Abstract
Advances in the field of tissue engineering have enhanced the potential of regenerative medicine, yet the efficacy of these strategies remains incomplete, and is limited by the innate and adaptive immune responses. The immune response associated with injury or disease combined with that mounted to biomaterials, transplanted cells, proteins, and gene therapies vectors can contribute to the inability to fully restore tissue function. Blocking immune responses such as with anti-inflammatory or immunosuppressive agents are either ineffective, as the immune response contributes significantly to regeneration, or have significant side effects. This review describes targeted strategies to modulate the immune response in order to limit tissue damage following injury, promote an anti-inflammatory environment that leads to regeneration, and induce antigen (Ag)-specific tolerance that can target degenerative diseases that destroy tissues and promote engraftment of transplanted cells. Focusing on targeted immuno-modulation, we describe local delivery techniques to sites of inflammation as well as systemic approaches that preferentially target subsets of immune populations.
Graphical abstract
1. INTRODUCTION
The immune system has been implicated in numerous aspects of tissue dysfunction and/or limited regeneration, such as injury, autoimmune diseases, and allograft rejection. More than 50 million injuries are reported to hospitals each year accruing over $80 billion in direct medical costs over the lives of these patients ranging from minor bone fractures to traumatic brain and spinal cord injuries [1]. An injury can activate the innate immune response leading to the recruitment of pro-inflammatory neutrophils and macrophages to prevent infection, yet their presence may lead to extensive secondary damage and persistent activity may result in chronic inflammation, both of which can limit tissue regeneration [2]. Dysregulation of the adaptive immune response, which is characterized by a shift in T cell phenotypes, can lead to autoimmunity or chronic inflammation after injury, both of which can compromise the function of tissues [3].
Strategies that are central to tissue engineering can also initiate an immune response that is detrimental to regeneration [4]. Biomaterial scaffolds are frequently used to mechanically support the regenerating tissue or as a vehicle for cell transplantation, for which the immune system initiates a response to the implantation procedure and a foreign body response targeting the implant. Transplantation of non-autologous cells will initiate an allogeneic or xenogeneic immune response, which leads to failure of the graft. Furthermore, protein or gene delivery systems have potential immunogenicity based on intrinsic properties of the bioactive agent or consequences of the processing. Taken together, the immune responses that develop due to injury or to therapeutic strategy can limit regeneration.
Herein, we review strategies for modulating the immune response, both locally and systemically, in order to promote tissue regeneration. Blocking immune responses has proven to be ineffective, as the immune response contributes to regeneration. Modulating the immune response has the ability to limit tissue damage following injury, promote an anti-inflammatory environment that leads to regeneration, and induce Ag-specific tolerance that can target degenerative diseases that destroy tissues and promote engraftment of transplanted cells. We discuss recent developments in the area of biomaterial design, protein and gene delivery systems, cell transplantation, and nanoparticle delivery systems for their ability to modulate immune responses associated with multiple regenerative medicine applications.
2. LOCALIZED STRATEGIES
Modulating the immune response locally at the injury site can promote regeneration and facilitate the engraftment of transplanted cells [2]. Systemic strategies for delivering therapeutics to affect local responses have led to concerns associated with side effects, such as hypersensitivity, the development of bacterial resistance, and gastrointestinal intolerance [5]. Conversely, delivering therapeutics into the local environment can modulate recruitment of immune cell types and their phenotype while avoiding adverse systemic side effects. Localized delivery of therapeutics also provides direct access to tissue-specific inflammatory cells. Within the central nervous system (CNS) microglia and astrocytes work in concert with traditional inflammatory cells to limit inflammation to the injury site and limit excitotoxic molecules such as glutamate released from damaged neurons. In other tissues there are tissue-specific subsets of traditional immune cells, such as Langerhan’s cells which are specialized dendritic cells (DCs) in the epidermis and Kuppfer cells that are specialized macrophages in the liver. Tissue specific immune cell populations must be considered when designing local delivery therapeutics, as they may have a different response than their systemically derived counterparts. The traditional early inflammatory response is characterized by macrophages and neutrophils responding to injury or non-autologous cells by secreting interleukin (IL)-1β, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-12, which can induce cell dysfunction and/or apoptosis [6]. These inflammatory cytokines can further amplify the adaptive immune response, leading to T cell activation and cell destruction. The traditional innate and adaptive immune response (Figure 1) following injury can be extended to spinal cord injury (SCI), however additional tissue-specific astrocytes and macrophage-like microglia also contribute to inflammation and distinguish the immune restrictive nerve tissue from other tissues. Activation of the innate immune system has been shown as an important barrier to induction of immune tolerance in autoimmune diseases and cell transplantation [7], through multiple mechanisms, including activation of toll-like receptors [8, 9], elaboration of chemokines [10], and negating local immunosuppressive mechanisms such as negative co-stimulatory molecules (programmed death ligand 1 (PD-L1), T cell immunoglobulin mucin-3 (TIM3)) [11] and anti-inflammatory factors (tryptophan, hemoxygenase (HO), carbon monoxide) [12]. Therefore, ameliorating innate immune responses associated with injured tissues and/or cell-based therapies will likely be a critical target to facilitating regeneration and promoting function of endogenous or exogenous progenitor cells. Strategies to achieve this goal are discussed below and reviewed in Table 1.
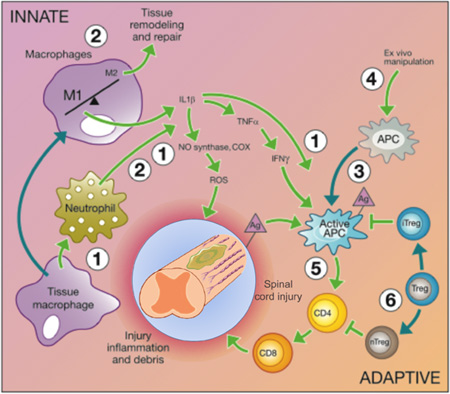
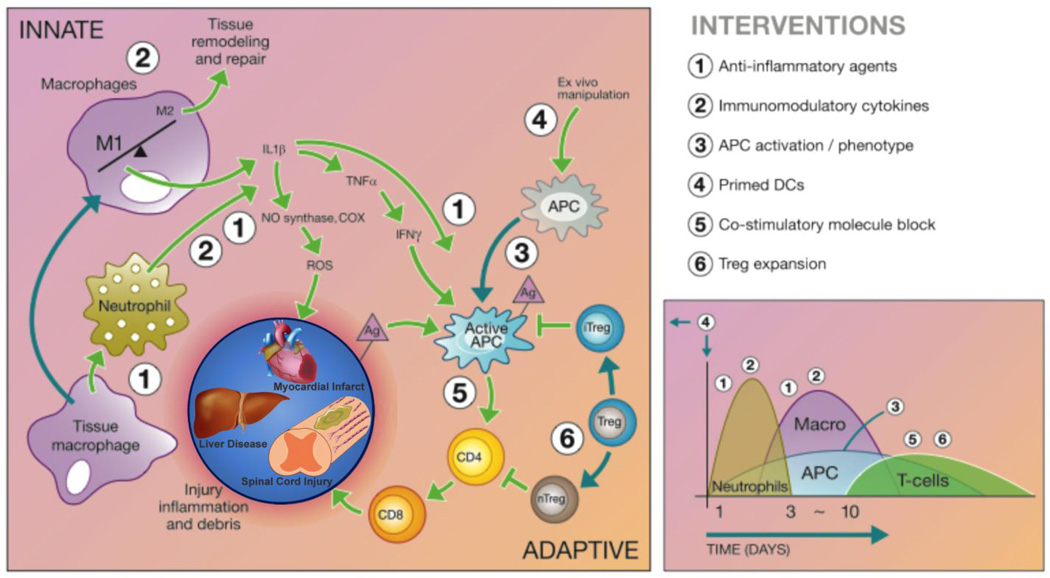
Figure 1.
Immunomodulatory intervention is time and cell dependent and tissue-specific considerations may need to be made. Tissue-specific immune cells can initiate the inflammatory cascade, followed by infiltration of traditional immune cells that work with local immune populations. The innate immune system responds to the initial trauma through macrophage and neutrophil activation to prevent infection, but results in increased inflammation and recruitment of other inflammatory cells through the release of inflammatory cytokines and reactive oxygen species. The release of inflammatory factors and cellular debris activates Ag presenting cells (APCs) and results in secondary damage in some tissues, such as the spinal cord. The adaptive immune response is characterized by APC activation of helper T cells (CD4+) that in turn activate cytotoxic T cells (CD8+) responsible for chronic inflammation following injury. Although cell transplantation is a promising treatment strategy, non-autologous cells can also activate the adaptive immune response. Regulatory T cells (Tregs) help to inactivate the APCs and helper T cells, preventing chronic inflammation following injury and protecting therapeutic cells delivered to treat the injury. Cell infiltration following injury is depicted to the right and the numbers correspond to therapeutic interventions that would modulate the immune response and promote tissue regeneration. Adapted from [6].
Table 1.
Local delivery of therapeutic agents utilizing biomaterials, protein and gene delivery, and cell delivery can modulate the response of target immune cell populations such as monocytes (m), neutrophils (N), macrophages (M), dendritic cells (DC), T cells (T), and tissue specific inflammatory cells such as microglia (μ) and astrocytes (a). Arrows indicate increased (↑) and decreased (↓) expression of factors or changes to cell expression that have been studied in vivo with further validation of the immunomodulatory mechanism studies in in vitro assays.
| AGENT | TARGET | IMMUNOMODULATORY EFFECT | REF. |
|---|---|---|---|
| BIOMATERIALS | |||
| Hydrophilicity | M, m | ↓ adhesion and differentiation; ↓ FBGC | [28, 29] |
| Porosity (30–40 µm) | m | M2 > M1 polarization; ↓ FBGC ↑angiogenesis, tissue repair |
[17, 18] |
| Nanotopography | m | M2 > M1 polarization | [35] |
| Electrospun fiber orientation | m a |
Aligned fibers ↓ inflammation and fibrous capsule size ↑ integration; ↓glutamate excitotoxicity to ↑Treg infiltration |
[32] [36, 37] |
| Electrospun fiber diameter | M | nanofibers ↓ release of pro-inflammatory cytokines compared to microfibers | [31] |
| FasL | T | ↑ T cell apoptosis | [38] |
| PROTEIN DELIVERY | |||
| Anti-MMP-9 | N, m | ↓ vascular permeability, infiltration | [39] |
| IL-1R antagonist nanoparticles | M | ↓ IL-1R/IL-1β induced NF-κB activation ↑ retention at injection site |
[40] |
| TGF-β1 | N, m | ↓ infiltration | [41] |
| IL-4 | T, M | M2 > M1 polarization; ↑ Th2; ↓Th1 cell recruitment; ↓TNF-α, RANKL, IL-1ra |
[42, 43] [44–46] |
| IL-10 | N, M | M2 > M1 polarization ; ↓ H2O2, NO, superoxide, MIP-2 | [44, 47] |
| CXCL12 | N, T | ↓ recruitment, Teff; ↑ Tregs, vascularization | [48] |
| CCL5-CXCL4 block | N | ↓ recruitment; ↑ vascularization | [49] |
| CXCL3 block | T | ↓ recruitment | [50] |
| CCL22 | Tregs, DC | ↑ targeted recruitment of Tregs, IDO; ↓ Teff | [51–54] |
| GM-CSF | M, DC, µ | ↑ recruitment; ↑ BDNF promoting M2 polarization |
[55–57] |
| SDF-1 | M | M2 > M1 polarization; ↑angiogenesis, IL-10 | [58, 59] |
| chABC | M | M2 > M1 polarization | [60] |
| GENE DELIVERY | |||
| BDNF | m | M2 > M1 polarization; ↑IL-10, IL-13; ↓ IL-1β, TNF-α | [61] |
| IL-1 + TNF-α | N, m | ↓ recruitment | [62] |
| IL-2 | T | ↑ Tregs | [63] |
| IL-4 | M, T | M2 > M1 polarization ↑ Tregs |
[64] |
| IL-10 | M, DC, T | M2 > M1 polarization; ↓ TNF-α, NF-κB, infiltration | [65–68] |
| IκB | M | M2 > M1 polarization; ↓ NF-κB | [69] |
| GFAP | a | Direct conversion to regenerative neuroblasts | [70] |
| CELL DELIVERY | |||
| MSCs | M | M2 > M1 polarization; ↑GM-CSF; ↓ infiltration, TNF-α, IL-6, MMP-9, CCL2, CCL5, CXCL10 |
[71, 72] |
| NSCs | T | Treg > Teff; ↓T cell proliferation ↑TGF-β1, PGE2, NO, HO-1 |
[73–75] |
| Tregs | T | ↑ tolerance of implanted cells and materials | [76, 77] |
| FasL-bound cells | T | ↑ T cell apoptosis | [78–80] |
2.1 Biomaterials
Biomaterials play a central role in many strategies for regenerative medicine, as they provide space for tissue growth, maintain mechanical stability, and support cell adhesion and migration. In addition, biomaterials also act as a delivery vehicle for cell transplantation, such as mesenchymal stem cells (MSC) and neural stem cells (NSCs) [13–16]. Porous scaffolds have been used for cell transplantation, which leads to vascularization and integration with the host, whereas encapsulation systems have been employed for allogeneic or xenogeneic cell transplantatation to isolate the cells from the host immune response [17, 18]. These scaffolds also provide a convenient platform to present biological factors such as extracellular matrix proteins that modulate the cellular microenvironment for stimulating and directing cell migration and adhesion.
The chemical composition and surface properties of the biomaterial affect protein adsorption, which influences interactions with immune cells and their activation. Blood-material interactions lead to protein adsorption on the biomaterial surfaces and the formation of a provisional matrix, consisting mainly of fibrin and fibronectin [19]. These events occur within minutes to hours after biomaterial implantation, and affect leukocyte interactions with biomaterials after implantation [20, 21]. Natural materials such as collagen [22], hyaluronic acid [23], and dextran [24] would generally be considered biocompatible, though their source, processing, and physical properties can influence the host response. Synthetic materials, such as poly(lactide coglycolide) (PLG) [25], poly(ethylene glycol) (PEG) [26], and poly(vinyl alcohol) (PVA) [27], can be modified in order to modulate the host immune response. The relative balance of hydrophobicity and hydrophilicity of the polymer surface influences biocompatibility, with increased monocyte adhesion observed with hydrophobic polymer surfaces, whereas a more hydrophilic chemistry decreased monocyte adhesion [28]. Additionally, hydrophilic/neutral material surfaces result in a significant decrease in monocytes and macrophages adhesion density and foreign body giant cell (FBGC) formation compare to hydrophobic surfaces in vitro [29]. FBGC formation can also be limited by the biomaterial dimensions. Spheres with increasing diameter (1.5– 2.5 mm) reduce the foreign body response compared to smaller diameter (<1 mm) hydrogels, ceramics, metals, and plastics [30]. Taken together these results indicate that the bulk charge and size characteristics of a biomaterial must be considered to limit the foreign body response.
The surface topography can also modulate immune responses at the host/implant interface [17, 18, 31–34]. Porous materials promote vascularization and less fibrous tissue encapsulation relative to non-porous biomaterials [17, 18]. Porosity on the scale of 30–40 µm also increased the ratio of anti-inflammatory M2 to pro-inflammatory M1 macrophages, leading to fewer FBGC and enhanced tissue repair [17]. Nanotopography has also been shown to modulate the immune response with reduced M1 macrophage polarization on titanium substrates [35]. Similarly, aligned electrospun nanofibers minimize the host immune reaction, enhance tissue-scaffold integration, and generate a thinner fibrous capsule compared to random fibers and films [32]. Diameter of electrospun fibers modulate the immune reaction, macrophage-mediated release of pro-inflammatory cytokines, and astrocyte-mediated reduction of excitotoxic glutamate [31, 36], the latter of which could promote Treg infiltration and prevent chronic inflammation [37].
Increasingly, biochemical modifications to biomaterials are being investigated as a means to modulate immune responses. Immobilization of adhesion peptides (RGD and PHSRN) are common to many synthetic materials to promote cell adhesion [81, 82]. While these peptides may be targeting transplanted cells, they can also influence adhesion and function of macrophages. Attachment of Fas ligand (FasL) onto biomaterials has been used to modulate autoreactive T cell populations. Fas, a cell surface receptor expressed on activated lymphocytes, interacting with FasL promotes apoptotic cell death contributing to the down-regulation of T and B lymphocytes and human neutrophils [83], which could prevent destructive alloreactive responses. Polymeric substrates conjugated with Fas antibodies promote T cell apoptosis, which can be enhanced further by conjugating a T cell adhesion ligand to the surface [38]. Taken together, biomaterials can regulate the host immune response through modulating immune cell responses, such as macrophage and T cell phenotype, toward the goal of promoting tissue regeneration.
2.2 Protein Delivery
Localized delivery systems provide the means to modulate immune responses. Several anti-inflammatory or regenerative cytokines are up-regulated following injury but are not sufficiently high to elicit an immunomodulatory response. Small molecules (i.e.., non-steroidal anti-inflammatory drugs (NSAIDS), methylprednisolone, and dexamethasone) have been widely investigated for their ability to down regulate leukocyte recruitment and phenotype (reviewed in [84]). In this section, we focus on protein delivery to promote and manage numerous cell processes in tissue regeneration, such as cell survival, proliferation, and differentiation. Local delivery of exogenous protein can initiate an immunomodulatory response, however, soluble proteins typically have an inherently short half-life. Proteins encapsulated within hydrogels often have a relatively rapid release, and may be more appropriate for resolution or dampening of acute inflammation. Alternatively, proteins can be encapsulated for sustained release from polymeric systems, or through immobilization of affinity based release strategies, thus extending their bioactivity to target cells involved in chronic inflammation [85–87]. These biomaterial (scaffold or particle) delivery systems for proteins limit contact with cell populations outside of the site of inflammation. Furthermore, the rate of release and duration can be tailored for each protein to maximize their efficacy. Growth factors, soluble growth factor receptors, cytokines, and monoclonal antibodies have all been delivered as a means to modulate the host immune response; we discuss their localized delivery from a biomaterial.
2.2.1 Leukocyte Recruitment
Modulating leukocyte infiltration can be one strategy for creating a local anti-inflammatory microenvironment, which can be achieved by preventing the infiltration into the site of inflammation by pro-inflammatory leukocyte phenotypes. Preventing early extravasation of neutrophils and monocytes into a site of injury or inflammation can be achieved by limiting the increase in vascular permeability through inhibition of matrix metalloproteinase-9 (MMP-9) [39]. Cytokines, such as transforming growth factor (TGF)-β1, IL-4, and IL-10, can also be used to limit monocyte, neutrophil, and T helper (Th)1cell recruitment independent of vascular permeability [41, 42, 47]. Chemokines are a class of cytokines that recruit immune cells to an inflammatory site and have the potential to effectively target specific immune cell populations. Targeted blocking of C-X-C ligand (CXCL)12 and C-C ligand (CCL)5-CXCL4 through soluble release of these molecules, blocking antibodies, or antagonists reduced neutrophil infiltration with increased vascularization seen following simultaneous biomaterial-release of CXCL12 and CCL5 [48, 49]. T cell recruitment can be limited with CXCL3 blocking antibodies or IL-4 delivery, however, IL-4 can stimulate Th2 recruitment [42, 43, 50].
Creating a local anti-inflammatory microenvironment can also be achieved by promoting anti-inflammatory leukocyte infiltration. CCL22 and CXCL12 have been reported to enhance recruitment of Tregs, and delivery within tissues has been shown to control inflammation, reduce disease progression, and limit immune cell-mediated destruction [51–54, 88, 89]. Local delivery of granulocyte macrophage colony-stimulating factor (GM-CSF), either soluble or within a biomaterial, promotes recruitment of macrophages, microglia, and DCs, and in a spinal cord model have reduced glial scar formation and improved motor recovery [55, 57]. While GM-CSF recruited more immune cells, those recruited cells likely had either an alternatively activated polarization or became alternatively polarized (i.e., M2 macrophages and microglia) upon entering the injury.
2.2.2 Leukocyte Phenotype
Modulating the phenotype of the recruited immune cells may be a valuable therapeutic option for promoting regeneration. In a SCI model, the relative ratio of pro-healing M2 to pro-inflammatory M1 macrophages demonstrated a linear relationship with the number of axons growing through the injury site [45], suggesting therapeutics that promote the M2 macrophage polarization would be advantageous for regeneration. Localized delivery of stromal derived factor (SDF)-1 or chondroitinase ABC (chABC) induced alternatively activated M2 macrophages with increased expression of angiogenic factors and IL-10 [58–60]. Direct delivery of known anti-inflammatory ILs, such as IL-10 and IL-4, can promote M2 macrophage polarization, yet few manuscripts describe local delivery of these cytokines (see gene delivery sections; [44, 45]). IL-4 has been incorporated into polymeric nerve guidance channels leading to increased pro-healing M2 macrophages which resulted in Schwann cell infiltration, reduced pro-inflammatory cytokine release (TNF-α, IL-1ra, receptor activator of nuclear factor κB ligand (RANKL)), and re-growth of axons in sciatic nerve injury model [45, 46].
Cytokine delivery can also indirectly modulate the immune response through tissue specific cells. Fibroblasts inhibit secretion of macrophage inflammatory protein (MIP)-1α from activated macrophages [90] and promote GM-CSF release from monocytes [91] following injury. Delivery of cytokines to promote fibroblast localization to the wound site could limit inflammatory myeloid phenotypes and result in improved tissue regeneration.
2.3 Gene Delivery
The delivery of gene therapy vectors represents a versatile alternative to the direct delivery of immunomodulatory factors. Vectors for gene delivery consist of DNA or RNA that may be packaged with proteins, polymers or lipids to create particles that can effectively overcome the extracellular and intracellular barriers to gene transfer [92]. The delivery of a gene can be employed to increase the expression of a target gene, or delivery of siRNA or miRNA can be used to decrease expression of a target gene [92, 93], with both strategies potentially useful in increasing or decreasing expression of immunomodulatory factors. Gene delivery is highly versatile, as nucleic acids have their “information” encoded in the linear sequence of bases, and not a three-dimensional conformation, like proteins [94]. Delivery systems can thus be developed based on the vector properties, which are relatively independent of the sequence, and thus distinct target genes can be readily delivered using the same delivery system. Furthermore, this versatility allows for the delivery of multiple constructs that can target various aspects of the immune response. Finally, the delivery of gene therapy vectors can provide protein expression for long periods of time, which can be challenging for some proteins that have relatively short half-lives, or whose stability limits their encapsulation into materials.
Gene delivery of viral and non-viral vectors from biomaterials has generally been categorized as occurring by release of genes that had initially been immobilized to a substrate. Sustained release is proposed as a means to maintain elevated concentrations of the vectors locally for extended periods of time, which may enhance gene transfer and enable targeting of cells beyond those that arrive immediately after implantation. The immobilization of vectors to biomaterials, which has been termed substrate-mediated delivery, solid phase delivery, or reverse transfection, mimics the natural process of virus binding to extracellular matrix proteins [95, 96]. Immobilization to the adhesive matrix co-localizes the vector and adhered cells [97, 98], which can overcome mass transport limitations. Importantly, the vector can be immobilized to the scaffold surface following fabrication, thereby providing a method for gene delivery from scaffolds formed by processes that would normally inactivate the vector during fabrication. The type of vector influences the cell types as well as the expression profile. The release of a non-viral vector has been associated with relatively rapid expression of the construct, particularly by immune cells attracted to the implant. Rapid gene expression with non-viral vectors would be appropriate for targeting early infiltrating cells involved in acute inflammation such as neutrophils, macrophages, and resident inflammatory cells, such as microglia in the CNS. Viral vectors, such as lentivirus, are more efficient than the non-viral vectors, transduce a broad range of cell types with localized delivery, and integrate into the genome for long-term expression. Many viral vectors can have a delay between delivery and expression that may limit their ability to influence the early events in an acute response [68]. Despite not integrating into the genome, non-viral vector delivery can lead to prolonged expression of the transgene, with expression observed rapidly and persisting for weeks to months [99]. Additionally, these vectors can transfect immune cells following implantation, which may provide a means to directly modulate immune responses.
Similar to protein delivery, gene delivery has been employed to express cytokines or chemokines to modulate immune cell infiltration and phenotypes. The sustained expression afforded with gene delivery may sustain immune cell phenotypes for longer periods relative to protein delivery [67]. Expression of IL-10 or IL-4 have reduced pro-inflammatory cytokine secretion, modulated leukocyte infiltration and phenotype, and enhanced the recruitment of Tregs to inhibit inflammation [64–66, 68]. IL-10 expression has also enhanced the survival of transplanted stem cells [100, 101]. Lentiviral delivery of the regenerative neurotrophic factor brain-derived neurotrophic factor (BDNF), was found to promote M2 macrophage polarization, increase IL-10 and IL-13 expression, and reduce pro-inflammatory IL-1β and TNF-α after SCI [61].
The versatility of gene delivery has enabled targeting of processes other than expression of soluble cytokines or chemokines. Inducing the expression of antagonists to the receptors for cytokines involved in disease progression (i.e.., IL-1, TNF-α) has decreased leukocyte infiltration and tissue degeneration [62]. Alternatively, gene delivery has been employed to reprogram progenitor cells towards a therapeutic response rather than an inflammatory response. Following SCI, glial fibrillary acidic protein (GFAP)+ astrocytes participate in the inflammatory response forming a glial scar. Expression of Sox2 under the GFAP promoter following SCI converted cells to neuroblasts that can promote regeneration and away from astrocytes that can develop into a glial scar [70]. In addition, expressing negative IκB to inhibit signaling of nuclear factor (NF)-κB in bone marrow derived macrophages resulted in pro-healing M2 macrophage phenotype activation after inflammation [69].
2.4 Cell Delivery
Cell-mediated therapies hold promise in both modulating the immune response and repopulating the injury site. Stem cells are widely used for regenerative medicine, as they can directly contribute to the regeneration of tissues by repopulating the injury site and differentiating into tissue-specific cells that will form the new tissue. Interestingly, these cells have the potential to either evade immune recognition or to locally modulate an immune response. Embryonic stem cells are less susceptible to immune rejection than adult cells due to an absence of major histocompatibility complex (MHC)-II and CD80/CD86 and very low levels of MHC-I expression [102, 103], however, these cells cannot confer this tolerance to local cells through secretion of anti-inflammatory cytokines. MSCs and NSCs have the ability to modulate the local immune response, such as macrophage polarization and T cell phenotype, in the context of inflammatory, autoimmune, and alloimmune responses [72, 74, 75, 104–110]. One caveat to cell mediated therapies is that there can be source-dependent variability in the efficacy of the immunomodulatory properties of the stem cells. For example, immortalized MSCs produce more IL-6 and are less effective at suppressing T cell proliferation than primary human MSCs [104]. MSCs taken from multiple donors can result in altered induction of M2 macrophage polarization, indicating significant variably in the efficacy of MSC immunomodulation in patients [111]. Similar variability in source has also been documented for NSCs [112]. Understanding this variation in efficacy could enhance the ability to modulate local environments. To address this variability, researchers are constraining the source of immunomodulatory cells, such as MSCs, and have proven clinical grade multipotent adult progenitor cells are equally efficacious as nonclinical grade (i.e., less constrained) MSC sources [113].
MSCs and NSCs modulate myeloid leukocytes and lymphocytes involved in the immune response via multiple mechanisms, including direct cell-cell contact and indirect contact via cytokines and signaling molecules [72–75, 104–110, 114, 115]. Relative to the drug delivery strategies, transplantation of MSCs or NSCs results in the secretion of numerous proteins that modulate a response [72, 74, 75, 105, 109, 110]. NSCs release soluble factors such as TGF-β1, prostaglandin E (PGE)2, nitric oxide (NO), and HO-1 that increase Treg populations at the expense of effector T cells (Teff) [73–75]. NSCs can also effect these changes directly through contact with T cell populations using intracellular adhesion molecule (ICAM) and B7 cell surface proteins [73]. Through direct contact and local cytokine release, NSCs promoted an increase in Treg populations, increased expression of anti-inflammatory cytokines, decreased pro-inflammatory cytokines, improved neurological function in the case of intracerebral hemorrhage, and supported long-term graft function in the case of cell transplantation [110, 116].
MSCs have also been demonstrated to reduce inflammation and confer tolerance to cell transplants (reviewed in [72, 114, 115]). Within the context of inflammation, MSCs injected into a contused spinal cord reduced macrophage infiltration, restored the blood spinal cord barrier, led to alternative polarization of macrophages and microglia, and improved hind-limb motor function [71]. A decrease in the pro-inflammatory cytokines (TNF-α, IL-6), mediators of vascular permeability (MMP-9), and macrophage recruitment factors (CCL2, CCL5, and CXCL10) coupled with an increase in GM-CSF within the first 24 hours after SCI likely contributed to the improved functional outcomes [71]. Similarly, MSCs co-transplanted with allogeneic cells suppressed T cell activity and improved graft survival [117].
Tregs have also been transplanted as a means to promote long-term survival and function of transplanted cells without systemic immunosuppression [76, 77]. Two types of CD4+ Tregs, thymic-derived natural Tregs (nTregs) or Tregs induced in the periphery (iTregs) in response to Ag, have been described in promoting peripheral tolerance [118]. The innate ability of Tregs to induce tolerance provides a viable platform on which to develop cell-based therapeutics for treatment of autoimmune and alloimmune responses. Multiple mechanisms are used by Tregs to reduce Teff and DC activity, including modulating DC activity with co-stimulatory receptors, competition for APCs with Teff, and release of cytokines. Tregs are reported to affect their immunosuppressive actions through secretion of TGF-β1, IL-10, IL-35, and galectin-1, and through cell-cell interactions involving glucocorticoid-induced TNFR related protein (GITR), cytotoxic T lymphocyte associated protein (CTLA)-4, CD39, CD73, and lymphocyte activation gene (LAG)-3 [118]. Tregs co-transplanted with islets in PLG scaffolds within diabetic mice prevented autoimmune rejection and allowed for restoration of normoglycemia [76]. Interestingly, the transplanted Tregs were Ag specific, yet led to the recruitment of Tregs with alternative specificities to islet grafts. Furthermore, the local delivery of Tregs also protected cells at distal sites, indicating the potential for systemic protection with localized delivery.
An alternative approach to protect cells has been modifying cells with FasL, either through genetic engineering or chemical modification of cell surfaces [78–80, 119]. The covalent modification of cells was accompanied with short-term rapamycin treatment to yield long-term engraftment of allogeneic and xenogeneic islets for the treatment of type 1 diabetes (T1D) [119]. Although the surface conjugated protein does not permanently reside on the cells, only transient immunosuppression is needed. The genetic modification of cells to express FasL has been reported to prevent CD4+ T cell-mediated rejection in cardiomyocyte and hematopoietic cell transplants [79, 80]. Additionally, FasL overexpressing myoblasts have been co-transplanted with islets, either in the ipsilateral or contralateral kidney, and induced site-specific and systemic tolerance to restore normoglycemia [78].
3. SYSTEMIC STRATEGIES
The long-term protection of allogeneic cells and tissues transplanted following injury or chronic inflammation is expected to require either a tolerogenic approach or systemic immunosuppression. Although immuno-privileged sites are naturally present in the body (brain, eye, testes), synthetic mimics of these sites that provide local immunomodulation cannot currently provide indefinite protection. Even mild local responses can induce adaptive responses such as effector cell priming, differentiation, and trafficking. Similarly, autoimmune responses can arise due to T cell dysfunction, resulting in chronic Ag-specific inflammation, a characteristic that has also been shown to develop following traumatic injuries such as SCI [3, 120–122]. Techniques to modulate the adaptive response often target T cells, either directly or indirectly through modifications to the innate immune response. Current strategies for autoimmune and alloimmune responses utilize non-specific down-regulation of the immune system through immunosuppressants such as rapamycin or blocking antibodies that reduce the ability of the body to fight infections as well as dampen the regenerative benefits of tissue remodeling by leukocytes after injury. The development of targeted approaches to modulate the immune response that can be administered systemically will alleviate these undesirable side effects while promoting Ag-specific immune tolerance. More recently, host-microbiome interactions have been identified as a powerful player in systemic responses, yet is beyond the scope and readers can be referred to a recent review [123]. Systemic strategies to target and modulate the immune response are described below and in Table 2.
Table 2.
Systemic delivery of therapeutic agents (proteins, cells, and nanoparticles) can modulate the response of target immune cell populations such as monocytes (m), neutrophils (N), macrophages (M), dendritic cells (DC), T cells (T), regulatory T cells (Tregs) and tissue specific inflammatory cells such as microglia (μ). Arrows indicate increased (↑) and decreased (↓) expression of factors or changes to cell expression that have occurred in vivo with further validation of the immunomodulatory mechanism studies in in vitro assays.
| AGENT | TARGET | IMMUNOMODULATORY EFFECT | REF. |
|---|---|---|---|
| PROTEIN DELIVERY | |||
| Anti-α4 | N | ↓cell infiltration | [124] |
| Anti-CD40L | T | ↓IFN-γ, T activation ↑CTLA-4 | [125, 126] |
| Anti-CXCL10 | N, M | ↓ cell infiltration | [127] |
| Anti-IL-6 | N, M, µ | ↓ cell infiltration; M2 > M1 polarization, IL-4+ µ | [128] |
| Anti-IL-18 | T | ↑CTLA-4, TGF-β1, ↓Th1, CD40L | [129] |
| CTLA-4 | T | ↓IFN-γ, T activation | [130, 131] |
| Dkk3 | T | ↑ MHC-1 mismatch protection; ↓ IFN-γ | [132] |
| EPO | DC, T | ↓DC, Th17, IL-6, TNF-α, IL-2; ↑Tregs | [133] |
| G-CSF | N, M, DC, T | ↑VEGF, AQP4, tolerogenic DCs, Tregs; ↓vascular permeability, cell infiltration |
[55, 134, 135] |
| Gremlin-1 | m, M | M2 > M1 polarization; ↓monocyte migration, MIF | [136] |
| HGF | DC, T | ↑tolerogenic DCs, TregsIL-10, IL-4; ↓IFN-γ, IL-12p70, Th17 |
[137] |
| IL-25 | M | M2 > M1 polarization; ↓IL-6, IL-23, TNF-α, IL-1β | [138] |
| IL-33 | N, M, DC, T | M2 > M1 polarization; ↑IL-2, Tregs, neutrophil infiltration; ↓TNF-α, gliosis, demyelination |
[139–142] |
| Statins | N, M | ↓vascular permeability, cell infiltration | [143] |
| CELL DELIVERY | |||
| MSCs | T | ↑IDO, PGE2, NO, Th17, Tregs | [72, 107, 108, 144] |
| Tregs | T, DC | ↓Teff, DC migration; ↑TGF-β1, IL-10 | [145] |
| Ag-DC + αCD3 | T | ↑Tregs; ↓Teff, T cell infiltration | [146] |
| ECDI-SP | DCs, T | ↑T anergy, Ag-tolerance | [147–151] |
| ERY1-OVA erythrocytes | DCs, T | Peptide targets erythrocytes: ↑tolerogenic DCs,PD-1+ T | [152] |
| NANOPARTICLES | |||
| IMPs | M, DC | ↓ monocyte migration and differentiation; ↑tolerogenic DCs |
[153, 154] |
| Ag-PS | DCs, T | ↑ MARCO uptake, autoimmune tolerance | [155, 156] |
| Ag-PLG | m, DCs, T | ↑ autoimmune tolerance ↓Th1, Th2, inflammatory m | [153, 155] |
| Ag-PLG + rapa | T | ↑ autoimmune tolerance, alloimmune tolerance; ↓Teff proliferation |
[147, 157] |
3.1 Inflammatory Response
A wave of inflammatory monocyte recruitment into inflamed tissues leads to differentiation into multiple effector cells, including DCs, macrophages, and microglia [158, 159], depending on the prevailing milieu (recall Figure 1). These cells normally play important house-keeping functions (i.e., digest and clear tissue debris prior to remodeling). However, these cells may express high levels of NO via induced expression of NO synthase (NOS)2, as well as increased levels of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, cathepsins, myeloperoxidase, all of which may demonstrably contribute to further tissue damage, such as the case with myocardial infarction and SCI [153, 160, 161]. Monocyte management has emerged as a treatment modality to limit damage associated with the acute phase of inflammation. Damage can also be limited by modulating the immune cells responsible for the early, acute, and chronic phases of inflammation leading to treatments that are applicable for each phase of diagnosis.
Early inflammation is characterized by increased vascular permeability leading to neutrophil infiltration and recruitment of other leukocytes through the release of IL-1β, TNF-α, IFN-γ, and IL-12 [6]. Vascular permeability and neutrophil infiltration can be limited in CNS injuries through the use of statins, granulocyte colony stimulating factor (G-CSF), CXCL10, or antibody blockades for IL-6 or the α4 integrin subunit necessary for α4β1-dependent neutrophil extravasation [124, 127, 128, 134, 143]. G-CSF results in increased vascular endothelial growth factor (VEGF) and aquaporin-4 (AQP4) via c-Jun and ERK pathways, respectively, that limited vascular permeability in a dose dependent manner [134].
Strategies to limit acute inflammation target monocyte recruitment, macrophage phenotype, and local cell response to prevent further damage and begin to promote a regenerative microenvironment. Administration of factors that inhibit inflammatory cytokines, such as macrophage migration inhibitory factor (MIF) and IL-6, have been implemented to reduce inflammation after injury. MIF inhibitors, such as gremlin-1 and liposome encapsulated Chicago sky blue can alleviate inflammation by limiting monocyte recruitment from the bone marrow/spleen through the vasculature to sites of inflammation and by promoting M2 macrophages [136, 162]. Intravenously administrated nanoparticles can also target monocytes responsible for acute inflammation. One approach, involving the combination of siRNA with liposomes has shown some promise in animals models [154]. Nanoparticles can target these cells through specific scavenger receptors, to reduce circulating inflammatory monocytes. Immune modifying nanoparticles (IMP), i.e., NPs with a highly negative surface charge, bind with high specificity to inflammatory monocytes, marking them for sequestration by the spleen and thereby preventing migration to sites of inflammation and subsequent differentiation and participation in pathogenic immune responses [153]. Sequestered monocytes either undergo caspase-3-mediated apoptosis or differentiate into CD11b+CD11c+CD103+ DCs [153, 154].
Therapies that target both the innate and adaptive immune responses to promote regenerative M2 macrophages and Tregs aim to remediate chronic inflammation. Directly injecting Tregs can alleviate the chronic inflammation characteristic of multiple sclerosis (MS) [163]. Non-cell based approaches to limit chronic inflammation include the delivery of various proteins. Within the context of SCI, G-CSF and GM-CSF have been shown to limit inflammation in the acute and chronic phases [55]. G-CSF promotes monocyte differentiation into tolerogenic DCs that promote Treg-dependent anergy, which may contribute to the reduced chronic inflammation seen following G-CSF infusion into SCI models [135]. An increase in M2 macrophages and IL-4+ microglia leading to enhanced axon sparing can be achieved following SCI with an IL-6 blockade, as increased IL-6 expression leads to inflammation following injury [128]. Conversely, an increase in some of the ILs can prevent secondary damage and promote regeneration. IL-25 has been shown to reduce the detrimental release of Il-6, IL-23, TNF-α, and IL-1β by local macrophages that shift towards an M2 polarization [138]. Interestingly, IL-33 is released by glia following injury and can promote M2 macrophages [140]. Use of exogenous IL-33 infusion reduces cavitation, demyelination, astrogliosis, and TNF-α expression leading to enhanced recovery of hind-limb locomotor function, as well as increase DC-derived IL-2 that induces Tregs to prevent SCI-induced experimental autoimmune encephalomyelitis (EAE; mouse MS model) [3, 140–142]. IL-33 can also recruit neutrophils, yet this has led to a decreased systemic immune response [139].
3.2 Ag specific tolerance
Allogeneic immunity following cell transplantation and autoimmunity arise in response to the presence of specific Ags leading to T cell activation. Numerous therapies have utilized intravenous delivery of soluble proteins and antibodies for broad immune suppression that are capable of dampening humoral immunity, primarily through manipulation of the DC and T cell populations (Table 2). Systemic delivery of proteins may result in off target effects in other cell populations, making the co-stimulatory factors necessary for complete T cell activation an attractive target for modulating Th1 and Th2 diseases. The co-stimulatory factor CD40L is necessary to activate T cells, while PD-1 and CTLA-4 are necessary to inhibit T cell activity. Infusion of soluble CTLA-4 and/or CD40L blocking antibodies can reduce pro-inflammatory cytokine expression and T cell activation, while increasing anti-inflammatory cytokine expression and inhibitory T cell co-stimulatory factors [125, 126, 129, 131, 164–166]. Delivery of proteins, whether pleotropic or T cell-specific, provides some reduction in disease progression and symptoms through the use of non-Ag)-specific approaches. Unfortunately, these therapies may elicit off-target effects and initiate a systemic decrease in humoral immunity, increasing the host to infections, much like the use of immunosuppressants.
3.2.1 Cell based approaches
Cell-mediated immunomodulation can be performed by intravenous delivery of ex vivo expanded Tregs. Tregs have been isolated and expanded, or can be produced from naïve CD4+ T cells that are induced and expanded in vitro. Intravenous infusion of Tregs following islet graft transplantation are capable of suppressing alloimmunity by first migrating to the injury site where they inhibit local Ag-specific Teff cell accumulation and proliferation [145]. A subset of these Tregs also migrates to the draining lymph nodes to suppress systemic Teff populations and inhibit DC migration through secretion of TGF-β1 and IL-10 [145].
T cells can also be regulated indirectly by either systemic or intraportal infusion of modified or primed DCs. Autologous tolerogenic DCs delivered with CD3 antibodies can promote islet allograft acceptance by preventing T cell infiltration into the graft and promoting an up-regulation of Tregs, both locally and systemically, that promote donor-specific suppression [146]. Furthermore, Ag-pulsed DCs have also been shown to improve motor function after SCI through a reduction in inflammation (as reviewed by [3]).
The induction of Ag-specific immune tolerance has also been investigated through the intravenous infusion of donor splenocytes (SP) chemically treated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (ECDI-SP) [147–149, 151, 167–169]. ECDI is employed to affix Ags to, and induce apoptosis of, donor splenic leukocytes to promote tolerance to the bound Ag following intravenous administration. This strategy derives, in part, from observations that intravenously delivered peptide is able to induce tolerance; however, free peptide in the blood has a risk of inducing anaphylaxis. Approaches such as Ag-SP can minimize the amount of free peptide in the blood, while also delivering the Ag to APCs that can mediate immune tolerance. Tolerance by this strategy is dependent on marginal zone (MZ) APCs that process the infused apoptotic ECDI-SP cells, their elaboration of negative co-stimulatory molecules (i.e., PD-L1), and subsequent phasic tolerogenic effects on Ag-specific T cells including[170] anergy, deletion, and induction of Tregs [147–150]. Ag-SP have been employed to prevent and treat the relapsing EAE model of MS [171], and T1D in the non-obese diabetic (NOD) mouse [172]. A recent publication presented the results of a phase I trial in MS patients in Germany using apoptotic ECDI-fixed peripheral blood mononuclear cells (PBMCs) pulsed with a cocktail of myelin peptides, illustrating the safety and efficacy of this procedure in human autoimmune disease [173]. Importantly, the mechanistic aspects of this study provided an important proof-of-principle that induced peripheral tolerance can be successfully employed to induce unresponsiveness in human autoreactive T cells as responses to 4 of the 7 tolerated myelin epitopes were significantly reduced (with no effect on tetanus responses) in the four MS patients treated with >109 autologous Ag-coupled PBMCs while having no effect in the nine patients receiving <5×108 Ag-PBMCs. These studies provided the first definitive demonstration of induced tolerance to autoantigens in humans and serve as the basis for Ag-PLG tolerance.
Within the context of allogeneic tolerance, intravenous delivery of ECDI-SP has shown robust efficacy in multiple murine models of allogeneic and xenogeneic islet cell transplant [168, 174]. Note that in these studies donor SPs are treated with ECDI to induce apoptosis. No Ags are coupled to the particles as the donor cells contain the allogeneic or xenogeneic Ags. This strategy was also effective for allogeneic islets transplanted on biomaterial scaffolds [167]. Interestingly, tolerance induction was more efficacious for islets transplanted on PLG scaffolds within the epididymal fat pad compared with those transplanted intra-portally [167], suggesting that the local environment influences the ability to promote tolerance.
Similar to apoptotic SPs, apoptotic erythrocytes are cleared from the blood fairly regularly and are a potential cell source to induce Ag-specific T cell deletion. Unlike intravenous delivery of ECDI-SP, erythrocytes do not need to be expanded ex vivo, but rather a small peptide (ERY1) conjugated to ovalbumin (OVA) Ag is delivered intravenously and binds specifically to glycophorin-A on erythrocytes [152]. The localization of OVA to erythrocytes using a targeting peptide resulted in increased tolerogenic DCs and PD-1+ T cells preventing the onset of T1D and promoting tolerance in OVA-expressing graft [152]. This “piggybacking” of Ag on the cells offers a distinctive cell-mediated therapy to deliver Ag and promote Ag-specific T cell deletion.
In addition to local MSC immune modulation, intravenous injection of MSCs immediately following transplantation has promoted allogeneic tolerance when delivered with the immunosuppressant mycophenolate mofetil (MMF) through an initial MSC-dependent increase in Th17 cells that are converted to Tregs by MMF [108]. Systemic delivery of MSCs 7 days prior to transplantation has been shown to promote allograft tolerance without immunosuppressants by up-regulating Tregs systemically rather than MSCs localizing to the injury site leading to increased inflammation with some local delivery techniques [144]. MSCs have also been used to confer autoimmune tolerance in mice with MS [107] and human patients suffering from systemic lupus erythematosus [175] and Crohn’s disease [176].
3.2.2 Nanoparticles
The translational challenges associated with Ag-SP cell-based therapy has motivated the development of nanoparticles for Ag-specific tolerance. Nanoparticles with properties similar to apoptotic cell debris may function as an alternative Ag carrier for tolerance induction. Intravenous injection of 500 nm ‘non-biodegradable’ carboxylated polystyrene (PS) particles coupled with peptides were able to prevent the onset of disease in EAE prevent epitope spreading, and to ameliorate progression of pre-established EAE [155]. Interestingly, these studies using PS nanoparticles identified that particles with diameters ranging from 500 to 1000 nm were most effective, and tolerance was dependent on particle uptake by the macrophage receptor with collagenous structures (MARCO) scavenger receptor. MARCO has been shown to be responsible for uptake of PS beads which have an anionically charged surface [156].
More recently, PLG particles were able to induce Ag-specific tolerance for prevention and treatment of EAE [155, 157, 177]. Administration of particles results in significantly reduced CNS infiltration of encephalitogenic Th1 (IFN-γ) and Th17 (IL-17a, GM-CSF) cells as well as inflammatory monocytes/MΦs. Tolerance is most effectively induced by intravenous infusion of Ag-PLG [155, 178], though intraperitoneal and subcutaneous delivery was able to attenuate disease scores. Efficacy of the route of administration is likely due to altered trafficking to the lymph tissue as particles delivered intravenously traffic directly to the spleen and liver, while subcutaneous particle delivery targeted the draining lymph nodes [150, 153, 157].
Similarly, PLG particles containing Ag peptides and rapamycin have been shown to induce Agspecific tolerance through inhibition of CD4+ and CD8+ T cell activation in autoimmune models [157]. Development of Ag-specific therapies for treatment of autoimmune diseases benefits from the Ag-specific response being well characterized, such as myelin peptides (myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP)) in EAE models. In the case of alloimmune responses, the Ags and their epitopes are not well characterized, thus making the development of alloimmune tolerance therapies more challenging. One approach is to lyse donor cells for coupling to PLG particles in order to confer tolerance to full MHC mismatch islet allografts [147]. Mechanistic studies investigating full MHC mismatch islet allografts with Ag-PLG have shown that while ECDI-SP can confer tolerance of direct- and indirect-activated T cells resulting in anergy and deletion, whereas Ag-PLG can only modulate T cells with indirect donor specificity and require transient immunosuppression using rapamycin (up to 2 days post-transplantation) to support long term graft survival [147].
4. CONCLUSION
This review describes multiples approaches for immunomodulation that are being employed within the field of tissue regeneration. Local and systemic approaches to modulate the immune response are being applied based on the type of host response incurred (injury, autoimmune, or alloimmune), stage of inflammation (early, acute, chronic), and extent of inflammatory response that is being targeted. Combinatorial approaches, such as the local delivery of cytokines and chemokines to synergize with systemic immunomodulation, may ultimately be needed to address the complex interplay of the innate and adaptive responses. This combination of local and systemic effects reflects a strategy currently being investigated for cancer therapies [179–181] that could be extended to tissue regeneration following injury or autoimmune inflammation. Figure 1 lists several interventions, and combinations of them may be useful in targeting multiple aspects of the immune response. For example, following SCI, the local delivery of therapeutic factors to promote alternative macrophage polarization and tissue regeneration, such as IL-33 and GM-CSF [55–57, 140–142], coupled with systemic nanoparticle approaches to modulate monocyte trafficking [153, 157] and limit the chronic myelin-specific T cell response may provide a synergistic approach to promote regeneration after SCI. Similarly, therapeutics that limit local inflammation while systemic therapies that promote Ag-specific tolerance could enable cell engraftment and long term function. Understanding the mechanisms by which the microbiome exerts immunomodulatory properties could lead to new therapies or complement the local and systemic approaches described in this review.
ACKNOWLEDGMENTS
Funding was provided by the National Institutes of Health (RO1EB005678, RO1EB013198, RO1CA173745, RO1EB009910).
Abbreviations
- Ag
Antigen
- APC
antigen presenting cell
- AQP4
aquaporin 4
- BDNF
brain-derived neurotrophic factor
- CD
cluster of differentiation
- CCL
C-C ligand
- chABC
chondroitinase ABC
- CNS
central nervous system
- COX
cyclooxygenase
- CTLA-4
cytotoxic T lymphocyte associated protein-4
- CXCL
C-X-C ligand
- DC
dendritic cell
- Dkk3
dickkopf related protein 3
- EAE
experimental autoimmune encephalomyelitis
- ECDI
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- EPO
erythropoietin
- FasL
Fas ligand
- FBGC
foreign body giant cell
- G-CSF
granulocyte colony-stimulating factor
- GFAP
glial fibrillary acidic protein
- GITR
glucocorticoid-induced TNF receptor related protein
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HGF
hepatocyte growth factor
- HO
hemoxygenase
- ICAM
intracellular adhesion molecule
- IDO
indoleamine 2,3-dioxygenase
- IMP
immune modifying nanoparticle
- IFN-γ
interferon gamma
- IκB
inhibitor of kappa B
- IL
interleukin
- iTreg
induced Treg
- LAG-3
lymphocyte activation gene 3
- M1
pro-inflammatory macrophage polarization
- M2
alternative macrophage polarization
- MARCO
macrophage receptor with collagenous structures
- MBP
myelin basic protein
- MHC
major histocompatibility complex
- MIF
migration inhibitory factor
- MIP-1α
macrophage inflammatory protein-1 alpha
- MMF
mycophenolate mofetil
- MMP
matrix metalloproteinase
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- MSC
mesenchymal stem cell
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa B
- NO
nitric oxide
- NOD
non-obese diabetic
- NOS
nitric oxide synthase
- NSAID
non-steroidal anti-inflammatory drug
- NSC
neural stem cell
- nTreg
natural Treg
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cell
- PD-L1
programmed death ligand-1
- PEG
poly(ethylene glycol)
- PGE
prostaglandin E
- PLG
poly(lactide coglycolide)
- PS
polystyrene
- PVA
poly(vinyl alcohol)
- RANKL
receptor activator of nuclear factor kappa B ligand
- ROS
reactive oxygen species
- SCI
spinal cord injury
- SDF-1
stromal derived factor 1
- SP
splenocyte
- T1D
type 1 diabetes
- Teff
T effector cell
- TGF-β1
transforming growth factor beta 1
- Th
T helper
- TIM3
T cell immunoglobulin mucin-3
- TNF-α
tumor necrosis factor alpha
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Eric PSC, Finkelstein A, Miller Ted R Associates. The Incidence and Economic Burden of Injuries in the United States. 1st ed. USA: Oxford University; 2006. [Google Scholar]
- 2.Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51:239–240. doi: 10.2144/000113754. 242, 244 passim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones TB. Lymphocytes and autoimmunity after spinal cord injury. Experimental neurology. 2014;258:78–90. doi: 10.1016/j.expneurol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 5.Schwach-Abdellaoui K, Vivien-Castioni N, Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm. 2000;50:83–99. doi: 10.1016/s0939-6411(00)00086-2. [DOI] [PubMed] [Google Scholar]
- 6.Gibly RF, Graham JG, Luo X, Lowe WL, Jr, Hering BJ, Shea LD. Advancing islet transplantation: from engraftment to the immune response. Diabetologia. 2011;54:2494–2505. doi: 10.1007/s00125-011-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nature reviews. Immunology. 2012;12:459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. The Journal of clinical investigation. 2003;111:1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, Najafian N, Kupiec-Weglinski JW. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorenza S, Kenna TJ, Comerford I, McColl S, Steptoe RJ, Leggatt GR, Frazer IH. A combination of local inflammation and central memory T cells potentiates immunotherapy in the skin. Journal of immunology. 2012;189:5622–5631. doi: 10.4049/jimmunol.1200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. Journal of immunology. 2007;179:3672–3679. doi: 10.4049/jimmunol.179.6.3672. [DOI] [PubMed] [Google Scholar]
- 12.Cobbold SP, Adams E, Graca L, Daley S, Yates S, Paterson A, Robertson NJ, Nolan KF, Fairchild PJ, Waldmann H. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol Rev. 2006;213:239–255. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. Journal of neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Lim E, Back S, Na H, Park Y, Sun K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A. 2010;93:1091–1099. doi: 10.1002/jbm.a.32519. [DOI] [PubMed] [Google Scholar]
- 15.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82:452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibly RF, Zhang X, Graham ML, Hering BJ, Kaufman DB, Lowe WL, Jr, Shea LD. Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials. 2011;32:9677–9684. doi: 10.1016/j.biomaterials.2011.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Underwood RA, Usui ML, Zhao G, Hauch KD, Takeno MM, Ratner BD, Marshall AJ, Shi X, Olerud JE, Fleckman P. Quantifying the effect of pore size and surface treatment on epidermal incorporation into percutaneously implanted sphere-templated porous biomaterials in mice. J Biomed Mater Res A. 2011;98:499–508. doi: 10.1002/jbm.a.33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 20.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sano A, Hojo T, Maeda M, Fujioka K. Protein release from collagen matrices. Adv Drug Deliv Rev. 1998;31:247–266. doi: 10.1016/s0169-409x(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 23.Vercruysse KP, Prestwich GD. Hyaluronate derivatives in drug delivery. Crit Rev Ther Drug Carrier Syst. 1998;15:513–555. [PubMed] [Google Scholar]
- 24.Draye JP, Delaey B, Van de Voorde A, Van Den Bulcke A, De Reu B, Schacht E. In vitro and in vivo biocompatibility of dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials. 1998;19:1677–1687. doi: 10.1016/s0142-9612(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 25.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 26.Espadas-Torre C, Meyerhoff ME. Thrombogenic properties of untreated and poly(ethylene oxide)-modified polymeric matrices useful for preparing intraarterial ion-selective electrodes. Anal Chem. 1995;67:3108–3114. doi: 10.1021/ac00114a003. [DOI] [PubMed] [Google Scholar]
- 27.Lejardi A, Hernandez R, Criado M, Santos JI, Etxeberria A, Sarasua JR, Mijangos C. Novel hydrogels of chitosan and poly(vinyl alcohol)-g-glycolic acid copolymer with enhanced rheological properties. Carbohydr Polym. 2014;103:267–273. doi: 10.1016/j.carbpol.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Hezi-Yamit A, Sullivan C, Wong J, David L, Chen M, Cheng P, Shumaker D, Wilcox JN, Udipi K. Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J Biomed Mater Res A. 2009;90:133–141. doi: 10.1002/jbm.a.32057. [DOI] [PubMed] [Google Scholar]
- 29.Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83:585–596. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 30.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacik I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG. Size- and shape-dependent foreign body immune response to materials implanted in rodents and nonhuman primates. Nature materials. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12:1900–1911. doi: 10.1021/bm200248h. [DOI] [PubMed] [Google Scholar]
- 32.Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93:1151–1159. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100:1375–1386. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barth KA, Waterfield JD, Brunette DM. The effect of surface roughness on RAW 264.7 macrophage phenotype. J Biomed Mater Res A. 2013;101:2679–2688. doi: 10.1002/jbm.a.34562. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Choi J, Shin S, Im YM, Song J, Kang SS, Nam TH, Webster TJ, Kim SH, Khang D. Analysis on migration and activation of live macrophages on transparent flat and nanostructured titanium. Acta biomaterialia. 2011;7:2337–2344. doi: 10.1016/j.actbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Zuidema JM, Hyzinski-Garcia MC, Van Vlasselaer K, Zaccor NW, Plopper GE, Mongin AA, Gilbert RJ. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-L-lactic acid fibers. Biomaterials. 2014;35:1439–1449. doi: 10.1016/j.biomaterials.2013.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beurel E, Harrington LE, Buchser W, Lemmon V, Jope RS. Astrocytes modulate the polarization of CD4+ T cells to Th1 cells. PloS one. 2014;9:e86257. doi: 10.1371/journal.pone.0086257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hume PS, Anseth KS. Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations. Biomaterials. 2010;31:3166–3174. doi: 10.1016/j.biomaterials.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, Garcia AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33:7665–7675. doi: 10.1016/j.biomaterials.2012.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuff CA, Martiney JA, Berman JW, Brosnan CF. Differential effects of transforming growth factor-beta 1 on interleukin-1-induced cellular inflammation and vascular permeability in the rabbit retina. J Neuroimmunol. 1996;70:21–28. doi: 10.1016/s0165-5728(96)00103-8. [DOI] [PubMed] [Google Scholar]
- 42.Lazarski CA, Ford J, Katzman SD, Rosenberg AF, Fowell DJ. IL-4 attenuates Th1-associated chemokine expression and Th1 trafficking to inflamed tissues and limits pathogen clearance. PloS one. 2013;8:e71949. doi: 10.1371/journal.pone.0071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 45.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao AJ, Nich C, Dhulipala LS, Gibon E, Valladares R, Zwingenberger S, Smith RL, Goodman SB. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J Biomed Mater Res A. 2013;101:1926–1934. doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu CL, Lin LY, Yang JS, Chan MC, Hsueh CM. Attenuation of lipopolysaccharide-induced acute lung injury by treatment with IL-10. Respirology. 2009;14:511–521. doi: 10.1111/j.1440-1843.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- 48.Projahn D, Simsekyilmaz S, Singh S, Kanzler I, Kramp BK, Langer M, Burlacu A, Bernhagen J, Klee D, Zernecke A, Hackeng TM, Groll J, Weber C, Liehn EA, Koenen RR. Controlled intramyocardial release of engineered chemokines by biodegradable hydrogels as a treatment approach of myocardial infarction. J Cell Mol Med. 2014;18:790–800. doi: 10.1111/jcmm.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grommes J, Alard JE, Drechsler M, Wantha S, Morgelin M, Kuebler WM, Jacobs M, von Hundelshausen P, Markart P, Wygrecka M, Preissner KT, Hackeng TM, Koenen RR, Weber C, Soehnlein O. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am J Respir Crit Care Med. 2012;185:628–636. doi: 10.1164/rccm.201108-1533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Boyle G, Fox CR, Walden HR, Willet JD, Mavin ER, Hine DW, Palmer JM, Barker CE, Lamb CA, Ali S, Kirby JA. Chemokine receptor CXCR3 agonist prevents human T-cell migration in a humanized model of arthritic inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4598–4603. doi: 10.1073/pnas.1118104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jhunjhunwala S, Raimondi G, Glowacki AJ, Hall SJ, Maskarinec D, Thorne SH, Thomson AW, Little SR. Bioinspired controlled release of CCL22 recruits regulatory T cells in vivo. Advanced materials. 2012;24:4735–4738. doi: 10.1002/adma.201202513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mailloux AW, Young MR. NK-dependent increases in CCL22 secretion selectively recruits regulatory T cells to the tumor microenvironment. Journal of immunology. 2009;182:2753–2765. doi: 10.4049/jimmunol.0801124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montane J, Bischoff L, Soukhatcheva G, Dai DL, Hardenberg G, Levings MK, Orban PC, Kieffer TJ, Tan R, Verchere CB. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. The Journal of clinical investigation. 2011;121:3024–3028. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin XJ, Shi HZ, Deng JM, Liang QL, Jiang J, Ye ZJ. CCL22 recruits CD4-positive CD25-positive regulatory T cells into malignant pleural effusion. Clin Cancer Res. 2009;15:2231–2237. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 55.Chung J, Kim MH, Yoon YJ, Kim KH, Park SR, Choi BH. Effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on glial scar formation after spinal cord injury in rats. Journal of neurosurgery. Spine. 2014;21:966–973. doi: 10.3171/2014.8.SPINE131090. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton TA, Zhao C, Pavicic PG, Jr, Datta S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Frontiers in immunology. 2014;5:554. doi: 10.3389/fimmu.2014.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi K, Ohta S, Kawakami Y, Toda M. Activation of dendritic-like cells and neural stem/progenitor cells in injured spinal cord by GM-CSF. Neurosci Res. 2009;64:96–103. doi: 10.1016/j.neures.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 58.Karin N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J Leukoc Biol. 2010;88:463–473. doi: 10.1189/jlb.0909602. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
- 60.Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ. Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:16424–16432. doi: 10.1523/JNEUROSCI.2927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji XC, Dang YY, Gao HY, Wang ZT, Gao M, Yang Y, Zhang HT, Xu RX. Local Injection of Lenti-BDNF at the Lesion Site Promotes M2 Macrophage Polarization and Inhibits Inflammatory Response After Spinal Cord Injury in Mice. Cellular and molecular neurobiology. 2015 doi: 10.1007/s10571-015-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghivizzani SC, Lechman ER, Kang R, Tio C, Kolls J, Evans CH, Robbins PD. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4613–4618. doi: 10.1073/pnas.95.8.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goudy KS, Johnson MC, Garland A, Li C, Samulski RJ, Wang B, Tisch R. Inducible adeno-associated virus-mediated IL-2 gene therapy prevents autoimmune diabetes. Journal of immunology. 2011;186:3779–3786. doi: 10.4049/jimmunol.1001422. [DOI] [PubMed] [Google Scholar]
- 64.Butti E, Bergami A, Recchia A, Brambilla E, Del Carro U, Amadio S, Cattalini A, Esposito M, Stornaiuolo A, Comi G, Pluchino S, Mavilio F, Martino G, Furlan R. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther. 2008;15:504–515. doi: 10.1038/gt.2008.10. [DOI] [PubMed] [Google Scholar]
- 65.Goudy K, Song S, Wasserfall C, Zhang YC, Kapturczak M, Muir A, Powers M, Scott-Jorgensen M, Campbell-Thompson M, Crawford JM, Ellis TM, Flotte TR, Atkinson MA. Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13913–13918. doi: 10.1073/pnas.251532298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cua DJ, Hutchins B, LaFace DM, Stohlman SA, Coffman RL. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. Journal of immunology. 2001;166:602–608. doi: 10.4049/jimmunol.166.1.602. [DOI] [PubMed] [Google Scholar]
- 67.Boehler RM, Kuo R, Shin S, Goodman AG, Pilecki MA, Gower RM, Leonard JN, Shea LD. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol Bioeng. 2014;111:1210–1221. doi: 10.1002/bit.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gower RM, Boehler RM, Azarin SM, Ricci CF, Leonard JN, Shea LD. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials. 2014;35:2024–2031. doi: 10.1016/j.biomaterials.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson HM, Chettibi S, Jobin C, Walbaum D, Rees AJ, Kluth DC. Inhibition of macrophage nuclear factor-kappaB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol. 2005;167:27–37. doi: 10.1016/s0002-9440(10)62950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nature communications. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WE, Baba H. Early Transplantation of Mesenchymal Stem Cells after Spinal Cord Injury Relieves Pain Hypersensitivity Through Suppression of Pain-Related Signaling Cascades and Reduced Inflammatory Cell Recruitment. Stem cells. 2015 doi: 10.1002/stem.2006. [DOI] [PubMed] [Google Scholar]
- 72.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunology and cell biology. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 73.Nazmi A, Mohamed Arif I, Dutta K, Kundu K, Basu A. Neural stem/progenitor cells induce conversion of encephalitogenic T cells into CD4+-CD25+- FOXP3+ regulatory T cells. Viral immunology. 2014;27:48–59. doi: 10.1089/vim.2013.0090. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, Shi J, van Ginkel FW, Lan L, Niemeyer G, Martin DR, Snyder EY, Cox NR. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Experimental neurology. 2009;216:177–183. doi: 10.1016/j.expneurol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 75.Bonnamain V, Mathieux E, Thinard R, Thebault P, Nerriere-Daguin V, Leveque X, Anegon I, Vanhove B, Neveu I, Naveilhan P. Expression of heme oxygenase-1 in neural stem/progenitor cells as a potential mechanism to evade host immune response. Stem cells. 2012;30:2342–2353. doi: 10.1002/stem.1199. [DOI] [PubMed] [Google Scholar]
- 76.Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, Gower RM, Luo X, Shea LD. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue engineering. Part A. 2013;19:1465–1475. doi: 10.1089/ten.tea.2012.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marek N, Krzystyniak A, Ergenc I, Cochet O, Misawa R, Wang LJ, Golab K, Wang X, Kilimnik G, Hara M, Kizilel S, Trzonkowski P, Millis JM, Witkowski P. Coating human pancreatic islets with CD4(+)CD25(high)CD127(−) regulatory T cells as a novel approach for the local immunoprotection. Annals of surgery. 2011;254:512–518. doi: 10.1097/SLA.0b013e31822c9ca7. discussion 518–519. [DOI] [PubMed] [Google Scholar]
- 78.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 79.Plenter RJ, Grazia TJ, Nelson DP, Zamora MR, Gill RG, Pietra BA. Ectopic expression of Fas Ligand on cardiomyocytes renders cardiac allografts resistant to CD4(+) T-cell mediated rejection. Cellular immunology. 2015;293:30–33. doi: 10.1016/j.cellimm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearl-Yafe M, Yolcu ES, Stein J, Kaplan O, Yaniv I, Shirwan H, Askenasy N. Fas ligand enhances hematopoietic cell engraftment through abrogation of alloimmune responses and nonimmunogenic interactions. Stem cells. 2007;25:1448–1455. doi: 10.1634/stemcells.2007-0013. [DOI] [PubMed] [Google Scholar]
- 81.Kao WJ, Lee D, Schense JC, Hubbell JA. Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J Biomed Mater Res. 2001;55:79–88. doi: 10.1002/1097-4636(200104)55:1<79::aid-jbm110>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 82.Kao WJ, Liu Y. Utilizing biomimetic oligopeptides to probe fibronectin-integrin binding and signaling in regulating macrophage function in vitro and in vivo. Front Biosci. 2001;6:D992–D999. doi: 10.2741/kao. [DOI] [PubMed] [Google Scholar]
- 83.Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 84.Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014;171:3988–4000. doi: 10.1111/bph.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zustiak SP, Wei Y, Leach JB. Protein-hydrogel interactions in tissue engineering: mechanisms and applications. Tissue Eng Part B Rev. 2013;19:160–171. doi: 10.1089/ten.teb.2012.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim HP, Tey BT, Chan ES. Particle designs for the stabilization and controlled-delivery of protein drugs by biopolymers: a case study on insulin. J Control Release. 2014;186:11–21. doi: 10.1016/j.jconrel.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 87.Jiang Y, Chen J, Deng C, Suuronen EJ, Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Bischoff L, Alvarez S, Dai DL, Soukhatcheva G, Orban PC, Verchere CB. Cellular mechanisms of CCL22-mediated attenuation of autoimmune diabetes. Journal of immunology. 2015;194:3054–3064. doi: 10.4049/jimmunol.1400567. [DOI] [PubMed] [Google Scholar]
- 89.Chen T, Yuan J, Duncanson S, Hibert ML, Kodish BC, Mylavaganam G, Maker M, Li H, Sremac M, Santosuosso M, Forbes B, Kashiwagi S, Cao J, Lei J, Thomas M, Hartono C, Sachs D, Markmann J, Sambanis A, Poznansky MC. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:618–627. doi: 10.1111/ajt.13049. [DOI] [PubMed] [Google Scholar]
- 90.Oshikawa K, Yamasawa H, Sugiyama Y. Human lung fibroblasts inhibit macrophage inflammatory protein-1alpha production by lipopolysaccharide-stimulated macrophages. Biochem Biophys Res Commun. 2003;312:650–655. doi: 10.1016/j.bbrc.2003.10.166. [DOI] [PubMed] [Google Scholar]
- 91.Chung AS, Kao WJ. Fibroblasts regulate monocyte response to ECM-derived matrix: the effects on monocyte adhesion and the production of inflammatory, matrix remodeling, and growth factor proteins. J Biomed Mater Res A. 2009;89:841–853. doi: 10.1002/jbm.a.32431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Middaugh CR, Evans RK, Montgomery DL, Casimiro DR. Analysis of plasmid DNA from a pharmaceutical perspective. J Pharm Sci. 1998;87:130–146. doi: 10.1021/js970367a. [DOI] [PubMed] [Google Scholar]
- 95.Bajaj B, Lei P, Andreadis ST. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol Prog. 2001;17:587–596. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 96.Proctor RA. Fibronectin: a brief overview of its structure, function, and physiology. Rev Infect Dis. 1987;9(Suppl 4):S317–S321. doi: 10.1093/clinids/9.supplement_4.s317. [DOI] [PubMed] [Google Scholar]
- 97.Levy RJ, Song C, Tallapragada S, DeFelice S, Hinson JT, Vyavahare N, Connolly J, Ryan K, Li Q. Localized adenovirus gene delivery using antiviral IgG complexation. Gene Ther. 2001;8:659–667. doi: 10.1038/sj.gt.3301452. [DOI] [PubMed] [Google Scholar]
- 98.Segura T, Shea LD. Surface-tethered DNA complexes for enhanced gene delivery. Bioconjug Chem. 2002;13:621–629. doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 99.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holladay C, Power K, Sefton M, O'Brien T, Gallagher WM, Pandit A. Functionalized scaffold-mediated interleukin 10 gene delivery significantly improves survival rates of stem cells in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:969–978. doi: 10.1038/mt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holladay CA, Duffy AM, Chen X, Sefton MV, O'Brien TD, Pandit AS. Recovery of cardiac function mediated by MSC and interleukin-10 plasmid functionalised scaffold. Biomaterials. 2012;33:1303–1314. doi: 10.1016/j.biomaterials.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 102.Drukker M. Immunogenicity of embryonic stem cells and their progeny. Methods in enzymology. 2006;420:391–409. doi: 10.1016/S0076-6879(06)20019-3. [DOI] [PubMed] [Google Scholar]
- 103.Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barbeau DJ, La KT, Kim DS, Kerpedjieva SS, Shurin GV, Tamama K. Early growth response-2 signaling mediates immunomodulatory effects of human multipotential stromal cells. Stem cells and development. 2014;23:155–166. doi: 10.1089/scd.2013.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gharibi T, Ahmadi M, Seyfizadeh N, Jadidi-Niaragh F, Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cellular immunology. 2015;293:113–121. doi: 10.1016/j.cellimm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Kwon MS, Noh MY, Oh KW, Cho KA, Kang BY, Kim KS, Kim YS, Kim SH. The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. Journal of neurochemistry. 2014 doi: 10.1111/jnc.12814. [DOI] [PubMed] [Google Scholar]
- 107.Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, Dahlke MH. Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. Journal of immunology. 2014;193:4988–4999. doi: 10.4049/jimmunol.1401776. [DOI] [PubMed] [Google Scholar]
- 109.Yan Z, Zhuansun Y, Chen R, Li J, Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Experimental cell research. 2014;324:65–74. doi: 10.1016/j.yexcr.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 110.Gao L, Lu Q, Huang LJ, Ruan LH, Yang JJ, Huang WL, ZhuGe WS, Zhang YL, Fu B, Jin KL, ZhuGe QC. Transplanted neural stem cells modulate regulatory T, gammadelta T cells and corresponding cytokines after intracerebral hemorrhage in rats. International journal of molecular sciences. 2014;15:4431–4441. doi: 10.3390/ijms15034431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 112.Liu J, Gotherstrom C, Forsberg M, Samuelsson EB, Wu J, Calzarossa C, Hovatta O, Sundstrom E, Akesson E. Human neural stem/progenitor cells derived from embryonic stem cells and fetal nervous system present differences in immunogenicity and immunomodulatory potentials in vitro. Stem cell research. 2013;10:325–337. doi: 10.1016/j.scr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Reading JL, Yang JH, Sabbah S, Skowera A, Knight RR, Pinxteren J, Vaes B, Allsopp T, Ting AE, Busch S, Raber A, Deans R, Tree TI. Clinical-grade multipotent adult progenitor cells durably control pathogenic T cell responses in human models of transplantation and autoimmunity. Journal of immunology. 2013;190:4542–4552. doi: 10.4049/jimmunol.1202710. [DOI] [PubMed] [Google Scholar]
- 114.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature reviews. Immunology. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 115.Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 116.Melzi R, Antonioli B, Mercalli A, Battaglia M, Valle A, Pluchino S, Galli R, Sordi V, Bosi E, Martino G, Bonifacio E, Doglioni C, Piemonti L. Co-graft of allogeneic immune regulatory neural stem cells (NPC) and pancreatic islets mediates tolerance, while inducing NPC-derived tumors in mice. PloS one. 2010;5:e10357. doi: 10.1371/journal.pone.0010357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and-9. Diabetes. 2009;58:1797–1806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caridade M, Graca L, Ribeiro RM. Mechanisms Underlying CD4+ Treg Immune Regulation in the Adult: From Experiments to Models. Frontiers in immunology. 2013;4:378. doi: 10.3389/fimmu.2013.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, Shirwan H. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. Journal of immunology. 2011;187:5901–5909. doi: 10.4049/jimmunol.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Experimental neurology. 2014;258:121–129. doi: 10.1016/j.expneurol.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kil K, Zang YC, Yang D, Markowski J, Fuoco GS, Vendetti GC, Rivera VM, Zhang JZ. T cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98:201–207. doi: 10.1016/s0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 122.Ibarra A, Jimenez A, Cortes C, Correa D. Influence of the intensity, level and phase of spinal cord injury on the proliferation of T cells and T-cell-dependent antibody reactions in rats. Spinal Cord. 2007;45:380–386. doi: 10.1038/sj.sc.3101972. [DOI] [PubMed] [Google Scholar]
- 123.Bromberg JS, Fricke WF, Brinkman CC, Simon T, Mongodin EF. Microbiota-implications for immunity and transplantation. Nat Rev Nephrol. 2015;11:342–353. doi: 10.1038/nrneph.2015.70. [DOI] [PubMed] [Google Scholar]
- 124.Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC. Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Experimental neurology. 2008;214:147–159. doi: 10.1016/j.expneurol.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 125.Howard LM, Miga AJ, Vanderlugt CL, Dal Canto MC, Laman JD, Noelle RJ, Miller SD. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. The Journal of clinical investigation. 1999;103:281–290. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Im SH, Barchan D, Maiti PK, Fuchs S, Souroujon MC. Blockade of CD40 ligand suppresses chronic experimental myasthenia gravis by down-regulation of Th1 differentiation and up-regulation of CTLA-4. Journal of immunology. 2001;166:6893–6898. doi: 10.4049/jimmunol.166.11.6893. [DOI] [PubMed] [Google Scholar]
- 127.Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Experimental neurology. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 128.Guerrero AR, Uchida K, Nakajima H, Watanabe S, Nakamura M, Johnson WE, Baba H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. Journal of neuroinflammation. 2012;9:40. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Im SH, Barchan D, Maiti PK, Raveh L, Souroujon MC, Fuchs S. Suppression of experimental myasthenia gravis, a B cell-mediated autoimmune disease, by blockade of IL-18. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:2140–2148. doi: 10.1096/fj.01-0072com. [DOI] [PubMed] [Google Scholar]
- 130.Israel-Assayag E, Fournier M, Cormier Y. Blockade of T cell costimulation by CTLA4-Ig inhibits lung inflammation in murine hypersensitivity pneumonitis. Journal of immunology. 1999;163:6794–6799. [PubMed] [Google Scholar]
- 131.Tang A, Judge TA, Nickoloff BJ, Turka LA. Suppression of murine allergic contact dermatitis by CTLA4Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. Journal of immunology. 1996;157:117–125. [PubMed] [Google Scholar]
- 132.Meister M, Papatriantafyllou M, Nordstrom V, Kumar V, Ludwig J, Lui KO, Boyd AS, Popovic ZV, Fleming TH, Moldenhauer G, Nawroth PP, Grone HJ, Waldmann H, Oelert T, Arnold B. Dickkopf-3, a tissue-derived modulator of local T-cell responses. Frontiers in immunology. 2015;6:78. doi: 10.3389/fimmu.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yuan R, Maeda Y, Li W, Lu W, Cook S, Dowling P. Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PloS one. 2008;3:e1924. doi: 10.1371/journal.pone.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chu H, Tang Y, Dong Q. Protection of granulocyte-colony stimulating factor to hemorrhagic brain injuries and its involved mechanisms: effects of vascular endothelial growth factor and aquaporin-4. Neuroscience. 2014;260:59–72. doi: 10.1016/j.neuroscience.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 135.Rossetti M, Gregori S, Roncarolo MG. Granulocyte-colony stimulating factor drives the in vitro differentiation of human dendritic cells that induce anergy in naive T cells. European journal of immunology. 2010;40:3097–3106. doi: 10.1002/eji.201040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muller II, Chatterjee M, Schneider M, Borst O, Seizer P, Schonberger T, Vogel S, Muller KA, Geisler T, Lang F, Langer H, Gawaz M. Gremlin-1 inhibits macrophage migration inhibitory factor-dependent monocyte function and survival. International journal of cardiology. 2014;176:923–929. doi: 10.1016/j.ijcard.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 137.Benkhoucha M, Santiago-Raber ML, Schneiter G, Chofflon M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu J, Zhou X, Zhan Z, Meng Q, Han Y, Shi Q, Tang J, Li J, Fan H, Liu Z. IL-25 regulates the polarization of macrophages and attenuates obliterative bronchiolitis in murine trachea transplantation models. International immunopharmacology. 2015;25:383–392. doi: 10.1016/j.intimp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 139.Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nature medicine. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- 140.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, Turnquist HR. IL-33 is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. Journal of immunology. 2014;193:4010–4020. doi: 10.4049/jimmunol.1400481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pomeshchik Y, Kidin I, Korhonen P, Savchenko E, Jaronen M, Lehtonen S, Wojciechowski S, Kanninen K, Koistinaho J, Malm T. Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain, behavior, and immunity. 2015;44:68–81. doi: 10.1016/j.bbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 143.Ifergan I, Wosik K, Cayrol R, Kebir H, Auger C, Bernard M, Bouthillier A, Moumdjian R, Duquette P, Prat A. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol. 2006;60:45–55. doi: 10.1002/ana.20875. [DOI] [PubMed] [Google Scholar]
- 144.Casiraghi F, Azzollini N, Todeschini M, Cavinato RA, Cassis P, Solini S, Rota C, Morigi M, Introna M, Maranta R, Perico N, Remuzzi G, Noris M. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:2373–2383. doi: 10.1111/j.1600-6143.2012.04115.x. [DOI] [PubMed] [Google Scholar]
- 145.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baas MC, Kuhn C, Valette F, Mangez C, Duarte MS, Hill M, Besancon A, Chatenoud L, Cuturi MC, You S. Combining autologous dendritic cell therapy with CD3 antibodies promotes regulatory T cells and permanent islet allograft acceptance. Journal of immunology. 2014;193:4696–4703. doi: 10.4049/jimmunol.1401423. [DOI] [PubMed] [Google Scholar]
- 147.Bryant J, Lerret NM, Wang JJ, Kang HK, Tasch J, Zhang Z, Luo X. Preemptive donor apoptotic cell infusions induce IFN-gamma-producing myeloid-derived suppressor cells for cardiac allograft protection. Journal of immunology. 2014;192:6092–6101. doi: 10.4049/jimmunol.1302771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, Houlihan JL, Miller SD, Zhang ZJ, Luo X. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. Journal of immunology. 2012;189:804–812. doi: 10.4049/jimmunol.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang S, Zhang X, Zhang L, Bryant J, Kheradmand T, Hering BJ, Miller SD, Luo X. Preemptive Tolerogenic Delivery of Donor Antigens for Permanent Allogeneic Islet Graft Protection. Cell transplantation. 2014 doi: 10.3727/096368914X681027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD. Tolerance induced by apoptotic antigencoupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. Journal of immunology. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E60–E68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kuhn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJ. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Science translational medicine. 2014;6:219ra217. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Getts DRK, C NJ. IMMUNE-MODIFYING PARTICLES FOR THE TREATMENT OF INFLAMMATION. United States: Cour Pharmaceutical Development Company; 2013. [Google Scholar]
- 155.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZH, Yap WT, Getts MT, Pleiss M, Luo X, King NJC, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nature Biotechnology. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kanno S, Furuyama A, Hirano S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicological sciences : an official journal of the Society of Toxicology. 2007;97:398–406. doi: 10.1093/toxsci/kfm050. [DOI] [PubMed] [Google Scholar]
- 157.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O'Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Getts DR. Editorial to special issue: monocytes in homeostasis and disease. Cellular immunology. 2014;291:1–2. doi: 10.1016/j.cellimm.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 159.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. The Journal of experimental medicine. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Deffrasnes C, Ashhurst TM, Radford J, Hofer M, Thomas S, Campbell IL, King NJ. Targeted blockade in lethal West Nile virus encephalitis indicates a crucial role for very late antigen (VLA)-4-dependent recruitment of nitric oxide-producing macrophages. Journal of neuroinflammation. 2012;9:246. doi: 10.1186/1742-2094-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saxena T, Loomis KH, Pai SB, Karumbaiah L, Gaupp E, Patil K, Patkar R, Bellamkonda RV. Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury. ACS nano. 2015;9:1492–1505. doi: 10.1021/nn505980z. [DOI] [PubMed] [Google Scholar]
- 163.Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, Harris RA, Magnusson PU, Brittebo E, Loskog AS. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. Journal of neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Haanstra KG, Dijkman K, Bashir N, Bauer J, Mary C, Poirier N, Baker P, Scobie L, t Hart BA, Vanhove B. Selective blockade of CD28-mediated T cell costimulation protects rhesus monkeys against acute fatal experimental autoimmune encephalomyelitis. Journal of immunology. 2015;194:1454–1466. doi: 10.4049/jimmunol.1402563. [DOI] [PubMed] [Google Scholar]
- 165.Szot GL, Yadav M, Lang J, Kroon E, Kerr J, Kadoya K, Brandon EP, Baetge EE, Bour-Jordan H, Bluestone JA. Tolerance induction and reversal of diabetes in mice transplanted with human embryonic stem cell-derived pancreatic endoderm. Cell stem cell. 2015;16:148–157. doi: 10.1016/j.stem.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 166.Krinzman SJ, De Sanctis GT, Cernadas M, Mark D, Wang Y, Listman J, Kobzik L, Donovan C, Nassr K, Katona I, Christiani DC, Perkins DL, Finn PW. Inhibition of T cell costimulation abrogates airway hyperresponsiveness in a murine model. The Journal of clinical investigation. 1996;98:2693–2699. doi: 10.1172/JCI119093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kheradmand T, Wang S, Gibly RF, Zhang X, Holland S, Tasch J, Graham JG, Kaufman DB, Miller SD, Shea LD, Luo X. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials. 2011;32:4517–4524. doi: 10.1016/j.biomaterials.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang S, Tasch J, Kheradmand T, Ulaszek J, Ely S, Zhang X, Hering BJ, Miller SD, Luo X. Transient B-cell depletion combined with apoptotic donor splenocytes induces xeno-specific T- and B-cell tolerance to islet xenografts. Diabetes. 2013;62:3143–3150. doi: 10.2337/db12-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen G, Kheradmand T, Bryant J, Wang S, Tasch J, Wang JJ, Zhang Z, Luo X. Intragraft CD11b(+) IDO(+) cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:2920–2929. doi: 10.1111/j.1600-6143.2012.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Frontiers in immunology. 2015;6:61. doi: 10.3389/fimmu.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Podojil JR, Miller SD. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunological reviews. 2009;229:337–355. doi: 10.1111/j.1600-065X.2009.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Prasad S, Kohm AP, McMahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9–23 epitope and involves functional epitope spreading. J Autoimmun. 2012;39:347–353. doi: 10.1016/j.jaut.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lutterotti A, Yousef S, Sputtek A, Sturner KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, Schippling S, Miller SD, Sospedra M, Martin R. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Science translational medicine. 2013;5:188ra175. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell transplantation. 2013;22:2267–2277. doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 177.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS nano. 2014 doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vredent C, Ashhurst TM, Chami B, McCarthy DP, Wu H, Ma J, Martin A, Wu B, Kansas GS, Kuhn J, Hafezi W, Campbell IL, Shea LD, Reilly D, Say J, Brown L, White MY, Witting P, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJC. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 2014;6:219ra217. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Agemy L, Sugahara KN, Kotamraju VR, Gujraty K, Girard OM, Kono Y, Mattrey RF, Park JH, Sailor MJ, Jimenez AI, Cativiela C, Zanuy D, Sayago FJ, Aleman C, Nussinov R, Ruoslahti E. Nanoparticle-induced vascular blockade in human prostate cancer. Blood. 2010;116:2847–2856. doi: 10.1182/blood-2010-03-274258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gu L, Ruff LE, Qin Z, Corr M, Hedrick SM, Sailor MJ. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Advanced materials. 2012;24:3981–3987. doi: 10.1002/adma.201200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.von Maltzahn G, Park JH, Lin KY, Singh N, Schwoppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nanoparticles that communicate in vivo to amplify tumour targeting. Nature materials. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]