMD and migraine headaches. IBS case status was determined using 4 abdominal pain questions in accordance with Rome III Criteria.17 Multicenter Criteria were used to classify FM, defined by widespread pain in combination with tenderness at 11 or more of the 18 specific tender point sites.18 Classification of TMD was based on the Research Diagnostic Criteria for temporomandibular disorders, with the defining characteristics being a history of facial pain and examiner-evoked pain reported in the cheeks, jaw muscles, temples or jaw joints.19 Migraine headaches (with or without aura) were classified by the International Classification of Headache Disorders (ICHD-2) criteria.20 As the majority of the VBD subjects with co-occurring pain syndromes had IBS, the following 3 groups were created: subjects with VBD alone, subjects with VBD and IBS (including those with or without additional co-occurring pain disorders) and pain-free healthy controls. Healthy controls were excluded if they reported any psychological or pain-related conditions.
Mucosal Pressure Pain Measurement
Twelve pressure points of the vestibule were defined with reference to a clinical drawing annotated with a conventional clock face. The 12 o’clock position marked the anterior midline and the 6 o’clock position marked the posterior (in dorsal lithotomy position). Six sites were assessed for pain: 3 on the upper vestibule at positions 2, 10 and 12; and 3 on the lower vestibule at points 5, 7 and 6, in that order.15 To account for subject-to-subject variability, anatomical landmarks were also used to standardize the location of vestibular sites between women. For each pelvic mucosal pain assay averaging the 6 site scores together created an aggregate score.
Intensity
In order to assess pain sensitivity in the vestibule the examiner applied pressure to each site with a Q-tip and instructed the subject to rate her pain on a scale of 0–10 (0=no pain, 10=worst imaginable). For mucosal intensity assays: HC N=21, VBD N=33 and VBD+IBS N=23.
Threshold
A digital vestibular pressure algometer designed by the Center for Pain Research and Innovation at UNC and first described by Zolnoun et al15 was used to record the threshold to pressure pain sensitivity in the vestibule. The algometer is attached to a cotton swab that was applied to each site beginning at 1N and increasing until the subject’s first sensation of pain, at which time the subject was instructed to click a computer mouse. The latency was recorded in real-time. This test was repeated on each site 3 times with an interstimulus interval of 2 seconds. Results report the average score of the 3 tests. For mucosal threshold assays: HC N=13, VBD N=31 and VBD+IBS N=20.
Muscle Pressure Pain Detection Measurement
In order to test muscle pressure pain of the vulvovaginal muscles, a pressure sensor was affixed to a plastic thimble that was worn over the investigator’s right index finger. The algometer pressure sensor, which measured forces ranging from <1N to >98N, was used for direct and isolated palpation of the right and left puborectalis levator muscles (sites 5 and 7) and perineal muscle complex (site 6). An aggregate score was calculated as the average of the 3 muscular sites. The exam to test for muscle pressure pain was conducted transvaginally in accordance with conventional clinical practice.15
Intensity
Examiner applied pressure to each site on the lower vestibule and had the subject rate her pain (0=pressure, 1=low, 2=moderate and 3=severe). For muscle intensity tests: HC N=21, VBD N=33 and VBD+IBS N=23.
Threshold
Subjects were instructed to click a computer mouse at the first sensation of pain to find the muscle pain perception threshold, which was recorded in real-time by the computer. The examiner terminated the pressure if she was unable to apply additional force to reach the subject’s threshold. For muscle threshold tests: HC N=16, VBD N=30 and VBD+IBS N=19.
Tolerance
The subject was then given the option to undergo tolerance testing, which was performed after a 5-minute rest period. 61/78, or 78% of subjects opted to participate. Subjects were instructed to click the computer mouse when they were no longer able to tolerate the pressure. For muscle tolerance tests: HC N=15, VBD N=28 and VBD+IBS N=18.
Remote Bodily Pressure Pain Threshold Measurement
A digital algometer (Wagner, Greenwich, CT, USA) was applied for 3 trials to each side of the trapezius and the temporomandibular joint (TMJ) beginning at a pressure of 1 N and increasing until the subject’s first sensation of pain, at which time the threshold force was recorded in Newtons. Final scores report the average of left and right thresholds for each site. For remote bodily pressure pain measurements: HC N=23, VBD N=30 and VBD+IBS N=20.
Remote Bodily Thermal Windup Measurement
Thermal stimuli of 50°C were applied repeatedly to the right hand to evaluate the temporal summation of thermal heat pain “windup”.21 Ratings of pain in response to 10 repeated stimuli were evaluated. Stimuli of 0.5 sec duration were repeated once every three seconds and pain was rated on a 0–100 numerical rating scale (NRS). The procedure was stopped if the participant reported a rating of 100 or if she asked that it be stopped. For thermal windup measurements: HC N=21, VBD N=30 and VBD+IBS N=19.
Assessment of Psychological and Self-reported Health Phenotypes
Prior to clinical examination, subjects completed questionnaires that collectively assessed affective components of pain, somatic symptoms related to pain, perceived control, self-rated health and mood. The McGill Pain Questionnaire (MPQ) assessed sensory components of pain using 11 verbal describers and affective qualities related to pain using 5 describers. Responses on 4-point scales were summed to compute scores for each section.6 The Short Form 12 version 2 (SF12v2) assessed 6 domains: global health, physical functioning, physical roles, emotional functioning, emotional roles and pain interference; using an algorithm22 based on answers to 12 physical and mental health related questions. Each scale ranged from 0 to 100, with higher values signifying better function and mood.6 Answers to 54 questions on the Pennebaker Index of Limbic Languidness (PILL) questionnaire were used to create a summary score of somatic symptoms (e.g. itchy eyes, dizziness). Frequency for each symptom was recorded on a five-point Likert scale ranging from “never or almost never” to “more than once a week”.6 The Comprehensive Pain and Symptom Questionnaire (CPSQ) assessed various components and effects of bodily pain (e.g. face, jaw, head and lower back pain) using individual scaled scores from answers to 55 questions.23 The Symptom Checklist 90-Revised (SCL-90R) used 90 questions in to assess a broad range of psychological symptoms such as anxiety and depression.24
Assessment of circulating cytokine protein levels
During the clinic visit, whole blood was collected into ethylenediaminetetraacetic acid-coated Vacutainer tubes (BD Biosciences, Franklin Lakes, NJ, USA) and placed on ice. Plasma was isolated by centrifuging at 1520 RPM for 10 minutes at 4°C and aliquoted into Cryotubes (Nalge Nunc International, Lima, OH, USA), frozen with liquid nitrogen and stored at −80°C. Samples were thawed on ice and the Fluorokine MAP Multiplex Human Cytokine Panel A (R&D Systems, Minneapolis, MN, USA) was used to measure the levels of 22 cytokines, including monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), macrophage inflammatory protein-1β (MIP-1β), regulated upon activation normal t-cell expressed and secreted (RANTES), epithelial-derived neutrophil-activating peptide 78 (ENA-78), fibroblast growth factor basic (FGF basic), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-1 receptor antagonist (IL-1ra), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-17 (IL-17), tumor necrosis factor α (TNFα), thrombopoietin (Tpo), and vascular endothelial growth factor (VEGF). A full list of cytokines including detection thresholds can be found here: http://www.rndsystems.com/product_detail_objectname_fmaphumansample.aspx.
Plasma samples were incubated first with a set of color-coded beads pre-coated with antibodies for each of the 22 cytokines and then with corresponding biotinylated secondary antibodies. Samples were then incubated with streptavidin-phycoerythrin conjugate and read with a Luminex dual laser analyzer (Luminex Corporation, Austin, TX, USA), which detects the magnitude of the phycoerythrin signal in order to determine cytokine levels. Standards were measured in duplicate and then the mean fluorescent intensity was calculated for each sample. The experimenter was blinded to experimental groups for all cytokine assays.
miRNA Profiling
During the clinical visit, whole blood was also collected in PAXgene Blood RNA tubes (BD Biosciences, Franklin Lakes, NJ, USA) and stored at −80°C. After thawing tubes, RNA was isolated from blood using PAXgene blood miRNA kits (Qiagen, Germantown, MD, USA). RNA concentrations were measured on a NanoDrop 1000 (NanoDrop Technologies, Wilmington, DE, USA) and normalized to 20ng/uL using TE buffer. cDNA synthesis was performed using the TaqMan Reverse Transcription Kit, along with human Pool A and B Megaplex RT primers. cDNA was preamplified using TaqMan Preamplification Master Mix and human primers that corresponded to the Pool A and B RT primers. Samples were prepared and loaded onto OpenArray plates by the AccuFill Robot System (Life Technologies, Grand Island, NY, USA) using the protocol recommended by the vendor. Loaded OpenArray plates were run through the QuantStudio 12K Flex Real-Time OpenArray PCR System (Life Technologies, Grand Island, NY, USA) in order to assess expression of 761 miRNAs. RT-PCR was used to verify results for 5 miRNAs of interest using the Taqman Universal PCS Master Mix, Taqman miRNA Assays (Life Technologies, Grand Island, NY, USA) and the protocol recommended by the vendor. The experimenter was blinded to experimental groups for all miRNA assays.
miRNA Pathway Analysis
MiRNA expression profiles in blood may inform us of both peripheral and central pain processes affected in subtypes of CCPCs. Samples of circulating blood provide a rich source of pain-relevant molecules, including neurochemicals released by sympathetic nerve terminals and inflammatory mediators released by circulating immune cells.25 Furthermore, studies have shown that whole blood shares significant gene26 and miRNA27 expression similarities with CNS tissues.
To determine the genes and pathways affected by miRNA expression in women with VBD and VBD+IBS, we performed pathway analysis using the in silico Multiple MicroRNA Analysis provided by the Diana Lab (http://diana.cslab.ece.ntua.gr/pathways/index_multiple.php). The analysis generated a list of genes affected by changes in miRNA expression in each group and organized them into Kyoto Encyclopedia of Genes and Genomes (Kegg) pathways. For each pathway, a union −ln(p-value) that accounts for all affected genes was also generated.
Statistical Analysis
Summary scores from questionnaires and clinical assessments were compared among cases and controls using 2-tailed t-tests with the Benjamini-Hochberg procedure for multiple testing corrections. For all mucosal, muscle and remote bodily pressure pain measurements, groups were compared using one-way ANOVA and the Dunnett correction method. The thermal windup data were analyzed using a linear mixed model for repeated measures. The participant dependent variable was the numeric rating of pain in response to each stimulus. Fixed effects were clinical case classification (3 groups, modeled as a categorical variable), the stimulus sequence (continuous variable, 1–10) and the square of the stimulus sequence (continuous variable, 1–100). Interaction terms between case-classification and each of the continuous variables were included to test for differences in the rate of windup among clinical sub-groups. The random effect was person (categorical variable) and a variance components covariance structure was specified for the random effects. Cytokine expression levels were compared among groups using one-way ANOVA and the Dunnett correction method. All comparison analyses were completed using GraphPad Prism (Prism, La Jolla, CA, USA).
miRNA Data Analysis
Raw data of miRNA Cycle Threshold (CT) were filtered by at least 3 expressed (i.e. CT<32) samples within either HC group or combined case group. This filtration process permitted assessment of miRNAs that were present in all groups, as well as those that were largely increased or decreased in either case or control groups. Of the 761 human miRNAs, 250 were excluded, leaving 511 miRNAs for analysis. The filtered data was imported into DataAssist (Life Technologies, Grand Island, NY, USA) in order to calculate fold changes using CT values and the 2ΔΔCT method. Data were normalized by the Global Normalization method28 using median CTs with maximum allowable CT of 32 and including max CT in the calculations option, then 2ΔΔCT values were exported from DataAssist software as relative expression values. Secondary normalization using Variance Stabilization method29 were performed with the vsn package in Bioconductor (http://www.bioconductor.org/) and the output is log2 transformed normalized relative expression values. Differential expression of miRNAs between case and control groups was tested by ANOVA or ANCOVA models using Partek GenomicSuite software (Partek, St. Louis, MO, USA). Including run and plate as fixed effect factors in the models controlled for batch effect between different runs and assay plates. Selected clinical variables were included in the models, either as fixed effect factor for nominal measures, or covariates in ANCOVA models for numeric measures. miRNAs of interest from the differential expression analyses were picked based on combination of p-value and fold change filters indicated in the report. Linear regression analyses between miRNAs and intermediate phenotypes were performed in Partek, with correlation p-values adjusted for multiple testing using the Benjamini-Hochberg (step-up) false discovery rate. Reported correlations are with r<−0.40 or r>0.40, and adjusted p<0.05.
Results
Presence of Comorbid Pain Conditions
Data were collected from 33 women with VBD, 23 with VBD+IBS and 22 HC. Of those with VBD+IBS, 8 displayed additional pain conditions: TMD (n=4) or TMD and FM (n=4) Subjects were demographically similar (Table 1).
Table 1.
Demographic Data
| Demographic Data | HC | VBD | VBD+IBS |
|---|---|---|---|
| N | 22 | 33 | 23 |
|
| |||
| Age | 26.09 (1.04) | 28.06 (1.10) | 28.13 (1.36) |
|
| |||
| Weight (kg) | 69.90 (0.66) | 63.63**** (0.45) | 60.88**** (0.66) |
|
| |||
| RACE | |||
| White | 16 | 25 | 16 |
| Black | 2 | 4 | 2 |
| Hispanic | 1 | 1 | 0 |
| Other | 3 | 3 | 5 |
|
| |||
| EDUCATION | |||
| High school | 1 | 1 | 1 |
| Some college | 3 | 4 | 4 |
| College grad | 6 | 13 | 10 |
| Post grad | 12 | 15 | 8 |
|
| |||
| INCOME | |||
| 0–39k | 7 | 12 | 10 |
| 40–79k | 7 | 9 | 6 |
| 80–149k | 2 | 6 | 2 |
| 150k+ | 3 | 2 | 1 |
| refused or left blank | 3 | 4 | 3 |
|
| |||
| COMORBID CONDITIONS | |||
| VBD + IBS only | N/A | N/A | 15 |
| VBD + IBS + TMD | N/A | N/A | 4 |
| VBD + IBS + TMD + FM | N/A | N/A | 4 |
Abbreviations: healthy control (HC), vestibulodynia (VBD), irritable bowel syndrome (IBS), temporomandibular joint disorder (TMD), fibromyalgia (FM). For age and weight, data are mean (SEM). For other categories, N is reported for each group.
p<0.0001 compared to HC.
Pelvic Pressure Pain Associated with Case Status
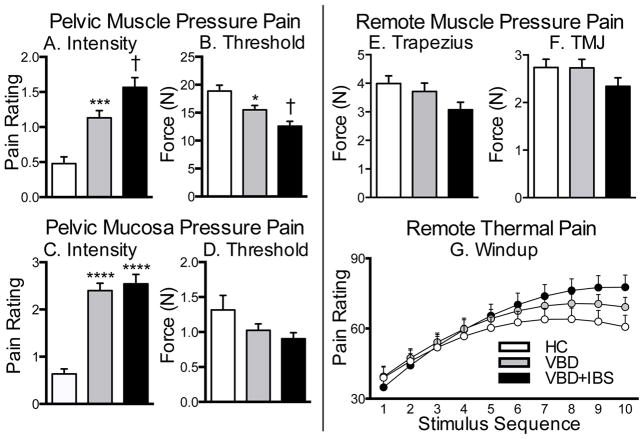
Pain evoked in response to pressure applied to the pelvic muscles and mucosa is a primary symptom of VBD. Average responses to pelvic muscle tests were similar at each site (See Supplemental Figure 1) therefore data were collapsed across all 3 muscle sites. Compared to HC, women with VBD and VBD+IBS had increased stimulus-evoked muscle pressure pain. Women with VBD+IBS reported the greatest evoked muscle pain intensity ratings (F2,228=19.74, p<0.0001; Fig. 1A) and the lowest muscle threshold scores (F2,191=10.02, p<0.0001; Fig. 1B). No differences in muscle pain tolerance were observed (See Supplemental Figure 1G–I). Average verbal intensity and threshold responses to pelvic mucosal stimulation were similar at each site (See Supplemental Figure 2); therefore data were collapsed across all 6 mucosal sites. Compared to HC, women with VBD and VBD+IBS reported enhanced pain intensity in response to pressure applied to the pelvic mucosa (F2,459 =37.26, p<0.0001; Fig. 1C). Women with VBD and VBD+IBS did not, however, exhibit reductions in pelvic mucosal pain thresholds (Fig. 1D) as compared to HC. These data suggest that muscular pain, which is characterized by increased pain intensity and decreased thresholds, is a primary feature of VBD and VBD+IBS. The magnitude of pain evoked by mucosal stimulation is less pronounced in VBD and VBD+IBS compared to pain evoked by muscle stimulation.
Fig. 1. Pelvic muscle and mucosa pressure pain is enhanced in women with VBD and VBD+IBS, while remote bodily muscle pain and thermal windup is enhanced only in women with VBD+IBS.
Women with VBD or VBD+IBS reported greater pain intensity and decreased pain thresholds in the pelvic muscle (A–B) and mucosa (C–D). Women with VBD+IBS demonstrated a trend towards decreased pressure pain thresholds in the trapezius (E) and temporomandibular joint (F) as compared to HC and VBD. (G) For the thermal data, there was a significant interaction between study group and stimulus sequence, although not between study group and the square of stimulus sequence. VBD+IBS women exhibit the greatest degree of pain in response to the windup thermal heat assay. Data are Mean ± SEM. *p<0.05, ***p<0.001, ****p<0.0001 compared to HC; †p<0.05 as compared to HC and VBD.
Remote Bodily Pain Associated with Case Status
Pain at remote bodily sites was evaluated to determine if pain was generalized to other body regions. When a pressure stimulus was applied to the trapezius (Fig. 1E) or the TMJ (Fig. 2F), women with VBD+IBS exhibited a tendency towards reduced pain thresholds compared to HC or women with VBD alone. Similar group differences were observed in response to thermal heat repeatedly applied to the forearm. The 1st stimulus elicited similar ratings for all groups. Successive stimuli then elicited greater pain among VBD+IBS subjects compared to controls whose pain ratings plateaued after the 6th pulse, indicating that women with VBD+IBS have a greater capacity to temporarily summate heat pain. Intermediate effects were observed for the VBD group (Fig. 2G). In sum, these data suggest that women with VBD+IBS experience enhanced pain in response to pressure and thermal stimuli at remote bodily regions, indicative of central sensitization.
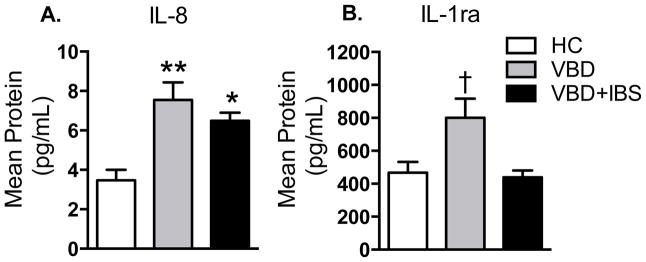
Fig. 2. Cytokine expression is altered in women with VBD and VBD+IBS.
Compared to HC, women with VBD exhibit elevated levels of pro-inflammatory cytokine IL-8 (A) and anti-inflammatory cytokine IL-1ra (B). Women with VBD+IBS exhibit elevated levels of IL-8, but no compensatory increase in IL-1ra. Data are Mean ± SEM. *p<0.05, **p<0.01 compared to HC; †p<0.05 compared to HC and VBD+IBS.
Self-reported Clinical Pain and Psychological Characteristics Associated with Case Status
Compared to HC, women with VBD+IBS displayed greater levels of affective, aching, tender and stabbing pain (Table 2). Women with VBD+IBS also demonstrated decreases in perceived general and physical health status, while women with VBD demonstrated lower perceived mental health status. Additionally, VBD+IBS subjects reported more somatization. Lastly, women with VBD+IBS demonstrated a greater number of headaches and impact of pain on daily activity in the past 6 months. In sum, VBD subjects were similar to HC in self-reported pain and psychological characteristics, whereas VBD+IBS subjects reported greater clinical pain and decreased perceived health. Although not tabulated, no differences in SCL-90R subscale scores were observed between groups. Significance is p<0.05 and overall p-values for all variables are reported (Table 2).
Table 2.
Self-reported pain, function and psychological characteristics associated with case status
| Phenotype | Questionnaire | HC | VBD | VBD + IBS | Overall P-value |
|---|---|---|---|---|---|
| Affective Pain | MPQ | 11.67 (0.41) | 12.50 (0.62) | 14.78*** (0.8) | 0.001 |
| Aching Pain | MPQ | 1.14 (0.10) | 1.23 (0.10) | 2.06†††† (0.17) | <0.0001 |
| Tender Pain | MPQ | 1.10 (0.06) | 1.37 (0.13) | 1.61** (0.16) | 0.003 |
| Stabbing Pain | MPQ | 1.00 (0.00) | 1.13 (0.10) | 1.39* (0.20) | 0.038 |
| General Health | SF12v2 | 4.51 (0.11) | 4.31 (0.15) | 4.01* (0.15) | 0.012 |
| Mental Health | SF12v2 | 51.82 (1.87) | 45.01* (1.96) | 46.41 (2.20) | 0.069 |
| Physical Health | SF12v2 | 55.83 (1.12) | 57.58 (0.85) | 50.54†† (1.26) | 0.003 |
| Somatization | PILL | 89.79 (4.15) | 98.44 (3.60) | 115.61†† (4.61) | 0.0002 |
| Headache Types | CPSQ | 1.37 (0.19) | 1.93 (0.19) | 2.37** (0.23) | 0.009 |
| Impact of Pain on Daily Activity | CPSQ | 0.30 (0.20) | 0.81 (0.37) | 2.05* (0.64) | 0.028 |
Data are expressed as Mean (SEM).
p<0.05,
p<0.01,
p<0.001,
p<0.0001 compared to HC.
p<0.01,
p<0.0001 as compared to HC and VBD.
Cytokines Associated with Case Status
Circulating cytokine levels were measured in subjects to determine if inflammatory mediators correlate with case status. Of the cytokines measured, IL-8 and IL-1ra were statistically significant between groups. Of the remaining 20 cytokines, 9 failed to exhibit differences between groups (MCP-1, MIP-1β, ENA-78, FGF basic, G-CSF, IL-6, TNFα, Tpo, and VEGF) and 11 were undetectable (MIP-1α, RANTES, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-10, and IL-17). Compared to HC, women with VBD and VBD+IBS had increased expression of pro-inflammatory cytokine IL-8 (F2,79=6.337, p=0.0028; Fig. 2A). Women with VBD, but not with VBD+IBS, displayed a compensatory increase in anti-inflammatory cytokine IL-1ra (Fig. 2B). These data suggest that women with VBD+IBS, but not VBD alone, have an impaired anti-inflammatory response.
miRNAs Associated with Case Status
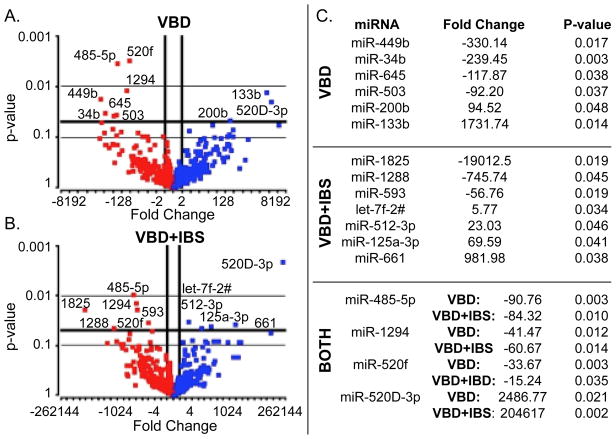
We measured miRNA expression profiles to determine if miRNAs map onto distinguishing clinical features of CCPCs. Compared to HC, VBD subjects had downregulation of 7 (miR-449b, miR-34b, miR-645, miR-503, miR-485-5p, miR-1294, miR-520f) and upregulation of 3 miRNAs (miR-200b, miR-133b, miR-520D-3p) at p-value<0.05 (Fig. 3A). VBD+IBS subjects had downregulation of 6 (miR-1825, miR-1288, miR-593, miR-485-5p, miR-1294, miR-520f) and upregulation of 5 miRNAs (let-7f-2#, miR-512-3p, miR-125a-3p, miR-661, miR-520D-3p) (Fig. 3B). Downregulated miRNAs (fold change < -2) are depicted by red dots and upregulated miRNAs (fold change > 2) by blue dots (Fig. 3A–B). All miRNAs are listed with corresponding fold change and p-value. miRNAs listed under “Both” were dysregulated in VBD and VBD+IBS subjects (Fig. 3C). Results were verified for 5 of the dysregulated miRNAs using RT-PCR (See Supplemental Figure 3).
Fig. 3. miRNA expression signatures are altered in women with VBD and VBD+IBS.
Compared to HC, women with VBD exhibit significant downregulation of 7 and upregulation of 3 miRNAs (A), while women with VBD+IBS exhibit significant downregulation of 6 and upregulation of 5 miRNAs (B). Red dots represent downregulated miRNAs and blue dots represent upregulated miRNAs compared to HC. Four miRNAs are dysregulated in both VBD and VBD+IBS women. Dysregulated miRNAs are listed with fold change and p values (C).
Specific miRNAs were associated with pain-relevant cytokines and intermediate phenotypes, with the direction of correlation varying according to miRNA and phenotype (See Supplemental Table 1). Specifically, 3 miRNAs were associated with IL-1ra expression and 2 miRNAs were associated with IL-8 expression. MiRNAs were also associated with self-reported pain and function, such that 12 miRNAs were associated with ‘stabbing pain’, 1 miRNA was associated with ‘affective pain’ and 2 miRNAs were associated with impact of pain on daily activity. Finally, miRNAs were associated with experimental pressure pain, such that 9 miRNAs were associated with pressure applied to the TMJ and 11 miRNAs were associated with pressure applied to the trapezius. Some miRNAs (miR-645, RNU44, miR-543 and miR-213) were associated with more than one phenotype and, thus, may play a key role in pain-relevant processes. All correlations with absolute values >0.40 and p<0.05 are reported (See Supplemental Table 1).
miRNA Pathways Associated with Case Status
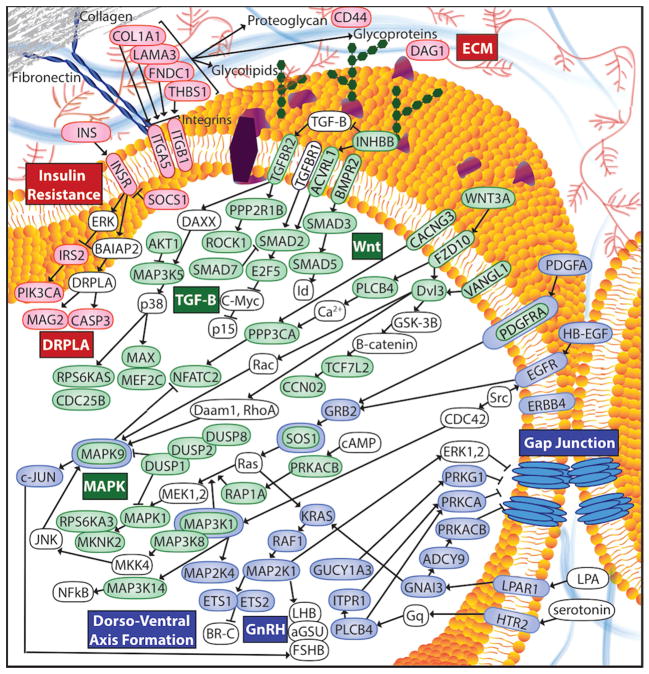
We performed in silico pathway analysis using DIANA-miRPath v2.0 to identify genes and downstream pathways affected by miRNA dysregulation in CCPCs.30 In both groups, miRNA dysregulation is predicted to disrupt transforming growth factor beta (TGF-β), mitogen-activated protein kinase (MAPK) and Wnt signaling pathways (Fig. 4, green). For example, upregulation of miR-520D-3p causes decreased levels of SMADs in the TGF- β pathway. Downregulation of miR-485-5p and miR-520f causes increased levels of MAPK genes such as protein kinase B (AKT1) and mitogen-activated protein 3-kinase 14 (MAP3K14). Downregulation of miR-485-5p results in increased levels of myc-associated factor X (MAX), another MAPK gene. Upregulation of miR-520D-3p causes increased levels of deubiquitinating enzymes (DUSPs), which act as MAPK phosphatases.31 MAPKs and SMADs are also involved in Wnt signaling.
Fig. 4. miRNA dysregulation affects multiple genes and pathways in women with VBD and VBD+IBS.
miRNA dysregulation in women with VBD and VBD+IBS leads to dysregulation of genes involved in pain-relevant pathways including the TGFβ, MAPK, and Wnt signaling pathways (shown in green). Pathways that do not overlap between women with VBD and VBD+IBS represent separate pathways of vulnerability. Pathways unique to VBD (shown in blue) include the dorso-ventral axis formation, GnRH signaling and gap junction pathways. Pathways unique to VBD+IBS (shown in red) include the ECM, DRPLA and insulin resistance pathways. A considerable amount of interaction and communication is demonstrated between genes and across pathways. See Supplemental Content for abbreviations.
In VBD, but not VBD+IBS, the dorsal-ventral axis formation, gonadotropin-releasing hormone (GnRH) and gap junction pathways may be affected (Fig. 4, blue). For example, downregulation of miR-34b and miR-449b leads to increased levels of dorsal-ventral axis proteins including receptor tyrosine-protein kinase erbB-4 (ERBB4), protein C-ets-1 (ETS1) and RAF proto-oncogene serine/threonine-protein kinase (RAF1). Downregulation of miR-200b causes increased levels of many gap junction proteins including: epidermal growth factor receptor (EGFR), GTPase KRas (KRAS), adenylate cyclase type 9 (ADCY9), phospholipase C beta-4 (PLCB4), cAMP-dependent protein kinase catalytic subunit beta (PRKACB), protein kinase C (PRKCA) and lysophosphatidic acid receptor 1 (LPAR1). Many of these proteins (i.e. RAF1, ADCY9, KRAS, PLCB4, PRKACB and PRKCA) are also vital to the GnRH pathway.
In VBD+IBS, but not VBD, the extracellular matrix (ECM), insulin resistance and dentatorubral-pallidoluysian atrophy (DRPLA) pathways may be affected (Fig. 4, red). Downregulation of miR-593 causes increased expression of ECM proteins including fibronectin type III domain containing 1 (FNDC1) and integrin alpha 5 (ITGA5). Upregulation of let-7f and miR-661 causes decreased levels of ECM proteins alpha-1 type I collagen (COL1A1) and dystroglycan 1 (DAG1), respectively. Upregulation of miR-125a-3p and miR-512-3p causes decreased levels of integrin beta 1 (ITGB1), another ECM protein; and insulin (INS), insulin receptor (INSR) and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA), each of which is involved with insulin resistance. The insulin resistance and DRPLA pathways greatly overlap, sharing proteins such as INS and INSR. Full lists of the top 20 affected pathways for each group are reported with names and union −ln(p-values) for target genes (See Supplemental Tables 2–3). Shared and unique pathways identified in VBD versus VBD+IBS may provide insight on the mechanisms underlying localized and widespread pain.
Discussion
Complex and chronic pain conditions are heterogeneous in nature and have limited effective therapeutic options. Here, we identify two subtypes of chronic pain patients, those with localized pain (VBD) and those with widespread pain (VBD+IBS) that differ with respect to pain phenotypes, psychological profiles, circulating cytokine expression and miRNA profiles. Pathway analysis of miRNA targets further suggests mechanisms that are common and unique to VBD and VBD+IBS.
Historically, VBD has been defined as a vulvar mucosa condition.15 Our results, however, demonstrate that enhanced pelvic pressure pain in VBD and VBD+IBS is experienced predominantly in the muscles, suggesting modest increases in mucosal pain may exist as a byproduct of muscle pain. These data are in line with recent evidence that proposes a complex pathophysiology for VBD, involving the muscles as well as the peripheral and central nervous systems.32 This suggests that VBD is, and should be treated as, a musculoskeletal pain condition.
Although VBD and VBD+IBS groups had similar pelvic pain phenotypes, they differed in remote bodily pain phenotypes and psychological profiles. Increased responses to mechanical and thermal stimuli at remote bodily sites in VBD+IBS indicates central enhancement in the processing of pain-evoking sensations.33 Our results are in line with those from studies that suggest chronic pain conditions such as VBD likely reflect localized sensory dysregulation in the affected area34 whereas widespread bodily pain often involves dysregulation in central pain processing, perhaps due to sensitization of spinal nociceptors35 or impairments in descending noxious control systems.36 This probably explains why there was co-occurrence of TMD or FM for several of the VBD+IBS subjects and it raises the possibility that those, or other, overlapping pain conditions might likewise be associated with circulating cytokine expression and miRNA profiles. Unfortunately, there were too few TMD and FM subjects to address that question with sufficient power in this study, although it merits investigation in future studies.
Women with VBD also differed from those with VBD+IBS in circulating cytokine expression. Women with VBD had elevated levels of pro-inflammatory cytokine IL-8 and anti-inflammatory cytokine IL-1ra. This is consistent with studies that have demonstrated elevated inflammatory cytokine levels in pain conditions such as TMD and FM.37, 38 Women with VBD+IBS had elevated levels of IL-8 with no compensatory increase in IL-1ra. IL-1ra, a negative regulator of inflammation, can suppress IL-8 expression and block IL-8-induced mechanical pain.39, 40 Our finding is similar to that of a study that indicates an imbalance between IL-8 and IL-1ra in individuals with TMD plus widespread pain.6 In sum, women in this study can be divided into two subsets: (1) those with localized pelvic pain, whose pain at other bodily sites resembles that of HC, who otherwise report good health and who have intact anti-inflammatory responses; and (2) those with co-occurring pain conditions, more pain at remote bodily sites, poorer self-reported health and impaired anti-inflammatory responses.
As miRNAs are key regulators of processes related to pain, psychological variables and inflammatory responses, we explored their expression in VBD and VBD+IBS by miRNA profiling. Shared dysregulation of miRNAs may help to elucidate the mechanisms underlying CCPCs. Both VBD and VBD+IBS groups in this study demonstrate dysregulation of miR-485-5p, miR-1294, miR-520f and miR-520D-3p. Previous studies have found the same miRNAs to be associated with other diseases. For example, miR-485-5p41, 42 and miR-520D-3p43 have been linked with cancer, and miR-1294 has been linked to Alzheimer’s 44 and Parkinson’s disease.45 While little is understood about the function and role of miR-520f in human disease, the miR-520 family is known to regulate IL-8.46 Downregulation of miR-520f, but not upregulation of miR-520D-3p, can explain the increased IL-8 levels measured here in women with VBD and VBD+IBS. It is important to note that miRNA regulation is dynamic and complex. MiRNA expression can be affected by the presence of downstream mRNAs or proteins, and can be indirectly influenced by miRNAs within the same family. Several interactions, particularly in the form of negative or positive feedback loops, have been established within miRNA families.47 Additional experiments are necessary in order to confirm the relationship between the miR-520 family and IL-8 in VBD and VBD+IBS, and to measure the concerted effect of these and other miRNAs on cytokine expression.
In order to further understand how these miRNAs could contribute to CCPCs, we performed pathway analysis of predicted downstream targets. Both the VBD and VBD+IBS groups share predicted disruption of pain-relevant pathways. For example SMADs, which are downregulated in CCPCs, are responsible for regulating anti-inflammatory cytokines of the TGF-β family and controlling inflammatory response.48, 49 Our results are in line with those from the TMD study previously mentioned that demonstrates suppression of the TGF-β pathway in individuals with either TMD or TMD plus widespread pain.6 We demonstrate potential upregulation of AKT1, MAP3K14, MAX and DUSPs in CCPCs, each of which may contribute to pain sensitization, pro-inflammatory mediator synthesis and the development of chronic pain via the MAPK pathway.31, 50–53 MAPKs and SMADs are also involved in Wnt signaling, a pathway that alters production of pro-inflammatory cytokines and sensitivity of peripheral sensory neurons, thereby contributing to development of neuropathic pain.54, 55 We conclude that dysregulation of miRNAs and downstream pathways may promote a constant state of inflammation and pain in women with VBD and VBD+IBS.
Dysregulated miRNAs that do not overlap between the two groups represent unique pathways of vulnerability. Women with VBD have dysregulation of miR-449b, miR-34b, miR-645, miR-503, miR-200b and miR-133b. MiR-449b,56 miR-34b,57 miR-645,58 miR-50359, miR-200b60 and miR-133b 61 have been implicated in cancer. Notably, miR-449b is known to regulate neurokinin-1 receptor (NK1R) in chronic bladder pain syndrome (BPS). 62 The NK1 pathway is important for pain transmission and neuroimmune modulation63 and may be affected similarly in VBD and BPS.
Based upon miRNA dysregulation, our pathway analysis predicts that women with VBD have increased levels of ERBB4, ETS1 and RAF1, each of which may contribute to inflammation or the development of neuropathic pain.64–67 Other proteins targeted in VBD include EGFR, KRAS, ADCY9, PLCB4, PRKACB, PRKCA and LPAR1, which are all linked to inflammation.68–70 Interestingly, another common denominator of the genes and pathways affected in VBD is estrogen.71–75 RAF1, ADCY9, KRAS, PLCB4, PRKACB and PRKCA are vital to the GnRH pathway, in which neuropeptide GnRH interacts with estrogen via a negative feedback loop to maintain hormone levels.76 Other affected targets, such as ETS1, ERBB4 and LPAR1 interact directly with estrogen or estrogen receptors.72, 77, 78 Our results predict that women with VBD will have blunted estrogen and GnRH activity. This provides a possible explanation for why estrogen and GnRH effectively reduce pain in endometriosis and other pelvic pain conditions.79, 80 From these results we conclude that miRNA dysregulation of estrogen-relevant genes contributes to localized pelvic pain characteristic of VBD.
We found that subjects with VBD+IBS show dysregulation of miR-1825, miR-1288, miR-593, let-7f-2#, miR-512-3p, miR-125a-3p and miR-661. MiR-1825,81 miR-593,82 let-7f-2#,83 miR-512-3p,84 miR-125a-3p85 and miR-66186 have all been shown to contribute to cancer. In addition, miR-1825 has been associated with avian influenza;87 miR-1288 with ectopic pregnancy;88 and let-7f-2# with lupus.89 Most relevant to our results, miR-125a-3p has been shown to promote orofacial pain through upregulation of p38 MAPK.90
In VBD+IBS, our pathway analysis predicts that miRNA dysregulation will affect genes of the ECM including COL1A1, ITGB1 and ITGA5. Alterations in the ECM, which provides structural and mechanical support to surrounding tissue, have been previously linked to many painful conditions.91, 92 INS and INSR, also affected in VBD+IBS, have been implicated in nociception via the insulin resistance pathway.93 In addition, each of the pathways and molecules affected by miRNA dysregulation in VBD+IBS share an involvement in both muscle function and sensory processing. For example, COL1A1 has been implicated in skeletal muscle atrophy.94 Decreases in ITGB1, PIK3CA and INS have all been linked to impairments in muscle development and function.95–97 ITGA5 interacts with fibronectins to influence astrocyte physiology98 and ITGB1 is necessary for normal axon formation.99 Decreased DAG1 expression is associated with abnormal myelin sheath folding and muscle impairment.100 In sum, dysfunction of pathways such as muscle and sensory nerve processes may lead to central sensitization and generalized body pain in VBD+IBS.
While the present study did not enroll women with IBS alone, an emerging literature demonstrates the contribution of miRNAs, particularly the miR-29 family, to IBS.101, 102 Interestingly, we did not observe an overlap between the miRNAs associated with IBS in other cohorts and those associated with VBD or VBD+IBS in our cohort. This might reflect the differential expression of miRNAs in different sample types (e.g., whole blood used in the present study versus colon tissue and microvesicles commonly used in IBS studies). Further, it might indicate that the pathophysiological processes that drive IBS are distinct from those that drive VBD or VBD+IBS.
In conclusion, we have identified separate pathways for localized versus widespread pain as predicted by miRNA profiles. miRNA dysregulation in VBD is predicted to affect estrogen-relevant pathways, explaining why pain is localized to the pelvis. In contrast, miRNA dysregulation in VBD+IBS may be linked to alterations in muscle, nerve and glial cell function, thereby contributing to widespread pain. While these results suggest that miRNAs hold promise as biomarkers for chronic pain, it is important to note that existing miRNA studies of human CCPCs are underpowered statistically and our knowledge of miR-1294, miR-645, miR-1825 and miR-1288 is extremely limited. Further studies are required to 1) identify miRNA profiles in a larger population of chronic pain patients; 2) observe miRNA expression profiles in sample types other than whole blood (e.g., plasma, tissue, specific cell types); 3) validate changes in the expression levels of the predicted targets (e.g., estrogen and insulin); 4) correlate miRNA profiles with response to treatments that target different pathophysiologic processes (e.g., topical estrogen therapy to improve local vestibular tone103 versus anticonvulsant medication to calm the activity of nociceptive neurons104–106); and 5) assess the effectiveness of targeting miRNAs and downstream pathways to reduce and manage symptoms of chronic pain patients. Accomplishing these next steps will facilitate the use of miRNA screening tools in the clinic to determine the most effective treatment with minimal risk for patients with chronic pain. Such studies should help inform the development of novel therapeutics targeted against one of several key elements along the canonical pathway from gene to protein.
Supplementary Material
Acknowledgments
This work was funded by NIH/NINDS P01 NS045685 to A.N.; NIH/OBSSR R24 DK067674 to A.N. and D.Z.; NIH/NINDS R01 NS072205 to A.N.; and an NVA grant to A.N. and D.Z. The authors thank Lawrence Yoon (GlaxoSmithKline, Inc, Research Triangle Park, NC, USA) for his assistance with miRNA methods.
Footnotes
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. Authors have no financial disclosures to make and no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zolnoun DA, Rohl J, Moore CG, Perinetti-Liebert C, Lamvu GM, Maixner W. Overlap between orofacial pain and vulvar vestibulitis syndrome. The Clinical journal of pain. 2008;24(3):187–91. doi: 10.1097/AJP.0b013e318159f976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wesselmann U, Bonham A, Foster D. Vulvodynia: Current state of the biological science. Pain. 2014 doi: 10.1016/j.pain.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RE, Hartmann KE, Sandler RS, Miller WC, Steege JF. Prevalence and characteristics of irritable bowel syndrome among women with chronic pelvic pain. Obstetrics and gynecology. 2004;104(3):452–8. doi: 10.1097/01.AOG.0000135275.63494.3d. [DOI] [PubMed] [Google Scholar]
- 4.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205–21. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 5.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123(3):226–30. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, et al. Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 2011;152(12):2802–12. doi: 10.1016/j.pain.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederberger E, Kynast K, Lotsch J, Geisslinger G. MicroRNAs as new players in the pain game. Pain. 2011;152(7):1455–8. doi: 10.1016/j.pain.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 9.Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, Barrett JE, et al. MicroRNA modulation in complex regional pain syndrome. Journal of translational medicine. 2011;9:195. doi: 10.1186/1479-5876-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor RM, Dinan TG, Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Molecular psychiatry. 2012;17(4):359–76. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- 11.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Translational research : the journal of laboratory and clinical medicine. 2011;157(4):163–79. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Schack D, Agostino MJ, Murray BS, Li Y, Reddy PS, Chen J, et al. Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PloS one. 2011;6(3):e17670. doi: 10.1371/journal.pone.0017670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borgonio Cuadra VM, Gonzalez-Huerta NC, Romero-Cordoba S, Hidalgo-Miranda A, Miranda-Duarte A. Altered expression of circulating microRNA in plasma of patients with primary osteoarthritis and in silico analysis of their pathways. PloS one. 2014;9(6):e97690. doi: 10.1371/journal.pone.0097690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PloS one. 2008;3(11):e3740. doi: 10.1371/journal.pone.0003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. The journal of pain : official journal of the American Pain Society. 2012;13(9):910–20. doi: 10.1016/j.jpain.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pukall CF, Binik YM, Khalife S. A new instrument for pain assessment in vulvar vestibulitis syndrome. Journal of sex & marital therapy. 2004;30(2):69–78. doi: 10.1080/00926230490275065. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research. 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin SF. Research Diagnostic criteria for Temporomandibular Disorders: current status & future relevance. Journal of oral rehabilitation. 2010;37(10):734–43. doi: 10.1111/j.1365-2842.2010.02090.x. [DOI] [PubMed] [Google Scholar]
- 20.Olesen J. The International Classification of Headache Disorders, 2nd edition: application to practice. Functional neurology. 2005;20(2):61–8. [PubMed] [Google Scholar]
- 21.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 22.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. Journal of clinical epidemiology. 1998;51(11):1171–8. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 23.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. The journal of pain : official journal of the American Pain Society. 2011;12(11 Suppl):T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy BL, Morris RL, Pedley LL, Schwab JJ. The ability of the Symptom Checklist SCL-90 to differentiate various anxiety and depressive disorders. The Psychiatric quarterly. 2001;72(3):277–88. doi: 10.1023/a:1010357216925. [DOI] [PubMed] [Google Scholar]
- 25.Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. The journal of pain : official journal of the American Pain Society. 2008;9(2):122–45. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2006;141(3):261–8. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Frontiers in bioscience. 2011;3:1265–72. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 28.Mestdagh P, Van Vlierberghe P, De Weer A, Muth D, Westermann F, Speleman F, et al. A novel and universal method for microRNA RT-qPCR data normalization. Genome biology. 2009;10(6):R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18 (Suppl 1):S96–104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- 30.Vlachos IS, Kostoulas N, Vergoulis T, Georgakilas G, Reczko M, Maragkakis M, et al. DIANA miRPath v.2.0: investigating the combinatorial effect of microRNAs in pathways. Nucleic acids research. 2012;40(Web Server issue):W498–504. doi: 10.1093/nar/gks494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. Journal of immunology. 2006;177(11):7497–504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- 32.Zolnoun D, Hartmann K, Lamvu G, As-Sanie S, Maixner W, Steege J. A conceptual model for the pathophysiology of vulvar vestibulitis syndrome. Obstetrical & gynecological survey. 2006;61(6):395–401. doi: 10.1097/01.ogx.0000219814.40759.38. quiz 23. [DOI] [PubMed] [Google Scholar]
- 33.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slade GD, Smith SB, Zaykin DV, Tchivileva IE, Gibson DG, Yuryev A, et al. Facial pain with localized and widespread manifestations: separate pathways of vulnerability. Pain. 2013;154(11):2335–43. doi: 10.1016/j.pain.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best practice & research Clinical rheumatology. 2003;17(4):593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 36.Pfau DB, Rolke R, Nickel R, Treede RD, Daublaender M. Somatosensory profiles in subgroups of patients with myogenic temporomandibular disorders and Fibromyalgia Syndrome. Pain. 2009;147(1–3):72–83. doi: 10.1016/j.pain.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Kaneyama K, Segami N, Nishimura M, Suzuki T, Sato J. Importance of proinflammatory cytokines in synovial fluid from 121 joints with temporomandibular disorders. The British journal of oral & maxillofacial surgery. 2002;40(5):418–23. [PubMed] [Google Scholar]
- 38.Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clinical and experimental rheumatology. 2007;25(2):225–30. [PubMed] [Google Scholar]
- 39.Hattar K, Fink L, Fietzner K, Himmel B, Grimminger F, Seeger W, et al. Cell density regulates neutrophil IL-8 synthesis: role of IL-1 receptor antagonist and soluble TNF receptors. Journal of immunology. 2001;166(10):6287–93. doi: 10.4049/jimmunol.166.10.6287. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz YA, Amin RS, Stark JM, Trapnell BC, Wilmott RW. Interleukin-1 receptor antagonist inhibits interleukin-8 expression in A549 respiratory epithelial cells infected in vitro with a replication-deficient recombinant adenovirus vector. American journal of respiratory cell and molecular biology. 1999;21(3):388–94. doi: 10.1165/ajrcmb.21.3.3549. [DOI] [PubMed] [Google Scholar]
- 41.He N, Zheng H, Li P, Zhao Y, Zhang W, Song F, et al. miR-485–5p binding site SNP rs8752 in HPGD gene is associated with breast cancer risk. PloS one. 2014;9(7):e102093. doi: 10.1371/journal.pone.0102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaman MS, Maher DM, Khan S, Jaggi M, Chauhan SC. Current status and implications of microRNAs in ovarian cancer diagnosis and therapy. Journal of ovarian research. 2012;5(1):44. doi: 10.1186/1757-2215-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura M, Jung EJ, Shah MY, Lu C, Spizzo R, Shimizu M, et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer discovery. 2013;3(11):1302–15. doi: 10.1158/2159-8290.CD-13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satoh J, Kino Y, Niida S. MicroRNA-Seq Data Analysis Pipeline to Identify Blood Biomarkers for Alzheimer’s Disease from Public Data. Biomarker insights. 2015;10:21–31. doi: 10.4137/BMI.S25132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgos K, Malenica I, Metpally R, Courtright A, Rakela B, Beach T, et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PloS one. 2014;9(5):e94839. doi: 10.1371/journal.pone.0094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-kappaB and TGF-beta signaling pathways. Oncogene. 2012;31(37):4150–63. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 47.Nazarov PV, Reinsbach SE, Muller A, Nicot N, Philippidou D, Vallar L, et al. Interplay of microRNAs, transcription factors and target genes: linking dynamic expression changes to function. Nucleic acids research. 2013;41(5):2817–31. doi: 10.1093/nar/gks1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horbelt D, Denkis A, Knaus P. A portrait of Transforming Growth Factor beta superfamily signalling: Background matters. The international journal of biochemistry & cell biology. 2012;44(3):469–74. doi: 10.1016/j.biocel.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. Journal of the American Society of Nephrology : JASN. 2005;16(5):1371–83. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- 50.Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain research reviews. 2009;60(1):135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Zhang L, Ma Y, Chen L, Tian Y, Mao J, et al. Nociceptive behavior following hindpaw burn injury in young rats: response to systemic morphine. Pain medicine. 2011;12(1):87–98. doi: 10.1111/j.1526-4637.2010.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. The Journal of clinical investigation. 2012;122(6):1973–90. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kulyte A, Belarbi Y, Lorente-Cebrian S, Bambace C, Arner E, Daub CO, et al. Additive effects of microRNAs and transcription factors on CCL2 production in human white adipose tissue. Diabetes. 2014;63(4):1248–58. doi: 10.2337/db13-0702. [DOI] [PubMed] [Google Scholar]
- 54.Itokazu T, Hayano Y, Takahashi R, Yamashita T. Involvement of Wnt/beta-catenin signaling in the development of neuropathic pain. Neuroscience research. 2014;79:34–40. doi: 10.1016/j.neures.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Simonetti M, Agarwal N, Stosser S, Bali KK, Karaulanov E, Kamble R, et al. Wnt-Fzd Signaling Sensitizes Peripheral Sensory Neurons via Distinct Noncanonical Pathways. Neuron. 2014;83(1):104–21. doi: 10.1016/j.neuron.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 56.Mortensen MM, Hoyer S, Orntoft TF, Sorensen KD, Dyrskjot L, Borre M. High miR-449b expression in prostate cancer is associated with biochemical recurrence after radical prostatectomy. BMC cancer. 2014;14:859. doi: 10.1186/1471-2407-14-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Feng J, Tang L, Liao L, Xu Q, Zhu S. The regulation and function of miR-21-FOXO3a-miR-34b/c signaling in breast cancer. International journal of molecular sciences. 2015;16(2):3148–62. doi: 10.3390/ijms16023148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lan H, Lu H, Wang X, Jin H. MicroRNAs as Potential Biomarkers in Cancer: Opportunities and Challenges. BioMed research international. 2015;2015:125094. doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng Y, Liu YM, Li LC, Wang LL, Wu XL. microRNA-503 inhibits gastric cancer cell growth and epithelial-to-mesenchymal transition. Oncology letters. 2014;7(4):1233–8. doi: 10.3892/ol.2014.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye F, Tang H, Liu Q, Xie X, Wu M, Liu X, et al. miR-200b as a prognostic factor in breast cancer targets multiple members of RAB family. Journal of translational medicine. 2014;12:17. doi: 10.1186/1479-5876-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen XN, Wang KF, Xu ZQ, Li SJ, Liu Q, Fu DH, et al. MiR-133b regulates bladder cancer cell proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer cell international. 2014;14:70. doi: 10.1186/s12935-014-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez Freire V, Burkhard FC, Kessler TM, Kuhn A, Draeger A, Monastyrskaya K. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. The American journal of pathology. 2010;176(1):288–303. doi: 10.2353/ajpath.2010.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Annals of the New York Academy of Sciences. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi L, Wang L, Wang B, Cretoiu SM, Wang Q, Wang X, et al. Regulatory mechanisms of betacellulin in CXCL8 production from lung cancer cells. Journal of translational medicine. 2014;12:70. doi: 10.1186/1479-5876-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dai DW, Xu Z, Chen X, Yuan L, Zhang AJ, Zhang PQ, et al. Distinct roles of neuregulin in different models of neuropathic pain. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2014;35(4):531–6. doi: 10.1007/s10072-013-1537-z. [DOI] [PubMed] [Google Scholar]
- 66.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286–93. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Jaggi AS, Singh N. Intrathecal delivery of farnesyl thiosalicylic acid and GW 5074 attenuates hyperalgesia and allodynia in chronic constriction injury-induced neuropathic pain in rats. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;34(3):297–304. doi: 10.1007/s10072-012-0991-3. [DOI] [PubMed] [Google Scholar]
- 68.Quinn SR, Mangan NE, Caffrey BE, Gantier MP, Williams BR, Hertzog PJ, et al. The role of Ets2 transcription factor in the induction of microRNA-155 (miR-155) by lipopolysaccharide and its targeting by interleukin-10. The Journal of biological chemistry. 2014;289(7):4316–25. doi: 10.1074/jbc.M113.522730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25(14):2105–12. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 70.McCullough SD, Duncan KE, Swanton SM, Dailey LA, Diaz-Sanchez D, Devlin RB. Ozone Induces a Pro-inflammatory Response in Primary Human Bronchial Epithelial Cells Through MAP Kinase Activation Without NF-kappaB Activation. American journal of respiratory cell and molecular biology. 2014 doi: 10.1165/rcmb.2013-0515OC. [DOI] [PubMed] [Google Scholar]
- 71.Miyagawa S, Katsu Y, Watanabe H, Iguchi T. Estrogen-independent activation of erbBs signaling and estrogen receptor alpha in the mouse vagina exposed neonatally to diethylstilbestrol. Oncogene. 2004;23(2):340–9. doi: 10.1038/sj.onc.1207207. [DOI] [PubMed] [Google Scholar]
- 72.Kalet BT, Anglin SR, Handschy A, O’Donoghue LE, Halsey C, Chubb L, et al. Transcription factor Ets1 cooperates with estrogen receptor alpha to stimulate estradiol-dependent growth in breast cancer cells and tumors. PloS one. 2013;8(7):e68815. doi: 10.1371/journal.pone.0068815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pratt MA, Satkunaratnam A, Novosad DM. Estrogen activates raf-1 kinase and induces expression of Egr-1 in MCF-7 breast cancer cells. Molecular and cellular biochemistry. 1998;189(1–2):119–25. doi: 10.1023/a:1006827015320. [DOI] [PubMed] [Google Scholar]
- 74.Aupperlee MD, Zhao Y, Tan YS, Leipprandt JR, Bennett J, Haslam SZ, et al. Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland. Endocrinology. 2014;155(6):2301–13. doi: 10.1210/en.2013-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horii J, Hiraoka S, Kato J, Saito S, Harada K, Fujita H, et al. Methylation of estrogen receptor 1 in colorectal adenomas is not age-dependent, but is correlated with K-ras mutation. Cancer science. 2009;100(6):1005–11. doi: 10.1111/j.1349-7006.2009.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radovick S, Levine JE, Wolfe A. Estrogenic regulation of the GnRH neuron. Frontiers in endocrinology. 2012;3:52. doi: 10.3389/fendo.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey AK, Marrero L, et al. Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer research. 2006;66(16):7991–8. doi: 10.1158/0008-5472.CAN-05-4397. [DOI] [PubMed] [Google Scholar]
- 78.Tanabe E, Shibata A, Inoue S, Kitayoshi M, Fukushima N, Tsujiuchi T. Regulation of cell motile activity through the different induction of LPA receptors by estrogens in liver epithelial WB-F344 cells. Biochemical and biophysical research communications. 2012;428(1):105–9. doi: 10.1016/j.bbrc.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Practice Committee of the American Society for Reproductive M. Treatment of pelvic pain associated with endometriosis. Fertility and sterility. 2006;86(5 Suppl 1):S18–27. doi: 10.1016/j.fertnstert.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 80.Bazin S, Lefebvre J, Fortier M, Brisson J, Brouillette F, Bujold E, et al. Evaluation of an estrogen vaginal cream for the treatment of dyspareunia: a double-blind randomized trial. Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2011;33(8):838–43. doi: 10.1016/S1701-2163(16)34987-8. [DOI] [PubMed] [Google Scholar]
- 81.Haj-Ahmad TA, Abdalla MA, Haj-Ahmad Y. Potential Urinary miRNA Biomarker Candidates for the Accurate Detection of Prostate Cancer among Benign Prostatic Hyperplasia Patients. Journal of Cancer. 2014;5(3):182–91. doi: 10.7150/jca.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ito T, Sato F, Kan T, Cheng Y, David S, Agarwal R, et al. Polo-like kinase 1 regulates cell proliferation and is targeted by miR-593* in esophageal cancer. International journal of cancer Journal international du cancer. 2011;129(9):2134–46. doi: 10.1002/ijc.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shigehara K, Yokomuro S, Ishibashi O, Mizuguchi Y, Arima Y, Kawahigashi Y, et al. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PloS one. 2011;6(8):e23584. doi: 10.1371/journal.pone.0023584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. MicroRNA expression variability in human cervical tissues. PloS one. 2010;5(7):e11780. doi: 10.1371/journal.pone.0011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ninio-Many L, Grossman H, Levi M, Zilber S, Tsarfaty I, Shomron N, et al. MicroRNA miR-125a-3p modulates molecular pathway of motility and migration in prostate cancer cells. Oncoscience. 2014;1(4):250–61. doi: 10.18632/oncoscience.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vetter G, Saumet A, Moes M, Vallar L, Le Bechec A, Laurini C, et al. miR-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers. Oncogene. 2010;29(31):4436–48. doi: 10.1038/onc.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Lam WY, Yeung AC, Ngai KL, Li MS, To KF, Tsui SK, et al. Effect of avian influenza A H5N1 infection on the expression of microRNA-141 in human respiratory epithelial cells. BMC microbiology. 2013;13:104. doi: 10.1186/1471-2180-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dominguez F, Moreno-Moya JM, Lozoya T, Romero A, Martinez S, Monterde M, et al. Embryonic miRNA profiles of normal and ectopic pregnancies. PloS one. 2014;9(7):e102185. doi: 10.1371/journal.pone.0102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hewagama A, Gorelik G, Patel D, Liyanarachchi P, McCune WJ, Somers E, et al. Overexpression of X-linked genes in T cells from women with lupus. Journal of autoimmunity. 2013;41:60–71. doi: 10.1016/j.jaut.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong Y, Li P, Ni Y, Zhao J, Liu Z. Decreased microRNA-125a-3p contributes to upregulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PloS one. 2014;9(11):e111594. doi: 10.1371/journal.pone.0111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. Journal of cell science. 2010;123(Pt 24):4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. The European journal of neuroscience. 2004;19(3):634–42. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- 93.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends in endocrinology and metabolism: TEM. 2012;23(3):133–41. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calura E, Cagnin S, Raffaello A, Laveder P, Lanfranchi G, Romualdi C. Meta-analysis of expression signatures of muscle atrophy: gene interaction networks in early and late stages. BMC genomics. 2008;9:630. doi: 10.1186/1471-2164-9-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwander M, Shirasaki R, Pfaff SL, Muller U. Beta1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(37):8181–91. doi: 10.1523/JNEUROSCI.1345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes research and clinical practice. 2011;93 (Suppl 1):S52–9. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 97.Luo J, Sobkiw CL, Hirshman MF, Logsdon MN, Li TQ, Goodyear LJ, et al. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cell metabolism. 2006;3(5):355–66. doi: 10.1016/j.cmet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Stenzel D, Lundkvist A, Sauvaget D, Busse M, Graupera M, van der Flier A, et al. Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development. 2011;138(20):4451–63. doi: 10.1242/dev.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lei WL, Xing SG, Deng CY, Ju XC, Jiang XY, Luo ZG. Laminin/beta1 integrin signal triggers axon formation by promoting microtubule assembly and stabilization. Cell research. 2012;22(6):954–72. doi: 10.1038/cr.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38(5):747–58. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Q, Verne GN. miRNA-based therapies for the irritable bowel syndrome. Expert opinion on biological therapy. 2011;11(8):991–5. doi: 10.1517/14712598.2011.577060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59(6):775–84. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goetsch MF. Unprovoked vestibular burning in late estrogen-deprived menopause: a case series. Journal of lower genital tract disease. 2012;16(4):442–6. doi: 10.1097/LGT.0b013e31825c2d28. [DOI] [PubMed] [Google Scholar]
- 104.Jeon Y, Kim Y, Shim B, Yoon H, Park Y, Shim B, et al. A retrospective study of the management of vulvodynia. Korean journal of urology. 2013;54(1):48–52. doi: 10.4111/kju.2013.54.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ventolini G, Barhan S, Duke J. Vulvodynia, a step-wise therapeutic prospective cohort study. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2009;29(7):648–50. doi: 10.1080/01443610903095882. [DOI] [PubMed] [Google Scholar]
- 106.Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia, unprovoked. The Journal of reproductive medicine. 2007;52(2):103–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.