Abstract
Background
Several prospective studies have evaluated the association between glycosylated hemoglobin (HbA1c) and death risk among diabetic patients. However, the results have been inconsistent.
Methods
We performed a prospective study which included 13,334 men and 21,927 women with type 2 diabetes. Cox proportional hazards regression models were used to estimate the association of different levels of HbA1c with all-cause mortality.
Results
During a mean follow up of 8.7 years, 4,199 (2,082 men and 2,117 women) patients died. The multivariable-adjusted hazard ratios (HRs) of all-cause mortality associated with different levels of HbA1c at baseline (<6.0%, 6.0-6.9% [reference], 7.0-7.9, 8.0-8.9%, 9.0-9.9%, 10.0-10.9%, and ≥11.0%) were 1.06, 1.00, 1.10, 0.93, 1.26, 1.18 and 1.31 (Pnon-linear =0.008) for men, and 1.21, 1.00, 1.01, 1.08, 1.30, 1.30 and 1.74 (Pnon-linear <0.001) for women, respectively. The J-shaped association of HbA1c with all-cause mortality was confirmed among African American and white diabetic patients, patients who were more than 50 years old, never smoked or used insulin. When we used an updated mean value of HbA1c, the J-shaped association of HbA1c with the risk of all-cause mortality did not change.
Conclusions
Our study demonstrated a J-shaped association between HbA1c and the risk of all-cause mortality among men and women with type 2 diabetes. Both high and low levels of HbA1c were associated with an increased risk of all-cause mortality.
Keywords: mortality, HbA1c, cohort study, type 2 diabetes
1. Introduction
Diabetes is one of the major public health problems worldwide, affecting about 24 million individuals in the US alone [1]. Individuals with diabetes have a 2-4 fold increased risk of all-cause mortality than those without diabetes [2]. Glycosylated hemoglobin (HbA1c) provides a measure of average blood glucose levels over the preceding two to three months and is considered the best measurement of long-term glycemic control among diabetic patients.
Previous observational studies have focused on the association of glycemic control with all-cause mortality, and the majority have showed a positive linear relationship between HbA1c and all-cause mortality [3-6]. However, several other studies suggested an increased risk of all-cause mortality associated with both low normal HbA1c level and high HbA1c level (J or U shaped curve) [7-9]. The UK prospective Diabetes Study (UKPDS) demonstrated a marginally significant lower risk of myocardial infarction (MI) in the intensively treated group [10] and a continued reduction in microvascular risk and the risk of MI and death after an extended post-trial follow-up of 10 years [11]; however, other randomized controlled trials (RCTs) did not show a benefit of intensive glucose control [12-14]. Thus, more epidemiological data are needed. In addition, most epidemiological studies only use a single baseline measurement of HbA1c to predict risk of mortality, which may produce bias. The aim of the present study was to examine the association between levels of HbA1c at baseline and during follow-up with the risk of all-cause mortality among patients with type 2 diabetes in the Louisiana State University Hospital-Based Longitudinal Study (LSUHLS).
2. Materials and Methods
2.1 Study Population
Between 1997 and 2012, the LSU Health Care Services Division (LSUHCSD) operated seven public hospitals and affiliated clinics in Louisiana providing quality medical care to the residents of Louisiana regardless of their income or insurance coverage [15-22]. Since 1997, administrative, anthropometric, laboratory (test code, test collection date, test result values, and abnormal flag), clinical diagnosis, and medication data collected at these facilities are available in electronic form for both inpatients and outpatients. Totally there were about 1.6 million patients (35% of the Louisiana population) in the LSU HCSD facilities. The LSUHLS was established in 2010 by using these data [15]. We established a cohort of diabetic patients who used LSU HCSD hospitals between January 1, 1999 and December 31, 2009, using the International Classification of Diseases (ICD)-9 (code 250). All diabetic patients in the LSU HCSD hospitals were diagnosed using the American Diabetes Association (ADA) criteria: a fasting plasma glucose ≥7.0 mmol/l, or 2-hour glucose ≥11.1 mmol/l after a 75-g 2-hour oral glucose tolerance test (OGTT); or a patient with one or more classic symptoms plus a random plasma glucose ≥11.1 mmol/l [23]. A validation study has been carried out for the diagnosis of diabetes in LSUHCSD hospitals. 20,919 patients from a sample of 21,566 with hospital discharge diagnoses based on ICD codes also had physician-confirmed diabetes using the ADA diabetes diagnosis criteria [23], and the agreement of diabetes diagnosis was 97%.
In the present study, we only included patients who had newly diagnosed diabetes. These patients had used the LSUHSCD system with a mean time of 5.0 years before the diagnosis of diabetes. After excluding patients without at least 2 measurements of any of the required variables for analysis (all variables listed in Table 1), the present study sample included 35,261 patients with type 2 diabetes (19,757 African Americans and 15,504 White Americans; 13,334 men and 21,927 women), who were 30-94 years of age. Among 35,261 participants, 77.4% of patients qualified for free care (by virtue of being low income and uninsured [any individual or family unit whose income is at or below 200% of Federal Poverty Level]), 4.9% of patients were self-pay (uninsured, but incomes not low enough to qualify for free care), 5.1% of patients were covered by Medicaid, 10.4% of patients had Medicare, and 2.2% of patients were covered by commercial insurance. The study and analysis plan were approved by Pennington Biomedical Research Center and LSU Health Sciences Center Institutional Review Boards. We did not obtain informed consent from participants involved in our study because we used anonymized data compiled from electronic medical records.
Table 1. Baseline characteristics according to HbA1c categories of patients with type 2 diabetes.
| HbA1c (%) (mmol/mol) | P | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| <6.0 (42) | 6.0-6.9 (42-52) | 7.0-7.9 (53-63) | 8.0-8.9 (64-74) | 9.0-9.9 (75-85) | 10.0-10.9 (86-96) | ≥11.0 (97) | ||
| No. of participants | 9,417 | 9,217 | 4,952 | 3,119 | 2,379 | 1,867 | 4,310 | - |
| No. of deaths | 1,127 | 1,039 | 646 | 368 | 283 | 231 | 505 | 0.114 |
| Race/Ethnicity (%) | <0.0 01 | |||||||
| African American | 47.3 | 56.4 | 56.1 | 57.0 | 56.5 | 59.3 | 71.8 | |
| White | 52.7 | 43.6 | 43.9 | 43.0 | 43.5 | 40.7 | 28.2 | |
| Male (%) | 37.5 | 33.3 | 35.5 | 36.7 | 41.4 | 46.6 | 45.9 | <0001 |
| Age, yr | 53.9 (0.1) | 54.3 (0.1) | 52.8 (0.1) | 50.8 (0.2) | 49.3 (0.2) | 48.5 (0.2) | 47.9 (0.2) | <0.001 |
| Income, $/family | 19,714 (280) | 19,684 (282) | 19,692 (383) | 19,056 (483) | 18,860 (554) | 18,815 (626) | 17,794 (418) | 0.005 |
| Body mass index, kg/m2 | 33.6 (0.1) | 35.3 (0.1) | 35.1 (0.1) | 34.3 (0.1) | 34.1 (0.2) | 33.8 (0.2) | 32.5 (0.1) | <0.001 |
| Systolic blood pressure, mm Hg | 141 (0.2) | 144 (0.2) | 146 (0.3) | 146 (0.4) | 147 (0.5) | 147 (0.6) | 145 (0.4) | <0.001 |
| Low-density lipoprotein cholesterol, mg/dL | 110 (0.4) | 110 (0.4) | 111 (0.6) | 112 (0.7) | 111 (0.8) | 115 (0.9) | 118 (0.6) | <0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) (%) | <0.001 | |||||||
| ≥90 | 37.9 | 40.8 | 45.0 | 51.9 | 56.0 | 57.9 | 60.9 | |
| 60-89 | 46.4 | 44.1 | 39.9 | 35.8 | 33.9 | 32.0 | 30.4 | |
| 30-59 | 13.7 | 13.6 | 13.3 | 10.8 | 8.6 | 9.2 | 7.9 | |
| 5-29 | 1.3 | 1.1 | 1.3 | 1.2 | 1.3 | 0.9 | 0.6 | |
| <15 | 0.8 | 0.5 | 0.5 | 0.3 | 0.3 | 0.2 | 0.2 | |
| Smoking status (%) | <0.001 | |||||||
| Never smoking | 63.6 | 69.1 | 68.4 | 66.2 | 64.5 | 63.5 | 62.3 | |
| Past smoking | 7.5 | 7.1 | 6.8 | 7.6 | 8.2 | 7.8 | 6.6 | |
| Current smoking | 29.0 | 23.8 | 24.8 | 26.2 | 27.3 | 28.7 | 31.1 | |
| Type of insurance (%) | <0.001 | |||||||
| Free | 74.9 | 76.3 | 76.1 | 79.9 | 79.2 | 81.5 | 81.4 | |
| Self-pay | 4.1 | 4.2 | 4.4 | 5.1 | 5.6 | 6.1 | 8.1 | |
| Medicaid | 5.4 | 5.0 | 5.4 | 4.1 | 5.5 | 5.3 | 5.3 | |
| Medicare | 13.2 | 12.3 | 11.8 | 8.5 | 7.2 | 5.7 | 3.9 | |
| Commercial | 2.4 | 2.4 | 2.3 | 2.5 | 2.6 | 1.5 | 1.4 | |
| Patient status (%) | <0.001 | |||||||
| Outpatient only | 47.7 | 49.5 | 48.1 | 48.0 | 44.0 | 43.1 | 41.0 | |
| Outpatient and inpatient | 52.3 | 50.5 | 51.9 | 52.0 | 56.0 | 56.9 | 59.1 | |
| Uses of medications (%) | ||||||||
| Glucose-lowering medication | <0.001 | |||||||
| Oral hypoglycemic agents | 33.7 | 44.1 | 39.0 | 29.1 | 24.3 | 23.2 | 20.1 | |
| Insulin | 8.5 | 20.4 | 35.1 | 45.2 | 53.5 | 53.1 | 58.0 | |
| Lipid-lowering medication | 53.9 | 58.3 | 58.2 | 56.7 | 58.2 | 57.1 | 54.8 | <0.001 |
| Antihypertensive medication | 72.3 | 73.6 | 72.8 | 71.5 | 74.3 | 72.4 | 72.9 | 0.140 |
Values represent means (SE) or percentages. All continuous data adjusted for age, gender and race (age was adjusted for gender and race).
2.2 Baseline and follow-up measurements
The patient's characteristics, including age of diabetes diagnosis, gender, race/ethnicity, family income, smoking status, types of health insurance, body weight, height, BMI, blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, HbA1c, estimated glomerular filtration rate (eGFR), and medication (antihypertensive drug, cholesterol lowering drug and anti-diabetic drug) within half a year after the diabetes diagnosis (baseline) and during follow-up after the diabetes diagnosis (follow-up) were extracted from the computerized hospitalization records. The updated mean values of HbA1c, LDL cholesterol, BMI, blood pressure and eGFR over time were calculated for each participant from baseline to each year of follow up. For example, at one year the updated mean is the average of the baseline and one year values and at three years it is the average of baseline, one year, two year, and three year values. In the case of an event during follow-up, the period for estimating the updated mean value was from baseline to the year before this event occurred [24]. The average number of HbA1c measurements during the follow-up period was 15.0.
2.3 Prospective follow-up
Follow-up information was obtained from the LSUHLS inpatient and outpatient database by using the unique number assigned to every patient who visits the LSUHCSD hospitals. The diagnosis of all-cause death was the primary endpoint of interest of the study. Mortality outcomes were assessed by linkage with the State Center for Health Statistics at Louisiana's Office of Public Health (the Louisiana Office of Public Health Vital Records Registry). Follow-up of each cohort member continued until the date of the death, or June 31, 2013.
2.4 Statistical analysis
The Cox proportional hazard model was used to estimate the association between HbA1c and all-cause mortality. HbA1c was divided into 7 categories (HbA1c <6.0% [42 mmol/mol], 6.0-6.9% [42-52 mmol/mol] [reference], 7.0-7.9% [53-63mmol/mol], 8.0-8.9% [64-74 mmol/mol], 9.0-9.9% [75-85 mmol/mol7], 10.0-10.9% [86-96 mmol/mol], and ≥11.0% ]97 mmol/mol]). All analyses were adjusted for age and race (Model 1); age, race, smoking, income, type of insurance, LDL cholesterol, systolic blood pressure, BMI, and eGFR at baseline (in the baseline analyses) and during follow-up (in the follow-up analyses) (Model 2); variables in Model 2, and also for use of antihypertensive drugs, glucose-lowering agents, and cholesterol-lowering agents (Model 3). The different categories of HbA1c were included in the models as dummy variables. To avoid the potential bias due to the presence of occult diseases at baseline, additional analyses were carried out excluding the subjects who died during the first two years of follow-up, or excluding patients with a history of coronary heart disease (CHD) or cancer at the time of diagnosis of diabetes. Restricted cubic splines in Cox models were used to test whether there was a dose-response or non-linear associate of HbA1c as a continuous variable with all-cause mortality risk [25]. Non-linear trends were assessed with likelihood-ratio tests of restricted cubic splines [25]. Statistical significance was considered to be p <0.05. All statistical analyses were performed with SAS for Windows, version 9.3 (SAS Institute, Cary, NC).
3. Results
General characteristics stratified by baseline HbA1c are presented in Table 1. During a mean follow-up period of 8.7 years, 4,199 (2,082 men and 2,117 women) patients died. The multivariable-adjusted (Model 3) hazard ratios (HRs) (95% CIs) of all-cause mortality associated with different levels of HbA1c at baseline (<6.0%, 6.0-6.9% [reference], 7.0-7.9%, 8.0-8.9%, 9.0-9.9%, 10.0-10.9%, and ≥11.0%) were 1.06 (0.92-1.24), 1.00, 1.10 (0.92-1.30), 0.93 (0.75-1.16), 1.26 (1.01-1.58), 1.18 (0.93-1.51) and 1.31 (1.08-1.60) (Pnon-linear =0.008) for men, and 1.21 (1.04-1.41), 1.00, 1.01 (0.85-1.20), 1.08 (0.87-1.34), 1.30 (1.03-1.64), 1.30 (1.00-1.69) and 1.74 (1.42-2.13) (Pnon-linear <0.001) for women, respectively (Table 2). When patients were divided into 7 groups based on HbA1c levels with the same sample size in each group, there was a J or U-shaped association between HbA1c levels and the risk of all-cause mortality (Online table 1). When HbA1c was considered as a continuous variable by using restricted cubic splines, a nadir of the J-shaped association of HbA1c with all-cause mortality was observed at HbA1c of 6.0-6.9% (Table 2 and Figure 1).
Table 2. Hazard ratios for all-cause mortality according to different levels of HbA1c at baseline and during follow-up among patients with type 2 diabetes.
| HbA1c (%) (mmol/mol) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| <6.0 (<42) | 6.0-6.9 (42-52) | 7.0-7.9 (53-63) | 8.0-8.9 (64-74) | 9.0-9.9 (75-85) | 10.0-10.9 (86-96) | ≥11.0 (≥97) | |
| Male | |||||||
| Baseline | |||||||
| No. of participants | 3,532 | 3,067 | 1,757 | 1,146 | 984 | 869 | 1,979 |
| No. of cases | 567 | 516 | 302 | 175 | 141 | 124 | 257 |
| Person-years | 26,526 | 25,390 | 15,176 | 10,193 | 8,455 | 7,527 | 16,093 |
| HR (95% CI) | |||||||
| Model 1 | 1.15 (1.02-1.29) | 1.00 | 1.03 (0.89-1.18) | 0.98 (0.83-1.17) | 1.11 (0.92-1.33) | 1.17 (0.96-1.42) | 1.32 (1.13-1.54) |
| Model 2 | 1.17 (1.01-1.36) | 1.00 | 1.09 (0.92-1.30) | 0.97 (0.79-1.21) | 1.24 (0.99-1.54) | 1.26 (0.99-1.60) | 1.41 (1.17-1.71) |
| Model 3 | 1.06 (0.92-1.24) | 1.00 | 1.10 (0.92-1.30) | 0.93 (0.75-1.16) | 1.26 (1.01-1.58) | 1.18 (0.93-1.51) | 1.31 (1.08-1.60) |
| Follow-up | |||||||
| No. of participants | 3,031 | 3,256 | 2,446 | 1,713 | 1,162 | 787 | 939 |
| No. of cases | 547 | 518 | 368 | 244 | 159 | 109 | 137 |
| Person-years | 21,525 | 26,372 | 21,446 | 15,244 | 10,316 | 6,862 | 7,596 |
| HR (95% CI) | |||||||
| Model 1 | 1.47 (1.30-1.66) | 1.00 | 0.93 (0.81-1.06) | 1.03 (0.88-1.20) | 1.15 (0.96-1.38) | 1.37 (1.10-1.69) | 1.81 (1.49-2.20) |
| Model 2 | 1.44 (1.25-1.67) | 1.00 | 0.91 (0.77-1.07) | 1.18 (0.98-1.42) | 1.32 (1.06-1.63) | 1.50 (1.16-1.94) | 1.82 (1.41-2.35) |
| Model 3 | 1.22 (1.05-1.42) | 1.00 | 0.89 (0.76-1.06) | 1.09 (0.90-1.32) | 1.19 (0.95-1.49) | 1.31 (1.01-1.71) | 1.48 (1.14-1.93) |
| Female | |||||||
| Baseline | |||||||
| No. of participants | 5,885 | 6,150 | 3,195 | 1,973 | 1,395 | 998 | 2,331 |
| No. of cases | 567 | 516 | 302 | 175 | 141 | 124 | 257 |
| Person-years | 26,526 | 25,390 | 15,176 | 10,193 | 8,455 | 7,527 | 16,093 |
| HR (95% CI) | |||||||
| Model 1 | 1.29 (1.15-1.46) | 1.00 | 1.16 (1.01-1.33) | 1.18 (1.00-1.39) | 1.36 (1.13-1.64) | 1.55 (1.25-1.91) | 1.92 (1.65-2.24) |
| Model 2 | 1.30 (1.12-1.50) | 1.00 | 1.02 (0.86-1.21) | 1.16 (0.94-1.43) | 1.43 (1.14-1.79) | 1.51 (1.16-1.96) | 1.95 (1.61-2.37) |
| Model 3 | 1.21 (1.04-1.41) | 1.00 | 1.01 (0.85-1.20) | 1.08 (0.87-1.34) | 1.30 (1.03-1.64) | 1.30 (1.00-1.69) | 1.74 (1.42-2.13) |
| Follow-up | |||||||
| No. of participants | 5,192 | 6,400 | 4,012 | 2,479 | 1,660 | 1,059 | 1,125 |
| No. of cases | 539 | 579 | 386 | 224 | 159 | 118 | 112 |
| Person-years | 40,144 | 55,568 | 38,516 | 24,504 | 16,743 | 10,619 | 10,428 |
| HR (95% CI) | |||||||
| Model 1 | 1.40 (1.24-1.57) | 1.00 | 1.07 (0.94-1.22) | 1.14 (0.97-1.33) | 1.34 (1.12-1.60) | 1.86 (1.52-2.28) | 2.16 (1.75-2.67) |
| Model 2 | 1.48 (1.28-1.72) | 1.00 | 1.11 (0.94-1.30) | 1.10 (0.90-1.34) | 1.35 (1.08-1.69) | 2.08 (1.62-2.68) | 2.27 (1.71-3.03) |
| Model 3 | 1.30 (1.11-1.51) | 1.00 | 1.04 (0.88-1.22) | 0.94 (0.77-1.16) | 1.16 (0.91-1.46) | 1.78 (1.37-2.31) | 1.80 (1.34-2.42) |
| Total | |||||||
| Baseline | |||||||
| No. of participants | 9,417 | 9,217 | 4,952 | 3,119 | 2,379 | 1,867 | 4,310 |
| No. of cases | 1134 | 1032 | 604 | 350 | 282 | 248 | 514 |
| Person-years | 53,052 | 50,780 | 30,352 | 20,386 | 16,910 | 15,054 | 32,186 |
| HR (95% CI)* | |||||||
| Model 1 | 1.23 (1.13-1.33) | 1.00 | 1.09 (0.99-1.21) | 1.08 (0.96-1.22) | 1.23 (1.08-1.41) | 1.35 (1.17-1.56) | 1.60 (1.43-1.78) |
| Model 2 | 1.24 (1.12-1.38) | 1.00 | 1.06 (0.93-1.19) | 1.07 (0.92-1.24) | 1.34 (1.14-1.57) | 1.39 (1.16-1.66) | 1.67 (1.45-1.91) |
| Model 3 | 1.15 (1.03-1.28) | 1.00 | 1.06 (0.93-1.19) | 1.01 (0.87-1.17) | 1.29 (1.10-1.52) | 1.25 (1.05-1.50) | 1.52 (1.32-1.75) |
| Follow-up | |||||||
| No. of participants | 8,223 | 9,656 | 6,458 | 4,192 | 2,822 | 1,846 | 2,064 |
| No. of cases | 1086 | 1097 | 754 | 468 | 318 | 227 | 249 |
| Person-years | 61,669 | 81,940 | 59,962 | 39,748 | 27,059 | 17,481 | 18,024 |
| HR (95% CI)* | |||||||
| Model 1 | 1.44 (1.32-1.57) | 1.00 | 1.00 (0.91-1.09) | 1.08 (0.97-1.21) | 1.24 (1.09-1.41) | 1.60 (1.38-1.85) | 1.98 (1.72-2.29) |
| Model 2 | 1.48 (1.34-1.64) | 1.00 | 1.01 (0.90-1.13) | 1.15 (1.01-1.32) | 1.36 (1.16-1.59) | 1.77 (1.48-2.12) | 2.05 (1.69-2.47) |
| Model 3 | 1.27 (1.14-1.42) | 1.00 | 0.96 (0.86-1.08) | 1.03 (0.89-1.18) | 1.19 (1.01-1.40) | 1.52 (1.26-1.83) | 1.64 (1.35-1.99) |
Model 1 adjusted for age and race.
Model 2 adjusted for age, race, types of insurance, income, smoking, body mass index, low-density lipoprotein cholesterol, systolic blood pressure, and glomerular filtration rate at baseline (in baseline analyses) and during follow-up (in the follow-up analyses).
Model 3 adjusted for variables in Model 2, and also for use of antihypertensive drugs, glucose-lowering agents, and cholesterol-lowering agents at baseline (in baseline analyses) and during follow-up (in the follow-up analyses).
Gender was additional adjusted in Model 1, 2, 3 among the total patients.
Figure 1.
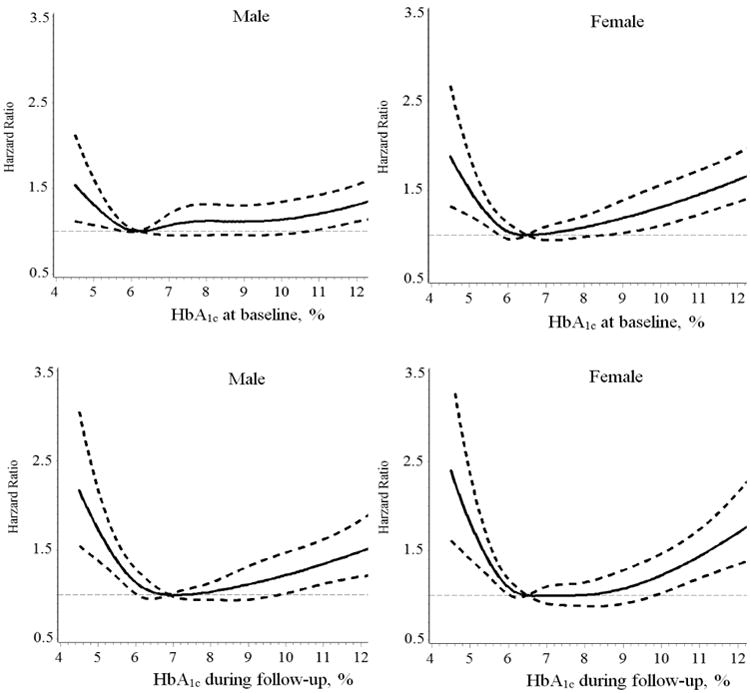
Hazard ratios for all-cause mortality based on different levels of HbA1c at baseline and during follow-up. Adjusted for age, race, types of insurance, income, smoking, body mass index, low-density lipoprotein cholesterol, systolic blood pressure, glomerular filtration rate, use of antihypertensive drugs, glucose-lowering agents, and cholesterol-lowering agents.
When we did an additional analysis by using an updated mean value of HbA1c, we found the same J-shaped association between HbA1c and the risk of all-cause mortality (Table 2 and Figure 1). The multivariable-adjusted (Model 3) HRs (95% CIs) of all-cause mortality associated with different levels of HbA1c during follow-up (<6.0%, 6.0-6.9%, 7.0-7.9%, 8.0-8.9%, 9.0- 9.9%, 10.0-10.9%, and ≥11.0%) were 1.22 (1.05-1.42), 1.00, 0.89 (0.76-1.06), 1.09 (0.90-1.32), 1.19 (0.95-1.49), 1.31 (1.01-1.71) and 1.48 (1.14-1.93) (Pnon-linear <0.001) for men, and 1.30 (1.11-1.51), 1.00, 1.04 (0.88-1.22), 0.94 (0.77-1.16), 1.16 (0.91-1.46), 1.78 (1.37-2.31) and 1.80 (1.34-2.42) (Pnon-linear <0.001) for women, respectively (Table 2).
When stratified by race, age, BMI, smoking status, and use of antidiabetic drugs, the trend of a J-shaped association of HbA1c with all-cause mortality was present in most subgroups. But this J-shaped association disappeared, which probably indicated that the effect of HbA1c weakened among patients who were currently smoking (Table 3). When we performed sensitivity analyses by excluding patients who died during the first two years of follow-up (n=435) or excluding patients with a history of CHD or cancer at the diagnosis of diabetes (n=5,608), the multivariable-adjusted J-shaped association of HbA1c with all-cause mortality risk did not change (Online table 2).
Table 3. Hazard ratio for all-cause mortality according to different levels of HbA1c at baseline and during follow-up among various subpopulations.
| HbA1c (%) (mmol/mol) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| <6.0(<42) | 6.0-6.9(42-52) | 7.0-7.9(53-63) | 8.0-8.9(64-74) | 9.0-9.9(75-85) | 10.0-10.9(86-96) | ≥11.0(≥97) | |
| Baseline | |||||||
| Race | |||||||
| African American | 1.30 (1.11-1.52) | 1.00 | 1.06 (0.88-1.27) | 1.06 (0.85-1.31) | 1.31 (1.05-1.64) | 1.31 (1.02-1.68) | 1.44 (1.20-1.74) |
| White American | 1.05 (0.91-1.21) | 1.00 | 1.07 (0.90-1.26) | 0.97 (0.78-1.20) | 1.29 (1.02-1.62) | 1.21 (0.94-1.57) | 1.67 (1.34-2.08) |
| Age groups, yr | |||||||
| <60 | 1.03 (0.89-1.19) | 1.00 | 1.07 (0.91-1.26) | 0.89 (0.73-1.07) | 1.18 (0.97-1.43) | 1.10 (0.89-1.36) | 1.33 (1.13-1.56) |
| 60-94 | 1.21 (1.04-1.40) | 1.00 | 1.00 (0.83-1.21) | 1.12 (0.87-1.44) | 1.16 (0.86-1.57) | 1.15 (0.81-1.65) | 1.16 (0.83-1.63) |
| Body mass index, kg/m2 | |||||||
| <30 | 1.09 (0.93-1.27) | 1.00 | 0.86 (0.71-1.05) | 0.95 (0.75-1.19) | 1.34 (1.05-1.71) | 0.95 (0.70-1.27) | 1.37 (1.12-1.68) |
| ≥30 | 1.13 (0.98-1.31) | 1.00 | 1.20 (1.02-1.40) | 1.07 (0.88-1.31) | 1.28 (1.03-1.58) | 1.55 (1.23-1.94) | 1.62 (1.34-1.97) |
| Smoking status | |||||||
| Never | 1.27 (1.11-1.45) | 1.00 | 1.16 (1.00-1.35) | 1.15 (0.95-1.39) | 1.43 (1.17-1.76) | 1.42 (1.13-1.77) | 1.59 (1.33-1.91) |
| Ever | 1.90 (1.21-.99) | 1.00 | 1.28 (0.76-2.15) | 0.90 (0.44-1.87) | 1.38 (0.74-2.55) | 1.81 (0.92-3.58) | 2.38 (1.35-4.21) |
| Current | 0.88 (0.73-1.06) | 1.00 | 0.83 (0.66-1.06) | 0.79 (0.60-1.04) | 1.07 (0.80-1.43) | 0.89 (0.64-1.23) | 1.28 (1.01-1.62) |
| Using glucose-lowering agents | |||||||
| No | 1.05 (0.91-1.22) | 1.00 | 1.23 (1.01-1.51) | 1.02 (0.78-1.33) | 1.49 (1.11-2.02) | 1.30 (0.95-1.79) | 1.74 (1.37-2.22) |
| Oral hypoglycemic agents | 1.11 (0.91-1.35) | 1.00 | 0.88 (0.69-1.11) | 0.95 (0.68-1.33) | 1.13 (0.77-1.66) | 1.12 (0.73-1.71) | 1.16 (0.82-1.66) |
| Insulin | 1.61 (1.26-2.06) | 1.00 | 1.11 (0.90-1.36) | 1.06 (0.84-1.34) | 1.32 (1.05-1.67) | 1.34 (1.03-1.75) | 1.60 (1.29-1.98) |
| Follow-up | |||||||
| Race | |||||||
| African American | 1.37 (1.17-1.61) | 1.00 | 0.89 (0.75-1.06) | 1.06 (0.87-1.29) | 1.18 (0.95-1.47) | 1.40 (1.09-1.79) | 1.33 (1.03-1.71) |
| White American | 1.20 (1.04-1.38) | 1.00 | 1.04 (0.89-1.22) | 0.98 (0.80-1.20) | 1.21 (0.94-1.55) | 1.66 (1.26-2.20) | 2.23 (1.63-3.06) |
| Age groups, yr | |||||||
| <60 | 1.27 (1.09-1.48) | 1.00 | 1.07 (0.91-1.25) | 0.95 (0.80-1.13) | 1.15 (0.95-1.39) | 1.32 (1.07-1.63) | 1.33 (1.07-1.65) |
| 60-94 | 1.20 (1.03-1.40) | 1.00 | 0.79 (0.66-0.95) | 1.04 (0.82-1.32) | 0.85 (0.59-1.23) | 1.38 (0.86-2.21) | 1.60 (0.92-2.80) |
| Body mass index kg/m2 | |||||||
| <30 | 1.23 (1.05-1.44) | 1.00 | 0.95 (0.79-1.14) | 1.03 (0.83-1.29) | 1.24 (0.97-1.59) | 1.04 (0.77-1.41) | 1.51 (1.15-1.98) |
| ≥30 | 1.21 (1.04-1.40) | 1.00 | 0.98 (0.84-1.14) | 1.04 (0.87-1.25) | 1.17 (0.94-1.45) | 2.01 (1.59-2.55) | 1.59 (1.20-2.12) |
| Smoking status | |||||||
| Never | 1.32 (1.15-1.51) | 1.00 | 1.00 (0.87-1.16) | 1.00 (0.83-1.19) | 1.29 (1.05-1.59) | 1.69 (1.34-2.14) | 1.65 (1.27-2.15) |
| Ever | 2.13 (1.33-3.41) | 1.00 | 0.85 (0.51-1.40) | 1.10 (0.64-1.92) | 1.74 (0.97-3.15) | 2.06 (0.97-4.38) | 2.01 (0.88-4.61) |
| Current | 1.09 (0.90-1.33) | 1.00 | 0.91 (0.73-1.14) | 1.07 (0.84-1.37) | 0.94 (0.70-1.26) | 1.19 (0.85-1.67) | 1.57 (1.14-2.16) |
| Using glucose-lowering agents | |||||||
| No | 1.09 (0.94-1.26) | 1.00 | 1.19 (0.98-1.44) | 1.16 (0.91-1.47) | 1.23 (0.90-1.68) | 1.93 (1.38-2.71) | 2.05 (1.48-2.84) |
| Oral hypoglycemic agents | 1.60 (1.32-1.94) | 1.00 | 1.03 (0.83-1.29) | 1.01 (0.72-1.44) | 1.38 (0.91-2.09) | 1.32 (0.74-2.36) | 0.69 (0.30-1.57)* |
| Insulin | 1.87 (1.40-2.50) | 1.00 | 0.77 (0.63-0.94) | 0.89 (0.72-1.10) | 1.03 (0.82-1.31) | 1.29 (0.99-1.67) | 1.49 (1.13-1.98) |
Adjusted for age, race, types of insurance, income, smoking, body mass index, low-density lipoprotein cholesterol, systolic blood pressure, glomerular filtration rate, use of antihypertensive drugs, glucose-lowering agents, and cholesterol-lowering agents at baseline (in baseline analyses) and during follow-up (in the follow-up analyses), other than the variable for stratification.
There were only 7 deaths in this group.
4. Discussion
Our study found a J-shaped association of HbA1c at baseline and during follow-up with the risk of all-cause mortality among men and women with type 2 diabetes. A significantly increased risk of all-cause mortality was observed among men with HbA1c <6.0% and HbA1c ≥11.0%, and among women with HbA1c <6.0% and HbA1c ≥10.0%, as compared with patients with HbA1c 6.0-6.9%.
Previous studies have focused on the association of HbA1c with all-cause mortality; however, the results were inconsistent. Most of the observational studies suggested a linear and positive association between HbA1c and all-cause mortality [3-6], while others suggested that both low normal HbA1c level and high HbA1c level were associated with an increased risk of all-cause mortality (J or U shaped curve) [7-9]. Major reasons for the inconsistent results of the above studies might include small sample size or small number of deaths, short follow-up time, and different participant characteristics across studies. Until now, there are two systematic reviews evaluating the association between HbA1c and the risk of all-cause mortality among diabetic patients in prospective cohort studies. One systematic review indicated that each 1% increase in HbA1c among diabetic patients might have a 1.15 fold increase in all-cause mortality risk [26]. However, whether low HbA1c would increase all-cause mortality was not suggested in this review because it only assessed HbA1c as a continuous variable for each 1-unit increase of HbA1c. Another systematic review found a significant J-shaped relationship between HbA1c and the risk of all-cause mortality among diabetic patients [27]. However, HbA1c was only divided into ≥7.5% and <7.5% in this meta-analysis. Our study, conducted in a real clinical population with low income, for the first time revealed a J -shaped relationship between HbA1c and all-cause mortality among patients with type 2 diabetes.
Although clinical trials are better than observational studies to evaluate the association between HbA1c and the risk of all-cause mortality among diabetic patients, whether there is a benefit of intensive glucose control was not confirmed by RCTs. The Sibutramine Cardiovascular OUTcomes (SCOUT) trial indicated that among cardiovascular high-risk patients with type 2 diabetes, increasing HbA1c concentrations increased the risks of cardiovascular adverse outcomes and all-cause mortality, and there was no evidence of increased risk associated with HbA1c ≤6.4% [3]. The UKPDS demonstrated a marginally significant lower risk of MI in the intensively treated group during the RCT period [10] and a continued reduction in microvascular risk and the risks of MI and death after an extended post-trial follow-up of 10 years [11]. However, other two RCTs, Action in Diabetes and Vascular Disease-Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT), did not find any benefits of intensive glucose control on CVD outcomes [13, 14], and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) found an unexpected increase in all-cause and CVD mortality in the intensive glycemic treatment (HbA1c <6.0%) [12]. Two meta-analyses of relevant RCTs indicated that intensive glucose control could reduce the risk of some cardiovascular diseases (such as nonfatal MI and coronary events), but did not reduce the risks of all-cause and CVD-related mortality [28, 29]. The present study, with large sample size and long follow-up time, supported that both low (<6.0% in men and women) and high (≥10% or 11% in men and women) levels of HbA1c were associated with an increased risk of all-cause mortality among men and women with type 2 diabetes. To make our methodology more rigorous, we have conducted sensitivity analyses by excluding patients who died during the first two years of follow-up or excluding patients with a history of CHD or cancer at the diagnosis of diabetes, and the J-shaped association of HbA1c with all-cause mortality risk did not change. When stratified by race, age, BMI, smoking status, and use of antidiabetic drugs, this J-shaped association of HbA1c with all-cause mortality tended to be present in most subgroups.
Despite no benefit of intensive glucose control from RCTs, the ADA, the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) kept the general blood control goal of HbA1c <7% (Class IIB, level of evidence A) for most diabetic patients [30]. The present study found that the lowest mortality rate was among diabetic patients with HbA1c 6.0-6.9% at baseline (new-onset diabetes), and HbA1c 6.0-7.9% during follow-up. Two other cohort studies demonstrating the similar J-shaped association between HbA1c and all-cause mortality also suggested that the lowest mortality rate was found among patients with HbA1c 6.5-6.9% for new-onset diabetes [9], and with a median HbA1c of 7.5% for long-standing diabetes (mean of diabetes duration >5 years) [8]. Based on results from the RCTs, the present study and above two epidemiological studies, the best range of HbA1c seems to be 6.0-6.9% for new-onset diabetes and 6.0-7.9% for long-standing diabetes.
The mechanism for the relationship between elevated blood glucose levels and high mortality has been well explained. Diabetic complications are the major causes of death in persons with diabetes and the theories of diabetic complications caused by hyperglycemia mainly include the toxic effects of hyperglycemia and its pathophysiological derivatives (such as oxidants, hyperosmolarity, or glycation products) on tissues directly and a sustained alteration in cell signaling pathways (such as changes in phospholipids or kinases) induced by the products of glucose metabolism [31]. Reasons for very low HbA1c associated with an increased risk of all-cause mortality are not clear now. The most likely culprit has been hypoglycemia, even though the post hoc analyses of ACCORD data have suggested that this is not the case [32, 33]. Indeed some other studies supported this hypothesis, such as the Veterans Affairs Study which suggested more than one episode of severe hypoglycemia was associated with an 88% rise in the risk for sudden death [34]. Future studies are needed to confirm or explore the mechanism of low HbA1c with an increased risk of all-cause mortality.
There were several strengths of our study, including the large sample size, long follow-up time, and the use of administrative databases to avoid differential recall bias. We used baseline HbA1c levels and updated mean values, which could avoid potential bias from a single baseline measurement. Besides, our study subjects were not volunteers, but rather represent a real clinical population, which makes the results very pertinent to the clinical population of people with low income and with type 2 diabetes. In addition, participants in the present study used the same public health care system which minimized the influence from the accessibility to health care. There were also some limitations. First, our analysis was not performed on a representative sample of the population which limited the generalizability of this study; however, LSUHCSD hospitals were public hospitals and covered over 1.6 million patients, most of whom were low income persons in Louisiana. The results of the present study would have wide applicability for the population with low income and without health insurance in the US. Second, we did not have information on cause-specific deaths and could not assess cardiovascular mortality as a separate end-point. Third, even though our analyses adjusted for an extensive set of confounding factors, residual confounding due to measurement error in the assessment of confounding factors, unmeasured factors such as physical activity, education, and dietary factors, cannot be excluded.
In conclusion, our study demonstrated a J-shaped association of HbA1c with the risk of all-cause mortality risk among men and women with type 2 diabetes. Both high and low levels of HbA1c were associated an increased risk of all-cause mortality.
Supplementary Material
Highlights.
We performed a prospective study including 35261 patients with type 2 diabetes.
Our study subjects represented a real clinical population with low income.
A J-shaped association of HbA1c with death was found among diabetic patients.
Both low and high HbA1c will increase death risk.
Acknowledgments
Funding Source: This work was supported by Louisiana State University's Improving Clinical Outcomes Network (LSU ICON), the Louisiana Clinical Data Research Network (LACDRN), and 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science (LA CaTS) Center.
Footnotes
The authors have reported they have no relationships to disclose.
Contributions: W.L. wrote the manuscript and researched data. P.T.K. reviewed and edited the manuscript. R.H. and Y.W. researched data. J.J. reviewed and edited the manuscript. G.H. wrote, reviewed, and edited the manuscript and researched data. G.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–94. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson C, van Gaal L, Caterson ID, Weeke P, James WP, Coutinho W, et al. Relationship between HbA1c levels and risk of cardiovascular adverse outcomes and all-cause mortality in overweight and obese cardiovascular high-risk women and men with type 2 diabetes. Diabetologia. 2012;55:2348–55. doi: 10.1007/s00125-012-2584-3. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso CR, Leite NC, Ferreira MT, Salles GF. Prognostic importance of baseline and serial glycated hemoglobin levels in high-risk patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Acta diabetologica. 2014 doi: 10.1007/s00592-014-0592-0. [DOI] [PubMed] [Google Scholar]
- 5.Sluik D, Boeing H, Montonen J, Kaaks R, Lukanova A, Sandbaek A, et al. HbA1c measured in stored erythrocytes is positively linearly associated with mortality in individuals with diabetes mellitus. PLoS One. 2012;7:e38877. doi: 10.1371/journal.pone.0038877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitan EB, Liu S, Stampfer MJ, Cook NR, Rexrode KM, Ridker PM, et al. HbA1c measured in stored erythrocytes and mortality rate among middle-aged and older women. Diabetologia. 2008;51:267–75. doi: 10.1007/s00125-007-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skriver MV, Stovring H, Kristensen JK, Charles M, Sandbaek A. Short-term impact of HbA1c on morbidity and all-cause mortality in people with type 2 diabetes: a Danish population-based observational study. Diabetologia. 2012;55:2361–70. doi: 10.1007/s00125-012-2614-1. [DOI] [PubMed] [Google Scholar]
- 8.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–9. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 9.Twito O, Ahron E, Jaffe A, Afek S, Cohen E, Granek-Catarivas M, et al. New-onset diabetes in elderly subjects: association between HbA1c levels, mortality, and coronary revascularization. Diabetes Care. 2013;36:3425–9. doi: 10.2337/dc12-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 11.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 14.Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58:2642–8. doi: 10.2337/db09-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Wang Y, Chen L, Horswell R, Xiao K, Besse J, et al. Increasing prevalence of diabetes in middle or low income residents in Louisiana from 2000 to 2009. Diabetes Res Clin Pract. 2011;94:262–8. doi: 10.1016/j.diabres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Li W, Wang Y, Chen L, Horswell R, Xiao K, et al. Increasing prevalence of hypertension in low income residents within Louisiana State University Health Care Services Division Hospital System. Eur J Intern Med. 2012;23:e179–84. doi: 10.1016/j.ejim.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Hu G, Horswell R, Wang Y, Li W, Besse J, Xiao K, et al. Body Mass Index and the Risk of Dementia among Louisiana Low Income Diabetic Patients. PLoS One. 2012;7:e44537. doi: 10.1371/journal.pone.0044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Katzmarzyk PT, Horswell R, Li W, Xiao K, Besse J, et al. Racial Disparities in Diabetic Complications in an Underinsured Population. J Clin Endocrinol Metab. 2012;97:4446–53. doi: 10.1210/jc.2012-2378. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Cefalu WT, et al. Blood Pressure and Stroke Risk among Diabetic Patients. J Clin Endocrinol Metab. 2013;98:3653–62. doi: 10.1210/jc.2013-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. HbA1c and Coronary Heart Disease Risk among Diabetic Patients HbA1c and CHD risk in diabetic patients. Diabetes Care. 2014;37:428–35. doi: 10.2337/dc13-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Li W, Johnson J, et al. Aggressive Blood Pressure Control Increases Coronary Heart Disease Risk Among Diabetic Patients. Diabetes Care. 2013;36:3287–96. doi: 10.2337/dc13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Heymsfield SB, et al. HbA1c and Lower-Extremity Amputation Risk in Low-Income Patients With Diabetes. Diabetes Care. 2013;36:3591–8. doi: 10.2337/dc13-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 24.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in medicine. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2012;7:e42551. doi: 10.1371/journal.pone.0042551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold LW, Wang Z. The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: a meta-analysis of observational studies. Rev Diabet Stud. 2014;11:138–52. doi: 10.1900/RDS.2014.11.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 29.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 30.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–7. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 31.Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288:2579–88. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 32.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–90. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraira C, Colwell J, Nuttall F, Sawin CT, Henderson W, Comstock JP, et al. Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Arch Intern Med. 1997;157:181–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


